Screening of Methanotrophic Strain for Scale Applications: Methane Emission Reduction and Resource Utilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Source and Model Strain
2.2. Medium
2.3. Isolation and Storage of Strains
2.3.1. Preliminary Enrichment of Strains
2.3.2. Strains Screening
2.4. Strain Identification
2.4.1. Morphological Observation
2.4.2. Physiological and Biochemical Characterization
2.4.3. 16S rDNA Sequencing and Phylogenetic Tree Construction
2.5. DNA Extraction and Whole-Genome Analysis
2.6. Optimization of Culture Conditions
2.7. Data Analysis
3. Results
3.1. Strain Screening and Metabolic Characterization
3.2. Traditional Taxonomic Characteristics of Strain M6
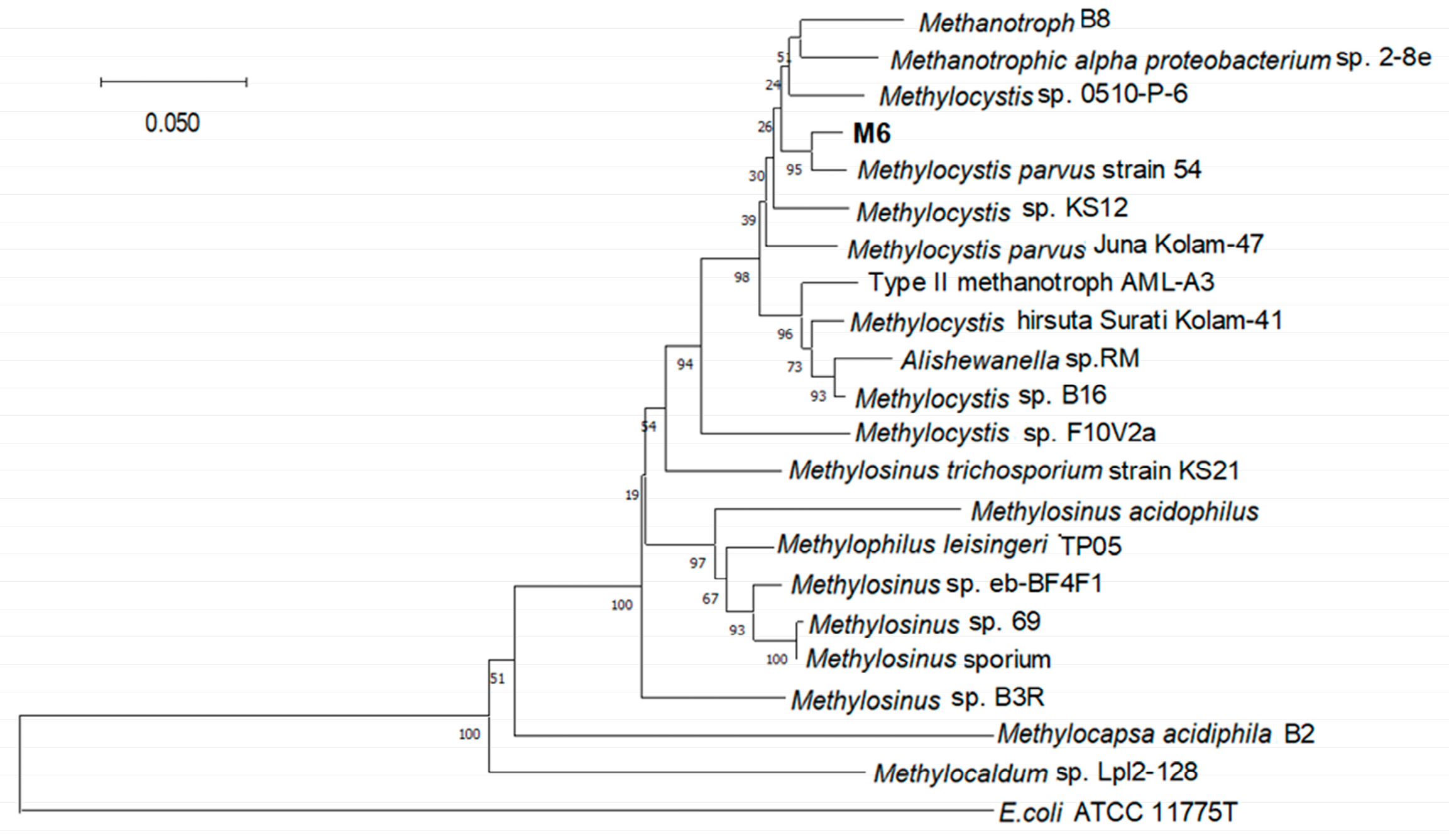
3.3. Phylogenetic Analysis of Strain M6
3.4. Metabolic Network Analysis of Strain M6
3.4.1. Genome Sequencing and Assembly
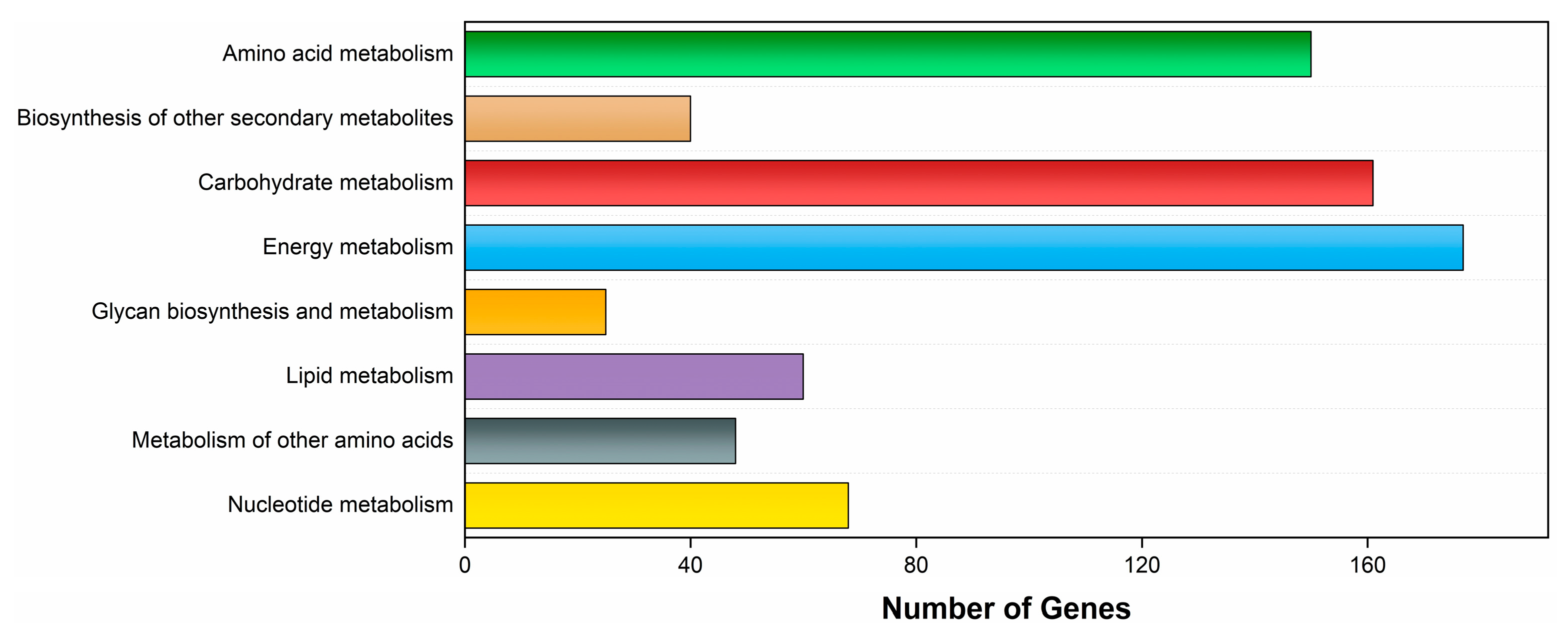
3.4.2. KEGG Database Annotation
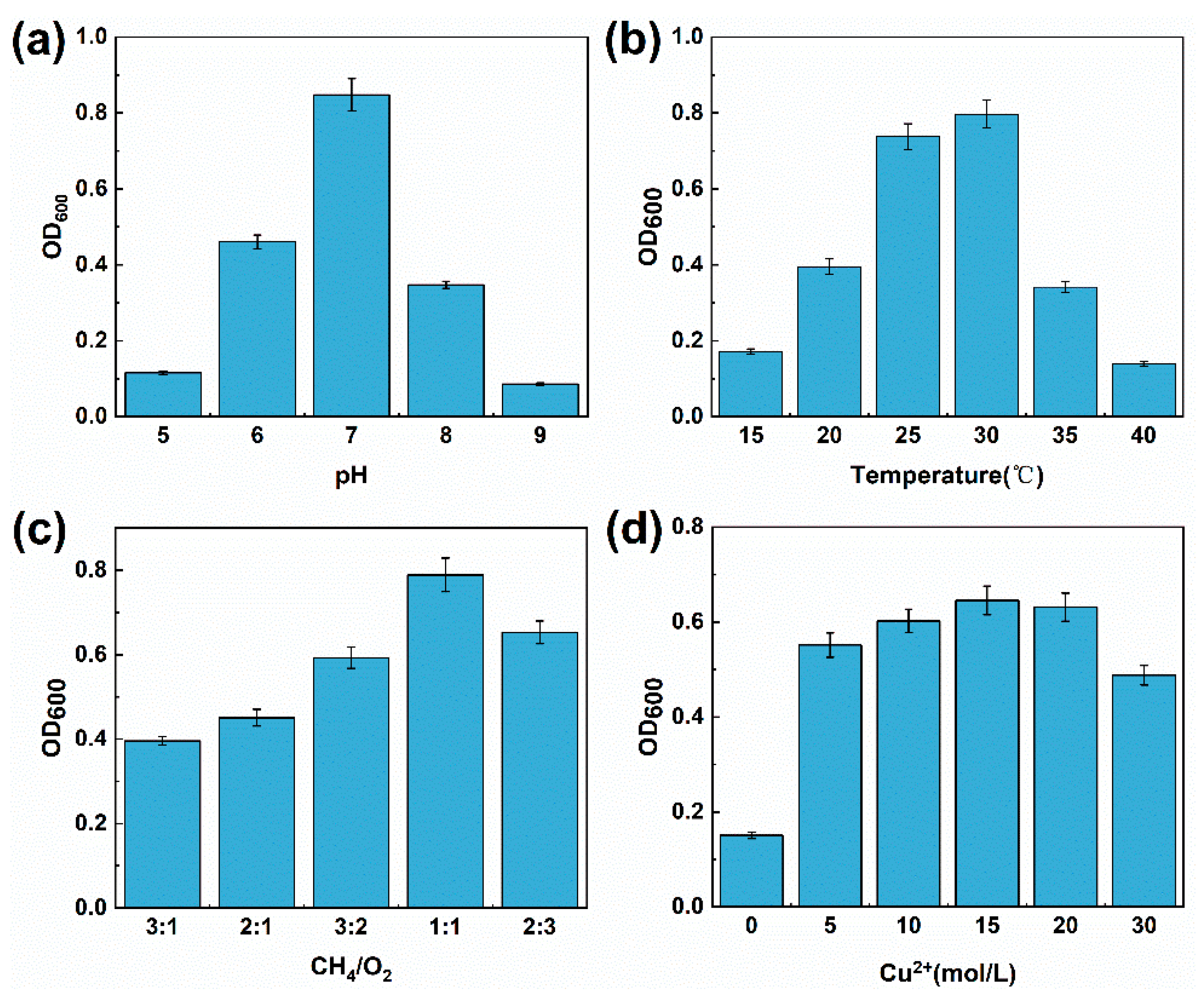
3.5. Optimization of Culture Conditions for Strain M6
4. Discussion
4.1. Rapid Proliferation and Nitrogen Assimilation Capability
4.2. Phylogenetic Affiliation and Metabolic Characteristic
4.3. Metabolic Functional Diversity
4.4. Mild Cultivation Conditions
4.5. Methane Emission Reduction and Resource Utilization
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| RuMP | Ribulose monophosphate |
| CBB | Calvin–Benson–Bassham |
| GO | Gene Ontology |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| COG | Clusters of Orthologous Groups |
| MMO | Methane monooxygenase |
| PHA | Polyhydroxyalkanoate |
| MDH | Methanol dehydrogenase |
References
- Kirschke, S.; Bousquet, P.; Ciais, P.; Saunois, M.; Canadell, J.G.; Dlugokencky, E.J.; Bergamaschi, P.; Bergmann, D.; Blake, D.R.; Bruhwiler, L.; et al. Three decades of global methane sources and sinks. Nat. Geosci. 2013, 6, 813–823. [Google Scholar] [CrossRef]
- Dean, J.F.; Middelburg, J.J.; Röckmann, T.; Aerts, R.; Blauw, L.G.; Egger, M.; Jetten, M.S.M.; de Jong, A.E.E.; Meisel, O.H.; Rasigraf, O.; et al. Methane Feedbacks to the Global Climate System in a Warmer World. Rev. Geophys. 2018, 56, 207–250. [Google Scholar] [CrossRef]
- Howarth, R.W. A bridge to nowhere: Methane emissions and the greenhouse gas footprint of natural gas. Energy Sci. Eng. 2014, 2, 47–60. [Google Scholar] [CrossRef]
- Lackner, K.S. Practical constraints on atmospheric methane removal. Nat. Sustain. 2020, 3, 357. [Google Scholar] [CrossRef]
- Chen, D.; Ma, M.Y.; Hu, L.T.; Du, Q.N.; Li, B.W.; Yang, Y.; Guo, L.Y.; Cai, Z.X.; Ji, M.R.; Zhu, R.Z.; et al. Characteristics of China’s coal mine methane emission sources at national and provincial levels. Environ. Res. 2024, 259, 119549. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.Y.; Ciais, P.; Smith, P.; Yan, X.Y.; Kuzyakov, Y.; Liu, S.W.; Li, T.T.; Zou, J.W. The role of rice cultivation in changes in atmospheric methane concentration and the Global Methane Pledge. Glob. Change Biol. 2023, 29, 2776–2789. [Google Scholar] [CrossRef]
- Chai, R.S.; Cao, H.J.; Huang, Q.Y.; Xie, L.H.; Yang, F.; Yin, H.B. Temporal and environmental factors drive community structure and function of methanotrophs in volcanic forest soils. J. For. Res. 2024, 35, 1–13. [Google Scholar] [CrossRef]
- Pan, J.R.; Wang, X.L.; Cao, A.X.; Zhao, G.Z.; Zhou, C.B. Screening methane-oxidizing bacteria from municipal solid waste landfills and simulating their effects on methane and ammonia reduction. Environ. Sci. Pollut. Res. 2019, 26, 37082–37091. [Google Scholar] [CrossRef]
- Qin, T.; Liu, Y.J.; Hu, R.W.; Yang, K.; Zheng, B.F.; Li, J.H.; Liu, Z.X.; Li, P.; Ma, T.T.; Xiong, K.L.; et al. Reduction of soil methane emissions from croplands with 20–40 years of cultivation mediated by methane-metabolizing microorganisms. J. Clean Prod. 2024, 435, 140489. [Google Scholar] [CrossRef]
- Whalen, S.C. Biogeochemistry of methane exchange between natural wetlands and the atmosphere. Environ. Eng. Sci. 2005, 22, 73–94. [Google Scholar] [CrossRef]
- Le, H.T.Q.; Lee, E.Y. Methanotrophs: Metabolic versatility from utilization of methane to multi-carbon sources and perspectives on current and future applications. Bioresour. Technol. 2023, 384, 129296. [Google Scholar] [CrossRef]
- Goyal, A.; Shukla, J.B. Can methane oxidising bacteria reduce global warming? A modelling study. Int. J. Glob. Warm. 2018, 15, 82–97. [Google Scholar] [CrossRef]
- Gesicka, A.; Oleskowicz-Popiel, P.; Lezyk, M. Recent trends in methane to bioproduct conversion by methanotrophs. Biotechnol. Adv. 2021, 53, 107861. [Google Scholar] [CrossRef] [PubMed]
- Khoshnevisan, B.; Tsapekos, P.; Zhang, Y.; Valverde-Perez, B.; Angelidaki, I. Urban biowaste valorization by coupling anaerobic digestion and single cell protein production. Bioresour. Technol. 2019, 290, 121743. [Google Scholar] [CrossRef]
- Sahoo, K.K.; Goswami, G.; Das, D. Biotransformation of Methane and Carbon Dioxide into High-Value Products by Methanotrophs: Current State of Art and Future Prospects. Front. Microbiol. 2021, 12, 636486. [Google Scholar] [CrossRef]
- Hanson, R.S.; Hanson, T.E. Methanotrophic bacteria. Microbiol. Rev. 1996, 60, 439–471. [Google Scholar] [CrossRef] [PubMed]
- Whittenbury, R.; Phillips, K.C.; Wilkinson, J.F. Enrichment, Isolation and Some Properties of Methane-Utilizing Bacteria. J. Gen. Microbiol. 1970, 61, 205–218. [Google Scholar] [CrossRef]
- Knief, C. Diversity and Habitat Preferences of Cultivated and Uncultivated Aerobic Methanotrophic Bacteria Evaluated Based on pmoA as Molecular Marker. Front. Microbiol. 2015, 6, 1346. [Google Scholar] [CrossRef]
- Lu, Y.Z.; Li, X.; Chen, Y.; Wang, Y.Z.; Zhu, G.C.; Zeng, R.J.X. The indispensable role of assimilation in methane driven nitrate removal. Sci. Total Environ. 2020, 746, 141089. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Kondaveeti, S.; Otari, S.V.; Pagolu, R.T.; Jeong, S.H.; Kim, S.C.; Cho, B.K.; Kang, Y.C.; Lee, J.K. Repeated batch methanol production from a simulated biogas mixture using immobilized Methylocystis bryophila. Energy 2018, 145, 477–485. [Google Scholar] [CrossRef]
- Koch, A.L. Turbidity measurements of bacterial cultures in some available commercial instruments. Anal. Biochem. 1970, 38, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Schill, N.; van Gulik, W.M.; Voisard, D.; von Stockar, U. Continuous cultures limited by a gaseous substrate: Development of a simple, unstructured mathematical model and experimental verification with Methanobacterium thermoautotrophicum. Biotechnol. Bioeng. 1996, 51, 645–658. [Google Scholar] [CrossRef]
- Ross, M.O.; Rosenzweig, A.C. A tale of two methane monooxygenases. J. Biol. Inorg. Chem. 2017, 22, 307–319. [Google Scholar] [CrossRef]
- Ge, X.; Yang, L.; Sheets, J.P.; Yu, Z.; Li, Y. Biological conversion of methane to liquid fuels: Status and opportunities. Biotechnol. Adv. 2014, 32, 1460–1475. [Google Scholar] [CrossRef] [PubMed]
- Dalton, H. The biochemistry of methylotrophs. Trends Biochem. Sci. 1983, 8, 342–343. [Google Scholar] [CrossRef]
- Beck, D.A.C.; McTaggart, T.L.; Setboonsarng, U.; Vorobev, A.; Kalyuzhnaya, M.G.; Ivanova, N.; Goodwin, L.; Woyke, T.; Lidstrom, M.E.; Chistoserdova, L. The Expanded Diversity of Methylophilaceae from Lake Washington Through Cultivation and Genomic Sequencing of Novel Ecotypes. PLoS ONE 2014, 9, e102458. [Google Scholar] [CrossRef]
- Chen, F.; Hu, X.; Hong, Z.; Duan, J.; Zhou, S.; Chen, J.; Wang, D.; Lin, H. Screening, Identification, and Fermentation Optimization of the Antagonistic Actinomycete Strain TCS21-117 Against Botrytis cinerea. Microorganisms 2025, 13, 379. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.Z.; Yang, Y.F.; Yan, X.; Zhang, T.Q.; Xiang, J.; Gao, Z.X.; Chen, Y.H.; Yang, S.H.; Fei, Q. Molecular Mechanism Associated with the Impact of Methane/Oxygen Gas Supply Ratios on Cell Growth of Methylomicrobium buryatense 5GB1 Through RNA-Seq. Front. Bioeng. Biotechnol. 2020, 8, 263. [Google Scholar] [CrossRef]
- Nguyen, A.D.; Lee, E.Y. Engineered Methanotrophy: A Sustainable Solution for Methane-Based Industrial Biomanufacturing. Trends Biotechnol. 2021, 39, 381–396. [Google Scholar] [CrossRef]
- Murrell, J.C.; McDonald, I.R.; Gilbert, B. Regulation of expression of methane monooxygenases by copper ions. Trends Microbiol. 2000, 8, 221–225. [Google Scholar] [CrossRef]
- Sun, J.; Yu, W.J.; Li, X.; Zhu, X.Y.; Pi, J.C.; Di, C.; Tan, X.Y.; Li, N.; Zhu, G.C.; Lu, Y.Z. Divergent effects of copper oxide nanoparticles on methanotrophs: Stimulation at low concentrations and inhibition at high concentrations. Biochem. Eng. J. 2024, 208, 109340. [Google Scholar] [CrossRef]
- Li, X.; Lu, Y.; Chen, Y.; Zhu, G.; Zeng, R.J. Constraining nitrification by intermittent aeration to achieve methane-driven ammonia recovery of the mainstream anaerobic effluent. J. Environ. Manag. 2021, 295, 113103. [Google Scholar] [CrossRef] [PubMed]
- Tsapekos, P.; Zhu, X.; Pallis, E.; Angelidaki, I. Proteinaceous methanotrophs for feed additive using biowaste as carbon and nutrients source. Bioresour. Technol. 2020, 313, 123646. [Google Scholar] [CrossRef]
- Zappi, A.; Fortela, D.L.; Holmes, W.E. An Assessment of Methanotrophs Producing Industrial-Grade Lipids for Biofuels and Other Commercial Chemicals. Energies 2020, 13, 3887. [Google Scholar] [CrossRef]
- Patel, S.K.S.; Singh, D.; Pant, D.; Gupta, R.K.; Busi, S.; Singh, R.V.; Lee, J.-K. Polyhydroxyalkanoate Production by Methanotrophs: Recent Updates and Perspectives. J. Microbiol. Biotechnol. 2016, 26, 717–724. [Google Scholar] [CrossRef]
- Mannina, G.; Presti, D.; Montiel-Jarillo, G.; Carrera, J.; Suárez-Ojeda, M.E. Recovery of polyhydroxyalkanoates (PHAs) from wastewater: A review. Bioresour. Technol. 2020, 297, 122478. [Google Scholar] [CrossRef]
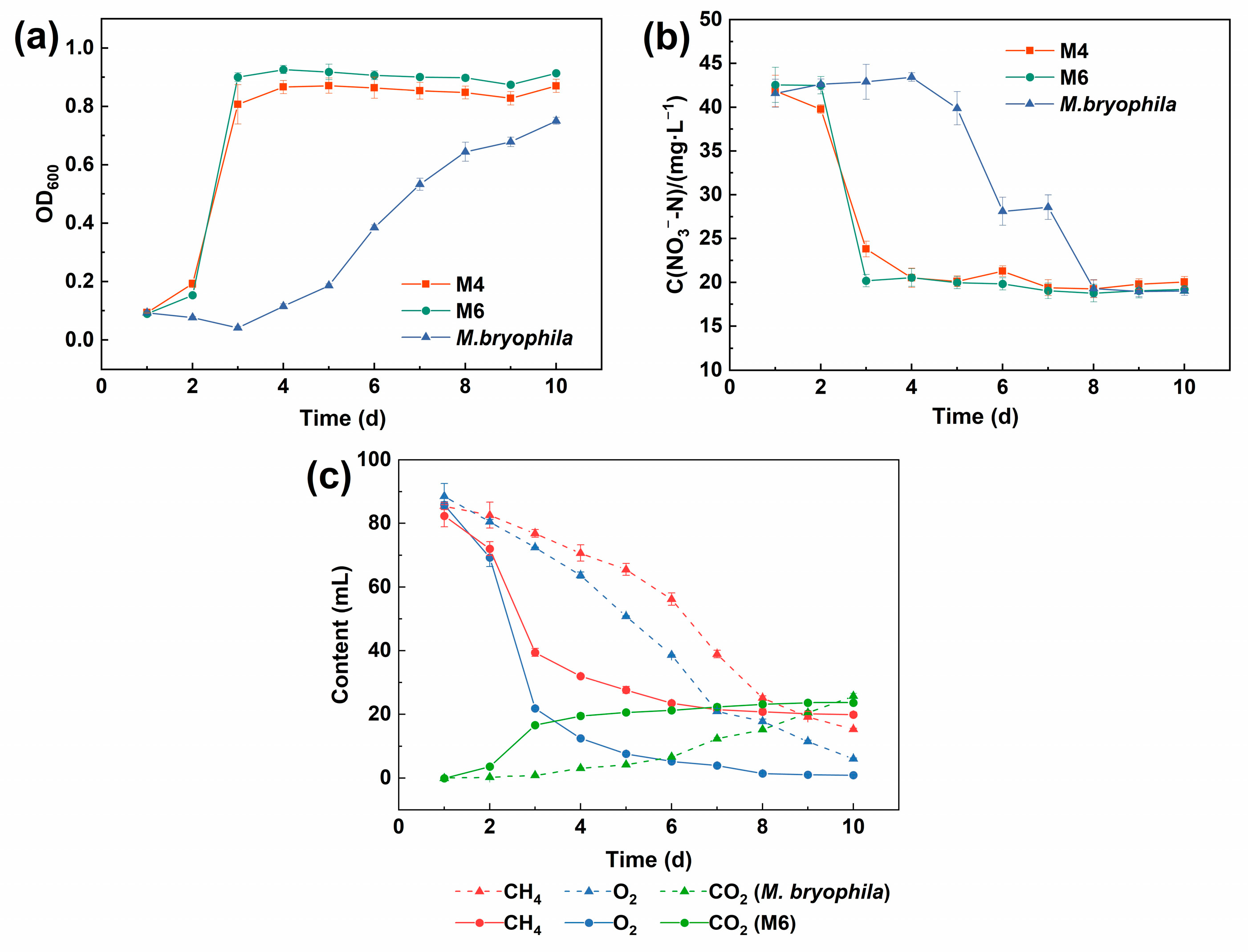

| Strain | V(O2)/V(CH4) | Y(CO2/CH4) | Y(Cells/CH4) * | μmax |
|---|---|---|---|---|
| M. bryophila | 1.11 | 0.33 | 1.28 × 108 | 0.28 |
| M6 | 1.35 | 0.38 | 1.71 × 108 | 0.78 |
| Indicator | Value |
|---|---|
| Number of scaffolds | 1 |
| Genome size (bp) | 4,148,822 |
| G + C content (mol%) | 64.15 |
| Number of genes | 4702 |
| Gene length/Genome length | 87.32 |
| tRNA | 51 |
| sRNA | 0 |
| rRNA | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Di, C.; Yu, W.; Lu, Y. Screening of Methanotrophic Strain for Scale Applications: Methane Emission Reduction and Resource Utilization. Sustainability 2025, 17, 3687. https://doi.org/10.3390/su17083687
Di C, Yu W, Lu Y. Screening of Methanotrophic Strain for Scale Applications: Methane Emission Reduction and Resource Utilization. Sustainability. 2025; 17(8):3687. https://doi.org/10.3390/su17083687
Chicago/Turabian StyleDi, Chen, Weijia Yu, and Yongze Lu. 2025. "Screening of Methanotrophic Strain for Scale Applications: Methane Emission Reduction and Resource Utilization" Sustainability 17, no. 8: 3687. https://doi.org/10.3390/su17083687
APA StyleDi, C., Yu, W., & Lu, Y. (2025). Screening of Methanotrophic Strain for Scale Applications: Methane Emission Reduction and Resource Utilization. Sustainability, 17(8), 3687. https://doi.org/10.3390/su17083687







