Abstract
The development of sustainable agriculture is critical in order to address the growing challenges of global food security while reducing environmental impact. This study focuses on the potential of Furcellaria lumbricalis, red algae found in the Baltic Sea, that can serve as a source of biostimulant. The research methodology included several consecutive steps combining qualitative and quantitative research methods: (1) an analysis of secondary data and literature review; (2) the production of algae digestate by anaerobic fermentation; (3) supervised laboratory experiments; (4) economic analysis; and (5) an assessment of the availability and prospects for use of algae biomass in the Baltic Sea region. The anaerobic fermentation process was used to produce algae digestate, the effectiveness of which was tested under controlled laboratory conditions. Experiments with basil (Ocimum basilicum) plants showed that 3% digestate concentrations significantly enhanced plant growth, increasing green mass by 52.7% to 85.4%. Economic analysis revealed the potential to increase gross profit for different crops in Latvian agriculture. The results indicate the potential of Furcellaria lumbricalis digestate as an effective and sustainable biostimulant that can contribute to the development of the green economy in the region. However, further research is needed to optimise production processes, explore long-term impacts on soil and ecosystems and conduct field trials on different crops under different climatic conditions. In addition, it is necessary to investigate precise mechanisms of action at the molecular level and develop standardised quality control processes for the production of biostimulants.
1. Introduction
The agricultural sector faces a number of major challenges in the 21st century, including the need to increase food production for the growing global population while reducing environmental impacts and adapting to climate change [1]. Traditional farming practices, which rely heavily on the use of chemical fertilisers and pesticides, have led to soil degradation, water pollution and loss of biodiversity. The European Union Green Deal and strategy “from field to table” address these challenges by promoting sustainable agricultural practices and reducing the use of chemicals [2].
In this context, biostimulants have emerged as a promising solution to improve crop productivity and resilience while reducing environmental impact. In particular, algae biostimulants have attracted considerable attention due to their rich composition of bioactive compounds that can improve plant growth, nutrient intake and stress tolerance [3]. Algae biostimulants can promote plant growth under difficult environmental conditions by improving nutrient intake and enhancing the natural defence mechanisms of plants [4].
Research shows that algae biostimulants can significantly improve crop yield and quality. For example, Hussein et al. [5] indicated that algae biostimulants can increase yields by 10–15% for different crops while improving their nutritional value and tolerance to environmental stresses. Moreover, Ali et al. [6] stressed that algae biostimulants can improve nutrient efficiency by 15–25%, potentially reducing the need for synthetic fertilisers.
Despite their potential, the full implementation and commercialisation of algae biostimulants face a number of challenges. Lähteenmäki-Uutela et al. [7] drew attention to the complex regulatory environment, especially in the European Union, where the new Regulation 2019/1009 on fertilisers provides a framework for the registration of biostimulants, but compliance processes remain complex. Furthermore, as Ronga et al. pointed out [8], there is still a lack of scientific understanding of the precise mechanisms of action of algae biostimulants and their long-term impact on soil and the ecosystem.
In the context of this study, particular attention is paid to Furcellaria lumbricalis, a species of red algae found in the Baltic Sea. The local species offer a sustainable source for biostimulant production, potentially delivering economic and ecological benefits for agriculture. The use of the digestate from anaerobic fermentation processes further improves the sustainability profile of this approach when assessing waste streams [8].
Focusing on local algae species and using a biorefinery business model approach for the biomass valorisation process, this study aims to develop and evaluate the potential of a prototype of the algae extract from Furcellaria lumbricalis as a biostimulant, measuring its impact on crop growth and green mass growth under controlled laboratory conditions. The hypothesis of the study is that Furcellaria lumbricalis extract will improve plant growth and increase biomass compared to a control group.
Objectives of the study:
- To conduct an analysis of the scientific literature (scoping review) on the potential of Furcellaria lumbricalis as an algae biostimulant in agriculture, collecting information on its composition, biomass availability and economic value in the Baltic Sea region and analysing gross cover data and international research results.
- Design and implement the experimental part:
- to carry out an anaerobic fermentation process for the production of algae digestate of Furcellaria lumbricalis under controlled conditions;
- to develop an automated environmental control system for growing crops under controlled conditions;
- to conduct experimental studies with different concentrations of algae extract (0%, 3%, 6%, 12%).
- To analyse the results obtained on the increase in green mass depending on the applied extract concentration and to draw conclusions on the effectiveness of the application of algae extract in sustainable agriculture.
This study contributes to the development of sustainable agriculture in the Baltic Sea Region while addressing global challenges related to food security and environmental protection.
2. Materials and Methods
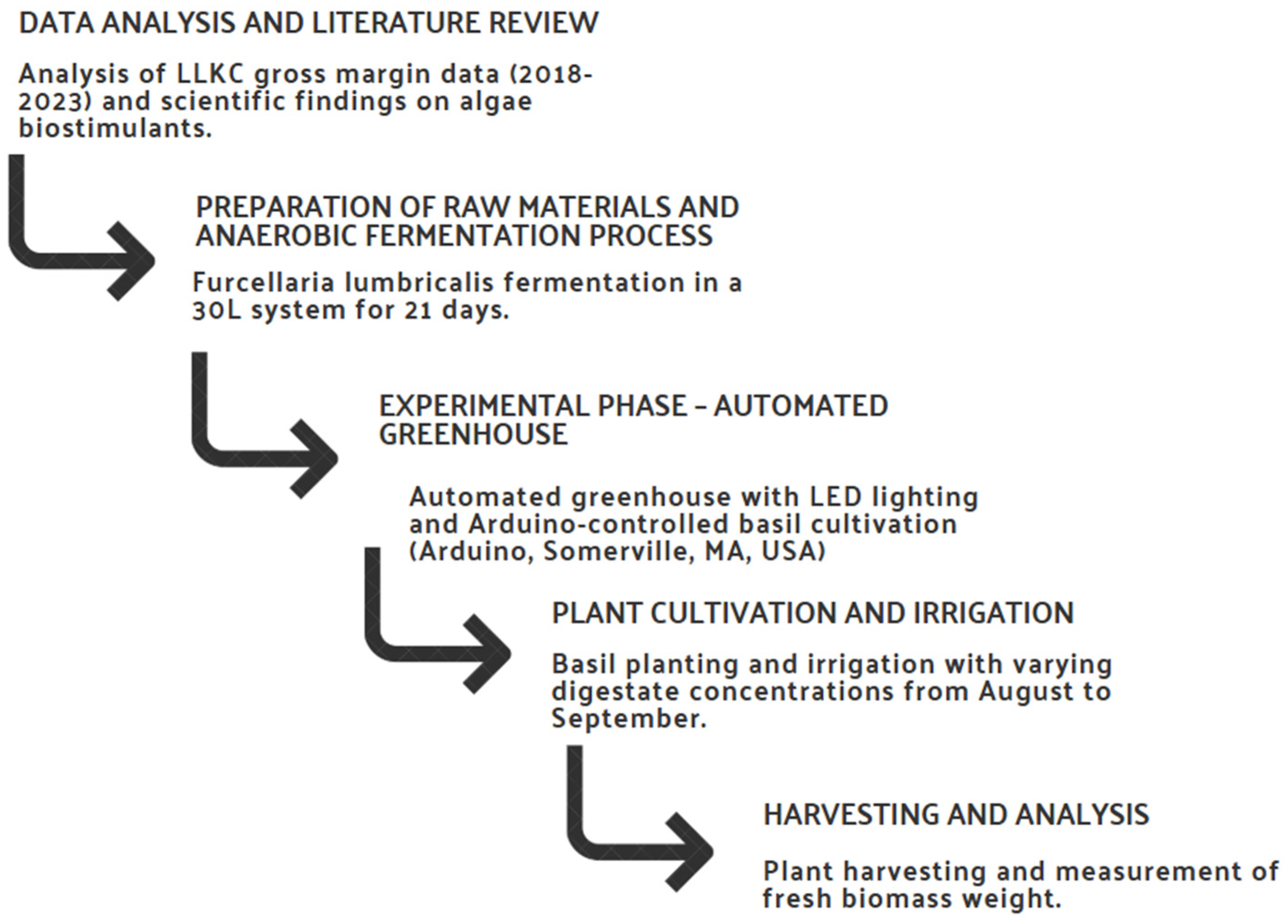
The research methodology was structured to provide a comprehensive approach for the development of a prototype algae extract from the digestate of Furcellaria lumbricalis and evaluate its potential as a biostimulant in agriculture. The methodological approach involved several consecutive and interrelated steps. Figure 1 illustrates the process of developing an algae extract prototype, including data analysis, raw material preparation, experimental stages, plant cultivation and final analysis.
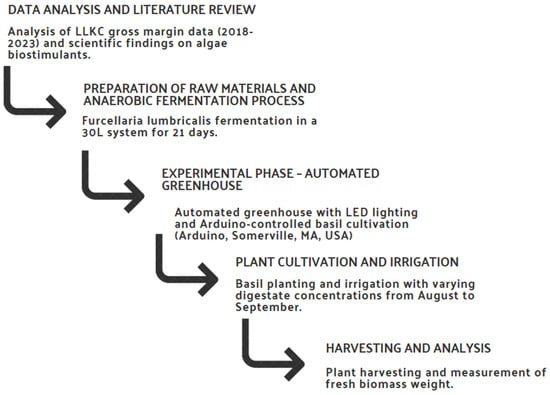
Figure 1.
Methodological framework for the development and evaluation of Furcellaria lumbricalis algae extract as an agricultural biostimulant.
Using a comprehensive approach, combining quantitative and qualitative data analysis, an in-depth analysis of the scientific literature and industry reports was initially carried out. Data on the availability, current use and potential of algae biomass in the Baltic Sea region were compiled. These data were extracted from published sources based on market analysis, expert assessments and statistical data, ensuring comparability between the Baltic Sea Region countries. In addition, laboratory analyses of a digestate sample were carried out to obtain accurate data on its composition and properties. These analyses provided valuable data that complemented the literature study and allowed for a more accurate assessment of the potential of Furcellaria lumbricalis as a biostimulant raw material.
The study analysed the Latvian Rural Advisory and Education Centre—LLKC gross cover data for 2018 and 2023 using the LLKC gross cover calculation tool (2023 version), which is available online and allows the input of farm-specific data for both crop and livestock production. These data were integrated with the latest scientific findings on the impact of algae biostimulants on different crops, taking into account their ability to improve nutrient uptake, promote root development and increase plant resistance to environment-related stressors.
The potential gross yield increase was calculated on the basis of yield increases documented in the scientific literature using the comparative analysis method. This approach ensured a common methodology for all the Baltic Sea Region countries considered.
The study also integrated data from the GRASS (Growing Algae Sustainably in the Baltic Sea) project, which provided a broader context of the potential for growing and using macroalgae in the region. The potential socio-economic benefits of macroalgae farming were analysed, including their impact on water quality and the potential for biomass use in different sectors.
2.1. Literature Review and Data Analysis
The research methodology started with a comprehensive analysis of the scientific literature and industry reports. This process included the selection of data sources using scientific databases, such as Web of Science, Scopus and Google Scholar, as well as industry reports from recognised organisations in the Baltic Sea region. The search strategy was based on keywords such as ’algal biostimulants’, ’Furcellaria lumbricalis’, ’sustainable agriculture’ and ’Baltic Sea region’, used in various combinations. The inclusion criteria gave preference to sources published in the last 10 years.
Of the 124 sources initially identified, 62 were included in the final analysis after a thorough review for relevance and quality. This means that 50% of the sources were excluded. The main reasons for exclusion were irrelevance to the topic of the study (25% of excluded sources), insufficient scientific quality or reliability (15%), outdated information (10%) and duplicated or too similar studies (5%). The data extraction process systematically collected and analysed information on the availability, use and economic potential of algal biomass in the Baltic Sea countries.
This comprehensive approach provided a broad and deep understanding of the topic under study, allowing for detailed analysis and sound conclusions on algal biostimulants and their potential role in sustainable agriculture in the Baltic Sea region.
2.2. Economic Analysis Methodology
The economic analysis used data from the Gross Profit Calculation Tool (2023 version) developed by the Latvian Rural Advisory and Education Centre (LRAC), which includes current market prices for agricultural raw materials and products. Yield growth projections were based on findings from peer-reviewed literature documenting the effects of algal biostimulants on different crops. The economic model used the following parameters: (1) baseline yield and income using current farming practices; (2) projected yield increase based on biostimulant use (conservative estimate: 10%, optimistic estimate: 20%); (3) additional costs associated with biostimulant production and use; (4) net change in gross profit taking into account both yield increase and additional costs.
2.3. Preparation of the Digestate
The raw material preparation and fermentation process was carried out using Furcellaria lumbricalis algae. Digestate is produced by a biogas extraction (digestion) process, which anaerobically extracts biogas from the biomass and produces a solid residue and a liquid fraction—digestate. No pre-treatment of the algae was carried out in order to maximise the approximation of the experimental conditions to a potential large-scale production, where each treatment step involves additional resources in terms of time and cost. Fermentation took place in a 30 litre sealed unit for 100 days. A custom-built methane generator system with two 30-litre containers, equipped with MQ-4 methane sensors and a temperature control of 38 ± 1 °C, was used for digestate production and analysis, providing an optimal environment for mesophilic microbial activity. In addition, the volume (flow) of biogas produced was monitored, and the data (temperature, methane content, gas volume and flow) were stored in the memory module of the device. Then, 1.227 kg of dry matter yielded about 23 litres of liquid digestate and 4.2 kg of solid residue.
2.4. Plant-Growing Equipment
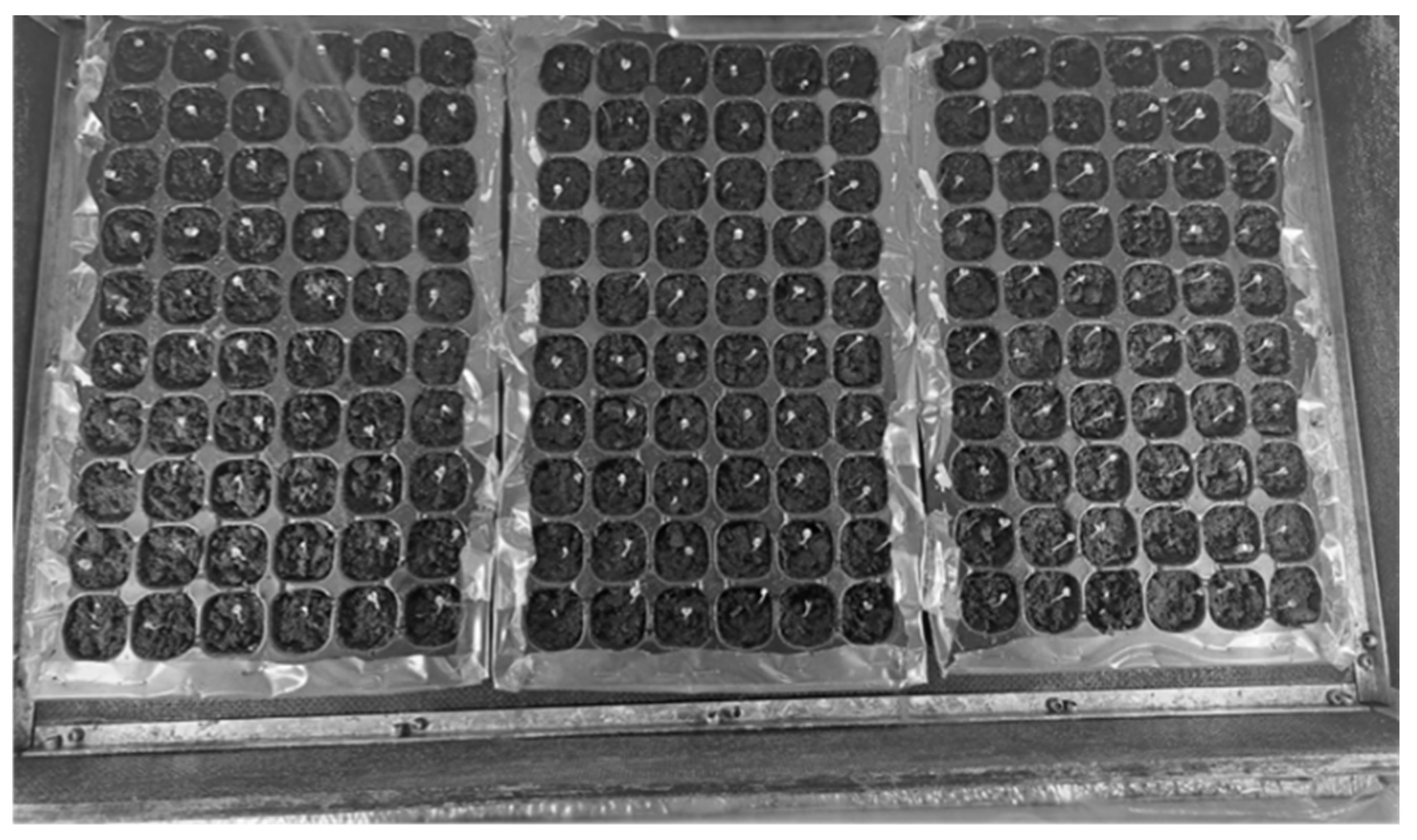
The experimental phase was carried out in a custom-built, specially designed automated greenhouse (900 × 550 × 410–570 mm) equipped with LED lighting and microcontroller-operated environmental regulation. The greenhouse equipment included programmable controls, specialised broadband (IR-UV) LED plant growth bulbs, air and soil humidity control and humidification equipment, ventilation and temperature control. Figure 2 shows the arrangement of the plants in the cassettes during the cotyledon development phase.
Figure 2.
Plant arrangement in the automated greenhouse; basil plants in the cotyledon stage.
Three containers with 60 sections each provide plenty of space for a variety of plants, making the growing box suitable for both hobby-level gardening and scientific research.
2.5. Plant Test Methodology
The test methodology was based on the use of digestate from the fermentation of Furcellaria lumbricalis at controlled concentrations in basil (Ocimum basilicum) growth trials. Anaerobic fermentation took place in a 30 litre closed fermenter where fresh algae were fermented to produce digestate—a solution of organic matter that can serve as a plant growth promoter.
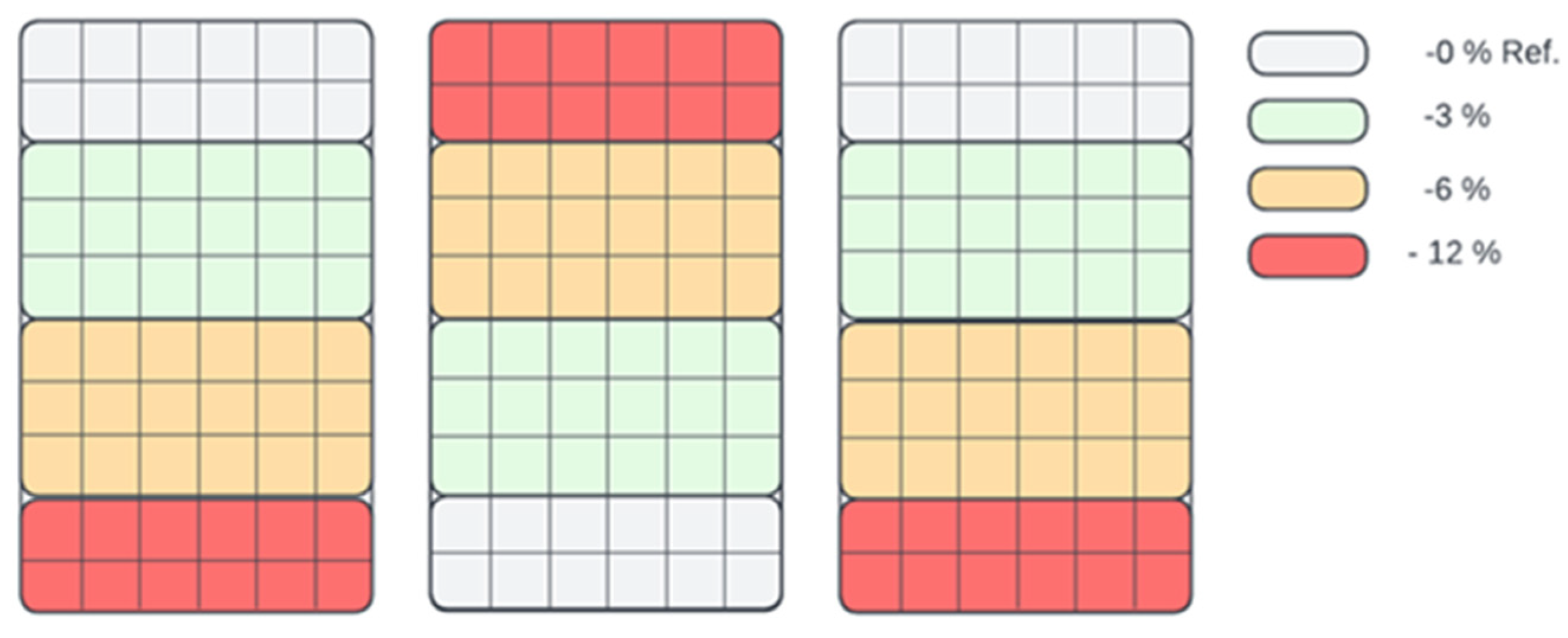
After the fermentation process, the digestate was used in a test experiment carried out in an automated greenhouse where constant conditions of light, temperature and humidity were maintained to ensure objective results. The greenhouse was divided into three zones, with each zone treating basil plants with a diluted digestate concentration of 0% (pure water—control group), 3%, 6% and 12%. Figure 3 shows the distribution zones of application for the different concentrations.
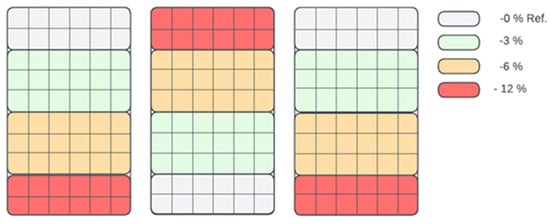
Figure 3.
Zonal distribution in the seedling boxes for fertilisation (stimulation) with different concentrations of biostimulant (0%, 3%, 6% and 12%).
Each zone was physically separated by 2 cm high plastic barriers between the different treatment zones to prevent the diffusion of liquid between them. In addition, each treatment zone was irrigated separately using precision volumetric pipettes to ensure that the digestate solution was only applied to the intended zone. Soil moisture levels were maintained below saturation, which also helped to prevent the horizontal movement of liquid between zones. After planting the basil plants, the first treatment with digestate solutions was carried out after 12 days during the true leaf stage. Subsequent irrigations were made four more times at weekly intervals. The plants were harvested on the 45th day after planting, and the weight of the green mass was measured. Additionally, the chemical composition of the digestate was analysed during the experiment to determine its potential effect.
This methodological approach provides a comprehensive and scientifically sound basis for the development of an algae extract prototype and the evaluation of its potential in agriculture. The detailed methodology allows the results of the study to be considered reliable and potentially relevant for the development of sustainable agriculture.
3. Results
3.1. The Potential of Algae Biostimulants for the Development of Sustainable Agriculture
Algae biostimulants have become an important subject of research in the agricultural sector due to their ability to stimulate plant growth and development. These natural preparations have shown significant potential to improve crop productivity, quality and tolerance to environmental stressors [9]. Considering the growing interest in sustainable agricultural practices, it is important to investigate the potential economic impact of algae biostimulants on the agricultural sector. The results presented in this section are organised in such a way as to clearly distinguish the results of our initial pilot study from the data presented in the literature review. Section 3.1.2, Section 3.1.3 and Section 3.1.4 present the original experimental data collected by the authors, while Section 3.1, Section 3.1.1 and Section 3.2 mainly synthesise data from the existing literature with analytical contributions from the authors. Table 1 illustrates the main categories of challenges affecting the economic potential of algae biostimulants and their successful market uptake.

Table 1.
Challenges and potential benefits of introducing algae biostimulants in agriculture.
Algae biostimulants face a number of challenges in agriculture but at the same time offer significant potential benefits. In terms of technological aspects, the main challenge is the optimisation of extraction and formulation, as well as the improvement of production processes, resulting in improved crop resistance and stress tolerance. In the context of variations in efficacy, it is essential to take into account the crop type, the environmental conditions and application particularities, which require further research for optimisation to ensure the effective adaptation of the biostimulant to specific crops and conditions. In terms of economic viability, the main challenge relates to the cost competitiveness of production and application compared to traditional means, but there is potential for cost reduction in the long term. In the area of scientific understanding, there is still a need for a deeper understanding of the precise operating mechanisms of biostimulants and their long-term effects on plants and the ecosystem. The regulatory framework is currently characterised by different regulations in different regions. This is especially notable in the context of the EU Fertilisers Regulation 2019/1009, which, despite certain limitations, promotes market development through the implementation of common regulations. While the Table 1 analysis reveals crucial aspects of the challenges and potential benefits of the introduction of algae biostimulants in agriculture, it is important to expand research.
3.1.1. Effects of Algae Extracts on Crop Yield
Algae biostimulants offer significant potential for improving the agricultural sector in the Baltic Sea region. They can promote plant growth, improve nutrient uptake and increase tolerance to abiotic stress in the form of increased yields, improved crop quality and reduced necessity for synthetic fertilisers and pesticides [5,9]. These benefits are particularly important for the development of sustainable agriculture in the region.
Agriculture is an important economic sector in the Baltic Sea region, with a diverse crop mix in different countries [12]:
- In Poland: cereals and potatoes;
- In Denmark: cereals and fodder crops;
- In Sweden and Finland: cereals and rapeseed;
- In the Baltic countries: cereals, rapeseed and legumes.
This diversity creates the need for tailored solutions across the region.
To illustrate the potential impact of algae biostimulants, a detailed economic analysis was carried out using Latvian data as an example. The analysis focused on strategically important crops, including winter and spring wheat, rapeseed, potatoes, oats and buckwheat, which are also common in other Baltic Sea countries [13]. The following economic analysis is a synthesis of existing literature data and agricultural statistics, combined with analytical projections made by the authors. The yield increase percentages are derived from peer-reviewed literature, while the economic calculations are original contributions applying these percentages to Baltic Sea agricultural data. Table 2 shows the results of the economic analysis carried out.

Table 2.
Projected impact of algae biostimulants on gross margins (€/ha) for major crops in Latvia based on literature-derived yield increases.
The results show that algae biostimulants have the potential to increase gross income for all crops analysed. The highest absolute increases were observed for potatoes (357.12–476.17 €/ha) and rapeseed (80.37–107.17 €/ha). In relative terms, the highest increases are forecast for rapeseed (15–20%) and potatoes (15–20%).
An analysis of scientific studies reveals the multiple impacts of algal biostimulants on agricultural sustainability and environmental quality. Studies show that algal biostimulants significantly reduce the need for chemical fertilisers while improving the efficiency of plant nutrient uptake [14]. Studies by García-González and Sommerfeld [20] show positive effects on soil health, soil structure and organic matter content. In the context of climate change, work by Khan et al. [21] and Calvo et al. [22] documented the ability of algal biostimulants to increase plant tolerance to abiotic stresses such as drought and salinity. In a study by Battacharyya et al. [23] and Craigie [24], the role of these products in enhancing soil microflora diversity was highlighted. In the area of environmental protection, studies by Win et al. [25] and Rathore et al. [26] have confirmed the positive impact of algal biostimulants on water quality, significantly reducing nitrate leaching. In addition, the study by Arias et al. [27] found significant reductions in greenhouse gas emissions, with up to a 24% CO2 equivalent reduction in vegetable production. The potential to improve ecosystem integrity was confirmed by du Jardin [28] and Rouphael et al. [29], who showed that the application of algal biostimulants activates soil biological processes, improving its structure and water-holding capacity. The study by Yakhin et al. [30], which contributes to the study of molecular mechanisms, showed how these products positively affect plant metabolism, reducing the need for chemicals. In the long term, Van Oosten et al. [11] and Kholssi et al. [31] showed that the systematic application of algal biostimulants contributes to a sustainable increase in soil fertility, in contrast to conventional farming practices. These effects include the stimulation of mycorrhizal fungal activity, which naturally improves nutrient availability to plants. To translate these findings into agricultural practice, Pereira et al. [32] and Chen et al. [33] developed models for the integration of algal biostimulants into sustainable cropping systems, while Tritean et al. [34] and Mutlu-Durak et al. [35] applied genomics and metabolomics to better understand the mechanisms of action. The analysis of economic aspects by Bulgari et al. [36] and LLKC et al. [37] confirmed that the introduction of algal biostimulants reduces agricultural costs in the long term while improving environmental quality.
Algae biostimulants can play a crucial role in the transition towards greener and more sustainable agricultural practices. By simultaneously addressing several aspects of agricultural sustainability, algae biostimulants offer promising solutions to balance productivity and preserve the environment in the face of the increasing challenges of climate change and the need to reduce the environmental impact of agriculture [38,39]. The potential of these biostimulants is particularly important in the Baltic Sea region, where the intensification of agriculture has had a significant impact on environmental quality. The results obtained provide a basis for further comparative studies across the Baltic Sea region to determine the effectiveness of algae biostimulants under different climatic and agricultural conditions. Such studies could make a valuable contribution to the sustainable development of agriculture in the region while promoting the use of local resources and reducing the impact of agriculture on the Baltic Sea ecosystem [40].
3.1.2. Anaerobic Digestate Production
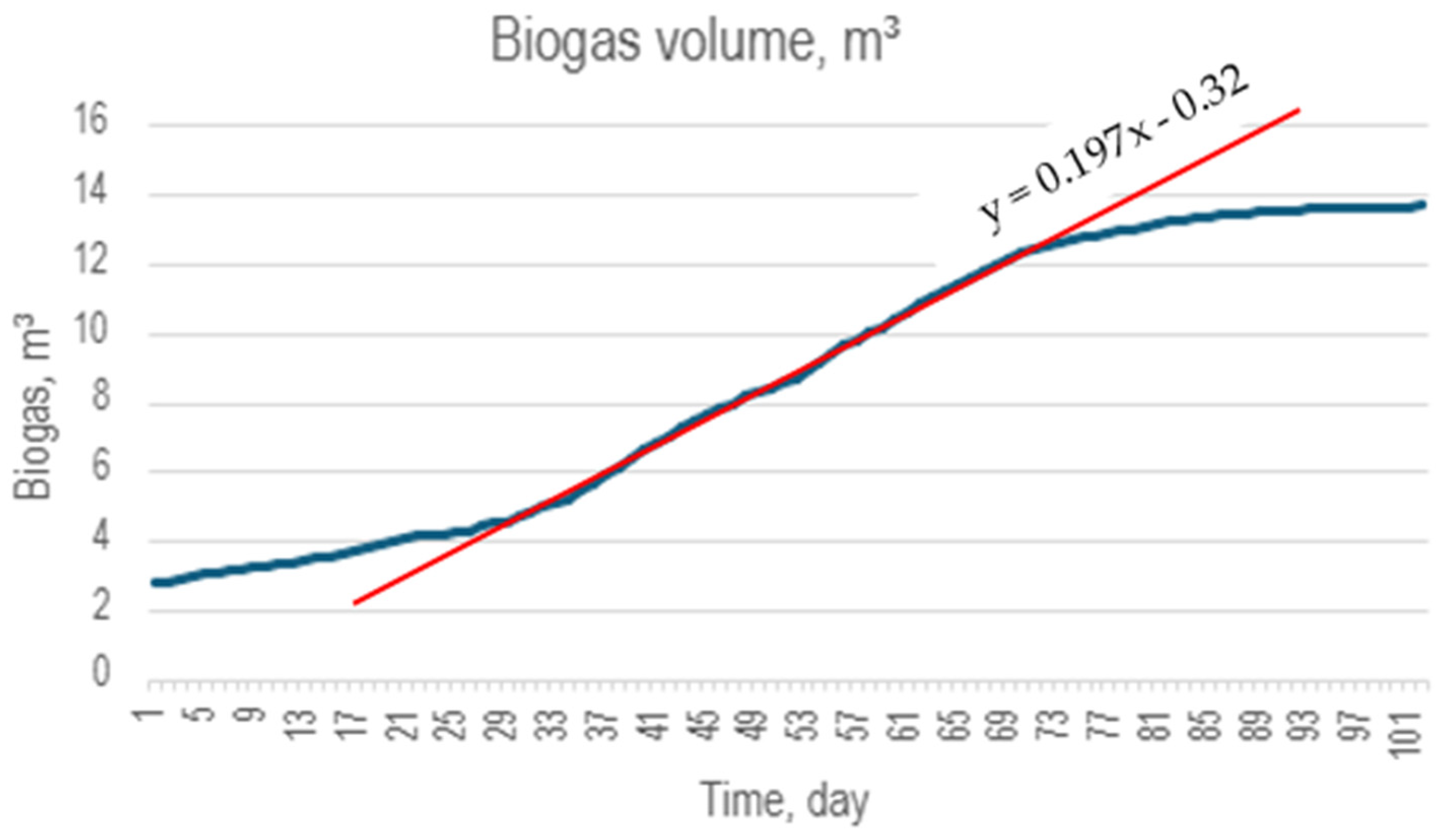
During the digestion process, biogas was obtained that can be used as an energy source for heating, thermal processes for algae processing or other purposes (storage for disposal). The following results present original experimental data from our anaerobic digestate production process using Furcellaria lumbricalis collected from the Baltic Sea. All production parameters, measurements and analyses were conducted by the authors. Figure 4 provides a graph of the volume of the biogas produced under normalised conditions—temperature, pressure. The duration of the process was about 100 days, and the active phase was started late due to low temperatures; however, the output of biogas between the 30th and the 70th day is estimated at 0.197 m3/day from the 1.227 kg of algae Furcellaria lumbricalis used in the process.
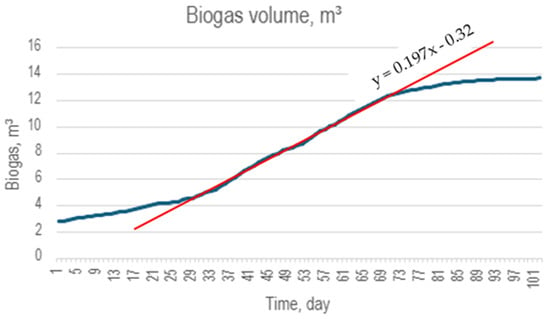
Figure 4.
Digestion biogas output from 1.227 kg of dry organic matter of algae. It can be observed that the biogas yield during the active digestion phase is 0.197 m3/day.
The methane content of the biogas was monitored with a dedicated CH4 sensor MQ4 designed for concentrations of 200–10,000 ppm, with its calibration at 1000 ppm. The results obtained 52 ± 5% methane content correlate well with the value commonly found in the industry. The temperature was kept constantly between 37–39 °C.
3.1.3. Characterisation of the Chemical Composition and Biostimulant Properties of Algae Digestate Extract
Seaweed extracts have become an important research subject in agriculture and horticulture due to their potential to improve plant growth, development and resistance to environmental stressors. This subsection provides a detailed insight into the chemical composition of algae digestate extract and its biostimulant properties based on a careful analysis and comparison with other extracts of algae that have been widely studied.
The results cover the composition of extracts from different algae species, their active substances and their effects on plant physiology. Special attention is paid to the chemical composition of the digestate obtained, its nutrient content and its potential impact on plant growth and development, using samples from two fermentation periods. This analysis aids in better understanding how algae biostimulants can contribute to sustainable agricultural practices while addressing environmental challenges in the Baltic Sea region.
Table 3 gives an overview of five extensively studied algae extracts, their main constituents and their benefits for plant growth and health. This comparison allows for an assessment of the potential of different algae species for biostimulant development and their possible application in agriculture in the Baltic Sea region.

Table 3.
Active substances and plant benefits of selected seaweed extracts.
The results provide a detailed overview of the composition and key active ingredients of five algae extracts: Kelp, Ascophyllum Nodosum, Sargassum, Chlorella and Spirulina. For each product, the main ingredients, their concentration and their benefits for plant growth and health are specified. Kelp extract is particularly effective in stimulating root development and increasing yield due to the presence of auxins and cytokinins [43]. Ascophyllum nodosum extract is notable for its ability to improve plant growth and stress tolerance due to its glycine betaine [44]. Sargassum extract enhances the immune response in plants primarily due to flavonoids [43]. Both Chlorella extract and Spirulina extract promote growth and nutritional value, with carotenoids and amino acids as their main components [44]. Based on the information presented in the table, it is clear that the amount and type of active substances significantly impact the effectiveness of the extract. The Fucillaria Lumbicalis algae species shows potential to compete with other extracts. It contains phlorotannins, which act as powerful antioxidants and anti-inflammatory agents, offering significant benefits in plant defence against stress and disease. These properties make Fucillaria Lumbicalis a promising candidate in the biostimulant market [43].
To fully evaluate the potential of the obtained digestate as a plant biostimulant, a detailed analysis was conducted, focusing not only on its growth-stimulating properties but also on other factors that may affect plant growth and development. For this purpose, detailed liquid fraction analyses were carried out for two biomass samples, with samples taken on the 25th and 20th days, respectively, after the start of the digestion process. Parameters such as environmental reaction pH, dry matter content, macroelement composition (total nitrogen, phosphorus, potassium) and organic matter content for the first sample, as well as microelement composition (calcium, magnesium, manganese, iron) for the second sample, were determined. Table 4 presents a summary of the obtained results.

Table 4.
Preliminary analysis of chemical composition and nutrient content of algae digestate extract from two fermentation periods.
The only significant difference between the samples is observed in the potassium content—it is twice as low in the second sample. The other comparable parameters (environmental reaction, dry matter, total nitrogen and phosphorus) are approximately the same within the uncertainty (possible error) of the result.
Such a comprehensive analysis allows not only for an assessment of the nutrient composition of the digestate but also its potential impact on soil microflora, plant hormone synthesis and stress resistance. For example, the organic matter content can indicate the ability of the digestate to improve soil structure and water retention capacity, while the presence of micronutrients can promote plant metabolism and enzyme activity [54].
The obtained results enable a comprehensive assessment of digestate as a biostimulant, considering its potential impact on plant physiological processes beyond its direct fertilisation function. This approach provides a broader insight on the applicability and effectiveness of digestate in different aspects of plant cultivation [55].
Studies have shown that the use of digestate can have a positive effect on plant growth by improving nutrient uptake and increasing yields [29]. Furthermore, the application of digestate can contribute to sustainable agricultural practices by reducing dependence on mineral fertilisers and improving soil quality [56].
A detailed summary of the analysis results allows for an evaluation of the potential of the digestate as an alternative or supplement to traditional fertilisers while providing insights into its biostimulant properties, which can enhance plant health and productivity [54].
The analysis of digestate from two biomass inputs provides initial compositional information on its potential use in agriculture. While these preliminary results show a relatively stable pH (5.4–5.6) and a significant organic matter content (47.5%), it is important to recognise that chemical composition alone cannot establish the digestate as an effective biostimulant. It can be seen that 52.5% of the dry matter of Sample 1 consists mainly of mineral components (ash content), structural polysaccharides and other inorganic elements. Macroelement analysis shows significant levels of nitrogen (0.091–0.087%) and phosphorus (0.020–0.013%), with significant variation in potassium levels between samples (0.051–0.025%). The presence of micronutrients such as calcium (1.68%), iron (19.3 mg/kg) and manganese (8.05 mg/kg) suggests potential nutritional value. However, these preliminary compositional data require validation through rigorous comparative studies with commercially established biostimulants to determine relative efficacy. Future research should include side-by-side growth trials, comparing our digestate with commercial products at equivalent application rates, comprehensive micronutrient profiling and phytohormone analysis to better characterise the bioactive compounds responsible for any growth-enhancing effects. In addition, long-term studies examining the effects on soil microbiome composition and health would provide valuable insights into the wider ecological effects of digestate application.
3.1.4. Crop Test Results
The basil cultivation experiment was conducted during the summer and autumn seasons of 2024 to evaluate the impact of an algae digestate biostimulant on crop growth under controlled greenhouse conditions. Basil (Ocimum basilicum) was selected as a model test crop to evaluate the biostimulant properties of Furcellaria lumbricalis digestate under controlled conditions. Although basil represents only one crop species, it serves as a useful indicator crop due to its responsiveness to nutrient amendments and rapid growth cycle, although results should be interpreted with appropriate caution regarding generalisability to other crop species. The experiment consisted of two consecutive trials, with the second one being conducted with an adjusted methodology based on the results of the first experiment.
In both experiments, the primary evaluation criterion was the green mass weight of the crops, which was used to compare the effectiveness of different biostimulant concentrations.
- First experiment.
In the first experiment, basil was planted on 7 August 2024. Irrigation with digestate at different concentrations (0%, 3%, 6%, 12%) was performed weekly, starting from the 12th day after planting (during the plant’s true leaf development phase) until the 40th day. The greenhouse was divided into three zones, with each zone treating basil plants with different concentrations of digestate. The crops were harvested two days later, and their green mass weight was measured.
During the first experiment, it was found that the 6% and 12% dilution rates were too high, causing plant damage and ultimately leading to crop death (Figure 5). This effect occurred when the irrigation with all concentrations was started simultaneously at the true leaf development stage. In contrast, the plants in the 3% dilution zone developed well. Only the results for 0% and 3% dilutions were included in the analysis.

Figure 5.
Biostimulant test in progress.
The first experiment compared basil plants irrigated with a 3% digestate concentration to the control group, which received pure water (0% digestate). The results showed that the application of 3% digestate concentration enhanced crop growth compared to the control group. Measurements of the total green mass of basil revealed the following results: (1) green mass weight of basil in the 3% digestate group: (35.3 ± 0.1) g; (2) green mass weight of basil in the control (0% digestate) group: (33.3 ± 0.1) g.
In the first zone (left), a smaller plant mass can be observed in the farthest area, while the nearest area, treated with 3% digestate, shows the largest plant mass. The plants in the 6% and 12% digestate solution areas are withered. On the right, the same is shown in reverse order (according to the layout described in Section 2.5: Plant test methodology). A one-factor analysis of variance (ANOVA) followed by a Tukey post-hoc test with a confidence level of p < 0.05 was used to determine whether the differences observed between the control group and the different digestate concentrations were statistically significant. The results confirm that the increase in green matter in the 3% digestate concentration group (52.7% to 85.4%) is statistically significant (p < 0.01) compared to the control group.
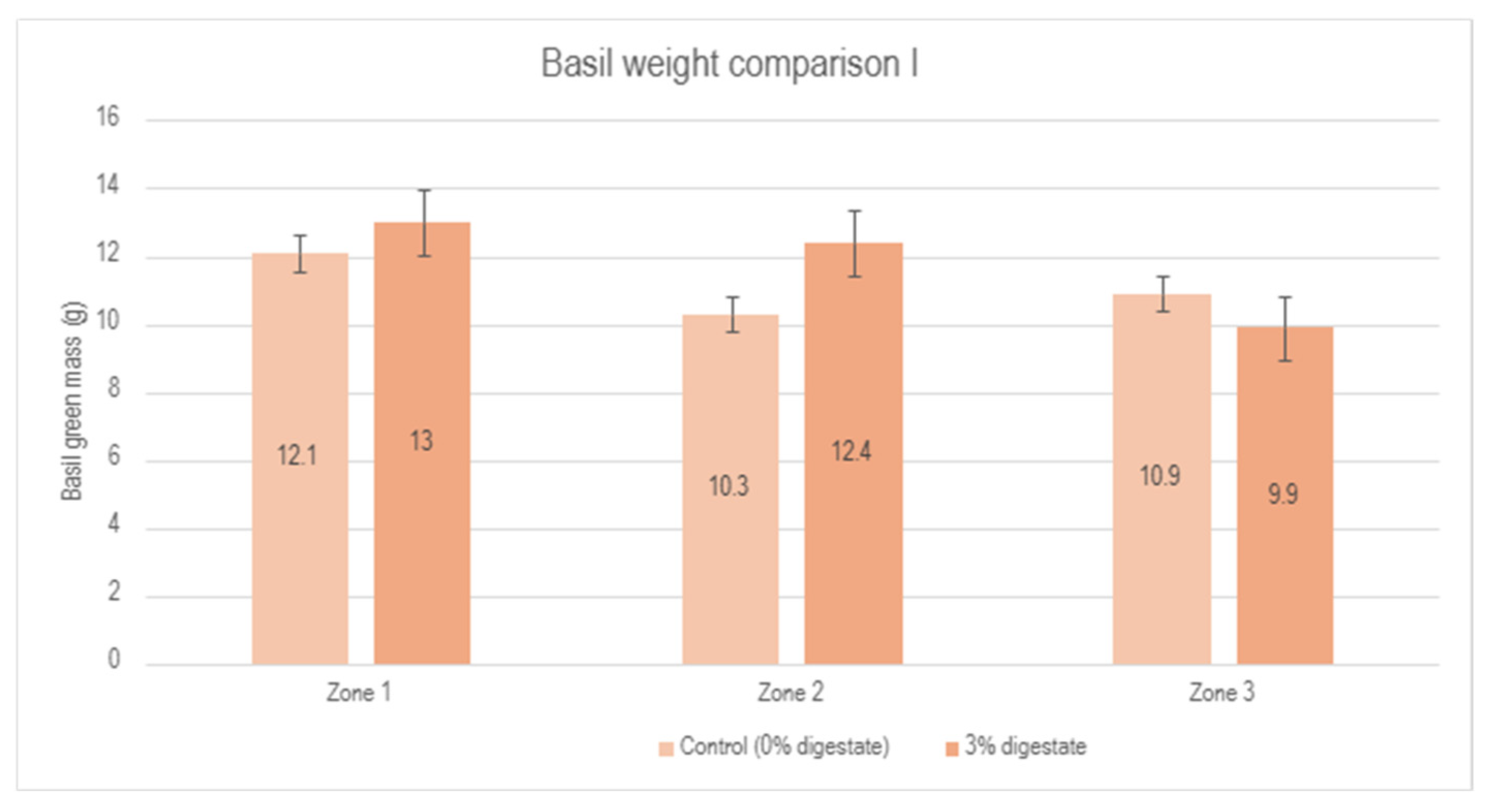
These results indicate a small but measurable positive effect of applying a 3% digestate concentration in basil cultivation. A detailed visual representation of these results, including a comparison of different zones and percentage changes between the control group and the 3% digestate group, is shown in Figure 6.
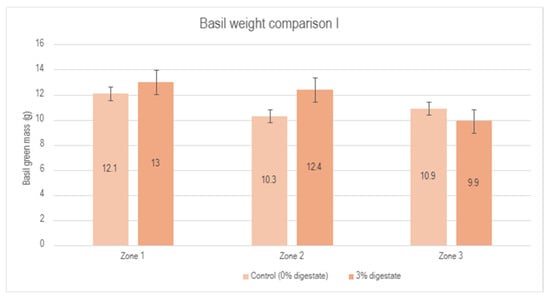
Figure 6.
Comparison of basil green mass between the control group (0% digestate) and the 3% digestate group across different zones, as well as percentage changes.
This graphical representation clearly demonstrates the impact of digestate on the increase in basil’s green mass across different experimental zones. Although two zones showed a slight increase in green mass, the results in zone 3 were inconsistent. This may be attributed to external factors, such as plant positioning in the greenhouse or other uncontrolled variations in experimental conditions. Overall, the average green mass of basil treated with a 3% digestate concentration was slightly higher than that of the control group, suggesting the potential effectiveness of the biostimulant. However, additional tests are necessary to fully confirm the stability of these results.
- Second experiment.
Based on the experience of the first experiment, the methodology for the second experiment was adjusted. In this trial, crops were treated with lower concentrations of the biostimulant—3% and 6%—while a control group with 0% concentration was maintained for comparison. The other experimental parameters, including planting, treatment and harvesting dates, remained the same as in the first experiment.
This improved experiment structure enabled a more accurate assessment of the impact of the algae digestate biostimulant on basil crop growth, ensuring more reliable results and avoiding damage caused by excessive concentrations.
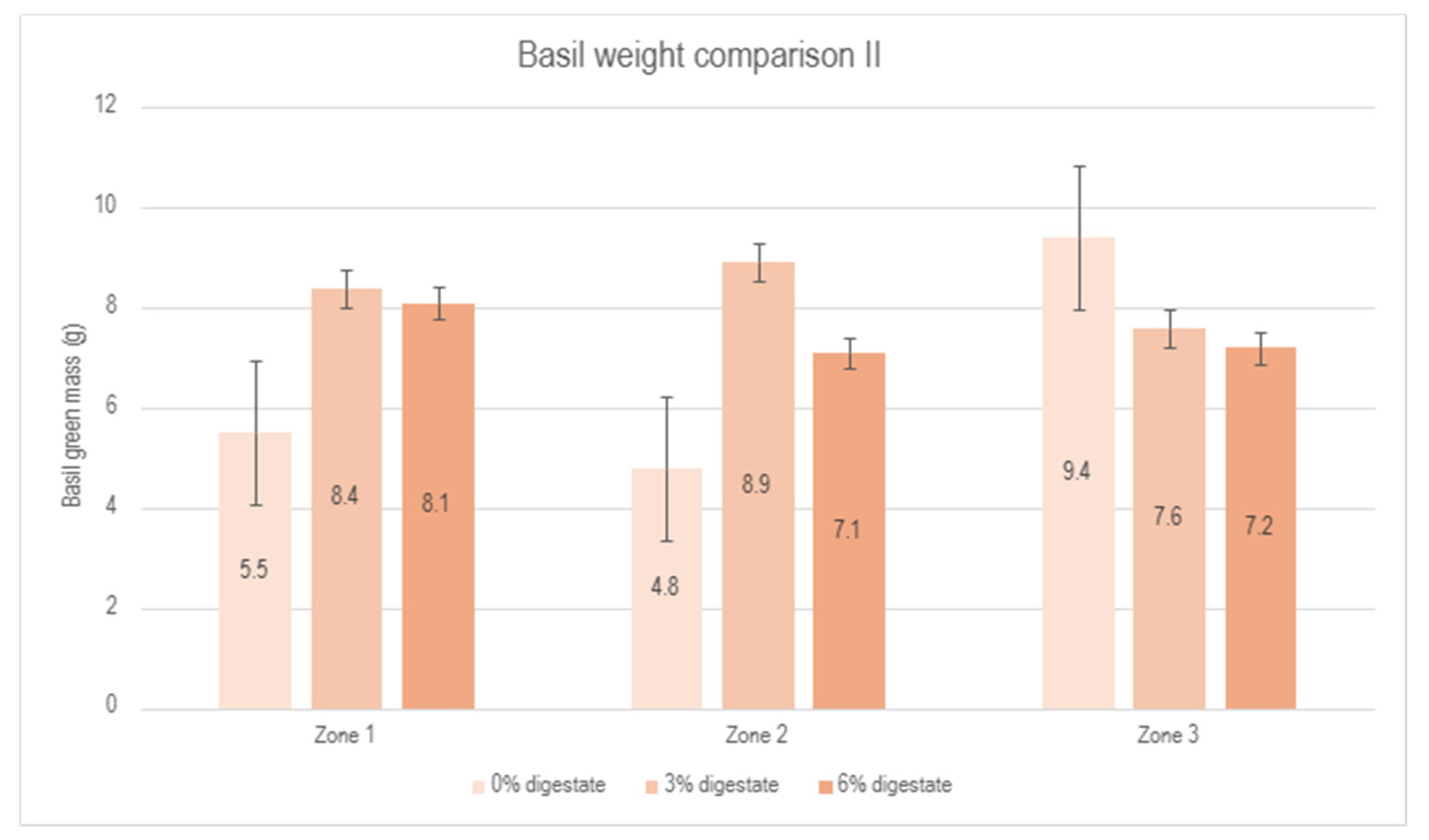
In the second experiment, plants of the same plant species (basil, Ocimum basilicum) were irrigated with digestate solutions at concentrations of 3% and 6%, while the control group received 0% (pure water). A comparative analysis of the different treatment groups yielded the following results: (1) the 3% digestate solution demonstrated a growth increase ranging from 52.7% to 85.4% across various zones (Figure 7), indicating a significant positive effect on basil growth; (2) the 6% digestate solution exhibited a moderate growth increase of 29.1% to 52.2%, suggesting that although this concentration promotes plant growth, its effect is less pronounced compared to the 3% concentration. These findings suggest that both 3% and 6% digestate concentrations enhance basil growth; however, the 3% concentration provides a more consistent and substantial growth increase across different zones. Figure 8 illustrates the distribution of plant biomass in zones treated with 0%, 3% and 6% biostimulant solutions.

Figure 7.
Plant development in the second experiment; explicit growth was observed in the central region of each zone treated with a 3% digestate solution.
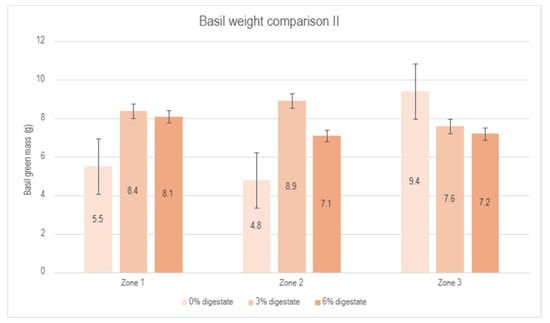
Figure 8.
Comparison of basil green mass between the control group (0% digestate), 3% digestate group and 6% digestate group across different zones.
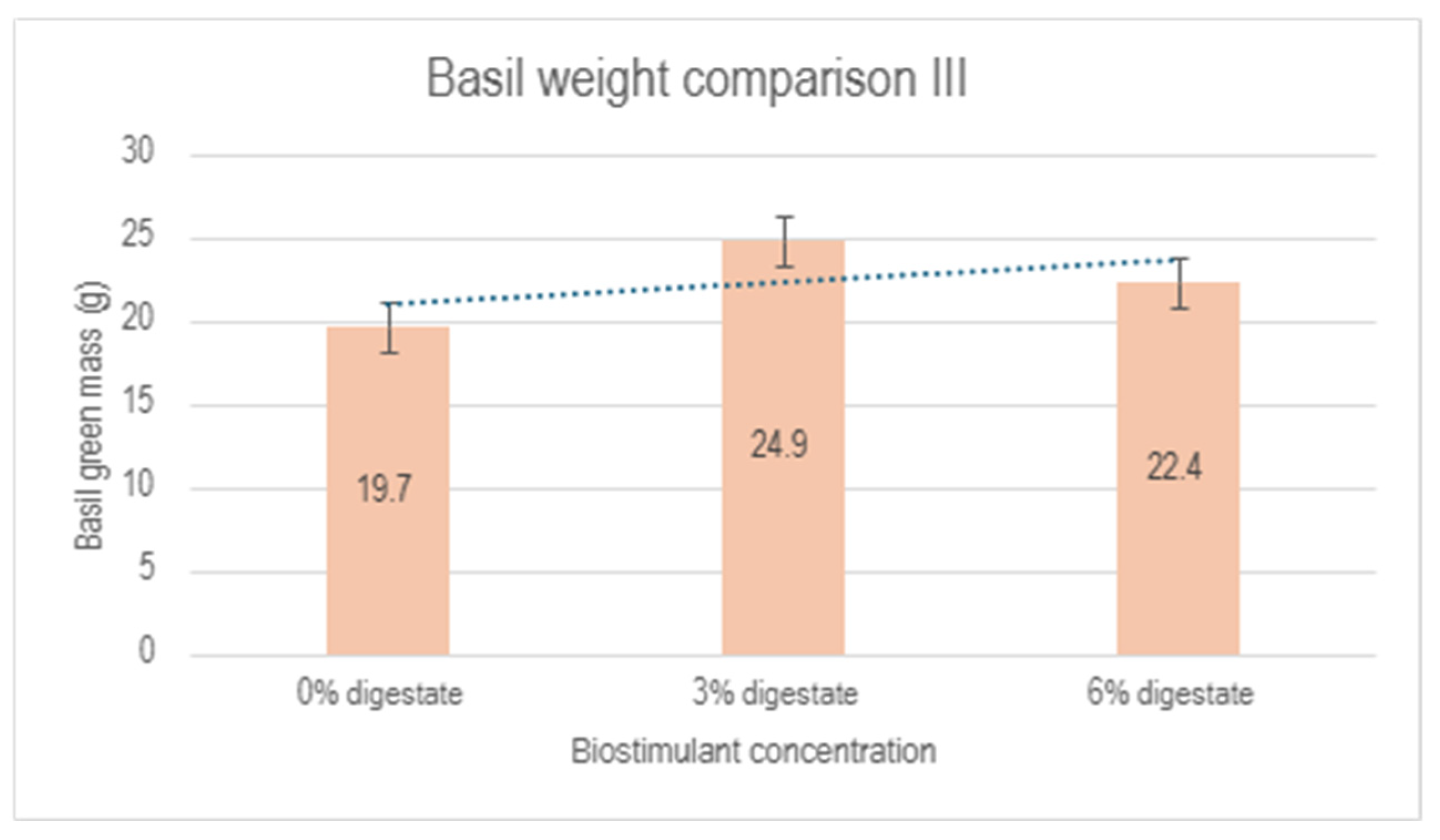
Similar to the first experiment, a reduction in biomass was observed in zone 3 compared to the control group, which could be attributed to specific greenhouse conditions and unresolved issues related to ventilation performance. A summary of the results from the second experiment is presented in Figure 9. Despite the negative influence of zone 3, a positive effect of the biostimulant on plant development is evident, reaching a maximum at a 3% biostimulant concentration.
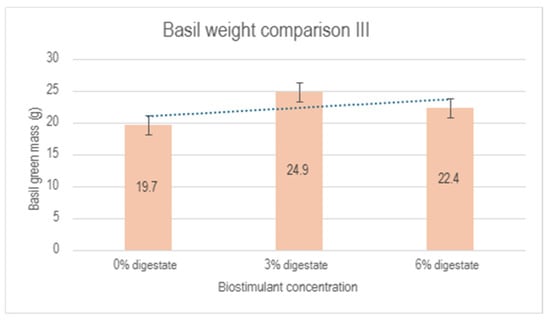
Figure 9.
Comparison of basil green mass between the control group (0% digestate), 3% digestate group and 6% digestate group.
Digestate obtained from the anaerobic fermentation of the seaweed Furcellaria lumbricalis can be used as an effective plant growth biostimulant. The developed biostimulant accelerates plant growth and increases plant green mass compared to the control group, irrigated with pure water under the same external growth conditions. The most effective results were observed with the 3% digestate concentration. However, it should be noted that at higher concentrations (above 5%), there is a risk of plant scorching. The results of the experiments indicate the successful application of the 3% digestate concentration for stimulating plant growth. It is recommended to apply the biostimulant periodically (once a week), avoiding direct contact with the aerial parts of the plant. It is recommended to apply or spray the solution on the soil near the plant rather than directly on the aerial parts (leaves, tops, stem) as this may lead to a risk of scorching. Based on current laboratory studies, it can be concluded that further research is required to investigate the effect of bright sunlight on plants treated with the biostimulant.
3.2. Availability of Algae Biomass and Technological Aspects
Following the research of various algae extracts and their active compounds affecting plant growth and development, it is crucial to focus on the availability and potential of algae biomass in the Baltic Sea region. This information is essential for evaluating the realistic possibilities of using local algae species, particularly Furcellaria lumbricalis, for the production of biostimulants in the Baltic Sea region. Table 5 provides an overview of the availability of algae biomass, its current applications and it future potential in Baltic Sea countries. This overview offers insights into regional opportunities and challenges in the development of algae biostimulants based on local resources. Such an assessment is key for evaluating the prospects of biostimulant production in the Baltic Sea region and its potential contribution to the development of sustainable agriculture. It helps identify both existing resources and areas where further research or investment is needed.

Table 5.
Algae biomass availability and economic potential in Baltic Sea Region countries.
Table 5 synthesises data from several literature sources as cited, with additional analytical interpretation by the authors. The economic values presented are compiled from published studies and reports and have been standardised by the authors to facilitate comparison across the Baltic Sea countries. These values represent estimates derived from the literature rather than original economic measurements.
The analysis carried out, the results of which are presented in Table 5, provides valuable insights into the potential of algae biomass in the Baltic Sea region. Estonia demonstrates the highest biomass of Furcellaria lumbricalis (150,000 t/year), but only 1.33% of it is used for furcellaran production. This indicates a significant untapped potential. In Latvia, the estimated biomass of Furcellaria lumbricalis (20,000–30,000 t/year) is considerably lower, yet the utilisation rate remains low—only about 2% is collected from washed-up algae. Estonia’s economic value from algae biomass (15–20 million EUR/year) is the highest in the region, reflecting development opportunities within the industry. Finland’s potential economic value (5–7 million EUR/year) is the second highest, despite the current limited exploitation, indicating significant growth potential in the future. The situation in Poland, with its significantly reduced Furcellaria lumbricalis biomass (<1000 t/year), underlines the need for active restoration measures. Sweden’s example, where biomass reduction has stabilised (5000–10,000 t/year), highlights the importance of sustainable management. The potential identified in Finland and Germany in the field of bioactive compounds and ecosystem services suggests opportunities for diversifying algae uses. The total algae biomass in the Baltic Sea region (236,000–276,000 t/year) and its potential economic value (24.1–34 million EUR/year) underline the industry’s considerable potential. The data and calculations presented in the analysis show that the Baltic Sea region’s algae biomass offers significant opportunities for sustainable economic development, particularly in biostimulant production, biorefineries and ecosystem service provision. However, to fully exploit this potential, further research, technological development and the establishment of appropriate policies for the sustainable use of algae resources are necessary. Table 6 reflects the potential applications of algae extracts in agriculture.

Table 6.
Application methods and effectiveness of algae extracts in agriculture.
The information summarised in Table 6 offers a detailed evaluation of the methods, forms and effectiveness of using algae extracts in agriculture: (1) algae extracts can be applied in various ways, each with specific benefits. Seed treatment with algae extracts can significantly improve germination rates (by 20–30%). Leaf treatment with extracts can increase the photosynthetic rate by up to 75%, which is a significant increase. Soil treatment enhances soil structure and microbial activity, both of which are crucial for sustainable agriculture. In hydroponic cultivation, adding algae extracts to the nutrient solution can accelerate plant growth by 15–25%; (2) each form has its advantages. Liquid extracts are easily absorbed and fast-acting, which can be particularly useful for quick adjustments. Powdered extracts provide longer-lasting effects and are easy to store. Granular products enable even distribution of the extract in the soil. The use of living algae cells provides long-lasting effects and improves soil structure; (3) efficacy depends on a number of factors, including the timing, frequency and concentration of application. Optimal results are achieved when extracts are applied 3–7 times per season. Concentrations between 0.2% and 2% are the most effective. (4) Combining algae extracts with other biostimulants or fertilisers can create synergistic effects, reducing the use of chemical fertilisers by 30–50%, which is an important step towards sustainable agriculture.
The results indicate that the use of algae extracts in agriculture offers versatile opportunities to improve crop growth, increase yields and promote sustainable farming practices. However, optimal application requires careful planning and adaptation to specific conditions and crops. Future research should focus on developing precise dosage methods and assessing the long-term impact on soil health and overall ecosystem stability.
4. Discussion
This study makes a significant contribution to the theoretical and practical discourse on the use of marine algae biostimulants in sustainable agriculture, with a particular focus on the potential of Furcellaria lumbricalis digestate extract in the Baltic Sea region. The results confirm both the effectiveness of the biostimulants and their economic and sustainability potential, while also highlighting several challenges that need to be addressed in order to fully realise the advantages of this technology.
It is important to recognise the limitations in the generalisability of our results. This study primarily used basil (Ocimum basilicum) as a model crop due to its rapid growth cycle, sensitivity to nutrient amendments and controlled greenhouse suitability. While our results show significant growth promotion in basil with the 3% digestate application (52.7–85.4% increase in green mass), caution must be exercised in extrapolating these results to other agricultural crops. Different plant species show different physiological responses to biostimulants due to species-specific differences in nutrient utilisation pathways, root architecture, phytohormone sensitivity and stress response mechanisms. For example, monocotyledonous plants such as cereals (wheat, barley, oats) may respond differently from dicotyledonous plants such as basil due to fundamental differences in their root systems and nutrient acquisition strategies. Similarly, perennial crops may respond differently to annual crops such as basil. While our economic projections (Table 2) incorporate literature-derived yield increases for various crops, empirical validation through field trials with regionally important Baltic Sea Region crops is essential. Future research should systematically evaluate the efficacy of Furcellaria lumbricalis digestate on major agricultural crops relevant to the Baltic Sea region, including cereals, oilseed rape, potatoes and legumes, under both controlled and field conditions in different soil types and climatic scenarios.
- The experimental results indicate that 3% Furcellaria lumbricalis digestate extract significantly enhances basil (Ocimum basilicum) growth, increasing green mass by 52.7% to 85.4%. These results are very promising and indicate the high effectiveness of the extract. However, it was observed that a higher concentration (6%) demonstrated lower effectiveness, highlighting the need for the careful optimisation of concentration depending on the crop and environmental conditions. The study demonstrates the economic potential of algal biostimulants in Latvian agriculture, with projections of gross profit increases of 10–20% for different crops. Using the Gross Profit Calculation Tool (2023 version) developed by the Latvian Rural Advisory and Education Centre (LRAC) and yield increase estimates derived from the peer-reviewed literature, it was calculated that gross profit could increase by 357–476 EUR/ha for potatoes and 80–107 EUR/ha for rapeseed (see Section 2.2 for detailed methodology and Table 2 for comprehensive results). These estimates take into account both yield improvements and additional costs associated with the use of biostimulants. However, it should be noted that these calculations are based on theoretical modelling and laboratory experiments and that practical results may vary depending on factors such as climatic conditions and soil characteristics.
- Furcellaria lumbricalis is a significant biomass resource in the Baltic Sea region, with different distributions in different countries. The study reveals that Estonia has the most significant biomass available, with 150,000 tonnes of wet weight per year, followed by Latvia with 20,000–30,000 tonnes per year. This resource base offers substantial development opportunities in the production of biostimulants and biorefining.
- The analysis of the enzyme composition (Table 4) provides valuable preliminary information on the potential nutritional value of the Furcellaria lumbricalis digestate. At the same time, it should be noted that these results are preliminary characterisations and not definitive proof of the efficacy of the biostimulant. The relatively stable pH and significant organic matter content suggest potential soil replenishing properties, while the presence of various macro- and micro-nutrients suggests possible nutritional benefits. However, in order to properly contextualise these findings, it is important to carry out comparative studies with established commercial biostimulants. Future studies should investigate not only the nutrient composition but also their bioavailability, the presence of plant growth-promoting compounds, such as phytohormones and signalling molecules, and possible synergistic effects between components. Particular attention should be paid to the relatively high calcium content (1.68%), as calcium plays a crucial role in cell wall formation and in signalling the plant stress response.
The utilisation of this resource base can reduce transportation costs and boost the local economy. Additionally, the production of digestate from anaerobic fermentation processes ensures waste stream recycling and helps reduce the environmental impact of agriculture.
The potential benefits and challenges of introducing algal biostimulants into agriculture, as outlined in Table 1, were considered throughout the study. Our results are consistent with several of these potential outcomes. For example, we observed improved plant resistance and stress tolerance, as evidenced by increased basil growth under controlled conditions. The economic analysis showed the potential for cost reduction in the long term, with projected increases in gross profit for various crops. However, our study also identified challenges in optimising extraction and formulation processes, as evidenced by the variable efficacy of different digestate concentrations. These findings highlight the need for further research to fully realise the potential benefits while addressing the challenges identified in Table 1.
While the study points to the potential of the Furcellaria lumbricalis digestate, several challenges and limitations were identified. Firstly, the standardisation of biostimulant production processes and quality control is needed to ensure a consistent and reliable product. Secondly, the new European Union regulation provides a framework for the registration of biostimulants, but this process remains complex and requires significant resources. Thirdly, further research is needed on the precise mechanisms of action at the molecular level to optimise the formulation and use of biostimulants. Fourthly, long-term field trials in different climatic zones should be conducted to test the effectiveness of the biostimulant under different environmental conditions.
One of the methodological limitations of this study is related to the lack of analysis of the organic matter content of Sample 2. Due to resource constraints, we took the strategic decision to prioritise different analytical parameters for the main nutrient elements of each sample to obtain a more comprehensive overall characterisation, rather than performing identical analyses for all samples. Given this constraint, more comprehensive analyses should be performed in future studies. Such studies would provide valuable information for optimising the biostimulant and improving the efficiency of its use in different areas of agriculture and horticulture. Future research should focus on field tests with different crops and regions and develop optimal application methods for specific crops.
The study results confirm the potential of Furcellaria lumbricalis digestate extract as a biostimulant for sustainable agriculture. Laboratory experiments demonstrate its ability to significantly increase the green mass of basil under controlled conditions, indicating potential benefits for agricultural production. Economic analyses show the potential to improve agricultural profitability in the Latvian context. However, the study also highlights the need for more in-depth research on several aspects, in particular on the mechanisms of action of biostimulants at the molecular level and their practical application in different field conditions and crop systems.
Overall, the results suggest that Furcellaria lumbricalis digestate extract offers promising opportunities to promote sustainable agriculture in the Baltic Sea region, while also addressing global issues related to food security and environmental protection. The study provides a foundation for further research and development in this area and could stimulate new research and innovations in the field of sustainable agriculture.
Author Contributions
Conceptualization, I.S. and U.Ž.; methodology, I.S. and U.Ž.; validation, I.S. and U.Ž.; formal analysis, I.S.; investigation, I.S.; resources, I.S.; data curation, I.S. and U.Ž.; writing—original draft preparation, I.S. and U.Ž.; writing—review and editing, G.G.-Z.; visualization, I.S. and U.Ž.; supervision, G.G.-Z.; project administration, G.G.-Z.; funding acquisition, G.G.-Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Research grant No. DG4 of Latvia University of Life Sciences and Technologies with Agreement No. 3.2.-10/206 from 13.11.2023 for research grant/scientific project “Assessment of the Resource Potential of Baltic Sea Macroalgae for agricultural production”.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data is contained within the article.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Drobek, M.; Frąc, M.; Cybulska, J. Plant biostimulants: Importance of the quality and yield of horticultural crops and the improvement of plant tolerance to abiotic stress—A review. Agronomy 2019, 9, 335. [Google Scholar] [CrossRef]
- European Commission. Farm to Fork Strategy—For a Fair, Healthy, and Environmentally-Friendly Food System. Available online: https://food.ec.europa.eu/horizontal-topics/farm-fork-strategy_en (accessed on 20 December 2024).
- Nanda, S.; Kumar, G.; Hussain, S. Utilization of seaweed-based biostimulants in improving plant and soil health: Current updates and future prospective. Int. J. Environ. Sci. Technol. 2022, 19, 12839–12852. [Google Scholar] [CrossRef]
- Carillo, P.; Colla, G.; Fusco, G.M.; Dell’Aversana, E.; El-Nakhel, C.; Giordano, M.; Pannico, A.; Cozzolino, E.; Mori, M.; Reynaud, H.; et al. Morphological and Physiological Responses Induced by Protein Hydrolysate-Based Biostimulant and Nitrogen Rates in Greenhouse Spinach. Agronomy 2019, 9, 450. [Google Scholar] [CrossRef]
- Hussein, H.-A.A.; Alshammari, S.O.; Kenawy, S.K.M.; Elkady, F.M.; Badawy, A.A. Grain-Priming with L-Arginine Improves the Growth Performance of Wheat (Triticum aestivum L.) Plants under Drought Stress. Plants 2022, 11, 1219. [Google Scholar] [CrossRef] [PubMed]
- Ali, O.; Ramsubhag, A.; Jayaraman, J. Biostimulant Properties of Seaweed Extracts in Plants: Implications towards Sustainable Crop Production. Plants 2021, 10, 531. [Google Scholar] [CrossRef]
- Lähteenmäki-Uutela, A.; Rahikainen, M.; Camarena-Gómez, M.T.; Piiparinen, J.; Spilling, K.; Yang, B. European Union legislation on macroalgae products. Aquacult. Int. 2021, 29, 487–509. [Google Scholar] [CrossRef]
- Ronga, D.; Biazzi, E.; Parati, K.; Carminati, D.; Carminati, E.; Tava, A. Microalgal biostimulants and biofertilisers in crop productions. Agronomy 2019, 9, 192. [Google Scholar] [CrossRef]
- Sanchez-Quintero, A.; Leca, M.-A.; Regnault, L. Innovative production of microalgae-based plant biostimulants from waste streams for a sustainable agriculture. In Proceedings of the Young Algaeneers Symposium (YAS 2023), Faro, Portugal, 23–25 May 2023. [Google Scholar]
- Rouphael, Y.; Colla, G. Synergistic Biostimulatory Action: Designing the Next Generation of Plant Biostimulants for Sustainable Agriculture. Front. Plant Sci. 2018, 9, 1655. [Google Scholar] [CrossRef]
- Van Oosten, M.J.; Pepe, O.; De Pascale, S.; Silletti, S.; Maggio, A. The role of biostimulants and bioeffectors as alleviators of abiotic stress in crop plants. Chem. Biol. Technol. Agric. 2017, 4, 5. [Google Scholar] [CrossRef]
- Eurostat. Agricultural Production-Crops. Available online: https://ec.europa.eu/eurostat/statistics-explained/index.php?title=Agricultural_production_-_crops (accessed on 20 December 2024).
- Ministry of Agriculture. Latvijas Lauksaimniecība 2021; Zemkopības Ministrija: Rīga, Latvia, 2021.
- Sharma, H.S.S.; Fleming, C.; Selby, C.; Rao, J.R.; Martin, T. Plant biostimulants: A review on the processing of macroalgae and use of extracts for crop management to reduce abiotic and biotic stresses. J. Appl. Phycol. 2014, 26, 465–490. [Google Scholar]
- Boutahiri, S.; Benrkia, R.; Tembeni, B.; Idowu, O.E.; Olatunji, O.J. Effect of Biostimulants on the Chemical Profile of Food Crops under Normal and Abiotic Stress Conditions. Curr. Plant Biol. 2024, 40, 100410. [Google Scholar] [CrossRef]
- Hamouda, M.M.; Saad-Allah, K.M.; Gad, D. Potential of Seaweed Extract on Growth, Physiological, Cytological and Biochemical Parameters of Wheat (Triticum aestivum L.) Seedlings. J. Soil. Sci. Plant Nutr. 2022, 22, 13. [Google Scholar] [CrossRef]
- Rouphael, Y.; Colla, G.; Giordano, M.; El-Nakhel, C.; Kyriacou, M.C.; De Pascale, S. Foliar Applications of a Legume-derived Protein Hydrolysate Elicit Dose-dependent Increases of Growth, Leaf Mineral Composition, Yield and Fruit Quality in Two Greenhouse Tomato Cultivars. Sci. Hortic. 2017, 226, 353–360. [Google Scholar] [CrossRef]
- Bartucca, M.L.; Cerri, M.; Del Buono, D.; Forni, C. Use of Biostimulants as a New Approach for the Phytoremediation of Contaminated Soils: Promises and Challenges. Plants 2022, 11, 1946. [Google Scholar] [CrossRef]
- Zarzecka, K.; Gugała, M. The effect of herbicides and biostimulants on sugars content in potato tubers. Plant Soil. Environ. 2018, 64, 82–87. [Google Scholar] [CrossRef]
- García-González, J.; Sommerfeld, M. Biofertilizer and biostimulant properties of the microalga Acutodesmus dimorphus. J. Appl. Phycol. 2016, 28, 1051–1061. [Google Scholar]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.T.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed extracts as biostimulants of plant growth and development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Calvo, P.; Nelson, L.; Kloepper, J.W. Agricultural uses of plant biostimulants. Plant Soil. 2014, 383, 3–41. [Google Scholar] [CrossRef]
- Battacharyya, D.; Babgohari, M.Z.; Rathor, P.; Prithiviraj, B. Seaweed extracts as biostimulants in horticulture. Sci. Hortic. 2015, 196, 39–48. [Google Scholar]
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Win, T.T.; Barone, G.D.; Secundo, F.; Fu, P. Algal Biofertilizers and Plant Growth Stimulants for Sustainable Agriculture. Ind. Biotechnol. 2018, 14, 203–211. [Google Scholar] [CrossRef]
- Rathore, S.S.; Chaudhary, D.; Boricha, G.; Ghosh, A.; Bhatt, B.; Zodape, S.; Patolia, J. Effect of seaweed extract on the growth, yield and nutrient uptake of soybean (Glycine max) under rainfed conditions. S. Afr. J. Bot. 2009, 75, 351–355. [Google Scholar] [CrossRef]
- Arias, A.; Feijoo, G.; Moreira, M.T. Macroalgae as a sustainable biostimulant for crop production according to techno-economic and environmental criteria. Sustain. Prod. Consum. 2024, 48, 169–180. [Google Scholar] [CrossRef]
- du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef]
- Rouphael, Y.; Cardarelli, M.; Bonini, P.; Colla, G. Synergistic action of a microbial-based biostimulant and a plant derived-protein hydrolysate enhances lettuce tolerance to alkalinity and salinity. Front. Plant Sci. 2018, 9, 1870. [Google Scholar] [CrossRef]
- Yakhin, O.I.; Lubyanov, A.A.; Yakhin, I.A.; Brown, P.H. Biostimulants in plant science: A global perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef]
- Kholssi, R.; Lougraimzi, H.; Grina, F.; Lorentz, J.; Silva, I.; Castaño Sánchez, O.; Marks, E.A.N. Green Agriculture: A Review of the Application of Micro- and Macroalgae and Their Impact on Crop Production on Soil Quality. J. Soil. Sci. Plant Nutr. 2022, 22, 4627–4641. [Google Scholar] [CrossRef]
- Pereira, R.V.; Filgueiras, C.C.; Dória, J.; Peñaflor, M.F.G.V.; Willett, D.S. The Effects of Biostimulants on Induced Plant Defense. Front. Agron. 2021, 3, 630596. [Google Scholar] [CrossRef]
- Chen, D.; Zhou, W.; Yang, J.; Ao, J.; Huang, Y.; Shen, D.; Jiang, Y.; Huang, Z.; Shen, H. Effects of Seaweed Extracts on the Growth, Physiological Activity, Cane Yield and Sucrose Content of Sugarcane in China. Agronomy 2021, 11, 1234. [Google Scholar] [CrossRef]
- Tritean, N.; Trică, B.; Dima, Ş.-O.; Capră, L.; Gabor, R.-A.; Cimpean, A.; Oancea, F.; Constantinescu-Aruxandei, D. Mechanistic Insights into the Plant Biostimulant Activity of a Novel Formulation Based on Rice Husk Nanobiosilica Embedded in a Seed Coating Alginate Film. Front. Plant Sci. 2024, 15, 1349573. [Google Scholar] [CrossRef]
- Mutlu-Durak, H.; Arikan-Algul, Y.; Bayram, E.; Haznedaroglu, B.Z.; Kutman, U.B.; Kutman, B.Y. Various Extracts of the Brown Seaweed Cystoseira barbata with Different Compositions Exert Biostimulant Effects on Seedling Growth of Wheat. Physiol. Plant. 2024, 176, e14503. [Google Scholar] [CrossRef] [PubMed]
- Bulgari, R.; Cocetta, G.; Trivellini, A.; Vernieri, P.; Ferrante, A. Biostimulants and crop responses: A review. Biol. Agric. Hortic. 2014, 31, 1–17. [Google Scholar] [CrossRef]
- Latvian Rural Advisory and Training Centre (LLKC). Gross Margins. Available online: https://new.llkc.lv/lv/nozares/ekonomika/bruto-segumi (accessed on 15 December 2024).
- Zalidis, G.; Stamatiadis, S.I.; Takavakoglou, V.; Eskridge, K.; Misopolinos, N. Impacts of Agricultural Practices on Soil and Water Quality in the Mediterranean Region and Proposed Assessment Methodology. Agric. Ecosyst. Environ. 2002, 88, 137–146. [Google Scholar] [CrossRef]
- Castiglione, A.M.; Mannino, G.; Contartese, V.; Bertea, C.M.; Ertani, A. Microbial Biostimulants as Response to Modern Agriculture Needs: Composition, Role and Application of These Innovative Products. Plants 2021, 10, 1533. [Google Scholar] [CrossRef]
- HELCOM. Baltic Sea Action Plan Implementation Report; Helsinki Commission: Helsinki, Finland, 2021.
- Chwastowska-Siwiecka, I.; Micinski, J. Characteristics and Applications of Marine Algae in the Agri-Food Industry and Animal Nutrition. J. Elem. 2023, 28, 855–874. [Google Scholar] [CrossRef]
- Siddhnath; Surasani, V.K.R.; Singh, A.; Singh, S.M.; Hauzoukim; Murthy, L.N.; Baraiya, K.G. Bioactive Compounds from Micro-Algae and Its Application in Foods: A Review. Discov. Food 2024, 4, 27. [Google Scholar] [CrossRef]
- Breton, É.; Juster, R.P.; Booij, L. Gender and sex in eating disorders: A narrative review of the current state of knowledge, research gaps, and recommendations. Brain Behav. 2023, 13, e2871. [Google Scholar] [CrossRef] [PubMed]
- O’Sullivan, R.; Lawlor, B.; Burns, A.; Leavey, G. Will the pandemic reframe loneliness and social isolation? Lancet Health Longev. 2021, 2, e54–e55. [Google Scholar] [CrossRef]
- ISO 10390:2021; Soil Quality—Determination of pH. International Organization for Standardization (ISO): Geneva, Switzerland, 2021.
- LVS EN 13039:2012; Soil Improvers and Growing Media—Determination of Organic Matter Content and Ash. Latvian Standards (LVS): Riga, Latvia, 2012.
- LVS EN 13040:2008; Soil Improvers and Growing Media—Sample Preparation for Chemical and Physical Tests, Determination of Dry Matter Content, Moisture Content, and Laboratory Compacted Bulk Density. Latvian Standards (LVS): Riga, Latvia, 2008.
- LVS EN 13654-1:2003; Soil Improvers and Growing Media—Determination of Nitrogen—Part 1: Modified Kjeldahl Method. Latvian Standards (LVS): Riga, Latvia, 2003.
- LVS 398:2002; Fertilizers—Determination of Phosphorus Content. Latvian Standards (LVS): Riga, Latvia, 2002.
- LVS ISO 11466:1995; Soil Quality—Extraction of Trace Elements Soluble in Aqua Regia & Water Quality—Determination of Potassium Content. Latvian Standards (LVS): Riga, Latvia, 1995.
- LVS EN ISO 7980:2000; Water Quality—Determination of Calcium and Magnesium Content by Atomic Absorption Spectrometry. Latvian Standards (LVS): Riga, Latvia, 2000.
- LVS ISO 11047:1998; Soil Quality—Determination of Trace Elements Using Flame and Electrothermal Atomic Absorption Spectrometry. Latvian Standards (LVS): Riga, Latvia, 1998.
- Stand.Method.3111B:2017; Standard Methods for the Examination of Water and Wastewater—Metals by Atomic Absorption Spectrometry. American Public Health Association (APHA): Washington, DC, USA, 2017.
- Guilayn, F.; Jimenez, J.; Rouez, M.; Crest, M.; Patureau, D. Digestate Mechanical Separation: Efficiency Profiles Based on Anaerobic Digestion Feedstock and Equipment Choice. Bioresour. Technol. 2019, 274, 180–189. [Google Scholar] [CrossRef]
- Erraji, H.; Asehraou, A.; Tallou, A.; Rokni, Y. Assessment of Biogas Production and Fertilizer Properties of Digestate from Cow Dung Using Household Biogas Digester. Biomass Convers. Biorefin. 2023, 14, 29001–29007. [Google Scholar] [CrossRef]
- Mora-Salguero, D.; Montenach, D.; Gilles, M.; Jean-Baptiste, V. Long-Term Effects of Combining Anaerobic Digestate with Other Organic Waste Products on Soil Microbial Communities. Front. Microbiol. 2024, 15, 1490034. [Google Scholar] [CrossRef]
- Guilayn, F.; Rouez, M.; Crest, M.; Patureau, D.; Jimenez, J. Valorization of Digestates from Urban or Centralized Biogas Plants: A Critical Review. Rev. Environ. Sci. Bio/Technol. 2020, 19, 419–462. [Google Scholar] [CrossRef]
- GRASS Project. Grazing Resilience and Sustainable Solutions Program Factsheet; Queensland Government: Brisbane, Australia, 2023.
- Orav-Kotta, H.; Kotta, J.; Herkül, K.; Kotta, I.; Paalme, T. Seasonal Variability in the Grazing Potential of the Invasive Amphipod Gammarus tigrinus and the Native Amphipod Gammarus salinus (Amphipoda: Crustacea) in the Northern Baltic Sea. Biol. Invasions 2009, 11, 597–608. [Google Scholar] [CrossRef]
- Weinberger, F.; Paalme, T.; Wikström, S.A. Seaweed resources of the Baltic Sea, Kattegat and German and Danish North Sea coasts. Bot. Mar. 2020, 63, 61–72. [Google Scholar] [CrossRef]
- Brion, N.; Jans, S.; Chou, L.; Rousseau, V. Nutrient Loads to the Belgian Coastal Zone. In Current Status of Eutrophication in the Belgian Coastal Zone; Rousseau, V., Lancelot, C., Cox, D., Eds.; Presses Universitaires de Bruxelles: Brussels, Belgium, 2006. [Google Scholar]
- O’Sullivan, L.; Murphy, B.; McLoughlin, P.; Duggan, P.; Lawlor, P.G.; Hughes, H.; Gardiner, G.E. Prebiotics from marine macroalgae for human and animal health applications. Mar. Drugs 2010, 8, 2038–2064. [Google Scholar] [CrossRef]
- Davis, R.; Markham, J.; Kinchin, C.; Grundl, N.; Tan, E.C.D.; Humbird, D. Process Design and Economics for the Production of Algal Biomass: Algal Biomass Production in Open Pond Systems and Processing Through Dewatering for Downstream Conversion; NREL Technical Report; National Renewable Energy Lab (NREL): Golden, CO, USA, 2024; pp. 1–128.
- Friedlingstein, P.; O’Sullivan, M.; Jones, M.W.; Andrew, R.M.; Gregor, L.; Hauck, J.; Le Quéré, C. Global Carbon Budget 2023. Earth Syst. Sci. Data 2023, 15, 5301–5369. [Google Scholar] [CrossRef]
- Prisa, D.; Fresco, R.; Jamal, A.; Saeed, M.F.; Spagnuolo, D. Exploring the Potential of Macroalgae for Sustainable Crop Production in Agriculture. Life 2024, 14, 1263. [Google Scholar] [CrossRef] [PubMed]
- Kocira, S.; Szparaga, A.; Kocira, A.; Czerwińska, E.; Wójtowicz, A.; Bronowicka-Mielniczuk, U.; Koszel, M.; Findura, P. Modeling Biometric Traits, Yield and Nutritional and Antioxidant Properties of Seeds of Three Soybean Cultivars Through the Application of Biostimulant Containing Seaweed and Amino Acids. Front. Plant Sci. 2018, 9, 388. [Google Scholar] [CrossRef]
- Di Filippo-Herrera, D.A.; Muñoz-Ochoa, M.; Hernández-Herrera, R.M.; Hernández-Carmona, G. Biostimulant activity of individual and blended seaweed extracts on the germination and growth of the mung bean. J. Appl. Phycol. 2019, 31, 2025–2037. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).