Challenges and Strategies for the Sustainable Environmental Management of Phosphogypsum
Abstract
1. Introduction
1.1. Physical and Chemical Characteristics of PG
1.2. Geographical Variability
1.3. Disposal Practices
2. Environmental Impacts and Regulatory Frameworks of PG Waste
2.1. Soil, Water, and Air Contamination
2.2. Health and Ecological Risks
| Contaminant | Exposure Pathway | Health Effects | References |
|---|---|---|---|
| Radium-226 | Airborne (dust) | Increased risk of bone cancer, leukemia, and anemia due to radioactive decay products (radon gas). | USEPA 1999: Radiation at Superfund Sites [125] IAEA, 2013 [5] |
| Water (leaching) | Elevated risk of internal radiation exposure, kidney damage, and soft tissue cancers. | WHO2011: Guidelines for Drinking-water Quality, 4th Edition [126] UNSCEAR, 2021: Report on Sources and Effects of Ionizing Radiation [7] | |
| Fluoride | Water | Dental and skeletal fluorosis with chronic exposure; neurological effects in high concentrations. | WHO 2011: Guidelines for Drinking-water Quality, 4th Edition [126] ATSDR 2012: Toxicological Profile for Cadmium [127] |
| Cadmium | Crops, Water | Kidney damage, skeletal demineralization, and increased risk of cancer through bioaccumulation. | WHO 2010: Guidelines for Drinking-water Quality, 4th Edition [126] Codex Alimentarius Commission, CXS 193-1995 [128] |
| Arsenic | Water, Crops | Increased risk of skin cancer, cardiovascular disease, and neurological toxicity. | WHO 2010: Guidelines for Drinking-water Quality, 4th Edition [126] Codex Alimentarius Commission: (CXS 193-1995) [128] |
| Lead | Airborne, Crops | Neurological impairment in children, kidney damage, and hypertension in adults. | USEPA 2006: Radiation at Superfund Sites [125] WHO 2011: Guidelines for Drinking-water Quality, 4th Edition [126] Codex Alimentarius Commission, CXS 193-1995 [128] |
| Uranium | Water, Crops | Nephrotoxicity, chemical toxicity in kidneys, and increased risk of cancer due to radiation. | ATSDR 2013: Toxicological Profile for Uranium [129] IAEA 2013: Safety Standards for the Management of NORM Residues [5] |
| Chromium | Water, Crops | Chromium VI exposure can cause lung cancer, kidney damage, and skin irritation. | ATSDR 2012: Toxicological Profile for Chromium [130] WHO 2010: Guidelines for Drinking-water Quality, 4th Edition [126] |
| Thorium | Airborne | Increased risk of lung and pancreatic cancers due to radioactive decay. | USEPA 2021: Radiation at Superfund Sites [125] WHO 2011: Guidelines for Drinking-water Quality, 4th Edition [126] |
| Zinc | Water, Crops | It can disrupt gastrointestinal function and immune response with chronic overexposure. | ATSDR 2005: Toxicological Profile for Zinc [131] |
| Nickel | Airborne, Water | Allergic reactions, respiratory issues, and increased cancer risk in high exposures. | ATSDR 2007: Toxicological Profile for Nickel [132] |
| Barium | Water | It can lead to gastrointestinal symptoms, muscle weakness, and cardiovascular toxicity. | ATSDR 2007: Toxicological Profile for Barium [133] |
2.3. Economic Consequences
2.4. Regulatory Frameworks and Challenges
2.4.1. International Guidelines on Phosphogypsum Management
- The International Atomic Energy Agency (IAEA) has developed detailed guidelines under the Safety Reports Series No. 78, focusing on managing Naturally Occurring Radioactive Materials (NORM) such as PG. The IAEA outlines protocols for radiological characterization, controlled storage, worker safety, and disposal. For example, PG stacks must include impermeable liners and leachate collection systems to prevent groundwater contamination, while reuse criteria ensure PG with low radioactivity is safely repurposed [5].
- The United Nations Environment Programme (UNEP) advocates a circular economy approach through its Sustainable Use of Industrial By-products framework. UNEP emphasizes resource recovery, such as extracting REEs, and promotes alternative uses of PG in construction and agriculture. It also highlights the importance of environmental risk assessments and policy support to encourage innovation and commercialization of PG-based products [139].
- The Food and Agriculture Organization (FAO) provides specific guidelines for PG use in agriculture, detailed in the FAO Soils Bulletin No. 62. These include strict thresholds for impurities like cadmium and lead to prevent soil and crop contamination. Application rates depend on soil and crop types, with safeguards to avoid areas with shallow groundwater. Farmer training on safe storage, handling, and application is also emphasized to balance agricultural benefits with environmental protection [140].
- The US Environmental Protection Agency (EPA) regulates PG management under frameworks like the Environmental Protection Authority Act (EPA)-1968. The EPA quantified ocean-dumping practices, including 4.5 million tons of industrial waste, and has established restrictions to mitigate PG’s environmental risks [134,141].
2.4.2. European Union Guidelines on Phosphogypsum Management
- The European Commission’s Radiological Protection Principles Concerning the Natural Radioactivity of Building Materials (RP-112, 1999) provides a foundation for PG management in the EU. These principles require radiological characterization of PG to ensure that its radioactivity levels, particularly from radium-226, thorium-232, and uranium-238, meet safety thresholds. The guidelines recommend limiting the gamma dose rate from building materials made with PG to a maximum of 1 millisievert per year [142].
- PG is classified as a waste material under the European Waste Framework Directive (2008/98/EC), which mandates proper handling to prevent environmental contamination. The directive emphasizes impermeable liners and leachate collection systems in PG storage facilities to prevent groundwater pollution. It also provides criteria for PG reuse, ensuring compliance with safety standards to minimize health and environmental risks. Member states are encouraged to develop strategies for reducing PG waste through recycling and reuse [143].
- The EU Circular Economy Action Plan (2020) aligns with the Sustainable Development Goals (SDGs) by promoting resource recovery, such as the extraction of REEs from PG. It advocates alternative uses for PG in construction materials (e.g., cement and plasterboard) and agriculture as a soil conditioner. These practices aim to reduce reliance on virgin resources and foster a sustainable waste management system [144].
- EU legislation mandates comprehensive environmental risk assessments to mitigate PG’s potential impacts. For instance, the Water Framework Directive (2000/60/EC) requires member states to monitor and manage the leachability of heavy metals and radionuclides from PG to protect water resources. Similarly, the Industrial Emissions Directive (2010/75/EU) sets strict emission limits for industries managing PG, requiring leachate control systems, emission monitoring, and advanced pollution abatement technologies [145,146].
- The Industrial Emissions Directive (2010/75/EU) specifically addresses phosphoric acid plants that produce PG, requiring the adoption of the Best Available Techniques (BAT) to reduce emissions and environmental impacts. It focuses on controlling dust, heavy metals, and radionuclides during PG production and storage, implementing impermeable liners for leachate containment, and ensuring regular reporting of emissions data to ensure compliance [145].
- The European Green Deal emphasizes a low-carbon, resource-efficient economy by 2050. It supports the reuse of PG in agriculture and construction, provided safety and environmental standards are met. The EU provides funding for research into innovative PG applications, such as carbon sequestration in construction and soil improvement techniques [62,147].
2.4.3. National Regulation of PG Management Practices by Countries in the EU
2.5. Gaps in Regulation and Monitoring Standards
2.5.1. Inconsistencies in Radiological Standards Across Countries
2.5.2. Lack of Harmonized Global Protocols for Long-Term Stack Monitoring
2.5.3. Gaps in EU Policy on Secondary Uses of PG in Non-Agricultural Sectors
2.5.4. Need for Integrated Policies Addressing Environmental Risks and Economic Opportunities
2.5.5. Policy Challenges for Reuse
3. Sustainable Management and Applications of PG
3.1. Waste Minimization
3.2. Safe Storage
3.3. Technological Innovations for Environmental Protection
3.4. Applications and Reuse Potential
3.4.1. Construction Materials (Cement, Concrete, Boards, Roads)
3.4.2. Agricultural and Land Reclamation Uses of PG
3.4.3. Recovery of Valuable Elements
3.4.4. Environmental and Industrial Uses of Phosphogypsum
- Mine Backfilling
- Wastewater Treatment
- Landfill Liner Material
- Sulfuric Acid Production
- Building Paints
- Plaster of Paris (POP)
- Energy Production
- Carbon Capture
- Decorative Tiles
- Production of Ammonium Sulfate and Calcium Carbonate
- Functional Filler in Composite Materials
- Oil–Water Separation Applications
3.5. Barriers to Reuse and Circular Economy Integration
3.5.1. Technological Barriers
- Processing Techniques
- Separation of Contaminants
3.5.2. Economic Feasibility and Market Acceptance
3.5.3. Managing Radioactive Elements
3.5.4. Circular Economy for PG
3.6. Case Studies: Phosphogypsum Recycling
4. Future Perspectives and Recommendations
4.1. Innovations in Material Recycling and Resource Recovery
4.2. Enhanced Monitoring and Characterization Techniques
4.3. Policy Recommendations
4.4. Emerging Technologies and Future Recommendations
4.5. Research and Policy Directions
Strategic Pathways for Collaboration
- Public–Private Partnerships (PPPs): Governments and industries can establish PPPs to co-fund research and development (R&D) initiatives to advance phosphogypsum recycling technologies. These partnerships can pool resources to scale up innovative solutions, such as extracting rare earth elements or converting phosphogypsum into sustainable construction materials [141].
- International Collaboration: International cooperation is essential given the global nature of phosphogypsum production and its environmental impacts. Collaborative efforts can focus on harmonizing safety standards, sharing best practices, and developing transboundary agreements for safe reuse [62,223].
- Academic–Industry Partnerships: Academic institutions and industries should work together to bridge knowledge gaps and accelerate the development of advanced technologies for phosphogypsum recycling. Joint research projects can focus on innovative applications, such as carbon capture, wastewater treatment, and advanced construction materials, aligning with circular economy principles [1,57,70].
- Stakeholder Engagement: Engaging local communities, environmental organizations, and policymakers is crucial for building public trust and ensuring socially responsible practices. Transparent communication about the safety and benefits of phosphogypsum reuse can increase market acceptance and address public concerns about radioactivity and environmental risks [5,204]
- Global Knowledge Networks: Establishing platforms for knowledge sharing among researchers, industries, and policymakers can drive innovation and reduce duplication of efforts. Conferences, workshops, and online repositories can facilitate the exchange of data, case studies, and research findings, fostering a collaborative environment for sustainable phosphogypsum management [235].
- Funding and Incentives: Governments and international bodies should create funding mechanisms and provide financial incentives to encourage collaboration on phosphogypsum projects. Subsidies for research, tax breaks for industries adopting recycling technologies, and grants for pilot projects can drive participation and innovation [2,3,15,20,62].
5. Conclusions
- Investing in advanced technologies for the extraction of REE with higher efficiency recovery and safe contaminant removal;
- Industrial integration incorporating phosphogypsum as raw material in sectors will enhance resource efficiency and support circular economy goals by offering sustainable alternatives and decreasing reliance on non-renewable materials in industrial processes;
- Regulatory frameworks and policymakers should establish harmonized global standards to ensure safe phosphogypsum reuse, address radiological concerns, and promote trade through financial incentives and awareness campaigns to enhance market acceptance;
- Public Awareness of the reuse of phosphogypsum encouraging its trade through financial incentives and awareness campaigns will help build market acceptance;
- Collaboration among industries, researchers, policymakers, and environmental organizations is essential to share best practices and overcome technical, regulatory, and market challenges through public–private partnerships;
- Academia should close knowledge gaps, improve the current industrial processes, and develop novel applications like carbon sequestration to expand sustainable phosphogypsum reuse;
- Global Cooperation and International collaboration are essential to standardizing practices, addressing radioactive contamination, and fostering knowledge exchange. This ensures consistent progress across regions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chernysh, Y.; Yakhnenko, O.; Chubur, V.; Roubík, H. Phosphogypsum Recycling: A Review of Environmental Issues, Current Trends, and Prospects. Appl. Sci. 2021, 11, 1575. [Google Scholar] [CrossRef]
- Pliaka, M.; Gaidajis, G. Potential uses of phosphogypsum: A review. J. Environ. Sci. Health—Part A Toxic/Hazard. Subst. Environ. Eng. 2022, 57, 746–763. [Google Scholar] [CrossRef] [PubMed]
- Tayibi, H.; Choura, M.; López, F.A.; Alguacil, F.J.; López-Delgado, A. Environmental impact and management of phosphogypsum. J. Environ. Manag. 2009, 90, 2377–2386. [Google Scholar] [CrossRef] [PubMed]
- Pérez-López, R.; Álvarez-Valero, A.M.; Nieto, J.M. Changes in mobility of toxic elements during the production of phosphoric acid in the fertilizer industry of Huelva (SW Spain) and environmental impact of phosphogypsum wastes. J. Hazard. Mater. 2007, 148, 745–750. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). Radiation Protection and Management of NORM Residues in the Phosphate Industry; Safety Reports Series No.78; International Atomic Energy Agency (IAEA): Vienna, Austria, 2013; p. 288. [Google Scholar]
- USEPA. Short-Term Methods for Estimating the Chronic Toxicity of Effluents and Receiving Waters to Marine and Estuarine Organisms; Report No.: EPA-821-R-02-014; US Environmental Protection Agency Office of Water: Washington, DC, USA, 2002; p. 486.
- UNSCEAR. Source and Effects of Ionizing Radiation: The United Nations Scientific Committee on the Effects of Atomic Radiation; United Nations: New York, NY, USA, 2021; Volume I, 14p. [Google Scholar]
- US Environmental Protection Agency (EPA). Radionuclides in Soil Guide for Incident Response—Radionuclides in Soil; Report No: EPA 402-R-12-006; US Environmental Protection Agency (EPA): Washington, DC, USA, 2012; p. 114.
- Saadaoui, E.; Ghazel, N.; Ben Romdhane, C.; Massoudi, N. Phosphogypsum: Potential uses and problems—A review. Int. J. Environ. Stud. 2017, 74, 558–567. [Google Scholar] [CrossRef]
- Grabas, K.; Pawełczyk, A.; Stręk, W.; Szełęg, E.; Stręk, S. Study on the Properties of Waste Apatite Phosphogypsum as a Raw Material of Prospective Applications. Waste Biomass Valorization 2019, 10, 3143–3155. [Google Scholar] [CrossRef]
- El Zrelli, R.; Rabaoui, L.; van Beek, P.; Castet, S.; Souhaut, M.; Grégoire, M.; Courjault-Radé, P. Natural radioactivity and radiation hazard assessment of industrial wastes from the coastal phosphate treatment plants of Gabes (Tunisia, Southern Mediterranean Sea). Mar. Pollut. Bull. 2019, 146, 454–461. [Google Scholar] [CrossRef]
- Bouargane, B.; Laaboubi, K.; Biyoune, M.G.; Bakiz, B.; Atbir, A. Effective and innovative procedures to use phosphogypsum waste in different application domains: Review of the environmental, economic challenges and life cycle assessment. J. Mater. Cycles Waste Manag. 2023, 25, 1288–1308. [Google Scholar] [CrossRef]
- Mesić, M.; Brezinščak, L.; Zgorelec, Ž.; Perčin, A.; Šestak, I.; Bilandžija, D.; Trdenić, M.; Lisac, H. The Application of Phosphogypsum in Agriculture. Agric. Conspec. Sci. 2016, 81, 7–13. [Google Scholar]
- Al-Hwaiti, M.; Al-Khashman, O. Potentially Utilizations of Jordan Phosphogypsum: A Review. Int. J. Curr. Res. 2019, 11, 3258–3262. [Google Scholar]
- Bituh, T.; Petrinec, B.; Skoko, B.; Babic, D.; Raseta, D. Phosphogypsum and its potential use in Croatia: Challenges and opportunities Fosfogips i njegovo potencijalno koristenje u Republici Hrvatskoj izazovi i prilike. Arh. Za Hig. Rada I Toksikol. 2021, 72, 93–100. [Google Scholar]
- Pérez-López, R.; Nieto, J.M.; de la Rosa, J.D.; Bolívar, J.P. Environmental tracers for elucidating the weathering process in a phosphogypsum disposal site: Implications for restoration. J. Hydrol. 2015, 529, 1313–1323. [Google Scholar] [CrossRef]
- Huang, X.; Zhao, X.; Bie, S.; Yang, C. Hardening Performance of Phosphogypsum-Slag-Based Material. Procedia Environ. Sci. 2016, 31, 970–976. [Google Scholar] [CrossRef]
- Fuleihan, N.F. Phosphogypsum disposal—The pros & cons of wet versus dry stacking. Procedia Eng. 2012, 46, 195–205. [Google Scholar]
- Silva, L.F.O.; Oliveira, M.L.S.; Crissien, T.J.; Santosh, M.; Bolivar, J.; Shao, L.; Dotto, G.L.; Gasparotto, J.; Schindler, M. A review on the environmental impact of phosphogypsum and potential health impacts through the release of nanoparticles. Chemosphere 2022, 286 Pt 1, 131513. [Google Scholar] [CrossRef]
- Rutherford, P.M.; Dudas, M.J.; Arocena, J.M. Radioactivity and elemental composition of phosphogypsum produced from three phosphate rock sources. Waste Manag. Res. 1995, 13, 407–423. [Google Scholar] [CrossRef]
- Nedelciu, C.E.; Ragnarsdottir, K.V.; Schlyter, P.; Stjernquist, I. Global phosphorus supply chain dynamics: Assessing regional impact to 2050. Glob. Food Secur. 2020, 26, 100426. [Google Scholar] [CrossRef]
- Villa, M.; Mosqueda, F.; Hurtado, S.; Mantero, J.; Manjón, G.; Periañez, R.; Vaca, F.; García-Tenorio, R. Contamination and restoration of an estuary affected by phosphogypsum releases. Sci. Total Environ. 2009, 408, 69–77. [Google Scholar] [CrossRef]
- Tovazhnyansky, L.L.; Meshalkin, V.P.; Kapustenko, P.O.; Bukhkalo, S.I.; Arsenyeva, O.P.; Perevertaylenko, O.Y. Energy efficiency of complex technologies of phosphogypsum conversion. Theor. Found. Chem. Eng. 2013, 47, 225–230. [Google Scholar] [CrossRef]
- Romero-Hermida, M.I.; Flores-Alés, V.; Hurtado-Bermúdez, S.J.; Santos, A.; Esquivias, L. Environmental impact of phosphogypsum-derived building materials. Int. J. Environ. Res. Public Health 2020, 17, 4248. [Google Scholar] [CrossRef]
- Macías, F.; Pérez-López, R.; Cánovas, C.R.; Carrero, S.; Cruz-Hernandez, P. Environmental Assessment and Management of Phosphogypsum According to European and United States of America Regulations. Procedia Earth Planet. Sci. 2017, 17, 666–669. [Google Scholar] [CrossRef]
- Guan, Q.; Wang, Z.; Zhou, F.; Yu, W.; Yin, Z.; Zhang, Z.; Chi, R.; Zhou, J. The Impurity Removal and Comprehensive Utilization of Phosphogypsum: A Review. Materials 2024, 17, 2067. [Google Scholar] [CrossRef] [PubMed]
- Chuan, L.M.; Zheng, H.G.; Zhao, J.J.; Wang, A.L.; Sun, S.F. Phosphogypsum production and utilization in China. IOP Conf. Ser. Mater. Sci. Eng. 2018, 382, 022099. [Google Scholar] [CrossRef]
- Florida Polytechnic University. Phosphogypsum Stacks; Florida Polytechnic University: Lakeland, FL, USA, 1992. [Google Scholar]
- Liang, H.; Zhang, P.; Jin, Z.; DePaoli, D. Rare earths recovery and gypsum upgrade from Florida phosphogypsum. Miner. Metall. Process. 2017, 34, 201–206. [Google Scholar] [CrossRef]
- Ennaciri, Y.; Zdah, I.; El Alaoui-Belghiti, H.; Bettach, M. Characterization and purification of waste phosphogypsum to make it suitable for use in the plaster and the cement industry. Chem. Eng. Commun. 2020, 207, 382–392. [Google Scholar] [CrossRef]
- Lutskiy, D.; Litvinova, T.; Ignatovich, A.; Fialkovskiy, I. Complex processing of phosphogypsum—A way of recycling dumps with reception of commodity production of wide application. J. Ecol. Eng. 2018, 19, 221–225. [Google Scholar] [CrossRef]
- Garbaya, H.; Jraba, A.; Khadimallah, M.A.; Elaloui, E. The development of a new phosphogypsum-based construction material: A study of the physicochemical, mechanical and thermal characteristics. Materials 2021, 14, 7369. [Google Scholar] [CrossRef]
- Samet, M.; Karray, F.; Mhiri, N.; Kamoun, L.; Sayadi, S.; Gargouri-Bouzid, R. Effect of phosphogypsum addition in the composting process on the physico-chemical proprieties and the microbial diversity of the resulting compost tea. Environ. Sci. Pollut. Res. 2019, 26, 21404–21415. [Google Scholar] [CrossRef]
- Maina, L.; Kiegiel, K.; Chajduk, E.; Zakrzewska-Kołtuniewicz, G. Chemical and radiochemical characterization of phosphogypsum from Poland. Nukleonika 2024, 69, 113–117. [Google Scholar] [CrossRef]
- Wędrychowicz, M.; Bydałek, A.W.; Skrzekut, T.; Noga, P.; Gabryelewicz, I.; Madej, P. Analysis of the mechanical strength, structure and possibilities of using waste phosphogypsum in aluminum powder composites. SN Appl. Sci. 2019, 1, 992. [Google Scholar] [CrossRef]
- Chaalal, O.; Madhuranthakam, C.M.R.; Moussa, B.; Hossain, M.M. Sustainable Approach for Recovery of Sulfur from Phophogypsum. ACS Omega 2020, 5, 8151–8157. [Google Scholar] [CrossRef] [PubMed]
- Marchi, G.; Spehar, C.R.; Sousa-Silva, J.C.; Guilherme, L.R.G.; Martins, E.S. Research Perspectives on the Use of Phosphogypsum in the Brazilian Cerrado. J. Agric. Food Dev. 2020, 6, 22–30. [Google Scholar] [CrossRef]
- IBM. Indian Minerals Yearbook 2022; Indian Bureau of Mines: Nagpur, India, 2024; Volume 2018, pp. 1–9.
- Alla, M.; Harrou, A.; Elhafiany, M.L.; Azerkane, D.; El Ouahabi, M.; Gharibi, E.K. Reduction of phosphogypsum to calcium sulfide (CaS) using metallic iron in a hydrochloric acid medium. Phosphorus Sulfur Silicon Relat. Elem. 2022, 197, 1026–1035. [Google Scholar] [CrossRef]
- Ennaciri, Y.; Bettach, M. Procedure to convert phosphogypsum waste into valuable products. Mater. Manuf. Process. 2018, 33, 1727–1733. [Google Scholar] [CrossRef]
- Folek, S.; Walawska, B.; Wilczek, B.; Miśkiewicz, J. Use of phosphogypsum in road construction. Pol. J. Chem. Technol. 2011, 13, 18–22. [Google Scholar] [CrossRef]
- Guan, Q.; Zhou, Y.; Yu, W.; Fan, R.; Sui, Y.; Bu, Y.; Yin, Z.; Chi, R.-a.; Gao, Z. Efficient removal of impurities from phosphogypsum during preparation of α-hemihydrate gypsum. Miner. Eng. 2023, 201, 108203. [Google Scholar] [CrossRef]
- Bouargane, B.; Biyoune, M.G.; Mabrouk, A.; Bachar, A.; Bakiz, B.; Ait Ahsaine, H.; Mançour Billah, S.; Atbir, A. Experimental Investigation of the Effects of Synthesis Parameters on the Precipitation of Calcium Carbonate and Portlandite from Moroccan Phosphogypsum and Pure Gypsum Using Carbonation Route. Waste Biomass Valorization 2020, 11, 6953–6965. [Google Scholar] [CrossRef]
- Lee, J.; Yi, S.C. Assessment of radiological impact on the surrounding environment and biota for phosphogypsum waste stockyard in Korean facility. Env. Monit Assess 2023, 195, 767. [Google Scholar] [CrossRef]
- Cárdenas-Escudero, C.; Morales-Flórez, V.; Pérez-López, R.; Santos, A.; Esquivias, L. Procedure to use phosphogypsum industrial waste for mineral CO2 sequestration. J. Hazard. Mater. 2011, 196, 431–435. [Google Scholar] [CrossRef]
- Al-Masri, M.S.; Amin, Y.; Ibrahim, S.; Al-Bich, F. Distribution of some trace metals in Syrian phosphogypsum. Appl. Geochem. 2004, 19, 747–753. [Google Scholar] [CrossRef]
- Sfar Felfoul, H.; Clastres, P.; Ben Ouezdou, M.; Arligué, C.-G. Proprietes et perspectives de valorisation du phosphogypse l’exemple de la Tunisie. In Proceedings of the International Symposium on Environmental Pollution Control and Waste Management, Tunis, Republic of Tunisia, 7–10 January 2002; Volume 3, pp. 510–520. [Google Scholar]
- Hassen, S.; Anna, Z.; Elaloui, E.E.; Belgacem, M.N.; Mauret, E. Study of the valorization of phosphogypsum in the region of Gafsaas filler in paper. IOP Conf. Ser. Mater. Sci. Eng. 2012, 28, 012018. [Google Scholar] [CrossRef]
- Deǧirmenci, N. Utilization of phosphogypsum as raw and calcined material in the manufacturing of building products. Constr. Build. Mater. 2008, 22, 1857–1862. [Google Scholar] [CrossRef]
- Guan, Q.; Sun, N.; Bu, Y.; Fan, R.; Zhang, Z.; Yu, W.; Chi, R.-a.; Gao, Z. Efficient extraction of impurities from phosphogypsum during crystal regulation of α-hemihydrate gypsum. J. Am. Ceram. Soc. 2023, 106, 7360–7374. [Google Scholar] [CrossRef]
- Dvorkin, L.; Lushnikova, N.; Sonebi, M. Application areas of phosphogypsum in production of mineral binders and composites based on them: A review of research results. MATEC Web Conf. 2018, 149, 1–9. [Google Scholar] [CrossRef]
- Burnett, W.C.; Schultz, M.K.; Hull, C.D. Radionuclide flow during the conversion of phosphogypsum to ammonium sulfate. J. Environ. Radioact. 1996, 32, 33–51. [Google Scholar] [CrossRef]
- Thakur, Y.; Tyagi, A.; Sarkar, S. Utilization of Industrial Waste Phosphogypsum as Geomaterial: A Review. J. Hazard. Toxic Radioact. Waste 2023, 27, 03123001. [Google Scholar] [CrossRef]
- Choura, M.; Maalouf, F.; Keskes, M.; Cherif, F. Sulphur matrix from phosphogypsum: A sustainable route to waste valorization. In Beneficiation of Phosphates: New Thought, New Technology, New Development; Society for Mining, Metallurgy, and Exploration: Englewood, CO, USA, 2012; pp. 297–302. [Google Scholar]
- Krishnamurthy, N.; Gupta, C.K. Extractive Metallurgy of Rare Earths, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Mashifana, T.P. Chemical treatment of phosphogypsum and its potential application for building and construction. Procedia Manuf. 2019, 35, 641–648. [Google Scholar] [CrossRef]
- Bilal, E.; Bellefqih, H.; Bourgier, V.; Mazouz, H.; Dumitraş, D.-G.; Bard, F.; Laborde, M.; Caspar, J.P.; Guilhot, B.; Iatan, E.-L.; et al. Phosphogypsum circular economy considerations: A critical review from more than 65 storage sites worldwide. J. Clean. Prod. 2023, 414, 137561. [Google Scholar] [CrossRef]
- Cuesta, E.; Barba-Lobo, A.; Lozano, R.L.; San Miguel, E.G.; Mosqueda, F.; Bolívar, J.P. A comparative study of alternative methods for 210Pb determination in environmental samples. Radiat. Phys. Chem. 2022, 191, 109840. [Google Scholar] [CrossRef]
- Wei, Z.; Deng, Z. Research hotspots and trends of comprehensive utilization of phosphogypsum: Bibliometric analysis. J. Environ. Radioact. 2022, 242, 106778. [Google Scholar] [CrossRef]
- Pérez-López, R.; Nieto, J.M.; López-Coto, I.; Aguado, J.L.; Bolívar, J.P.; Santisteban, M. Dynamics of contaminants in phosphogypsum of the fertilizer industry of Huelva (SW Spain): From phosphate rock ore to the environment. Appl. Geochem. 2010, 25, 705–715. [Google Scholar] [CrossRef]
- Borges, R.C.; Ribeiro, F.C.A.; Lauria, D.; da Costa Lauria, D.; Bernedo, A.V.B. Radioactive characterization of phosphogypsum from Imbituba, Brazil. J. Environ. Radioact. 2013, 126, 188–195. [Google Scholar] [CrossRef] [PubMed]
- Haneklaus, N.; Barbossa, S.; Basallote, M.D.; Bertau, M.; Bilal, E.; Chajduk, E.; Chernysh, Y.; Chubur, V.; Cruz, J.; Dziarczykowski, K.; et al. Closing the upcoming EU gypsum gap with phosphogypsum. Resour. Conserv. Recycl. 2022, 182, 106328. [Google Scholar] [CrossRef]
- Outbakat, M.B.; El Mejahed, K.; El Gharous, M.; El Omari, K.; Beniaich, A. Effect of Phosphogypsum on Soil Physical Properties in Moroccan Salt-Affected Soils. Sustainability 2022, 14, 13087. [Google Scholar] [CrossRef]
- Pinto, S.R.; Angulski da Luz, C.; Munhoz, G.S.; Medeiros-Junior, R.A. Resistance of phosphogypsum-based supersulfated cement to carbonation and chloride ingress. Constr. Build. Mater. 2020, 263, 120640. [Google Scholar] [CrossRef]
- Jha, M.K.; Kumari, A.; Panda, R.; Kumar, J.R.; Yoo, K.; Lee, J.Y. Review on hydrometallurgical recovery of rare earth metals. Hydrometallurgy 2016, 165 Pt 1, 2–26. [Google Scholar] [CrossRef]
- Haneklaus, N.H.; Mwalongo, D.A.; Lisuma, J.B.; Amasi, A.I.; Mwimanzi, J.; Bituh, T.; Ćirić, J.; Nowak, J.; Ryszko, U.; Rusek, P.; et al. Rare earth elements and uranium in Minjingu phosphate fertilizer products: Plant food for thought. Resour. Conserv. Recycl. 2024, 207, 107694. [Google Scholar] [CrossRef]
- Hentati, O.; Abrantes, N.; Caetano, A.L.; Bouguerra, S.; Gonçalves, F.; Römbke, J.; Pereira, R. Phosphogypsum as a soil fertilizer: Ecotoxicity of amended soil and elutriates to bacteria, invertebrates, algae and plants. J. Hazard. Mater. 2015, 294, 80–89. [Google Scholar] [CrossRef]
- Li, W.; Zhang, Y.; Li, X.; Liu, C. Weathering and Transformation of Phosphogypsum: Implications for Waste Management. Waste Manag. 2020, 106, 232–240. [Google Scholar]
- Rashad, A.M. Phosphogypsum as a construction material. J. Clean. Prod. 2017, 166, 732–743. [Google Scholar] [CrossRef]
- Rajkovic, M.B.; Tošković, D. A new procedure of phosphogypsum purification in order to diminish the content of radionuclides. Environ. Prot. Eng. 2003, 29, 45–63. [Google Scholar]
- Bouargane, B.; Pérez-Moreno, S.M.; Barba-Lobo, A.; Bakiz, B.; Atbir, A.; Pedro Bolívar, J. Behavior of heavy metals and natural radionuclides along the Moroccan phosphogypsum carbonation process with several alkaline reagents. Chem. Eng. Sci. 2023, 280, 119013. [Google Scholar] [CrossRef]
- IAEA. Extent of Environmental Contamination by Naturally Occurring Radioactive Material (Norm) and Technological Options for Mitigation; Report Trs-419; IAEA: Vienna, Austria, 2003; p. 208. [Google Scholar]
- CNEN. Ministério da Ciência, Tecnologia e Inovação. 2015. [Spanish]. Available online: https://www.gov.br/cnen/pt-br/acesso-a-informacao/atos-normativos-cnen/comissao_deliberativa/resolucoes/2015/rs_cnencd_189_2015.pdf (accessed on 2 April 2025).
- International Atomic Energy Agency (IAEA). Regulatory Control of Exposure Due to Radionuclides in Building Materials and Construction Materials. Safety Reports Series; International Atomic Energy Agency (IAEA): Vienna, Austria, 2023; p. 85. [Google Scholar]
- Maina, L.; Kiegiel, K.; Haneklaus, N.; Samczy, Z. Sulfuric Acid Leaching Recovery of Rare Earth Elements from Wizów ’s Phosphogypsum in Poland. Sustainability 2024, 16, 9059. [Google Scholar] [CrossRef]
- Al-Hwaiti, M.; Ibrahim, K.A.; Harrara, M. Removal of heavy metals from waste phosphogypsum materials using polyethylene glycol and polyvinyl alcohol polymers. Arab. J. Chem. 2019, 12, 3141–3150. [Google Scholar] [CrossRef]
- El Afifi, E.M.; Hilal, M.A.; Attallah, M.F.; EL-Reefy, S.A. Characterization of phosphogypsum wastes associated with phosphoric acid and fertilizers production. J. Environ. Radioact. 2009, 100, 407–412. [Google Scholar] [CrossRef]
- Santos, A.J.G.; Mazzilli, B.P.; Favaro, D.I.T. Characterization of stockpiled phosphogypsum waste in Santos basin, Brazil. Radioprotection 2002, 37, C1-1307–C1-1315. [Google Scholar] [CrossRef]
- Tayibi, H.; Gascó, C.; Navarro, N.; López-Delgado, A.; Choura, M.; Alguacil, F.J.; López, F.A. Radiochemical Characterization of Phosphogypsum for Engineering Use. J. Environ. Prot. 2011, 2, 168–174. [Google Scholar] [CrossRef]
- Máduar, M.F.; Mazzilli, B.P.; Nisti, M.B. Radiation hazard indices in the application of phosphogypsum mixtures as a building material: Proposal for a Brazilian regulation. Braz. J. Radiat. Sci. 2019, 7. [Google Scholar] [CrossRef]
- Men, J.; Li, Y.; Cheng, P.; Zhang, Z. Recycling phosphogypsum in road construction materials and associated environmental considerations: A review. Heliyon 2022, 8, e11518. [Google Scholar] [CrossRef]
- US-EPA. EPA Regulation of Phosphogypsum; US-EPA: Washington, DC, USA, 2024.
- Owens, C.L.; Nash, G.R.; Hadler, K.; Fitzpatrick, R.S.; Anderson, C.G.; Wall, F. Apatite enrichment by rare earth elements: A review of the effects of surface properties. Adv. Colloid Interface Sci. 2019, 265, 14–28. [Google Scholar] [CrossRef]
- Moshynskyi, V.; Zhomyruk, R.; Vasylchuk, O.; Semeniuk, V.; Okseniuk, R.; Rysbekov, K.; Yelemessov, K. Investigation of technogenic deposits of phosphogypsum dumps. E3S Web Conf. 2021, 280, 08008. [Google Scholar] [CrossRef]
- Robinson, M.J.C.; Dhar, A.; Naeth, M.A.; Nichol, C.K. Phosphogypsum Stack Reclamation Using Soil Amendments and Short-Rotational Woody Species. Land 2022, 11, 2003. [Google Scholar] [CrossRef]
- Indelicato, S.; Orecchio, S.; Avellone, G.; Bellomo, S.; Ceraulo, L.; Di Leonardo, R.; Di Stefano, V.; Favara, R.; Gagliano Candela, E.; La Pica, L.; et al. Effect of solid waste landfill organic pollutants on groundwater in three areas of Sicily (Italy) characterized by different vulnerability. Environ. Sci. Pollut. Res. 2017, 24, 16869–16882. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, J.L.; Pérez-Moreno, S.M.; Gutiérrez-Álvarez, I.; Gázquez, M.J.; Bolívar, J.P. Behaviour of heavy metals and natural radionuclides in the mixing of phosphogypsum leachates with seawater. Environ. Pollut. 2021, 268, 115843. [Google Scholar] [CrossRef]
- Kumar, S. Guidelines for Management and Handling of Phosphogypsum Generated from Phosphoric Acid Plants; Ministry of the Environment, Forests, and Climate Change: Dheli, India, 2014; pp. 1–64.
- Yang, X.; Werner, J.; Honaker, R.Q. Leaching of rare Earth elements from an Illinois basin coal source. J. Rare Earths 2019, 37, 312–321. [Google Scholar] [CrossRef]
- Kateb AEl Stalder, C.; Rüggeberg, A.; Neururer, C.; Spangenberg, J.E.; Spezzaferri, S. Impact of industrial phosphate waste discharge on the marine environment in the Gulf of Gabes (Tunisia). PLoS ONE 2018, 13, e0197731. [Google Scholar]
- Koukouliou, V. Phosphogypsum Disposal in Greece; Greek Atomic Energy Commission: Paraskeví, Greece, 2006.
- Li, C.; Dong, Y.; Yi, Y.; Tian, J.; Xuan, C.; Wang, Y.; Wen, Y.; Cao, J. Effects of phosphogypsum on enzyme activity and microbial community in acid soil. Sci. Rep. 2023, 13, 6189. [Google Scholar] [CrossRef]
- Belahbib, L.; Arhouni, F.E.; Boukhair, A.; Essadaoui, A.; Ouakkas, S.; Hakkar, M.; Abdo, M.A.S.; Benjelloun, M.; Bitar, A.; Nourreddine, A. Impact of Phosphate Industry on Natural Radioactivity in Sediment, Seawater, and Coastal Marine Fauna of El Jadida Province, Morocco. J. Hazard. Toxic Radioact. Waste 2021, 25, 04020064. [Google Scholar] [CrossRef]
- Nahiun, K.M.; Sarker, B.; Keya, K.N.; Mahir, F.I.; Shahida, S.; Khan, R.A. A Review on the Methods of Industrial Waste Water Treatment. Sci. Rev. 2021, 73, 20–31. [Google Scholar] [CrossRef]
- Zmemla, R.; Sdiri, A.; Naifar, I.; Benjdidia, M.; Elleuch, B. Tunisian phosphogypsum tailings: Assessment of leaching behavior for an integrated management approach. Environ. Eng. Res. 2020, 25, 345–355. [Google Scholar] [CrossRef]
- Silva, N.C.; Fernandes, E.A.D.N.; Cipriani, M.; Taddei, M.H.T. Leaching Assessment of Radioactive and Non-Radioactive Elements from Brazilian Phosphogypsum; International Atomic Energy Agency: Vienna, Austria, 2022; pp. 142–148. [Google Scholar]
- Dahnoun, K.; Djadouni, F. Effects of Heavy-Metal Pollution on Soil Microbial Community, Plants, and Human Health. Jordan J. Earth Environ. Sci. 2020, 11, 234–240. [Google Scholar]
- Yücel, H.; Demirel, H.; Parmaksiz, A.; Karadeniz, H.; Çakir, İ.T.; Çetiner, B.; Zararsiz, A.; Kaplan, M.; Özgür, S.; Kislal, H.; et al. Measurement of Natural Radioactivity in Phosphogypsum by High Resolution Gamma Ray Spectrometry. In Radiation Safety Problems in the Caspian Region, Proceedings of the NATO Advanced Research Workshop on Radiation Safety Problems in the Caspian Region, Baku, Azerbaijan,11–14 September 2003; Springer: Dordrecht, The Netherlands, 2006; pp. 197–204. [Google Scholar] [CrossRef]
- Wang, K.; Zhang, N.; Tan, F.; He, J.; Li, J.; Bao, L. The influence of passivating agent on soil pollution. MethodsX 2021, 8, 101321. [Google Scholar] [CrossRef] [PubMed]
- Michael, A. Heavy Metals in Soil: A Review. Chem. Eng. Process Tech. 2023, 8, 1076. [Google Scholar]
- Xie, Y.; Huang, J.; Wang, H.; Lv, S.; Jiang, F.; Pan, Z.; Liu, J. Simultaneous and efficient removal of fluoride and phosphate in phosphogypsum leachate by acid-modified sulfoaluminate cement. Chemosphere 2022, 305, 135422. [Google Scholar] [CrossRef] [PubMed]
- Jin, H.; Wang, Y.; An, X.; Wang, S.; Wang, Y.; Yang, G.; Shi, L.; Sun, Y. A Review of Fluoride Removal from Phosphorous Gypsum: A Quantitative Analysis via a Machine Learning Approach. Materials 2024, 17, 3606. [Google Scholar] [CrossRef]
- Msila, X.; Labuschagne, F.; Barnard, W.; Billing, D.G. Radioactive nuclides in phosphogypsum from the lowveld region of South Africa. S. Afr. J. Sci. 2016, 112, 1–5. [Google Scholar] [CrossRef]
- Idboufrade, A.; Bouargane, B.; Ennasraoui, B.; Biyoune, M.G.; Bachar, A.; Bakiz, B.; Atbir, A.; Mançour-Billah, S. Phosphogypsum Two-Step Ammonia-Carbonation Resulting in Ammonium Sulfate and Calcium Carbonate Synthesis: Effect of the Molar Ratio OH−/Ca2+ on the Conversion Process. Waste Biomass Valorization 2022, 13, 1795–1806. [Google Scholar] [CrossRef]
- Rentería-Villalobos, M.; Vioque, I.; Mantero, J.; Manjón, G. Radiological, chemical and morphological characterizations of phosphate rock and phosphogypsum from phosphoric acid factories in SW Spain. J. Hazard. Mater. 2010, 181, 193–203. [Google Scholar] [CrossRef]
- Akter, S.; Muniruzzaman, M. Industrial Waste Management and Environment: A Study in Kamrangirchar (Raised Land), Dhaka. Environ. Manag. Sustain. Dev. 2020, 10, 24. [Google Scholar] [CrossRef]
- Kozłowski, R.; Szwed, M.; Żelezik, M. Environmental aspect of the cement manufacturing in the Świętokrzyskie mountains (Southeastern Poland). Minerals 2021, 11, 277. [Google Scholar] [CrossRef]
- Chuan, M.C.; Shu, G.Y.; Liu, J.C. Solubility of heavy metals in a contaminated soil: Effects of redox potential and pH. Water Air Soil Pollut. 1996, 90, 543–556. [Google Scholar] [CrossRef]
- Zielinski, R.A.; Al-Hwaiti, M.S.; Budahn, J.R.; Ranville, J.F. Radionuclides, trace elements, and radium residence in phosphogypsum of Jordan. Environ. Geochem. Health 2011, 33, 149–165. [Google Scholar] [CrossRef] [PubMed]
- Lei, L.; Gu, J.; Wang, X.; Song, Z.; Yu, J.; Guo, H.; Xie, J.; Wang, J.; Sun, W. Effects and Microbial Mechanisms of Phosphogypsum and Medical Stone on Organic Matter Degradation and Methane Emissions During Swine Manure Composting. J. Environ. Manag. 2022, 315, 115139. [Google Scholar] [CrossRef]
- Ali, M.A.; Lee, C.-H.; Kim, P.J. Effect of Phosphogypsum on Reduction of Methane Emission from Rice Paddy Soil. Korean J. Environ. Agric. 2007, 26, 131–136. [Google Scholar] [CrossRef]
- US-EPA. EPA Assessment of Risks from Radon in Homes; Office of Radiation and Indoor Air: Washington, DC, USA, 2003; p. 88.
- Chen, T.M.; Kuschner, W.G.; Gokhale, J.; Shofer, S. Outdoor air pollution: Nitrogen dioxide, sulfur dioxide, and carbon monoxide health effects. Am. J. Med. Sci. 2007, 333, 249–256. [Google Scholar] [CrossRef]
- USEPA. Sulfur Dioxide Basics What is SO2 and How Does It Get in the Air; EPA: Washington, DC, USA, 2017.
- Saueia, C.H.R.; Mazzilli, B.P. Distribution of natural radionuclides in the production and use of phosphate fertilizers in Brazil. J. Environ. Radioact. 2006, 89, 229–239. [Google Scholar] [CrossRef]
- Al Attar, L.; Al-Oudat, M.; Kanakri, S.; Budeir, Y.; Khalily, H.; Al Hamwi, A. Radiological impacts of phosphogypsum. J. Environ. Manag. 2011, 92, 2151–2158. [Google Scholar] [CrossRef]
- Njoku, P.O.; Edokpayi, J.N.; Odiyo, J.O. Health and environmental risks of residents living close to a landfill: A case study of thohoyandou landfill, Limpopo province, South Africa. Int. J. Environ. Res. Public Health 2019, 16, 2125. [Google Scholar] [CrossRef]
- Muhammad Zaffar Hashmi, V.S. Dust and Health Challenges and Solutions; Al-Dousari, A., Safat, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2023; pp. 1–259. [Google Scholar]
- Cross, F.L.; Ross, R.W. New developments in fluoride emissions from phosphate processing plants. J. Air Pollut. Control. Assoc. 1969, 19, 15–17. [Google Scholar] [CrossRef]
- Burge, F.; Burns, G.J.; Burridge, R.A.; Findlay, D.J.S.; Haynes, D.J.; Kershaw, A.H.; Masterson, P.A.; McCrohon, R.; Škoro, G.P.; Wright, P.N.M. Monitoring radioactive gaseous emissions from the ISIS Spallation Neutron and Muon Source. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2021, 1013, 165640. [Google Scholar] [CrossRef]
- Saravanakumar, R.; Gopi, R.; Elango, K.S.; Balai, D. Industrial Waste Phosphogypsum’s Impact on CO2 Reduction and Global Warming. Glob. NEST J. 2023, 25, 127–132. [Google Scholar]
- Wang, Z.; Ma, X.; Pan, H.; Yang, X.; Zhang, X.; Lyu, Y.; Liao, W.; Shui, W.; Wu, J.; Xu, M.; et al. Investigating Effects of Phosphogypsum Disposal Practices on the Environmental Performance of Phosphate Fertilizer Production Using Emergy Analysis and Carbon Emission Amounting: A Case Study from China. J. Clean. Prod. 2023, 409, 137248. [Google Scholar] [CrossRef]
- Hao, X.; Larney, F.J.; Chang, C.; Travis, G.R.; Nichol, C.K.; Bremer, E. The Effect of Phosphogypsum on Greenhouse Gas Emissions During Cattle Manure Composting. J. Environ. Qual. 2005, 34, 774–781. [Google Scholar] [CrossRef]
- Gezerman, A.O. Effect of Phosphogypsum Use as a Waste Recycling on GHG Emissions by Mineral Carbonisation Method. Int. J. Chem. Technol. 2022, 6, 102–107. [Google Scholar] [CrossRef]
- U.S. EPA. Radiation at Superfund Sites. 2018. Available online: https://www.epa.gov/superfund/radiation-superfund-sites (accessed on 2 April 2025).
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; WHO: Geneva, Switzerland, 2017. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) and the U.S. Environmental Protection Agency (EPA). Department of Health and Human Services; Agency for Toxic Substances and Disease Registry. Toxicological Profile for Cadmium. ATSDR’s Toxicological Profiles. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp5.pdf (accessed on 2 April 2025).
- CODEX. Codex Alimentarius; FAO: Rome, Italy, 2023; Volume VIII, pp. 1–19. [Google Scholar]
- Agency for Toxic Substances and Disease Registry (ATSDR) and the U.S. Environmental Protection Agency (EPA). Department of Health and Human Services; Agency for Toxic Substances and Disease Registry. Toxicological Profile for Uranium. ATSDR’s Toxicological Profiles. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp150.pdf (accessed on 2 April 2025).
- Agency for Toxic Substances and Disease Registry (ATSDR) and the U.S. Environmental Protection Agency (EPA).Department of Health and Human Services; Agency for Toxic Substances and Disease Registry. Toxicological Profile for Chromium. ATSDR’s Toxicological Profiles. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp7.pdf (accessed on 2 April 2025).
- Agency for Toxic Substances and Disease Registry (ATSDR) and the U.S. Environmental Protection Agency (EPA). Department of Health and Human Services; Agency for Toxic Substances and Disease Registry. Toxicological Profile for Zinc. ATSDR’s Toxicological Profiles. Available online: http://www.atsdr.cdc.gov/toxprofiles/tp60.pdf (accessed on 2 April 2025).
- Agency for Toxic Substances and Disease Registry (ATSDR) and the U.S. Environmental Protection Agency (EPA). Department of Health and Human Services; Agency for Toxic Substances and Disease Registry. Toxicological Profile for Nickel. ATSDR’s Toxicological Profiles. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp15.pdf (accessed on 2 April 2025).
- Moffet, D.; Smith, C.; Stevens, Y.; Ingerman, L.; Swarts, S.; Chappell, L. Toxicological Profile for Barium and Barium Compounds; Agency for Toxic Substances and Disease Registry: Atlanta, GA, USA, 2007; pp. 1–231.
- US-Environmental Protection Agency (EPA). Potentail Uses of Phoshogypsum and Assoscited Risks; US-EPA: Washington, DC, USA, 1992; pp. 1–124.
- Environmental Health and Safety’s Radiation Control Office University of Florida. RSSC Radiation Protection 07/11 3-1. Chapter 3 Radiation Protection. pp. 1–52. Available online: https://dl.icdst.org/pdfs/files4/ad825dab00c0435d5b32d0d1fd2a928b.pdf (accessed on 2 April 2025).
- Lachehab, A.; Mertah, O.; Kherbeche, A.; Hassoune, H. Utilization of phosphogypsum in CO2 mineral sequestration by producing potassium sulphate and calcium carbonate. Mater. Sci. Energy Technol. 2020, 3, 611–625. [Google Scholar] [CrossRef]
- The Fertilizer Institute, on Behalf of Its Members in the United States Environmental Protection Agency, Revised Its Request for Approval of Additional Uses. Volume 20024, US Environmental Protection Agency. 2020. Available online: www.epa.gov (accessed on 2 April 2025).
- Yuan, P.; Li, M.; Chen, S.; Xiang, W. Advances in Phosphogypsum Calcination and Decomposition Processes in Circulating Fluidized Beds. ACS Omega 2024, 9, 39307–39325. [Google Scholar] [CrossRef]
- United Nations Environment Programme (UNEP). Circular Economy: From Indicators and Data to Policy-Making; United Nations Environment Programme (UNEP): Nairobi, Kenya, 2024. [Google Scholar]
- FAO (Food and Agriculture Organisation). Management of Gypsiferous Soils; Soils Bulletin 62; FAO (Food and Agriculture Organization of the United Nations): Rome, Italy, 1990; Available online: https://www.fao.org/4/t0323e/t0323e00.htm#Contents (accessed on 2 April 2025).
- US Environmental Protection Agency (EPA). National Environmental Policy Act of 1969. In Encyclopedia of the U.S. Government and the Environment: History, Policy, and Politics; US Environmental Protection Agency (EPA): Washington, DC, USA, 2010; Volume 2. [Google Scholar]
- European Commission. Radiological Protection Principles. Report RP-112. 1999. Available online: https://op.europa.eu/en/home (accessed on 2 April 2025).
- European Union. DIRECTIVE 2008/98/EC. 2008. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/PDF/?uri=CELEX:02008L0098-20180705 (accessed on 2 April 2025).
- European Commission. Brussels, 16.4.2013 SWD (2013) 134 Final Commission Staff Working Document Guidelines on Developing Adaptation Strategies Accompanying the Document Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions. An EU Strategy on Adaptation to Climate Change. 2013. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=swd:SWD_2013_0134 (accessed on 16 April 2013).
- European Union. Industrial Emissions Directive (2010/75/EU of the European Parliament and of the Council of 24 November 2010 on industrial emissions (integrated pollution prevention and control). Off. J. Eur. Union 2011, 119, 17–119. [Google Scholar]
- European Commission- Statement. Water Framework Directive (2000/60/EC) of the European Parliament and of the council. Off. J. Eur. Communities 2000, 21, 196. [Google Scholar]
- European Commission. The European Green Deal; European Union: Brussels, Belgium, 2019. [Google Scholar]
- European Commission. Council Directive 2013/59/Euratom: Basic Safety Standards for Protection Against the Dangers Arising from Exposure to Ionising Radiation; European Commission: Brussels, Belgium, 2014; Volume 73, pp. 1–73. [Google Scholar]
- European Commission. A New Circular Economy Action Plan; European Commission: Brussels, Belgium, 2020; pp. 1–23. [Google Scholar]
- European Commission—Directorate-General Environment. Guidance on the implementation of key provisions of Directive 2008/98/EC on waste. Off. J. Eur. Communities 2012. Available online: https://ec.europa.eu/environment/pdf/waste/framework/guidance_doc.pdf (accessed on 2 April 2025).
- US-EPA. Resource Conservation and Recovery Act (RCRA) Laws and Regulations US EPA; United States Government: Washington, DC, USA, 2022.
- Bituh, T.; Petrinec, B.; Skoko, B.; Vučić, Z.; Marović, G. Measuring and modeling the radiological impact of a phosphogypsum deposition site on the surrounding environment. Arh. Za Hig. Rada I Toksikol. 2015, 66, 31–40. [Google Scholar] [CrossRef]
- Lütke, S.F.; Oliveira, M.L.S.; Silva, L.F.O.; Cadaval, T.R.S.; Dotto, G.L. Nanominerals assemblages and hazardous elements assessment in phosphogypsum from an abandoned phosphate fertilizer industry. Chemosphere 2020, 256, 127138. [Google Scholar] [CrossRef] [PubMed]
- Pereira, V.M.; Geraldo, R.H.; Cruz, T.A.M.; Camarini, G. Valorization of Industrial By-Product: Phosphogypsum Recycling as Green Binding Material. Clean. Eng. Technol. 2021, 5, 100310. [Google Scholar] [CrossRef]
- Liu, S.; Ouyang, J.; Ren, J. Mechanism of Calcination Modification of Phosphogypsum and Its Effect on the Hydration Properties of Phosphogypsum-Based Supersulfated Cement. Constr. Build. Mater. 2020, 243, 118226. [Google Scholar] [CrossRef]
- Levickaya, K.; Alfimova, N.; Nikulin, I.; Kozhukhova, N.; Buryanov, A. The Use of Phosphogypsum as a Source of Raw Materials for Gypsum-Based Materials. Resources 2024, 13, 69. [Google Scholar] [CrossRef]
- Murali, G.; Azab, M. Recent Research in Utilization of Phosphogypsum as Building Materials: Review. J. Mater. Res. Technol. 2023, 25, 960–987. [Google Scholar] [CrossRef]
- Bumanis, G.; Vaičiukynienė, D.; Tambovceva, T.; Puzule, L.; Sinka, M.; Nizevičienė, D.; Fornés, I.V.; Bajare, D. Circular Economy in Practice: A Literature Review and Case Study of Phosphogypsum Use in Cement. Recycling 2024, 9, 63. [Google Scholar] [CrossRef]
- Calderón-Morales, B.R.S.; García-Martínez, A.; Pineda, P.; García-Tenório, R. Valorization of phosphogypsum in cement-based materials: Limits and potential in eco-efficient construction. J. Build. Eng. 2021, 44, 102506. [Google Scholar] [CrossRef]
- Anouar, I.; Jouraiphy, R.; Essallaki, H.; Mazouz, H.; Mahfoud, T.; Yousfi, S. Treatment of Phosphogypsum Waste for Use as an Additive in the Manufacture of Cement and Ceramics. Int. J. Sci. Res. 2020, 9, 496–501. [Google Scholar]
- Li, B.; Li, L.; Chen, X.; Ma, Y.; Zhou, M. Modification of phosphogypsum using circulating fluidized bed fly ash and carbide slag for use as cement retarder. Constr. Build. Mater. 2022, 338, 127630. [Google Scholar] [CrossRef]
- Ahmed, H.U.; Rashid, M.M.; Haque, M.N.; Alam, M.M. Development and Optimization of Phosphogypsum-Based Geopolymer Cement. Constr. Build. Mater. 2023, 369, 130577. [Google Scholar]
- Wang, Y.; Chen, B.; Liu, N.; Jiang, Z. Utilization of Waste Phosphogypsum in High-Strength Geopolymer Concrete: Performance Optimization and Mechanistic Exploration. J. Build. Eng. 2024, 98, 111253. [Google Scholar] [CrossRef]
- Pratap, B.; Mondal, S.; Rao, B.H. Development of Geopolymer Concrete Using Fly Ash and Phosphogypsum as a Pavement Composite Material. Mater. Today Proc. 2023, 93 Pt 3, 35–40. [Google Scholar] [CrossRef]
- Gong, Y.; Dong, S.; Liu, L.; Wu, F. A Sustainable Composite Cementitious Material Manufactured by Phosphogypsum Waste. Appl. Sci. 2022, 12, 12718. [Google Scholar] [CrossRef]
- Singh, M. Treating waste phosphogypsum for cement and plaster manufacture. Cem. Concr. Res. 2002, 32, 1033–1038. [Google Scholar] [CrossRef]
- Du, M.; Wang, J.; Dong, F.; Wang, Z.; Yang, F.; Tan, H.; Fu, K.; Wang, W. The study on the effect of flotation purification on the performance of α-hemihydrate gypsum prepared from phosphogypsum. Sci. Rep. 2022, 12, 95. [Google Scholar] [CrossRef]
- Borowski, G.; Hycnar, J.J. The effect of granulated fly ashes with phosphogypsum on the hardening of cement mortar. Czas. Tech. 2016, 2016, 37–45. [Google Scholar]
- Mehta, S.; Faraz, M.I.; Goliya, H.S. Soil Stabilization by Phosphogypsum: A Review. Int. J. Sci. Eng. Res. 2017, 8, 777–780. [Google Scholar]
- Tomašević Pilipović, D.; Slijepčević, N.; Veselić, D.R.; Šešlija, M.; Bulatović, V.; Duduković, N. Utilization of Phosphogypsum and Sediment in Subgrade Material for Pavement Construction. Appl. Sci. 2025, 15, 347. [Google Scholar] [CrossRef]
- Lloyd, G.M., Jr.; Nifong, G.D.; Robertson, D.J.; El-Shal, H.; Akins, R.S. Phosphophogypsum for Seconday Road Construction; Florida Institute of Phosphate Research: Bartow, FL, USA, 2022; pp. 91–103. [Google Scholar]
- Meskini, S.; Samdi, A.; Ejjaouani, H.; Remmal, T. Valorization of phosphogypsum as a road material: Stabilizing effect of fly ash and lime additives on strength and durability. J. Clean. Prod. 2021, 323, 10–15. [Google Scholar] [CrossRef]
- Anamika, B.; Debabrata, G. Utilisation of phosphogypsum along with other additives in geo-engineering- A review. Mater. Constr. 2022, 72, e288. [Google Scholar] [CrossRef]
- Gharaibeh, M.A.; Rusan, M.J.; Eltaif, N.I.; Shunnar, O.F. Reclamation of highly calcareous saline-sodic soil using low quality water and phosphogypsum. Appl. Water Sci. 2014, 4, 223–230. [Google Scholar] [CrossRef]
- Wang, J.; Dong, F.; Wang, Z.; Yang, F.; Du, M.; Fu, K.; Wang, Z. A novel method for purification of phosphogypsum. Physicochem. Probl. Miner. Process. 2020, 56, 975–983. [Google Scholar] [CrossRef]
- Gabsi, H.; Tallou, A.; Aziz, F.; Boukchina, R.; Karbout, N.; Caceres, L.A.; García-Tenorio, R.; Boudabbous, K.; Moussa, M. Application of Phosphogypsum and Organic Amendment for Bioremediation of Degraded Soil in Tunisia Oasis: Targeting Circular Economy. Sustainability 2023, 15, 4769. [Google Scholar] [CrossRef]
- Plyatsuk, L.; Balintova, M.; Chernysh, Y.; Demcak, S.; Holub, M.; Yakhnenko, E. Influence of phosphogypsum dump on the soil ecosystem in the sumy region (Ukraine). Appl. Sci. 2019, 9, 5559. [Google Scholar] [CrossRef]
- Smith, C.J.; Peoples, M.B.; Keerthisinghe, G.; James, T.R.; Garden, D.L. Effect of surface applications of lime, gypsum and phosphogypsum on the alleviating of surface and subsurface acidity in soil under pasture. Aust. J. Soil Res. 1994, 32, 995–1008. [Google Scholar] [CrossRef]
- Zhang, Z.; Han, C.; Tao, C.; Fan, X.; Liu, R. Preparation of Phosphogypsum–Bentonite-Based Slow-Release Potassium Magnesium Sulfate Fertilizer. Agriculture 2025, 15, 692. [Google Scholar] [CrossRef]
- Tian, D.; Xia, J.; Zhou, N.; Xu, M.; Li, X.; Zhang, L.; Du, S.; Gao, H. The Utilization of Phosphogypsum as a Sustainable Phosphate-Based Fertilizer by Aspergillus niger. Agronomy 2022, 12, 646. [Google Scholar] [CrossRef]
- Ramirez, J.D.; Diwa, R.R.; Palattao, B.L.; Tabora, E.U. Economic Potential of Rare Earth Elements in the Philippine Phosphogypsum. Preprints 2021. [Google Scholar] [CrossRef]
- Statista. Mining, Metals & Minerals. Distribution of Rare Earths Production Worldwide as of 2024, by Country. Available online: https://www.statista.com/statistics/270277/mining-of-rare-earths-by-country/ (accessed on 2 April 2025).
- Hammas-Nasri, I.; Horchani-Naifer, K.; Férid, M.; Barca, D. Rare Earths Concentration from Phosphogypsum Waste by Two-Step Leaching Method. Int. J. 2016, 149, 78–83. [Google Scholar] [CrossRef]
- Kulczycka, J.; Kowalski, Z.; Smol, M.; Wirth, H. Evaluation of the recovery of Rare Earth Elements (REE) from phosphogypsum waste—Case study of the WIZÓW Chemical Plant (Poland). J. Clean. Prod. 2016, 113, 345–354. [Google Scholar] [CrossRef]
- Samsonov, M.D.; Trofimov, T.I.; Kulyako, Y.M.; Malikov, D.A.; Myasoedov, B.F. Supercritical fluid extraction of rare earth elements, thorium and uranium from monazite concentrate and phosphogypsum using carbon dioxide containing tributyl phosphate and di-(2-ethylhexyl)phosphoric acid. Russ. J. Phys. Chem. B 2016, 10, 1078–1084. [Google Scholar] [CrossRef]
- Kiegiel, K.; Gajda, D.; Zakrzewska-Kołtuniewicz, G. Recovery of uranium and other valuable metals from substrates and waste from copper and phosphate industries. Sep. Sci. Technol. 2020, 55, 2099–2107. [Google Scholar] [CrossRef]
- Bailey, G.; Joyce, P.J.; Schrijvers, D.; Schulze, R.; Sylvestre, A.M.; Sprecher, B.; Vahidi, E.; Dewulf, W.; Van Acker, K. Review and New Life Cycle Assessment for Rare Earth Production from Bastnäsite, Ion Adsorption Clays and Lateritic Monazite. Resour. Conserv. Recycl. 2020, 155, 104675. [Google Scholar] [CrossRef]
- Chen, L.; Luan, X.; Han, F.; Zhao, Y.; Yang, H.; Zhang, L.; Yin, Y.; Liu, W.; Cui, Z. Life Cycle Environmental and Economic Assessment of Phosphogypsum Utilization in China. Resour. Conserv. Recycl. 2025, 212, 107938. [Google Scholar] [CrossRef]
- International Atomic Energy Agency (IAEA). The Recovery of Uranium from Phosphoric Acid; International Atomic Energy Agency: Vienna, Austria, 1989. [Google Scholar]
- World Nuclear Association. Uranium from Phosphates; World Nuclear Association: London, UK, 2024; pp. 11–14. [Google Scholar]
- IAEA. IAEA Nuclear Energy Series No. NG-T-3.20; IAEA: Vienna, Austria, 2019; Volume 24. [Google Scholar]
- Dong, W.; Deng, X.; Chai, L.; Zhang, Y.; Chen, H.; Wu, H.; Chi, R. Leaching Characteristics and Mechanisms of Fluorine and Phosphorus from Phosphogypsum. Molecules 2025, 30, 5. [Google Scholar] [CrossRef]
- Kiegiel, K.; Miśkiewicz, A.; Herdzik-Koniecko, I.; Gajda, D.; Zakrzewska-Kołtuniewicz, G. Perspective of Obtaining Rare Earth Elements in Poland. In Lanthanides; IntechOpen: London, UK, 2019. [Google Scholar]
- Huang, Y.; Shi, K.; Su, S.; Liu, B.; Sun, H.; Han, G. Selectively Stepwise Separation and Recovery of Molybdenum and Vanadium from Spent Hydrodesulfurization Catalysts Through Solvent Extraction. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=4150674 (accessed on 2 April 2025).
- Cheng, C.Y.; Boddy, G.; Zhang, W.; Godfrey, M.; Robinson, D.J.; Pranolo, Y.; Zhu, Z.; Wang, W. Recovery of nickel and cobalt from laterite leach solutions using direct solvent extraction: Part 1—Selection of a synergistic SX system. Hydrometallurgy 2010, 104, 45–52. [Google Scholar] [CrossRef]
- Chen, W.S.; Ho, H.J.; Lin, K.Y. Hydrometallurgical process for tantalum recovery from epoxy-coated solid electrolyte tantalum capacitors. Materials 2019, 12, 1220. [Google Scholar] [CrossRef]
- Rasheed, R.; Tahir, F.; Afzaal, M.; Ahmad, S.R. Decomposition analytics of carbon emissions by cement manufacturing—A way forward towards carbon neutrality in a developing country. Environ. Sci. Pollut. Res. 2022, 29, 49429–49438. [Google Scholar] [CrossRef]
- Zhong, Y.; Shi, T.; Chen, Q.; Yang, X.; Xu, D.; Zhang, Z.; Wang, X.; Zhong, B. Leaching calcium from phosphogypsum desulfurization slag by using ammonium chloride solution: Thermodynamics and kinetics study. Chin. J. Chem. Eng. 2020, 28, 208–215. [Google Scholar] [CrossRef]
- Zhao, S.; Zhang, H.; Liu, Q.; He, Y.; Xiao, H.; Ma, L. Study on the Recycling of P in Phosphogypsum Leachate. J. Geosci. Environ. Prot. 2023, 11, 209–225. [Google Scholar] [CrossRef]
- Yang, J.; Dong, S.; Ma, L.; Dai, Q.; Zheng, D.; Huang, B.; Sun, M.; Hu, B.; Du, W.; Xie, L.; et al. Review on high-value utilization of phosphogypsum: Utilization of calcium and oxygen resources present in phosphogypsum. Sep. Purif. Technol. 2024, 344, 127246. [Google Scholar] [CrossRef]
- Dong, S.; Yu, S.; Chen, L.; Zhuo, Q.; Wu, F.; Xie, L.; Liu, L. Effects of Pretreated Phosphogypsum and Granulated Blast-Furnace Slag on the Rheological Properties of the Paste Excited by NaOH. Molecules 2023, 28, 2662. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.S.; Wang, C.Q.; Mei, X.D.; Zhang, C. An effective treatment method for phosphogypsum. Environ. Sci. Pollut. Res. 2019, 26, 30533–30539. [Google Scholar] [CrossRef]
- Xu, P.; Li, H.; Chen, Y. Experimental study on optimization of phosphogypsum suspension decomposition conditions under double catalysis. Materials 2021, 14, 1120. [Google Scholar] [CrossRef]
- Florida Institute of Phosphate Research. Microbiology and Radiochemistry of Phosphogypsum; Florida Institute of Phosphate Research: Bartow, FL, USA, 1996. [Google Scholar]
- European Patent Application. Process for Recovering Sulphuric Acid from Natural Gypsum or Phosphogypsum: EP0004568A1; European Patent Office: Munich, Germany, 2019; Volume 11, pp. 1–14. [Google Scholar]
- Gui, M.M.; Cong, G. A study on plaster of paris made from phosphogypsum. J. Civ. Environ. Eng. 2000, 22, 33–36. [Google Scholar]
- Madu, M.J.; Ndaliman, M.B. Development of Plaster of Paris from Gypsum Deposit of North. In Proceedings of the 1st AGM and Conference of the Nigerian Institution of Mechanical Engineers, Minna, Nigeria, 9–10 September 2016; pp. 1–9. [Google Scholar]
- European Union. Critical Raw Materials Act Consultation; European Union: Brussels, Belgium, 2022. [Google Scholar]
- Geraldo, R.H.; Costa, A.R.D.; Kanai, J.; Silva, J.S.; Souza, J.D.; Andrade, H.M.C.; Gonçalves, J.P.; Fontanini, P.S.P.; Camarini, G. Calcination parameters on phosphogypsum waste recycling. Constr. Build. Mater. 2020, 256, 119450. [Google Scholar] [CrossRef]
- Zhang, W.; Zhang, F.; Ma, L.; Yang, J.; Wei, Y.; Kong, D. CO2 capture and process reinforcement by hydrolysate of phosphogypsum decomposition products. J. CO2 Util. 2020, 36, 253–262. [Google Scholar] [CrossRef]
- Fornés, I.V.; Vaičiukynienė, D.; Nizevičienė, D.; Tamošaitis, G.; Pupeikis, D. The improvement of the thermal and acoustic insulation properties of phosphogypsum specimens by adding waste wood fibre. Constr. Build. Mater. 2022, 331, 127341. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, J.; Shu, Z.; Wang, Y.; Yakubu, Y.; Zhang, Y.; Li, X. Fabrication of PMMA/phosphogypsum non-fired ceramic composites with improved mechanical and waterproof properties. J. Aust. Ceram. Soc. 2021, 57, 81–90. [Google Scholar] [CrossRef]
- Liu, H.; Nie, C.; Li, H.; Xie, G.; Cao, J. Hydrophobically Modified Phosphogypsum and Its Application in Polypropylene Composites. Constr. Build. Mater. 2022, 347, 128500. [Google Scholar] [CrossRef]
- Nie, C.; Zhong, J.; Liu, H.; Yang, X.; Xie, G.; Bi, J.; Yang, C.; Shi, Y.; Yi, Y. Surface Modification of Anhydrous Phosphogypsum and Its Application in Isotactic Polypropylene. J. Thermoplast. Compos. Mater. 2023, 36, 5078–5093. [Google Scholar] [CrossRef]
- Essabir, H.; Nekhlaoui, S.; Bensalah, M.O.; Rodrigue, D.; Bouhfid, R.; Qaiss, A.E.K. Phosphogypsum Waste Used as Reinforcing Fillers in Polypropylene-Based Composites: Structural, Mechanical and Thermal Properties. J. Polym. Environ. 2017, 25, 658–666. [Google Scholar] [CrossRef]
- Xu, M.; Pan, G.; Guo, Y.; Liang, Q.; Yu, Z.; Cao, Y.; Wang, Y. Highly Efficient Oil–Water Separation and Oil Adsorption with Hydrophobic Hydrotalcite/Polyurethane Porous Composite Foam. J. Water Process Eng. 2024, 60, 105211. [Google Scholar] [CrossRef]
- Cao, W.; Yi, W.; Li, J.; Peng, J.; Yin, S. A facile approach for large-scale recovery of phosphogypsum: An insight from its performance. Constr. Build. Mater. 2021, 309, 125190. [Google Scholar] [CrossRef]
- Chen, X.; Gao, J.; Zhao, Y. Investigation on the hydration of hemihydrate phosphogypsum after post treatment. Constr. Build. Mater. 2019, 229, 116864. [Google Scholar] [CrossRef]
- Zhu, G.; Yang, Y.; He, L.; Li, H.; Meng, Z.; Zheng, G.; Li, F.; Su, X.; Xi, B.; Li, Z. Novel Synergistic Process of Impurities Extraction and Phosphogypsum Crystallization Control in Wet-Process Phosphoric Acid. ACS Omega 2023, 8, 28122–28132. [Google Scholar] [CrossRef]
- Moalla, R.; Gargouri, M.; Khmiri, F.; Kamoun, L.; Zairi, M. Phosphogypsum purification for plaster production: A process optimization using full factorial design. Environ. Eng. Res. 2018, 23, 36–45. [Google Scholar] [CrossRef]
- Awad, S.; Essam, M.; Boukhriss, A.; Kamar, M.; Midani, M. Properties, Purification, and Applications of Phosphogypsum: A Comprehensive Review Towards Circular Economy. Mater. Circ. Econ. 2024, 6, 9. [Google Scholar] [CrossRef]
- Royen, H.; Fortkamp, U. Rare Earth Elements—Purification, Separation and Recycling; Technical Report No. C211; IVL Swedish Environmental Research Institute: Stockholm, Sweden, 2016. [Google Scholar]
- Papageorgiou, F.; Godelitsas, A.; Mertzimekis, T.J.; Xanthos, S.; Voulgaris, N.; Katsantonis, G. Environmental impact of phosphogypsum stockpile in remediated Schistos waste site (Piraeus, Greece) using a combination of γ-ray spectrometry with geographic information systems. Env. Monit Assess 2016, 188, 133. [Google Scholar] [CrossRef]
- Millán-Becerro, R.; Pérez-López, R.; Cánovas, C.R.; Macías, F.; León, R. Phosphogypsum weathering and implications for pollutant discharge into an estuary. J. Hydrol. 2023, 617, 128943. [Google Scholar] [CrossRef]
- Akfas, F.; Elghali, A.; Aboulaich, A.; Munoz, M.; Benzaazoua, M.; Bodinier, J.L. Exploring the potential reuse of phosphogypsum: A waste or a resource? Sci. Total Environ. 2024, 908, 168196. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.R.; Rajak, D.K.; Ilyas, S.; Kim, H.; Pathak, P. Challenges, Regulations, and Case Studies on Sustainable Management of Industrial Waste. Minerals 2023, 13, 51. [Google Scholar] [CrossRef]
- Cánovas, C.R.; Macías, F.; Pérez-López, R.; Nieto, J.M. Mobility of rare earth elements, yttrium and scandium from a phosphogypsum stack: Environmental and economic implications. Sci. Total Environ. 2018, 618, 847–857. [Google Scholar] [CrossRef] [PubMed]
- Srinivasalu, K.; Raghava, P. A Study on Influence of Phosphogypsum on Durability of the Concrete. Int. J. Emerg. Technol. Eng. Res. (IJETER) 2017, 5, 41–47. [Google Scholar]
- Peng, X.; Zhu, J.; Guo, T.; Wu, X.; Sun, H. Technology improvement of production of sulfuric acid integrated with cement from phosphogypsum. ZKG Int. 2015, 68, 66–71. [Google Scholar]
- Meng, J.; Liao, W.; Zhang, G. Emerging CO2-mineralization technologies for co-utilization of industrial solid waste and carbon resources in China. Minerals 2021, 11, 274. [Google Scholar] [CrossRef]
- Binnemans, K.; Pontikes, Y.; Jones, P.T.; Van Gerven, T.; Blanpain, B. Recovery of rare earths from industrial waste residues: A concise review. In Proceedings of the 3rd International Slag Valorisation Symposium, Leuven, Belgium, 19–20 March 2013; pp. 191–205. [Google Scholar]
- Costis, S.; Mueller, K.K.; Coudert, L.; Neculita, C.M.; Reynier, N.; Blais, J.F. Recovery potential of rare earth elements from mining and industrial residues: A review and case studies. J. Geochem. Explor. 2021, 221, 106699. [Google Scholar] [CrossRef]
- Zhang, W.; Noble, A.; Yang, X.; Honaker, R. A comprehensive review of rare earth elements recovery from coal-related materials. Minerals 2020, 10, 451. [Google Scholar] [CrossRef]
- Korcak, R.F. Chapter 7 Agricultural Uses of Phosphogypsum, Gypsum, and Other Industrial Byproducts. 1986, pp. 120–126. Available online: https://p2infohouse.org/ref/45/44601.pdf (accessed on 2 April 2025).
- Andrade Neto, J.S.; Bersch, J.D.; Silva, T.S.M.; Rodríguez, E.D.; Suzuki, S.; Kirchheim, A.P. Influence of phosphogypsum purification with lime on the properties of cementitious matrices with and without plasticizer. Constr. Build. Mater. 2021, 299, 123935. [Google Scholar] [CrossRef]
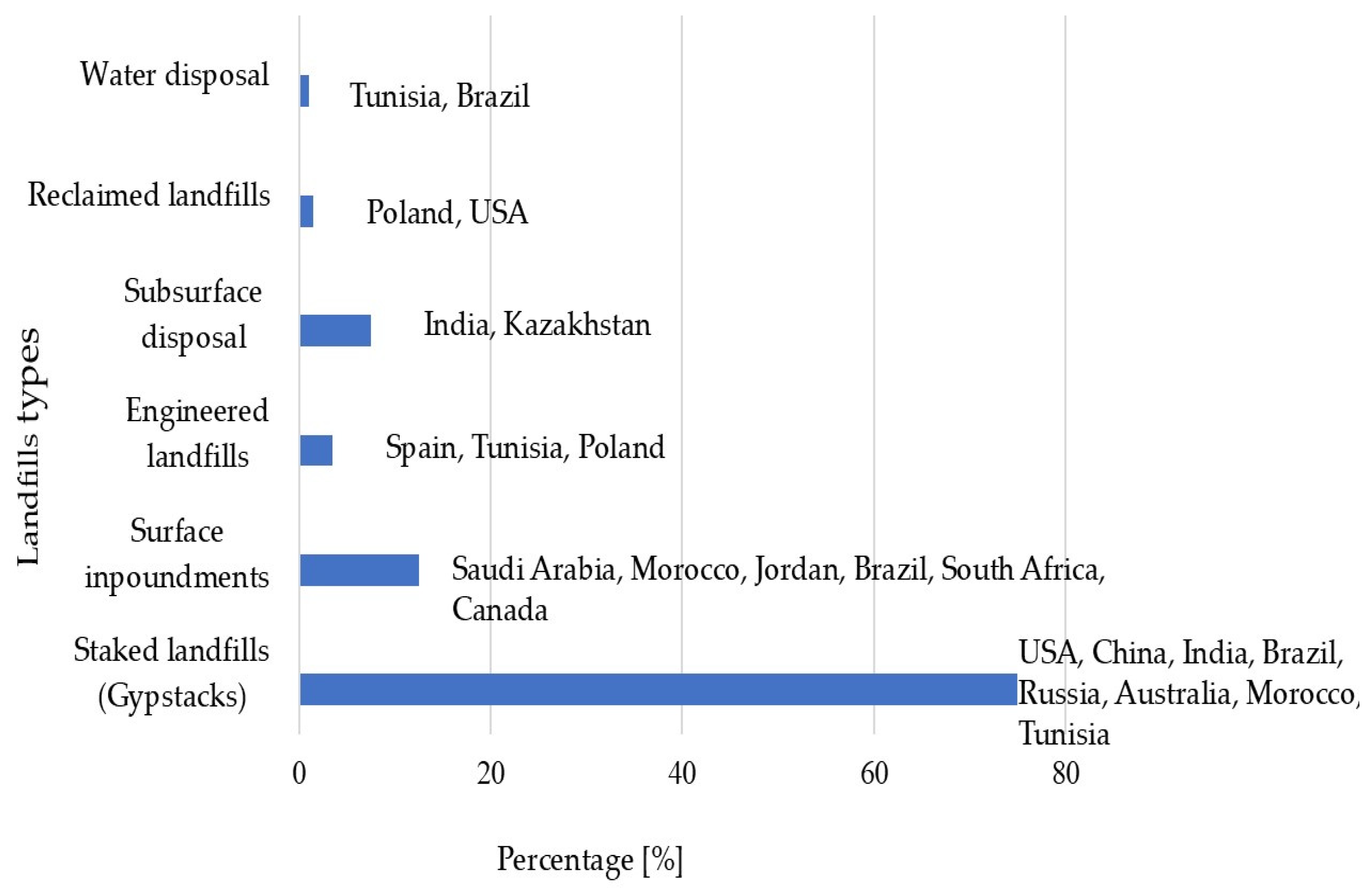

| Country | Estimated PG Production (Million Tons/Year) | References |
|---|---|---|
| Algeria | 1.0 | [36] |
| Brazil | 5.6–10.0 | [25,26,37] |
| China | 22.0–75.0 | [26,27] |
| Croatia | 8.5 | [15] |
| India | 5.0–12.0 | [38] |
| Jordan | 3.0 | [14] |
| Morocco | 14.0–15.0 | [39,40] |
| Poland | 1.5–2.5 | [34,35,41] |
| Russia | 14.0 | [31,42] |
| South Africa | 5 | [43] |
| South Korea | 11.0 | [44] |
| Spain | 2.5–3.0 | [16,45] |
| Syria | 0.35 | [46] |
| Netherlands | 4.0 | [15] |
| Tunisia | 10.0–12.0 | [32,42,47,48] |
| Turkey | 3.0 | [49,50] |
| Ukraine | 10.0 | [35,51] |
| USA | 30.0–50.0 | [50,52] |
| Vietnam | 1.2 | [53] |
| Worldwide Total | 280–300 | [1,25,54] |
| Method | Advantages | Disadvantages |
|---|---|---|
| Stacked Landfills |
|
|
|
| |
| ||
| Surface Impoundment |
|
|
|
| |
|
| |
| Engineered Landfills |
|
|
|
| |
| ||
| Subsurface Disposal |
|
|
|
| |
| ||
| Reclaimed Landfills |
|
|
|
| |
| ||
| Water Disposal Landfills |
|
|
|
| |
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maina, L.; Kiegiel, K.; Zakrzewska-Kołtuniewicz, G. Challenges and Strategies for the Sustainable Environmental Management of Phosphogypsum. Sustainability 2025, 17, 3473. https://doi.org/10.3390/su17083473
Maina L, Kiegiel K, Zakrzewska-Kołtuniewicz G. Challenges and Strategies for the Sustainable Environmental Management of Phosphogypsum. Sustainability. 2025; 17(8):3473. https://doi.org/10.3390/su17083473
Chicago/Turabian StyleMaina, Linda, Katarzyna Kiegiel, and Grażyna Zakrzewska-Kołtuniewicz. 2025. "Challenges and Strategies for the Sustainable Environmental Management of Phosphogypsum" Sustainability 17, no. 8: 3473. https://doi.org/10.3390/su17083473
APA StyleMaina, L., Kiegiel, K., & Zakrzewska-Kołtuniewicz, G. (2025). Challenges and Strategies for the Sustainable Environmental Management of Phosphogypsum. Sustainability, 17(8), 3473. https://doi.org/10.3390/su17083473







