Studies on Grass Germination and Growth on Post-Flotation Sediments
Abstract
1. Introduction
2. Materials and Methods
2.1. Seed Germination Test
2.2. Seedling Growth
2.3. Statistical Analysis
3. Results
3.1. Germination Test
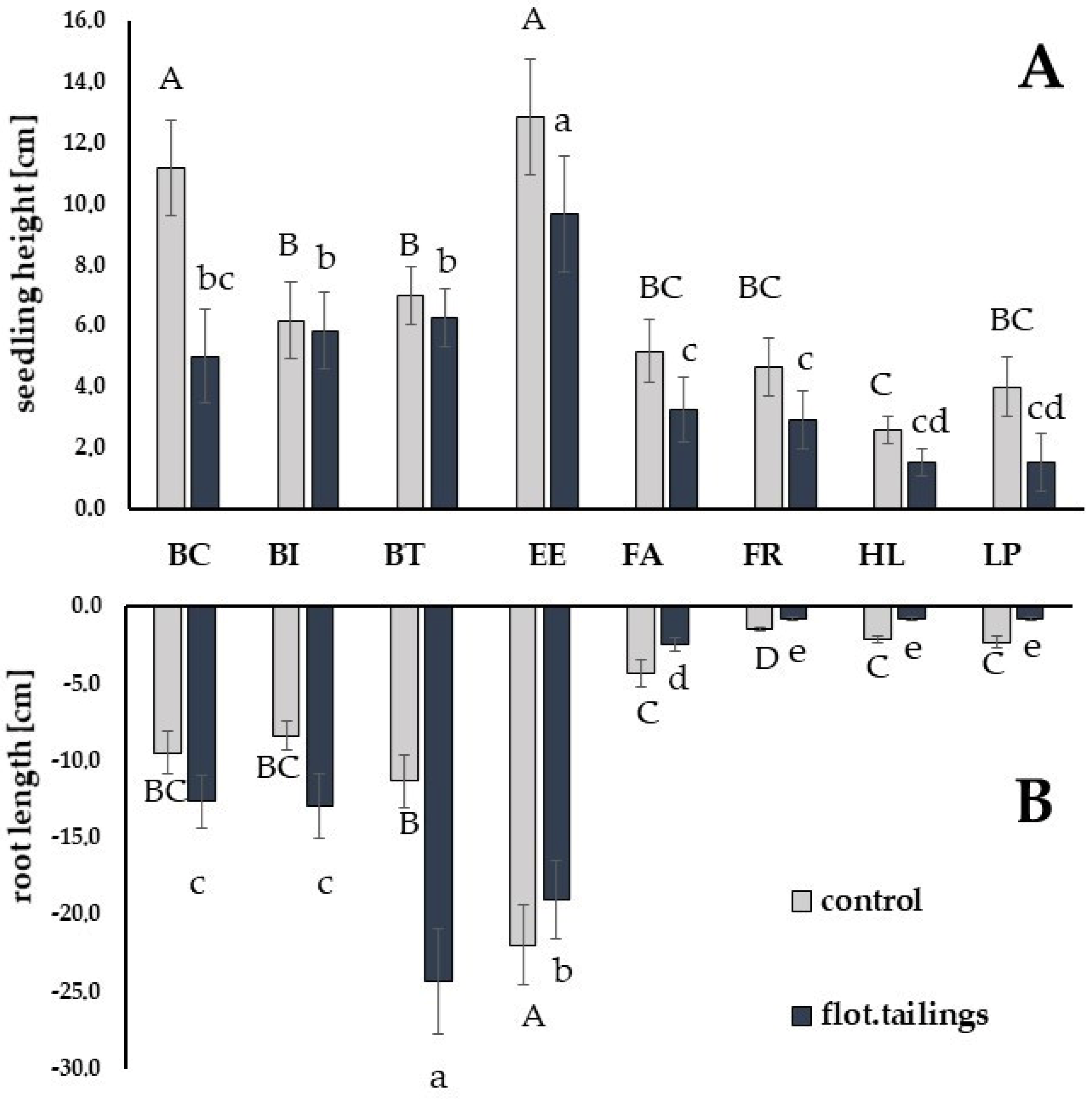
3.2. Seedling Growth and Development
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Leopold, A. A Sand County Almanac and Sketches Here and There; Penguin Random House: London, UK, 2020; p. 173. [Google Scholar]
- Oldeman, L.R. Global Extent of Soil Degradation. In ISRIC Bi-Annual Report 1991–1992; International Soil Reference and Information Centre: Wageningen, The Netherlands, 1992; pp. 19–36. [Google Scholar]
- Baumhardt, R.L.; Stewart, B.A.; Sainju, U.M. North American Soil Degradation: Processes, Practices, and Mitigating Strategies. Sustainability 2015, 7, 2936–2960. [Google Scholar] [CrossRef]
- Biegańska, J. Remediation and revitalization of industrial areas—A method of promotion. IOP Conf. Ser. Mater. Sci. Eng. 2019, 545, 012002. [Google Scholar] [CrossRef]
- Pasieczna, A. Soil contamination induced by historical zinc-lead ore mining and iron and zinc smelting in the central part of the upper silesian industrial region (Southern Poland). Biul. Państwowego Inst. Geol. 2018, 473, 49–66. [Google Scholar] [CrossRef]
- Pasieczna, A.; Konon, A.; Nadłonek, W. Sources of anthropogenic contamination of soil in the upper silesian agglomeration (Southern Poland). Geol. Quaterly 2020, 64, 988–1003. [Google Scholar] [CrossRef]
- Gondek, K.; Mierzwa-Hersztek, M.; Kopeć, M.; Bajda, T. Phytostabilisation on post-flotation sediment waste: Mobility of heavy metals and stimulation of biochemical processes by mineral-organic mixtures. J. Soils Sediments 2020, 20, 3502–3513. [Google Scholar] [CrossRef]
- Michalski, K. Analysis of the Features of a Degraded Land in Terms of Revitalization—Case Study on the Example of a Post-Smelter Area. Multidiscip. Asp. Prod. Eng. MAPE 2020, 3, 559–569. [Google Scholar] [CrossRef]
- Ciesiółka, P. Urban regeneration as a new trend in the development policy in Poland. Quaestionnes Geogr. 2018, 37, 109–123. [Google Scholar] [CrossRef]
- Hilson, G. An overview of land use conflicts in mining communities. Land Use Policy 2002, 19, 65–73. [Google Scholar] [CrossRef]
- Pietrzyk-Sokólska, E.; Uberman, R.; Kulczycka, J. Wpływ górnictwa na środowisko w Polsce—Mity i rzeczywistość. Gospod. Surowcami Miner. Miner. Resour. Manag. 2015, 31, 45–64. [Google Scholar] [CrossRef]
- Cacciuttolo, C.; Cano, D.; Custodio, M. Socio-Environmental Risks Linked with Mine Tailings Chemical Composition: Promoting Responsible and Safe Mine Tailings Management Considering Copper and Gold Mining Experiences from Chile and Peru. Toxics 2023, 11, 462. [Google Scholar] [CrossRef]
- Łuszczkiewicz, A. Koncepcje wykorzystania odpadów flotacyjnych z przeróbki rud miedzi w regionie legnicko-głogowskim. Inżynieria Miner. 2000, 1, 25–35. [Google Scholar]
- Haneef Mian, M.; Yanful, E. Tailings erosion and resuspension in two mine tailings ponds due to wind waves. Adv. Environ. Res. 2003, 7, 745–765. [Google Scholar] [CrossRef]
- Jelea, O.-C.; Baciu, C. Effects of heavy metals, contained in flotation tailings, on plants used in revegetation. Carpathian J. Earth Environ. Sci. 2023, 18, 89–103. [Google Scholar] [CrossRef]
- Slingerland, N.; Sommerville, A.; O’Leary, D.; Beier, N.A. Identification and quantification of erosion on a sand tailings dam. Geosystem Eng. 2018, 23, 131–145. [Google Scholar] [CrossRef]
- van Wyk, S.J.; Hatting, J.; Haagner, A.S.H. Wind erosion design considerations for closure of tailings storage facilities in South Africa: A case study. In Mine Closure 2019: Proceedings of the 13th International Conference on Mine Closure, Australian Centre for Geomechanics, Perth; ACG: Crawley, Australia, 2019; pp. 1185–1200. [Google Scholar] [CrossRef]
- Dentoni, V.; Grosso, B.; Pinna, F. Experimental evaluation of PM emission from Red Mud Basins exposed to wind erosion. Minerals 2021, 11, 405. [Google Scholar] [CrossRef]
- Namdar-Khojasteh, D.; Bazgir, M.; Hashemi Babaheidari, S.A.; Akwasi BAsmudu-Sakyi, A.B. Application of biocementation technique using Bacillus sphaericus for stabilization of soil surface and dust storm control. J. Arid Land 2002, 14, 537–549. [Google Scholar] [CrossRef]
- Maureira, A.; Zapata, M.; Olave, J.; Jeison, D.; Wong, L.-S.; Panico, A.; Hernández, P.; Cisternas, L.A.; Rivas, M. MICP mediated by indigenous bacteria isolated from tailings for biocementation for reduction of wind erosion. Front. Bioeng. Biotechnol. 2024, 12, 1393334. [Google Scholar] [CrossRef]
- Mohebbi, H.R.; Javadi, A.A.; Azizkandi, A.S. The Effects of Soil Porosity and Mix Design of Volcanic Ash-Based Geopolymer on the Surface Strength of Highly Wind Erodible Soils. Minerals 2022, 12, 984. [Google Scholar] [CrossRef]
- Andrejić, G.; Kovaćević, M.; Dzeletović, Z.; Aleksić, U.; Grdović, I.; Rakić, T. Potentially toxic element accumulation in two Equisetum species spontaneously grown in the flotation tailings. J. Serbian Chem. Soc. 2023, 88, 1055–1064. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, Z.; Li, Q.; He, L.; Zhao, X.; Wang, L.; Han, D. Determine the Optimal Vegetation Type for Soil Wind Erosion Prevention and Control in the Alpine Sandy Land of the Gonghe Basin on the Qinghai Tibet Plateau. Forests 2023, 14, 2342. [Google Scholar] [CrossRef]
- Antonelli, P.M.; Coghill, M.G.; Gardner, W.C.; Fraser, L.H. Semiarid bunchgrasses accumulate molybdenum on alkaline copper mine tailings: Assessing phytostabilization in the greenhouse. SN Appl. Sci. 2021, 3, 747. [Google Scholar] [CrossRef]
- El Berkaoui, M.; El Adnani, M.; Hakkou, R.; Ouhammou, A.; Bendaou, N.; Smouni, A. Assessment of the Transfer of Trace Metals to Spontaneous Plants on Abandoned Pyrrhotite Mine: Potential Application for Phytostabilization of Phosphate Wastes. Plants 2022, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Puffer, M.; Limb, R.F.; Daigh, A.L.M.; Sedivec, K.K. Mechanical and biotic reclamation strategies for a post-mine temperate grassland. Land Degrad. Dev. 2023, 34, 3114–3129. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; The International Seed Testing Association: Wallisellen, Switzerland, 2023. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and, Evolution of Dormancy and Germination, 2nd ed.; Academic Press, Elsevier Inc.: Amsterdam, The Netherlands, 2014; p. 1586. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; p. 392. [Google Scholar] [CrossRef]
- Nascimento, C.E.d.S.; da Silva, C.A.D.; Leal, I.R.; Tavares, W.d.S.; Serrão, J.E.; Zanuncio, J.C.; Tabarelli, M. Seed germination and early seedling survival of the invasive species Prosopis juliflora (Fabaceae) depend on habitat and seed dispersal mode in the Caatinga dry forest. PeerJ 2020, 8, e9607. [Google Scholar] [CrossRef]
- Gilani, M.M.; Ahmad, I.; Farooq, T.H.; Wu, P.; Yousaf, M.S.; Khan, M.W.; Yousuf Bin, T.; Ma, X. Effects of pre-sowing treatments on seed germination and morphological growth of Acacia nilotica and Faidherbia albida. Sci. For. Piracicba 2019, 47, 374–382. [Google Scholar] [CrossRef]
- Vogel, K.P.; Moore, K.J.; Moser, L.E. Bromegrass. In Cool-Season Forage Grasses; Agronomy Monograph 34; Moser, L.E., Buxton, D.R., Casler, M.D., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, MI, USA, 1996; pp. 535–567. [Google Scholar]
- Salesman, J.B.; Thomsen, M. Smooth Brome (Bromus inermis) in Tallgrass Prairies: A Review of Control Methods and Future Research Directions. Ecol. Restor. 2011, 29, 374–381. [Google Scholar] [CrossRef]
- Klimont, K.; Bulińska-Radomska, Z. Badanie Rozwoju Wybranych Gatunków Traw Do Umacniania Składowisk Popiołów Paleniskowych z Elektrociepłowni. Probl. Inżynierii Rol. 2009, 17, 135–145. [Google Scholar]
- Raawe, H. About Grass and Legume Species Suitability for Recultivation of Semi-Coke Dumps. Agronomy 2004, 219, 154–156. [Google Scholar]
- Hume, D.E. Primary growth and quality characteristics of Bromus willdenowii and Lolium multiflorum. Grass Forage Sci. 1990, 46, 313–326. [Google Scholar] [CrossRef]
- Yi, L.; Dong, Z.; Lei, Y.; Zhao, J.; Xiong, Y.; Yang, J.; Xiong, Y.; Gou, W.; Ma, X. Genetic Diversity and Molecular Characterization of Worldwide Prairie Grass (Bromus catharticus Vahl) Accessions Using SRAP Markers. Agronomy 2021, 11, 2054. [Google Scholar] [CrossRef]
- Belesky, D.P.; Ruckle, J.M.; Abaye, A.O. Seasonal distribution of herbage mass and nutritive value of Prairiegrass (Bromus catharticus Vahl). Grass Forage Sci. 2007, 62, 301–311. [Google Scholar] [CrossRef]
- Asay, K.H.; Jensen, K.B. Wheatgrasses. In Cool-Season Forage Grasses; Agronomy Monograph 34; Moser, L.E., Buxton, D.R., Casler, M.D., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, MI, USA, 1996; pp. 691–724. [Google Scholar]
- Taboada, M.A.; Rubio, G.; Lavado, R. The deterioration of tall wheatgrass pastures on saline sodic soils. J. Rangel. Manag. 1998, 51, 241–246. [Google Scholar] [CrossRef]
- Nichols, C. Establishing and Managing Tall Wheat Grass in Saline Soils for Productivity; Agricultural Notes; VGLS: Werribee, Australia, 1998; pp. 1–2. [Google Scholar]
- Csete, S.; Stranczinger, S.; Szalontai, B.; Farkas, A. Tall wheatgrass cultivar Szarvasi-1 (Elymus elongatus subsp. ponticus cv. Szarvasi-1) as a potential energy crop for semi-arid lands of Eastern Europe. In Sustainable Growth and Applications in Renewable Energy Sources; Nayeripour, M., Kheshti, M., Eds.; InTech: Rijeka, Croatia, 2011; pp. 269–294. [Google Scholar]
- Vergiev, S. Comparative Study of the capacity of three plant species from the Poaceae family for erosion and flooding control of coastal areas. Sustainable Development and Innovations in Marine Technologies. In Proceedings of the 18th International Congress of the International Maritime Association of the Mediterranean, IMAM 2019, Varna, Bulgaria, 9–11 September 2019; pp. 597–602. [Google Scholar]
- Beckstead, J.; Meyer, S.E.; Allen, P.S. Bromus tectorum seed germination: Between-population and between-year variation. Can. J. Bot. 1996, 75, 875–882. [Google Scholar] [CrossRef]
- Andersson, L.; Milberg, P.; Schütz, W.; Steinmetz, O. Germination characteristics and emergence time of annual Bromus species of differing weediness in Sweden. Weed Res. 2002, 42, 135–147. [Google Scholar] [CrossRef]
- Franzese, J.; di Virgilio, A.; Pirk, G.; Lescano, M.N.; Speziale, K.L. Low biotic resistance to cheatgrass invasion in Patagonia: Evidence from competition experiments. Biol. Invasions 2022, 24, 235–246. [Google Scholar] [CrossRef]
- Howel, A.; Winkler, D.E.; Phillips, M.L.; McNellis, B.; Reed, S.C. Experimental Warming Changes Phenology and Shortens Growing Season of the Dominant Invasive Plant Bromus tectorum (Cheatgrass). Front. Plant Sci. 2020, 11, 570001. [Google Scholar] [CrossRef]
- Mack, R.N. Invasion of Bromus tectorum L. into Western North America: An ecological chronicle. Agro-Ecosystems 1981, 7, 145–165. [Google Scholar] [CrossRef]
- Emam, T.M.; Espeland, E.K.; Rinella, M.J. Soil sterilization alters interactions between the native grass Bouteloua gracilis and invasive Bromus tectorum. J. Arid. Environ. 2014, 111, 91–97. [Google Scholar] [CrossRef]
- Seastedt, T.R.; Pysek, P. Mechanisms of plant invasions of North American and European grasslands. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 133–153. [Google Scholar] [CrossRef]
- Pyšek, P.; Manceur, A.M.; Alba, C.; McGregor, K.F.; Pergl, J.; Štajerová, K.; Chytrý, M.; Danihelka, J.; Kartesz, J.; Klimešová, J.; et al. Naturalization of central European plants in North America: Species traits, habitats, propagule pressure, residence time. Ecology 2015, 96, 762–774. [Google Scholar] [CrossRef]
- Sheley, R.L.; Krueger-Mangold, J. Principles for restoring invasive plant-infested rangeland. Weed Sci. 2003, 51, 260–265. Available online: http://www.jstor.org/stable/4046729 (accessed on 15 January 2025). [CrossRef]
- Hulme, P.E.; Bacher, S.; Kenis, M.; Klotz, S.; Kühn, I.; Minchin, L.; Nentwig, W.; Olenin, S.; Panov, J.; Pergl, J.; et al. Grasping at the routes of biological invasions: A framework for integrated biosecurity. J. Appl. Ecol. 2008, 45, 403–414. [Google Scholar] [CrossRef]
- Wong, M.H. Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere 2003, 50, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Krawczyńska, M.; Kołwzan, B.; Gediga, K.; Dziubek, A.M.; Grabas, K.; Karpenko, E. Evaluation of the possibility of phytostabilization of post-flotation tailings pond. Environ. Prot. Eng. 2015, 41, 157–167. [Google Scholar] [CrossRef]
- van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Wardle, D.A. Plant-soil feedback cycles as drivers of plant community assembly. Trends Ecol. Evol. 2016, 31, 589–601. [Google Scholar]
| Parameter | Substrate: | Accredited Procedure: | |
|---|---|---|---|
| Flotation Tailing | Sand (Control) | ||
| Soil composition: | laser diffraction method | ||
| sand (0.05–2.0 mm) | 94.96 | 100.0 | |
| silt (0.002–0.05 mm) | 5.04 | 0.0 | |
| clay (<0.002 mm) | 0 | 0.0 | |
| organic matter content (%) | <0.30 * | <0.30 * | titration method |
| pH (KCl) | 8.4± | 8.1 | potentiometric method |
| Macro- and micronutrients (in mg kg−1) | |||
| N-NO3 | <5 * | <5 * | Continuous Flow Analysis (CFA) method with spectrophotometric detection |
| N-NH4 | 5 | 8 | |
| Cl | <10 * | 11 | potentiometric method |
| P | 23 | 5 | Flame Atomic Absorption Spectrometry (FAAS) method |
| K | 20 | 10 | |
| Ca | 5350 | 440 | |
| Mg | 60 | 25 | |
| Cu | 24.0 | 1.1 | |
| Fe | 13.4 | 15.2 | |
| Mn | 9.2 | 9.9 | |
| Zn | 1.8 | 3.9 | |
| Genus Species | Germination: | ||
|---|---|---|---|
| acc. to the ISTA Rules | In Sand | In Flotation Tailing | |
| % [Mean ± std.dev.] | |||
| Bromus inermis LINCOLN | 83.3 ± 1.51 B b | 93.3 ± 4.08 A a | 57.2 ± 3.99 C cd |
| Bromus tectorum, ecotype | 85.5 ± 1.87 B ab | 93.0 ± 3.58 A a | 93.5 ± 3.08 A a |
| Bromus catharticus BROMA | 80.6 ± 7.37 AB b | 91.7 ± 6.38 A ab | 81.2 ± 10.17 AB ab |
| Elytrigia elongata BAMAR | 94.0 ± 1.55 A a | 91.0 ± 3.58 A ab | 79.7 ± 9.33 B b |
| Festuca arundinacea BARFELIX | 87.5 ± 4.10 A ab | 54.0 ± 4.81 B e | 60.7 ± 8.61 B c |
| Festuca rubra ARETA | 83.2 ± 1.47 A b | 69.0 ± 9.96 B c | 31.8 ± 1.94 C e |
| Holcus lanatus, ecotype | 84.2 ± 3.49 A ab | 61.0 ± 3.03 B d | 52.2 ± 2.13 C d |
| Lolium perenne BOKSER | 90.0 ± 3.90 A a | 34.1 ± 3.39 B f | 26.3 ± 0.75 C f |
| Genus Species | Growing Substrate | Seedling Height [cm] | Length of the Root System [cm] | ||
|---|---|---|---|---|---|
| Mean ± std.dev. | T-Test | Mean ± std.dev. | T-Test | ||
| Bromus inermis LINCOLN | Sand | 6.2 ± 1.47 | 0.43 ns | 8.4 ± 0.80 | −6.91 *** |
| flot. tailing | 5.8 ± 1.17 | 13.0 ± 1.41 | |||
| Bromus tectorum ecotype | Sand | 7.0 ± 1.90 | 0.84 ns | 11.3 ± 1.21 | −15.66 *** |
| flot. tailing | 6.3 ± 1.08 | 24.3 ± 1.63 | |||
| Bromus catharticus BROMA | Sand | 11.2 ± 1.17 | 7.78 *** | 9.5 ± 1.05 | −3.48 *** |
| flot. tailing | 5.0 ± 1.55 | 12.7 ± 1.97 | |||
| Elytrigia elongata BAMAR | Sand | 12.8 ± 1.94 | 3.39 ** | 22.0 ± 1.41 | 4.11 *** |
| flot. tailing | 9.7 ± 1.21 | 19.0 ± 1.09 | |||
| Festuca arundinacea BARFELIX | Sand | 5.2 ± 1.47 | 2.31 ** | 4.3 ± 1.75 | 2.20 ns |
| flot. tailing | 3.2 ± 1.40 | 2.5 ± 1.05 | |||
| Festuca rubra ARETA | Sand | 4.7 ± 1.03 | 2.95 ** | 1.5 ± 1.05 | 1.51 ns |
| flot. tailing | 2.9 ± 1.02 | 0.8 ± 0.26 | |||
| Holcus lanatus ecotype | Sand | 2.6 ± 1.43 | 1.52 ns | 2.2 ± 1.17 | 2.73 ** |
| flot. tailing | 1.5 ± 1.00 | 0.8 ± 0.26 | |||
| Lolium perenne BOKSER | Sand | 4.0 ± 0.89 | 5.68 *** | 2.3 ± 0.82 | 4.29 *** |
| flot. tailing | 1.4 ± 0.66 | 0.8 ± 0.26 | |||
| Mean | Sand | 6.3 ± 1.42 | 3.32 *** | 7.6 ± 1.11 | 0.82 ns |
| flot. tailing | 4.3 ± 1.09 | 8.8 ± 0.97 | |||
| Difference between species | Sand | *** | *** | ||
| in substrates used: | flot. tailing | *** | *** | ||
| Germination Test: | Germination Test in: | Growing Substrate: | ||||
|---|---|---|---|---|---|---|
| Sand | Flotation | Sand | Flotation Tailing | |||
| Tailings | Seedling Height | Length of Roots | Seedling Height | Length of Roots | ||
| ISTA rules | 0.89 *** | 0.78 ** | 0.43 ns | 0.49 ns | 0.59 ns | 0.52 ns |
| sand | x | 0.88 ** | 0.59 ns | 0.60 ns | 0.71 ** | 0.74 ** |
| flot.tailings | x | x | 0.67 ns | 0.72 ** | 0.74 ** | 0.87 ** |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Żurek, G.; Martyniak, D. Studies on Grass Germination and Growth on Post-Flotation Sediments. Sustainability 2025, 17, 3438. https://doi.org/10.3390/su17083438
Żurek G, Martyniak D. Studies on Grass Germination and Growth on Post-Flotation Sediments. Sustainability. 2025; 17(8):3438. https://doi.org/10.3390/su17083438
Chicago/Turabian StyleŻurek, Grzegorz, and Danuta Martyniak. 2025. "Studies on Grass Germination and Growth on Post-Flotation Sediments" Sustainability 17, no. 8: 3438. https://doi.org/10.3390/su17083438
APA StyleŻurek, G., & Martyniak, D. (2025). Studies on Grass Germination and Growth on Post-Flotation Sediments. Sustainability, 17(8), 3438. https://doi.org/10.3390/su17083438







