Efficiency Evaluation of a Photovoltaic-Powered Water Treatment System with Natural Sedimentation Pretreatment for Arsenic Removal in High Water Vulnerability Areas: Application in La Yarada Los Palos District, Tacna, Peru
Abstract
1. Introduction
1.1. Context and Background
1.2. Local Problem
1.3. Existing Technologies and Knowledge Gaps
1.4. Combined Technologies for Arsenic Treatment
1.5. Study Objectives
1.5.1. Main Objective
1.5.2. Specific Objectives
- Quantify the efficiency of arsenic removal under conditions with and without natural sedimentation pretreatment, highlighting its impact on the initial water quality.
- Analyze the performance of the photovoltaic system under local climatic conditions, identifying key and efficiency influencing factors such as solar radiation, and evaluate the system’s energy consumption, identifying how pretreatment reduces energy costs and improves system operation.
2. Materials and Methods
2.1. Study Area and Sampling
2.2. Analytical Techniques and Experimental Setup
2.2.1. Analytical Techniques
2.2.2. Natural Sedimentation Pretreatment
2.2.3. Water Treatment System
2.2.4. Photovoltaic System
2.3. Experimental Procedure and Data Analysis
3. Results
3.1. Physicochemical Characteristics of Water Samples
3.2. System Performance Under Local Conditions
3.3. Energy Efficiency of the Photovoltaic System
4. Discussion
4.1. Evaluation of Treatment System Efficiency Under Different Conditions
4.2. Statistical Analysis of Results on Arsenic Removal Efficiency and Energy Consumption
4.3. Environmental and Climatic Factors
4.4. Operational Feasibility and Long-Term Sustainability
4.5. Toxicological and Environmental Implications
4.6. Sensitivity Analysis of Operational Variables
4.7. System Limitations and Strategic Recommendations
4.7.1. Technical and Operational Challenges
4.7.2. Economic Feasibility and Cost Analysis
4.7.3. Considerations for As(III) Oxidation in Water Treatment
4.7.4. Recommendations for Optimization and Implementation
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Arsenic. Fact Sheet. 2022. Available online: https://www.who.int/es/news-room/fact-sheets/detail/arsenic (accessed on 2 January 2025).
- Agency for Toxic Substances and Disease Registry (ATSDR). Arsenic Toxicity. Available online: https://www.atsdr.cdc.gov/es/csem/arsenic/docs/arsenic_csem_spanish.pdf (accessed on 2 January 2025).
- Ahmad, A.; van Genuchten, C.M. Deep-Dive into Iron-Based Co-Precipitation of Arsenic: A Review of Mechanisms Derived from Synchrotron Techniques and Implications for Groundwater Treatment. Water Res. 2024, 249, 120970. Available online: https://pub.geus.dk/en/publications/deep-dive-into-iron-based-co-precipitation-of-arsenic-a-review-of (accessed on 2 January 2025). [PubMed]
- Alcocer Zuñiga, J.A.; Córdova Alarcón, E.J.; Hernández-Zavala, A. Arsenic in Water and Its Impact on Mexican Health. Epistemus 2024, 18, 37. Available online: https://epistemus.unison.mx/index.php/epistemus/article/view/374 (accessed on 2 January 2025).
- Rahimi, B.; Shirvani, H.; Alamolhoda, A.A.; Farhadi, F.; Karimi, M. A Feasibility Study of Solar-Powered Reverse Osmosis Processes. Desalination 2021, 500, 114885. [Google Scholar] [CrossRef]
- Zahmatkesh, S.; Amesho, K.T.T.; Sillanpaa, M.; Wang, C. Integration of Renewable Energy in Wastewater Treatment During the COVID-19 Pandemic: Challenges, Opportunities, and Progressive Research Trends. Clean. Chem. Eng. 2022, 3, 100036. [Google Scholar] [CrossRef]
- Ad Hoc Group on Arsenic in Water. Arsenic in Water—Final Report; National Scientific and Technical Research Council (CONICET), Food Safety Network. 2018. Available online: https://rsa.conicet.gov.ar/wp-content/uploads/2018/08/Informe-Arsenico-en-agua-RSA.pdf (accessed on 2 January 2025).
- Gómez, J.A. Integration of Floating Photovoltaic Solar Energy and Water Supply Systems. CAVECON. 2020. Available online: https://cavecon.org.ve/wp-content/uploads/2020/09/Integracion-Energia-Solar-FV-flotante-y-sistemas-abastecimiento-de-agua.pdf (accessed on 2 January 2025).
- Esquivel-Mayorga, R.; Luna-Hernández, F.; Carrasco-Montoya, A.; Pavón-Silva, T.B. Arsenic Removal from Well Water Using an Electrochemical Reactor Powered by Direct Solar Energy. Trends Renew. Sustain. Energy 2023, 2, 387. [Google Scholar] [CrossRef]
- Ciencia y Desarrollo Journal. Study on Water Quality in the Tacna Region. Available online: https://revistas.unjbg.edu.pe/index.php/cyd/article/view/743 (accessed on 2 January 2025).
- National Water Authority (ANA). Technical Report on Water Contamination in the Tacna Region. Available online: https://repositorio.ana.gob.pe/bitstream/handle/20.500.12543/5465/ANA0004032_2.pdf (accessed on 2 January 2025).
- Diario Correo. Tacna: Population Consumes Water with High Arsenic Levels. Available online: https://diariocorreo.pe/edicion/tacna/tacna-poblacion-consume-agua-con-alto-niveles-de-arsenico-638243/ (accessed on 2 January 2025).
- National Institute of Statistics and Informatics (INEI). Population and Housing Census 2017: REDATAM System. Available online: https://censos2017.inei.gob.pe/redatam/ (accessed on 2 January 2025).
- Ministry of the Environment of Peru. Supreme Decree No. 004-2017-MINAM: Environmental Quality Standards (ECA) for Water. Available online: https://www.minam.gob.pe/wp-content/uploads/2017/06/DS-004-2017-MINAM.pdf (accessed on 10 March 2025).
- Ministry of Energy and Mines of Peru. National Rural Electrification Plan (PNER) 2024–2033. Available online: https://cdn.www.gob.pe/uploads/document/file/5644885/5003343-plan-nacional-de-electrificacion-rural-pner-2024-2033.pdf (accessed on 10 March 2025).
- Provincial Municipality of Tacna. 2024–2026 Gap Diagnosis Report. Available online: https://cdn.www.gob.pe/uploads/document/file/4638249/diagnostico%20de%20brechas%202024-2026.pdf (accessed on 10 March 2025).
- National Institute of Statistics and Informatics (INEI). Statistical Compendium, Tacna 2022. Available online: https://www.gob.pe/institucion/inei/informes-publicaciones/4134044-compendio-estadistico-tacna-2022 (accessed on 2 January 2025).
- Diario Correo. Only 15% of the Population Was Surveyed in Los Palos. Available online: https://diariocorreo.pe/edicion/tacna/solo-15-de-poblacion-fue-censada-en-los-palos-785169/ (accessed on 2 January 2025).
- Peña Laureano, F.; Cotrina Chávez, G.J.; Acosta Pereira, H. Hydrogeology of the Caplina River Basin—Tacna Region; Geological, Mining and Metallurgical Institute (INGEMMET): Lima, Peru, 2009; Available online: https://repositorio.ingemmet.gob.pe/handle/20.500.12544/368?locale=es (accessed on 2 January 2025).
- Huapaya AI, A.; Sotelo, P.C.; Benites, C.C.; Philipps, C.R.; Bejarano, G.V. Variation of Agricultural Area in the District of La Yarada Los Palos, Tacna, Peru. Espac. Desarro. 2020, 35, 99–120. Available online: https://revistas.pucp.edu.pe/index.php/espacioydesarrollo/article/view/23815 (accessed on 2 January 2025).
- Mohan, D.; Pittman, C.U. Arsenic Removal from Water/Wastewater Using Adsorbents—A Critical Review. J. Hazard. Mater. 2007, 142, 1–53. Available online: https://www.sciencedirect.com/science/article/abs/pii/S0304389407000349?via%3Dihub (accessed on 2 January 2025).
- Lozano Rivera, A.I. Experimental Study of Alternatives for the Oxidation of As(III) to As(V) in Leaching Solutions from Acid Plant Residues. Master’s Thesis, University of Concepción, Concepción, Chile, 2018. Available online: https://repositorio.udec.cl/items/a79bc3c3-f736-4cdd-a8e6-0af8077a79ca (accessed on 2 January 2025).
- Francisca, F.M.; Carro Pérez, M.E. Arsenic Removal from Water Using Coagulation-Flocculation Processes. Rev. Int. Contam. Ambient. 2014, 30, 149–157. Available online: https://www.scielo.org.mx/scielo.php?pid=S0188-49992014000200005&script=sci_arttext (accessed on 2 January 2025).
- Delaire, C.; Amrose, S.; Zhang, M.; Hake, J.; Gadgil, A. How do operating conditions affect As(III) removal by iron electrocoagulation? Water Res. 2017, 112, 185–194. [Google Scholar] [CrossRef]
- Gude, J.C.J.; Joris, K.; Huysman, K.; Rietveld, L.C.; van Halem, D. Effect of supernatant water level on As removal in biological rapid sand filters. Water Res. X 2018, 1, 100013. [Google Scholar] [CrossRef]
- Zheng, Q.; Hou, J.; Hartley, W.; Ren, L.; Wang, M.; Tu, S.; Tan, W. As(III) adsorption on Fe-Mn binary oxides: Are Fe and Mn oxides synergistic or antagonistic for arsenic removal? Chem. Eng. J. 2020, 389, 124470. [Google Scholar] [CrossRef]
- Cheng, Y.; Zhang, S.; Huang, T.; Li, Y. Arsenite removal from groundwater by iron–manganese oxides filter media: Behavior and mechanism. Water Environ. Res. 2019, 91, 1837–1844. [Google Scholar] [CrossRef]
- Bandaru, S.R.S.; van Genuchten, C.M.; Kumar, A.; Glade, S.; Hernández, D.; Nahata, M.; Gadgil, A. Rapid and efficient arsenic removal by iron electrocoagulation enabled with in situ generation of hydrogen peroxide. Environ. Sci. Technol. 2020, 54, 4474–4483. [Google Scholar] [CrossRef]
- Shafiquzzaman, M.; Ahsan, A.; Hasan, M.M.; Ahmed, T.A.; Bari, Q.H. The role of naturally occurring Fe(II) in removing arsenic from groundwater: Batch experiments and field studies. Water 2023, 15, 4081. [Google Scholar] [CrossRef]
- Khan, Z.H.; Gao, M.; Qiu, W.; Song, Z. Efficient As(III) removal by novel MoS2-impregnated Fe-oxide–biochar composites: Characterization and mechanisms. ACS Omega 2020, 5, 21878–21888. [Google Scholar] [CrossRef]
- Catrouillet, C.; Hirosue, S.; Manetti, N.; Boureau, V.; Peña, J. Coupled As and Mn redox transformations in an Fe(0) electrocoagulation system: Competition for reactive oxidants and sorption sites. Environ. Sci. Technol. 2020, 54, 411–420. [Google Scholar] [CrossRef]
- International Renewable Energy Agency (IRENA). World Energy Transitions Outlook 2024. Available online: https://www.irena.org/-/media/Files/IRENA/Agency/Publication/2024/Nov/IRENA_World_energy_transitions_outlook_2024.pdf (accessed on 2 January 2025).
- Morales Cabrera, D.U.; Avendaño Cáceres, E.; Zevallos Ramos, D.; Fernández Prado, J.; Mendoza Rodas, Z.L.; Torres Ventura, A. Undesired Total Arsenic at Reference pH Values in Surface Water, Sama Watershed, Tacna Region, Peru. Rev. Investig. Altoandin. 2017, 19, 305–312. Available online: https://www.researchgate.net/publication/320139801_Arsenico_total_no_deseado_ante_valores_referenciales_de_ph_en_agua_superficial_cuenca_hidrografica_sama_Region_Tacna-Peru/ (accessed on 2 January 2025).
- Ministry of the Environment. Manual of Good Practices: Groundwater; Ministry of the Environment: Lima, Perú, 2016; Available online: https://www.minam.gob.pe/calidadambiental/wp-content/uploads/sites/22/2018/08/MANUAL-DE-BUENAS-PRACTICAS-MUESTREO-AGUA.pdf (accessed on 2 January 2025).
- Hanna Instruments. User Manual for the HI 98194 Multiparameter Meter; Hanna Instruments: Woonsocket, RI, USA, 2019; Available online: https://www.hannainst.com/hubfs/product-manuals/MAN98194_05_19_crop.pdf (accessed on 2 January 2025).
- Lindemann, T.; Hamester, M.; Hinrichs, J.; Wills, J.D. High Sensitivity Arsenic Speciation: HPLC Sector Field ICP-MS; Thermo Fisher Scientific: Waltham, MA, USA, 2003; Available online: https://assets.thermofisher.com/TFS-Assets/CMD/Application-Notes/AN-30012-HPLC-Sector-Field-ICP-MS-Arsenic-Speciation-AN30012-EN.pdf (accessed on 2 January 2025).
- Komorowicz, I.; Barałkiewicz, D. Arsenic Speciation in Water by High-Performance Liquid Chromatography/Inductively Coupled Plasma Mass Spectrometry—Method Validation and Uncertainty Estimation. Rapid Commun. Mass Spectrom. 2014, 28, 159–168. [Google Scholar] [CrossRef]
- Bansal, R.C.; Goyal, M. Activated Carbon Adsorption; Taylor & Francis: Boca Raton, FL, USA, 2005; ISBN 9780824753443. [Google Scholar] [CrossRef]
- Servicio Nacional de Meteorología e Hidrología del Perú (SENAMHI). Atlas de Energía Solar del Perú; SENAMHI: Lima, Perú, 2003; Available online: https://repositorio.senamhi.gob.pe/handle/20.500.12542/343 (accessed on 2 January 2025).
- Tofigh Rihani, A.; Ghandchi, M. Increasing the Efficiency of Photovoltaic Systems by Using Maximum Power Point Tracking (MPPT). arXiv 2021, arXiv:2201.00403. [Google Scholar]
- Carmin Silva, E.R.; Tarma Inche, Y.N.; Larrea Cerna, C.O.; Neri Ayala, A.C. Photovoltaic Energy for Improving Energy Efficiency in Homes and Buildings: RSL. Llamkasun 2023, 4, 37–48. Available online: https://llamkasun.unat.edu.pe/index.php/revista/article/view/123 (accessed on 2 January 2025).
- World Health Organization. Guidelines for Drinking-Water Quality, 4th ed.; Incorporating the 1st Addendum; World Health Organization: Geneva, Switzerland, 2017; Available online: https://www.who.int/publications/i/item/9789241549950 (accessed on 2 January 2025).
- Water Institute. Optimization of Coagulation-Flocculation in Drinking Water Treatment Plants. Available online: https://institutodelagua.es/aguas-residuales/coagulacion-y-floculacion-en-aguas-residualesaguas-residuales/ (accessed on 2 January 2025).
- University and Society Journal. Energy Optimization in a Drinking Water Treatment Plant in Manabí Province, Ecuador. 2021. Available online: https://dialnet.unirioja.es/servlet/articulo?codigo=9709772 (accessed on 2 January 2025).
- Langmuir, I. The Adsorption of Gases on Plane Surfaces of Glass, Mica, and Platinum. J. Am. Chem. Soc. 1918, 40, 1361–1403. [Google Scholar] [CrossRef]
- González, B. Design and Analysis of Experiments; Faculty of Agronomy, Universidad de San Carlos de Guatemala: Ciudad de Guatemala, Guatemala, 2016; Available online: https://archive.org/details/diseno-y-analisis-de-experimentos-2016a-fausac (accessed on 2 January 2025).
- Chen, Y.; Wei, W.; Wang, C.; Shafie-khah, M.; Catalão, J.P.S. Storage and Transmission Capacity Requirements of a Remote Solar Power Generation System. arXiv 2021, arXiv:2109.05766. [Google Scholar] [CrossRef]
- Environmental Protection Agency (EPA). Water Treatment Manuals: Coagulation, Flocculation & Clarification. Available online: https://www.epa.ie/publications/compliance--enforcement/drinking-water/advice--guidance/water-treatment-manuals---coagulation-floculation--clarification.php (accessed on 2 January 2025).
- Mohammadi, K.; Mohammadi, M. Intelligent Optimization of a Hybrid Renewable Energy System-Powered Reverse Osmosis Water Desalination System: A Case Study. Int. J. Environ. Sci. Technol. 2020, 17, 3485–3500. Available online: https://link.springer.com/article/10.1007/s13762-020-03107-y (accessed on 2 January 2025).
- Barrientos, E. Arsenic Removal from Water Using Magnetic Nanoparticles Obtained by ACCVD. Momento 2013, 46, 37–44. Available online: https://revistas.unal.edu.co/index.php/momento/article/view/41554 (accessed on 2 January 2025).
- International Energy Agency (IEA). CO2 Emissions in 2023. 2024. Available online: https://www.iea.org/reports/co2-emissions-in-2023 (accessed on 2 January 2025).
- Bang, S.; Pena, M.E.; Patel, M.; Lippincott, L.; Meng, X. Removal of Arsenate from Water by Adsorbents: A Comparative Case Study. Environ. Geochem. Health 2011, 33, 133–141. Available online: https://link.springer.com/article/10.1007/s10653-010-9349-z?utm_source=chatgpt.com (accessed on 2 January 2025). [CrossRef] [PubMed]
- Machaca-Rodríguez, A.; Pizarro-Rabanal, J.C.; Cornejo-Ponce, L.; Morales-Cabrera, D.; Avendaño-Cáceres, E. Arsenic Removal from the Locumba River Water (Ite District, Tacna Region, Peru) Using Ferric Chloride. Rev. Soc. Quím. Perú 2022, 88, 333–350. Available online: https://revistas.sqperu.org.pe/index.php/revistasqperu/article/view/410 (accessed on 2 January 2025).
- Valenzuela-Antezana, R.N.; Yucra-Limahuaya, Y. Groundwater Quality Assessment of the Taparachi Industrial Park in Juliaca District. Ñawparisun 2022, 3, 4. Available online: https://unaj.edu.pe/revista/index.php/vpin/article/view/205 (accessed on 2 January 2025).
- Rojas-Chaves, P.; Vargas-Benavides, M.J.; Araya-Obando, A.; Valverde-Cerdas, J.; Romero-Esquivel, L.G. Study on Arsenic Removal in Drinking Water at the Household Level Using Solar Oxidation and Coagulation-Flocculation. Tecnol. Marcha 2015, 28, 54–65. Available online: https://revistas.tec.ac.cr/index.php/tec_marcha/article/view/2443 (accessed on 2 January 2025).
- Wang, Y.; Guo, C.; Zhang, L.; Lu, X.; Liu, Y.; Li, X.; Wang, Y.; Wang, S. Arsenic Oxidation and Removal from Water via Core–Shell MnO2@La(OH)3 Nanocomposite Adsorption. Int. J. Environ. Res. Public Health 2022, 19, 10649. [Google Scholar] [CrossRef]
- Zhu, H.; Shi, M.; Zhang, X.; Liu, B.; Yao, D. Adsorption Kinetics of Arsenic (V) on Nanoscale Zero-Valent Iron Supported by Activated Carbon. Nanomaterials 2020, 10, 1791. [Google Scholar] [CrossRef]
- Rathi, B.; Neidhardt, H.; Berg, M.; Siade, A.; Prommer, H. Processes governing arsenic retardation on Pleistocene sediments: Adsorption experiments and model-based analysis. Water Resour. Res. 2017, 53, 6453–6469. [Google Scholar] [CrossRef]
- Hilbrandt, I.; Lehmann, V.; Zietzschmann, F.; Ruhl, A.S.; Jekel, M. Quantification and isotherm modelling of competitive phosphate and silicate adsorption onto micro-sized granular ferric hydroxide. RSC Adv. 2019, 9, 29044–29054. [Google Scholar] [CrossRef]
- Kraal, P.; van Genuchten, C.M.; Behrends, T.; Rose, A.L. Sorption of phosphate and silicate alters dissolution kinetics of poorly crystalline iron (oxyhydr)oxide. Chemosphere 2019, 229, 541–550. [Google Scholar] [CrossRef]
- Mukhopadhyay, R.; Sarkar, B.; Barman, A.; Datta, S.C.; Manjaiah, K.M. Arsenic Adsorption on Modified Clay Minerals in Contaminated Soil and Water: Impact of pH and Competitive Anions. Clean Soil Air Water 2021, 49, 2000259. [Google Scholar] [CrossRef]
- Aftabtalab, A.; Moreno-Jiménez, E.; Henschel, J.; Nowak, S.; Schaller, J.; Knorr, K.-H. The Impact of Dissolved Organic Matter on Arsenic Mobilization from Goethite in the Presence of Silicic Acid and Phosphate under Reducing Conditions. Water 2022, 14, 2975. [Google Scholar] [CrossRef]
- Ministry of Energy and Mines of Peru. Technical Report Supporting the Expansion of Solar Trackers and Photovoltaic Modules at the Tacna Solar 20T Plant; Ministry of Energy and Mines of Peru: Lima, Peru, 2023; Available online: https://www.gob.pe/institucion/minem/informes-publicaciones/4955296-informe-tecnico-sustentatorio-para-la-ampliacion-de-seguidores-solares-y-modulos-fotovoltaicos-en-la-planta-solar-tacna-solar-20t (accessed on 10 March 2025).
- United States Environmental Protection Agency (EPA). Complying With the Revised Drinking Water Standard for Arsenic: Small Entity Compliance Guide. Available online: https://www.epa.gov/system/files/documents/2021-08/compliance-dw_arsenic.pdf (accessed on 2 January 2025).
- Valles-Aragón, M.C. Modeling Arsenic Removal Processes in Water Using Constructed Wetlands. Ph.D. Thesis, Advanced Materials Research Center (CIMAV), Chihuahua, Mexico, 2013. Available online: https://cimav.repositorioinstitucional.mx/jspui/handle/1004/2343 (accessed on 2 January 2025).
- International Renewable Energy Agency (IRENA). Renewable Energy Statistics 2024. Available online: https://www.irena.org/publications/2024/Jul/Renewable-Energy-Statistics-2024 (accessed on 2 January 2025).
- United States Environmental Protection Agency (EPA). Best Practices for Siting Solar Photovoltaics on Municipal Solid Waste Landfills. Available online: https://www.epa.gov/re-powering/best-practices-siting-solar-photovoltaics-municipal-solid-waste-landfills (accessed on 2 January 2025).
- Akgül, S.T.; Yıldıran, I.; Erdem, T.; Ateş, N.; Bekaroğlu, Ş.Ş.K. A Systematic Study on Adsorptive Removal of Arsenic Using Low-Cost Adsorbents with or Without the Presence of NOM. Int. J. Energy Stud. 2023, 8, 117–130. [Google Scholar] [CrossRef]
- Rahmani, A.; Khamutian, S.; Doosti-Irani, A.; Shokoohizadeh, M.J.; Shirmohammadi-Khorram, N.; Sahraeei, F.; Khodabakhshi, M.; Ahangaran, N. The association of arsenic exposure with mortality due to cancer, diabetes, Alzheimer’s and congenital anomalies using Poisson regression. Sci. Rep. 2023, 13, 15456. [Google Scholar] [CrossRef]
- Jat Baloch, M.Y.; Zhang, W.; Zhang, D.; Al Shoumik, B.A.; Iqbal, J.; Li, S.; Farooq, M.A.; Parkash, A. Evolution Mechanism of Arsenic Enrichment in Groundwater and Associated Health Risks in Southern Punjab, Pakistan. Int. J. Environ. Res. Public Health 2022, 19, 13325. [Google Scholar] [CrossRef]
- Podgorski, J.; Berg, M. Global threat of arsenic in groundwater. Science 2020, 368, 845–850. [Google Scholar] [CrossRef]
- Haldar, D.; Duarah, P.; Purkait, M.K. Metal–Organic Frameworks for the Treatment of Arsenic, Fluoride, and Iron Contaminated Drinking Water: A Review. Chemosphere 2020, 251, 126388. [Google Scholar] [CrossRef]
- Agency for Toxic Substances and Disease Registry (ATSDR). Toxicological Profile for Arsenic. Available online: https://www.atsdr.cdc.gov/toxprofiles/tp2.pdf (accessed on 2 January 2025).
- Mohammad, N.; Athar, M. Analytical Strategies for Arsenic Estimation. In Arsenic Toxicity: Challenges and Solutions; Springer: Singapore, 2020; pp. 123–145. Available online: https://link.springer.com/chapter/10.1007/978-981-15-2039-6_7 (accessed on 2 January 2025).
- Saint-Jacques, N.; Parker, L.; Brown, P.; Dummer, T.J.B. Arsenic in Drinking Water and Urinary Tract Cancers: A Systematic Review of 30 Years of Epidemiological Evidence. Environ. Health 2014, 13, 44. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency (EPA). Arsenic Compounds Hazard Summary. Available online: https://www.epa.gov/sites/default/files/2021-04/documents/arsenic_april_2021.pdf (accessed on 2 January 2025).
- World Health Organization (WHO). Safe Management of Wastes from Health-Care Activities, 2nd ed.; WHO Press: Geneva, Switzerland, 2014; Available online: https://www.who.int/publications/i/item/9789241548564 (accessed on 2 January 2025).
- Duffie, J.A.; Beckman, W.A. Solar Engineering of Thermal Processes, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- pS-Eau. Sludge at WWTPs: Global Trends of Treatment and Disposal/Reuse; pS-Eau: Paris, France, 2019; Available online: https://www.pseau.org/outils/ouvrages/world_bank_sludge_at_wwtps_global_trends_of_treatment_and_disposal_reuse_2019.pdf (accessed on 2 January 2025).
- Vasudevan, S.; Mohan, S.; Sozhan, G.; Raghavendran, N.S. Studies on the Oxidation of As(III) to As(V) by In-Situ-Generated Hypochlorite. Ind. Eng. Chem. Res. 2006, 45, 7726–7732. [Google Scholar] [CrossRef]
- Hug, S.J.; Canonica, L.; Wegelin, M.; Gechter, D.; von Gunten, U. Solar Oxidation and Removal of Arsenic at Circumneutral pH in Iron-Containing Waters. Environ. Sci. Technol. 2001, 35, 2114–2121. [Google Scholar] [CrossRef] [PubMed]
- Sorlini, S.; Gialdini, F. Conventional Oxidation Treatments for the Removal of Arsenic with Chlorine Dioxide, Hypochlorite, Potassium Permanganate, and Monochloramine. Water Res. 2010, 44, 5653–5659. [Google Scholar] [CrossRef] [PubMed]
- Clifford, D.; Ghurye, G. Oxidizing Arsenic III to Arsenic V for Better Removal. Watewater Digest. 2021. Available online: https://www.wwdmag.com/wastewater-treatment/article/10916990/oxidizing-arsenic-iii-to-arsenic-v-for-better-removal (accessed on 2 January 2025).
- Pires, V.G.R.; Lima, D.R.S.; Aquino, S.F.; Libânio, M. Evaluating Arsenic and Manganese Removal from Water by Chlorine Oxidation Followed by Clarification. Braz. J. Chem. Eng. 2015, 32, 355–367. [Google Scholar]
- Mori Sosa, L.J.P.; Morales Cabrera, D.U.; Florez Ponce De León, W.D.; Hinojosa Ramos, E.A.; Torres Ventura, A.Y. Study of Arsenic Contamination in the Caplina Basin, Tacna, Peru: Arsenite and Arsenate Analysis Using Inductively Coupled Plasma Mass Spectrometry and High-Performance Liquid Chromatography. Sustainability 2025, 17, 611. [Google Scholar] [CrossRef]
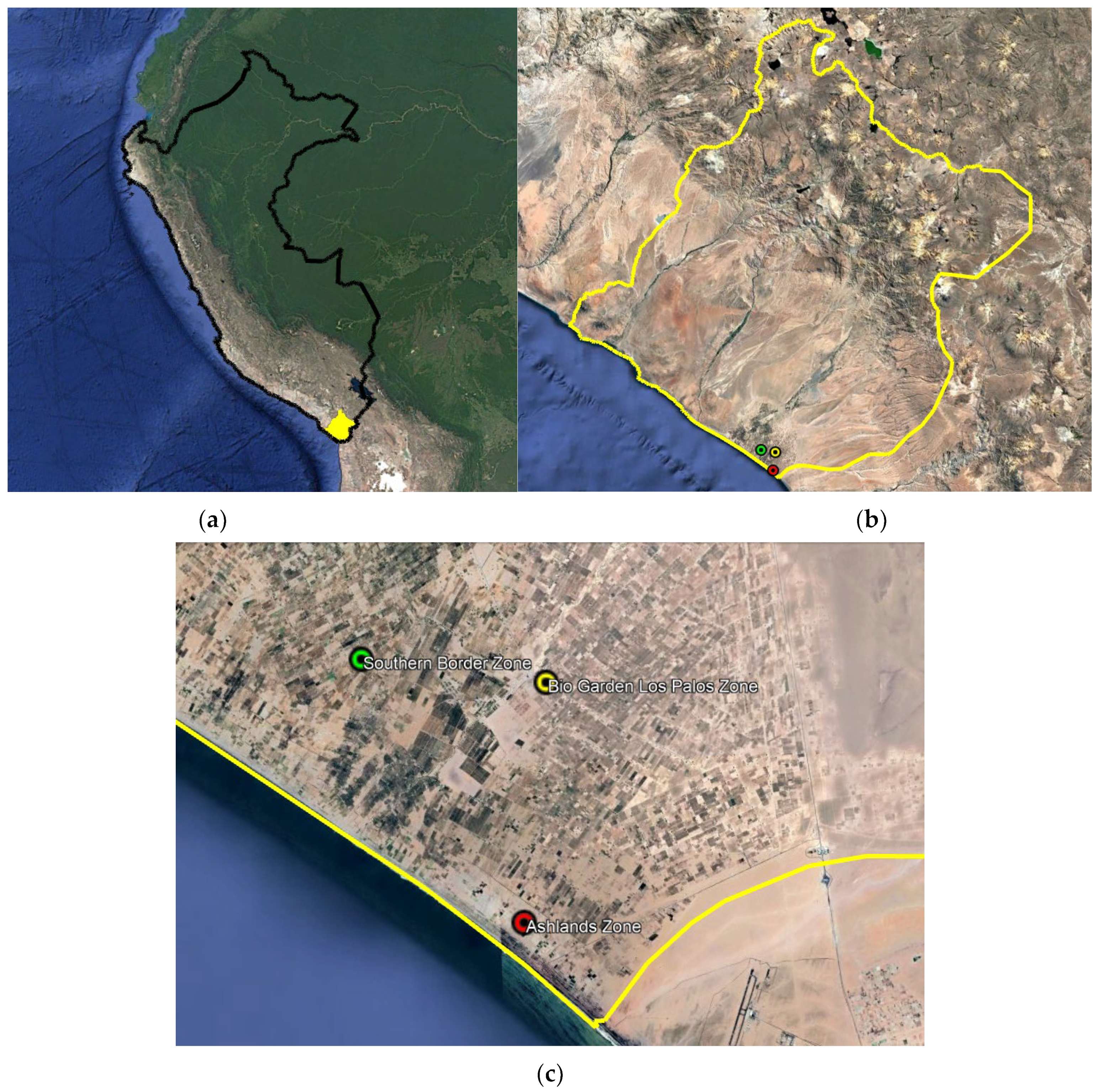




| UTM Coordinates WGS 84 | ||||
|---|---|---|---|---|
| Sampling Point | Zone | North | East | Altitude (m.a.s.l.) |
| Point 1 | Bio Garden Los Palos Zone | 353,151.00 m E | 7,980,461.00 m S | 68 |
| Point 2 | Southern Border Zone | 347,704.00 m E | 7,981,265.00 m S | 45 |
| Point 3 | Ashlands Zone | 352,342.00 m E | 7,973,450.00 m S | 19 |
| Sampling Point | Zone | Sample Code | Sampling Date | Temperature (°C) | pH | Conductivity (uS/cm) | TDS (mg/L) | TSS (mg/L) | Turbidity (NTU) |
|---|---|---|---|---|---|---|---|---|---|
| Point 1 | Bio Garden Los Palos Zone | SP1-1 | 10 February 2024 | 23.32 | 7.24 | 3418 | 1579 | 19 | 9 |
| SP1-2 | 15 February 2024 | 24.54 | 7.41 | 4732 | 2258 | 26 | 13 | ||
| SP1-3 | 20 February 2024 | 24.89 | 7.42 | 5031 | 2431 | 28 | 14 | ||
| SP1-4 | 26 February 2024 | 22.68 | 7.12 | 2684 | 1253 | 16 | 8 | ||
| SP1-5 | 4 March 2024 | 23.77 | 7.36 | 3975 | 1804 | 21 | 10 | ||
| SP1-6 | 11 March 2024 | 24.41 | 7.27 | 4593 | 2102 | 22 | 11 | ||
| SP1-7 | 21 March 2024 | 24.88 | 7.45 | 4849 | 2324 | 26 | 13 | ||
| SP1-8 | 28 March 2024 | 26.42 | 7.62 | 2815 | 1327 | 15 | 8 | ||
| SP1-9 | 2 April 2024 | 23.15 | 7.19 | 3296 | 1512 | 17 | 9 | ||
| SP1-10 | 8 April 2024 | 27.15 | 7.85 | 3452 | 1624 | 19 | 10 | ||
| SP1-11 | 13 April 2024 | 27.89 | 8.21 | 3518 | 1650 | 20 | 10 | ||
| Point 2 | Southern Border Zone | SP2-1 | 10 February 2024 | 28.04 | 8.02 | 2908 | 1386 | 16 | 9 |
| SP2-2 | 15 February 2024 | 28.12 | 8.25 | 3105 | 1456 | 18 | 9 | ||
| SP2-3 | 20 February 2024 | 26.54 | 7.81 | 2947 | 1381 | 16 | 8 | ||
| SP2-4 | 26 February 2024 | 25.88 | 7.76 | 3152 | 1495 | 18 | 9 | ||
| SP2-5 | 4 March 2024 | 26.89 | 7.94 | 3021 | 1415 | 15 | 8 | ||
| SP2-6 | 11 March 2024 | 27.65 | 8.14 | 3607 | 1690 | 20 | 10 | ||
| SP2-7 | 21 March 2024 | 28.05 | 8.15 | 3408 | 1601 | 19 | 10 | ||
| SP2-8 | 28 March 2024 | 22.85 | 7.16 | 2875 | 1334 | 17 | 9 | ||
| SP2-9 | 2 April 2024 | 22.75 | 7.11 | 2754 | 1286 | 16 | 8 | ||
| SP2-10 | 8 April 2024 | 23.57 | 7.35 | 3846 | 1746 | 21 | 11 | ||
| SP2-11 | 13 April 2024 | 25.92 | 7.72 | 2895 | 1378 | 16 | 8 | ||
| Point 3 | Ashlands Zone | SP3-1 | 10 February 2024 | 26.73 | 7.95 | 2984 | 1401 | 17 | 9 |
| SP3-2 | 15 February 2024 | 25.63 | 7.68 | 4135 | 1895 | 23 | 11 | ||
| SP3-3 | 20 February 2024 | 22.56 | 7.16 | 3067 | 1432 | 16 | 8 | ||
| SP3-4 | 26 February 2024 | 23.65 | 7.21 | 3728 | 1738 | 21 | 10 | ||
| SP3-5 | 4 March 2024 | 24.33 | 7.44 | 4973 | 2316 | 26 | 13 | ||
| SP3-6 | 11 March 2024 | 25.12 | 7.54 | 4512 | 2156 | 25 | 12 | ||
| SP3-7 | 21 March 2024 | 24.78 | 7.47 | 5238 | 2497 | 29 | 15 | ||
| SP3-8 | 28 March 2024 | 22.98 | 7.36 | 3152 | 1467 | 19 | 9 | ||
| SP3-9 | 2 April 2024 | 26.12 | 7.62 | 6723 | 3224 | 31 | 16 | ||
| SP3-10 | 8 April 2024 | 24.25 | 7.38 | 4289 | 2061 | 21 | 11 | ||
| SP3-11 | 13 April 2024 | 27.14 | 7.84 | 3275 | 1537 | 18 | 9 |
| Sampling Point | Zone | Sample Code | Sampling Date | Total As (mg/L) | Organic As | Inorganic As | |||
|---|---|---|---|---|---|---|---|---|---|
| AB (mg/L) | DMA (mg/L) | MMA (mg/L) | As(III) (mg/L) | As(V) (mg/L) | |||||
| Point 1 | Bio Garden Los Palos Zone | SP1-1 | 10 February 2024 | 0.0094 | 0.0024 | 0.0013 | 0.0022 | 0.0013 | 0.0022 |
| SP1-2 | 15 February 2024 | 0.0111 | 0.0003 | 0.0040 | 0.0023 | 0.0027 | 0.0018 | ||
| SP1-3 | 20 February 2024 | 0.0110 | 0.0011 | 0.0003 | 0.0003 | 0.0003 | 0.0090 | ||
| SP1-4 | 26 February 2024 | 0.0085 | 0.0014 | 0.0017 | 0.0011 | 0.0024 | 0.0019 | ||
| SP1-5 | 4 March 2024 | 0.0099 | 0.0016 | 0.0018 | 0.0014 | 0.0030 | 0.0021 | ||
| SP1-6 | 11 March 2024 | 0.0124 | 0.0033 | 0.0019 | 0.0015 | 0.0031 | 0.0026 | ||
| SP1-7 | 21 March 2024 | 0.0105 | 0.0030 | 0.0017 | 0.0015 | 0.0024 | 0.0019 | ||
| SP1-8 | 28 March 2024 | 0.0098 | 0.0020 | 0.0017 | 0.0015 | 0.0027 | 0.0019 | ||
| SP1-9 | 2 April 2024 | 0.0071 | 0.0014 | 0.0012 | 0.0010 | 0.0021 | 0.0014 | ||
| SP1-10 | 8 April 2024 | 0.0102 | 0.0019 | 0.0016 | 0.0013 | 0.0032 | 0.0022 | ||
| SP1-11 | 13 April 2024 | 0.0095 | 0.0020 | 0.0017 | 0.0013 | 0.0026 | 0.0019 | ||
| Point 2 | Southern Border Zone | SP2-1 | 10 February 2024 | 0.0087 | 0.0018 | 0.0015 | 0.0013 | 0.0023 | 0.0018 |
| SP2-2 | 15 February 2024 | 0.0072 | 0.0014 | 0.0013 | 0.0011 | 0.0019 | 0.0015 | ||
| SP2-3 | 20 February 2024 | 0.0094 | 0.0019 | 0.0017 | 0.0014 | 0.0024 | 0.0020 | ||
| SP2-4 | 26 February 2024 | 0.0077 | 0.0016 | 0.0013 | 0.0011 | 0.0021 | 0.0016 | ||
| SP2-5 | 4 March 2024 | 0.0088 | 0.0017 | 0.0015 | 0.0013 | 0.0024 | 0.0019 | ||
| SP2-6 | 11 March 2024 | 0.0092 | 0.0019 | 0.0016 | 0.0014 | 0.0024 | 0.0019 | ||
| SP2-7 | 21 March 2024 | 0.0097 | 0.0019 | 0.0017 | 0.0014 | 0.0026 | 0.0021 | ||
| SP2-8 | 28 March 2024 | 0.0085 | 0.0016 | 0.0014 | 0.0012 | 0.0019 | 0.0024 | ||
| SP2-9 | 2 April 2024 | 0.0088 | 0.0018 | 0.0015 | 0.0013 | 0.0023 | 0.0019 | ||
| SP2-10 | 8 April 2024 | 0.0092 | 0.0018 | 0.0016 | 0.0014 | 0.0024 | 0.0020 | ||
| SP2-11 | 13 April 2024 | 0.0081 | 0.0016 | 0.0014 | 0.0012 | 0.0022 | 0.0017 | ||
| Point 3 | Ashlands Zone | SP3-1 | 10 February 2024 | 0.0086 | 0.0017 | 0.0015 | 0.0013 | 0.0023 | 0.0018 |
| SP3-2 | 15 February 2024 | 0.0105 | 0.0016 | 0.0019 | 0.0017 | 0.0030 | 0.0023 | ||
| SP3-3 | 20 February 2024 | 0.0134 | 0.0027 | 0.0023 | 0.0020 | 0.0035 | 0.0029 | ||
| SP3-4 | 26 February 2024 | 0.0183 | 0.0043 | 0.0051 | 0.0054 | 0.0022 | 0.0013 | ||
| SP3-5 | 4 March 2024 | 0.0250 | 0.0043 | 0.0007 | 0.0001 | 0.0116 | 0.0083 | ||
| SP3-6 | 11 March 2024 | 0.0101 | 0.0015 | 0.0018 | 0.0016 | 0.0030 | 0.0022 | ||
| SP3-7 | 21 March 2024 | 0.0271 | 0.0059 | 0.0047 | 0.0046 | 0.0010 | 0.0109 | ||
| SP3-8 | 28 March 2024 | 0.0147 | 0.0031 | 0.0022 | 0.0017 | 0.0045 | 0.0032 | ||
| SP3-9 | 2 April 2024 | 0.0417 | 0.0071 | 0.0081 | 0.0060 | 0.0118 | 0.0087 | ||
| SP3-10 | 8 April 2024 | 0.0125 | 0.0033 | 0.0020 | 0.0016 | 0.0032 | 0.0024 | ||
| SP3-11 | 13 April 2024 | 0.0105 | 0.0023 | 0.0017 | 0.0015 | 0.0030 | 0.0020 | ||
| Sampling Date | Influent Condition | Temperature (°C) | pH | Conductivity (uS/cm) | Turbidity (NTU) | TSS (mg/L) |
|---|---|---|---|---|---|---|
| 1 June 2024 | Without Pretreatment | 25.24 | 7.48 | 5832 | 15 | 29 |
| 1 June 2024 | With Pretreatment | 25.30 | 7.45 | 5800 | 5 | 9 |
| 15 June 2024 | Without Pretreatment | 23.11 | 7.18 | 3387 | 10 | 19 |
| 15 June 2024 | With Pretreatment | 23.15 | 7.2 | 3370 | 4 | 6 |
| 1 July 2024 | Without Pretreatment | 24.12 | 7.41 | 4672 | 12 | 24 |
| 1 July 2024 | With Pretreatment | 24.05 | 7.38 | 4650 | 6 | 10 |
| 15 July 2024 | Without Pretreatment | 23.89 | 7.22 | 4517 | 11 | 23 |
| 15 July 2024 | With Pretreatment | 23.95 | 7.19 | 4500 | 4 | 7 |
| 1 August 2024 | Without Pretreatment | 25.54 | 7.67 | 6218 | 14 | 28 |
| 1 August 2024 | With Pretreatment | 25.60 | 7.65 | 6200 | 5 | 9 |
| 15 August 2024 | Without Pretreatment | 23.87 | 7.35 | 4892 | 13 | 27 |
| 15 August 2024 | With Pretreatment | 23.85 | 7.37 | 4870 | 5 | 8 |
| Sampling Date | Influent Condition | As Cinitial (mg/L) | As Cfinal (mg/L) | Removal Efficiency (%) | Fe (mg/L) |
|---|---|---|---|---|---|
| 1 June 2024 | Without Pretreatment | 0.0323 | 0.0047 | 85.50 | 1.92 |
| 1 June 2024 | With Pretreatment | 0.0274 | 0.0001 | 99.78 | 0.91 |
| 15 June 2024 | Without Pretreatment | 0.0157 | 0.0022 | 86.30 | 1.57 |
| 15 June 2024 | With Pretreatment | 0.0133 | <0.0001 | 99.72 | 0.85 |
| 1 July 2024 | Without Pretreatment | 0.0241 | 0.0031 | 87.10 | 1.64 |
| 1 July 2024 | With Pretreatment | 0.0200 | <0.0001 | 99.85 | 0.78 |
| 15 July 2024 | Without Pretreatment | 0.0212 | 0.0026 | 87.60 | 1.59 |
| 15 July 2024 | With Pretreatment | 0.0180 | <0.0001 | 99.78 | 0.71 |
| 1 August 2024 | Without Pretreatment | 0.0344 | 0.0041 | 88.20 | 1.89 |
| 1 August 2024 | With Pretreatment | 0.0293 | <0.0001 | 99.83 | 0.97 |
| 15 August 2024 | Without Pretreatment | 0.0302 | 0.0034 | 88.90 | 1.68 |
| 15 August 2024 | With Pretreatment | 0.0257 | <0.0001 | 99.85 | 0.87 |
| Groups | Count | Sum | Mean | Variance |
|---|---|---|---|---|
| Removal Efficiency (%) Without Pretreatment | 6 | 523.60 | 87.27 | 1.55 |
| Removal Efficiency (%) With Pretreatment | 6 | 598.81 | 99.80 | 0.0026 |
| Source of Variation | Sum of Squares | Degrees of Freedom | Mean Square | F | Probability | Critical Value for F |
|---|---|---|---|---|---|---|
| Between Groups | 471.38 | 1 | 471.38 | 608.51 | p < 0.001 | 4.96 |
| Within Groups | 7.75 | 10 | 0.77 | - | - | - |
| Total | 479.13 | 11 | - | - | - | - |
| Average Parameter | Energy Consumption of Backwash (kWh) | Frequency (per Month) | Number of Months | Total Energy Consumption in the Backwash Process (kWh) |
|---|---|---|---|---|
| Without Pretreatment | 2.54 | 6 | 3 | 45.72 |
| With Pretreatment | 2.54 | 4 | 3 | 30.48 |
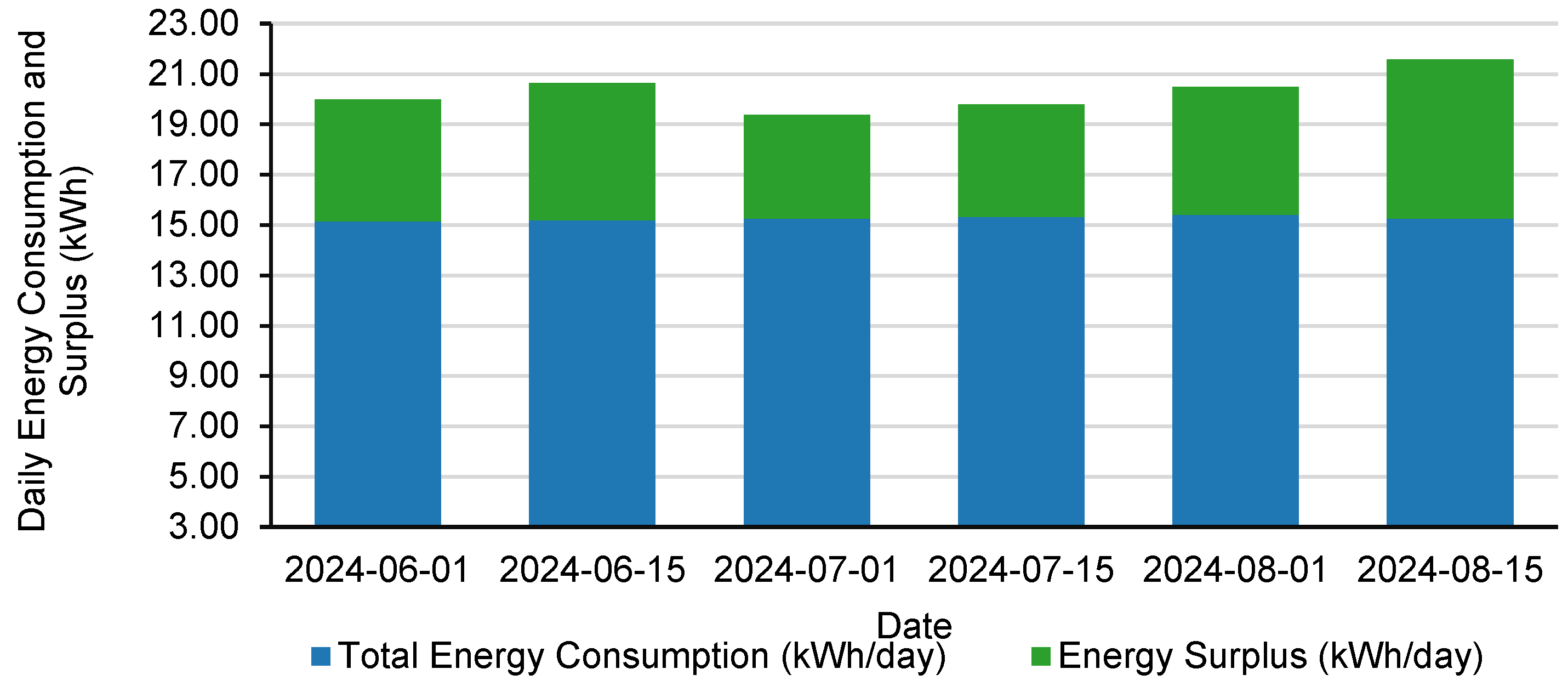
| Date | Solar Radiation (W/m2) | Total Energy Consumption (kWh/Day) | Energy Generation (kWh/Day) | Energy Surplus (kWh/Day) | Energy Surplus (%) |
|---|---|---|---|---|---|
| 1 June 2024 | 1001 | 15.15 | 19.98 | 4.83 | 31.89% |
| 15 June 2024 | 1034 | 15.19 | 20.64 | 5.45 | 35.88% |
| 1 July 2024 | 991 | 15.25 | 19.38 | 4.13 | 27.09% |
| 15 July 2024 | 1012 | 15.32 | 19.79 | 4.47 | 29.18% |
| 1 August 2024 | 1049 | 15.41 | 20.49 | 5.08 | 32.95% |
| 15 August 2024 | 1080 | 15.25 | 21.58 | 6.33 | 41.51% |
| Period | Total Monthly Generation (kWh) | Monthly Avoided Emissions (tCO2) | Global Standard Emissions (tCO2) | Reduction Percentage (%) |
|---|---|---|---|---|
| June 24 | 40.62 | 0.016 | 0.03656 | 44.34% |
| July 24 | 39.17 | 0.016 | 0.03525 | 44.53% |
| August 24 | 42.07 | 0.017 | 0.03786 | 44.37% |
| Sampling Date | Influent Condition | Total Arsenic Concentration Before Treatment (mg/L) | Total Arsenic Concentration After Treatment (mg/L) | Arsenic Species After Treatment | Arsenic Removal Efficiency (%) | |
|---|---|---|---|---|---|---|
| As(III) (mg/L) | As(V) (mg/L) | |||||
| 1 June 2024 | Without Pretreatment | 0.0323 | 0.0047 | <0.0001 | 0.0010 | 85.50 |
| 1 June 2024 | With Pretreatment | 0.0274 | 0.0001 | <0.0001 | <0.0001 | 99.78 |
| 15 June 2024 | Without Pretreatment | 0.0157 | 0.0022 | <0.0001 | 0.0004 | 86.30 |
| 15 June 2024 | With Pretreatment | 0.0133 | <0.0001 | <0.0001 | <0.0001 | 99.72 |
| 1 July 2024 | Without Pretreatment | 0.0241 | 0.0031 | <0.0001 | 0.0006 | 87.10 |
| 1 July 2024 | With Pretreatment | 0.0200 | <0.0001 | <0.0001 | <0.0001 | 99.85 |
| 15 July 2024 | Without Pretreatment | 0.0212 | 0.0026 | <0.0001 | 0.0005 | 87.60 |
| 15 July 2024 | With Pretreatment | 0.0180 | <0.0001 | <0.0001 | <0.0001 | 99.78 |
| 1 August 2024 | Without Pretreatment | 0.0344 | 0.0041 | <0.0001 | 0.0008 | 88.20 |
| 1 August 2024 | With Pretreatment | 0.0293 | <0.0001 | <0.0001 | <0.0001 | 99.83 |
| 15 August 2024 | Without Pretreatment | 0.0302 | 0.0034 | <0.0001 | 0.0006 | 88.90 |
| 15 August 2024 | With Pretreatment | 0.0257 | <0.0001 | <0.0001 | <0.0001 | 99.85 |
| Parameter | F | p-Value | Average with Pretreatment | Average without Pretreatment |
|---|---|---|---|---|
| Removal Efficiency (%) | 486.32 | <0.001 | 99.80 | 87.27 |
| Total Energy Consumption in the Backwash Process (kWh) | 15.27 | <0.001 | 30.48 | 45.72 |
| Date | Solar Radiation (W/m2) | Temperature (°C) |
|---|---|---|
| 1 January 2024 | 1193 | 26.8 |
| 15 January 2024 | 1189 | 26.5 |
| 1 February 2024 | 1181 | 26.0 |
| 15 February 2024 | 1172 | 25.5 |
| 1 March 2024 | 1165 | 24.8 |
| 15 March 2024 | 1145 | 24.0 |
| 1 April 2024 | 1117 | 22.5 |
| 15 April 2024 | 1103 | 21.8 |
| 1 May 2024 | 1078 | 17.5 |
| 15 May 2024 | 1045 | 17.2 |
| 1 June 2024 | 1001 | 15.3 |
| 15 June 2024 | 1034 | 16.5 |
| 1 July 2024 | 991 | 12.5 |
| 15 July 2024 | 1012 | 13.2 |
| 1 August 2024 | 1049 | 14.8 |
| 15 August 2024 | 1054 | 15.2 |
| 1 September 2024 | 1066 | 16.0 |
| 15 September 2024 | 1070 | 16.8 |
| 1 October 2024 | 1079 | 18.5 |
| 15 October 2024 | 1083 | 19.0 |
| 1 November 2024 | 1173 | 23.5 |
| 15 November 2024 | 1181 | 24.8 |
| 1 December 2024 | 1187 | 25.5 |
| 15 December 2024 | 1191 | 26.2 |
| Date | Ambient Temperature (°C) | Solar Radiation (W/m2) | Energy Generation (kWh/day) |
|---|---|---|---|
| 1 June 2024 | 28.00 | 1001 | 19.98 |
| 15 June 2024 | 28.50 | 1034 | 20.64 |
| 1 July 2024 | 27.30 | 991 | 19.38 |
| 15 July 2024 | 27.50 | 1012 | 19.79 |
| 1 August 2024 | 29.10 | 1049 | 20.49 |
| 15 August 2024 | 29.50 | 1080 | 21.58 |
| As Initial (mg/L) | pH | Turbidity (NTU) | TSS (mg/L) | Fe2(SO4)3 Dose (mg/L) | Removal Efficiency (%) | Pretreatment | S As | S pH | S Turbidity | S TSS | S Dose |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0.032 | 7.48 | 15 | 29 | 16 | 85.50 | No | N/A | N/A | N/A | N/A | N/A |
| 0.027 | 7.45 | 5 | 9 | 12 | 99.78 | Yes | −1.101 | −41.643 | −0.251 | −0.242 | −0.668 |
| 0.016 | 7.18 | 10 | 19 | 14 | 86.30 | No | 0.316 | 3.728 | −0.135 | −0.122 | −0.811 |
| 0.013 | 7.2 | 4 | 6 | 10 | 99.72 | Yes | −1.017 | 55.826 | −0.259 | −0.227 | −0.544 |
| 0.024 | 7.41 | 12 | 24 | 15 | 87.10 | No | −0.156 | −4.339 | −0.063 | −0.042 | −0.253 |
| 0.02 | 7.38 | 6 | 10 | 12 | 99.85 | Yes | −0.860 | −36.157 | −0.293 | −0.251 | −0.732 |
| 0.021 | 7.22 | 11 | 23 | 15 | 87.60 | No | −2.045 | 5.659 | −0.147 | −0.094 | −0.491 |
| 0.018 | 7.19 | 4 | 7 | 10 | 99.78 | Yes | −0.921 | −33.463 | −0.218 | −0.200 | −0.417 |
| 0.034 | 7.67 | 14 | 28 | 17 | 88.20 | No | −0.127 | −1.738 | −0.046 | −0.039 | −0.166 |
| 0.029 | 7.65 | 5 | 9 | 12 | 99.83 | Yes | −0.889 | −50.568 | −0.205 | −0.194 | −0.448 |
| 0.03 | 7.35 | 13 | 27 | 16 | 88.90 | No | −3.564 | 2.792 | −0.068 | −0.055 | −0.328 |
| 0.026 | 7.37 | 5 | 8 | 12 | 99.85 | Yes | −0.827 | 45.266 | −0.200 | −0.175 | −0.493 |
| Implementation Costs | Cost (USD) |
|---|---|
| 5 kW Photovoltaic System | 12,000.00 |
| |
| |
| |
| |
| Natural Sedimentation Pretreatment | 14,200.00 |
| |
| Water Treatment System (0.5 m3/h) | 15,500.00 |
| |
| Water Treatment System Consumables | 4500.00 |
| |
| |
| |
| |
| |
| |
| Total Implementation Costs | 46,200.00 |
| Annual Operation and Maintenance Costs | Cost (USD) |
|---|---|
| Operation and Maintenance of Photovoltaic System | 1500.00 |
| |
| Operation and Maintenance of Water Treatment System | 2500.00 |
| |
| Replacement of Water Treatment System Consumables | 4500.00 |
| Sludge Management | 2000.00 |
| Total Annual Operation and Maintenance Costs | 10,500.00 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mori Sosa, L.J.P. Efficiency Evaluation of a Photovoltaic-Powered Water Treatment System with Natural Sedimentation Pretreatment for Arsenic Removal in High Water Vulnerability Areas: Application in La Yarada Los Palos District, Tacna, Peru. Sustainability 2025, 17, 2987. https://doi.org/10.3390/su17072987
Mori Sosa LJP. Efficiency Evaluation of a Photovoltaic-Powered Water Treatment System with Natural Sedimentation Pretreatment for Arsenic Removal in High Water Vulnerability Areas: Application in La Yarada Los Palos District, Tacna, Peru. Sustainability. 2025; 17(7):2987. https://doi.org/10.3390/su17072987
Chicago/Turabian StyleMori Sosa, Luis Johnson Paúl. 2025. "Efficiency Evaluation of a Photovoltaic-Powered Water Treatment System with Natural Sedimentation Pretreatment for Arsenic Removal in High Water Vulnerability Areas: Application in La Yarada Los Palos District, Tacna, Peru" Sustainability 17, no. 7: 2987. https://doi.org/10.3390/su17072987
APA StyleMori Sosa, L. J. P. (2025). Efficiency Evaluation of a Photovoltaic-Powered Water Treatment System with Natural Sedimentation Pretreatment for Arsenic Removal in High Water Vulnerability Areas: Application in La Yarada Los Palos District, Tacna, Peru. Sustainability, 17(7), 2987. https://doi.org/10.3390/su17072987






