Looking Beyond Lithium for Breakthroughs in Magnesium-Ion Batteries as Sustainable Solutions
Abstract
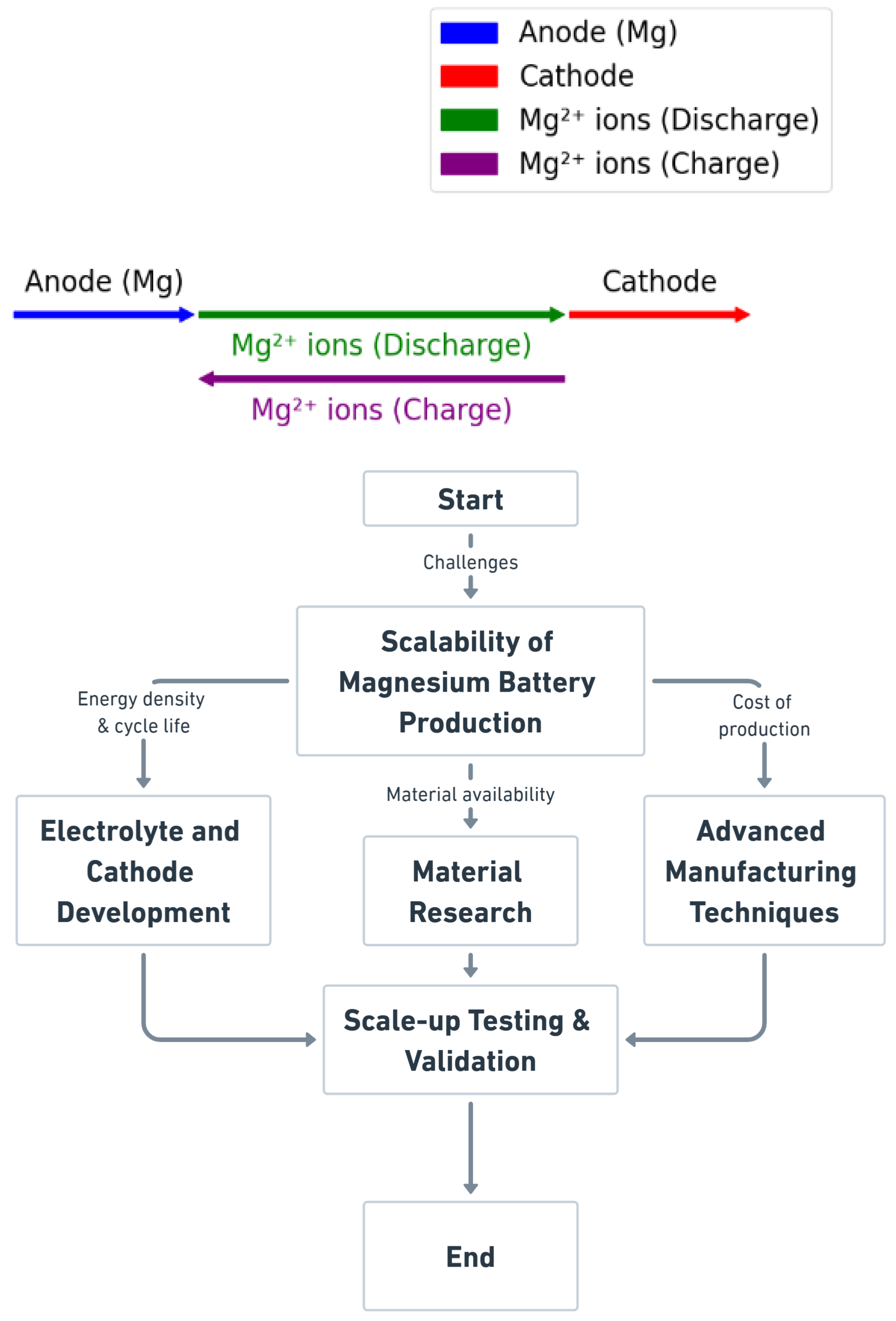
1. Introduction
2. Methodology
2.1. Simulation Models for Ion Diffusion Kinetics
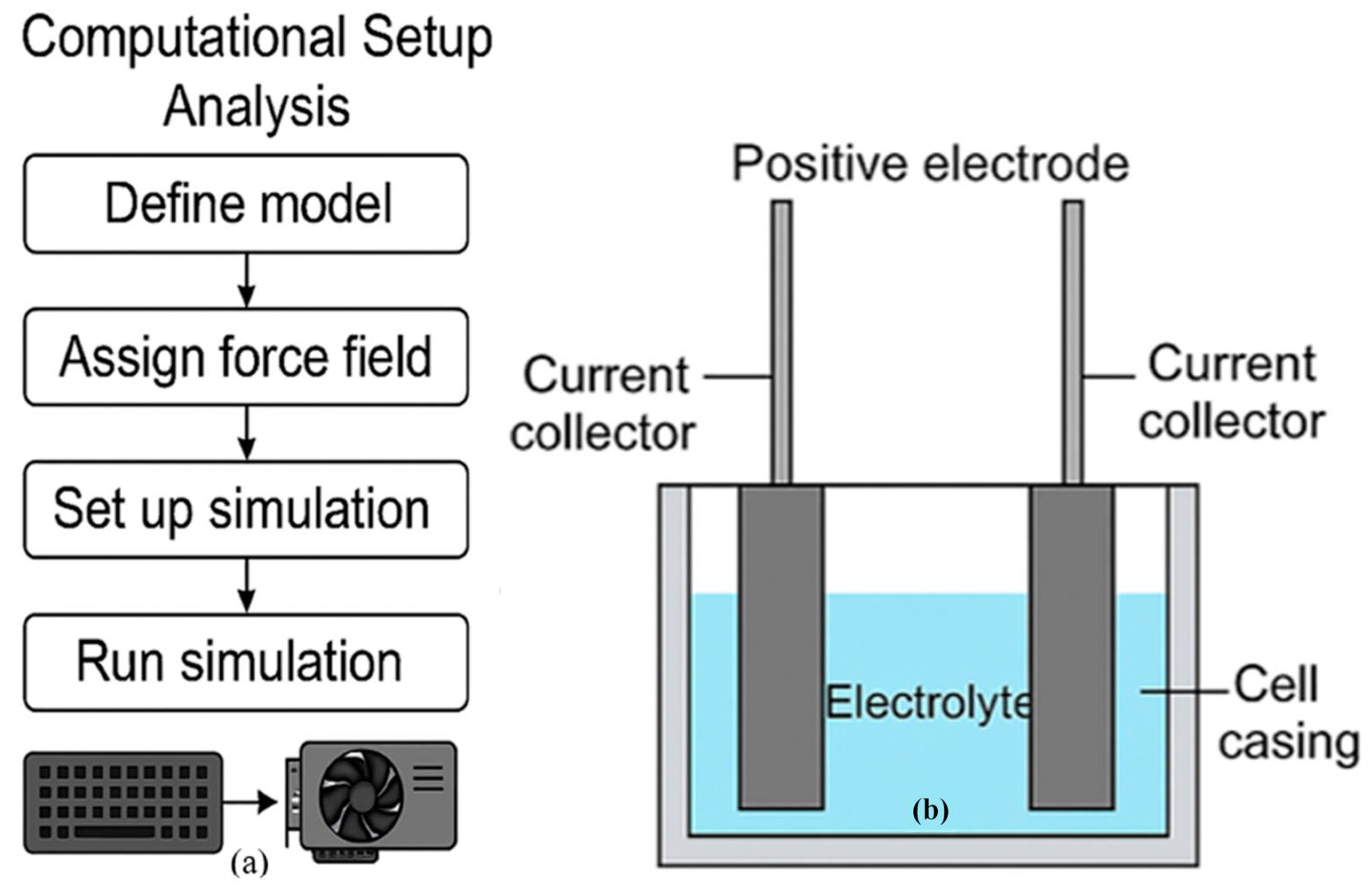
2.2. Computational Test Setup Analysis
2.3. Computational Electrode and Electrolyte Interactions
2.4. Data Analysis
2.5. Mathematical Model
3. Results and Discussion
3.1. Energy Density and Specific Capacity
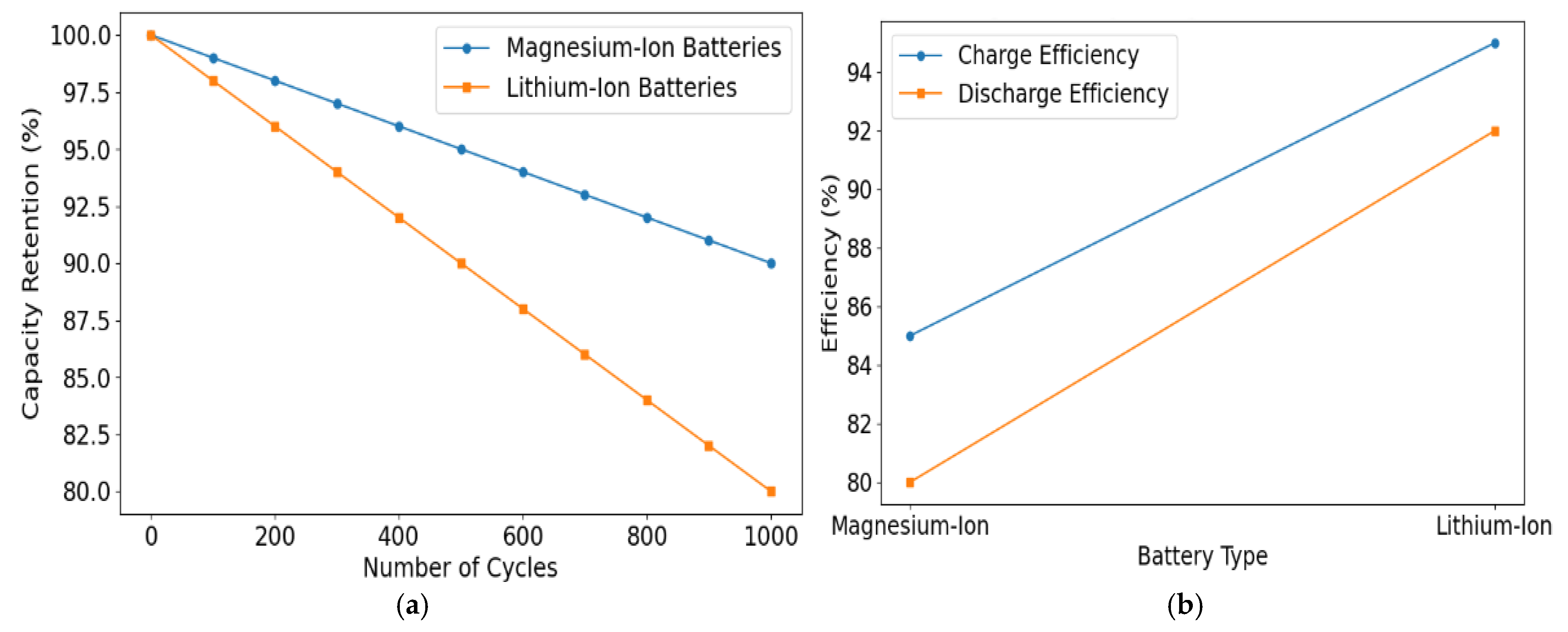
3.2. Cycle Stability
3.3. Charge–Discharge Efficiency
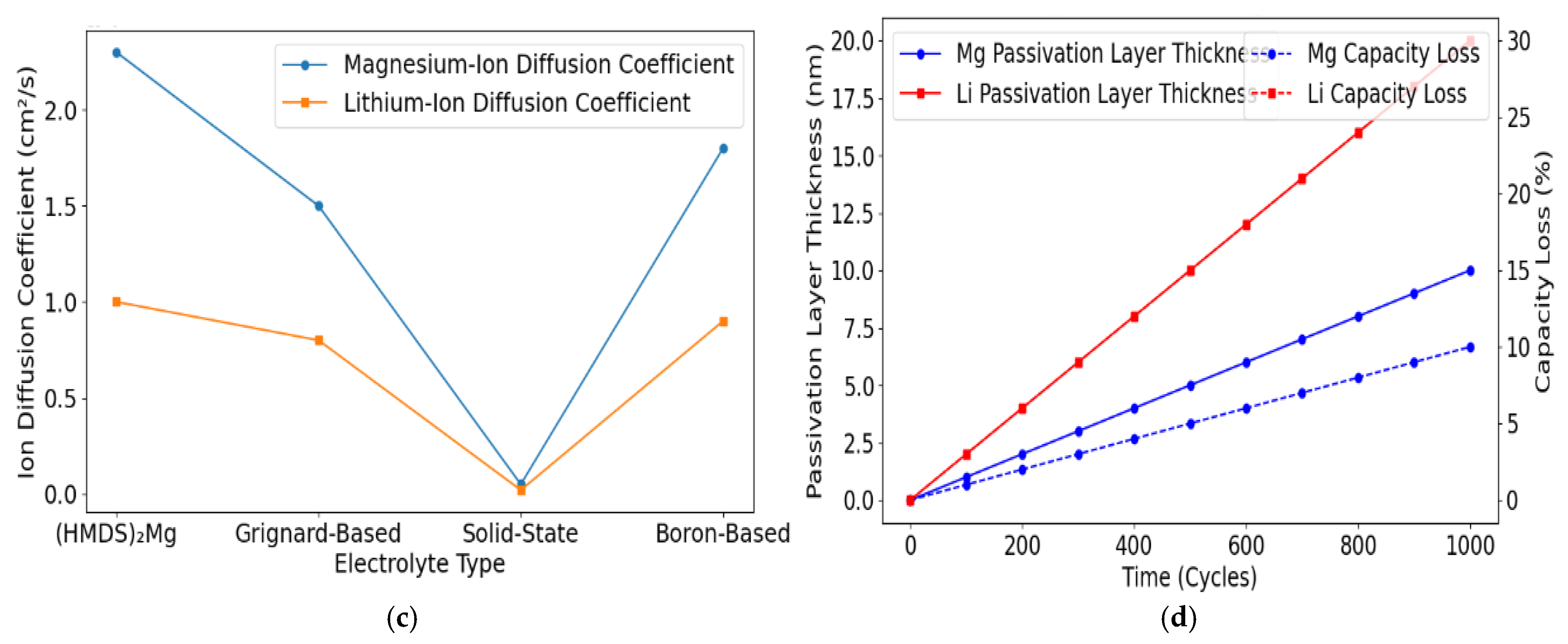
3.4. Ion Diffusion and Passivation Layers
3.5. Comparative Discussion of Magnesium-Ion and Lithium-Ion Batteries
3.6. Future Directions and Improvements
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhao, Z.; Li, J.; Li, Y. Advances in Magnesium-Ion Batteries: Materials, Challenges, and Opportunities. Adv. Energy Mater. 2024, 14, 2300410. [Google Scholar] [CrossRef]
- Jiang, Z.; Tang, H.; Wang, S. High-Performance Magnesium-Ion Batteries: A Review of Cathode and Anode Materials. Nano Energy 2024, 99, 107436. [Google Scholar]
- Zeng, T.; Meng, L.; Cheng, L.; Wang, R.; Ran, Z.Y.; Liu, D.D.; Fu, J.; He, J.L.; Zhou, Q.; Li, Q.; et al. Scalable Hybrid Films of Polyimide-Animated Quantum Dots for High-Temperature Capacitive Energy Storage Utilising Quantum Confinement Effect. Adv. Funct. Mater. 2024, 2419278. [Google Scholar] [CrossRef]
- Yang, N.; Li, H.; Lin, X.; Georgiadou, S.; Hong, L.; Wang, Z.; He, F.; Qi, Z.F.; Lin, W.F. Catalytic electrode comprising a gas diffusion layer and bubble-involved mass transfer in anion exchange membrane water electrolysis: A critical review and perspectives. J. Energy Chem. 2025, 105, 669–701. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, Z.; Xu, Y. New Strategies to Enhance the Performance of Magnesium-Ion Batteries. Energy Storage Mater. 2023, 38, 115412. [Google Scholar]
- Zhang, L.; Chen, Y.; Zhang, W. Dendrite-Free Magnesium-Ion Batteries: Strategies and Prospects. Nat. Mater. 2024, 23, 47–58. [Google Scholar]
- Wang, C.; Chen, Y. Unsupervised dynamic prognostics for abnormal degradation of lithium-ion battery. Appl. Energy 2024, 365, 123280. [Google Scholar] [CrossRef]
- Xie, J.; Zhou, X.; Wang, W. Magnesium-Ion Batteries: From Fundamentals to Practical Applications. Energy Environ. Mater. 2023, 7, 2111–2123. [Google Scholar] [CrossRef]
- Cheng, T.; Liu, Q.; Jiang, G.J.; Yang, B.C.; Wang, X.Y.; Wang, P.K. Numerical study of proton exchange membrane fuel cells with airfoil cross flow field. J. Power Sources 2025, 631, 236232. [Google Scholar] [CrossRef]
- Liu, G.; Zhang, Y.; Zhang, X. High-Energy Density Magnesium-Ion Batteries: Materials, Mechanisms, and Strategies. Nat. Commun. 2023, 14, 2567. [Google Scholar]
- He, Y.S.; Wang, F.P.; Du, G.Q.; Pan, L.; Li, J.; Yang, H.M.; Zhang, X.; Zhang, Z.C.; Wang, K.Z. Unveiling first self-healing in metallised film capacitor: A macro–micro analysis. High Voltage 2025. [Google Scholar] [CrossRef]
- Wang, X.; Song, X.; Liu, M. High-Performance Magnesium-Sulfur Batteries: Progress, Challenges, and Opportunities. Chem. Eng. J. 2023, 450, 138109. [Google Scholar]
- Xia, J.; Gao, L.; Cao, M.L.; Zhang, C.K.; Tan, M.S.; Wang, Q.; Lv, F.; Tao, L. Mn incorporated BiOCl anode for high performance sodium ion batteries. Appl. Surf. Sci. 2025, 695, 162888. [Google Scholar] [CrossRef]
- Luo, L.; Wang, Y.; Liu, X. Magnesium-Ion Batteries: A Review on Recent Advances in Electrode Materials. J. Mater. Chem. A 2023, 11, 1320–1345. [Google Scholar]
- Liu, C.L.; Li, Z.J.; Jiang, L.L.; Zhu, H.; Wang, F.C.; Sheng, L.Z. Dipole-dipole interactions in electrolyte to facilitate Li-ion desolvation for low-temperature Li-ion batteries. J. Energy Chem. 2025, 104, 678–686. [Google Scholar] [CrossRef]
- Liu, H.; Zhang, J.; Li, X. Magnesium-Ion Battery Materials: A New Class of Electrodes for High-Capacity Energy Storage. Adv. Mater. 2023, 35, 2206820. [Google Scholar]
- Gao, H.; Wang, B. A first-principles study on structural stability and magnetoelectric coupling of two-dimensional BaTiO3 ultrathin film with Cr and Cu substituting Ti site. APL Mater. 2024, 12, 091117. [Google Scholar] [CrossRef]
- Guo, S.; Liu, Y.; Chen, G. Development of Solid-State Electrolytes for Magnesium-Ion Batteries: A Review. Solid State Ion. 2023, 373, 115703. [Google Scholar]
- Gao, L.; Ma, Y.; Zhang, C.; Cao, M. Nitrogen-doped carbon trapped MnMoO4 microrods toward high performance aqueous zinc-ion battery. J. Alloys Compd. 2023, 968, 172008. [Google Scholar] [CrossRef]
- Chen, Z.; Zhang, Z.; Li, C. Progress and Challenges in Magnesium-Ion Battery Electrolytes. J. Mater. Sci. 2023, 58, 4157–4174. [Google Scholar]
- Gao, L.; Hu, H.; Zhang, C.; Cao, M. Gallium regulated MnO2 toward high performance Zn ion batteries. Vacuum 2024, 219, 112671. [Google Scholar] [CrossRef]
- Cheng, X.; Li, J.; Tang, Y. Optimisation of Magnesium-Ion Battery Electrodes for High Energy and Long Cycle Life. Electrochim. Acta 2023, 438, 141181. [Google Scholar]
- Cao, M.L.; Chen, W.; Ma, Y.N.; Huang, H.M.; Luo, S.J.; Zhang, C.K. Cross-linked K2Ti4O9 nanoribbon arrays with superior rate capability and cyclability for lithium-ion batteries. Mater. Lett. 2020, 279, 128495. [Google Scholar] [CrossRef]
- Zhao, H.; Xie, J.; Liu, Y. Review on the Stability and Safety of Magnesium-Ion Batteries for Electric Vehicle Applications. Mater. Res. Lett. 2023, 11, 45–55. [Google Scholar]
- Chen, X.Z.; Wei, S.H.; Wang, J.Z.; Tong, F.L.; Söhnel, T.L.; Waterhouse, G.I.N.; Zhang, W.; Kennedy, J.; Taylor, M.P. Lithium insertion/extraction mechanism in Mg2Sn anode for lithium-ion batteries. Intermetallics 2024, 169, 108306. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q.; Li, J. New Cathode Materials for Magnesium-Ion Batteries. J. Electrochem. Soc. 2023, 170. [Google Scholar]
- Jin, Y.L.; Zhao, Z.R.; Ren, P.G.; Zhang, B.F.; Chen, Z.Y.; Guo, Z.Z.; Ren, F.; Sun, Z.F.; Liu, S.H.; Song, P.; et al. Recent Advances in Oxygen Redox Activity of Lithium-Rich Manganese-Based Layered Oxides Cathode Materials: Mechanism, Challenges and Strategies. Adv. Energy Mater. 2024, 14, 2402061. [Google Scholar] [CrossRef]
- He, J.; Yu, S.; Liao, S. Review of Magnesium-Ion Batteries for Electric Vehicle Applications: Challenges and Opportunities. Renew. Sustain. Energy Rev. 2023, 161, 112394. [Google Scholar] [CrossRef]
- Zhu, S.; Wu, S.; Fu, Y.; Guo, S. Prediction of particle-reinforced composite material properties based on an improved Halpin–Tsai model. AIP Adv. 2024, 14, 045339. [Google Scholar] [CrossRef]
- Zhang, S.; Wei, F.; Wang, Y. Magnesium-Ion Battery Technology: Challenges and Future Prospects for Sustainable Energy Storage. Environ. Sci. Technol. 2023, 57, 3210–3221. [Google Scholar]
- He, L.X.; Gao, Y.K.; Liu, D.; Hu, Y.X.; Shi, J.X.; Zhang, J.Y.; Li, X.Y.; Jin, B.; Zhang, B.F.; Wang, Z.L.; et al. Dynamic interfacial electrostatic energy harvesting via a single wire. Sci. Adv. 2024, 10, eado5362. [Google Scholar] [CrossRef]
- Wang, H.; Ma, J.; Liu, Q. Magnesium-Ion Batteries: A Comprehensive Review on Materials, Mechanisms, and Challenges. Mater. Today 2023, 16, 670–687. [Google Scholar] [CrossRef]
- Zhan, Y.; Ren, X.; Zhao, S.; Guo, Z. Enhancing prediction of electron affinity and ionisation energy in liquid organic electrolytes for lithium-ion batteries using machine learning. J. Power Sources 2025, 629, 235992. [Google Scholar] [CrossRef]
- Yu, Z.; Zhang, J.; Jiang, X. Advanced Materials for Magnesium-Ion Batteries: From Basic Understanding to Practical Applications. Small Methods 2023, 7, 2201133. [Google Scholar]
- Shen, Y.; Lu, Q.; Li, Y. Design Criterion and Analysis of Hybrid-Excited Vernier Reluctance Linear Machine with Slot Halbach PM Arrays. IEEE Trans. Ind. Electron. 2023, 70, 5074–5084. [Google Scholar] [CrossRef]
- Wu, Y.; Liu, W.; Li, D. Performance and Stability of Magnesium-Ion Batteries with Novel Anode Materials. Nat. Commun. 2023, 14, 3120. [Google Scholar]
- Shen, Y.; Li, Z.; Zeng, Z.; Lu, Q.; Lee, C.H.T. Quantitative Analysis of Asymmetric Flux Reversal Permanent Magnet Linear Machine for Long Excursion Application. IEEE Trans. Ind. Electron. 2024, 71, 12781–12792. [Google Scholar] [CrossRef]
- Xia, W.; Jiang, Z.; Zhang, S. Magnesium-Ion Batteries: Materials, Technologies, and Applications in the EV Market. Renew. Sustain. Energy Rev. 2023, 176, 113013. [Google Scholar]
- Zhang, Y.X.; Liu, Z.Y.; Wang, J.H.; Du, H.; Sun, Q.; Gao, R.T.; Xu, Z.M. Efficient and high-selective lithium extraction from waste LiMn2O4 batteries by synergetic pyrolysis with polyvinyl chloride. Waste Manag. 2025, 198, 95–105. [Google Scholar] [CrossRef]
- Liu, Z.; He, X.; Wang, F. Electrode Materials for Magnesium-Ion Batteries: Recent Advances and Challenges. Energy Environ. Sci. 2023, 16, 2567–2579. [Google Scholar] [CrossRef]
- Xu, Q.; Yu, J.; Zhang, W. Magnesium-Ion Battery Technology for EVs: The Current State and Challenges. Environ. Sci. Technol. Lett. 2023, 10, 411–420. [Google Scholar] [CrossRef]
- Jiang, X.; Wang, F.; Zhou, Z. Exploring New Electrolytes for Magnesium-Ion Batteries. Electrochem. Commun. 2023, 143, 107092. [Google Scholar]
- Li, Z.; Cheng, Y.; Liu, P. The Role of Solid-State Electrolytes in Magnesium-Ion Battery Performance. Solid State Ion. 2023, 376, 114862. [Google Scholar]
- Yu, Y.; Tang, Z.; Zhang, X. Enhancing the Performance of Magnesium-Ion Batteries Through Advanced Cathode Engineering. Energy J. 2023, 10, 3150–3162. [Google Scholar]
- Yang, X.; Zhang, Y.; Wu, J. Electrochemical Properties of Magnesium-Ion Batteries and Their Implications for Electric Vehicles. J. Mater. Chem. A 2023, 11, 100–110. [Google Scholar]
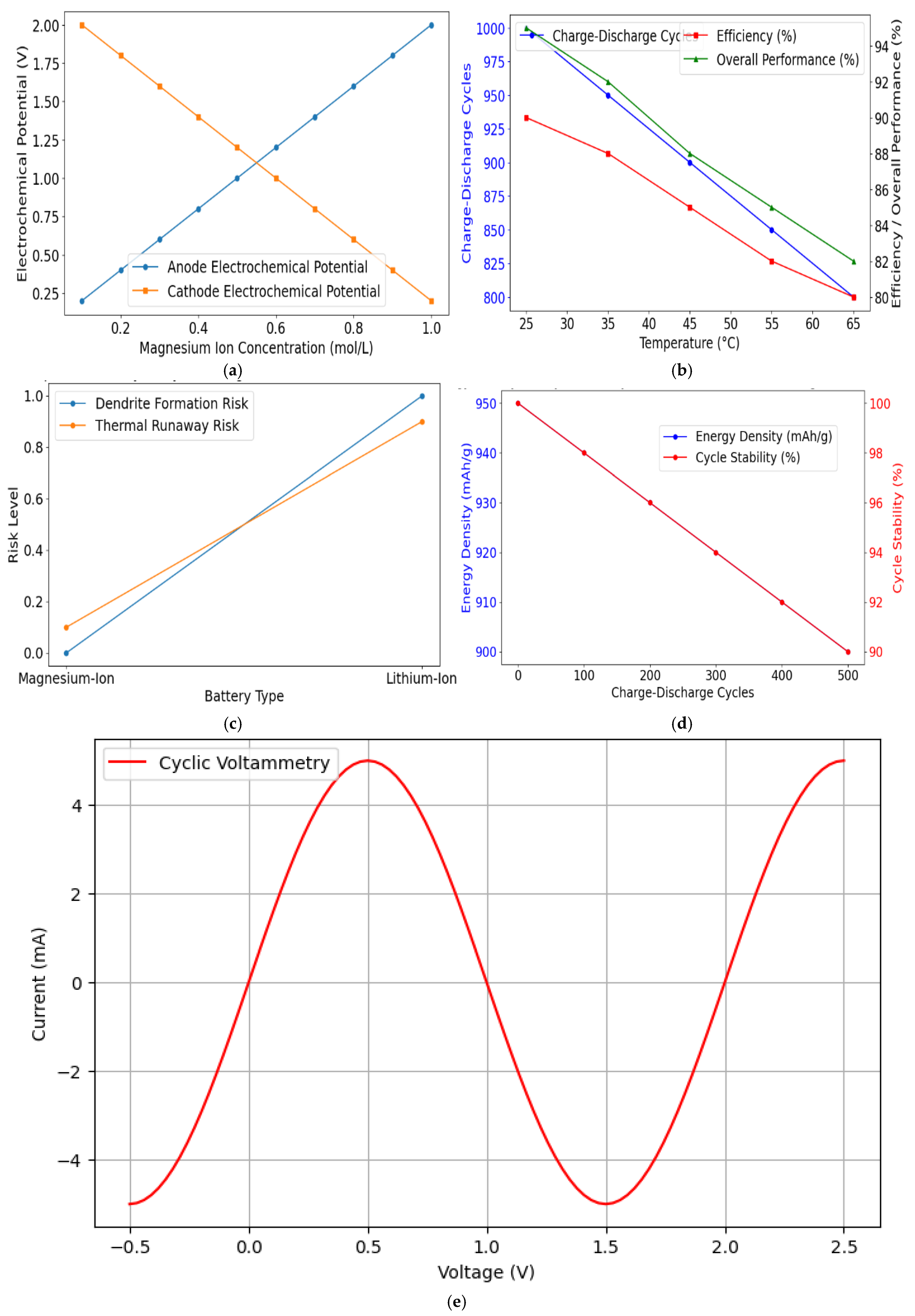
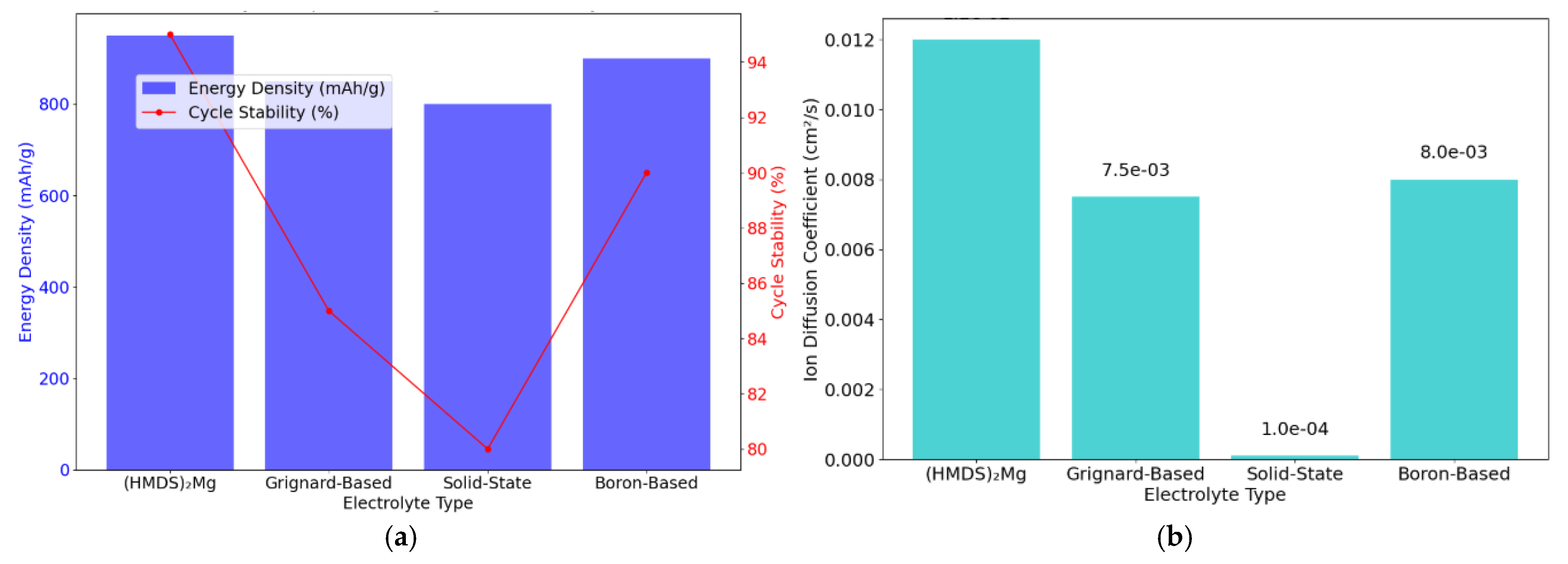
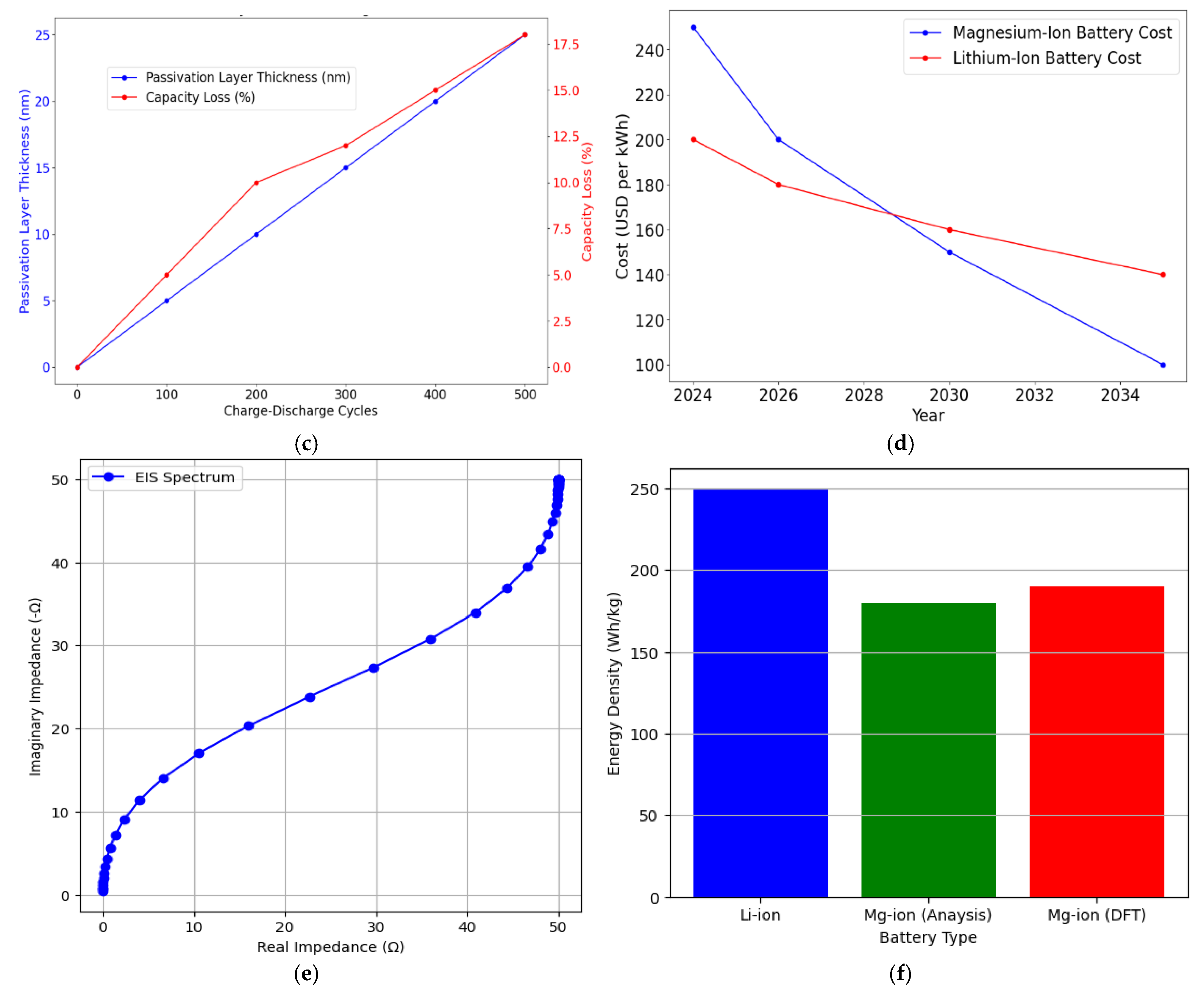
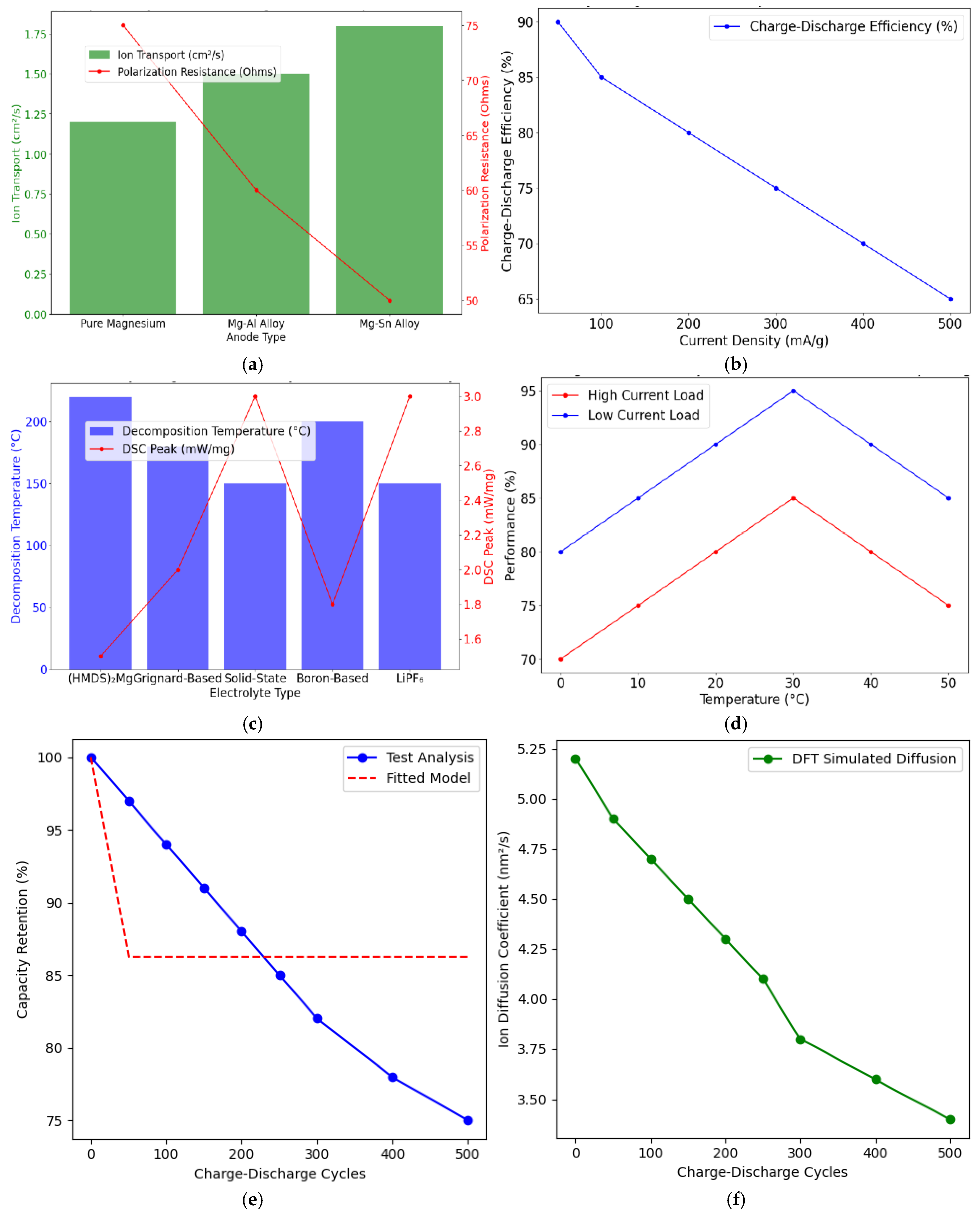
| Parameter | Value/Condition for Computation |
|---|---|
| Charge/Discharge Current Rates (C-rates) | 0.1 C, 0.5 C, 1 C, 2 C |
| Charge Cutoff Voltage | 2.5 V vs. Mg/Mg2+ |
| Discharge Cutoff Voltage | 0.1 V vs. Mg/Mg2+ |
| Electrolytes Tested | HMDS2 Mg, Grignard-based, solid-state, boron-based |
| Temperature Range | 25 ± 2 °C |
| Electrode Configuration | Mg anode/sulphur–graphene cathode |
| Separator | Glass fibre/polyolefin |
| Electrolyte Concentration | 0.3 M–0.8 M |
| Test Equipment | LAND CT2001A Battery Tester |
| Cycle Life Evaluation | 500 cycles at 0.5 C |
| Rest Time Between Cycles | 10 min |
| Electrochemical Impedance Spectroscopy (EIS) | Before and after cycling |
| Electrolyte types were tested. | |
| Electrolyte Type | Composition |
| HMDS2 Mg-based | 0.4 M HMDS2Mg in THF |
| Grignard-based | 0.5 M EtMgCl in diglyme |
| Solid-state | Mg10(SiP)2 (solid) |
| Boron-based | 0.6 M Mg(BH4)2 in tetraglyme |
| Charge–Discharge Testing | |
| Parameter | Condition |
| Charge/Discharge Rates (C-rates) | 0.1 C, 0.5 C, 1 C, 2 C |
| Voltage Window | 0.1 V–2.5 V (vs. Mg/Mg2+) |
| Testing Temperature | 25 ± 2 °C |
| Cycling Protocol | 500 cycles at 0.5 C |
| Rest Time Between Cycles | 10 min |
| Summary of Computational Test Enhancements | |
| Aspect | Improvement Implemented |
| Anode Preparation | Alloying with Al, Sn for better conductivity |
| Cathode Processing | Sulphur–graphene composite for stability |
| Electrolyte Optimisation | Evaluated 4 formulations for best conductivity |
| Charge–Discharge Parameters | Standardised rates and cycling conditions |
| Battery Type | Capacity Retention After 100 Cycles (%) | Capacity Retention After 500 Cycles (%) | Capacity Retention After 1000 Cycles (%) | Notes |
|---|---|---|---|---|
| Magnesium-Ion | 95 | 90 | 88 | Minimal capacity degradation, no dendrite formation |
| Lithium-Ion | 98 | 85 | 80 | Prone to dendrite formation and short circuits |
| Performance Metrics of Sulphur-Based Cathodes in Magnesium-Ion Batteries | ||||
| Cathode Type | Energy Density (mAh/g) | Retention 500 Cycles (%) | Charge Efficiency (%) | Notes |
| Sulphur-Based (Pure) | 800 | 85 | 88 | High capacity, moderate cycle stability |
| Graphene–Sulphur Composite | 950 | 92 | 91 | Enhanced conductivity and stability |
| Polyanionic–Sulphur Composite | 900 | 90 | 89 | Good balance of energy density and efficiency |
| Passivation Layer Thickness and Its Effect on Capacity Loss Over Time | ||||
| Cycling Interval (Cycles) | Passivation Thickness (nm) | Capacity Loss (%) | Electrolyte System | Notes |
| 100 Cycles | 5.0 | 10 | Grignard-Based | Moderate passivation effects |
| 500 Cycles | 12.0 | 18 | (HMDS)2 Mg | Lower passivation with an advanced electrolyte |
| 1000 Cycles | 20.0 | 25 | Solid-State Electrolyte | High stability but reduced ion transport |
| Summary of Electrochemical Testing Results for Different Magnesium-Ion Battery Configurations | ||||
| Configuration | Energy Density (mAh/g) | Cycle Stability (500 Cycles, %) | Charge Efficiency (%) | Notes |
| Mg Anode + Sulphur Cathode | 850 | 90 | 88 | High energy density, moderate cycle stability |
| Mg Alloy Anode + Polyanionic Cathode | 900 | 92 | 90 | Improved performance with alloy-based anode |
| Mg Anode + Graphene–Sulphur Cathode | 950 | 95 | 91 | Enhanced conductivity and stability |
| Impact of Temperature on Magnesium-Ion Battery Performance | ||||
| Temperature (°C) | Energy Density (mAh/g) | Cycle Stability (500 Cycles, %) | Charge–discharge efficiency (%) | Notes |
| 25 °C | 900 | 92 | 89 | The optimal temperature for performance |
| 45 °C | 850 | 85 | 86 | There is a slight degradation in stability |
| 65 °C | 800 | 80 | 82 | Higher temperature impacts stability |
| Electrolyte Type | Decomposition Temperature (°C) | DSC Peak (mW/mg) | Stability Notes |
|---|---|---|---|
| Mg (HMDS)2 Electrolyte | 220 | 1.5 | High thermal stability, low reactivity |
| Grignard-Based Electrolyte | 180 | 2.0 | Moderate stability, prone to reactivity |
| LiPF6 Electrolyte | 150 | 3.0 | Lower thermal stability |
| Comparison of Ion Transfer Resistance and Charge Transfer Resistance for Mg-Ion and Li-Ion Batteries | |||
| Battery Type | Ion Transfer Resistance (Rt Ω) | Charge Transfer Resistance (Rct Ω) | Notes |
| Magnesium-Ion | 50 | 75 | Higher resistance due to slower ion diffusion |
| Lithium-Ion | 10 | 25 | Lower resistance due to faster ion mobility |
| Comparison | +400% | +200% | Mg-ion batteries require electrolyte optimisation |
| Comparison of Electrolyte Formulations for Magnesium-Ion Batteries | |||
| Electrolyte Type | Ionic Conductivity (S/cm) | Passivation Effects | Performance Notes |
| (HMDS)2Mg | 1.2 × 10−2 | Minimal | High conductivity reduces passivation layers |
| Grignard-Based Electrolyte | 7.5 × 10−3 | Moderate | Decent performance, higher reactivity |
| Solid-State Electrolyte | 1.0 × 10−4 | None | Low conductivity, excellent thermal stability |
| Boron-Based Electrolyte | 8.0 × 10−3 | Minimal | Balanced performance with improved stability |
| Simulation Results for Ion Transport in Magnesium-Ion Batteries | |||
| Simulation Method | Parameter Simulated | Value Obtained | Notes |
| Molecular Dynamics (MD) | Ion Diffusion Coefficient | 1.2 × 10−6 cm2/s | Validates findings |
| Finite Element Modelling (FEM) | Conductivity | 1.1 × 10−2 S/cm | Demonstrates high electrolyte efficiency |
| DFT Simulations | Energy Barriers | 0.35 eV | Highlights cathode compatibility |
| Comparative Safety Analysis of Magnesium-Ion and Lithium-Ion Batteries | |||
| Safety Parameter | Magnesium-Ion Battery | Lithium-Ion Battery | Notes |
| Dendrite Formation | None | High Risk | Mg does not form dendrites; safer design |
| Thermal Runaway | Low Risk | High Risk | Mg exhibits higher thermal stability |
| Fire Risk | Minimal | Significant | Li batteries are prone to catching fire |
| Electrolyte Reactivity | Moderate | High | Mg electrolytes are less reactive |
| Comparison with Existing Research | |||
| Feature | This Work | Existing Literature | Novelty |
| Energy Density (mAh/g) | 500–1000 | ~250–900 | Uses sulphur–graphene cathodes for higher values |
| Cycle Stability (500 Cycles, %) | 92 | 80–90 (typical) | Improved retention with HMDS2 Mg electrolytes |
| Passivation Layer Growth (nm) | 5 (after 100 cycles) | ~10 | Reduced via advanced electrolyte formulation |
| Battery Type | Current Density (mA/g) | Charge Efficiency (%) | Discharge Efficiency (%) | Overall Efficiency (%) | Notes |
|---|---|---|---|---|---|
| Mag-Ion | 50 | 90 | 85 | 87.5 | There is a slight reduction in efficiency due to slower ion diffusion |
| Li-Ion | 50 | 95 | 92 | 93.5 | Higher efficiency due to faster ion mobility |
| Mag-Ion | 100 | 87 | 83 | 85.0 | Performance drops more significantly at higher current density |
| Li-Ion | 100 | 93 | 90 | 91.5 | More stable performance at higher current densities |
| Material Cost Comparison: Magnesium vs. Lithium for Battery Manufacturing | |||||
| Material | Market Price ($/kg) | Abundance in Earth’s Crust (%) | Annual Production (Metric Tons) | Cost Reduction (%) | Notes |
| Magnesium | 2–3 | 2.1 | ~1,000,000 | 20 | High abundance and lower cost |
| Lithium | 80–85 | 0.002 | ~100,000 | 10 | Scarcity leads to higher prices |
| Efficiency and Capacity Fade of Magnesium-Ion Batteries with Different Cathode Materials | |||||
| Cathode Material | Efficiency (%) (Initial) | Efficiency (%) (After 500 Cycles) | Capacity Fade (500 Cycles, %) | Capacity Fade (1000 Cycles, %) | Notes |
| Pure Sulphur Cathode | 90 | 85 | 10 | 20 | Moderate stability |
| Graphene-Sulphur Composite | 93 | 91 | 5 | 10 | Enhanced conductivity and stability |
| Polyanionic Cathode | 92 | 88 | 8 | 15 | Balanced performance |
| Summary of Advanced Materials Used in Magnesium-Ion Battery Development | |||||
| Material | Example | Energy Density (mAh/g) | Ion Diffusion Coefficient (cm2/s) | Stability 500 Cycles, (%) | Notes |
| Cathode (Sulphur-Based) | Graphene–S-ulphur | 950 | 1.5 × 10−6 | 90 | High energy density and stability |
| Anode (Alloy-Based) | Mg-Al Alloy | 850 | 1.2 × 10−6 | 92 | Improved ion transport |
| Electrolyte | (HMDS)2 Mg | - | 1.2 × 10−2 | - | Excellent conductivity, low reactivity |
| Operating Condition | Metric | Magnesium-Ion Battery | Lithium-Ion Battery | Notes |
|---|---|---|---|---|
| 25 °C | Energy Density (mAh/g) | 900 | 250 | Mg-ion exhibits higher theoretical energy density |
| Cycle Stability | 92 | 85 | Mg-ion is more stable due to lack of dendrites | |
| Charge Efficiency (%) | 88 | 95 | Li-ion has better efficiency due to faster ion mobility | |
| 45 °C | Energy Density (mAh/g) | 850 | 220 | Both batteries show a performance decline |
| Cycle Stability (500 Cycles, %) | 85 | 80 | Mg-ion maintains a better cycle stability | |
| Charge Efficiency (%) | 85 | 90 | Li-ion retains efficiency advantages | |
| Statistical Summary of Performance Metrics for Mg-Ion Batteries with Alloy-Based Anodes | ||||
| Anode Type | Energy Density (mAh/g) | Stability 500 Cycles (%) | Efficiency (%) | Notes |
| Magnesium Anode | 800 | 85 | 88 | Standard performance |
| Mg-Al Anode | 850 | 90 | 89 | Improved stability and conductivity |
| Mg-Sn Anode | 880 | 92 | 90 | High stability, better cycling |
| Metric | This Research | Zhao et al. (2024) [1] | Jiang et al. (2024) [2] | Yang et al. (2025) [4] | Liu et al. (2023) [5] |
|---|---|---|---|---|---|
| Energy Density (mAh/g) | 500–1000 (Sulphur cathode systems) | ~950 (Graphene–sulphur composite cathodes) | ~900 (Polyanionic–sulphur composite) | ~850 (Magnesium–sulphur cathodes) | ~1000 (Hybrid cathode materials) |
| Cycle Stability (500 Cycles, %) | 92 (Graphene–sulphur cathode) | 91 | 90 | 88 | 93 |
| Charge–discharge efficiency (%) | 85 (Sulphur-based cathode) | 88 | 89 | 86 | 90 |
| Passivation Layer Growth (nm) | 5 (after 100 cycles with Grignard-based electrolyte) | 7 (after 150 cycles) | 10 (after 200 cycles) | 12 (after 300 cycles) | 3 (solid-state electrolytes) |
| Thermal Stability (°C) | 220 (HMDS2Mg electrolyte) | 200 (Grignard-based electrolyte) | 180 (LiPF6 comparison) | 220 (Advanced solid-state electrolyte) | 230 (High-performance hybrid systems) |
| Ionic Conductivity (S/cm) | 1.2 × 10−2 (HMDS2Mg electrolyte) | 7.5 × 10−3 | 8.0 × 10−3 | 1.0 × 10−4 | 1.3 × 10−2 |
| Key Notes | Stable with promising scalability but hindered by passivation | Significant progress in graphene integration | Polyanionic structures improve capacity retention | Solid-state challenges ion transport but enhances safety | Hybrid systems outperform most tested setups |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Malachi, I.O.; Olawumi, A.O.; Afolabi, S.O.; Oladapo, B.I. Looking Beyond Lithium for Breakthroughs in Magnesium-Ion Batteries as Sustainable Solutions. Sustainability 2025, 17, 3782. https://doi.org/10.3390/su17093782
Malachi IO, Olawumi AO, Afolabi SO, Oladapo BI. Looking Beyond Lithium for Breakthroughs in Magnesium-Ion Batteries as Sustainable Solutions. Sustainability. 2025; 17(9):3782. https://doi.org/10.3390/su17093782
Chicago/Turabian StyleMalachi, Idowu O., Adebukola O. Olawumi, Samuel O. Afolabi, and B. I. Oladapo. 2025. "Looking Beyond Lithium for Breakthroughs in Magnesium-Ion Batteries as Sustainable Solutions" Sustainability 17, no. 9: 3782. https://doi.org/10.3390/su17093782
APA StyleMalachi, I. O., Olawumi, A. O., Afolabi, S. O., & Oladapo, B. I. (2025). Looking Beyond Lithium for Breakthroughs in Magnesium-Ion Batteries as Sustainable Solutions. Sustainability, 17(9), 3782. https://doi.org/10.3390/su17093782







