A Sustainable Development Strategy for Municipal Solid Waste Incineration Bottom Ash: Adsorption Performance and Mechanism in Removing Heavy Metals from Water
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
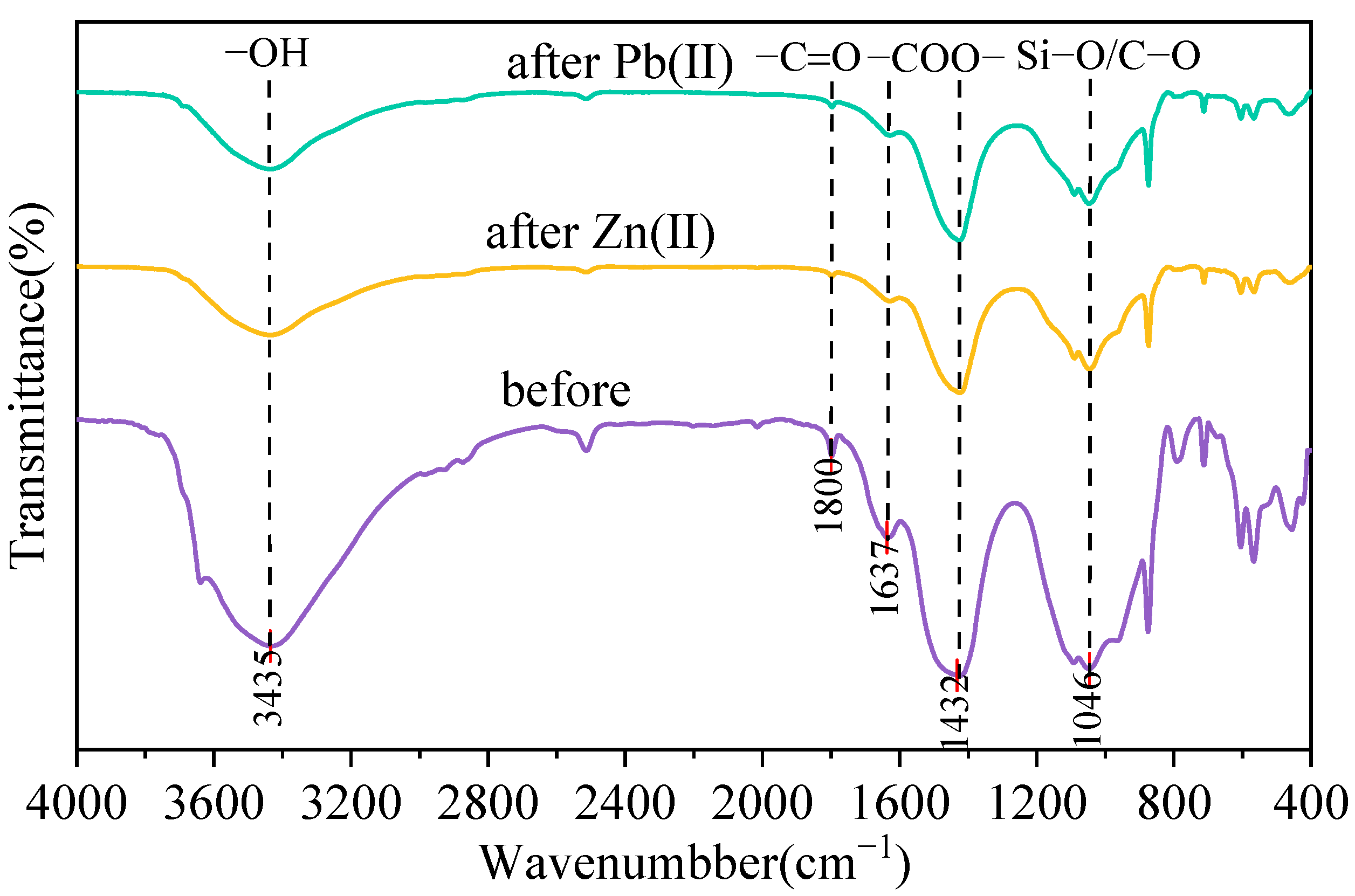
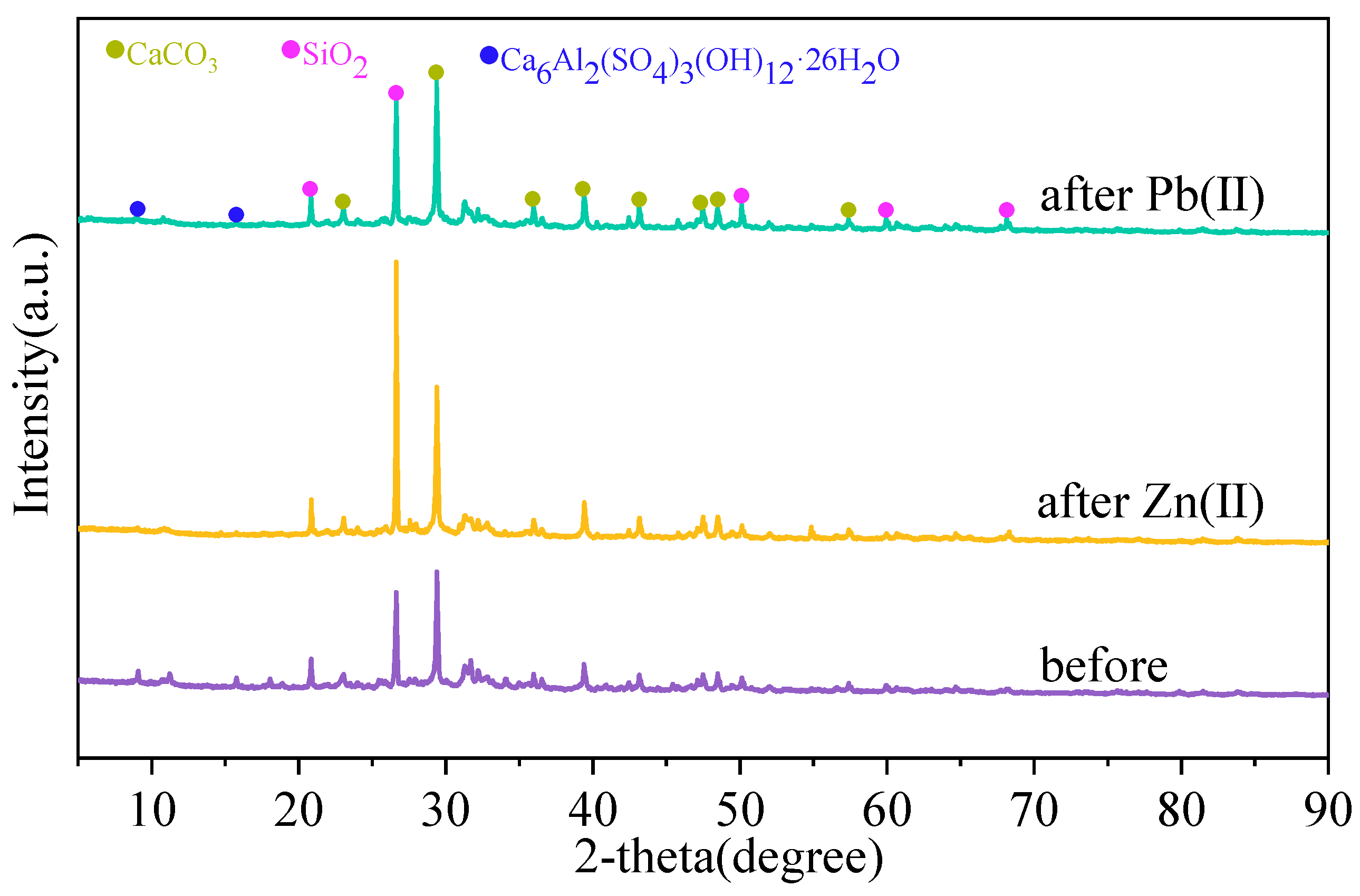
3.1. Characterization of MSWI-BA
3.2. Adsorption Behavior Pb(II) and Zn(II) onto MSWI-BA
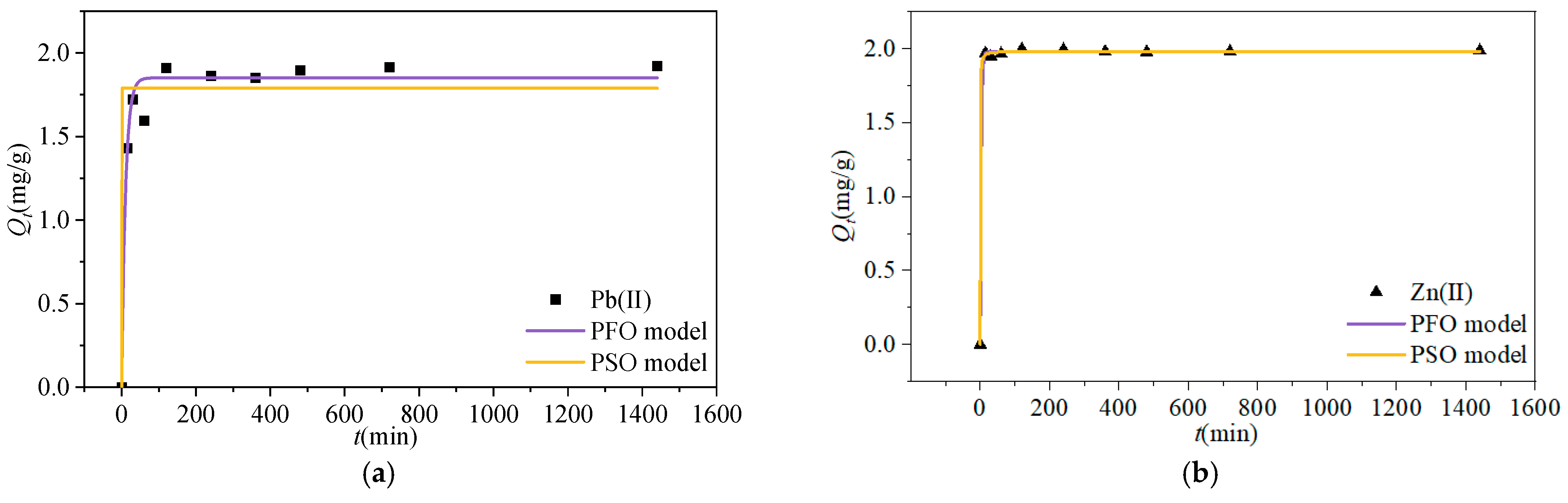
3.2.1. Kinetic Adsorption Properties
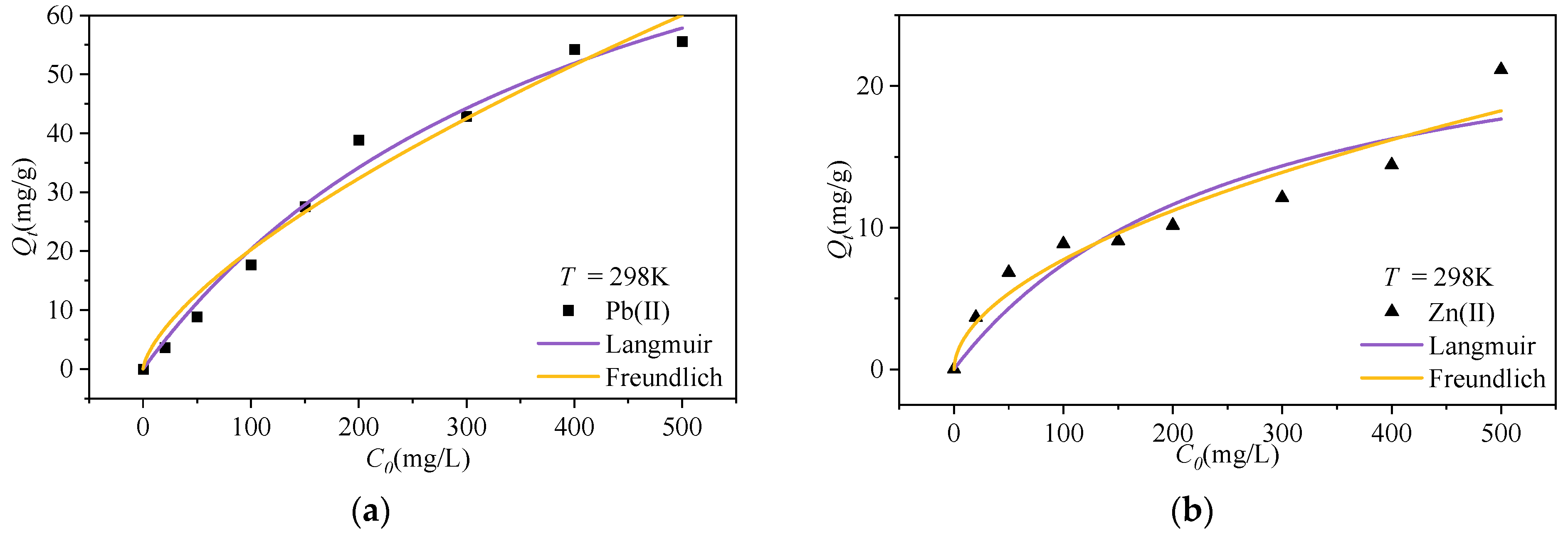
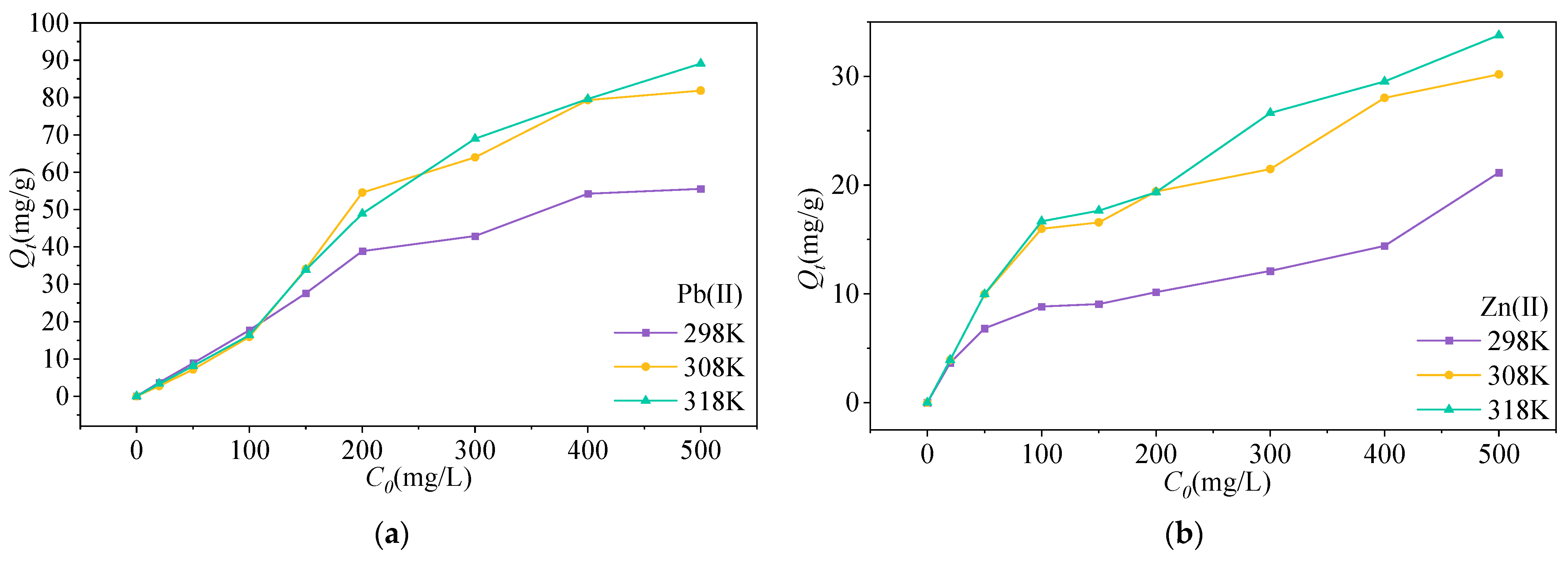
3.2.2. Isothermal Adsorption Properties
3.3. Factors Affecting Pb(II) and Zn(II) Adsorption onto MSWI-BA
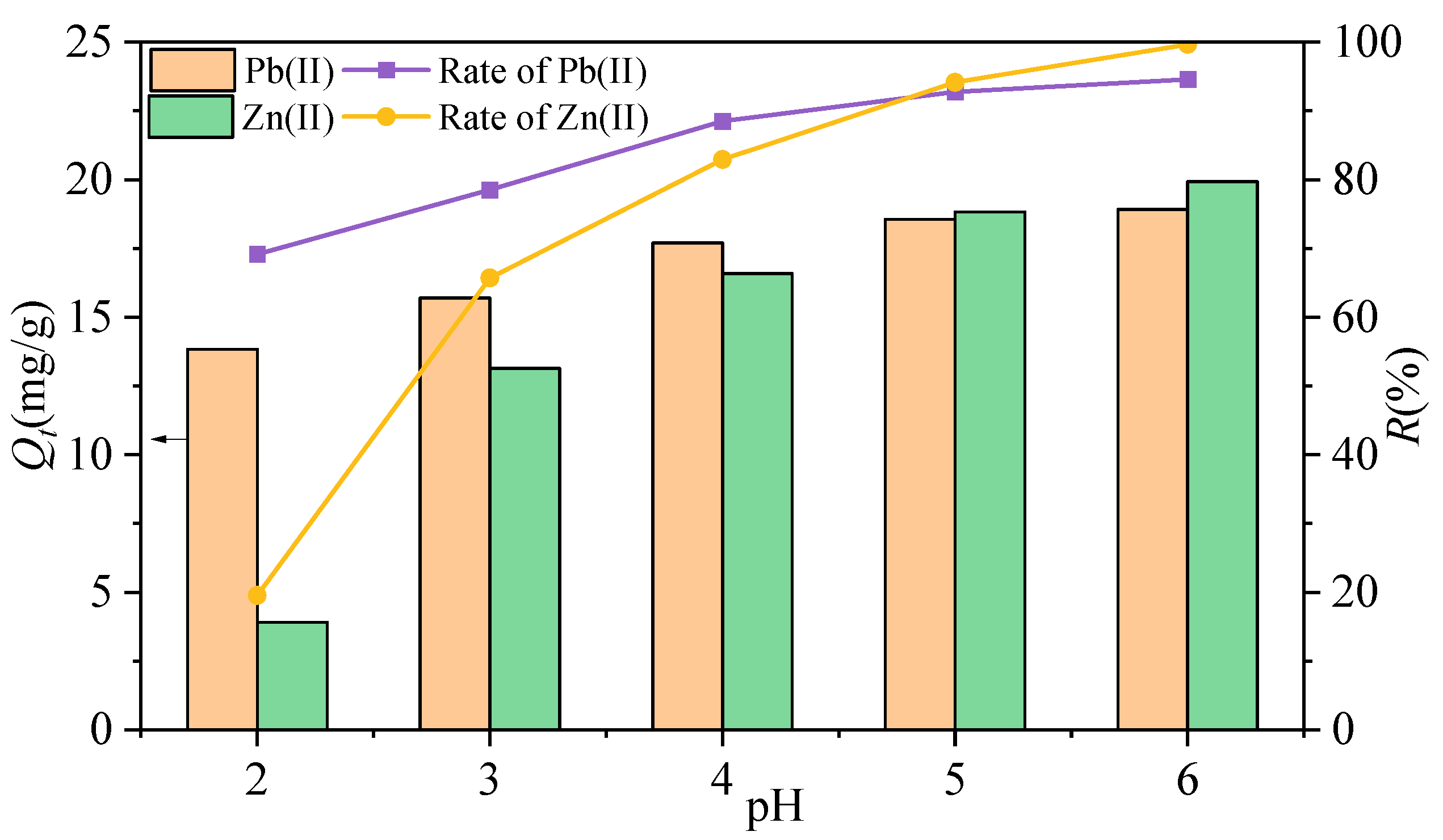
3.3.1. The Effect of Solution pH
3.3.2. The Effect of MSWI-BA Dosage
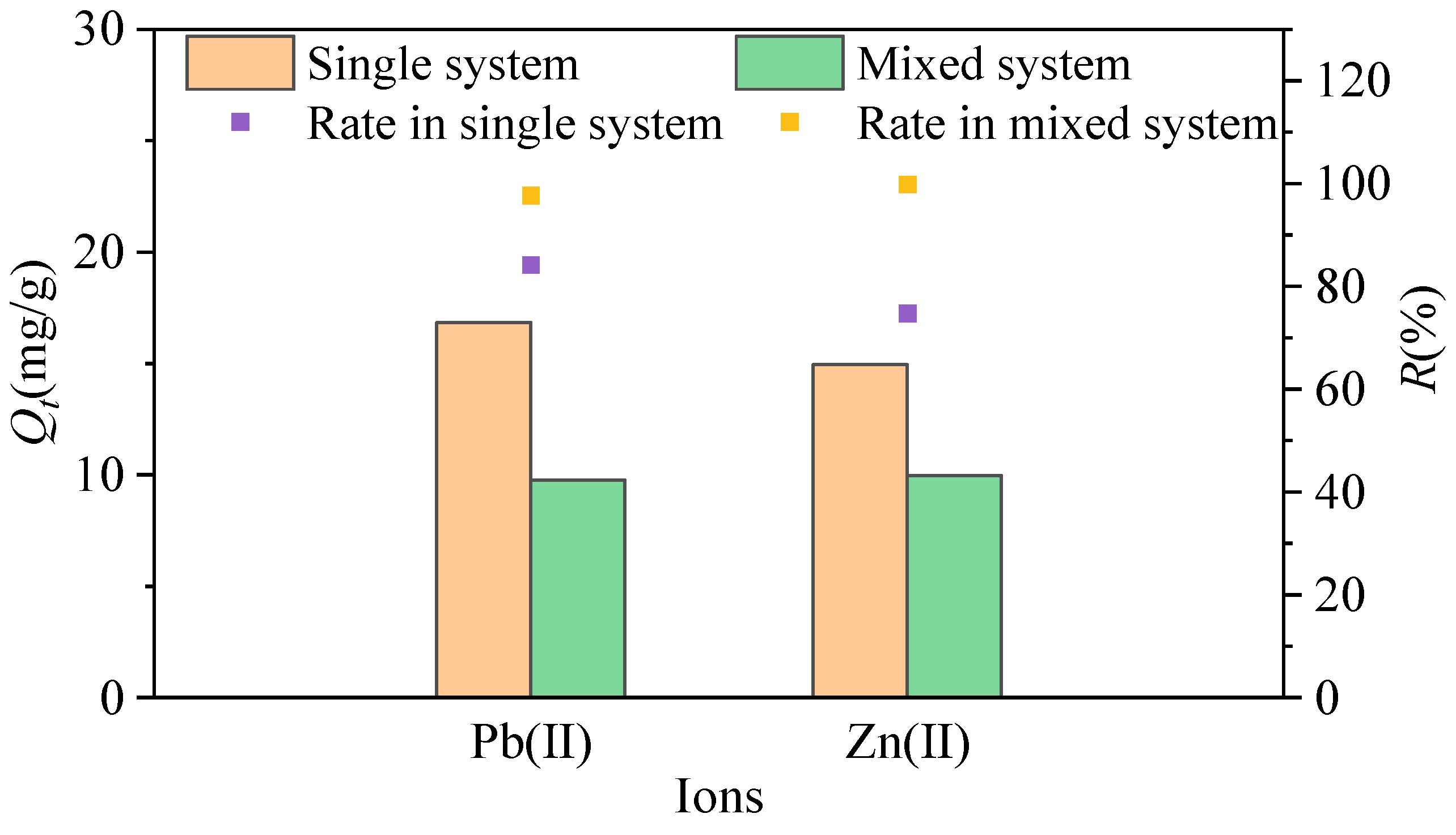
3.4. Competitive Adsorption Behavior in Mixed Systems
3.5. Results and Discussion on the Regeneration Performance of MSWI-BA
4. Adsorption Mechanism of MSWI-BA on Pb(II) and Zn(II)
5. Conclusions
- (1)
- MSWI-BA exhibited excellent adsorption capacity for Pb(II) and Zn(II) removal from aqueous solutions. The adsorption of Pb(II) on MSWI-BA fits the PFO and Langmuir models better; at pH = 4 and T = 318 K, the maximum adsorption capacity is 89.09 mg/g. The adsorption of Zn(II) on MSWI-BA fits well with the PFO, PSO, Langmuir, and Freundlich models; at pH = 5 and T = 318 K, the maximum adsorption capacity is 33.77 mg/g.
- (2)
- The adsorption efficiency of MSWI-BA for Pb(II) and Zn(II) is influenced by the initial pH of the solution, the amount of ash, the competition between heavy metal ions, and the number of regeneration cycles. At pH = 2, the removal rates of Pb(II) and Zn(II) by MSWI-BA are only 69.18% and 19.55%, respectively. When the pH value is in the range of 4–6 and the amount of ash is between 0.1 g/20 mL and 1.8 g/20 mL, the R values for the ash are all greater than or equal to 90%. In mixed systems, competitive adsorption between Pb(II) and Zn(II) reduced overall adsorption compared to single systems. After four cycles, the removal rates for Pb(II) and Zn(II) were recorded at 74.8% and 83.5%, respectively.
- (3)
- Characterization via ESEM-EDS, FTIR, and XRD revealed structural and compositional changes in MSWI-BA after adsorption. Chemical precipitation, ion exchange, and surface complexation were identified as key mechanisms.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiang, J.; Qiu, J.; Li, Z.; Chen, J.; Song, Y. Eco-Friendly Treatment for MSWI Bottom Ash Applied to Supplementary Cementing: Mechanical Properties and Heavy Metal Leaching Concentration Evaluation. Constr. Build. Mater. 2022, 327, 127012. [Google Scholar] [CrossRef]
- Qasem, N.A.; Mohammed, R.H.; Lawal, D.U. Removal of Heavy Metal Ions from Wastewater: A Comprehensive and Critical Review. NPJ Clean Water 2021, 4, 52. [Google Scholar] [CrossRef]
- He, Y.; Zhang, P.; Wang, L. Adsorption and Removal of Cr6+, Cu2+, Pb2+, and Zn2+ from Aqueous Solution by Magnetic Nano-Chitosan. Molecules 2023, 28, 2607. [Google Scholar] [CrossRef] [PubMed]
- Mao, M.; Yan, T.; Shen, J.; Zhang, J.; Zhang, D. Capacitive Removal of Heavy Metal Ions from Wastewater via an Electro-Adsorption and Electro-Reaction Coupling Process. Environ. Sci. Technol. 2021, 55, 3333–3340. [Google Scholar] [CrossRef]
- Xu, Y.-N.; Chen, Y. Advances in Heavy Metal Removal by Sulfate-Reducing Bacteria. Water Sci. Technol. J. Int. Assoc. Water Pollut. Res. 2020, 81, 1797–1827. [Google Scholar] [CrossRef]
- Liu, Y.; Hu, L.; Tan, B.; Li, J.; Gao, X.; He, Y.; Du, X.; Zhang, W.; Wang, W. Adsorption Behavior of Heavy Metal Ions from Aqueous Solution onto Composite Dextran-Chitosan Macromolecule Resin Adsorbent. Int. J. Biol. Macromol. 2019, 141, 738–746. [Google Scholar] [CrossRef] [PubMed]
- Belova, T.P. Adsorption of Heavy Metal Ions (Cu2+, Ni2+, Co2+ and Fe2+) from Aqueous Solutions by Natural Zeolite. Heliyon 2019, 5, e02320. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, L.; Zhang, D.; Zhang, F.; Zhou, S.; Ma, Y.; Guo, J.; Zhang, Y.; Xing, B. Stabilization of Pb, Cd, and Zn in Soil by Modified-Zeolite: Mechanisms and Evaluation of Effectiveness. Sci. Total Environ. 2022, 814, 152746. [Google Scholar] [CrossRef]
- Lv, Y.; Ma, B.; Liu, Y.; Wang, C.; Chen, Y. Adsorption Behavior and Mechanism of Mixed Heavy Metal Ions by Zeolite Adsorbent Prepared from Lithium Leach Residue. Microporous Mesoporous Mater. 2022, 329, 111553. [Google Scholar] [CrossRef]
- Wei, L.; Huang, Y.; Huang, L.; Li, Y.; Huang, Q.; Xu, G.; Müller, K.; Wang, H.; Ok, Y.S.; Liu, Z. The Ratio of H/C Is a Useful Parameter to Predict Adsorption of the Herbicide Metolachlor to Biochars. Environ. Res. 2020, 184, 109324. [Google Scholar] [CrossRef]
- Fang, Y.; Yang, L.; Rao, F.; Zheng, Y.; Song, Z. Adsorption behavior and mechanism of MB, Pb(II) and Cu(II) on porous geopolymers. Ceram. Int. 2025, 12, 564. [Google Scholar] [CrossRef]
- National Statistical Yearbook, 2024. Environmental and Resource Conditions. Available online: https://www.stats.gov.cn/sj/ndsj/2023/html/C08-18.jpg (accessed on 13 October 2024).
- Lu, Y.; Tian, A.; Zhang, J.; Tang, Y.; Shi, P.; Tang, Q.; Huang, Y. Physical and Chemical Properties, Pretreatment, and Recycling of Municipal Solid Waste Incineration Fly Ash and Bottom Ash for Highway Engineering: A Literature Review. Adv. Civ. Eng. 2020, 1, 17. [Google Scholar] [CrossRef]
- Alam, Q.; Schollbach, K.; van Hoek, C.; van der Laan, S.; de Wolf, T.; Brouwers, H.J. In-Depth Mineralogical Quantification of MSWI Bottom Ash Phases and Their Association with Potentially Toxic Elements. Waste Manag. 2019, 87, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Cho, B.H.; Nam, B.H.; An, J.; Youn, H. Municipal Solid Waste Incineration (MSWI) Ashes as Construction Materials—A Review. Materials 2020, 13, 3143. [Google Scholar] [CrossRef]
- Czop, M.; Łaźniewska-Piekarczyk, B. Use of Slag from the Combustion of Solid Municipal Waste as a Partial Replacement of Cement in Mortar and Concrete. Materials 2020, 13, 1593. [Google Scholar] [CrossRef]
- Tian, A.; Zhou, Y.; Chen, Y.; Kan, D.; Lu, Y.; Tang, Q. Use of Municipal Solid Waste Incineration (MSWI) Bottom Ash as a Permeable Subgrade Material: An Experimental and Mechanism Study. J. Air Waste Manag. Assoc. 2024, 74, 291–303. [Google Scholar] [CrossRef]
- Zhou, T.; Zhong, W.; Shen, Y.; Yu, Q.; Luo, S.; Feng, Y.; Zhang, W.; Ren, D. Study on the Co-pyrolysis Behavior of Copper Slag and Pine Sawdust and the Adsorption of Chromium. BioEnergy Res. 2024, 17, 2050–2061. [Google Scholar] [CrossRef]
- Czuma, N.; Zarębska, K. The use of mathematical models for modelling sulphur dioxide sorption on materials produced from fly ashes. Energetika 2018, 64, 1–10. [Google Scholar] [CrossRef]
- JTG 3432-2024; Test Specifications for Highway Engineering Aggregates. Ministry of Transport of the People’s Republic of China; People’s Transport Press: Beijing, China, 2024.
- Cheng, X.; Deng, J.; Li, X.; Wei, X.; Shao, Y.; Zhao, Y. Layered Double Hydroxides Loaded Sludge Biochar Composite for Adsorptive Removal of Benzotriazole and Pb(II) from Aqueous Solution. Chemosphere 2022, 287, 131966. [Google Scholar] [CrossRef]
- Li, P. Modification and Adsorption Studies of Bamboo-Based Biochar on Dyes and Heavy Metals. Master’s Thesis, Zhejiang University of Science and Technology, Zhejiang, China, 2023. (In Chinese). [Google Scholar]
- Cortés, J.C.; Navarro-Quiles, A.; Santonja, F.J.; Sferle, S.-M. Statistical Analysis of Randomized Pseudo-First/Second Order Kinetic Models. Application to Study the Adsorption of Cadmium Ions onto Tree Fern. Chemom. Intell. Lab. Syst. 2023, 240, 104910. [Google Scholar] [CrossRef]
- Ajiboye, T.O.; Oyewo, O.A.; Onwudiwe, D.C. Simultaneous Removal of Organics and Heavy Metals from Industrial Wastewater: A Review. Chemosphere 2021, 262, 128379. [Google Scholar] [CrossRef]
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption Isotherm Models: Classification, Physical Meaning, Application and Solving Method. Chemosphere 2020, 258, 127279. [Google Scholar] [CrossRef]
- Ruan, X.; Li, R.; Ding, Z.; Luo, J.; Liu, Q.; Deng, C.; Li, D. Removal of Pb(II) Ions from Aqueous Solutions by Spherical Nanocomposites Synthesized Through Immobilization of Paecilomyces Lilacinus in Silica Nanoparticles Coated with Ca-Alginate. J. Nanosci. Nanotechnol. 2020, 20, 1907–1916. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.Q. Preparation of Biomass Charcoal from Wetland Plants and Its Adsorption of Ibuprofen in Water. Master’s Thesis, Shandong Normal University, Jinan, China, 2020. (In Chinese). [Google Scholar]
- Lu, Y.; Luo, Z.Q.; Zhou, X.T.; Zhao, X.T.; Lan, X. Adsorption of Pb²⁺, Cu²⁺, Zn²⁺ by Copper Slag Iron-Based Zeolite Geopolymer and Its Mechanism. Fine Chem. 2023, 40, 1003–5214. (In Chinese) [Google Scholar]
- Zhao, M.; Reda, A.T.; Zhang, D. Reduced Graphene Oxide/ZIF-67 Aerogel Composite Material for Uranium Adsorption in Aqueous Solutions. ACS Omega 2020, 5, 8012–8022. [Google Scholar] [CrossRef]
- Shi, W.; Wang, Z.; Li, F.; Xu, Y.; Chen, X. Multilayer Adsorption of Lead (Pb) and Fulvic Acid by Chlorella Pyrenoidosa: Mechanism and Impact of Environmental Factors. Chemosphere 2023, 329, 138596. [Google Scholar] [CrossRef]
- Zhang, Q.; Zhao, Z.; He, L.; Tan, G.; Guo, L.; Zheng, W. Adsorption Capacity and Mechanism of MgO Nanomaterials Prepared by Ultrasonic Electrodeposition for Pb(II). J. Mater. Eng. Perform. 2024, 33, 09556–09557. [Google Scholar] [CrossRef]
- Es-Said, A.; El Hamdaoui, L.; Ennoukh, F.E.; Nafai, H.; Zerki, N.; Lamzougui, G.; Bchitou, R. Chemometrics Approach for Adsorption Multi-Response Optimization of Cu(II), Zn(II), and Cd(II) Ions from Phosphoric Acid Solution Using Natural Clay. Phosphorus Sulfur Silicon Relat. Elem. 2023, 198, 424–434. [Google Scholar] [CrossRef]
- Wu, Y.; Qiu, X.; Cao, S.; Chen, J.; Shi, X.; Du, Y.; Deng, H. Adsorption of Natural Composite Sandwich-like Nanofibrous Mats for Heavy Metals in Aquatic Environment. J. Colloid Interface Sci. 2019, 539, 533–544. [Google Scholar] [CrossRef]
- Badawy, A.A.; Ibrahim, S.M.; Essawy, H.A. Enhancing the Textile Dye Removal from Aqueous Solution Using Cobalt Ferrite Nanoparticles Prepared in Presence of Fulvic Acid. J. Inorg. Organomet. Polym. Mater. 2020, 30, 1798–1813. [Google Scholar] [CrossRef]
- Yin, W.; Zhao, C.; Xu, J. Enhanced adsorption of Cd(II) from aqueous solution by a shrimp bran modified Typha orientalis biochar. Environ. Sci. Pollut. Res. 2019, 26, 37092–37100. [Google Scholar] [CrossRef]
- Fadhel, S.R. Chitosan-NiFe2O4 Nanocomposite Synthesis for Effective Removal of Pb(II) and Zn(II) from Aqueous Solution. Results Eng. 2024, 24, 103293. [Google Scholar] [CrossRef]
- Zhang, Z.; Niu, Y. Feasible One-Pot Sequential Synthesis of Aminopyridine Functionalized Magnetic Fe3 O4 Hybrids for Robust Capture of Aqueous Hg(II) and Ag(I). ACS Sustain. Chem. Eng. 2019, 7, 7324–73371. [Google Scholar] [CrossRef]
- Gusain, R.; Kumar, N.; Fosso-Kankeu, E.; Ray, S.S. Efficient Removal of Pb(II) and Cd(II) from Industrial Mine Water by a Hierarchical MoS2/SH-MWCNT Nanocomposite. ACS Omega 2019, 4, 13922–13935. [Google Scholar] [CrossRef] [PubMed]
- Mo, W.; He, C. Adsorption Behavior of Mg–Al Layered Double Hydroxide on Pb(II), Zn(II), Cd(II), and As(V) Coexisting in Aqueous Solution. Mater. Today Sustain. 2024, 27, 100861. [Google Scholar]
- Myung, E.; Kim, H.; Choi, N.; Cho, K. The Biochar Derived from Spirulina platensis for the Adsorption of Pb and Zn and Enhancing the Soil Physicochemical Properties. Chemosphere 2024, 364, 143203. [Google Scholar] [CrossRef]
- Wijeyawardana, P.; Nanayakkara, N.; Law, D.; Gunasekara, C.; Karunarathna, A.; Pramanik, B.K. Evaluating the Performance of Cement-Modified Biochar Adsorbent for Cu, Pb, and Zn Removal from Urban Stormwater. Process Saf. Environ. Prot 2024, 186, 1419–1431. [Google Scholar] [CrossRef]
- Chen, Y.; Shi, C. One-Pot Synthesis of High-Efficiency Hydroxyapatite-Hydrochar Composites Derived from Phytoremediation Biomass and Bone Meal: Heavy Metal Stabilization, Adsorption Performance and Mechanism. J. Alloys Compd. 2025, 1022, 179859. [Google Scholar] [CrossRef]
- Meng, F.; Tang, X.; Kadja, G.T.; Yi, H.; Zhao, S.; Wu, W.; Zhang, Y.; Gao, F.; Yu, Q. A Systematic Review with Improving Activity and Stability in VOCs Elimination by Oxidation of Noble Metals: Starting from Active Sites. Sep. Purif. Technol. 2025, 354, 129222. [Google Scholar] [CrossRef]
- Das, D.; Prakash, J.; Bandyopadhyay, A.; Balhara, A.; Goutam, U.K.; Acharya, R.; Gupta, S.K.; Sudarshan, K. Modulating the Effective Ionic Radii of Trivalent Dopants in Ceria Using a Combination of Dopants to Improve Catalytic Efficiency for the Oxygen Evolution Reaction. RSC Adv. 2024, 14, 17801–17813. [Google Scholar] [CrossRef] [PubMed]
- Xie, H.; Xiao, G.; Song, Q.; Meng, Q. La3⁺ and Al3⁺ Loaded D151 as Salt-Resistant Resins for Efficient Adsorption of Glyphosate: Effects of Ionic Radius. J. Appl. Polym. Sci. 2023, 141, e54756. [Google Scholar] [CrossRef]
- Wang, H.; Lin, T.; Song, Z.; Huang, M.; Chai, R.; An, S.; Song, Y.-F. Simultaneous Mineralization of Cd(II), Pb(II) and As(V) Using MgAl-NO3: Performance and Mechanism. Sep. Purif. Technol. 2025, 362, 131853. [Google Scholar] [CrossRef]
- Fan, C.; Wang, B.; Xu, Y. Solidification/Stabilization and Immobilization Mechanism of Pb(II) and Zn(II) in Ettringite. Cem. Concr. Res. 2023, 174, 107350. [Google Scholar] [CrossRef]
- Asim, N.; Badiei, M. Inorganic-Based Adsorbent Materials for the Removal of Gaseous Pollutants. Int. J. Environ. Sci. Technol. 2022, 19, 5731–5752. [Google Scholar] [CrossRef]

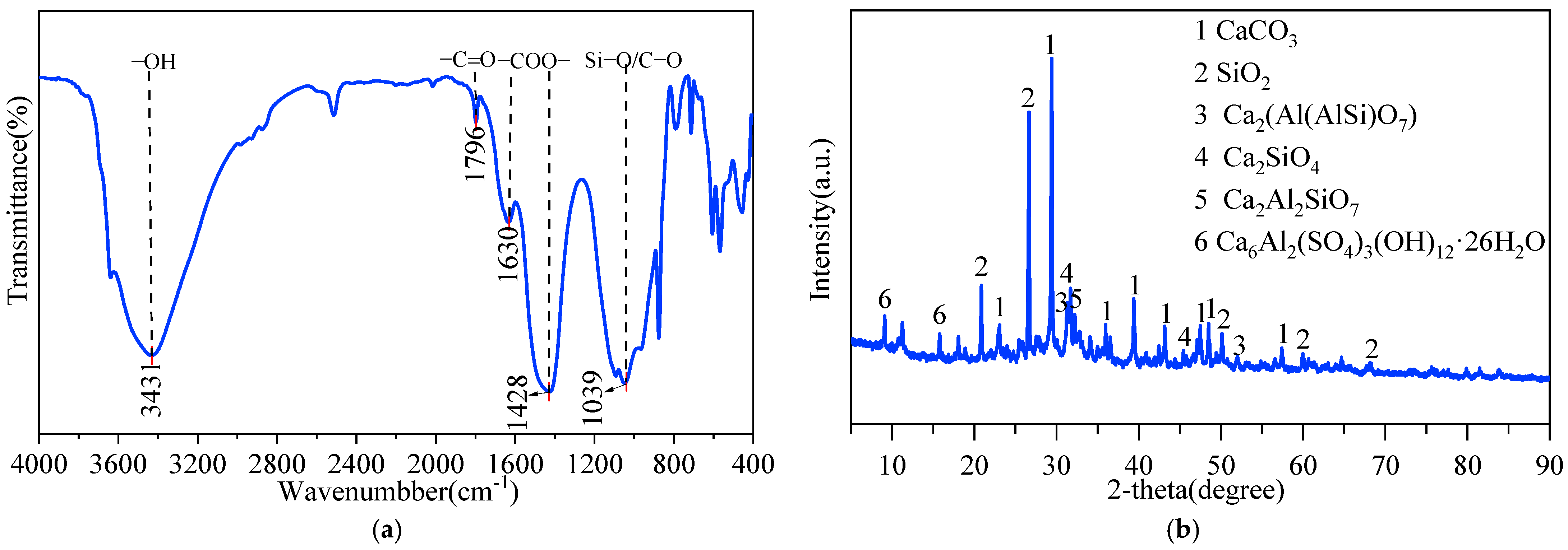
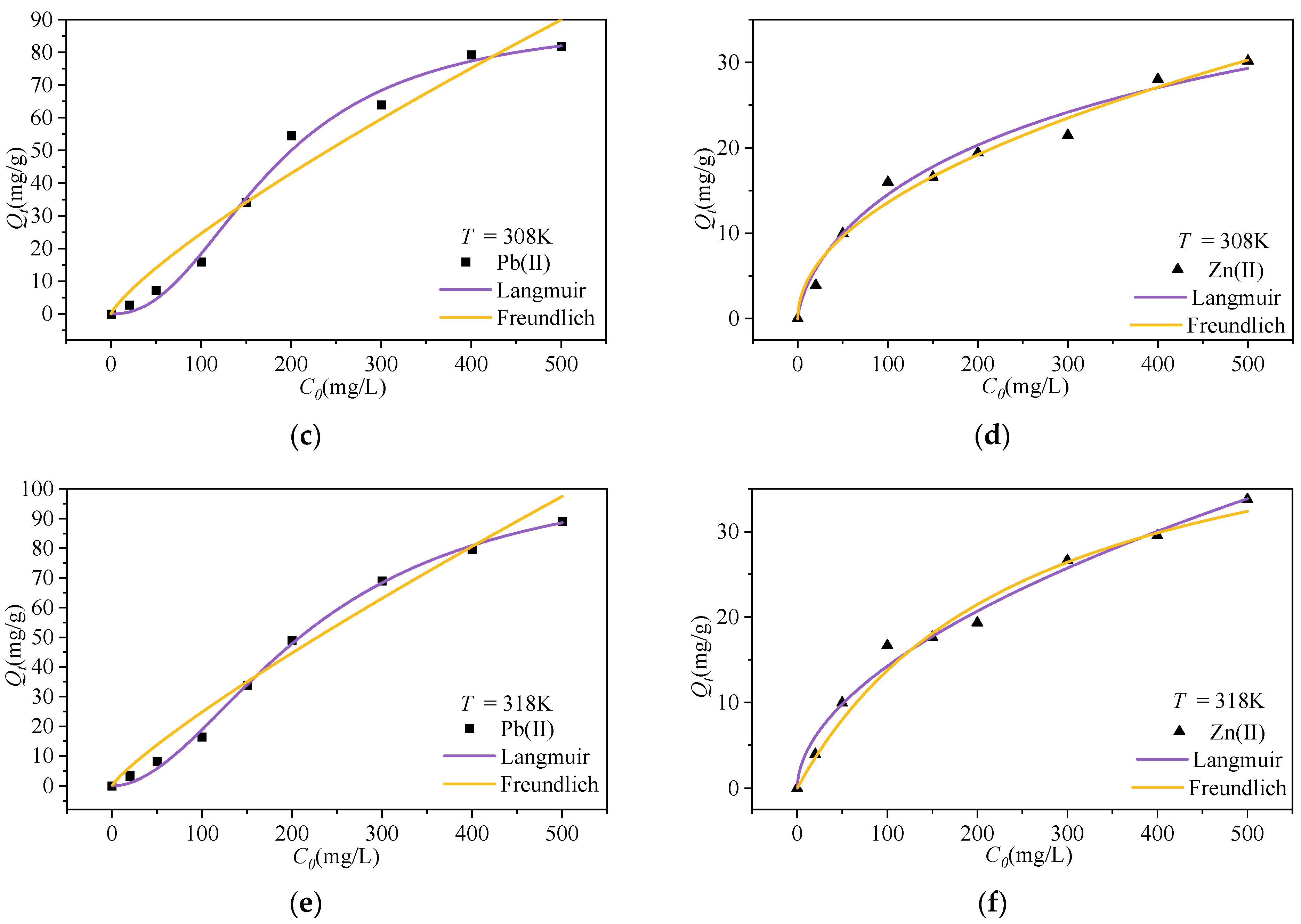

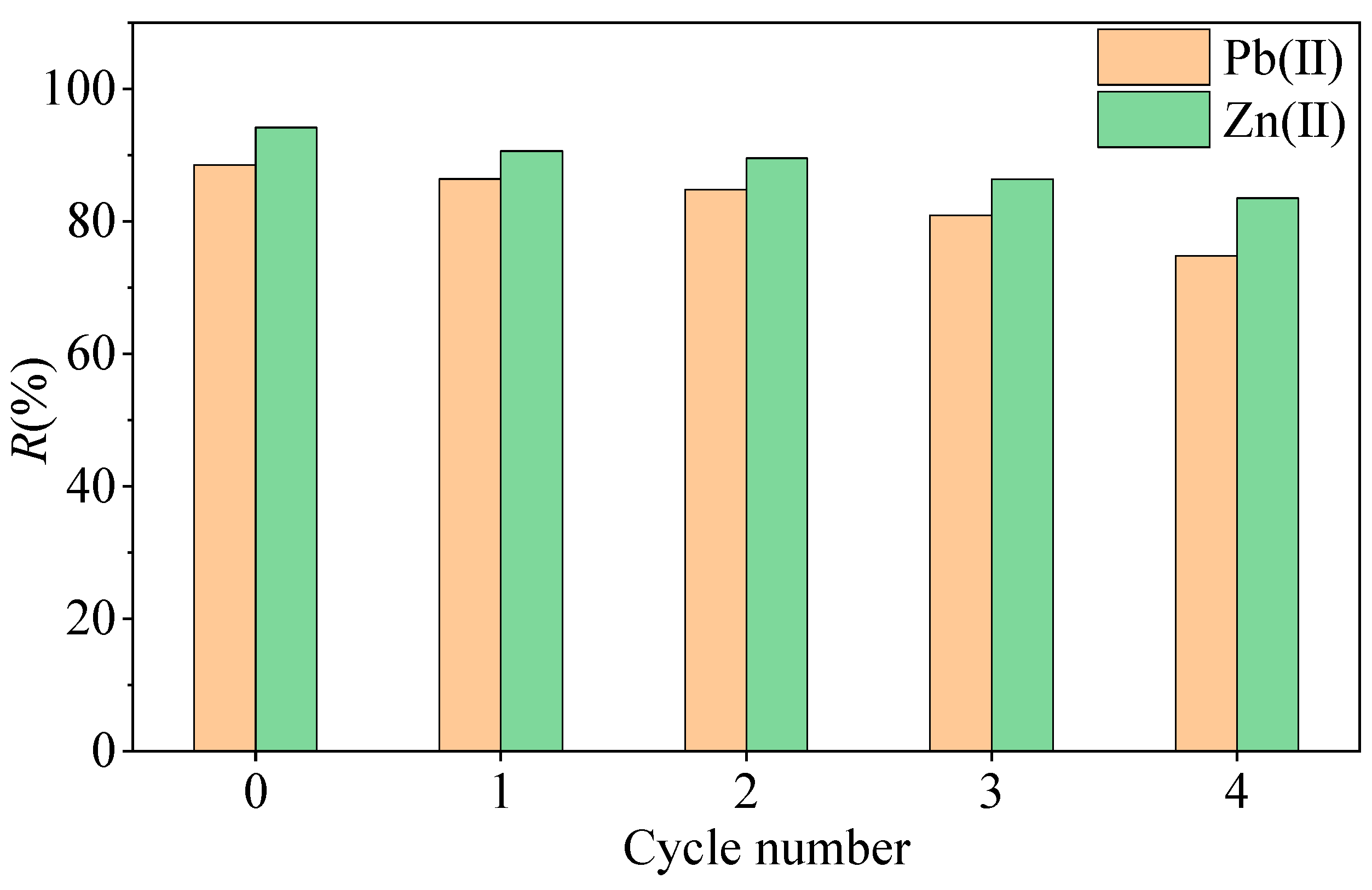


| Element | O | Na | Mg | Al | Si | K | Ca |
| Atomic/% | 61.5 | 0.9 | 0.8 | 2.0 | 6.9 | 0.8 | 26.9 |
| MSWI-BA Sample | BET-Specific Surface Area/m2·g−1 | Pore Volume /10−3 cm3·g−1 | Pore Diameter /nm |
|---|---|---|---|
| (1) | 7.48 | 0.027 | 3.25 |
| (2) | 7.66 | 0.031 | 3.45 |
| (3) | 7.24 | 0.029 | 3.42 |
| Average value | 7.46 | 0.029 | 3.36 |
| Ions | Qe/mg·g−1 | PFO Model (R2) | PSO Model (R2) |
|---|---|---|---|
| Pb(II) | 1.98 | 0.97 | 0.91 |
| Zn(II) | 1.92 | 0.99 | 0.99 |
| Ions | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|
| Qm/mg·g−1 | KL/L·mg−1 | R2 | 1/n | KF/L·mg−1 | R2 | |
| Pb(II) | 107.88 | 3.17 × 10−4 | 0.99 | 0.40 | 0.81 | 0.97 |
| Zn(II) | 60.29 | 3.78 × 10−4 | 0.98 | 0.46 | 0.53 | 0.98 |
| Ions | ΔH°/K·mol−1 | ΔS°/J·mol−1·K−1 | ΔG°/KJ·mol−1 | ||
|---|---|---|---|---|---|
| 298 K | 308 K | 318 K | |||
| Pb(II) | 9.9313 | 36.7638 | −0.4134 | −0.54357 | −0.6655 |
| Zn(II) | 23.4652 | 80.8864 | −0.25786 | −0.56536 | −0.8535 |
| Adsorbent | Pb(II)/mg·g−1 | Zn(II)/mg·g−1 | References |
|---|---|---|---|
| MgAl-layered double hydroxide | 10.88 | 7.30 | [40] |
| Spirulina platensis 600 | 26.9 | 27.8 | [41] |
| Cement-modified biochar composites | 38.76 | 26.53 | [42] |
| High-efficiency hydroxyapatite–hydrochar composites | -- | 24.99 | [43] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhao, Y.; Li, W.; Wang, J.; Hu, Z. A Sustainable Development Strategy for Municipal Solid Waste Incineration Bottom Ash: Adsorption Performance and Mechanism in Removing Heavy Metals from Water. Sustainability 2025, 17, 3466. https://doi.org/10.3390/su17083466
Zhao Y, Li W, Wang J, Hu Z. A Sustainable Development Strategy for Municipal Solid Waste Incineration Bottom Ash: Adsorption Performance and Mechanism in Removing Heavy Metals from Water. Sustainability. 2025; 17(8):3466. https://doi.org/10.3390/su17083466
Chicago/Turabian StyleZhao, Yao, Wenqian Li, Jiaqing Wang, and Zekunyun Hu. 2025. "A Sustainable Development Strategy for Municipal Solid Waste Incineration Bottom Ash: Adsorption Performance and Mechanism in Removing Heavy Metals from Water" Sustainability 17, no. 8: 3466. https://doi.org/10.3390/su17083466
APA StyleZhao, Y., Li, W., Wang, J., & Hu, Z. (2025). A Sustainable Development Strategy for Municipal Solid Waste Incineration Bottom Ash: Adsorption Performance and Mechanism in Removing Heavy Metals from Water. Sustainability, 17(8), 3466. https://doi.org/10.3390/su17083466






