Abstract
The sediments remaining after copper flotation (here referred to as to flotation tailings) are generally characterized by nutrient deficiency and heavy metal enrichment, presenting significant obstacles to vegetation establishment and the development of sustainable ecosystems. This research aimed to evaluate the germination and growth performance of eight grass species on these tailings compared to reference conditions. Seed germination was assessed across flotation tailings, sand, and controlled laboratory environments. Initial establishment success was determined by measuring seedling height and root length three weeks after sowing in a glasshouse pot experiment. The findings revealed that Bromus inermis, Bromus catharticus, and Elytrigia elongata were capable of germinating and growing successfully in the challenging substrate, indicating their potential role in sustainable land management. Despite demonstrating excellent germination and seedling growth under test conditions, Bromus tectorum was deemed unsuitable due to its potential for invasive behavior, which could threaten the sustainability of revegetation efforts. Furthermore, species commonly employed for the revegetation of difficult terrains, including Festuca arundinacea, Festuca rubra, and Lolium perenne, proved entirely ineffective for the sustainable treatment of these flotation tailings.
1. Introduction
Industry and intensive agriculture contribute significantly to the depletion of soil resources. These changes are often irreversible. Human-induced changes differ fundamentally from natural changes and have more extensive effects than anticipated or intended [1]. The primary drivers of land degradation include human activities such as deforestation, overgrazing, improper agricultural practices, and industrial emissions. These activities lead to soil erosion, loss of soil fertility, and consequently, biodiversity loss [2,3]. Degraded areas, for example, in Poland are particularly characterized by post-industrial sites, abandoned mines, un-reclaimed mining heaps, and urban regions suffering from socio-economic decline [4,5,6].
Heavy industries, especially in the Upper Silesian Industrial Region, have resulted in the widespread contamination of land with harmful substances and physical degradation due to mining and smelting activities [7,8]. Urban areas face degradation due to socio-economic decline, inadequate infrastructure, and high concentrations of negative social phenomena [9]. Despite the amount of scientific research and practical activities carried out to date, there are still issues that require urgent solution. One such issue is the growing amount of un-reclaimed remains of the mining industry, which are a clear example of unsustainable mining practices [10,11]. Due to the specificity of different mining processes, these remains are very different and, as such, require separate, specific approaches to their recultivation [12]. One of the many specific types of mining remains are flotation tailings of copper sulfide ores. The ore is of sedimentary origin and the tailing contains mainly quartz, dolomite, clay minerals, traces of sulfides, and some accessory minerals, as well as heavy metals. The waste handling and disposal system includes traditional ‘slurry’ transport combined with a hydraulic placement in an impoundment, which creates long-term environmental liabilities [12,13].
The problem of wind erosion from flotation tailings disposal sites poses significant environmental and health risks, particularly due to the high sand and silt content of these tailings [13,14]. After drying, they are very volatile due to their high content of sand and silt fractions. The dust from flotation tailing can be carried by the wind to surrounding areas, depositing heavy metals and other harmful substances onto soil and water bodies [15]. This can degrade air quality, harm vegetation, and pose respiratory risks to nearby communities [12,16,17]. Experimental research has demonstrated that wind erosion of dry tailings surfaces leads to increased emissions of particulate matter [12,18]. These examples underscore the critical need for effective erosion control measures and the careful design of tailings disposal sites to protect the environment and public health.
Scientific findings provide various methods for the surface stabilization of flotation tailings disposal sites to mitigate wind erosion. A range of biological, chemical, and physical approaches to stabilize the surfaces of flotation tailings disposal sites, thereby reducing the impact of wind erosion and improving environmental safety, has been applied. In addition to the application of Bacillus sphaericus for soil surface stabilization [19], or indigenous bacteria isolated from tailings soils with the potential to form biocement [20], volcanic ash-based geopolymers have shown notable performance in stabilizing silty and sandy soils [21]. Natural plant covers have been identified as an optimal and sustainable method of preventing and controlling wind erosion on flotation tailings disposal sites [15,22,23]. Among many plant species, grasses have been studied for their effectiveness in tailings stabilization, wind erosion reduction, and biomass accumulation during their growth [22,24,25].
The key characteristics of grass species that make them suitable for the sustainable reclamation of degraded land include their adaptation to extreme habitat conditions and their ability to increase biomass production. However, the challenges and limitations associated with using grass species for land reclamation include the possible negative impact of certain grass species on species richness and diversity, as well as the potential for establishment limitations hindering the recolonization of target species. Therefore, careful consideration of environmental suitability and proper management practices is essential for successful reclamation using perennial grasses. The increase in the abundance of certain grass species, including invasive species, can have a negative impact on species richness and diversity, as well as soil structure and moisture content, highlighting the need for the careful selection of grass species for reclamation [26].
This research aimed to determine the feasibility of using grass species for the sustainable restoration of natural vegetation cover on flotation tailings from an unrehabilitated post-mining heap. To our current knowledge, this is the first research report testing plant survival on pure flotation tailing as a growing medium.
2. Materials and Methods
The study used flotation tailings from a copper ore mine near Polkowice, Poland (51.520 N, 16.200 E). The tailings were taken from the tailings beach boundaries (sample 0–20 cm deep), which were dry and had a finely fragmented structure. River sand was used as the control. The analysis of both substrates was performed at the Regional Chemical-Agricultural Station in Warsaw, from the moment of sample collection until the presentation of the results (Table 1).

Table 1.
The physico-chemical characteristics of the substrate used in the experiment and the procedure applied.
Eight grass species were used as plant material for the study. Three of them were cultivars commonly used for forage or lawn purposes with a significant share of the grass seed market (tall fescue (Festuca arundinacea Schreb., FA) cv. BARFELIX, red fescue (Festuca rubra L., FR) cv. ARETA, and perennial ryegrass (Lolium perenne L., LP) cv. BOKSER. The next two were forage forms from the genus brome: smooth brome (Bromus inermis Leyss. BI) cv. LINCOLN, and rescue grass (Bromus catharticus Vahl., BC) cv. BROMA. The next one was a cultivar of tall wheatgrass (Elytrigia elongata Vahl., EE) cv. BAMAR, a species confirmed to be suitable for growing in saline and alkaline areas. The remaining two were grass species commonly found in degraded, ruderal areas: annual species-drooping brome or cheatgrass (Bromus tectorum L., BT) and velvet grass (Holcus lanatus L., HL). The seed of the above-mentioned cultivars were kindly provided by breeders and the seeds of the latter two species were collected from ruderal areas by authors.
2.1. Seed Germination Test
In March 2022, seeds of the grass varieties mentioned above were sown in the above-described substrates. Plastic trays (12 × 23 × 3 cm) were filled with sand or flotation tailings with a layer of about 3 cm thick. For each cultivar and each type of substrate, 2 trays were used and 50 seeds of each cultivar were placed in the substrate. Then, after watering with distilled water, trays were covered with foil to avoid the rapid drying of the substrate. After this, they were kept in air-conditioned glasshouse with the temperature set at 25–30 °C (daytime) and 10–15 °C (nighttime), with a 12 h day/night cycle. Substrates were sprayed with water at 2-day intervals. The experiment was repeated for 2 runs.
After 5 and 14 days, seedlings classified as normal were counted. Further, seedlings in trays were irrigated with 0.02% KNO2 for a further 5 days to ensure that all potentially dormant seeds germinated.
At the same time, to assess the germination capacity, seeds of the same varieties were sown on a Jacobsen-type germination device (3 × 100 seed per cultivar), using the lighting and temperature parameters as above, in line with the ISTA recommendations [27]. Three hundred seeds (3 × 100) from each selected cultivar were placed on filter paper on the germination device, with light and temperature regimes as provided above. After 5 and 14 days, seedling classification and counting were performed.
2.2. Seedling Growth
Seeds of the above-mentioned grass species were placed in test substrates in metal pots (50 seeds per pot, 3 pots per species per each substrate, 5000 cm3, 20 cm in diameter, with a drainage hole in the bottom). Pots were further also kept in an air-conditioned glasshouse with temperature set at 25–30 °C (daytime) and 10–15 °C (nighttime), with a 12 h day/night cycle. Pots were sprayed with water at 2-day intervals. This experiment was also repeated for 2 runs. The height of the seedlings was assessed after 14 days of seedling growth in test substrates, and after 20 days of growth the length of the root system after removing the plants from the substrate was measured.
2.3. Statistical Analysis
All statistical calculations were made with STATISTCA ver. 12.0 PL. Mean values from two runs were used for further calculations, as there were no significant differences between runs. The significance of differences was determined with 95% probability. Least significant differences (LSD) were calculated according to the Fisher test and values were shown only if statistically significant with accepted probability. To calculate the significance of the difference between germination in sand and flotation tailings, T-test was used. Homogeneity groups were determined using the Duncan test, with the probability of difference between means being greater than 95%.
3. Results
3.1. Germination Test
Seeds of all the studied species were germinated above 80% under ISTA-compliant conditions (Table 2). On the other hand, germination in sand and flotation tailings indicated considerable variability among the studied forms. Notably, species belonging to the Bromus genus exhibited superior germination rates in sand compared to the germination tray used in the ISTA test and in the flotation tailings. This observation was particularly evident in the case of Bromus tectorum (BT), which demonstrated equally impressive germination rates in flotation tailings (93.5%). Among the studied species, only BT, Bromus catharticus (BC), and Elyrigia elongata (EE) achieved germination rates of at least 80% in flotation tailings. The remaining species exhibited germination rates below 60% in this medium. Festuca rubra (FR) and Lolium perenne (LP), two species commonly utilized for sowing lawns, roadsides, and wasteland due to their rapid emergence and sod formation, were identified as the poorest germinators in flotation tailings (31.8 and 26.3%, respectively).

Table 2.
Seed germination rates (%) of eight grass species under three different conditions.
3.2. Seedling Growth and Development
In general, grass seedlings grown in flotation tailing were ca. 32% shorter than seedlings grown in pure sand (Table 3, Figure 1).

Table 3.
Results of measurements of seedling characteristics for grass species grown in two different growing substrates.
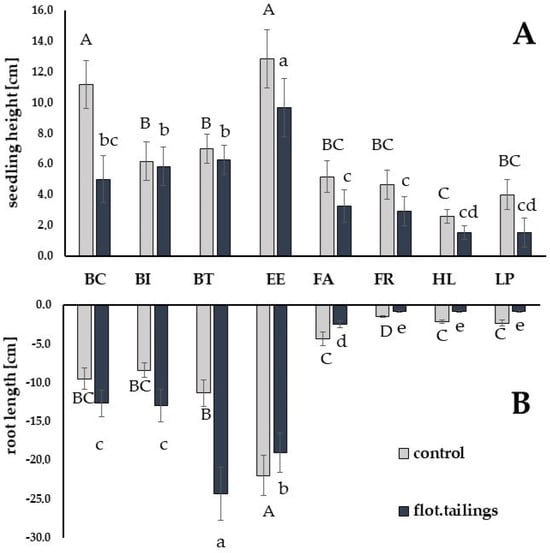
Figure 1.
Differences in seedling height (A) and seedling root length (B) of grass species grown in flotation tailings and control media. Upper-case letters (A, B, BC, C, D) refer to homogeneity groups (p > 95%) acc to Duncan test for control substrate; lower-case letters (a, b, bc, c, d, cd, e) refer to flotation tailings as a substrate. Bars indicate standard deviation from the mean value. Species code: BC—Bromus carinathus; BI—Bromus inermis; BT—Bromus tectorum; EE—Elytrigia elongata; FA—Festuca arundinacea; FR—Festuca rubra; HL—Holcus lanatus; LP—Lolium perenne.
In the case of Bromus inermis (BI), BT and Holcus lanatus (HL) no statistical difference was calculated between seedling height measured in both growing media. The highest reduction in seedling height grown in flotation tailings (ca. 65%) was noted for LP.
Seedlings root development in flotation tailings was promoted for grass species from genus Bromus, while it was significantly reduced for EE, HL and LP. The length of seedling roots grown in flotation tailings as compared to those grown in sand was 215% for BT, 154% for BI, and 133% for BC.
Despite the statistically significant effect of the seedling root length on the reduction in EE when grown in flotation tailings as compared to control, the previously mentioned species performed much better than FA, FR, and LP. Within the time frame provided in the experiment, during the growth in flotation tailings, EE produced the longest seedlings with the second (after BT) longest roots.
The seed germination of the investigated cultivars in test substrates was, as anticipated, strongly conditioned by the initial germination, which was conducted following the ISTA rules (Table 4).

Table 4.
Results of the Pearson correlation analysis between seed germination under different conditions and seedling growth in two different growing media.
Notably, the highest, positive, and statistically significant Pearson correlation coefficient was observed between germination in sand and germination in flotation tailings (r = 0.89, p > 0.001). Nevertheless, no significant correlation was observed between germination test results according to ISTA standards and seedling growth parameters in the tested substrates. Seed germination in sand was a good predictor of seedling growth in flotation tailings; however, this correlation did not extend to sand substrate.
4. Discussion
Seed germination in laboratory conditions tends to be more controlled and predictable, while, in contrast, germination in sand or soil is subject to a range of environmental and biotic factors that can influence the outcomes, making it more representative of natural conditions [28,29]. Our research has shown that seed germination in laboratory conditions, such as Petri dishes, can sometimes be lower than that in natural substrates like sand or soil. In this experiment, grasses from the genus Bromus were used. Generally, germination in sand is a reliable predictor of germination in tailings, but germination according to ISTA recommendations is not helpful for this specific application.
For example, studies on the seeds of desert plants such as Prosopis juliflora and Acacia nilotica demonstrated that germination rates are often higher in sandy soils compared to controlled laboratory conditions [30,31]. This is primarily because natural substrates provide more consistent moisture retention, better aeration, and appropriate microflora that enhance germination, conditions that are challenging to replicate accurately in a laboratory setting.
The species that is potentially most useful for restoring vegetation on a post-flotation sediment landfill should be characterized by the ability to germinate rapidly in such a substrate and to effectively continue the initial growth, i.e., produce seedlings with a developed root system. In light of the obtained results, Bromus inermis, B. catharticus, and Elytrigia elongata can be considered promising species.
Bromus inermis (smooth bromegrass), has been described as the most important grass for soil erosion purposes in the north central states of USA [32]. As a cool-season, rhizomatous grass of Eurasian origin, smooth bromegrass escaped from intentional plantings in North America and spread widely in natural areas [33]. It is recommended for the reclamation of open-pit mines and fly ash landfills [33,34]. Its extensive root system promotes adaptation to various soil types and environments, including dryland, weak and wet soils, waterlogged sites, embankments, and escarpments [35].
B. catharticus is a valuable grass, supporting livestock production through high-quality forage and seed, while also contributing to soil and water conservation and improved pasture health due to its robust growth [36,37]. Due to its strong regeneration and ability to adapt to diverse environments, B. catharticus holds potential for soil and water conservation and cultivated pasture improvement [38]. However, information concerning the suitability of this species for reclamation is lacking.
Last, of the mentioned species, tall wheatgrass (Elytrigia elongata) exhibits exceptional tolerance to soil salinity and alkalinity, withstanding soluble salt concentrations of up to 1%. In its natural habitat, it is often found in saline or alkaline soils [39]. This plant thrives in moderately to severely saline areas and tolerates winter waterlogging and summer drought in both acidic and alkaline soils [40,41,42]. Our experiment also confirmed that tall wheatgrass produces extensive root systems that help to stabilize soil and prevent erosion [43].
The next species that germinated and grew very well in flotation sediments was Bromus tectorum (BT). However, its potential usefulness in revegetation processes requires further in-depth analysis. The superior germination and seedling development of BT could be the combined effect of the nature of this species (annual species) and other factors. Adaptive germination responses in BT populations in contrasting habitats may have both genetic and environmental components, thus explaining why this species can become established in such a variety of habitats [44]. It has been suggested that, on the basis of the germination responses of seed from contrasting environments, BT, together with Bromus arvensis, seem to require a disturbed habitat to grow and establish a population, in contrast to an agricultural landscape, where they are quite infrequent [45]. Annuals, such as BT, often exhibit strategies such as a rapid growth, early maturation, and high seed production, which allows them to quickly utilize the available resources in unstable environments [46,47]. In such environments, natural selection favors organisms that reproduce and spread rapidly.
On the other hand, BT negatively impacts grass and shrublands in arid and semiarid regions, outcompeting native species and leading to shifts in ecosystem function and plant community composition [47,48,49]. Despite Bromus tectorum obtaining the best results regarding germination and initial seedling growth in flotation tailings, considering the aggressive and invasive nature of this species, no practical suggestions related to its use in reclamation issues can be made [50,51]. The result of the flotation tailings reclamation should not be the introduction of potentially aggressive weeds that can easily spread to areas adjacent to the reclaimed sites, thereby hindering ecological restoration efforts [52,53].
Grass varieties used in our experiment, such as Festuca arundinacea cv BARFELIX, Festuca rubra cv ARETA, and Lolium perenne cv BOKSER, were found to exhibit pronounced sensitivity to flotation tailings as a substrate for initial growth and development. The experimental evidence indicates that the conditions associated with flotation tailings, which are characterized by chemical and physical properties that deviate from those of conventional soils, adversely affect both the germination processes and the subsequent growth dynamics of plants [7,54,55]. The germination rates under these suboptimal conditions were significantly lower than expected, and the subsequent developmental stages failed to show the vigor typically observed in more conducive environments. Similar conclusions concerning the unsuitability of Lolium perenne to germinate and grow on flotation tailings were suggested by Jelea and Baciu [15]. Such an effect could be the result of the inhibitory factors of a physical and chemical nature (pH, Eh, heavy metals), as well as the granulometry of tailings (germination layer density, air presence, capillary force, etc.).
5. Conclusions
A comprehensive understanding of the biological growth and development of vegetation targeted for reclamation, in areas contaminated by, e.g., flotation sediments, is crucial for the strategic planning of sustainable reclamation initiatives. This understanding allows for the leveraging of natural processes, which culminates in the creation of a durable vegetative layer, preventing both water and wind erosion. The selection of suitable plant varieties, including grasses, stands as a cornerstone in determining the efficacy of the reclamation endeavor. The results of the studies documented herein identify a mere three cultivars of three perennial grass species demonstrating potential efficacy for the restoration of natural vegetation cover on a substrate of flotation tailings originating from an unrehabilitated heap: Bromus inermis cv. LINCOLN, Bromus catharticus cv. BROMA, and Elytrigia elongata cv. BAMAR.
This sensitivity raises concerns about the viability and effectiveness of these species in practical field applications, where soil quality is compromised by industrial residues. Consequently, the revegetation of post-industrial landscapes with flotation tailings as a primary medium for the restoration may require additional amendments or alternative species selections to achieve successful vegetative cover and long-term ecosystem stabilization. By evaluating a range of grass species directly on the challenging substrate of copper flotation tailings, this study provides valuable methodological insights for selecting appropriate vegetation that can not only tolerate harsh conditions but will also contribute to the long-term stability and ecological integrity of the restored landscape.
The problem of flotation tailings’ stabilization and reclamation is a microcosm of the larger environmental sustainability challenge. It highlights the interconnectedness of soil degradation, biodiversity loss, environmental contamination, and unsustainable industrial practices. The research being conducted represents an effort to mitigate these negative impacts and restore degraded ecosystems, emphasizing the importance of finding sustainable solutions to industrial waste management.
Future research could further explore the physiological mechanisms underlying the tolerance of successful species and investigate their impact on soil microbial communities and nutrient cycling, thereby deepening our understanding of sustainable soil reclamation in such specific degraded environments [56].
Author Contributions
Conceptualization: G.Ż. and D.M., methodology: G.Ż. and D.M.; formal analysis: D.M.; investigation: D.M.; resources: G.Ż.; data curation: G.Ż. and D.M.; writing—original draft preparation: G.Ż.; writing—review and editing: G.Ż. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external founding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The original contributions presented in this study are included in the article. Further inquiries can be directed to the corresponding author.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Leopold, A. A Sand County Almanac and Sketches Here and There; Penguin Random House: London, UK, 2020; p. 173. [Google Scholar]
- Oldeman, L.R. Global Extent of Soil Degradation. In ISRIC Bi-Annual Report 1991–1992; International Soil Reference and Information Centre: Wageningen, The Netherlands, 1992; pp. 19–36. [Google Scholar]
- Baumhardt, R.L.; Stewart, B.A.; Sainju, U.M. North American Soil Degradation: Processes, Practices, and Mitigating Strategies. Sustainability 2015, 7, 2936–2960. [Google Scholar] [CrossRef]
- Biegańska, J. Remediation and revitalization of industrial areas—A method of promotion. IOP Conf. Ser. Mater. Sci. Eng. 2019, 545, 012002. [Google Scholar] [CrossRef]
- Pasieczna, A. Soil contamination induced by historical zinc-lead ore mining and iron and zinc smelting in the central part of the upper silesian industrial region (Southern Poland). Biul. Państwowego Inst. Geol. 2018, 473, 49–66. [Google Scholar] [CrossRef]
- Pasieczna, A.; Konon, A.; Nadłonek, W. Sources of anthropogenic contamination of soil in the upper silesian agglomeration (Southern Poland). Geol. Quaterly 2020, 64, 988–1003. [Google Scholar] [CrossRef]
- Gondek, K.; Mierzwa-Hersztek, M.; Kopeć, M.; Bajda, T. Phytostabilisation on post-flotation sediment waste: Mobility of heavy metals and stimulation of biochemical processes by mineral-organic mixtures. J. Soils Sediments 2020, 20, 3502–3513. [Google Scholar] [CrossRef]
- Michalski, K. Analysis of the Features of a Degraded Land in Terms of Revitalization—Case Study on the Example of a Post-Smelter Area. Multidiscip. Asp. Prod. Eng. MAPE 2020, 3, 559–569. [Google Scholar] [CrossRef]
- Ciesiółka, P. Urban regeneration as a new trend in the development policy in Poland. Quaestionnes Geogr. 2018, 37, 109–123. [Google Scholar] [CrossRef]
- Hilson, G. An overview of land use conflicts in mining communities. Land Use Policy 2002, 19, 65–73. [Google Scholar] [CrossRef]
- Pietrzyk-Sokólska, E.; Uberman, R.; Kulczycka, J. Wpływ górnictwa na środowisko w Polsce—Mity i rzeczywistość. Gospod. Surowcami Miner. Miner. Resour. Manag. 2015, 31, 45–64. [Google Scholar] [CrossRef]
- Cacciuttolo, C.; Cano, D.; Custodio, M. Socio-Environmental Risks Linked with Mine Tailings Chemical Composition: Promoting Responsible and Safe Mine Tailings Management Considering Copper and Gold Mining Experiences from Chile and Peru. Toxics 2023, 11, 462. [Google Scholar] [CrossRef]
- Łuszczkiewicz, A. Koncepcje wykorzystania odpadów flotacyjnych z przeróbki rud miedzi w regionie legnicko-głogowskim. Inżynieria Miner. 2000, 1, 25–35. [Google Scholar]
- Haneef Mian, M.; Yanful, E. Tailings erosion and resuspension in two mine tailings ponds due to wind waves. Adv. Environ. Res. 2003, 7, 745–765. [Google Scholar] [CrossRef]
- Jelea, O.-C.; Baciu, C. Effects of heavy metals, contained in flotation tailings, on plants used in revegetation. Carpathian J. Earth Environ. Sci. 2023, 18, 89–103. [Google Scholar] [CrossRef]
- Slingerland, N.; Sommerville, A.; O’Leary, D.; Beier, N.A. Identification and quantification of erosion on a sand tailings dam. Geosystem Eng. 2018, 23, 131–145. [Google Scholar] [CrossRef]
- van Wyk, S.J.; Hatting, J.; Haagner, A.S.H. Wind erosion design considerations for closure of tailings storage facilities in South Africa: A case study. In Mine Closure 2019: Proceedings of the 13th International Conference on Mine Closure, Australian Centre for Geomechanics, Perth; ACG: Crawley, Australia, 2019; pp. 1185–1200. [Google Scholar] [CrossRef]
- Dentoni, V.; Grosso, B.; Pinna, F. Experimental evaluation of PM emission from Red Mud Basins exposed to wind erosion. Minerals 2021, 11, 405. [Google Scholar] [CrossRef]
- Namdar-Khojasteh, D.; Bazgir, M.; Hashemi Babaheidari, S.A.; Akwasi BAsmudu-Sakyi, A.B. Application of biocementation technique using Bacillus sphaericus for stabilization of soil surface and dust storm control. J. Arid Land 2002, 14, 537–549. [Google Scholar] [CrossRef]
- Maureira, A.; Zapata, M.; Olave, J.; Jeison, D.; Wong, L.-S.; Panico, A.; Hernández, P.; Cisternas, L.A.; Rivas, M. MICP mediated by indigenous bacteria isolated from tailings for biocementation for reduction of wind erosion. Front. Bioeng. Biotechnol. 2024, 12, 1393334. [Google Scholar] [CrossRef]
- Mohebbi, H.R.; Javadi, A.A.; Azizkandi, A.S. The Effects of Soil Porosity and Mix Design of Volcanic Ash-Based Geopolymer on the Surface Strength of Highly Wind Erodible Soils. Minerals 2022, 12, 984. [Google Scholar] [CrossRef]
- Andrejić, G.; Kovaćević, M.; Dzeletović, Z.; Aleksić, U.; Grdović, I.; Rakić, T. Potentially toxic element accumulation in two Equisetum species spontaneously grown in the flotation tailings. J. Serbian Chem. Soc. 2023, 88, 1055–1064. [Google Scholar] [CrossRef]
- Zhang, J.; Jia, Z.; Li, Q.; He, L.; Zhao, X.; Wang, L.; Han, D. Determine the Optimal Vegetation Type for Soil Wind Erosion Prevention and Control in the Alpine Sandy Land of the Gonghe Basin on the Qinghai Tibet Plateau. Forests 2023, 14, 2342. [Google Scholar] [CrossRef]
- Antonelli, P.M.; Coghill, M.G.; Gardner, W.C.; Fraser, L.H. Semiarid bunchgrasses accumulate molybdenum on alkaline copper mine tailings: Assessing phytostabilization in the greenhouse. SN Appl. Sci. 2021, 3, 747. [Google Scholar] [CrossRef]
- El Berkaoui, M.; El Adnani, M.; Hakkou, R.; Ouhammou, A.; Bendaou, N.; Smouni, A. Assessment of the Transfer of Trace Metals to Spontaneous Plants on Abandoned Pyrrhotite Mine: Potential Application for Phytostabilization of Phosphate Wastes. Plants 2022, 11, 179. [Google Scholar] [CrossRef] [PubMed]
- Puffer, M.; Limb, R.F.; Daigh, A.L.M.; Sedivec, K.K. Mechanical and biotic reclamation strategies for a post-mine temperate grassland. Land Degrad. Dev. 2023, 34, 3114–3129. [Google Scholar] [CrossRef]
- ISTA. International Rules for Seed Testing; The International Seed Testing Association: Wallisellen, Switzerland, 2023. [Google Scholar] [CrossRef]
- Baskin, C.C.; Baskin, J.M. Seeds: Ecology, Biogeography, and, Evolution of Dormancy and Germination, 2nd ed.; Academic Press, Elsevier Inc.: Amsterdam, The Netherlands, 2014; p. 1586. [Google Scholar] [CrossRef]
- Bewley, J.D.; Bradford, K.J.; Hilhorst, H.W.; Nonogaki, H. Seeds: Physiology of Development, Germination and Dormancy; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2013; p. 392. [Google Scholar] [CrossRef]
- Nascimento, C.E.d.S.; da Silva, C.A.D.; Leal, I.R.; Tavares, W.d.S.; Serrão, J.E.; Zanuncio, J.C.; Tabarelli, M. Seed germination and early seedling survival of the invasive species Prosopis juliflora (Fabaceae) depend on habitat and seed dispersal mode in the Caatinga dry forest. PeerJ 2020, 8, e9607. [Google Scholar] [CrossRef]
- Gilani, M.M.; Ahmad, I.; Farooq, T.H.; Wu, P.; Yousaf, M.S.; Khan, M.W.; Yousuf Bin, T.; Ma, X. Effects of pre-sowing treatments on seed germination and morphological growth of Acacia nilotica and Faidherbia albida. Sci. For. Piracicba 2019, 47, 374–382. [Google Scholar] [CrossRef]
- Vogel, K.P.; Moore, K.J.; Moser, L.E. Bromegrass. In Cool-Season Forage Grasses; Agronomy Monograph 34; Moser, L.E., Buxton, D.R., Casler, M.D., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, MI, USA, 1996; pp. 535–567. [Google Scholar]
- Salesman, J.B.; Thomsen, M. Smooth Brome (Bromus inermis) in Tallgrass Prairies: A Review of Control Methods and Future Research Directions. Ecol. Restor. 2011, 29, 374–381. [Google Scholar] [CrossRef]
- Klimont, K.; Bulińska-Radomska, Z. Badanie Rozwoju Wybranych Gatunków Traw Do Umacniania Składowisk Popiołów Paleniskowych z Elektrociepłowni. Probl. Inżynierii Rol. 2009, 17, 135–145. [Google Scholar]
- Raawe, H. About Grass and Legume Species Suitability for Recultivation of Semi-Coke Dumps. Agronomy 2004, 219, 154–156. [Google Scholar]
- Hume, D.E. Primary growth and quality characteristics of Bromus willdenowii and Lolium multiflorum. Grass Forage Sci. 1990, 46, 313–326. [Google Scholar] [CrossRef]
- Yi, L.; Dong, Z.; Lei, Y.; Zhao, J.; Xiong, Y.; Yang, J.; Xiong, Y.; Gou, W.; Ma, X. Genetic Diversity and Molecular Characterization of Worldwide Prairie Grass (Bromus catharticus Vahl) Accessions Using SRAP Markers. Agronomy 2021, 11, 2054. [Google Scholar] [CrossRef]
- Belesky, D.P.; Ruckle, J.M.; Abaye, A.O. Seasonal distribution of herbage mass and nutritive value of Prairiegrass (Bromus catharticus Vahl). Grass Forage Sci. 2007, 62, 301–311. [Google Scholar] [CrossRef]
- Asay, K.H.; Jensen, K.B. Wheatgrasses. In Cool-Season Forage Grasses; Agronomy Monograph 34; Moser, L.E., Buxton, D.R., Casler, M.D., Eds.; American Society of Agronomy, Crop Science Society of America, Soil Science Society of America: Madison, MI, USA, 1996; pp. 691–724. [Google Scholar]
- Taboada, M.A.; Rubio, G.; Lavado, R. The deterioration of tall wheatgrass pastures on saline sodic soils. J. Rangel. Manag. 1998, 51, 241–246. [Google Scholar] [CrossRef]
- Nichols, C. Establishing and Managing Tall Wheat Grass in Saline Soils for Productivity; Agricultural Notes; VGLS: Werribee, Australia, 1998; pp. 1–2. [Google Scholar]
- Csete, S.; Stranczinger, S.; Szalontai, B.; Farkas, A. Tall wheatgrass cultivar Szarvasi-1 (Elymus elongatus subsp. ponticus cv. Szarvasi-1) as a potential energy crop for semi-arid lands of Eastern Europe. In Sustainable Growth and Applications in Renewable Energy Sources; Nayeripour, M., Kheshti, M., Eds.; InTech: Rijeka, Croatia, 2011; pp. 269–294. [Google Scholar]
- Vergiev, S. Comparative Study of the capacity of three plant species from the Poaceae family for erosion and flooding control of coastal areas. Sustainable Development and Innovations in Marine Technologies. In Proceedings of the 18th International Congress of the International Maritime Association of the Mediterranean, IMAM 2019, Varna, Bulgaria, 9–11 September 2019; pp. 597–602. [Google Scholar]
- Beckstead, J.; Meyer, S.E.; Allen, P.S. Bromus tectorum seed germination: Between-population and between-year variation. Can. J. Bot. 1996, 75, 875–882. [Google Scholar] [CrossRef]
- Andersson, L.; Milberg, P.; Schütz, W.; Steinmetz, O. Germination characteristics and emergence time of annual Bromus species of differing weediness in Sweden. Weed Res. 2002, 42, 135–147. [Google Scholar] [CrossRef]
- Franzese, J.; di Virgilio, A.; Pirk, G.; Lescano, M.N.; Speziale, K.L. Low biotic resistance to cheatgrass invasion in Patagonia: Evidence from competition experiments. Biol. Invasions 2022, 24, 235–246. [Google Scholar] [CrossRef]
- Howel, A.; Winkler, D.E.; Phillips, M.L.; McNellis, B.; Reed, S.C. Experimental Warming Changes Phenology and Shortens Growing Season of the Dominant Invasive Plant Bromus tectorum (Cheatgrass). Front. Plant Sci. 2020, 11, 570001. [Google Scholar] [CrossRef]
- Mack, R.N. Invasion of Bromus tectorum L. into Western North America: An ecological chronicle. Agro-Ecosystems 1981, 7, 145–165. [Google Scholar] [CrossRef]
- Emam, T.M.; Espeland, E.K.; Rinella, M.J. Soil sterilization alters interactions between the native grass Bouteloua gracilis and invasive Bromus tectorum. J. Arid. Environ. 2014, 111, 91–97. [Google Scholar] [CrossRef]
- Seastedt, T.R.; Pysek, P. Mechanisms of plant invasions of North American and European grasslands. Annu. Rev. Ecol. Evol. Syst. 2011, 42, 133–153. [Google Scholar] [CrossRef]
- Pyšek, P.; Manceur, A.M.; Alba, C.; McGregor, K.F.; Pergl, J.; Štajerová, K.; Chytrý, M.; Danihelka, J.; Kartesz, J.; Klimešová, J.; et al. Naturalization of central European plants in North America: Species traits, habitats, propagule pressure, residence time. Ecology 2015, 96, 762–774. [Google Scholar] [CrossRef]
- Sheley, R.L.; Krueger-Mangold, J. Principles for restoring invasive plant-infested rangeland. Weed Sci. 2003, 51, 260–265. Available online: http://www.jstor.org/stable/4046729 (accessed on 15 January 2025). [CrossRef]
- Hulme, P.E.; Bacher, S.; Kenis, M.; Klotz, S.; Kühn, I.; Minchin, L.; Nentwig, W.; Olenin, S.; Panov, J.; Pergl, J.; et al. Grasping at the routes of biological invasions: A framework for integrated biosecurity. J. Appl. Ecol. 2008, 45, 403–414. [Google Scholar] [CrossRef]
- Wong, M.H. Ecological restoration of mine degraded soils, with emphasis on metal contaminated soils. Chemosphere 2003, 50, 775–780. [Google Scholar] [CrossRef] [PubMed]
- Krawczyńska, M.; Kołwzan, B.; Gediga, K.; Dziubek, A.M.; Grabas, K.; Karpenko, E. Evaluation of the possibility of phytostabilization of post-flotation tailings pond. Environ. Prot. Eng. 2015, 41, 157–167. [Google Scholar] [CrossRef]
- van der Putten, W.H.; Bardgett, R.D.; Bever, J.D.; Bezemer, T.M.; Casper, B.B.; Fukami, T.; Wardle, D.A. Plant-soil feedback cycles as drivers of plant community assembly. Trends Ecol. Evol. 2016, 31, 589–601. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).