Comparison of Crude Soybean Urease- and Pure Urease-Induced Carbonate Precipitation on Wind-Induced Erosion Resistance of Desert Sand
Abstract
1. Introduction
2. Materials and Methods
2.1. Urease Solution
2.2. Desert Sand
2.3. Test Program and Sample Preparation
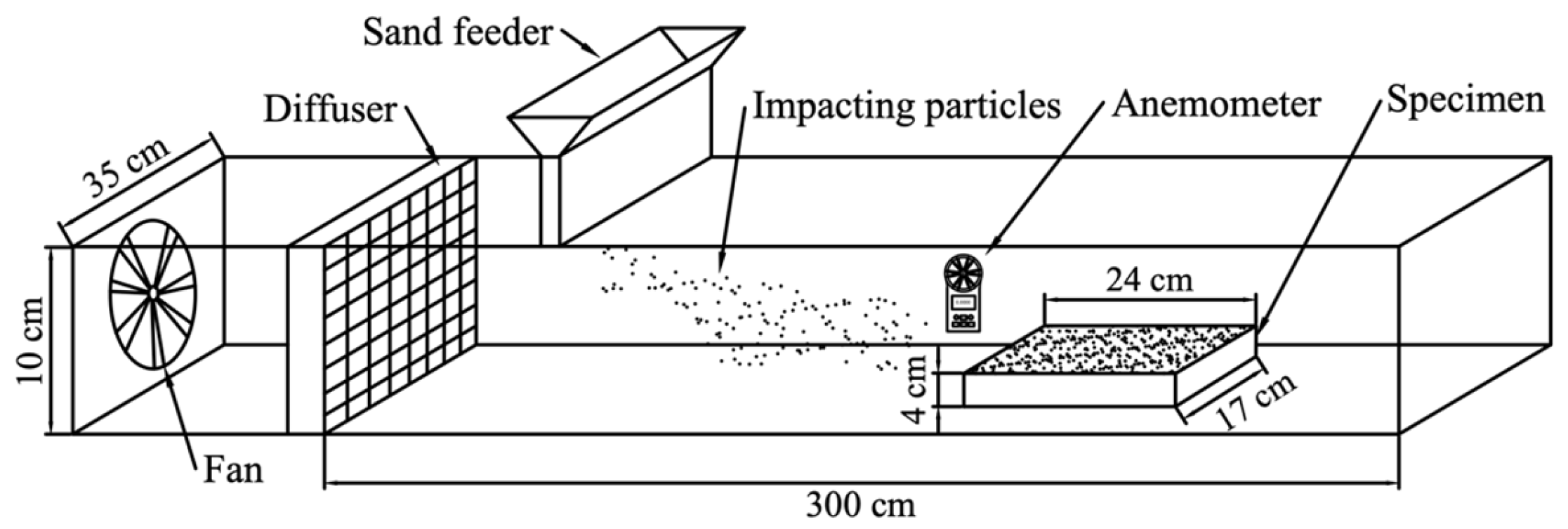
2.4. Wind Erosion Test
2.5. Measurement of CaCO3 and Organic Matter Contents in the Soil
3. Results
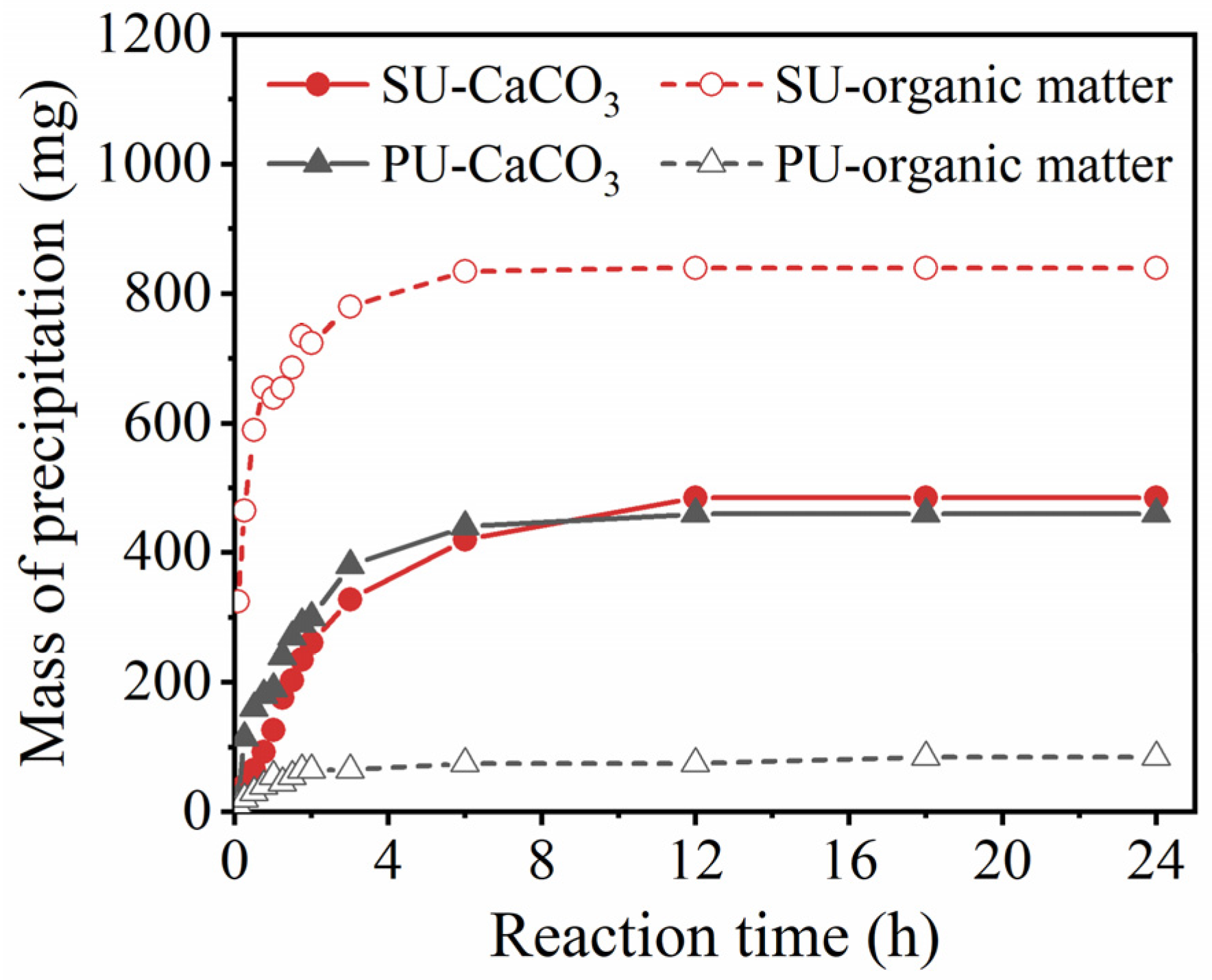
3.1. Precipitation Rates of CaCO3 and Organic Matter in the Tube Tests
3.2. Organic Matter Precipitation Distribution in Soil
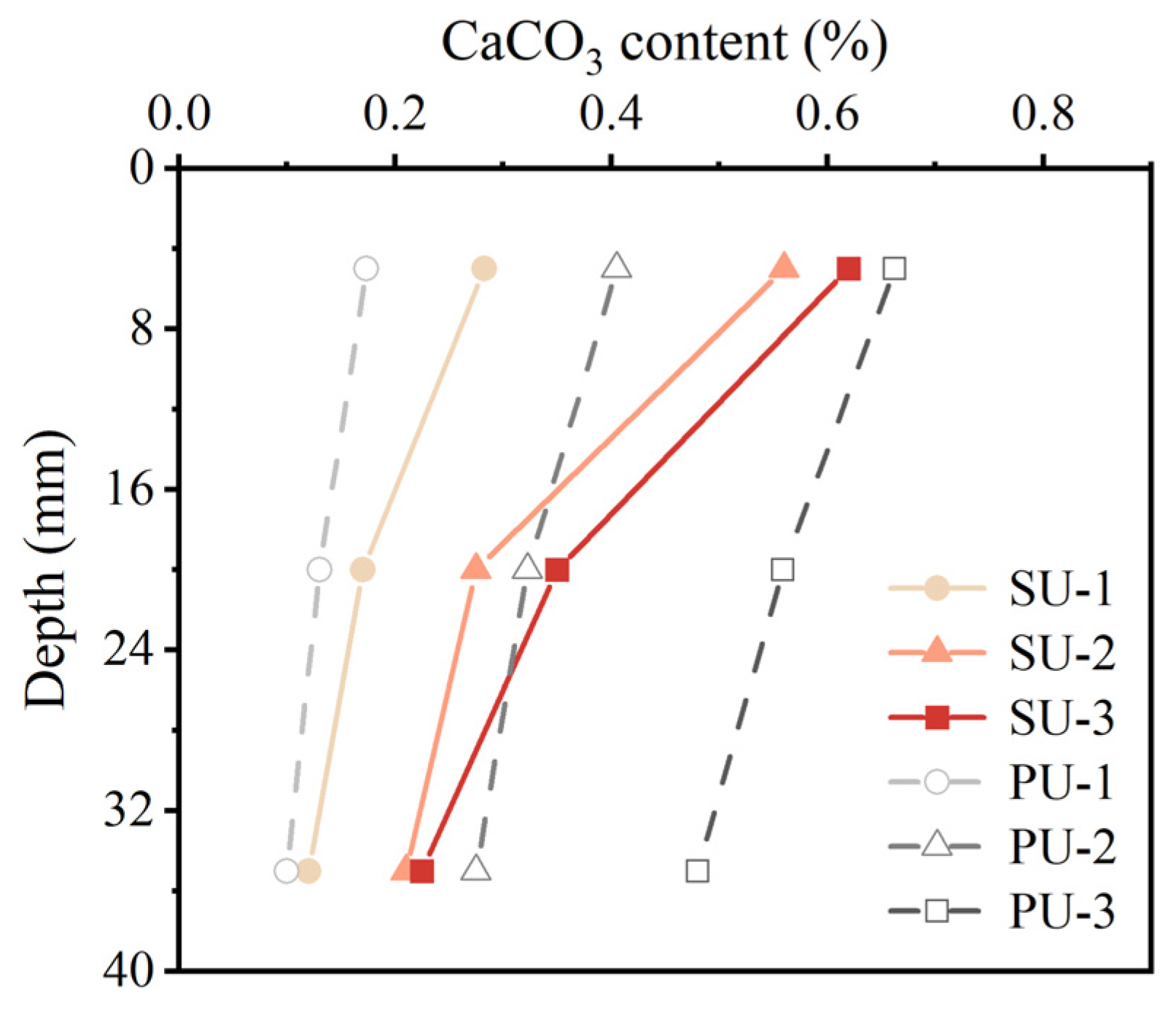
3.3. CaCO3 Precipitation Distribution in Soil
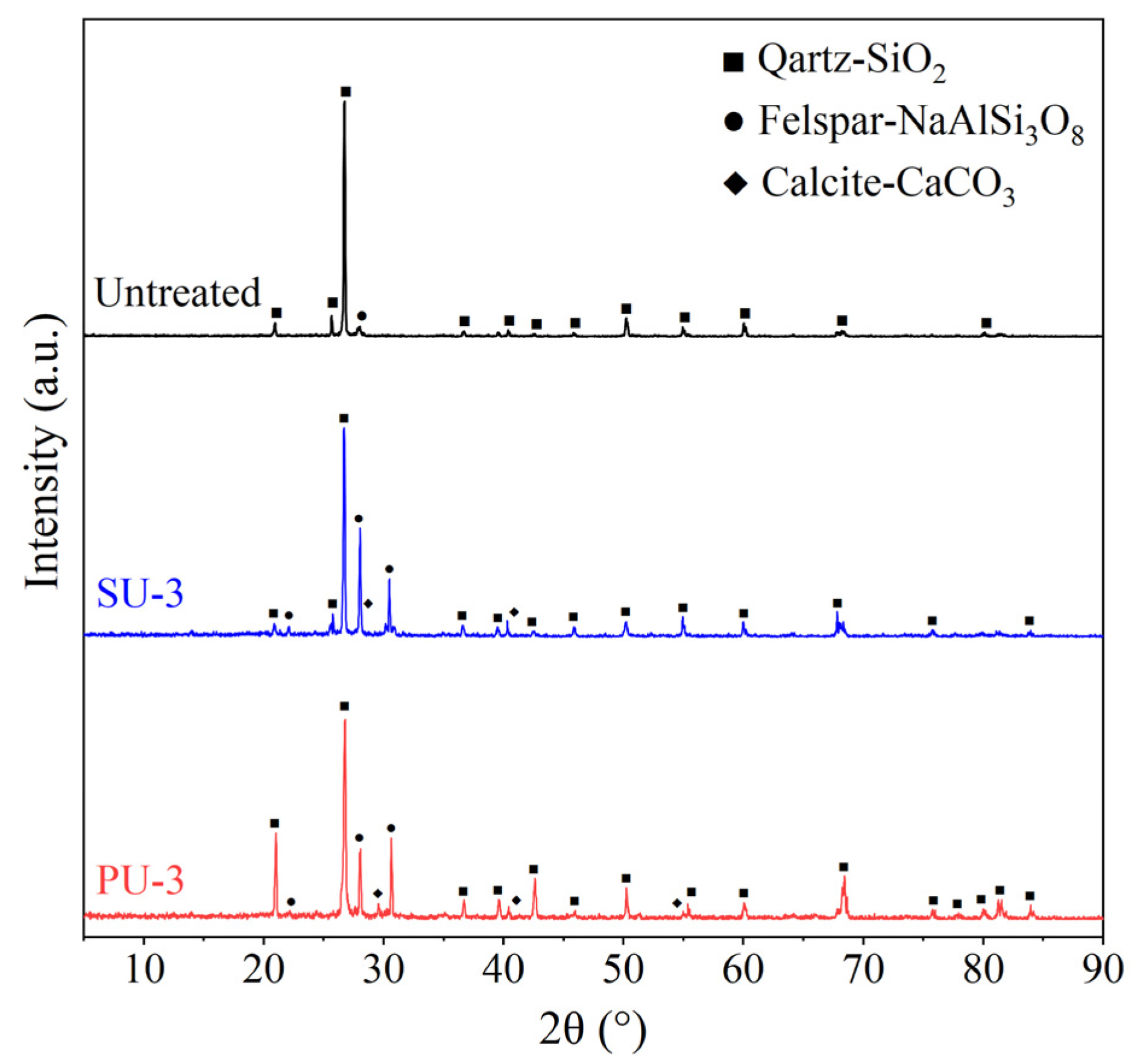
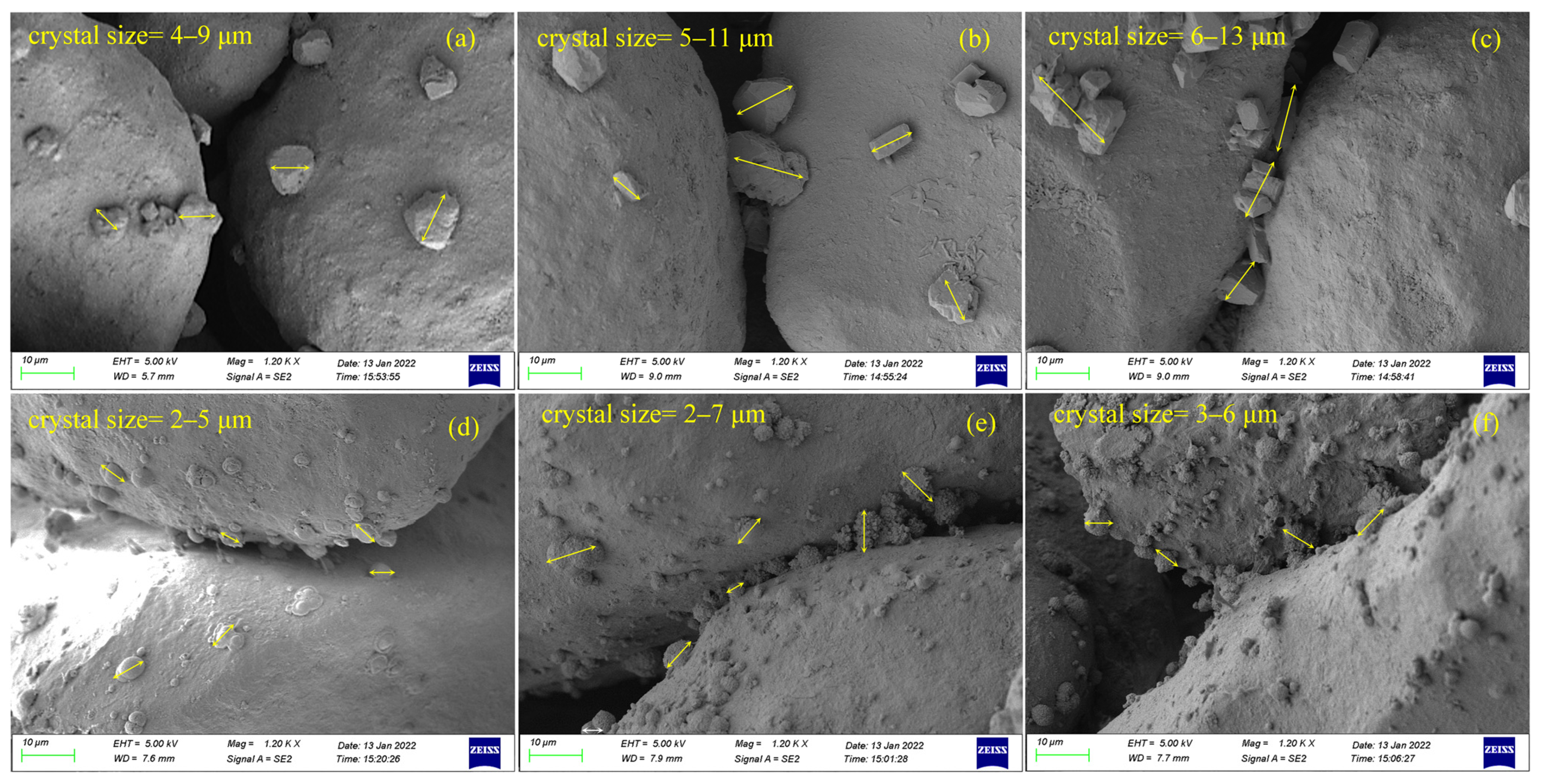
3.4. Microstructure of EICP-Treated Soil
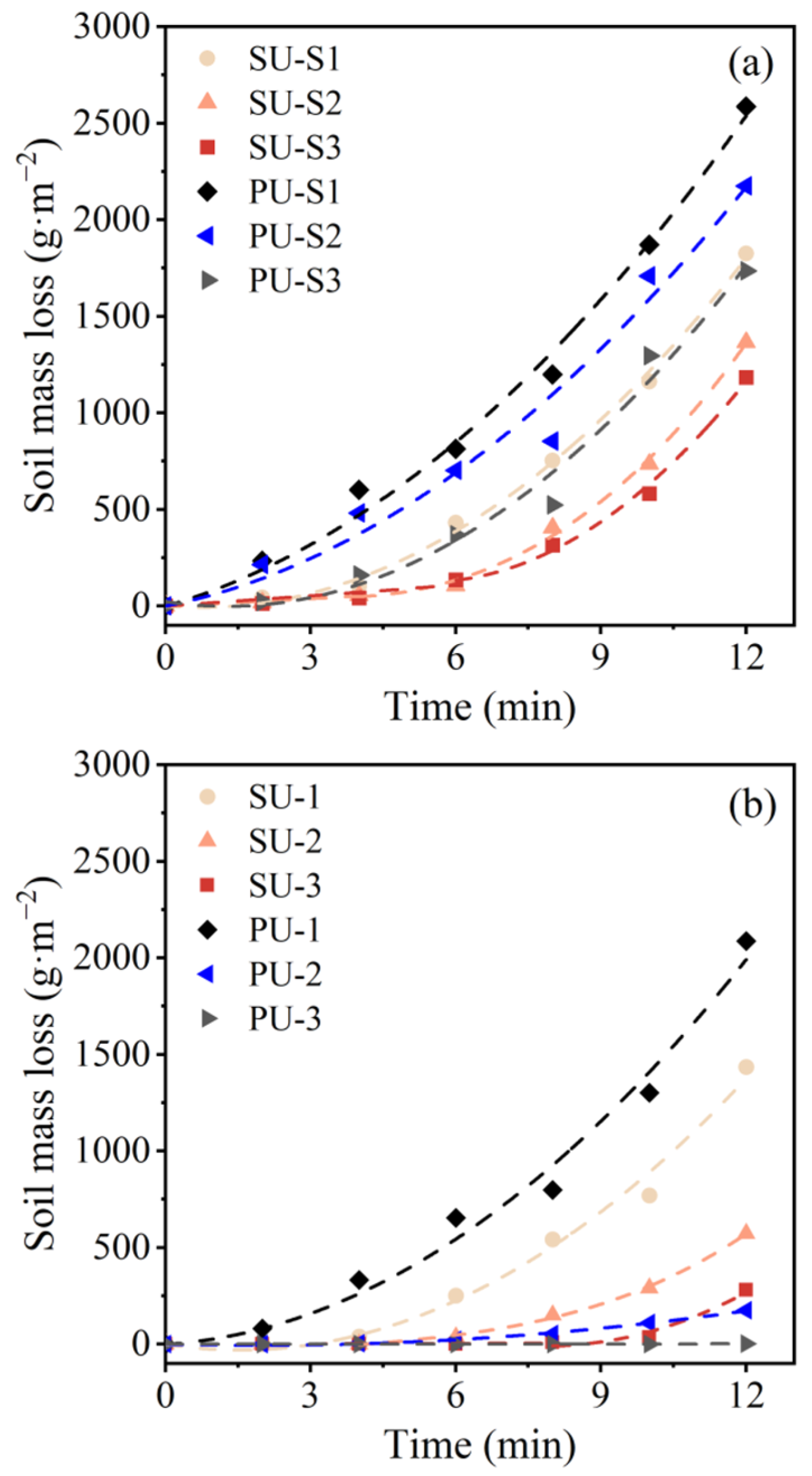
3.5. Wind Erosion Resistance
4. Conclusions
- (1)
- With the same enzyme activity of 5 mmol·L−1·min−1, more organic matter in SU was introduced into the soil than with PU. Due to the salting-out effect, the organic matter in SU rapidly precipitated as the urease solution mixed with the cementation solution. The mass of organic matter precipitation produced by SU was 9.88 times higher than that produced by PU.
- (2)
- The spatial distribution of CaCO3 in sand treated with SU and PU was different. SU-treated sand under multiple cycle treatment had significant non-uniformity in CaCO3 distribution. This was due to the relatively high viscosity of SU and the rapid precipitation of organic matter in the soil pores, which reduced the permeability of the top layer of SU-treated sand.
- (3)
- There were significant differences in the morphology and size of CaCO3 crystals in PU-treated specimens and SU-treated specimens. The CaCO3 crystals in PU-treated specimens were rhombic and became larger gradually with the increase in the number of treatment cycles. The relatively high concentration of organic matter in SU influenced the morphology and growth of CaCO3 crystals, resulting in spherical crystals in SU-treated specimens and the crystal size remained almost unchanged under multiple cycle treatment.
- (4)
- Under a single EICP-treatment cycle, SU is recommended to enhance the wind erosion resistance of desert sand. SU can induce more CaCO3 and organic matter precipitation in soil than PU, and the organic matter in soil can also act as part of the cement, leading to lower erosion mass. However, under multiple EICP-treatment cycles, the high concentration of organic matter in SU caused the inhomogeneous distribution of CaCO3 (more precipitation at the top and less at the bottom), leading to low wind erosion resistance in the middle and bottom parts of the soil. Once the top of SU-treated sand was eroded, the sand below the top layer was lost rapidly, resulting in the final erosion mass of PU-3 (13 g·m−2) being about 95% lower than that of SU-3 (280 g·m−2).
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Z.H.; Huisingh, D. Combating desertification in China: Monitoring, control, management and revegetation. J. Clean. Prod. 2018, 182, 765–775. [Google Scholar] [CrossRef]
- Salifu, E.; MacLachlan, E.; Iyer, K.R.; Knapp, C.W.; Tarantino, A. Application of microbially induced calcite precipitation in erosion mitigation and stabilisation of sandy soil foreshore slopes: A preliminary investigation. Eng. Geol. 2016, 201, 96–105. [Google Scholar] [CrossRef]
- Cardoso, R.; Pedreira, R.; Duarte, S.O.D.; Monteiro, G.A. About calcium carbonate precipitation on sand biocementation. Eng. Geol. 2020, 271, 105612. [Google Scholar] [CrossRef]
- Matsubara, H. Stabilisation of weathered limestone surfaces using microbially enhanced calcium carbonate deposition. Eng. Geol. 2021, 284, 106044. [Google Scholar] [CrossRef]
- Zeng, H.; Yin, L.Y.; Tang, C.S.; Zhu, C.; Cheng, Q.; Li, H.; Lv, C.; Shi, B. Tensile behavior of bio-cemented, fiber-reinforced calcareous sand from coastal zone. Eng. Geol. 2021, 294, 106390. [Google Scholar] [CrossRef]
- Liu, B.; Xie, Y.H.; Tang, C.S.; Pan, X.H.; Jiang, N.J.; Singh, D.N.; Cheng, Y.J.; Shi, B. Bio-mediated method for improving surface erosion resistance of clayey soils. Eng. Geol. 2021, 19, 106295. [Google Scholar] [CrossRef]
- Hamdan, N.M. Applications of Enzyme Induced Carbonate Precipitation (EICP) for Soil Improvement. Ph.D. Thesis, Arizona State University, Tempe, AZ, USA, 2015. [Google Scholar]
- Dilrukshi, R.A.N.; Nakashima, K.; Kawasaki, S. Soil improvement using plant-derived urease-induced calcium carbonate precipitation. Soils Found. 2018, 58, 894–910. [Google Scholar] [CrossRef]
- Kavazanjian, E.; Hamdan, N. Enzyme induced carbonate precipitation (EICP) columns for ground improvement. In Proceedings of the IFCEE 2015, San Antonio, TX, USA, 17 March 2015. [Google Scholar] [CrossRef]
- Almajed, A.; Lateef, M.A.; Moghal, A.A.B.; Lemboye, K. State-of-the-art review of the applicability and challenges of microbial-induced calcite precipitation (MICP) and enzyme-induced calcite precipitation (EICP) techniques for geotechnical and geoenvironmental applications. Crystals 2021, 11, 370. [Google Scholar] [CrossRef]
- Shu, S.; Chen, H.; Meng, H. Modelling microbially induced carbonate precipitation (MICP) in microfluidic porous chips. Geofluids 2022, 2022, 3616473. [Google Scholar] [CrossRef]
- Liu, J.; Chen, Z.H.; Kanungo, D.P.; Song, Z.Z.; Bai, Y.X.; Wang, Y.; Li, D.; Qian, W. Topsoil reinforcement of sandy slope for preventing erosion using water-based polyurethane soil stabilizer. Eng. Geol. 2019, 252, 125–135. [Google Scholar] [CrossRef]
- Cheng, Y.; Tang, C.; Pan, X.; Liu, B.; Xie, Y.; Cheng, Q.; Shi, B. Application of microbial induced carbonate precipitation for loess surface erosion control. Eng. Geol. 2021, 294, 106387. [Google Scholar] [CrossRef]
- Hamdan, N.M.; Kavazanjian, E. Enzyme-induced carbonate mineral precipitation for fugitive dust control. Géotechnique 2016, 66, 546–555. [Google Scholar] [CrossRef]
- Woolley, M.A.; Van Paassen, L.; Kavazanjian, E. Impact on surface hydraulic conductivity of EICP treatment for fugitive dust mitigation. In Proceedings of the Geo-Congress 2020, Minneapolis, MN, USA, 25–28 February 2020. [Google Scholar] [CrossRef]
- Almajed, A.; Tirkolaei, H.K.; Kavazanjian, E. Baseline investigation on enzyme-induced calcium carbonate precipitation. J. Geotech. Geoenviron. Eng. 2018, 144, 04018081. [Google Scholar] [CrossRef]
- Gao, Y.; He, J.; Tang, X.; Chu, J. Calcium carbonate precipitation catalyzed by soybean urease as an improvement method for fine-grained soil. Soils Found. 2019, 59, 1631–1637. [Google Scholar] [CrossRef]
- Ahenkorah, I.; Rahman, M.M.; Karim, M.R.; Beecham, S. Enzyme induced calcium carbonate precipitation and its engineering application: A systematic review and meta-analysis. Constr. Build. Mater. 2021, 308, 125000. [Google Scholar] [CrossRef]
- Lee, S.; Kim, J. An experimental study on enzymatic-induced carbonate precipitation using yellow soybeans for soil stabilization. KSCE J. Civ. Eng. 2020, 24, 2026–2037. [Google Scholar] [CrossRef]
- Gao, Y.; Meng, H.; He, J.; Qi, Y.; Hang, L. Field trial on use of soybean crude extract for carbonate precipitation and wind-induced erosion control of sandy soil. J. Cent. South Univ. 2020, 27, 3320–3333. [Google Scholar] [CrossRef]
- Miao, L.; Wu, L.; Sun, X. Enzyme-catalysed mineralisation experiment study to solidify desert sands. Sci. Rep. 2020, 10, 10611. [Google Scholar] [CrossRef]
- Dilrukshi, R.A.N.; Kawasaki, S. Effective use of plant-derived urease in the field of geoenvironmental/ geotechnical engineering. J. Civ. Environ. Eng. 2016, 6, 1000207. [Google Scholar] [CrossRef]
- Tirkolaei, H.K.; Javadi, N.; Krishnan, V.; Hamdan, N.; Kavazanjian, E. Crude urease extract for biocementation. J. Mater. Civ. Eng. 2020, 32, 04020374. [Google Scholar] [CrossRef]
- Arakawa, T.; Timasheff, S.N. Preferential interactions of proteins with salts in concentrated solutions. Biochemistry 1982, 21, 6545–6552. [Google Scholar] [CrossRef] [PubMed]
- Novák, P.; Havlíek, V. Protein extraction and precipitation. In Proteomic Profiling and Analytical Chemistry, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 51–62. [Google Scholar] [CrossRef]
- Liu, L.X.; Chen, Y.B.; Gao, Y.F.; Liu, B.; Zhou, Y.D.; Li, C. Effect of urease enrichment degree of multiple sources of urease on bio-cementation efficacy via enzyme-induced carbonate precipitation. Can. Geotech. J. 2023, 60, 1923–1937. [Google Scholar] [CrossRef]
- Agarwal, P.; Kaur, S. Effect of bio-enzyme stabilization on unconfined compressive strength of expansive soil. Int. J. Res. Eng. Technol. 2014, 3, 30–33. [Google Scholar] [CrossRef]
- Becker, A.; Vrettos, C. Study on erosion stability enhancement of sands using enzymes. Environ. Geotech. 2015, 2, 301–308. [Google Scholar] [CrossRef]
- Shafii, I.; Shidlovskaya, A.; Briaud, J.-L. Investigation into the effect of enzymes on the erodibility of a low-plasticity silt and a silty sand by EFA testing. J. Geotech. Geoenviron. Eng. 2019, 145, 04019001. [Google Scholar] [CrossRef]
- He, J.; Chu, J.; Gao, Y.; Liu, H. Research advances and challenges in biogeotechnologies. Geotech. Res. 2019, 6, 144–155. [Google Scholar] [CrossRef]
- Wu, C.Z.; Chu, J.; Wu, S.F.; Hong, Y. 3D characterization of microbially induced carbonate precipitation in rock fracture and the resulted permeability reduction. Eng. Geol. 2019, 249, 23–30. [Google Scholar] [CrossRef]
- Song, C.P.; Elsworth, D.; Jia, Y.Z.; Lin, J.Z. Permeable rock matrix sealed with microbially-induced calcium carbonate precipitation: Evolutions of mechanical behaviors and associated microstructure. Eng. Geol. 2022, 304, 106697. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, Y.; He, J.; Zhou, Y.; Geng, W. An experimental investigation of wind erosion resistance of desert sand cemented by soybean-urease induced carbonate precipitation. Geoderma 2023, 429, 116231. [Google Scholar] [CrossRef]
- Martinez, B.C.; DeJong, J.T.; Ginn, T.R.; Montoya, B.M.; Barkouki, T.H.; Hunt, C.; Tanyu, B.; Major, D. Experimental optimization of microbial-induced carbonate precipitation for soil improvement. J. Geotech. Geoenviron. Eng. 2013, 139, 587–598. [Google Scholar] [CrossRef]
- Cheng, L.; Cord-Ruwisch, R. Upscaling effects of soil improvement by microbially induced calcite precipitation by surface percolation. Geomicrobiol. J. 2014, 31, 396–406. [Google Scholar] [CrossRef]
- Cheng, L.; Shahin, M.A.; Chu, J. Soil bio-cementation using a new one-phase low-pH injection method. Acta Geotech. 2019, 14, 615–626. [Google Scholar] [CrossRef]
- Whiffin, V.S.; Van Paassen, L.A.; Harkes, M.P. Microbial carbonate precipitation as a soil improvement technique. Geomicrobiol. J. 2007, 24, 417–423. [Google Scholar] [CrossRef]
- Chu, J.; Ivanov, V.; Stabnikov, V.; Li, B. Microbial method for construction of an aquaculture pond in sand. Géotechnique 2013, 63, 871–875. [Google Scholar] [CrossRef]
- Shu, S.; Yan, B.; Ge, B.; Li, S.; Meng, H. Factors affecting soybean crude urease extraction and biocementation via enzyme-induced carbonate precipitation (EICP) for soil improvement. Energies 2022, 15, 5566. [Google Scholar] [CrossRef]
- Fu, K.; Zhang, P.; Fu, D. Experiment and model for the viscosity of carbonated 3-(dimethylamino) propan-1-ol and 2-(ethylamino) ethanol blended aqueous solutions. J. Chem. Thermodyn. 2019, 134, 61–68. [Google Scholar] [CrossRef]
- Bian, Y.; Chen, Y.; Zhan, L.; Ke, H.; Gao, Y.; Wang, Q.; Qi, G. An enzyme-induced carbonate precipitation method for Zn2+, Ni2+, and Cr (VI) remediation: An experimental and simulation study. Appl. Sci. 2024, 14, 6559. [Google Scholar] [CrossRef]
- Gao, Y.; Hua, C.; Ke, T. Field test on soybean-urease induced calcite precipitation (SICP) for desert sand stabilization against the wind-induced erosion. Sustainability 2022, 14, 15474. [Google Scholar] [CrossRef]
- ASTM. Standard Particle for Classification of Soils for Engineering Purposes (Unified Soil Classification System); ASTM International: West Conshohocken, PA, USA, 2018. [Google Scholar]
- ISO 6058; Water Quality: Determination of Calcium Content: EDTA Titrimetric Method. ISO: Geneva, Switzerland, 1984.
- Fattahi, S.M.; Soroush, A.; Huang, N. Biocementation control of sand against wind erosion. J. Geotech. Geoenviron. Eng. 2020, 146, 04020045. [Google Scholar] [CrossRef]
- Miao, L.; Wu, L.; Sun, X.; Li, X.; Zhang, J. Method for solidifying desert sands with enzyme-catalysed mineralization. Land Degrad. Dev. 2019, 31, 1317–1324. [Google Scholar] [CrossRef]
- Song, J.Y.; Sim, Y.; Jang, J.; Hong, W.T.; Yun, T.S. Near-surface soil stabilization by enzyme-induced carbonate precipitation for fugitive dust suppression. Acta Geotech. 2020, 15, 1967–1980. [Google Scholar] [CrossRef]
- Almajed, A.; Lemboye, K.; Arab, M.G.; Alnuaim, A. Mitigating wind erosion of sand using biopolymer-assisted EICP technique. Soils Found. 2020, 60, 356–371. [Google Scholar] [CrossRef]
- Bagnold, R.A. The Physics of Blown Sand and Desert Dunes; Methuen: London, UK, 1941. [Google Scholar]
- Yan, Y.; Wang, X.; Guo, Z.; Chen, J.; Xin, X.; Xu, D.; Yan, R.; Chen, B.; Xu, L. Influence of wind erosion on dry aggregate size distribution and nutrients in three steppe soils in northern China. Catena 2018, 170, 159–168. [Google Scholar] [CrossRef]
- Shahabinejad, N.; Mahmoodabadi, M.; Jalalian, A.; Chavoshi, E. In situ field measurement of wind erosion and threshold velocity in relation to soil properties in arid and semiarid environments. Environ. Earth Sci. 2019, 78, 501. [Google Scholar] [CrossRef]
- Navarro, A.F.; Cegarra, J.; Roig, A.; Garcia, D. Relationships between organic matter and carbon contents of organic wastes. Bioresour. Technol. 1993, 44, 203–207. [Google Scholar] [CrossRef]
- Cihacek, L.J.; Jacobson, K.A. Effects of soil sample grinding intensity on carbon determination by high-temperature combustion. Commun. Soil Sci. Plant Anal. 2007, 38, 1733–1739. [Google Scholar] [CrossRef]
- Wen, K.; Li, Y.; Amini, F.; Li, L. Impact of bacteria and urease concentration on precipitation kinetics and crystal morphology of calcium carbonate. Acta Geotech. 2019, 15, 17–27. [Google Scholar] [CrossRef]
- Cui, M.J.; Lai, H.J.; Wu, S.F.; Chu, J. Comparison of soil improvement methods using crude soybean enzyme, bacterial enzyme or bacteria-induced carbonate precipitation. Géotechnique 2022, 74, 18–26. [Google Scholar] [CrossRef]
- Orme, C.; Noy, A.; Wierzbicki, A.; Mcbride, M. Formation of chiral morphologies through selective binding of amino acids to calcite surface steps. Nature 2001, 411, 775–779. [Google Scholar] [CrossRef]
- Picker, A.; Kellermeier, M.; Seto, J.; Gebauer, D.; Cölfen, H. The multiple effects of amino acids on the early stages of calcium carbonate crystallization. Z. Krist.—Cryst. Mater. 2012, 227, 744–757. [Google Scholar] [CrossRef]
- Montanari, G.; Lakshtanov, L.Z.; Tobler, D.J.; Dideriksen, K.; Dalby, K.N.; Bovet, N.; Stipp, S.L.S. Effect of aspartic acid and glycine on calcite growth. Cryst. Growth Des. 2016, 16, 4813–4821. [Google Scholar] [CrossRef]
- Borman, A.H.; De Jong, E.; Huizinga, M.; Kok, D.J.; Westbroek, P.; Bosch, L. The role in CaCO3 crystallization of an acid Ca2+-binding polysaccharide associated with coccoliths of Emiliania huxleyi. Eur. J. Biochem. 1982, 129, 179–183. [Google Scholar] [CrossRef] [PubMed]
- Henriksen, K.; Stipp, S.L.S.; Young, J.R.; Marsh, M.E. Biological control on calcite crystallization: AFM investigation of coccolith polysaccharide function. Am. Mineral. 2004, 89, 1709–1716. [Google Scholar] [CrossRef]
- Treccani, L.; Mann, K.; Heinemann, F.; Fritz, M. Perlwapin, an abalone nacre protein with three four-disulfide core (whey acidic protein) domains, inhibits the growth of calcium carbonate crystals. Biophys. J. 2006, 91, 2601–2608. [Google Scholar] [CrossRef]
- Nielsen, J.W.; Sand, K.K.; Pedersen, C.S.; Lakshtanov, L.Z.; Winther, J.R.; Willemoës, M.; Stipp, S.L.S. Polysaccharide effects on calcite growth: The influence of composition and branching. Cryst. Growth Des. 2012, 12, 4906–4910. [Google Scholar] [CrossRef]


| Type | Crude Soybean Urease (g·L−1) | Pure Urease (g·L−1) | |
|---|---|---|---|
| Amino acid | Glu | 2.232 | <DL 1 |
| Asp | 1.300 | <DL | |
| Arg | 0.760 | <DL | |
| Leu | 0.760 | <DL | |
| Lys | 0.698 | <DL | |
| Pro | 0.590 | <DL | |
| Ser | 0.575 | <DL | |
| Phe | 0.537 | <DL | |
| Ile | 0.421 | <DL | |
| Gly | 0.414 | <DL | |
| Ala | 0.414 | <DL | |
| Val | 0.414 | <DL | |
| Thr | 0.399 | <DL | |
| Tyr | 0.391 | <DL | |
| His | 0.276 | <DL | |
| Met | <DL | <DL | |
| Protein | 9.795 | 3.432 | |
| Soluble polysaccharide | <DL | <DL | |
| Property | Value |
|---|---|
| Grain size distribution (%) | |
| 0.4–0.25 mm | 1.18 |
| 0.25–0.2 mm | 8.82 |
| 0.2–0.15 mm | 55.67 |
| 0.15–0.1 mm | 30.59 |
| 0.1–0.05 mm | 3.74 |
| Bulk density (g·cm−3) | 1.51 |
| Specific Gravity, Gs | 2.66 |
| Uniformity coefficient, Cu | 1.73 |
| Coefficient of curvature, Cc | 1.04 |
| Maximum void ratio, emax | 0.847 |
| Minimum void ratio, emin | 0.546 |
| Sets | Test ID | Urease Type | Cementation Solution | Treatment Cycle |
|---|---|---|---|---|
| Tube tests | SU | Soybean urease | 0.2 mol·L−1 CaCl2-CO(NH2)2 | 1 |
| PU | Pure urease | 0.2 mol·L−1 CaCl2-CO(NH2)2 | 1 | |
| Wind erosion tests | SU-S1 | Soybean urease | 0.2 mol·L−1 CaCl2 | 1 |
| PU-S1 | Pure urease | 0.2 mol·L−1 CaCl2 | 1 | |
| SU-S2 | Soybean urease | 0.2 mol·L−1 CaCl2 | 2 | |
| PU-S2 | Pure urease | 0.2 mol·L−1 CaCl2 | 2 | |
| SU-S3 | Soybean urease | 0.2 mol·L−1 CaCl2 | 3 | |
| PU-S3 | Pure urease | 0.2 mol·L−1 CaCl2 | 3 | |
| SU-1 | Soybean urease | 0.2 mol·L−1 CaCl2-CO(NH2)2 | 1 | |
| PU-1 | Pure urease | 0.2 mol·L−1 CaCl2-CO(NH2)2 | 1 | |
| SU-2 | Soybean urease | 0.2 mol·L−1 CaCl2-CO(NH2)2 | 2 | |
| PU-2 | Pure urease | 0.2 mol·L−1 CaCl2-CO(NH2)2 | 2 | |
| SU-3 | Soybean urease | 0.2 mol·L−1 CaCl2-CO(NH2)2 | 3 | |
| PU-3 | Pure urease | 0.2 mol·L−1 CaCl2-CO(NH2)2 | 3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, Y.; Liu, Y.; Gao, Y.; Zhou, Y.; Liu, B.; Wang, L.; Hang, L.; Zhang, S. Comparison of Crude Soybean Urease- and Pure Urease-Induced Carbonate Precipitation on Wind-Induced Erosion Resistance of Desert Sand. Sustainability 2025, 17, 2968. https://doi.org/10.3390/su17072968
Chen Y, Liu Y, Gao Y, Zhou Y, Liu B, Wang L, Hang L, Zhang S. Comparison of Crude Soybean Urease- and Pure Urease-Induced Carbonate Precipitation on Wind-Induced Erosion Resistance of Desert Sand. Sustainability. 2025; 17(7):2968. https://doi.org/10.3390/su17072968
Chicago/Turabian StyleChen, Yanbo, Yang Liu, Yufeng Gao, Yundong Zhou, Bin Liu, Liya Wang, Lei Hang, and Shijia Zhang. 2025. "Comparison of Crude Soybean Urease- and Pure Urease-Induced Carbonate Precipitation on Wind-Induced Erosion Resistance of Desert Sand" Sustainability 17, no. 7: 2968. https://doi.org/10.3390/su17072968
APA StyleChen, Y., Liu, Y., Gao, Y., Zhou, Y., Liu, B., Wang, L., Hang, L., & Zhang, S. (2025). Comparison of Crude Soybean Urease- and Pure Urease-Induced Carbonate Precipitation on Wind-Induced Erosion Resistance of Desert Sand. Sustainability, 17(7), 2968. https://doi.org/10.3390/su17072968






