Upcycled Composite Derived from Polyacrylonitrile and Elemental Sulfur: Thermomechanical Properties and Microstructural Insight
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instrumentation
2.3. PANS90
2.4. General Procedure for 8 h Reaction of Model Compounds
- Glutaronitrile–Sulfur
- Succinonitrile–Sulfur
- Adiponitrile–Sulfur
- 2–Methyloctanenitrile–Sulfur
2.5. Procedure for 4 h Reaction of Model Compounds
- Glutaronitrile–Sulfur
- Succinonitrile–Sulfur
- Adiponitrile–Sulfur
2.6. Procedure for Reaction of Model Compound/Sulfur Products with NaBH4
3. Results and Discussion
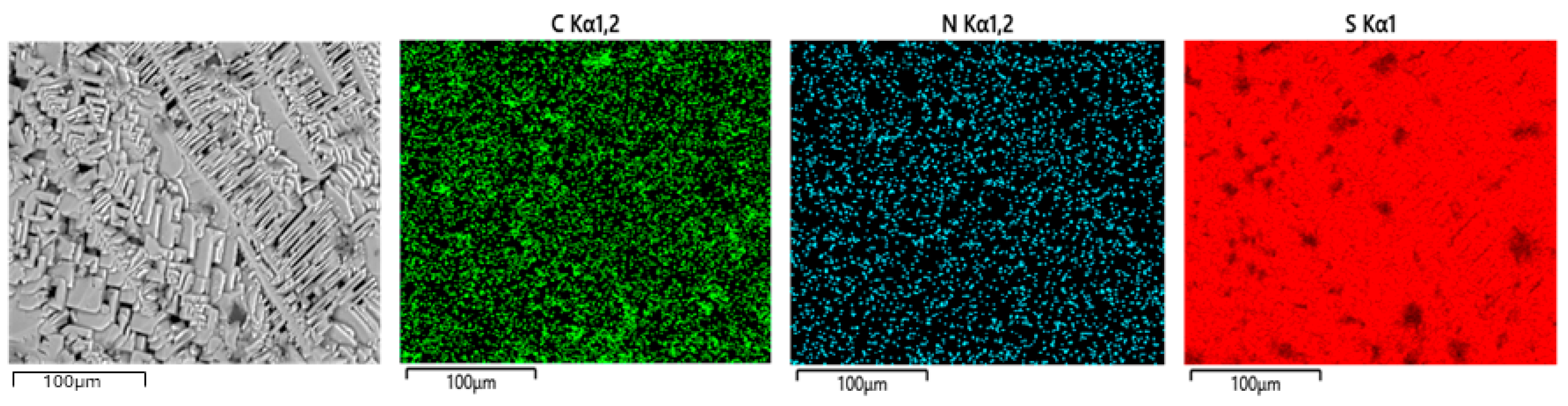
3.1. Preparation and Compositional Characterization of Composite from PAN
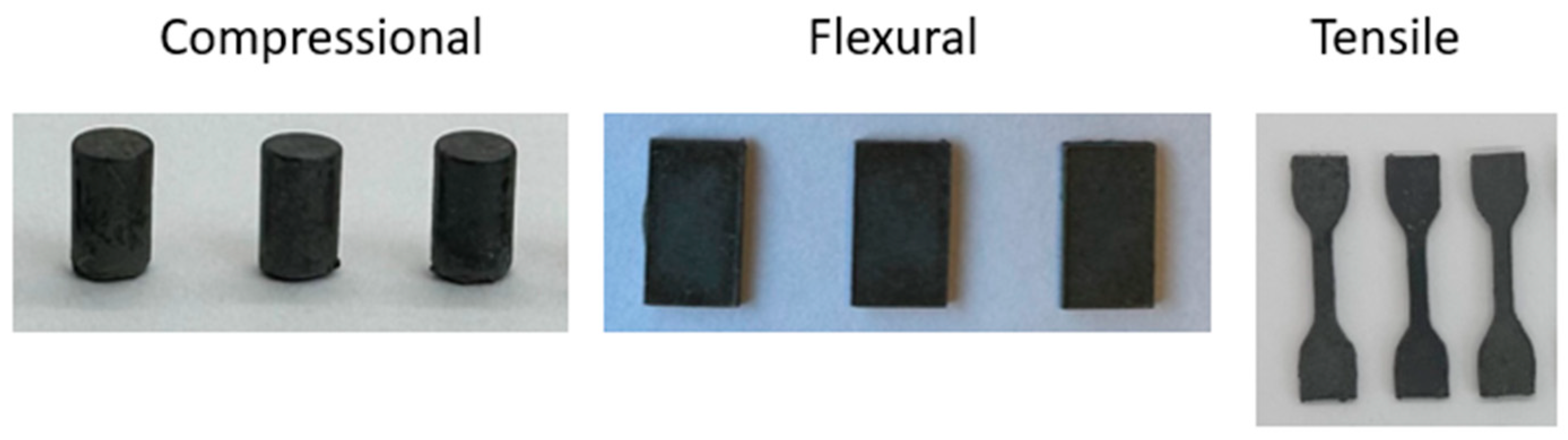
3.2. Thermal and Mechanical Properties of PANS90
3.3. Preliminary Environmental Impact Estimates
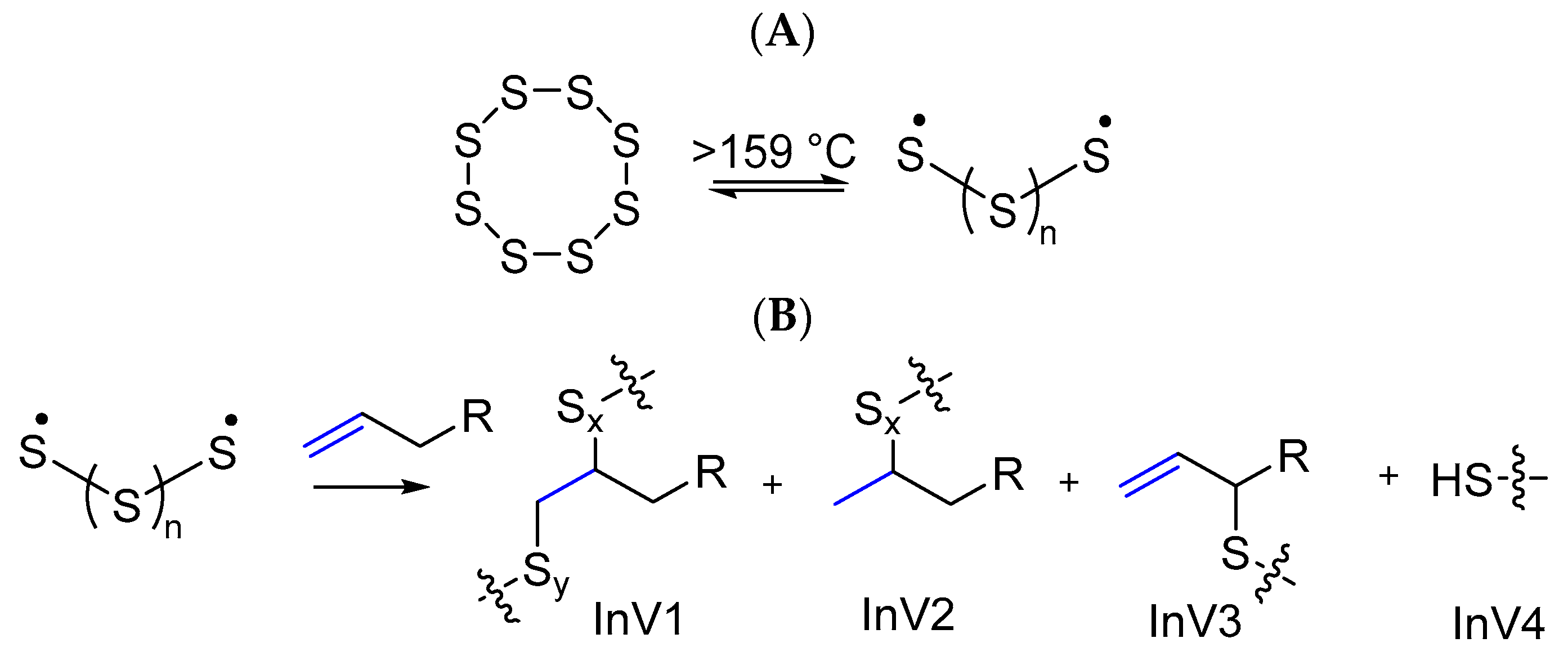
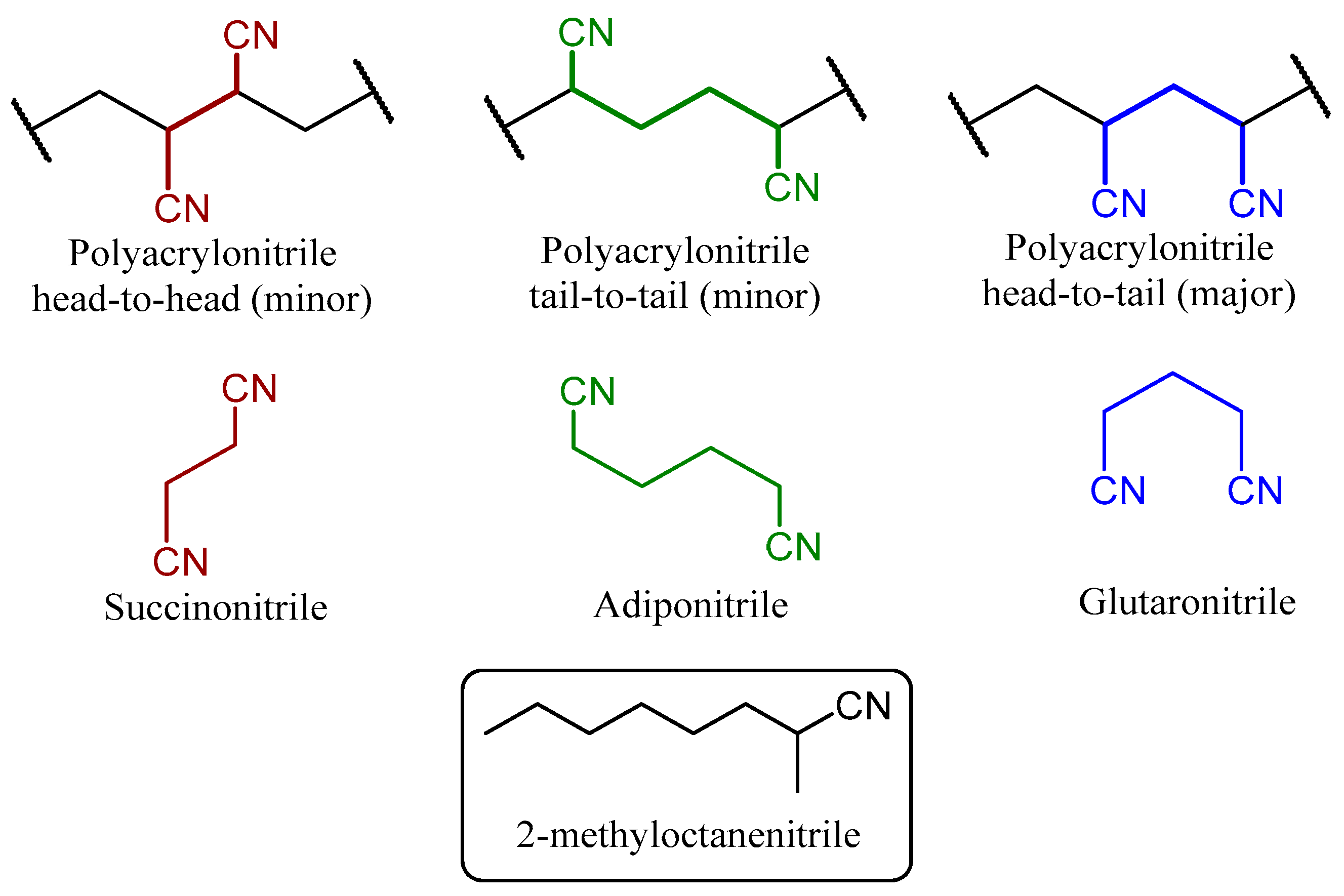
3.4. Microstructural Insight from Model Studies
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| PAN | Polyacrylonitrile |
| HSM | High-sulfur-content material |
| PANS90 | Composite made from 90 wt. % sulfur and 10 wt. % PAN |
| InV | Inverse vulcanization |
| SEM–EDX | Scanning electron microscopy with energy dispersive X-ray analysis |
| DSC | Differential scanning calorimetry |
| PSP90 | Composite made from 90 wt. % sulfur and 10 wt. % polystyrene packaging material particulate |
| mPES | Composite made from 90 wt. % sulfur and 10 wt. % esterified PET |
| PGMA–S | Composite made from 90 wt. % sulfur and 10 wt. % geraniol-transesterified PMMA |
| SPC90 | Composite made from 90 wt.% sulfur 10 wt. % poly(bisphenol A carbonate). |
| TGA | Thermogravimetric analysis |
| GWP | Global warming potential |
References
- Geyer, R. Production, use, and fate of synthetic polymers. In Plastic Waste and Recycling; Letcher, T.M., Ed.; Academic Press: New York, NY, USA, 2020; pp. 13–32. [Google Scholar]
- Patti, A.; Cicala, G.; Acierno, D. Eco-sustainability of the textile production: Waste recovery and current recycling in the composites world. Polymers 2021, 13, 134. [Google Scholar] [CrossRef] [PubMed]
- Athanasopoulos, P.; Zabaniotou, A. Post-consumer textile thermochemical recycling to fuels and biocarbon: A critical review. Sci. Total Environ. 2022, 834, 155387. [Google Scholar] [CrossRef] [PubMed]
- Xue, T.J.; McKinney, M.A.; Wilkie, C.A. The thermal degradation of polyacrylonitrile. Polym. Degrad. Stab. 1997, 58, 193–202. [Google Scholar] [CrossRef]
- Harpp, D.N.; Smith, R.A. Organic sulfur chemistry. 42. Sulfur-sulfur bond cleavage processes. Selective desulfurization of trisulfides. J. Am. Chem. Soc. 1982, 104, 6045–6053. [Google Scholar] [CrossRef]
- Marshall, C.M.; Molineux, J.; Kang, K.-S.; Kumirov, V.; Kim, K.-J.; Norwood, R.A.; Njardarson, J.T.; Pyun, J. Synthesis of Polycyclic Olefinic Monomers from Norbornadiene for Inverse Vulcanization: Structural and Mechanistic Consequences. J. Am. Chem. Soc. 2024, 146, 24061–24074. [Google Scholar] [CrossRef]
- Orme, K.; Fistrovich, A.H.; Jenkins, C.L. Tailoring Polysulfide Properties through Variations of Inverse Vulcanization. Macromolecules 2020, 53, 9353–9361. [Google Scholar] [CrossRef]
- Wickramasingha, Y.A.; Stojcevski, F.; Eyckens, D.J.; Hayne, D.J.; Chalker, J.M.; Henderson, L.C. Exploring Inverse Vulcanized Dicyclopentadiene As a Polymer Matrix for Carbon Fiber Composites. Macromol. Mater. Eng. 2024, 309, 2300298. [Google Scholar] [CrossRef]
- Wu, X.; Smith, J.A.; Petcher, S.; Zhang, B.; Parker, D.J.; Griffin, J.M.; Hasell, T. Catalytic inverse vulcanization. Nat. Commun. 2019, 10, 647. [Google Scholar] [CrossRef]
- Molineux, J.; Lee, T.; Kim, K.J.; Kang, K.-S.; Lyons, N.P.; Nishant, A.; Kleine, T.S.; Durfee, S.W.; Pyun, J.; Norwood, R.A. Fabrication of Plastic Optics from Chalcogenide Hybrid Inorganic/Organic Polymers for Infrared Thermal Imaging. Adv. Opt. Mater. 2024, 12, 2301971. [Google Scholar] [CrossRef]
- Qureshi, M.H.; Bao, J.; Kleine, T.S.; Kim, K.-J.; Carothers, K.J.; Molineux, J.; Cho, E.; Kang, K.-S.; Godman, N.P.; Coropceanu, V.; et al. Synthesis of Deuterated and Sulfurated Polymers by Inverse Vulcanization: Engineering Infrared Transparency via Deuteration. J. Am. Chem. Soc. 2023, 145, 27821–27829. [Google Scholar] [CrossRef]
- Bischoff, D.J.; Lee, T.; Kang, K.-S.; Molineux, J.; O’Neil Parker, W., Jr.; Pyun, J.; Mackay, M.E. Unraveling the rheology of inverse vulcanized polymers. Nat. Commun. 2023, 14, 7553. [Google Scholar] [CrossRef] [PubMed]
- Bao, J.; Martin, K.P.; Cho, E.; Kang, K.-S.; Glass, R.S.; Coropceanu, V.; Bredas, J.-L.; Parker, W.O.N., Jr.; Njardarson, J.T.; Pyun, J. On the Mechanism of the Inverse Vulcanization of Elemental Sulfur: Structural Characterization of Poly(sulfur-random-(1,3-diisopropenylbenzene)). J. Am. Chem. Soc. 2023, 145, 12386–12397. [Google Scholar] [CrossRef] [PubMed]
- Lee, T.; Dirlam, P.T.; Njardarson, J.T.; Glass, R.S.; Pyun, J. Polymerizations with Elemental Sulfur: From Petroleum Refining to Polymeric Materials. J. Am. Chem. Soc. 2022, 144, 5–22. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.-S.; Iyer, K.A.; Pyun, J. On the Fundamental Polymer Chemistry of Inverse Vulcanization for Statistical and Segmented Copolymers from Elemental Sulfur. Chem.-A Eur. J. 2022, 28, e202200115. [Google Scholar] [CrossRef]
- Lim, J.; Pyun, J.; Char, K. Recent Approaches for the Direct Use of Elemental Sulfur in the Synthesis and Processing of Advanced Materials. Angew. Chem. Int. Ed. 2015, 54, 3249–3258. [Google Scholar] [CrossRef]
- Eder, M.L.; Call, C.B.; Jenkins, C.L. Utilizing Reclaimed Petroleum Waste to Synthesize Water-Soluble Polysulfides for Selective Heavy Metal Binding and Detection. ACS Appl. Polym. Mater. 2022, 4, 1110–1116. [Google Scholar] [CrossRef]
- Smith, J.A.; Green, S.J.; Petcher, S.; Parker, D.J.; Zhang, B.; Worthington, M.J.H.; Wu, X.; Kelly, C.A.; Baker, T.; Gibson, C.T.; et al. Crosslinker Copolymerization for Property Control in Inverse Vulcanization. Chem.-A Eur. J. 2019, 25, 10433–10440. [Google Scholar] [CrossRef]
- Herrera, C.; Ysinga, K.J.; Jenkins, C.L. Polysulfides Synthesized from Renewable Garlic Components and Repurposed Sulfur Form Environmentally Friendly Adhesives. ACS Appl. Mater. Interfaces 2019, 11, 35312–35318. [Google Scholar] [CrossRef]
- Westerman, C.R.; Jenkins, C.L. Dynamic Sulfur Bonds Initiate Polymerization of Vinyl and Allyl Ethers at Mild Temperatures. Macromolecules 2018, 51, 7233–7238. [Google Scholar] [CrossRef]
- Newman, B.; Doeven, E.H.; Francis, P.S.; Stojcevski, F.; Hayne, D.J.; Chalker, J.M.; Henderson, L.C. A high value application of reclaimed carbon fibers: Environmental remediation and redeployment in structural composites. Sustain. Mater. Technol. 2023, 35, e00546. [Google Scholar] [CrossRef]
- Yan, P.; Zhao, W.; Tonkin, S.J.; Chalker, J.M.; Schiller, T.L.; Hasell, T. Stretchable and Durable Inverse Vulcanized Polymers with Chemical and Thermal Recycling. Chem. Mater. 2022, 34, 1167–1178. [Google Scholar] [CrossRef]
- Stojcevski, F.; Stanfield, M.K.; Hayne, D.J.; Mann, M.; Lundquist, N.A.; Chalker, J.M.; Henderson, L.C. Inverse Vulcanisation of canola oil as a route to recyclable chopped carbon fibre composites. Sustain. Mater. Technol. 2022, 32, e00400. [Google Scholar] [CrossRef]
- Mann, M.; Pauling, P.J.; Tonkin, S.J.; Campbell, J.A.; Chalker, J.M. Chemically Activated S-S Metathesis for Adhesive-Free Bonding of Polysulfide Surfaces. Macromol. Chem. Phys. 2022, 223, 2100333. [Google Scholar] [CrossRef]
- Gupta, A.; Worthington, M.J.H.; Patel, H.D.; Johnston, M.R.; Puri, M.; Chalker, J.M. Reaction of sulfur and sustainable algae oil for polymer synthesis and enrichment of saturated triglycerides. ACS Sust. Chem. Eng. 2022, 10, 9022–9028. [Google Scholar] [CrossRef]
- Mann, M.; Luo, X.; Tikoalu, A.D.; Gibson, C.T.; Yin, Y.; Al-Attabi, R.; Andersson, G.G.; Raston, C.L.; Henderson, L.C.; Pring, A.; et al. Carbonisation of a polymer made from sulfur and canola oil. Chem. Commun. 2021, 57, 6296–6299. [Google Scholar] [CrossRef]
- Bu Najmah, I.; Lundquist, N.A.; Stanfield, M.K.; Stojcevski, F.; Campbell, J.A.; Esdaile, L.J.; Gibson, C.T.; Lewis, D.A.; Henderson, L.C.; Hasell, T.; et al. Insulating Composites Made from Sulfur, Canola Oil, and Wool. ChemSusChem 2021, 14, 2352–2359. [Google Scholar] [CrossRef]
- Tonkin, S.J.; Gibson, C.T.; Campbell, J.A.; Lewis, D.A.; Karton, A.; Hasell, T.; Chalker, J.M. Chemically induced repair, adhesion, and recycling of polymers made by inverse vulcanization. Chem. Sci. 2020, 11, 5537–5546. [Google Scholar] [CrossRef]
- Lundquist, N.A.; Tikoalu, A.D.; Worthington, M.J.H.; Shapter, R.; Tonkin, S.J.; Stojcevski, F.; Mann, M.; Gibson, C.T.; Gascooke, J.R.; Karton, A.; et al. Reactive Compression Molding Post-Inverse Vulcanization: A Method to Assemble, Recycle, and Repurpose Sulfur Polymers and Composites. Chem.-A Eur. J. 2020, 26, 10035–10044. [Google Scholar] [CrossRef]
- Hanna, V.; Graysmark, M.; Willcock, H.; Hasell, T. Liquid polybutadiene reinforced inverse vulcanised polymers. J. Mater. Chem. A 2024, 12, 1211–1217. [Google Scholar] [CrossRef]
- Dop, R.A.; Neill, D.R.; Hasell, T. Sulfur-Polymer Nanoparticles: Preparation and Antibacterial Activity. ACS Appl. Mater. Interfaces 2023, 15, 20822–20832. [Google Scholar] [CrossRef]
- Dale, J.J.; Hanna, V.; Hasell, T. Manipulating Inverse Vulcanization Comonomers to Generate High-Tensile-Strain Polymers. ACS Appl. Polym. Mater. 2023, 5, 6761–6765. [Google Scholar] [CrossRef]
- Zhang, B.; Petcher, S.; Dop, R.A.; Yan, P.; Zhao, W.; Wang, H.; Dodd, L.J.; McDonald, T.O.; Hasell, T. Inverse vulcanised sulfur polymer nanoparticles prepared by antisolvent precipitation. J. Mater. Chem. A 2022, 10, 13704–13710. [Google Scholar] [CrossRef]
- Yan, P.; Zhao, W.; McBride, F.; Cai, D.; Dale, J.; Hanna, V.; Hasell, T. Mechanochemical synthesis of inverse vulcanized polymers. Nat. Commun. 2022, 13, 4824. [Google Scholar] [CrossRef]
- Jia, J.; Liu, J.; Wang, Z.-Q.; Liu, T.; Yan, P.; Gong, X.-Q.; Zhao, C.; Chen, L.; Miao, C.; Zhao, W.; et al. Photoinduced inverse vulcanization. Nat. Chem. 2022, 14, 1249–1257. [Google Scholar] [CrossRef]
- Dop, R.A.; Neill, D.R.; Hasell, T. Antibacterial Activity of Inverse Vulcanized Polymers. Biomacromolecules 2021, 22, 5223–5233. [Google Scholar] [CrossRef]
- Dodd, L.J.; Omar, O.; Wu, X.; Hasell, T. Investigating the Role and Scope of Catalysts in Inverse Vulcanization. ACS Catal. 2021, 11, 4441–4455. [Google Scholar] [CrossRef]
- Zhang, B.; Gao, H.; Yan, P.; Petcher, S.; Hasell, T. Inverse vulcanization below the melting point of sulfur. Mater. Chem. Front. 2020, 4, 669–675. [Google Scholar] [CrossRef]
- Anderson, L.E.; Kleine, T.S.; Zhang, Y.; Phan, D.D.; Namnabat, S.; LaVilla, E.A.; Konopka, K.M.; Ruiz Diaz, L.; Manchester, M.S.; Schwiegerling, J.; et al. Chalcogenide Hybrid Inorganic/Organic Polymers: Ultrahigh Refractive Index Polymers for Infrared Imaging. ACS Macro Lett. 2017, 6, 500–504. [Google Scholar] [CrossRef]
- Hanna, V.; Yan, P.; Petcher, S.; Hasell, T. Incorporation of fillers to modify the mechanical performance of inverse vulcanised polymers. Polym. Chem. 2022, 13, 3930–3937. [Google Scholar] [CrossRef]
- Meredith, H.J.; Jenkins, C.L.; Wilker, J.J. Enhancing the Adhesion of a Biomimetic Polymer Yields Performance Rivaling Commercial Glues. Adv. Funct. Mater. 2014, 24, 3259–3267. [Google Scholar] [CrossRef]
- Davis, A.E.; Sayer, K.B.; Jenkins, C.L. A comparison of adhesive polysulfides initiated by garlic essential oil and elemental sulfur to create recyclable adhesives. Polym. Chem. 2022, 13, 4634–4640. [Google Scholar] [CrossRef]
- Wickramasingha, Y.A.; Simon, Z.; Hayne, D.J.; Eyckens, D.J.; Dharmasiri, B.; Chalker, J.M.; Henderson, L.C. Adhesives made from sulfur copolymer composites reinforced with recycled carbon fiber. Ind. Eng. Chem. Res. 2024, 63, 12490–12501. [Google Scholar] [CrossRef]
- Mann, M.; Zhang, B.; Tonkin, S.J.; Gibson, C.T.; Jia, Z.; Hasell, T.; Chalker, J.M. Processes for coating surfaces with a copolymer made from sulfur and dicyclopentadiene. Polym. Chem. 2022, 13, 1320–1327. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Kucera, R.L.; Albuquerque, I.S.; Gibson, C.T.; Sibley, A.; Slattery, A.D.; Campbell, J.A.; Alboaiji, S.F.K.; Muller, K.A.; Young, J.; et al. Laying Waste to Mercury: Inexpensive Sorbents Made from Sulfur and Recycled Cooking Oils. Chem.-Eur. J. 2017, 23, 16106. [Google Scholar] [CrossRef]
- Chalker, J.M.; Mann, M.; Worthington, M.J.H.; Esdaile, L.J. Polymers Made by Inverse Vulcanization for Use as Mercury Sorbents. Org. Mater. 2021, 3, 362–373. [Google Scholar] [CrossRef]
- Worthington, M.J.H.; Mann, M.; Muhti, I.Y.; Tikoalu, A.D.; Gibson, C.T.; Jia, Z.; Miller, A.D.; Chalker, J.M. Modelling mercury sorption of a polysulfide coating made from sulfur and limonene. Phys. Chem. Chem. Phys. 2022, 24, 12363–12373. [Google Scholar] [CrossRef]
- Crockett, M.P.; Evans, A.M.; Worthington, M.J.H.; Albuquerque, I.S.; Slattery, A.D.; Gibson, C.T.; Campbell, J.A.; Lewis, D.A.; Bernardes, G.J.L.; Chalker, J.M. Sulfur-Limonene Polysulfide: A Material Synthesized Entirely from Industrial By-Products and Its Use in Removing Toxic Metals from Water and Soil. Angew. Chem. Int. Ed. 2016, 55, 1714–1718. [Google Scholar] [CrossRef]
- Lauer, M.K.; Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Recyclable, Sustainable, and Stronger than Portland Cement: A Composite from Unseparated Biomass and Fossil Fuel Waste. Mater. Adv. 2020, 1, 590–594. [Google Scholar] [CrossRef]
- Smith, A.D.; Smith, R.C.; Tennyson, A.G. Sulfur-Containing Polymers Prepared from Fatty Acid-Derived Monomers: Application of Atom-Economical Thiol-ene/Thiol-yne Click Reactions and Inverse Vulcanization Strategies. Sustain. Chem. 2020, 1, 209–237. [Google Scholar] [CrossRef]
- Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Green Synthesis of Thermoplastic Composites from a Terpenoid-Cellulose Ester. ACS Appl. Polym. Mater. 2020, 2, 3761–3765. [Google Scholar] [CrossRef]
- Lauer, M.K.; Karunarathna, M.S.; Tennyson, A.G.; Smith, R.C. Robust, remeltable and remarkably simple to prepare biomass-sulfur composites. Mater. Adv. 2020, 1, 2271–2278. [Google Scholar] [CrossRef]
- Maladeniya, C.P.; Karunarathna, M.S.; Lauer, M.K.; Lopez, C.V.; Thiounn, T.; Smith, R.C. A Role for Terpenoid Cyclization in the Atom Economical Polymerization of Terpenoids with Sulfur to Yield Durable Composites. Mater. Adv. 2020, 1, 1665–1674. [Google Scholar] [CrossRef]
- Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Inverse vulcanization of octenyl succinate-modified corn starch as a route to biopolymer-sulfur composites. Mater. Adv. 2021, 2, 2391–2397. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Lauer, M.K.; Smith, R.C. Facile route to an organosulfur composite from biomass-derived guaiacol and waste sulfur. J. Mater. Chem. A 2020, 8, 20318–20322. [Google Scholar] [CrossRef]
- Lopez, C.V.; Smith, A.D.; Smith, R.C. High strength composites from low-value animal coproducts and industrial waste sulfur. RSC Adv. 2022, 12, 1535–1542. [Google Scholar] [CrossRef]
- Pyun, J.; Carrozza, C.F.; Silvano, S.; Boggioni, L.; Losio, S.; de Angelis, A.R.; O’Neil Parker, W., Jr. Nuclear magnetic resonance structural characterization of sulfur-derived copolymers from inverse vulcanization. Part 1: Styrene. J. Polym. Sci. 2022, 60, 3471–3477. [Google Scholar] [CrossRef]
- Dodd, L.J.; Sandy, W.; Dop, R.A.; Zhang, B.; Lunt, A.; Neill, D.R.; Hasell, T. Inverse vulcanisation of self-activating amine and alkyne crosslinkers. Polym. Chem. 2023, 14, 4064–4078. [Google Scholar] [CrossRef]
- Sayer, K.B.; Miller, V.L.; Merrill, Z.; Davis, A.E.; Jenkins, C.L. Allyl sulfides in garlic oil initiate the formation of renewable adhesives. Polym. Chem. 2023, 14, 3091–3098. [Google Scholar] [CrossRef]
- Lai, Y.-S.; Liu, Y.-L. Reaction between 1,3,5-Triisopropylbenzene and Elemental Sulfur Extending the Scope of Reagents in Inverse Vulcanization. Macromol. Rapid Commun. 2023, 44, e2300014. [Google Scholar] [CrossRef]
- Wijeyatunga, S.K.; Tennyson, A.G.; Smith, R.C. High-Sulfur-Content Materials Derived from Postconsumer Polystyrene Wastes: Thermomechanical Properties, Environmental Impacts, and Microstructural Insights. ACS Sustain. Resour. Manag. 2024, 1, 2173–2183. [Google Scholar] [CrossRef]
- Karunarathna, M.S.; Maladeniya, C.P.; Lauer, M.K.; Tennyson, A.G.; Smith, R.C. Durable composites by vulcanization of oleyl-esterified lignin. RSC Adv. 2023, 13, 3234–3240. [Google Scholar] [CrossRef] [PubMed]
- Lopez, C.V.; Smith, R.C. Chemical recycling of poly(ethylene terephthalate) via sequential glycolysis, oleoyl chloride esterification and vulcanization to yield durable composites. Mater. Adv. 2023, 4, 2785–2793. [Google Scholar] [CrossRef]
- Derr, K.M.; Smith, R.C. One-Pot Method for Upcycling Polycarbonate Waste to Yield High-Strength, BPA-Free Composites. J. Polym. Sci. 2023, 62, 1115–1122. [Google Scholar] [CrossRef]
- Wijeyatunga, S.K.; Derr, K.M.; Maladeniya, C.P.; Sauceda-Oloño, P.Y.; Tennyson, A.G.; Smith, R.C. Upcycling waste PMMA to durable composites via a transesterification-inverse vulcanization process. J. Polym. Sci. 2024, 62, 554–563. [Google Scholar] [CrossRef]
- ASTM D695−23; Standard Test Method for Compressive Properties of Rigid Plastics. ASTM International: West Conshohocken, PA, USA, 2023.
- ASTM D638-22; Standard Test Method for Tensile Properties of Plastics. ASTM International: West Conshohocken, PA, USA, 2022.
- ASTM D790-03; Standard Test Methods for Flexural Properties of Unreinforced and Reinforced Plastics and Electrical Insulating Materials. ASTM International: West Conshohocken, PA, USA, 2003.
- Eom, T.; Khan, A. Disulfides as mercapto-precursors in nucleophilic ring opening reaction of polymeric epoxides: Establishing equimolar stoichiometric conditions in a thiol–epoxy ‘click’ reaction. Chem. Commun. 2020, 56, 7419–7422. [Google Scholar] [CrossRef]
- Griebel, J.J.; Li, G.; Glass, R.S.; Char, K.; Pyun, J. Kilogram scale inverse vulcanization of elemental sulfur to prepare high capacity polymer electrodes for Li-S batteries. J. Polym. Sci. Part A Polym. Chem. 2015, 53, 173–177. [Google Scholar] [CrossRef]
- Dale, J.J.; Petcher, S.; Hasell, T. Dark Sulfur: Quantifying Unpolymerized Sulfur in Inverse Vulcanized Polymers. ACS Appl. Polym. Mater. 2022, 4, 3169–3173. [Google Scholar] [CrossRef]
- Dale, J.J.; Stanley, J.; Dop, R.A.; Chronowska-Bojczuk, G.; Fielding, A.J.; Neill, D.R.; Hasell, T. Exploring inverse vulcanization mechanisms from the perspective of dark sulfur. Eur. Polym. J. 2023, 195, 112198. [Google Scholar] [CrossRef]
- Derr, K.M.; Lopez, C.V.; Maladeniya, C.P.; Tennyson, A.G.; Smith, R.C. Transesterification-vulcanization route to durable composites from post-consumer poly(ethylene terephthalate), terpenoids, and industrial waste sulfur. J. Polym. Sci. 2023, 61, 3075–3086. [Google Scholar] [CrossRef]
- Hoefling, A.; Lee, Y.J.; Theato, P. Sulfur-Based Polymer Composites from Vegetable Oils and Elemental Sulfur: A Sustainable Active Material for Li-S Batteries. Macromol. Chem. Phys. 2017, 218, 1600303. [Google Scholar] [CrossRef]
- Wreczycki, J.; Bielinski, D.M.; Anyszka, R. Sulfur/organic copolymers as curing agents for rubber. Polymers 2018, 10, 870. [Google Scholar] [CrossRef] [PubMed]
- Furushima, Y.; Nakada, M.; Takahashi, H.; Ishikiriyama, K. Study of melting and crystallization behavior of polyacrylonitrile using ultrafast differential scanning calorimetry. Polymer 2014, 55, 3075–3081. [Google Scholar] [CrossRef]
- Sheldon, R.A. The E Factor: Fifteen years on. Green Chem. 2007, 9, 1273–1283. [Google Scholar] [CrossRef]
- Sheldon, R.A. Green chemistry, catalysis and valorization of waste biomass. J. Mol. Catal. A Chem. 2016, 422, 3–12. [Google Scholar] [CrossRef]
- Sheldon, R.A. Recent advances in green catalytic oxidations of alcohols in aqueous media. Catal. Today 2015, 247, 4–13. [Google Scholar] [CrossRef]
- Clark, J.H.; Matharu, A.S. Waste to wealth using green chemistry. Issues Environ. Sci. Technol. 2013, 37, 66–82. [Google Scholar]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Anastas, P.T.; Warner, J.C. Green Chemistry: Theory and Practice; Oxford University Press: New York, NY, USA, 1998. [Google Scholar]
- Farquharson, D.; Jaramillo, P.; Schivley, G.; Klima, K.; Carlson, D.; Samaras, C. Beyond Global Warming Potential: A Comparative Application of Climate Impact Metrics for the Life Cycle Assessment of Coal and Natural Gas Based Electricity. J. Ind. Ecol. 2017, 21, 857–873. [Google Scholar] [CrossRef]
- Joos, F.; Roth, R.; Fuglestvedt, J.S.; Peters, G.P.; Enting, I.G.; von Bloh, W.; Brovkin, V.; Burke, E.J.; Eby, M.; Edwards, N.R.; et al. Carbon dioxide and climate impulse response functions for the computation of greenhouse gas metrics: A multi-model analysis. Atmos. Chem. Phys. 2013, 13, 2793. [Google Scholar] [CrossRef]
- Peters, G.P.; Aamaas, B.; Lund, M.T.; Solli, C.; Fuglestvedt, J.S. Alternative “Global Warming” Metrics in Life Cycle Assessment: A Case Study with Existing Transportation Data. Environ. Sci. Technol. 2011, 45, 8633–8641. [Google Scholar] [CrossRef]
- Fuglestvedt, J.S.; Shine, K.P.; Berntsen, T.; Cook, J.; Lee, D.S.; Stenke, A.; Skeie, R.B.; Velders, G.J.M.; Waitz, I.A. Transport impacts on atmosphere and climate: Metrics. Atmos. Environ. 2010, 44, 4648–4677. [Google Scholar] [CrossRef]
- Yacout, D.M.; Abd El-Kawi, M.; Hassouna, M. Cradle to gate environmental impact assessment of acrylic fiber manufacturing. Int. J. Life Cycle Assess. 2016, 21, 326–336. [Google Scholar] [CrossRef]
- Passarini, F.; Ciacci, L.; Santini, A.; Vassura, I.; Morselli, L. Auto shredder residue LCA: Implications of ASR composition evolution. J. Clean. Prod. 2012, 23, 28–36. [Google Scholar] [CrossRef]
- Arena, U.; Ardolino, F. Technical and environmental performances of alternative treatments for challenging plastics waste. Resour. Conserv. Recycl. 2022, 183, 106379. [Google Scholar] [CrossRef]
- Jeswani, H.; Krüger, C.; Russ, M.; Horlacher, M.; Antony, F.; Hann, S.; Azapagic, A. Life cycle environmental impacts of chemical recycling via pyrolysis of mixed plastic waste in comparison with mechanical recycling and energy recovery. Sci. Total Environ. 2021, 769, 144483. [Google Scholar] [CrossRef]
- Wüstenberg, D.; Kasper, J. Required energy and structural breakdown at the process of dynamic cutting—Comminution of polypropylene and aluminium. Int. J. Miner. Process. 2004, 74, S417–S424. [Google Scholar] [CrossRef]
- Schubert, G.; Bernotat, S. Comminution of non-brittle materials. Int. J. Miner. Process. 2004, 74, S19–S30. [Google Scholar] [CrossRef]
- Peukert, W. Material properties in fine grinding. Int. J. Miner. Process. 2004, 74, S3–S17. [Google Scholar] [CrossRef]
- Lewis, G.N.; Randall, M. The heat content of the various forms of sulfur. J. Am. Chem. Soc. 1911, 33, 476–488. [Google Scholar] [CrossRef]
- Scrivener, K.L.; John, V.M.; Gartner, E.M. Eco-efficient cements: Potential economically viable solutions for a low-CO2 cement-based materials industry. Cem. Concr. Res. 2018, 114, 2–26. [Google Scholar] [CrossRef]
- Scrivener, K.L.; Fullmann, T.; Gallucci, E.; Walenta, G.; Bermejo, E. Quantitative study of Portland cement hydration by X-ray diffraction/Rietveld analysis and independent methods. Cem. Concr. Res. 2004, 34, 1541–1547. [Google Scholar] [CrossRef]
- Tayebee, R.; Ahmadi, S.J.; Rezaei Seresht, E.; Javadi, F.; Yasemi, M.A.; Hosseinpour, M.; Maleki, B. Commercial Zinc Oxide: A Facile, Efficient, and Eco-friendly Catalyst for the One-Pot Three-Component Synthesis of Multisubstituted 2-Aminothiophenes via the Gewald Reaction. Ind. Eng. Chem. Res. 2012, 51, 14577–14582. [Google Scholar] [CrossRef]
- Kapuge Dona, N.L.; Maladeniya, C.P.; Smith, R.C. Reactivity of Biomass-Derived Olefins with Elemental Sulfur: Mechanistic Insight. Eur. J. Org. Chem. 2024, 27, e202301269. [Google Scholar] [CrossRef]
- Tisdale, K.A.; Kapuge Dona, N.L.; Smith, R.C. The Influence of the Comonomer Ratio and Reaction Temperature on the Mechanical, Thermal, and Morphological Properties of Lignin Oil–Sulfur Composites. Molecules 2024, 29, 4209. [Google Scholar] [CrossRef]
- Priebbenow, D.L.; Bolm, C. Recent advances in the Willgerodt–Kindler reaction. Chem. Soc. Rev. 2013, 42, 7870–7880. [Google Scholar] [CrossRef]
- Monascal, Y.; Cartaya, L.; Álvarez-Aular, Á.; Maldonado, A.; Chuchani, G. The ion pair mechanism in the thermal deamination of primary amines catalyzed by HBr in the gas phase: DFT and AIM analysis. Chem. Phys. Lett. 2018, 703, 117–123. [Google Scholar] [CrossRef]
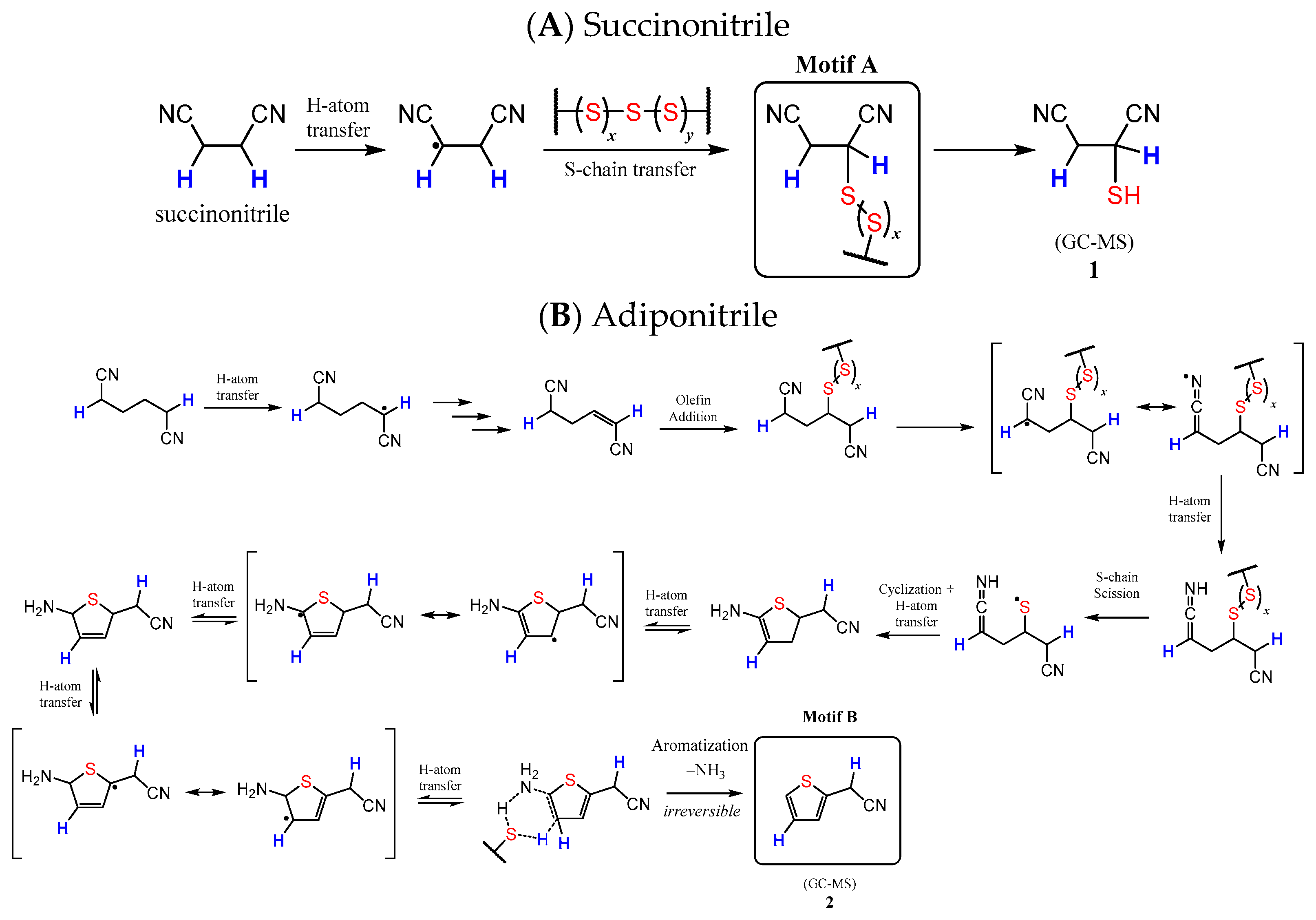
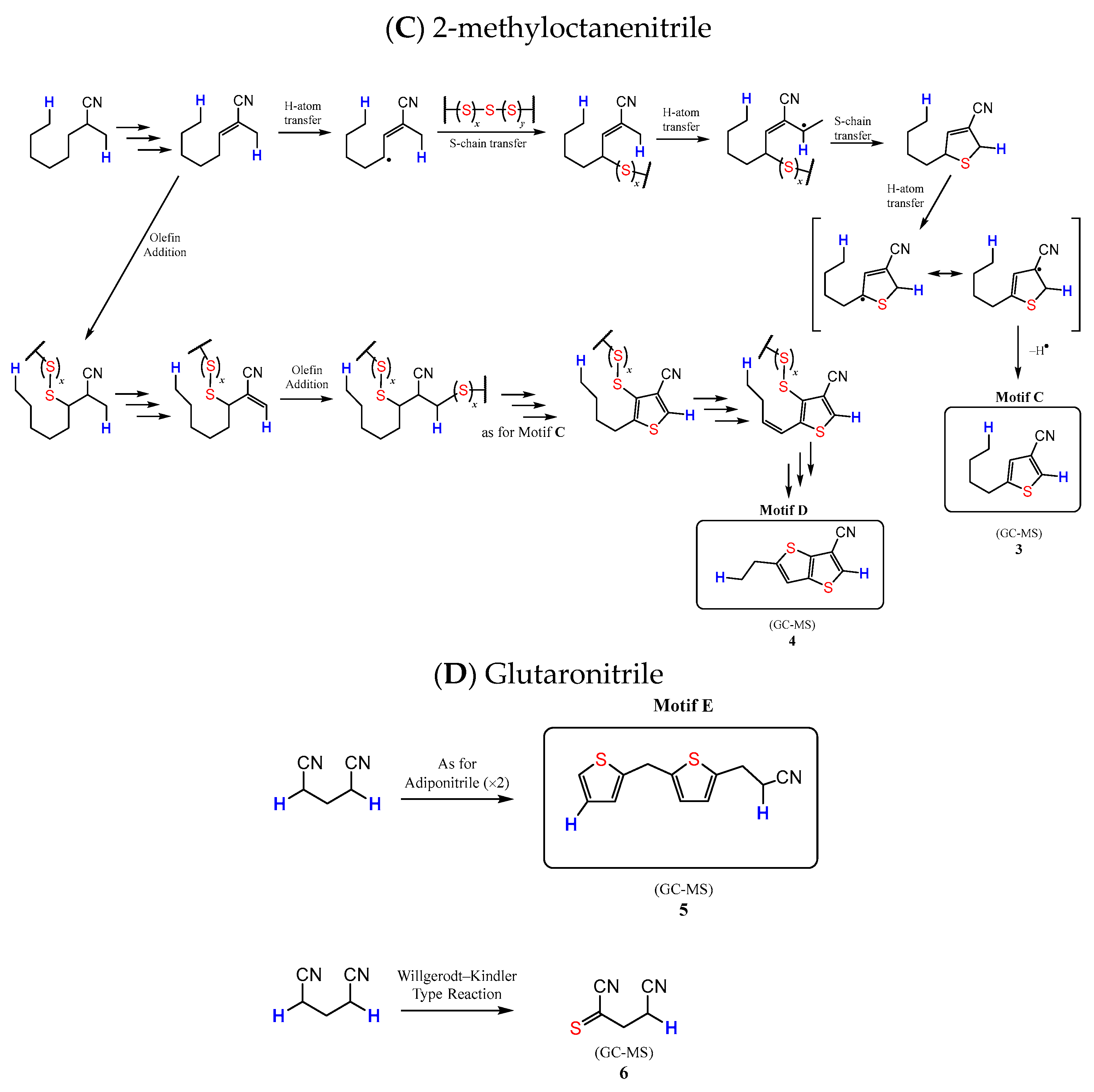

| Materials | Td [a] (°C) | Tm [b] (°C) | Tg, DSC [c] (°C) | Dark Sulfur (wt. %) |
|---|---|---|---|---|
| PANS90 | 209 | 119 | NA | 30 [d] |
| PSP90 [g] | 220 | 118 | −39 | 42 [d] |
| mPES [h] | 215 | 117 | −36 | 20 [e] |
| PGMA–S [i] | 211 | 117 | NA | 79 [f] |
| SPC90 [j] | 219 | 116 | −34 | 62 [f] |
| S8 | 229 | 118 | NA | NA |
| Compressional Strength (MPa) | Flexural Strength/Modulus (MPa) | Ultimate Tensile Strength (MPa) | |
|---|---|---|---|
| PANS90 | 11.4 ± 0.7 | 3.6 ± 0.4/975 ± 95 | 2.5 ± 0.2 |
| PSP90 [a] | 9.8 ± 1.2 | 2.4 ± 0.1/341 ± 23 | 2.0 ± 0.2 |
| mPES [b] | 26.9 ± 0.6 | 7.7 ± 0.2/320 ± 5 | 0.2 ± 0.0 |
| PGMA–S [c] | 17.5 ± 2.8 | 4.8 ± 0.7/642 ± 49 | 3.9 ± 1.2 |
| SPC90 [d] | 12.8 ± 1.6 | 3.1 ± 0.5 | ND |
| C62 Brick | 8.6 | ND [e] | ND |
| Portland Cement | 17.0 | 3.7/580 | ND |
| Process | Cost (+) or Credit (–)? | Value (kg CO2e) |
|---|---|---|
| Make PAN (0.10 kg × 5.4 kg CO2e/kg) | + | 0.54 |
| Grinding (0.100 kg of PAN × 0.092 kg CO2e/kg) | + | 0.0092 |
| Heating (1.00 kg mixture × 0.025 kg CO2e/kg) | + | 0.025 |
| Prevent Incineration of PAN (0.100 kg × 2.5 kg CO2e/kg) | – | 0.25 |
| Total | 0.33 kg CO2e/kg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wijeyatunga, S.K.; Smith, R.C. Upcycled Composite Derived from Polyacrylonitrile and Elemental Sulfur: Thermomechanical Properties and Microstructural Insight. Sustainability 2025, 17, 3702. https://doi.org/10.3390/su17083702
Wijeyatunga SK, Smith RC. Upcycled Composite Derived from Polyacrylonitrile and Elemental Sulfur: Thermomechanical Properties and Microstructural Insight. Sustainability. 2025; 17(8):3702. https://doi.org/10.3390/su17083702
Chicago/Turabian StyleWijeyatunga, Shalini K., and Rhett C. Smith. 2025. "Upcycled Composite Derived from Polyacrylonitrile and Elemental Sulfur: Thermomechanical Properties and Microstructural Insight" Sustainability 17, no. 8: 3702. https://doi.org/10.3390/su17083702
APA StyleWijeyatunga, S. K., & Smith, R. C. (2025). Upcycled Composite Derived from Polyacrylonitrile and Elemental Sulfur: Thermomechanical Properties and Microstructural Insight. Sustainability, 17(8), 3702. https://doi.org/10.3390/su17083702







