Impact of Ozone Exposure on Chlorophyll Fluorescence, Pigment Content and Leaf Gas Exchange on Lonicera caerulea var. kamtschatica and Lonicera caerulea var. emphyllocalyx
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Ozone Treatments
2.3. Content of Chlorophyl, Flavanols and Anthocyanins
2.4. Chlorophyll Fluorescence
2.5. Gas Exchange Parameters of Lonicera caeruelea L.
2.6. Statistical Analysis
3. Results and Discussion
3.1. The Value of Individual Pigments and the Nitrogen–Flavonol Index of Leaves
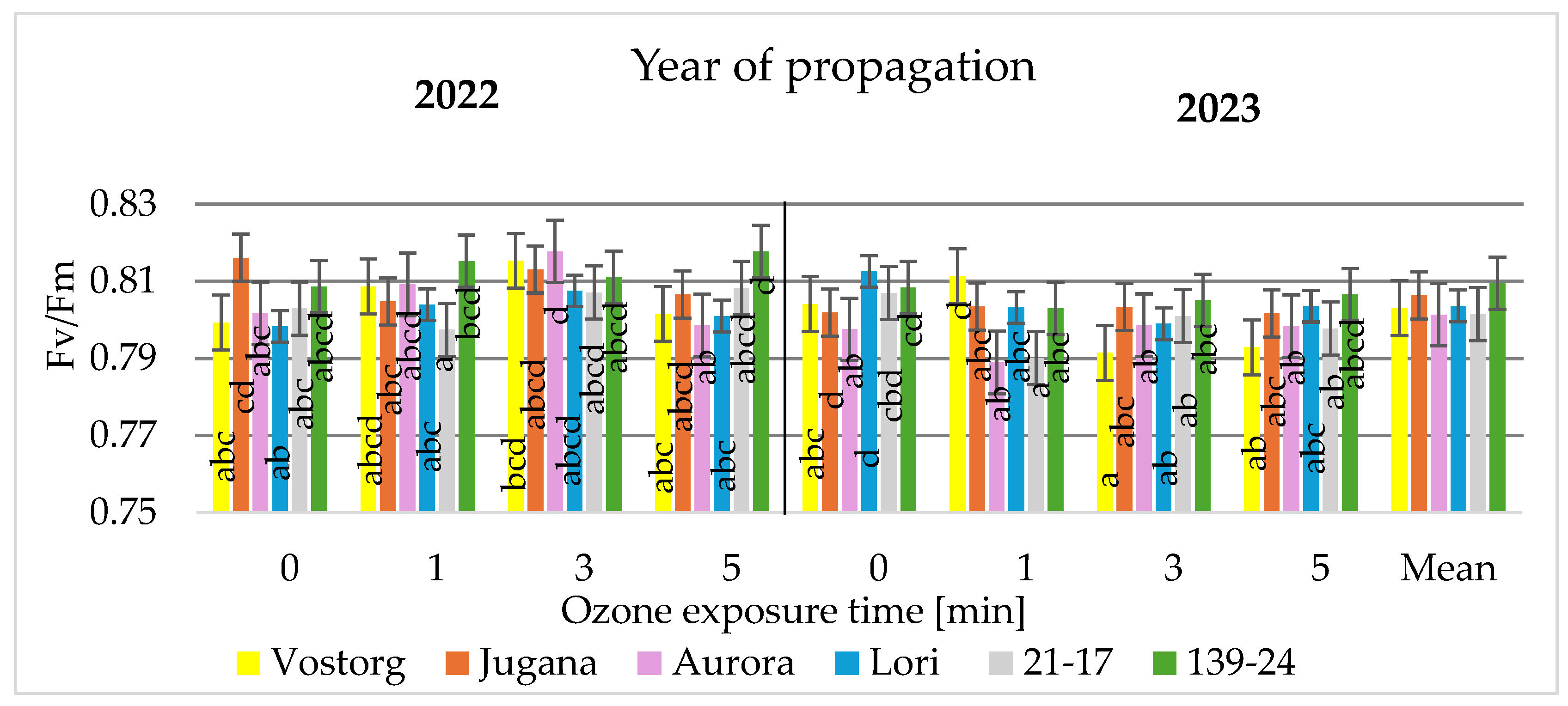
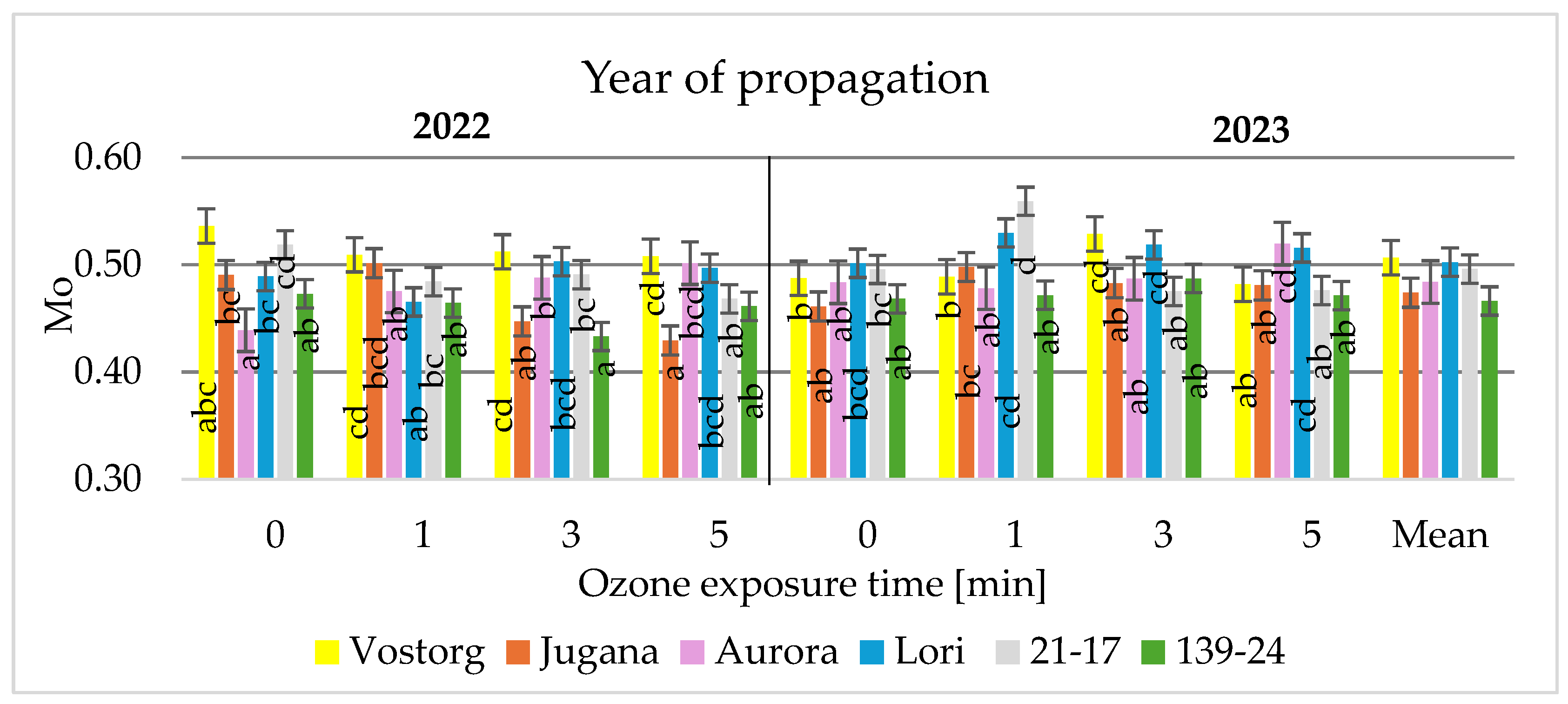
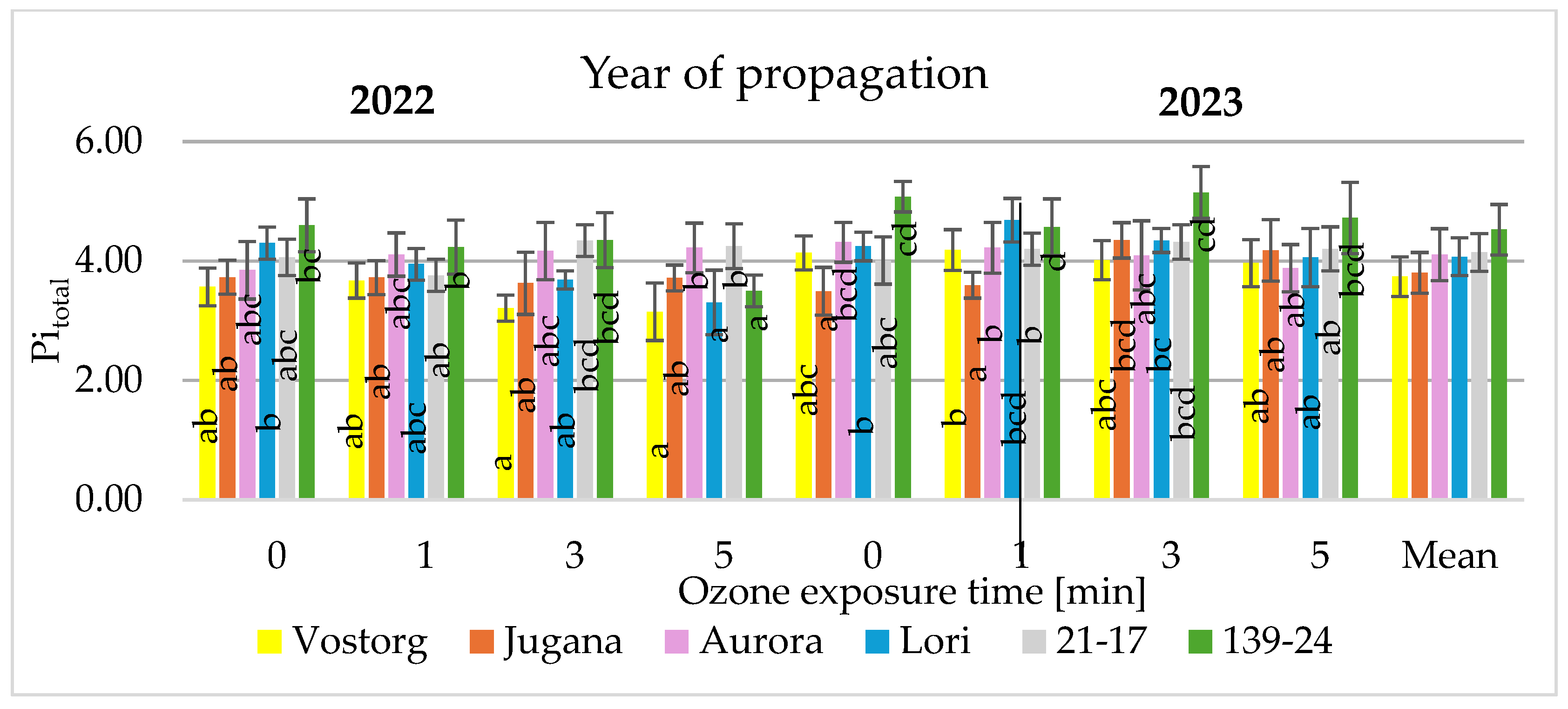
3.2. Physiological Parameters of Leaves
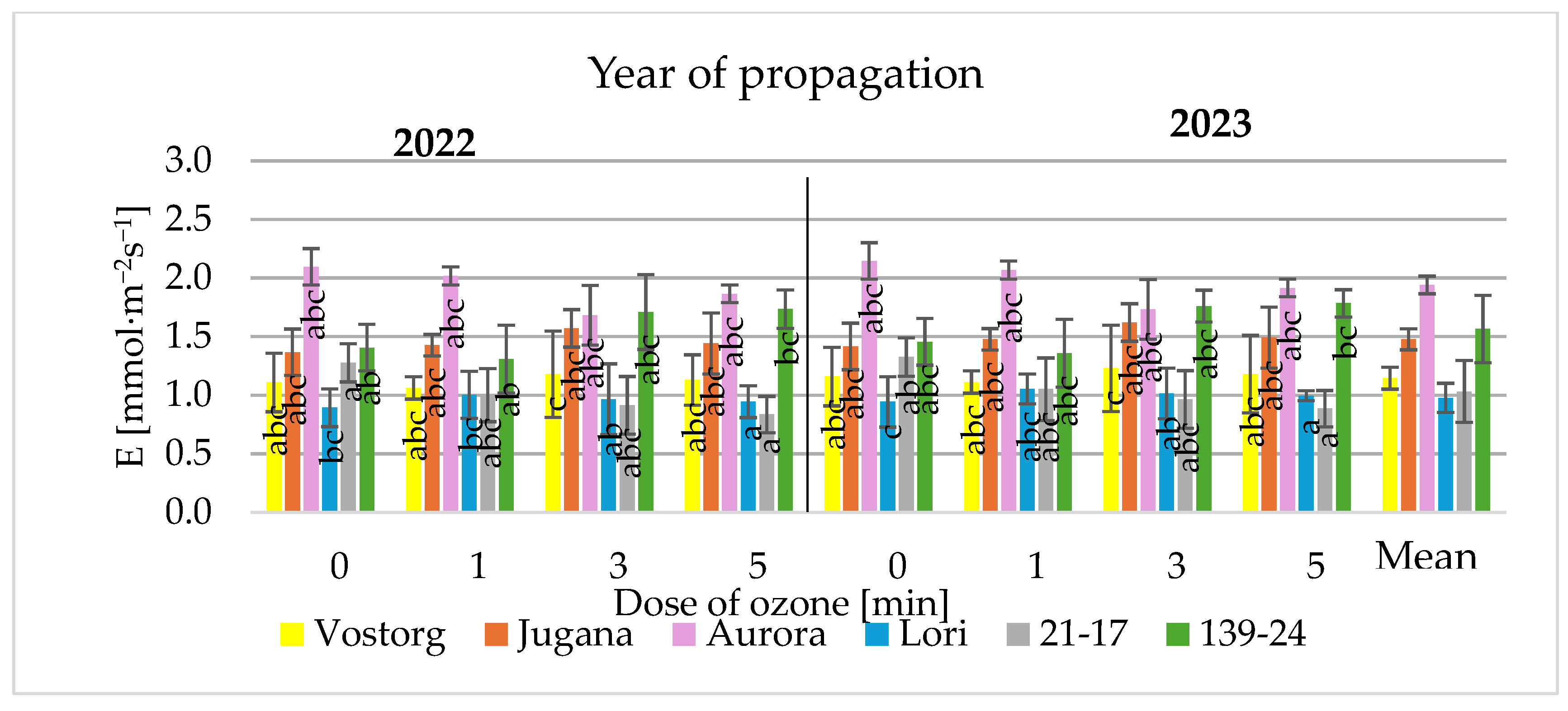
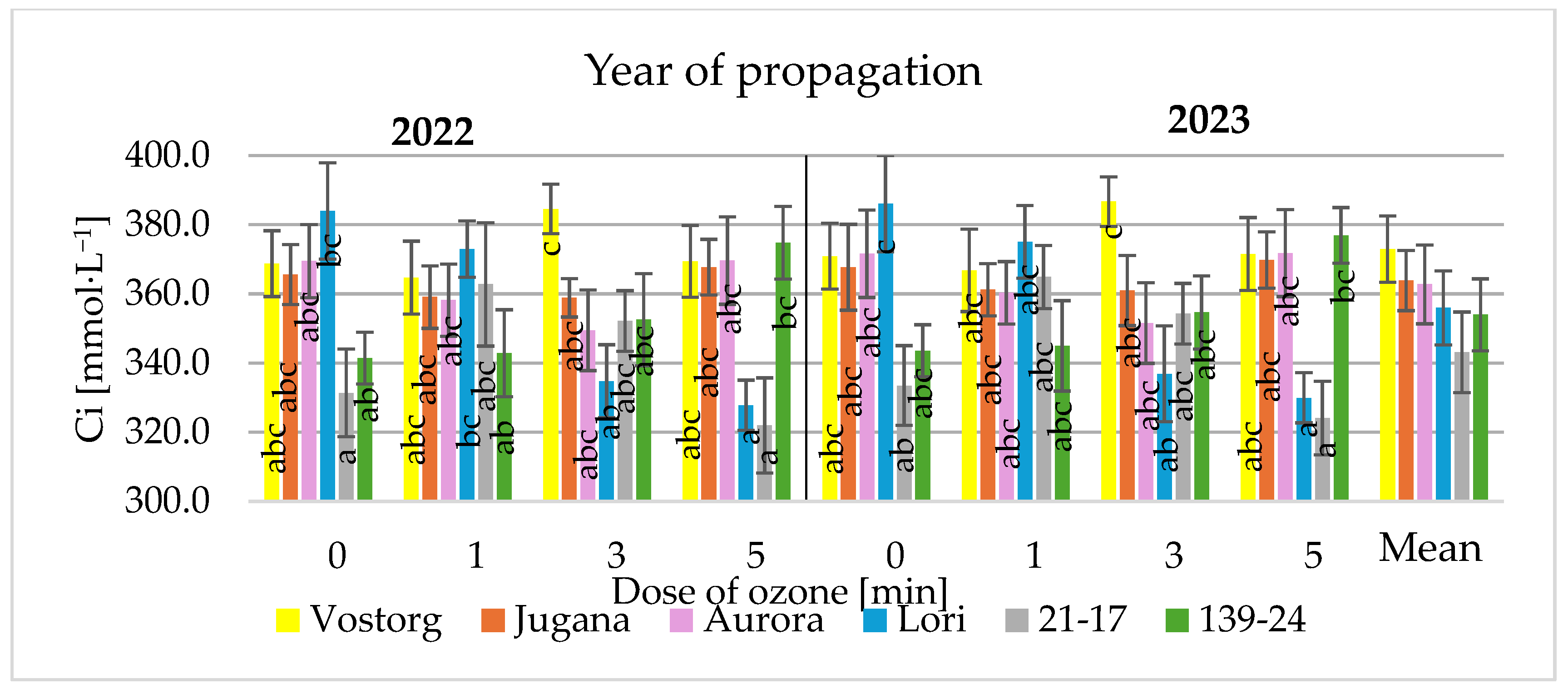
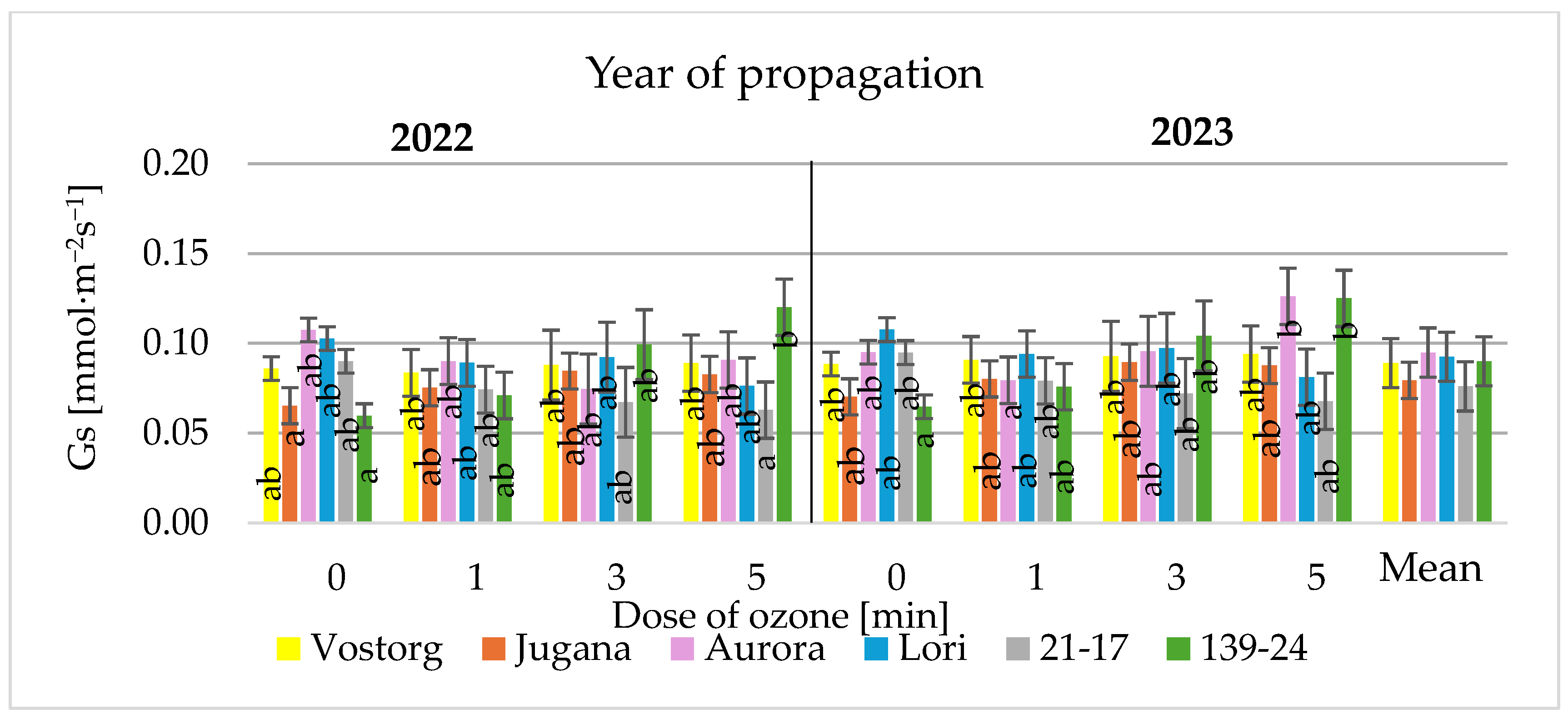
3.3. Gas Exchange Parameters of Leaves
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bieniek, A.A.; Grygorieva, O.; Bielska, N. Biological Properties of Honeysuckle (Lonicera caerulea L.): A Review: The Nutrition, Health Properties of Honeysuckle. Agrobiodivers. Improv. Nutr. Health Life Qual. 2021, 5. [Google Scholar] [CrossRef]
- Gawronski, J.; Hortynski, J.; Kaczmarska, E.; Dyduch-Sieminska, M.; Marecki, W.; Witorozec, A. Evaluation of phenotypic and genotypic diversity of some Polish and Russian blue honeysuckle (Lonicera caerulea L.) cultivars and clones. Acta Sci. Polonorum. Hortorum Cultus 2014, 13, 157–169. [Google Scholar]
- Kucharska, A.Z.; Sokół-Łętowska, A.; Oszmiański, J.; Piórecki, N.; Fecka, I. Iridoids, Phenolic Compounds and Antioxidant Activity of Edible Honeysuckle Berries (Lonicera caerulea var. kamtschatica Sevast.). Molecules 2017, 22, 405. [Google Scholar] [CrossRef]
- Thompson, M.M.; Barney, D.L. Evaluation and breeding of haskap in North America. J. Am. Pomol. Soc. 2007, 1, 25. [Google Scholar]
- Ochmian, I.D.; Skupień, K.; Grajkowski, J.; Smolik, M.; Ostrowska, K. Chemical Composition and Physical Characteristics of Fruits of Two Cultivars of Blue Honeysuckle (Lonicera caerulea L.) in Relation to their Degree of Maturity and Harvest Date. Not. Bot. Horti Agrobot. Cluj-Napoca 2012, 40, 155–162. [Google Scholar]
- Kula, M.; Krauze-Baranowska, M. Blue Honeysuckle (Lonicera caerulea L.)—The current state of phytochemical research and biological activity. Post. Fitoter. 2016, 17, 111–118. [Google Scholar]
- Jia, L.; He, X.; Chen, W.; Liu, Z.; Huang, Y.; Yu, S. Hormesis phenomena under Cd stress in a hyperaccumulator—Lonicera japonica Thunb. Ecotoxicology 2013, 22, 476–485. [Google Scholar]
- Nawaz, M.A.; Razaganova, M.P.; Rusakova, E.A.; Petrusha, E.N.; Sabitov, A.S.; Chunikhina, O.A.; Ercisli, S.; Tikhonova, N.G.; Golokhvast, K.S. Global metabolome profiles of Lonicera caerulea L. and Lonicera caerulea ssp. kamtschatica (Sevast.) Gladkova. Turk. J. Agric. For. 2024, 48, 745–759. [Google Scholar]
- Miller, F.A.; Silva, C.L.M.; Brandão, T.R.S. A review on ozone-based treatments for fruit and vegetables preservation. Food Eng. Rev. 2013, 5, 77–106. [Google Scholar]
- Fan, X. Gaseous ozone to preserve quality and enhance microbial safety of fresh produce: Recent developments and research needs. Compr. Rev. Food Sci. Food Saf. 2021, 20, 4993–5014. [Google Scholar] [CrossRef]
- Kuźniar, P.; Belcar, J.; Zardzewiały, M.; Basara, O.; Gorzelany, J. Effect of Ozonation on the Mechanical, Chemical, and Microbiological Properties of Organically Grown Red Currant (Ribes rubrum L.) Fruit. Molecules 2022, 27, 8231. [Google Scholar] [CrossRef]
- Fan, X.; Shang, D.L.; Lan, Y.B.; Bai, J.B.; Han, X. Application of ozone technology and ozone plant protection devices in agriculture. J. Agric. Sci. Technol. 2020, 22, 71–77. [Google Scholar]
- Msayleb, N.; Ibrahim, S. Treatment of nematodes with ozone gas: A sustainable alternative to nematicides. Phys. Procedia 2011, 21, 187–192. [Google Scholar] [CrossRef]
- Grulke, N.E.; Heath, R.L. Ozone effects on plants in natural ecosystems. Plant Biol. 2020, 22, 12–37. [Google Scholar] [CrossRef]
- Afsah-Hejri, L.; Hajeb, P.; Ehsani, R.J. Application of ozone for degradation of mycotoxins in food: A review. Compr. Rev. Food Sci. Food Saf. 2020, 19, 1777–1808. [Google Scholar] [CrossRef]
- Bussotti, F.; Strasser, R.J.; Schaub, M. Photosynthetic behavior of woody species under high ozone exposure probed with the JIP-test: A review. Environ. Pollut. 2007, 147, 430–437. [Google Scholar] [CrossRef]
- Díaz-López, M.; Siles, J.A.; Ros, C.; Bastida, F.; Nicolás, E. The effects of ozone treatments on the agro-physiological parameters of tomato plants and the soil microbial community. Sci. Total Environ. 2022, 812, 151429. [Google Scholar] [CrossRef]
- Zardzewiały, M.; Matłok, N.; Piechowiak, T.; Saletnik, B.; Balawejder, M.; Gorzelany, J. Preliminary Tests of Tomato Plant Protection Method with Ozone Gas Fumigation Supported with Hydrogen Peroxide Solution and Its Effect on Some Fruit Parameters. Sustainability 2024, 16, 3481. [Google Scholar] [CrossRef]
- Baskakov, I.V.; Orobinsky, V.I.; Gievsky, A.M.; Gulevsky, V.A.; Chernyshov, A.V. Ozone pest control of grain. IOP Conf. Ser. Earth Environ. Sci. 2023, 1138, 012026. [Google Scholar] [CrossRef]
- Aidoo, O.F.; Osei-Owusu, J.; Chia, S.Y.; Dofuor, A.K.; Antwi-Agyakwa, A.K.; Okyere, H.; Gyan, M.; Edusei, G.; Ninsin, K.D.; Duker, R.Q.; et al. Remediation of pesticide residues using ozone: A comprehensive overview. Sci. Total Environ. 2023, 894, 164933. [Google Scholar] [CrossRef]
- Di Vittori, L.; Mazzoni, L.; Battino, M.; Mezzetti, B. Pre-harvest factors influencing the quality of berries. Sci. Hortic. 2018, 233, 310–322. [Google Scholar]
- Remondino, M.; Valdenassi, L. Different Uses of Ozone: Environmental and Corporate Sustainability. Literature Review and Case Study. Sustainability 2018, 10, 4783. [Google Scholar] [CrossRef]
- Cheng, S.; Wang, Y.; Sun, M.; Zheng, J.; Zhu, X.; Zhao, T. Effects of Elevated Surface Ozone Concentration on Photosynthetic Fluorescence Characteristics and Yield of Soybean Parents and Offspring. Atmosphere 2023, 14, 1368. [Google Scholar] [CrossRef]
- Cheng, Z.; Bao, Y.; Li, Z.; Wang, J.; Wang, M.; Wang, S.; Li, B. Lonicera caerulea (Haskap berries): A review of development traceability, functional value, product development status, future opportunities, and challenges. Crit. Rev. Food Sci. Nutr. 2023, 63, 8992–9016. [Google Scholar] [CrossRef]
- Basara, O.; Gorzelany, J. Assessment of Selected Chemical and Morphological Properties of Lonicera var. kamtschatica and Lonicera var. emphyllocalyx Treated with Gaseous Ozone. Molecules 2024, 29, 3616. [Google Scholar] [CrossRef]
- Cerovic, Z.; Moise, N.; Agati, G.; Latouche, G.; Ben Ghozlen, N.; Meyer, S. New portable optical sensors for the assessment of winegrape phenolic maturity based on berry fluorescence. J. Food Compos. Anal. 2008, 21, 650–654. [Google Scholar] [CrossRef]
- Baker Neil, R.; Oxborough, K. Chlorophyll fluorescence as a probe of photosynthetic productivity. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Springer: Dordrecht, The Netherlands, 2004; pp. 65–82. [Google Scholar]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar]
- Strasser, B.J.; Strasser, R.J. Measuring fast fluorescence transients to address environmental questions: The JIP-test. In Photosynthesis: From Light to Biosphere; Springer: Berlin/Heidelberg, Germany, 1995; pp. 977–980. [Google Scholar]
- Joshi, N.C.; Yadav, D.; Ratner, K.; Kamara, I.; Aviv-Sharon, E.; Irihimovitch, V.; Charuvi, D. Sodium hydrosulfide priming improves the response of photosynthesis to overnight frost and day high light in avocado (Persea americana Mill, cv. ‘Hass’). Physiol. Plant. 2020, 168, 394–405. [Google Scholar] [PubMed]
- Sulkiewicz, M.; Ciereszko, I. Fluorescencja chlorofilu a-historia odkrycia i zastosowanie w badaniach roślin. Kosmos 2016, 65, 103–115. [Google Scholar]
- Muñoz-Huerta, F.R.; Guevara-Gonzalez, G.R.; Contreras-Medina, M.L.; Torres-Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velazquez, R.V. A Review of Methods for Sensing the Nitrogen Status in Plants: Advantages, Disadvantages and Recent Advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef]
- Hu, H.; Zhang, G.; Zheng, K. Modeling Leaf Image, Chlorophyll Fluorescence, Reflectance from SPAD Readings. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2014, 7, 4368–4373. [Google Scholar]
- Boublin, F.; Cabassa-Hourton, C.; Leymarie, J.; Leitao, L. Potential involvement of proline and flavonols in plant responses to ozone. Environ. Res. 2022, 207, 112214. [Google Scholar] [CrossRef]
- Brazaitytė, A.; Duchovskis, P.; Urbonavičiūtė, A.; Samuolienė, G.; Jankauskienė, J.; Kasiulevičiūtė-Bonakėrė, A.; Bliznikas, Z.; Novičkovas, A.; Breivė, K.; Žukauskas, A. The effect of light-emitting diodes lighting on cucumber transplants and after-effect on yield. Zemdirb.-Agric. 2009, 96, 102–118. [Google Scholar]
- Fujiwara, K.; Fujii, T.; Park, J.-S. Comparison of Foliar Spray Efficacy of Electrolytically Ozonated Water and Acidic Electrolyzed Oxidizing Water for Controlling Powdery Mildew Infection on Cucumber Leaves. Ozone Sci. Eng. 2009, 31, 10–14. [Google Scholar] [CrossRef]
- Antos, P.; Piechowicz, B.; Gorzelany, J.; Matlok, N.; Migut, D.; Jozefczyk, R.; Balawejder, M. Effect of Ozone on Fruit Quality and Fungicide Residue Degradation in Apples during Cold Storage. Ozone Sci. Eng. 2009, 40, 482–486. [Google Scholar] [CrossRef]
- Heleno, F.F.; Queiroz, M.E.L.R.D.; Neves, A.A.; Freitas, R.S.; Faroni, L.R.A.; Oliveira, A.F.D. Effects of ozone fumigation treatment on the removal of residual difenoconazole from strawberries and on their quality. J. Environ. Sci. Health Part B 2014, 49, 94–101. [Google Scholar] [CrossRef] [PubMed]
- Wu, Q.; Zhang, Y.; Xie, M.; Zhao, Z.; Yang, L.; Liu, J.; Hou, D. Estimation of Fv/Fm in Spring Wheat Using UAV-Based Multispectral and RGB Imagery with Multiple Machine Learning Methods. Agronomy 2023, 13, 1003. [Google Scholar] [CrossRef]
- Kalaji, H.M.; Schansker, G.; Brestic, M.; Bussotti, F.; Calatayud, A.; Ferroni, L.; Goltsev, V.; Guidi, L.; Jajoo, A.; Li, P.; et al. Frequently asked questions about chlorophyll fluorescence, the sequel. Photosynth. Res. 2017, 132, 13–66. [Google Scholar] [CrossRef]
- Zardzewiały, M.; Matłok, N.; Turan, J.; Balawejder, M.; Gorzelany, J. Yeast solution spray and ozone gas fumigation as methods of protecting tomato plants against diseases. Agric. Eng. 2024, 28, 215–233. [Google Scholar] [CrossRef]
- Thwe, A.A.; Kasemsap, P. Quantification of OJIP fluorescence transient in tomato plants under acute ozone stress. Agric. Nat. Resour. 2014, 48, 665–675. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. Probing Photosynth. Mech. Regul. Adapt. 2000, 25, 445–483. [Google Scholar]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. Analysis of the fluorescence transient. In Chlorophyll a Fluorescence: A Signature of Photosynthesis; Papageogiou, G., Govindjee, Eds.; Springer: Dordrecht, The Netherlands, 2000; Volume 19, pp. 321–362. [Google Scholar]
- Hermans, C.; Smeyers, M.; Rodriguez, R.M.; Eyletters, M.; Strasser, R.; Dehaye, J.P. Quality assessment of urban’s trees: A comparative study of physiological characterisation, airborne imaging and on site fluorescence monitoring by the OJIP test. J. Plant Physiol. 2003, 160, 81–90. [Google Scholar] [PubMed]
- Strauss, A.; Krüger, G.; Strasser, R.; Van Heerden, P. Ranking of dark chilling tolerance in soybean genotypes probed by the chlorophyll a fluorescence transient O-J-I-P. Environ. Exp. Bot. 2006, 56, 147–157. [Google Scholar]
- Ballarin-Denti, A.; Bussotti, F.; Cascio, C.; Desotgiu, R.; Gerosa, G.; Marzuoli, R.; Strasser, R.J.; Tagliaferri, A. An open-top chamber experiment at Curno (Northern Italy): Results of the first year of activity. Workshop: Critical levels of ozone: Further applying and developing the flux-based concept. In Proceedings of the Background Document of the ICP Vegetation Meeting, Obergurgl, Austria, 15–19 November 2005. [Google Scholar]
- Hasanuzzaman, M.; Hossain, M.A.; da Silva, J.A.T.; Fujita, M. Plant response and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor. In Crop Stress and Its Management: Perspectives and Strategies; Springer: Dordrecht, The Netherlands, 2012; pp. 261–315. [Google Scholar]
- Lombardozzi, D.; Levis, S.; Bonan, G.; Sparks, J.P. Predicting photosynthesis and transpiration responses to ozone: Decoupling modeled photosynthesis and stomatal conductance. Biogeosciences 2012, 9, 3113–3130. [Google Scholar] [CrossRef]
- Yu, H.; Blande, J.D. A potential ozone defense in intercellular air space: Clues from intercellular BVOC concentrations and stomatal conductance. Sci. Total Environ. 2022, 852, 158456. [Google Scholar]
- Hoshika, Y.; Paoletti, E.; Centritto, M.; Gomes, M.T.G.; Puértolas, J.; Haworth, M. Species-specific variation of photosynthesis and mesophyll conductance to ozone and drought in three Mediterranean oaks. Physiol. Plant. 2022, 174, e13639. [Google Scholar]
- Matłok, N.; Piechowiak, T.; Szostek, M.; Kuboń, M.; Neuberger, P.; Kapusta, I.; Balawejder, M. Modification of Fungicide Treatment Needs and Antioxidant Content as a Result of Real-Time Ozonation of Raspberry Plants. Molecules 2024, 29, 3949. [Google Scholar] [CrossRef]

| Parameter | Formula Explanation |
|---|---|
| Fo | Minimum fluorescence intensity (50 µs) |
| F0 | Fluorescense intensity on O step (20 µs) |
| FJ | Fluorescense intensity on J step (2 ms) |
| FI | Fluorescense intensity on I step (60 ms) |
| Fm | Maximal fluorescence also known as P step |
| Fv (Fm/Fo) | Variable fluorescence of PSII (Fv = Fm − Fo) |
| Fv/Fm | Variable fluorescence to maximal fluorescence (Fv/Fm = (Fm − Fo)/Fm) |
| Fv/Fo | The stress-detecting parameter (Fv/Fo = (Fm − Fo)/Fo) |
| Vj | Vj = (Fj − Fo)/Fm − Fo |
| Mo | Mo = (F300 − F50)/(Fm − F50)/0.25 ms |
| Pitotal | PItotal = PIAbs × {(1 − Vi)/(1 − Vj)}/[1 − {(1 − Vi)/(1 − Vj)}] |
| Variable | ChlM | FlvM | AnthM | NFI | |
|---|---|---|---|---|---|
| Lonicera caerulea var. kamstchatica | |||||
| Variety | Vostorg | 0.47 ± 0.09 bc | 1.36 ± 0.12 a | 0.12 ± 0.06 ab | 0.33 ± 0.09 ab |
| Jugana | 0.50 ± 0.09 c | 1.42 ± 0.09 b | 0.11 ± 0.04 a | 0.33 ± 0.07 ab | |
| Aurora | 0.50 ± 0.10 c | 1.41 ± 0.11 b | 0.12 ± 0.06 ab | 0.34 ± 0.07 b | |
| Lonicera caerulea var. emphyllocalyx | |||||
| Lori | 0.44 ± 0.07 a | 1.40 ± 0.11 ab | 0.11 ± 0.05 a | 0.31 ± 0.06 a | |
| 21-17 | 0.41 ± 0.10 a | 1.41 ± 0.10 b | 0.13 ± 0.04 b | 0.31 ± 0.07 a | |
| 139-24 | 0.46 ± 0.11 b | 1.30 ± 0.12 a | 0.10 ± 0.06 a | 0.36 ± 0.08 b | |
| Average | 0.45 ± 0.09 | 1.41 ± 0.11 | 0.11 ± 0.06 | 0.33 ± 0.08 | |
| p value | 0.0054 | 0.0036 | 0.0058 | 0.0003 | |
| Year of study | 2022 | 0.45 ± 0.08 a | 1.40 ± 0.10 a | 0.12 ± 0.05 a | 0.34 ± 0.07 a |
| 2023 | 0.43 ± 0.10 a | 1.39 ± 0.12 a | 0.11 ± 0.08 a | 0.32 ± 0.08 a | |
| Average | 0.44 ± 0.10 | 1.40 ± 0.11 | 0.12 ± 0.06 | 0.33 ± 0.07 | |
| p value | 0.1636 | 0.2441 | 0.1845 | 0.2054 | |
| Interaction | V | V | V | V | |
| Lenght of Ozonation | Time After Ozonation [Days] | ChlM | FlvM | AnthM | NFI |
|---|---|---|---|---|---|
| Control | 1 | 0.45 ± 0.10 cd | 1.36 ± 0.10 a | 0.13 ± 0.06 b | 0.33 ± 0.08 bc |
| 3 | 0.44 ± 0.09 b | 1.39 ± 0.11 bc | 0.12 ± 0.04 ab | 0.34 ± 0.08 c | |
| 5 | 0.46 ± 0.10 d | 1.43 ± 0.09 c | 0.12 ± 0.03 ab | 0.34 ± 0.06 c | |
| Average | 0.44 ± 0.09 | 1.39 ± 0.10 | 0.13 ± 0.08 | 0.34 ± 0.08 | |
| 5 ppm·1 min | 1 | 0.44 ± 0.09 c | 1.38 ± 0.11 abc | 0.15 ± 0.08 c | 0.30 ± 0.07 a |
| 3 | 0.44 ± 0.09 c | 1.41 ± 0.11 bc | 0.11 ± 0.03 a | 0.31 ± 0.06 b | |
| 5 | 0.45 ± 0.09 c | 1.43 ± 0.12 c | 0.13 ± 0.03 ab | 0.32 ± 0.06 b | |
| Average | 0.44 ± 0.10 | 1.41 ± 0.11 | 0.13 ± 0.05 | 0.31 ± 0.07 | |
| 5p pm·3 min | 1 | 0.43 ± 0.10 bc | 1.38 ± 0.10 abc | 0.14 ± 0.08 bc | 0.33 ± 0.09 bc |
| 3 | 0.45 ± 0.11 c | 1.36 ± 0.12 a | 0.09 ± 0.03 a | 0.34 ± 0.09 c | |
| 5 | 0.45 ± 0.07 c | 1.43 ± 0.14 c | 0.12 ± 0.04 a | 0.32 ± 0.06 b | |
| Average | 0.44 ± 0.09 | 1.39±0.12 | 0.12 ± 0.06 | 0.33 ± 0.08 | |
| 5 ppm·5 min | 1 | 0.43 ± 0.11 bc | 1.37 ± 0.10 ab | 0.15 ± 0.05 c | 0.30 ± 0.08 a |
| 3 | 0.39 ± 0.08 a | 1.30 ± 0.09 abc | 0.09 ± 0.06 a | 0.32 ± 0.06 b | |
| 5 | 0.40 ± 0.09 a | 1.39 ± 0.10 c | 0.11 ± 0.03 a | 0.31 ± 0.09 b | |
| Average | 0.41 ± 0.13 | 1.43 ± 0.10 | 0.12 ± 0.03 | 0.31 ± 0.08 | |
| p value | 0.0008 | 0.0035 | 0.0024 | 0.0042 | |
| Interaction | L × T | L × T | L × T | L × T |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Basara, O.; Gorzelany, J. Impact of Ozone Exposure on Chlorophyll Fluorescence, Pigment Content and Leaf Gas Exchange on Lonicera caerulea var. kamtschatica and Lonicera caerulea var. emphyllocalyx. Sustainability 2025, 17, 2820. https://doi.org/10.3390/su17072820
Basara O, Gorzelany J. Impact of Ozone Exposure on Chlorophyll Fluorescence, Pigment Content and Leaf Gas Exchange on Lonicera caerulea var. kamtschatica and Lonicera caerulea var. emphyllocalyx. Sustainability. 2025; 17(7):2820. https://doi.org/10.3390/su17072820
Chicago/Turabian StyleBasara, Oskar, and Józef Gorzelany. 2025. "Impact of Ozone Exposure on Chlorophyll Fluorescence, Pigment Content and Leaf Gas Exchange on Lonicera caerulea var. kamtschatica and Lonicera caerulea var. emphyllocalyx" Sustainability 17, no. 7: 2820. https://doi.org/10.3390/su17072820
APA StyleBasara, O., & Gorzelany, J. (2025). Impact of Ozone Exposure on Chlorophyll Fluorescence, Pigment Content and Leaf Gas Exchange on Lonicera caerulea var. kamtschatica and Lonicera caerulea var. emphyllocalyx. Sustainability, 17(7), 2820. https://doi.org/10.3390/su17072820





