Environmentally Sustainable Anode Material for Lithium-Ion Batteries Derived from Cattle Bone Waste: A Full-Cell Analysis with a LiFePO4 Cathode
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Cattle Bone Waste (CBW) Biochar Preparation
2.3. CBW8 Anode and LFP Cathode Electrode Preparation
2.4. Material Characterizations
2.5. Electrochemical Measurements
3. Results
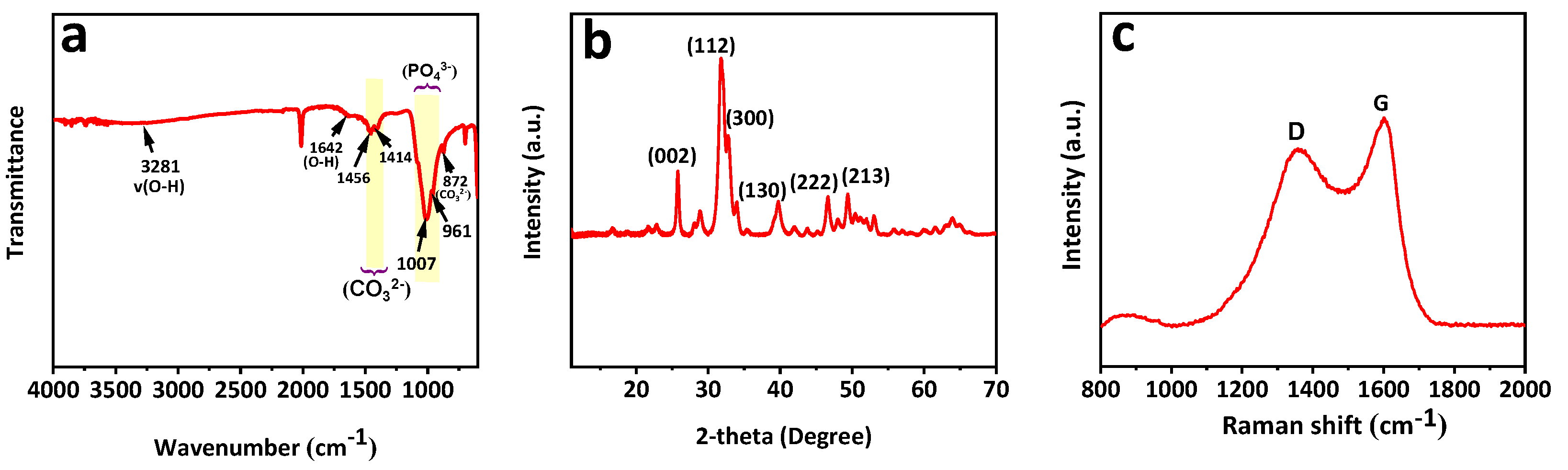
3.1. Structural Characterization
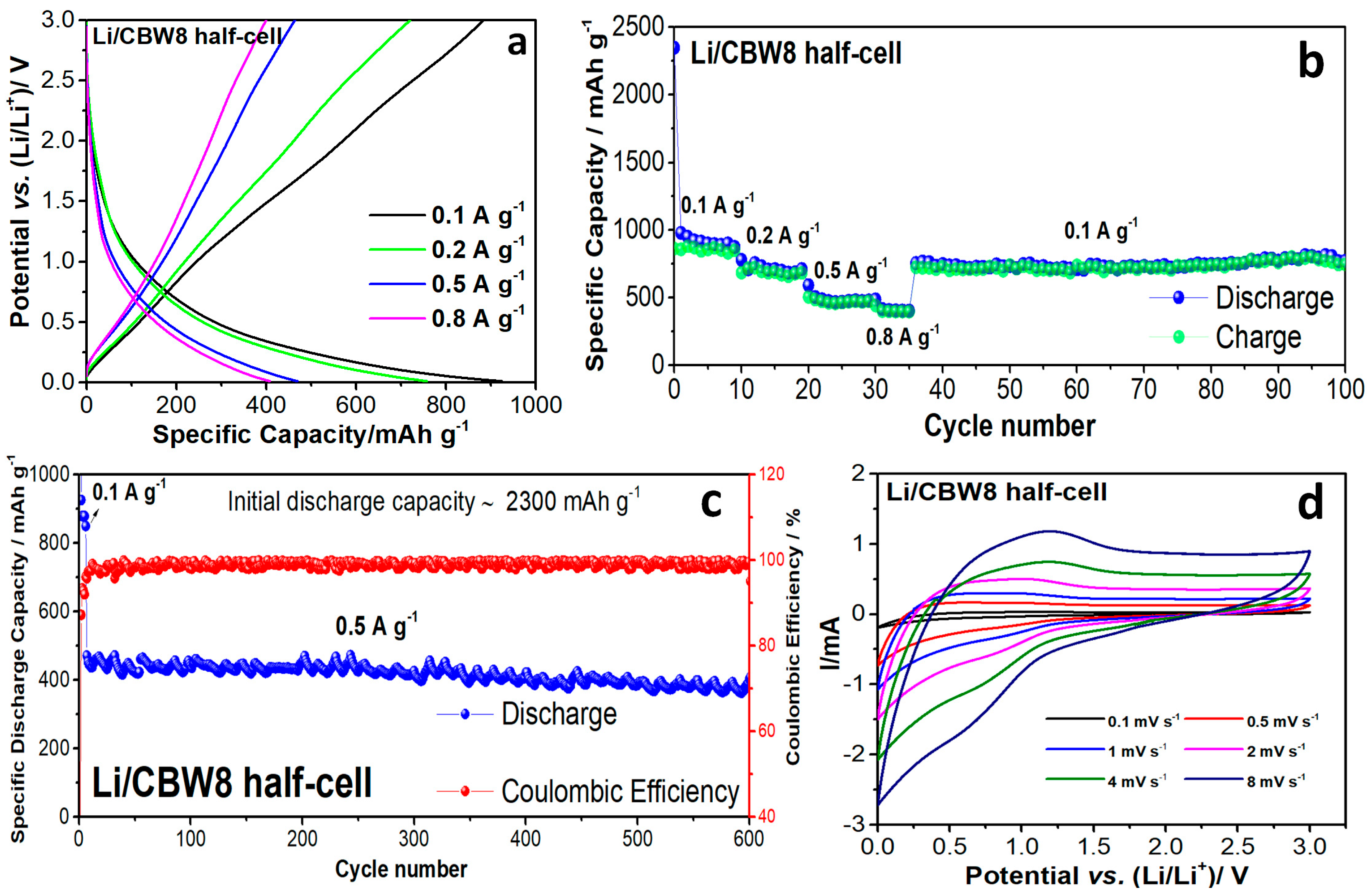
3.2. Electrochemical Performance of CBW8 in Half-Cell and Full-Cell Configurations
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- International Renewable Energy Agency (IRENA). Available online: https://www.irena.org/Digital-Report/World-Energy-Transitions-Outlook-2023 (accessed on 28 February 2025).
- International Energy Agency (Bioenergy). Available online: https://www.iea.org/energy-system/renewables/bioenergy (accessed on 28 February 2025).
- Cherubini, F. The Biorefinery Concept: Using Biomass Instead of Oil for Producing Energy and Chemicals. Energy Convers. Manag. 2010, 51, 1412–1421. [Google Scholar] [CrossRef]
- Papadis, E.; Tsatsaronis, G. Challenges in the Decarbonization of the Energy Sector. Energy 2020, 205, 118025. [Google Scholar] [CrossRef]
- Reijnders, L. Conditions for the Sustainability of Biomass Based Fuel Use. Energy Policy 2006, 34, 863–876. [Google Scholar] [CrossRef]
- Plavniece, A.; Volperts, A.; Dobele, G.; Zhurinsh, A.; Kaare, K.; Kruusenberg, I.; Kaprans, K.; Knoks, A.; Kleperis, J. Wood and Black Liquor-Based N-Doped Activated Carbon for Energy Application. Sustainability 2021, 13, 9237. [Google Scholar] [CrossRef]
- Mensah-Darkwa, K.; Zequine, C.; Kahol, P.K.; Gupta, R.K. Supercapacitor Energy Storage Device Using Biowastes: A Sustainable Approach to Green Energy. Sustainability 2019, 11, 414. [Google Scholar] [CrossRef]
- Salimi, P.; Venezia, E.; Taghavi, S.; Tieuli, S.; Carbone, L.; Prato, M.; Signoretto, M.; Qiu, J.; Proietti Zaccaria, R. Lithium-Metal Free Sulfur Battery Based on Waste Biomass Anode and Nano-Sized Li2S Cathode. Energy Environ. Mater. 2022, 7, e12567. [Google Scholar] [CrossRef]
- Salimi, P.; Askari, K.; Norouzi, O.; Kamali, S. Improving the Electrochemical Performance of Carbon Anodes Derived from Marine Biomass by Using Ionic-Liquid-Based Hybrid Electrolyte for LIBs. J. Electron. Mater. 2019, 48, 951–963. [Google Scholar] [CrossRef]
- Dai, Z.; Hou, H.; Liu, X.; Yao, Y.; Liao, Q.; Yu, C.; Li, D. Feasibility of Expired Waste Aspirin for Use as Lithium-Ion Battery Anode. Waste Biomass Valorization 2020, 11, 357–365. [Google Scholar] [CrossRef]
- Minakshi, M.; Mujeeb, A.; Whale, J.; Evans, R.; Aughterson, R.; Shinde, P.A.; Ariga, K.; Shrestha, L.K. Synthesis of Porous Carbon Honeycomb Structures Derived from Hemp for Hybrid Supercapacitors with Improved Electrochemistry. Chempluschem 2024, 89, e202400408. [Google Scholar] [CrossRef]
- Minakshi, M.; Samayamanthry, A.; Whale, J.; Aughterson, R.; Shinde, P.A.; Ariga, K.; Kumar Shrestha, L. Phosphorous—Containing Activated Carbon Derived From Natural Honeydew Peel Powers Aqueous Supercapacitors. Chem. Asian J. 2024, 19, e202400622. [Google Scholar] [CrossRef]
- Yao, Y.; Wu, F. Naturally Derived Nanostructured Materials from Biomass for Rechargeable Lithium/Sodium Batteries. Nano Energy 2015, 17, 91–103. [Google Scholar] [CrossRef]
- Norouzi, O.; Salimi, P.; Di Maria, F.; Pourhosseini, S.E.M.; Safari, F. Synthesis and Design of Engineered Biochars as Electrode Materials in Energy Storage Systems BT. In Production of Materials from Sustainable Biomass Resources; Fang, Z., Smith Richard, L.J., Tian, X.-F., Eds.; Springer: Singapore, 2019; pp. 233–265. ISBN 978-981-13-3768-0. [Google Scholar]
- Marzeddu, S.; Cappelli, A.; Ambrosio, A.; Décima, M.A.; Viotti, P.; Boni, M.R. A Life Cycle Assessment of an Energy-Biochar Chain Involving a Gasification Plant in Italy. Land 2021, 10, 1256. [Google Scholar] [CrossRef]
- Alexa Teigiserova, D.; Hamelin, L.; Thomsen, M. Towards Transparent Valorization of Food Surplus, Waste and Loss: Clarifying Definitions, Food Waste Hierarchy, and Role in the Circular Economy. Sci. Total Environ. 2020, 706, 136033. [Google Scholar] [CrossRef]
- Niu, J.; Shao, R.; Liu, M.; Zan, Y.; Dou, M.; Liu, J.; Zhang, Z.; Huang, Y.; Wang, F. Porous Carbons Derived from Collagen-Enriched Biomass: Tailored Design, Synthesis, and Application in Electrochemical Energy Storage and Conversion. Adv. Funct. Mater. 2019, 29, 1905095. [Google Scholar] [CrossRef]
- Wang, R.; Wang, K.; Wang, Z.; Song, H.; Wang, H.; Ji, S. Pig Bones Derived N-Doped Carbon with Multi-Level Pores as Electrocatalyst for Oxygen Reduction. J. Power Sources 2015, 297, 295–301. [Google Scholar] [CrossRef]
- Niu, J.; Shao, R.; Liu, M.; Liang, J.; Zhang, Z.; Dou, M.; Huang, Y.; Wang, F. Porous Carbon Electrodes with Battery-Capacitive Storage Features for High Performance Li-Ion Capacitors. Energy Storage Mater. 2018, 12, 145–152. [Google Scholar] [CrossRef]
- Shan, B.; Cui, Y.; Liu, W.; Zhang, Y.; Liu, S.; Wang, H.; Sun, L.; Wang, Z.; Wu, R. Fibrous Bio-Carbon Foams: A New Material for Lithium-Ion Hybrid Supercapacitors with Ultrahigh Integrated Energy/Power Density and Ultralong Cycle Life. ACS Sustain. Chem. Eng. 2018, 6, 14989–15000. [Google Scholar] [CrossRef]
- Wei, S.; Zhang, H.; Huang, Y.; Wang, W.; Xia, Y.; Yu, Z. Pig Bone Derived Hierarchical Porous Carbon and Its Enhanced Cycling Performance of Lithium–Sulfur Batteries. Energy Environ. Sci. 2011, 4, 736–740. [Google Scholar] [CrossRef]
- Niu, J.; Shao, R.; Liang, J.; Dou, M.; Li, Z.; Huang, Y.; Wang, F. Biomass-Derived Mesopore-Dominant Porous Carbons with Large Specific Surface Area and High Defect Density as High Performance Electrode Materials for Li-Ion Batteries and Supercapacitors. Nano Energy 2017, 36, 322–330. [Google Scholar] [CrossRef]
- Zafar, M.S.; Zahid, M.; Athanassiou, A.; Fragouli, D. Biowaste-Derived Carbonized Bone for Solar Steam Generation and Seawater Desalination. Adv. Sustain. Syst. 2021, 5, 2100031. [Google Scholar] [CrossRef]
- Shahid, M.K.; Kim, J.Y.; Choi, Y.G. Synthesis of Bone Char from Cattle Bones and Its Application for Fluoride Removal from the Contaminated Water. Groundw. Sustain. Dev. 2019, 8, 324–331. [Google Scholar] [CrossRef]
- Müller, M.; Obuz, H.E.; Keber, S.; Tekmanli, F.; Mettke, L.N.; Yagmurlu, B. Concepts for the Sustainable Hydrometallurgical Processing of End-of-Life Lithium Iron Phosphate (LFP) Batteries. Sustainability 2024, 16, 11267. [Google Scholar] [CrossRef]
- Park, J.-K. (Ed.) Principles and Applications of Lithium Secondary Batteries; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Fortune Business Insights Lithium Iron Phosphate Battery Market Size, Share & Industry Analysis, By Type (Portable Battery, Stationary Battery), By Application (Automotive, Industrial, Energy Storage System, Consumer Electronics, and Others), and Regional Forecast, 2024–2032. Available online: https://www.fortunebusinessinsights.com/amp/lithium-ion-li-ion-phosphate-batteries-market-102152 (accessed on 28 February 2025).
- Seroka, N.S.; Luo, H.; Khotseng, L. Biochar-Derived Anode Materials for Lithium-Ion Batteries: A Review. Batteries 2024, 10, 144. [Google Scholar] [CrossRef]
- Parviz, Z.; Salimi, P.; Javadian, S.; Gharibi, H.; Morsali, A.; Bayat, E.; Leoncino, L.; Lauciello, S.; Proietti Zaccaria, R. Fabrication of Sustainable Hybrid MOF/Silica Electrodes for Current Lithium-Ion Batteries and Beyond. ACS Appl. Energy Mater. 2022, 5, 15155–15165. [Google Scholar] [CrossRef]
- Chine, M.K.; Sediri, F.; Gharbi, N. Hydrothermal Synthesis of V3O7.H2O Nanobelts and Study of Their Electrochemical Properties. Mater. Sci. Appl. 2011, 2, 964–970. [Google Scholar] [CrossRef][Green Version]
- Yang, C.; Zhang, J.; Han, S.; Wang, X.; Wang, L.; Yu, W.; Wang, Z. Compositional Controls on Pore-Size Distribution by Nitrogen Adsorption Technique in the Lower Permian Shanxi Shales, Ordos Basin. J. Nat. Gas Sci. Eng. 2016, 34, 1369–1381. [Google Scholar] [CrossRef]
- ALOthman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Zhao, S.; Liao, Z.; Fane, A.; Li, J.; Tang, C.; Zheng, C.; Lin, J.; Kong, L. Engineering Antifouling Reverse Osmosis Membranes: A Review. Desalination 2021, 499, 114857. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Beg, M.D.H.; Ghazali, S.; Akindoyo, E.O.; Jeyaratnam, N. Synthesis of Hydroxyapatite through Ultrasound and Calcination Techniques. In IOP Conference Series: Materials Science and Engineering; IOP Publishing: Bristol, UK, 2017; Volume 203. [Google Scholar] [CrossRef]
- Akindoyo, J.O.; Ghazali, S.; Beg, M.D.H.; Jeyaratnam, N. Characterization and Elemental Quantification of Natural Hydroxyapatite Produced from Cow Bone. Chem. Eng. Technol. 2019, 42, 1805–1815. [Google Scholar] [CrossRef]
- Boskey, A.; Pleshko Camacho, N. FT-IR Imaging of Native and Tissue-Engineered Bone and Cartilage. Biomaterials 2007, 28, 2465–2478. [Google Scholar] [CrossRef]
- Liu, F.; Wang, R.; Cheng, Y.; Jiang, X.; Zhang, Q.; Zhu, M. Polymer Grafted Hydroxyapatite Whisker as a Filler for Dental Composite Resin with Enhanced Physical and Mechanical Properties. Mater. Sci. Eng. C 2013, 33, 4994–5000. [Google Scholar] [CrossRef] [PubMed]
- Zahid, M.; Del Río Castillo, A.E.; Thorat, S.B.; Panda, J.K.; Bonaccorso, F.; Athanassiou, A. Graphene Morphology Effect on the Gas Barrier, Mechanical and Thermal Properties of Thermoplastic Polyurethane. Compos. Sci. Technol. 2020, 200, 108461. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, F.; Wei, K.; Zhai, B.; Wang, X. Porous Carbon Microspheres with Controlled Porosity and Graphitization Degree for High-Performance Supercapacitor. J. Electroanal. Chem. 2022, 918, 116449. [Google Scholar] [CrossRef]
- Muraleedharan Pillai, M.; Kalidas, N.; Zhao, X.; Lehto, V.-P. Biomass-Based Silicon and Carbon for Lithium-Ion Battery Anodes. Front. Chem. 2022, 10, 882081. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, Y.; Chao, D.; Li, W.; Zhao, D. Recent Advances in Hard Carbon Anodes with High Initial Coulombic Efficiency for Sodium-Ion Batteries. Nano Mater. Sci. 2023, 5, 189–201. [Google Scholar] [CrossRef]
- Salimi, P.; Vercruysse, W.; Chauque, S.; Yari, S.; Venezia, E.; Lataf, A.; Ghanemnia, N.; Zafar, M.S.; Safari, M.; Hardy, A.; et al. Lithium-Metal-Free Sulfur Batteries with Biochar and Steam-Activated Biochar-Based Anodes from Spent Common Ivy. Energy Environ. Mater. 2024, 7, e12758. [Google Scholar] [CrossRef]
- Deng, J.; Li, M.; Wang, Y. Biomass-Derived Carbon: Synthesis and Applications in Energy Storage and Conversion. Green Chem. 2016, 18, 4824–4854. [Google Scholar] [CrossRef]
- Zhao, W.; Zhao, C.; Wu, H.; Li, L.; Zhang, C. Progress, Challenge and Perspective of Graphite-Based Anode Materials for Lithium Batteries: A Review. J. Energy Storage 2024, 81, 110409. [Google Scholar] [CrossRef]
- Toki, G.F.I.; Hossain, M.K.; Rehman, W.U.; Manj, R.Z.A.; Wang, L.; Yang, J. Recent Progress and Challenges in Silicon-Based Anode Materials for Lithium-Ion Batteries. Ind. Chem. Mater. 2024, 2, 226–269. [Google Scholar] [CrossRef]
- Barbosa Nogueira, M.J.; Chauque, S.; Sperati, V.; Savio, L.; Divitini, G.; Pasquale, L.; Marras, S.; Franchi, P.; Paciornik, S.; Proietti Zaccaria, R.; et al. Untreated Bamboo Biochar as Anode Material for Sustainable Lithium Ion Batteries. Biomass Bioenergy 2025, 193, 107511. [Google Scholar] [CrossRef]
- Han, S.-W.; Jung, D.-W.; Jeong, J.-H.; Oh, E.-S. Effect of Pyrolysis Temperature on Carbon Obtained from Green Tea Biomass for Superior Lithium Ion Battery Anodes. Chem. Eng. J. 2014, 254, 597–604. [Google Scholar] [CrossRef]
- Christensen, J.; Newman, J. Stress Generation and Fracture in Lithium Insertion Materials. J. Solid State Electrochem. 2006, 10, 293–319. [Google Scholar] [CrossRef]
- Feng, J.; Chernova, N.A.; Omenya, F.; Tong, L.; Rastogi, A.C.; Stanley Whittingham, M. Effect of Electrode Charge Balance on the Energy Storage Performance of Hybrid Supercapacitor Cells Based on LiFePO4 as Li-Ion Battery Electrode and Activated Carbon. J. Solid State Electrochem. 2018, 22, 1063–1078. [Google Scholar] [CrossRef]
- Xu, J.; Deshpande, R.D.; Pan, J.; Cheng, Y.-T.; Battaglia, V.S. Electrode Side Reactions, Capacity Loss and Mechanical Degradation in Lithium-Ion Batteries. J. Electrochem. Soc. 2015, 162, A2026. [Google Scholar] [CrossRef]
- Brutti, S.; Hassoun, J.; Scrosati, B.; Lin, C.-Y.; Wu, H.; Hsieh, H.-W. A High Power Sn–C/C–LiFePO4 Lithium Ion Battery. J. Power Sources 2012, 217, 72–76. [Google Scholar] [CrossRef]
- Balogun, M.-S.; Qiu, W.; Luo, Y.; Meng, H.; Mai, W.; Onasanya, A.; Olaniyi, T.K.; Tong, Y. A Review of the Development of Full Cell Lithium-Ion Batteries: The Impact of Nanostructured Anode Materials. Nano Res. 2016, 9, 2823–2851. [Google Scholar] [CrossRef]
- Zhang, X.; Aravindan, V.; Kumar, P.S.; Liu, H.; Sundaramurthy, J.; Ramakrishna, S.; Madhavi, S. Synthesis of TiO2 Hollow Nanofibers by Co-Axial Electrospinning and Its Superior Lithium Storage Capability in Full-Cell Assembly with Olivine Phosphate. Nanoscale 2013, 5, 5973–5980. [Google Scholar] [CrossRef]
- Zhu, G.-N.; Chen, L.; Wang, Y.-G.; Wang, C.-X.; Che, R.-C.; Xia, Y.-Y. Binary Li4Ti5O12-Li2Ti3O7 Nanocomposite as an Anode Material for Li-Ion Batteries. Adv. Funct. Mater. 2013, 23, 640–647. [Google Scholar] [CrossRef]
- Varzi, A.; Bresser, D.; von Zamory, J.; Müller, F.; Passerini, S. Lithium-Ion Batteries: ZnFe2O4-C/LiFePO4-CNT: A Novel High-Power Lithium-Ion Battery with Excellent Cycling Performance (Adv. Energy Mater. 10/2014). Adv. Energy Mater. 2014, 4, 1400054. [Google Scholar] [CrossRef]
- Ma, Y.; Younesi, R.; Pan, R.; Liu, C.; Zhu, J.; Wei, B.; Edström, K. Constraining Si Particles within Graphene Foam Monolith: Interfacial Modification for High-Performance Li+ Storage and Flexible Integrated Configuration. Adv. Funct. Mater. 2016, 26, 6797–6806. [Google Scholar] [CrossRef]
- Choi, S.; Cho, Y.-G.; Kim, J.; Choi, N.-S.; Song, H.-K.; Wang, G.; Park, S. Mesoporous Germanium Anode Materials for Lithium-Ion Battery with Exceptional Cycling Stability in Wide Temperature Range. Small 2017, 13, 1603045. [Google Scholar] [CrossRef]
- Yang, C.; Tartaglino, U.; Persson, B.N.J. Influence of Surface Roughness on Superhydrophobicity. Phys. Rev. Lett. 2006, 97, 116103. [Google Scholar] [CrossRef]
- Zafar, M.S.; Gatto, F.; Mancini, G.; Lauciello, S.; Pompa, P.; Athanassiou, A.; Fragouli, D. Biocomposite Cryogels for Photothermal Decontamination of Water. Langmuir 2023, 39, 7793–7803. [Google Scholar] [CrossRef]
- Liu, M.; Wang, S.; Wei, Z.; Song, Y.; Jiang, L. Bioinspired Design of a Superoleophobic and Low Adhesive Water/Solid Interface. Adv. Mater. 2009, 21, 665–669. [Google Scholar] [CrossRef]
- Liu, X.; Zhou, J.; Xue, Z.; Gao, J.; Meng, J.; Wang, S.; Jiang, L. Clam’s Shell Inspired High-Energy Inorganic Coatings with Underwater Low Adhesive Superoleophobicity. Adv. Mater. 2012, 24, 3401–3405. [Google Scholar] [CrossRef]




| Anode | Mass Ratio (Anode/Cathode) | ICE% | Working Voltage (V) | Initial Discharge Capacity (mAh g−1) | Specific Capacity (mAh g−1) | Ref. |
|---|---|---|---|---|---|---|
| Sn-C | 1:2 | - | 2.8 | 150 | 120 at 3 C | [51] |
| Graphene | 1:4 | 89 | 3 | 165 | 160 at 1 C | [52] |
| TiO2 hollow nanofibers | 1:1.3 | 68 | 1.4 | 140 | 110 at 0.1 A g−1, 80 at 0.2 A g−1, 50 at 0.5 A g−1, and 30 at 1 A g−1 | [53] |
| Binary Li4 Ti5O12-Li2 Ti3O7 | - | - | 1.8 | 125 | 75 at 0.08 A g−1 | [54] |
| ZnFe2O4-C | 1:1.5 | - | 2.1 | 120 | 80 at 9.6 C | [55] |
| Si-Graphene | - | 83.2 | 3 | 157 | 130 at 1 C | [56] |
| Ge | - | 93 | 2.8 | 105 | 90 at 0.5 C | [57] |
| CBW8 | 1:1.5 | 80 | 3 | 167 | 165 at 0.5 C, 155 at 1 C, 142 at 2 C, 95 at 5 C, 65 at 10 C | This work |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zafar, M.S.; Salimi, P.; Ricci, M.; Zia, J.; Zaccaria, R.P. Environmentally Sustainable Anode Material for Lithium-Ion Batteries Derived from Cattle Bone Waste: A Full-Cell Analysis with a LiFePO4 Cathode. Sustainability 2025, 17, 3005. https://doi.org/10.3390/su17073005
Zafar MS, Salimi P, Ricci M, Zia J, Zaccaria RP. Environmentally Sustainable Anode Material for Lithium-Ion Batteries Derived from Cattle Bone Waste: A Full-Cell Analysis with a LiFePO4 Cathode. Sustainability. 2025; 17(7):3005. https://doi.org/10.3390/su17073005
Chicago/Turabian StyleZafar, Muhammad Shajih, Pejman Salimi, Marco Ricci, Jasim Zia, and Remo Proietti Zaccaria. 2025. "Environmentally Sustainable Anode Material for Lithium-Ion Batteries Derived from Cattle Bone Waste: A Full-Cell Analysis with a LiFePO4 Cathode" Sustainability 17, no. 7: 3005. https://doi.org/10.3390/su17073005
APA StyleZafar, M. S., Salimi, P., Ricci, M., Zia, J., & Zaccaria, R. P. (2025). Environmentally Sustainable Anode Material for Lithium-Ion Batteries Derived from Cattle Bone Waste: A Full-Cell Analysis with a LiFePO4 Cathode. Sustainability, 17(7), 3005. https://doi.org/10.3390/su17073005







