Investigating the Impact of Salinity on Soil Organic Matter Dynamics Using Molecular Biomarkers and Principal Component Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Sampling Strategy
2.3. Salinity
2.4. Molecular Analysis
2.5. Gas Chromatography Coupled with Mass Spectrometry (GC-MS)
2.6. Molecular Biomarker Ratios
2.7. Data Analysis Method
3. Results and Discussion
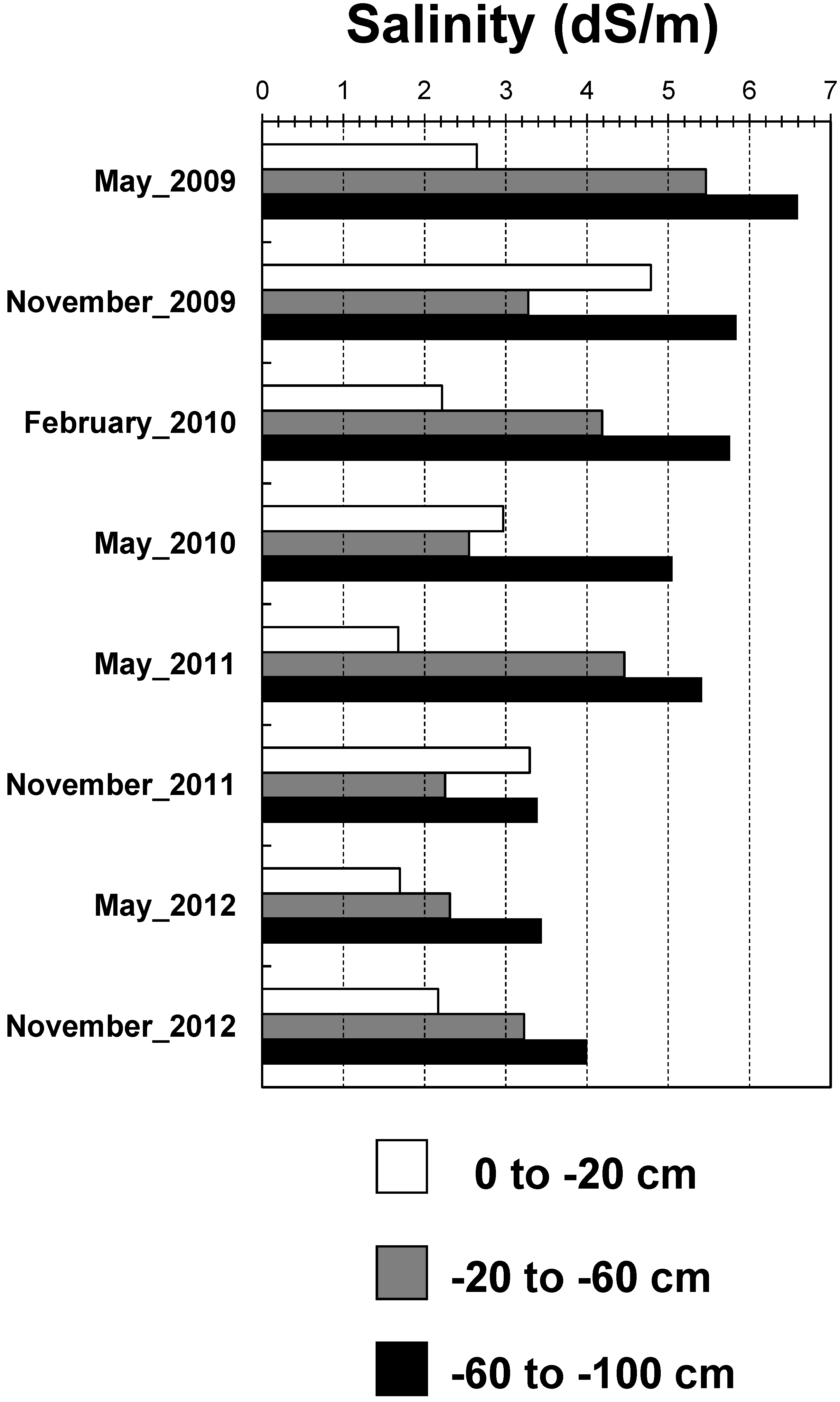
3.1. Temporal and Depth-Dependent Variations in Soil Salinity
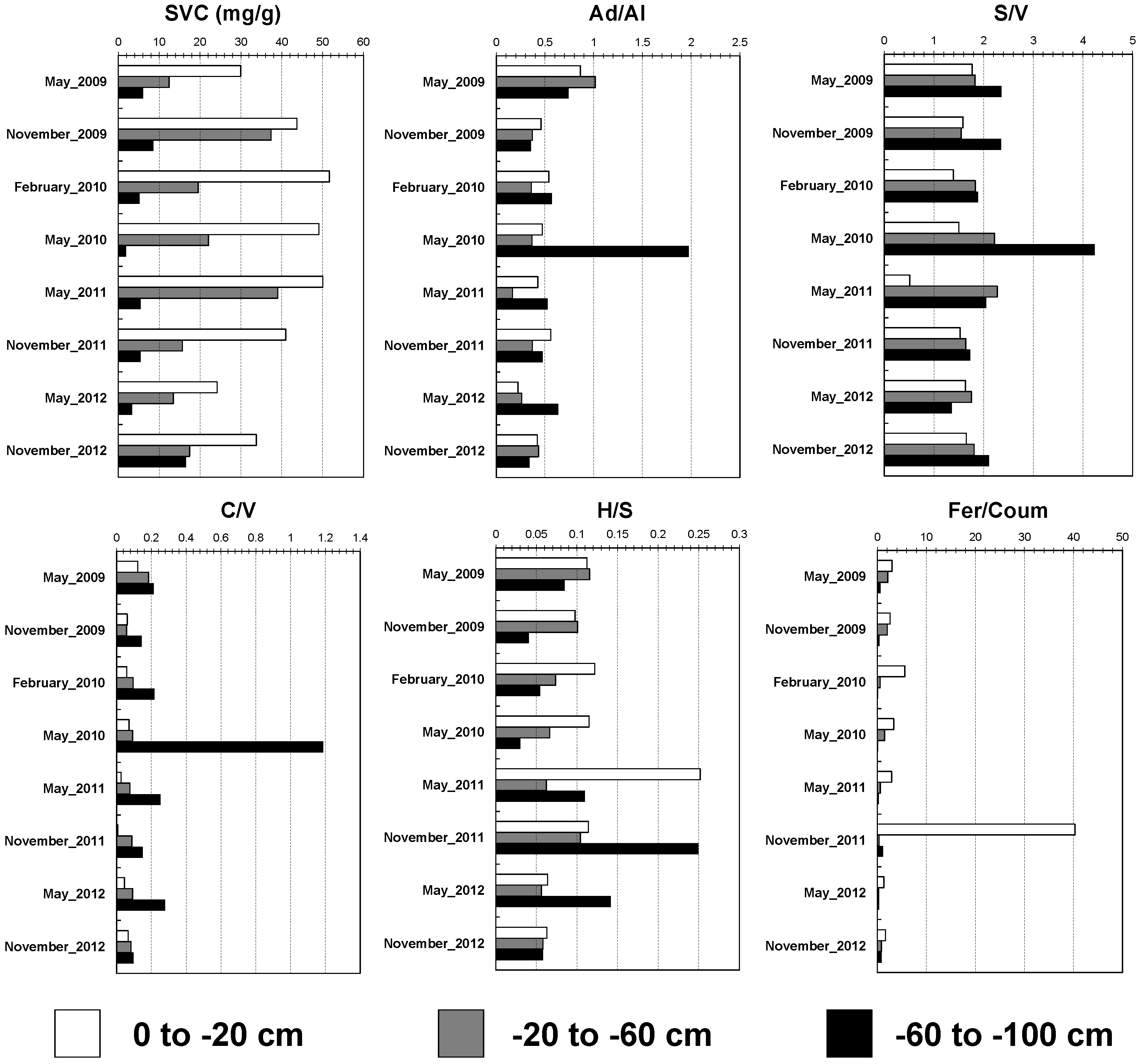
3.2. Depth-Dependent Dynamics of Lignin Biomarkers: Insights into Decomposition and Preservation in Soil Profiles
3.3. Depth and Temporal Variability of Lipid Biomarkers: Insights into Microbial Activity and Organic Matter Dynamics in Soil Profiles
3.4. Influence of Salinity on Lipid and Lignin Biomarkers: Implications for Microbial Activity and Organic Matter Stabilization
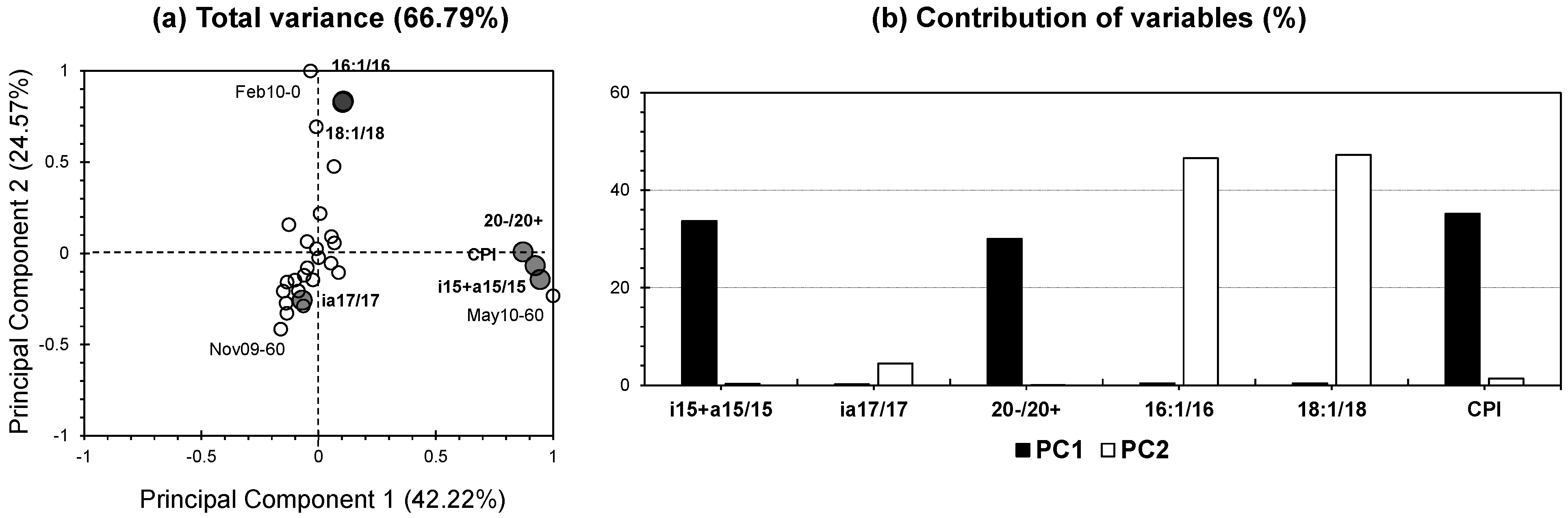
3.5. PCA for Molecular Biomarkers
3.5.1. Linking Lignin and Lipid Dynamics with Soil Biochemical Processes
3.5.2. PCA Analysis of Lignin Biomarkers: Enhanced Insights into Decomposition and Preservation Dynamics
3.5.3. PCA Analysis of Lipid Biomarkers: Insights into Microbial Activity and Lipid Dynamics in Soil Profiles
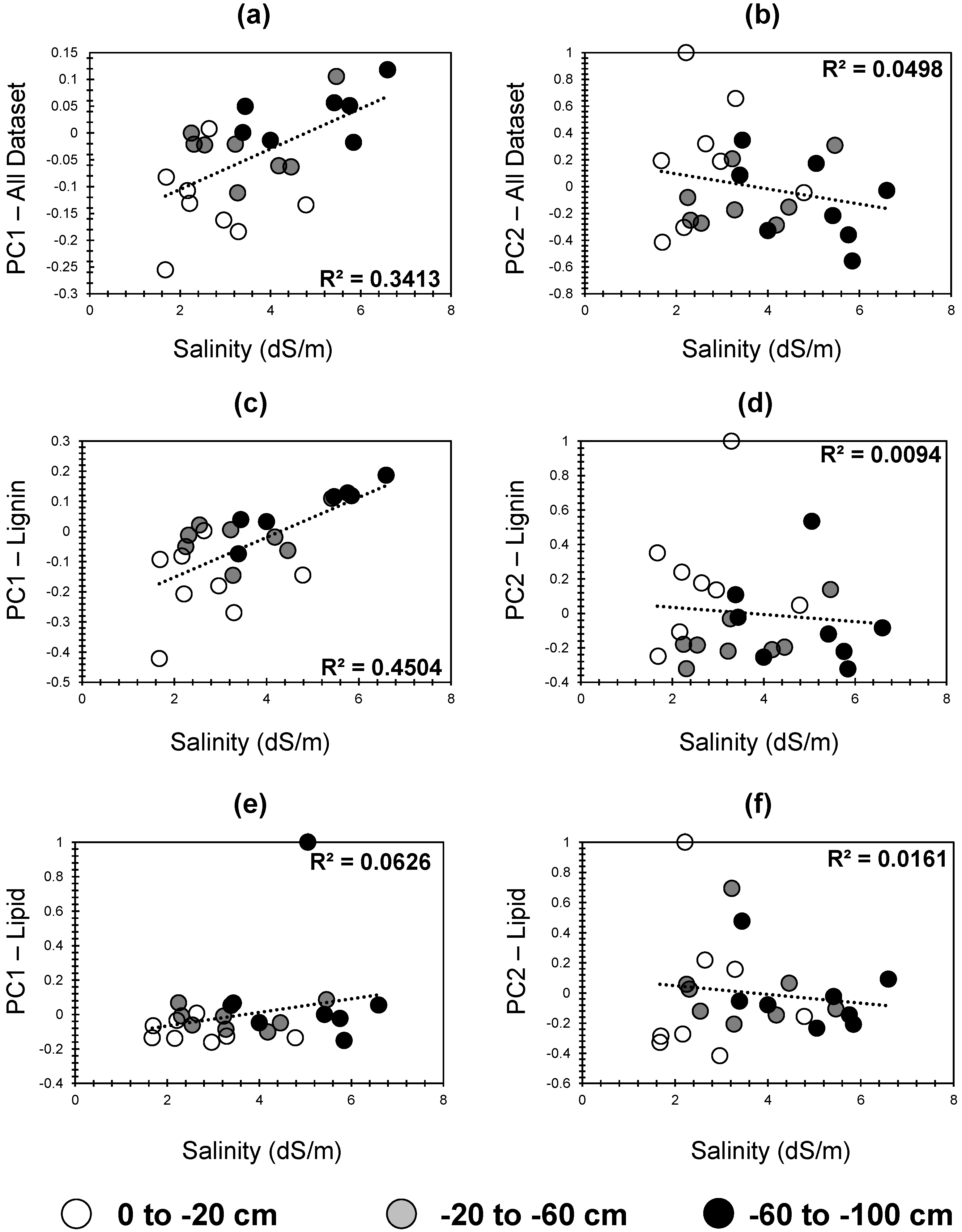
3.5.4. Correlation Between Principal Components and Salinity: Insights into Temporal and Depth-Based Soil Organic Matter Dynamics
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahri, H.; Dignac, M.-F.; Rumpel, C.; Rasse, D.P.; Chenu, C.; Mariotti, A. Lignin Turnover Kinetics in an Agricultural Soil Is Monomer Specific. Soil Biol. Biochem. 2006, 38, 1977–1988. [Google Scholar] [CrossRef]
- Thevenot, M.; Dignac, M.-F.; Rumpel, C. Fate of Lignins in Soils: A Review. Soil Biol. Biochem. 2010, 42, 1200–1211. [Google Scholar]
- Assembly, G. Sustainable Development Goals. SDGs Transform. Our World 2015, 2030, 6–28. [Google Scholar]
- Shahid, S.A.; Zaman, M.; Heng, L. Soil Salinity: Historical Perspectives and a World Overview of the Problem. In Guideline for Salinity Assessment, Mitigation and Adaptation Using Nuclear and Related Techniques; Springer International Publishing: Cham, Switzerland, 2018; pp. 43–53. ISBN 978-3-319-96189-7. [Google Scholar]
- Collard, F.-X.; Blin, J. A Review on Pyrolysis of Biomass Constituents: Mechanisms and Composition of the Products Obtained from the Conversion of Cellulose, Hemicelluloses and Lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar]
- Corwin, D.L. Climate Change Impacts on Soil Salinity in Agricultural Areas. Eur. J. Soil Sci. 2021, 72, 842–862. [Google Scholar] [CrossRef]
- Boulaine, J. Carte Des Sols Des Plaines Du Cheliff Au 1/50.000 e, Feuilles 1 à 5. Inspection générale de l’Agriculture du Gouvernement Général de l’Algérie 1956. [Google Scholar]
- Douaoui, A. Variabilité Spatiale de la Salinité et Sa Relation Avec Certaines Caractéristiques Des Sols de la Plaine du Bas-Chéliff. Ph.D. Thesis, University Center of Tipaza Morsli Abdallah, Tipaza, Algeria, 2005. [Google Scholar]
- Abd El Kader Douaoui, H.N.; Walter, C. Detecting Salinity Hazards within a Semiarid Context by Means of Combining Soil and Remote-Sensing Data. Geoderma 2006, 134, 217–230. [Google Scholar] [CrossRef]
- Younes, K.; Moghrabi, A.; Moghnie, S.; Mouhtady, O.; Murshid, N.; Grasset, L. Assessment of the Efficiency of Chemical and Thermochemical Depolymerization Methods for Lignin Valorization: Principal Component Analysis (PCA) Approach. Polymers 2022, 14, 194. [Google Scholar] [CrossRef]
- Schellekens, J.; Bindler, R.; Martínez-Cortizas, A.; McClymont, E.L.; Abbott, G.D.; Biester, H.; Pontevedra-Pombal, X.; Buurman, P. Preferential Degradation of Polyphenols from Sphagnum–4-Isopropenylphenol as a Proxy for Past Hydrological Conditions in Sphagnum-Dominated Peat. Geochim. Cosmochim. Acta 2015, 150, 74–89. [Google Scholar]
- Bahreininejad, B.; Allahdadi, M. Effects of Saline Irrigated Water on Forage Quality of Globe Artichoke (Cynara cardunculus var. scolymus L.). Iran Agric. Res. 2020, 39, 59–66. [Google Scholar]
- Hardie, M.; Doyle, R. Measuring Soil Salinity. In Plant Salt Tolerance; Shabala, S., Cuin, T.A., Eds.; Methods in Molecular Biology; Humana Press: Totowa, NJ, USA, 2012; Volume 913, pp. 415–425. ISBN 978-1-61779-985-3. [Google Scholar]
- Wang, J.J.; Provin, T.; Zhang, H. Measurement of Soil Salinity and Sodicity. In Soil Test Methods from the Southeastern United States; University of Kentucky: Lexington, KY, USA; Clemson University: Clemson, SC, USA, 2014; p. 185. Available online: https://aesl.ces.uga.edu/Sera6/PUB/Methodsmanualfinalsera6.Pdf#page=193 (accessed on 26 February 2025).
- Ertel, J.R.; Hedges, J.I. The Lignin Component of Humic Substances: Distribution among Soil and Sedimentary Humic, Fulvic, and Base-Insoluble Fractions. Geochim. Cosmochim. Acta 1984, 48, 2065–2074. [Google Scholar]
- Jørgensen, P.-E.; Eriksen, T.; Jensen, B.K. Estimation of Viable Biomass in Wastewater and Activated Sludge by Determination of ATP, Oxygen Utilization Rate and FDA Hydrolysis. Water Res. 1992, 26, 1495–1501. [Google Scholar]
- Hedges, J.I.; Mann, D.C. The Characterization of Plant Tissues by Their Lignin Oxidation Products. Geochim. Cosmochim. Acta 1979, 43, 1803–1807. [Google Scholar] [CrossRef]
- Otto, A.; Simpson, M.J. Evaluation of CuO Oxidation Parameters for Determining the Source and Stage of Lignin Degradation in Soil. Biogeochemistry 2006, 80, 121–142. [Google Scholar] [CrossRef]
- Collard, M.; Teychené, B.; Lemée, L. Comparison of Three Different Wastewater Sludge and Their Respective Drying Processes: Solar, Thermal and Reed Beds–Impact on Organic Matter Characteristics. J. Environ. Manag. 2017, 203, 760–767. [Google Scholar]
- Qadir, M.; Quillérou, E.; Nangia, V.; Murtaza, G.; Singh, M.; Thomas, R.J.; Drechsel, P.; Noble, A.D. Economics of Salt-induced Land Degradation and Restoration. Nat. Resour. Forum 2014, 38, 282–295. [Google Scholar] [CrossRef]
- Massoud, F.I. Salt Affected Soils at a Global Scale and Concepts for Control; FAO: Rome, Italy, 1981. [Google Scholar]
- Bourg, I.C.; Ajo-Franklin, J.B. Clay, Water, and Salt: Controls on the Permeability of Fine-Grained Sedimentary Rocks. Acc. Chem. Res. 2017, 50, 2067–2074. [Google Scholar] [CrossRef]
- Corwin, D.L.; Lesch, S.M.; Oster, J.D.; Kaffka, S.R. Short-Term Sustainability of Drainage Water Reuse: Spatio-Temporal Impacts on Soil Chemical Properties. J. Environ. Qual. 2008, 37, S-8-S-24. [Google Scholar] [CrossRef]
- Wichelns, D.; Qadir, M. Achieving Sustainable Irrigation Requires Effective Management of Salts, Soil Salinity, and Shallow Groundwater. Agric. Water Manag. 2015, 157, 31–38. [Google Scholar]
- Younes, K.; Laduranty, J.; Descostes, M.; Grasset, L. Molecular Biomarkers Study of an Ombrotrophic Peatland Impacted by an Anthropogenic Clay Deposit. Org. Geochem. 2017, 105, 20–32. [Google Scholar] [CrossRef]
- Kaal, J.; Pérez-Rodríguez, M.; Biester, H. Molecular Probing of DOM Indicates a Key Role of Spruce-Derived Lignin in the DOM and Metal Cycles of a Headwater Catchment: Can Spruce Forest Dieback Exacerbate Future Trends in the Browning of Central European Surface Waters? Environ. Sci. Technol. 2022, 56, 2747–2759. [Google Scholar] [CrossRef]
- Grünewald, G.; Kaiser, K.; Jahn, R.; Guggenberger, G. Organic Matter Stabilization in Young Calcareous Soils as Revealed by Density Fractionation and Analysis of Lignin-Derived Constituents. Org. Geochem. 2006, 37, 1573–1589. [Google Scholar]
- Gordon, E.S.; Goñi, M.A. Sources and Distribution of Terrigenous Organic Matter Delivered by the Atchafalaya River to Sediments in the Northern Gulf of Mexico. Geochim. Cosmochim. Acta 2003, 67, 2359–2375. [Google Scholar]
- Hedges, J.I.; Clark, W.A.; Come, G.L. Organic Matter Sources to the Water Column and Surficial Sediments of a Marine Bay: Organic Matter Sources. Limnol. Oceanogr. 1988, 33, 1116–1136. [Google Scholar] [CrossRef]
- Hedges, J.I.; Blanchette, R.A.; Weliky, K.; Devol, A.H. Effects of Fungal Degradation on the CuO Oxidation Products of Lignin: A Controlled Laboratory Study. Geochim. Cosmochim. Acta 1988, 52, 2717–2726. [Google Scholar]
- Jeffries, T.W. Biodegradation of Lignin-Carbohydrate Complexes. In Physiology of Biodegradative Microorganisms; Ratledge, C., Ed.; Springer Netherlands: Dordrecht, The Netherlands, 1991; pp. 163–176. ISBN 978-94-010-5527-7. [Google Scholar]
- Skyba, O.; Douglas, C.J.; Mansfield, S.D. Syringyl-Rich Lignin Renders Poplars More Resistant to Degradation by Wood Decay Fungi. Appl. Environ. Microbiol. 2013, 79, 2560–2571. [Google Scholar] [CrossRef]
- Chang, B.V.; Chang, S.W.; Yuan, S.Y. Anaerobic Degradation of Polycyclic Aromatic Hydrocarbons in Sludge. Adv. Environ. Res. 2003, 7, 623–628. [Google Scholar]
- Orem, W.H.; Colman, S.M.; Lerch, H.E. Lignin Phenols in Sediments of Lake Baikal, Siberia: Application to Paleoenvironmental Studies. Org. Geochem. 1997, 27, 153–172. [Google Scholar]
- Tortosa-Díaz, L.; Saura-Martínez, J.; Taboada-Rodríguez, A.; Martínez-Hernández, G.B.; López-Gómez, A.; Marín-Iniesta, F. Influence of Industrial Processing of Artichoke and By-Products on The Bioactive and Nutritional Compounds. Food Eng. Rev. 2025, 1–24. [Google Scholar] [CrossRef]
- Elouaqoudi, F.Z.; El Fels, L.; Amir, S.; Merlina, G.; Meddich, A.; Lemee, L.; Ambles, A.; Hafidi, M. Lipid Signature of the Microbial Community Structure during Composting of Date Palm Waste Alone or Mixed with Couch Grass Clippings. Int. Biodeterior. Biodegrad. 2015, 97, 75–84. [Google Scholar]
- Kögel, I.; Hempfling, R.; Zech, W.; Hatcher, P.G.; Schulten, H.-R. Chemical Composition of the Organic Matter in Forest Soils: 1. Forest Litter. Soil Sci. 1988, 146, 124–136. [Google Scholar]
- Kögel-Knabner, I. The Macromolecular Organic Composition of Plant and Microbial Residues as Inputs to Soil Organic Matter: Fourteen Years On. Soil Biol. Biochem. 2017, 105, A3–A8. [Google Scholar] [CrossRef]
- Eckmeier, E.; Wiesenberg, G.L. Short-Chain n-Alkanes (C16–20) in Ancient Soil Are Useful Molecular Markers for Prehistoric Biomass Burning. J. Archaeol. Sci. 2009, 36, 1590–1596. [Google Scholar] [CrossRef]
- Wiesenberg, G.L.; Lehndorff, E.; Schwark, L. Thermal Degradation of Rye and Maize Straw: Lipid Pattern Changes as a Function of Temperature. Org. Geochem. 2009, 40, 167–174. [Google Scholar] [CrossRef]
- Bhattarai, S.P.; Su, N.; Midmore, D.J. Oxygation Unlocks Yield Potentials of Crops in Oxygen-Limited Soil Environments. Adv. Agron. 2005, 88, 313–377. [Google Scholar]
- Balesdent, J.; Chenu, C.; Balabane, M. Relationship of Soil Organic Matter Dynamics to Physical Protection and Tillage. Soil Tillage Res. 2000, 53, 215–230. [Google Scholar] [CrossRef]
- Estournel-Pelardy, C.; El-Mufleh Al Husseini, A.; Doskočil, L.; Grasset, L. A Two-Step Thermochemolysis for Soil Organic Matter Analysis. Application to Lipid-Free Organic Fraction and Humic Substances from an Ombrotrophic Peatland. J. Anal. Appl. Pyrolysis 2013, 104, 103–110. [Google Scholar] [CrossRef]
- Akkermans, A.D.L.; Van Elsas, J.D.; De Bruijn, F.J. (Eds.) Molecular Microbial Ecology Manual; Springer Netherlands: Dordrecht, The Netherlands, 1996; ISBN 978-94-011-7660-6. [Google Scholar]
- Tremblay, L.; Benner, R. Microbial Contributions to N-Immobilization and Organic Matter Preservation in Decaying Plant Detritus. Geochim. Cosmochim. Acta 2006, 70, 133–146. [Google Scholar] [CrossRef]
- Ma, Y.; Dias, M.C.; Freitas, H. Drought and Salinity Stress Responses and Microbe-Induced Tolerance in Plants. Front. Plant Sci. 2020, 11, 591911. [Google Scholar] [CrossRef]
- Robert, M.; Chenu, C. Interactions between Soil Minerals and Microorganisms. In Soil Biochemistry; CRC Press: Boca Raton, FL, USA, 2021; pp. 307–404. [Google Scholar]
- Quinn, P.J.; Joo, F.; Vigh, L. The Role of Unsaturated Lipids in Membrane Structure and Stability. Prog. Biophys. Mol. Biol. 1989, 53, 71–103. [Google Scholar] [CrossRef]
- Younes, K.; Grasset, L. Carbohydrates as Proxies in Ombrotrophic Peatland: DFRC Molecular Method Coupled with PCA. Chem. Geol. 2022, 606, 120994. [Google Scholar] [CrossRef]
- Harji, R.R.; Bhosle, N.B.; Garg, A.; Sawant, S.S.; Venkat, K. Sources of Organic Matter and Microbial Community Structure in the Sediments of the Visakhapatnam Harbour, East Coast of India. Chem. Geol. 2010, 276, 309–317. [Google Scholar]
- El Fels, L.; Lemee, L.; Ambles, A.; Hafidi, M. Identification and Biotransformation of Aliphatic Hydrocarbons during Co-Composting of Sewage Sludge-Date Palm Waste Using Pyrolysis-GC/MS Technique. Environ. Sci. Pollut. Res. 2016, 23, 16857–16864. [Google Scholar]
- Srivastava, M.; Mishra, A.K. Comparative Analysis of Paddy Soil Denitrifying Bacteria with Soil Phospholipid Fatty Acid Profile. Geomicrobiol. J. 2021, 38, 404–414. [Google Scholar] [CrossRef]
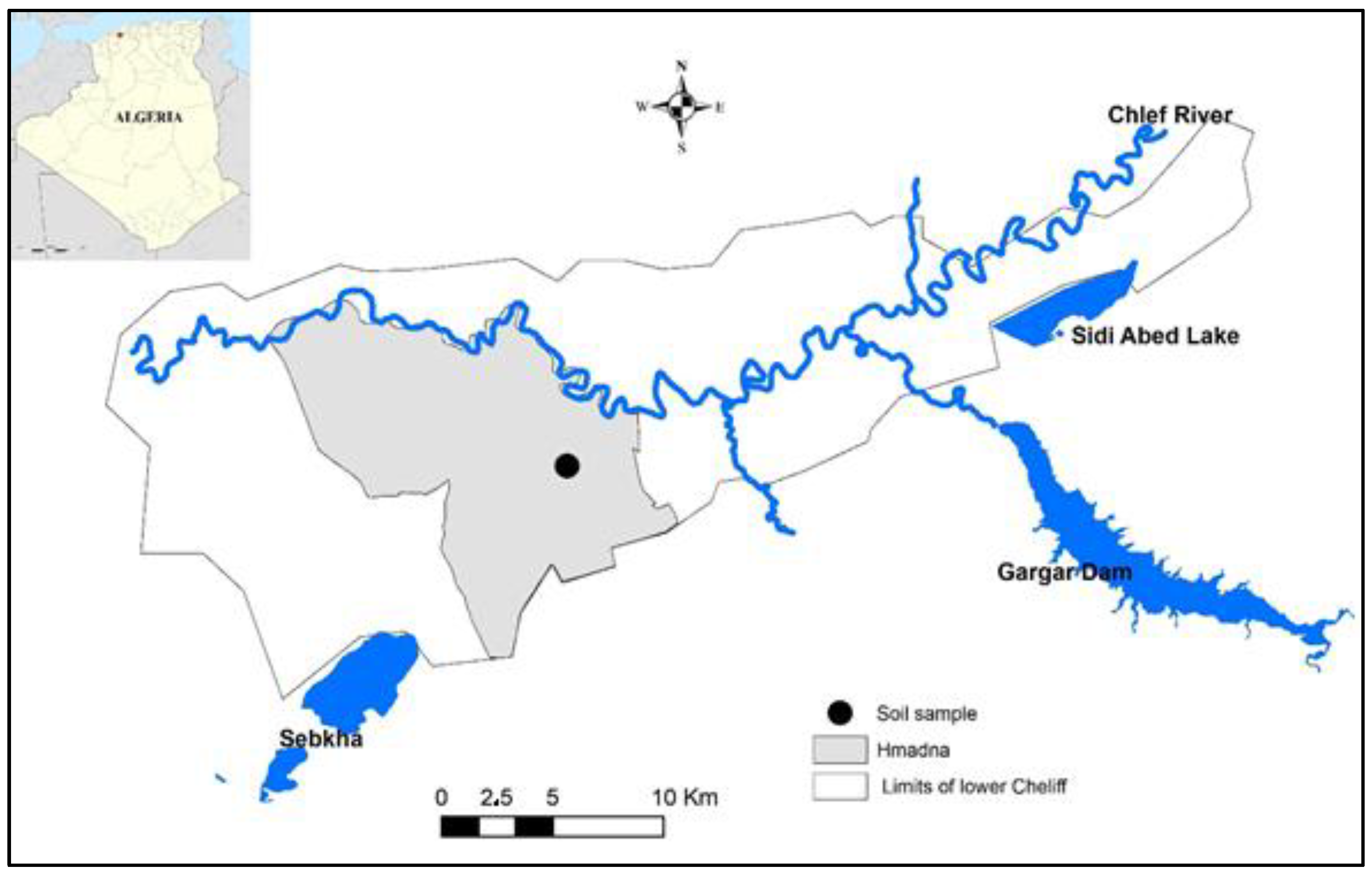




| Location | Geographical Coordinates | Surface | Agricultural Acitvity | Overall Salinity EC (dS/m) | Soil Texture | ||
|---|---|---|---|---|---|---|---|
| Type | Periods | ||||||
| X | Y | ||||||
| El Hmadna | 296,645 | 3,979,661 | 2 ha | Artichoke and Cereals | 2009–2012 | 4.17 | Clay |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akkacha, A.; Douaoui, A.; Younes, K.; El Sawda, C.; Alsyouri, H.; El-Zahab, S.; Grasset, L. Investigating the Impact of Salinity on Soil Organic Matter Dynamics Using Molecular Biomarkers and Principal Component Analysis. Sustainability 2025, 17, 2940. https://doi.org/10.3390/su17072940
Akkacha A, Douaoui A, Younes K, El Sawda C, Alsyouri H, El-Zahab S, Grasset L. Investigating the Impact of Salinity on Soil Organic Matter Dynamics Using Molecular Biomarkers and Principal Component Analysis. Sustainability. 2025; 17(7):2940. https://doi.org/10.3390/su17072940
Chicago/Turabian StyleAkkacha, Abderrhamen, Abdelkader Douaoui, Khaled Younes, Christina El Sawda, Hatem Alsyouri, Samer El-Zahab, and Laurent Grasset. 2025. "Investigating the Impact of Salinity on Soil Organic Matter Dynamics Using Molecular Biomarkers and Principal Component Analysis" Sustainability 17, no. 7: 2940. https://doi.org/10.3390/su17072940
APA StyleAkkacha, A., Douaoui, A., Younes, K., El Sawda, C., Alsyouri, H., El-Zahab, S., & Grasset, L. (2025). Investigating the Impact of Salinity on Soil Organic Matter Dynamics Using Molecular Biomarkers and Principal Component Analysis. Sustainability, 17(7), 2940. https://doi.org/10.3390/su17072940








