Advancing Biochar Applications: A Review of Production Processes, Analytical Methods, Decision Criteria, and Pathways for Scalability and Certification
Abstract
1. Introduction
Prospective Study on Biochar Research
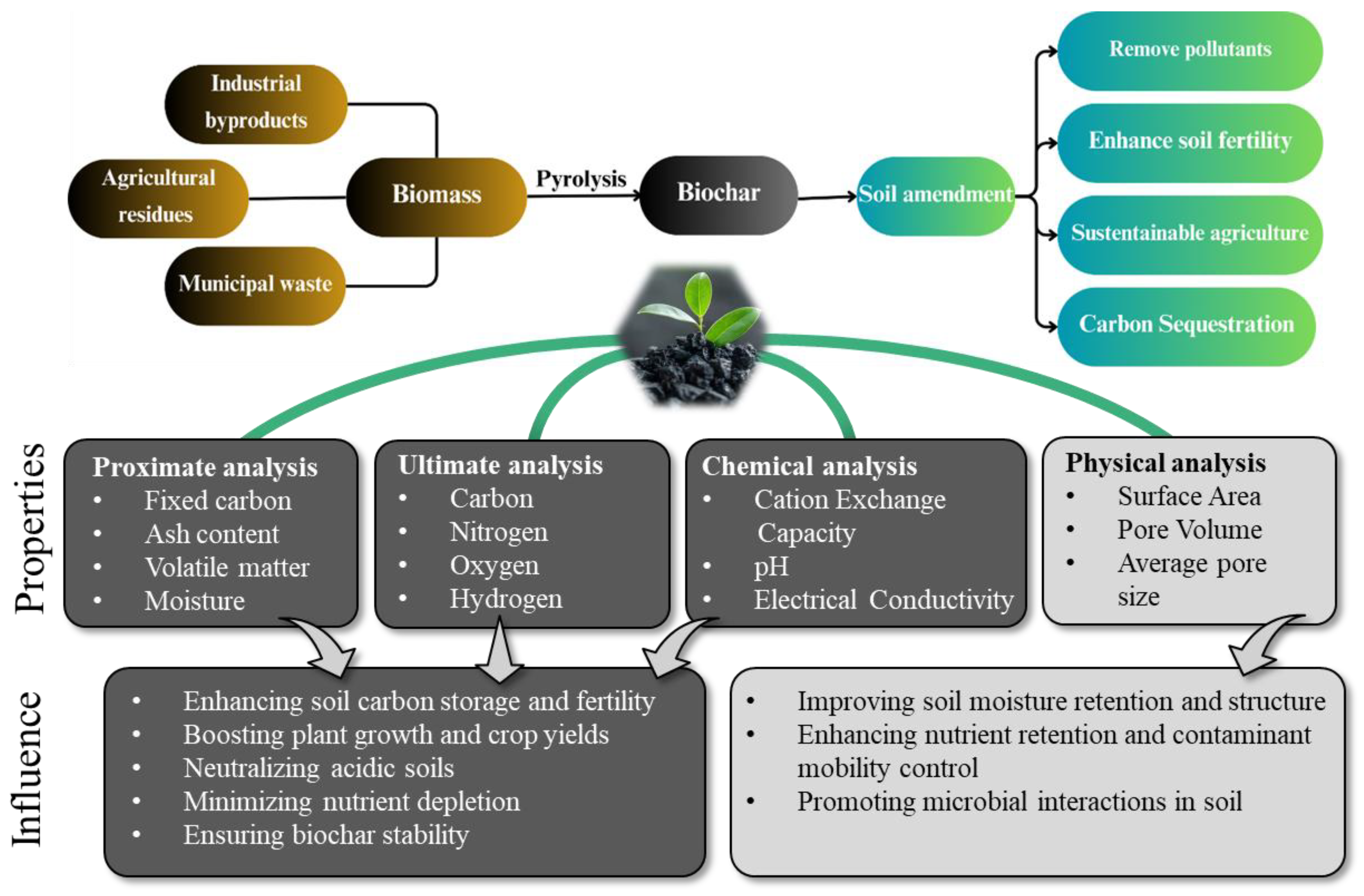
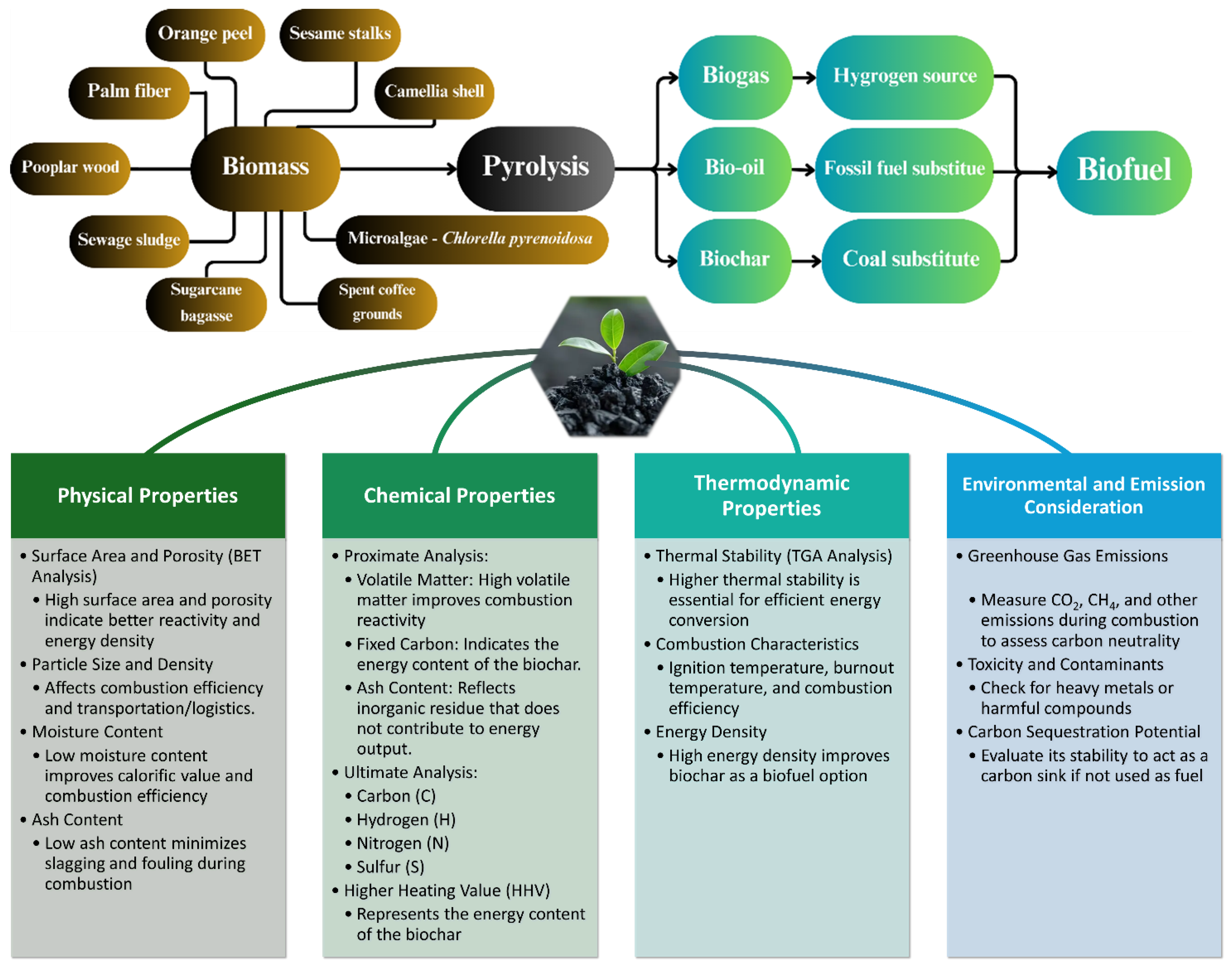
2. Biochar Production
3. Physicochemical Characterization of Biochar
3.1. Main Standard Biochar Characterization
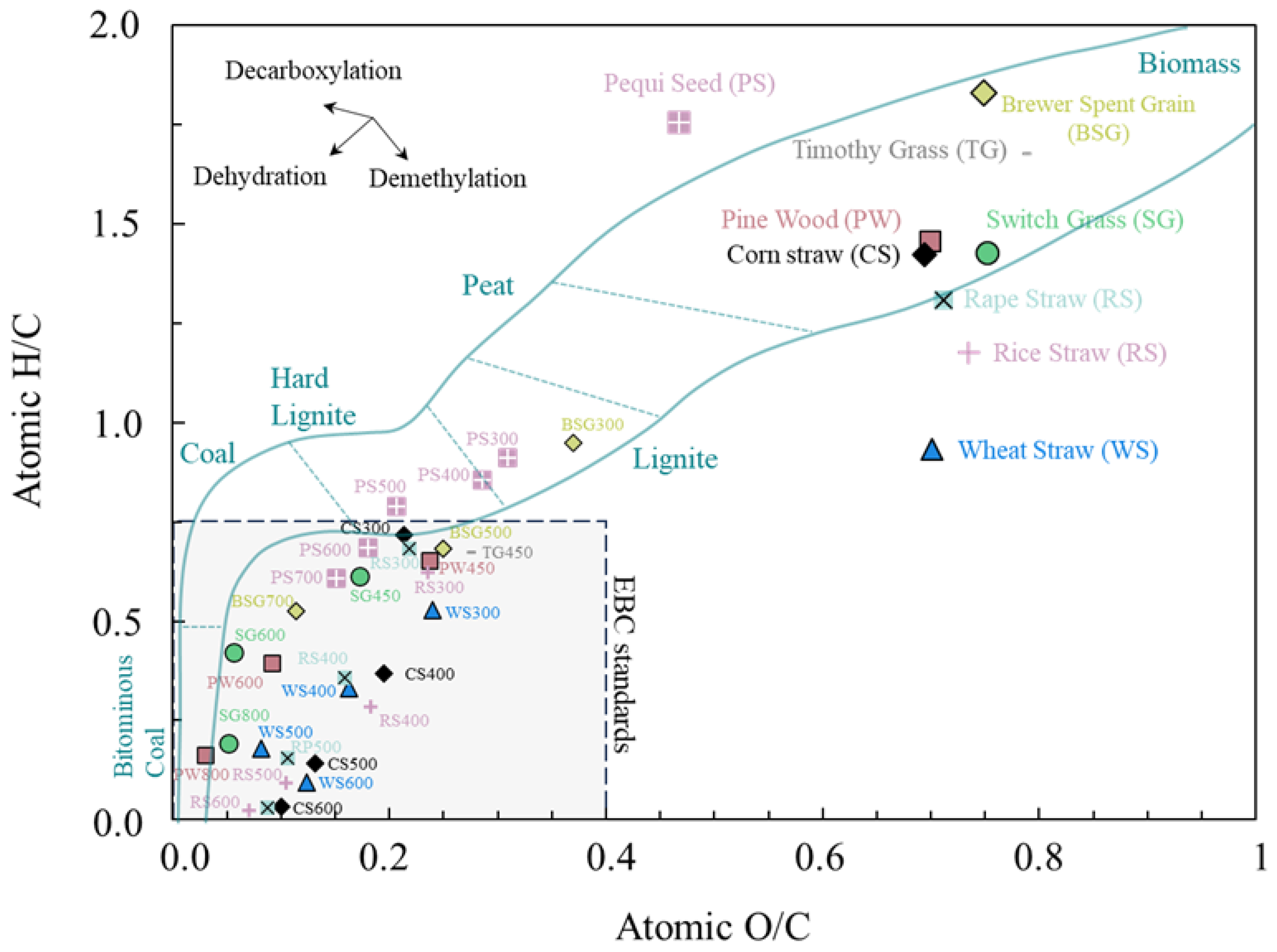
3.2. Atomic H/C and O/C Ratios
| Feedstock/Biochar | %C | %H | %O | H/C * | O/C * | HHV | Ref. |
|---|---|---|---|---|---|---|---|
| Pinewood | [67] | ||||||
| PW | 48.90 | 6.20 | 42.50 | 1.51 | 0.65 | 18.10 a/18.66 b | |
| PW450 | 75.50 | 3.70 | 17.00 | 0.58 | 0.17 | 27.80 a/27.94 b | |
| Timothy grass | |||||||
| TG | 43.40 | 6.10 | 45.40 | 1.68 | 0.79 | 15.90 a/16.23 b | |
| TG450 | 63.70 | 3.60 | 23.10 | 0.67 | 0.27 | 22.30 a/22.90 b | |
| Wheat straw | |||||||
| WS | 44.10 | 6.00 | 45.00 | 1.62 | 0.77 | 15.60 a/16.38 b | |
| WS450 | 64.80 | 3.10 | 23.00 | 0.57 | 0.27 | 22.00 a/22.58 b | |
| Pinewood-2 | [68] | ||||||
| PW-2 | 48.50 | 5.92 | 45.16 | 1.45 | 0.70 | 21.18 b | |
| PW450-2 | 71.80 | 3.94 | 22.66 | 0.65 | 0.24 | 28.08 b | |
| PW600-2 | 84.66 | 2.81 | 10.25 | 0.40 | 0.09 | 31.80 b | |
| PW800-2 | 89.70 | 1.24 | 3.61 | 0.16 | 0.03 | 31.81 b | |
| Switchgrass | |||||||
| SW | 45.58 | 5.45 | 45.65 | 1.43 | 0.75 | 20.67 b | |
| SW450 | 66.54 | 3.43 | 15.31 | 0.61 | 0.17 | 26.34 b | |
| SW600 | 71.52 | 2.53 | 5.39 | 0.42 | 0.06 | 26.81 b | |
| SW800 | 71.62 | 1.16 | 4.85 | 0.19 | 0.05 | 25.64 b | |
| Wheat straw-2 | [58] | ||||||
| WS-2 | 45.53 | 3.56 | 42.53 | 0.93 | 0.70 | 17.30 b | |
| WS300-2 | 61.48 | 2.73 | 19.61 | 0.53 | 0.24 | 22.34 b | |
| WS400-2 | 64.18 | 1.78 | 13.93 | 0.33 | 0.16 | 22.90 b | |
| WS500-2 | 67.39 | 1.01 | 7.35 | 0.18 | 0.08 | 22.35 b | |
| WS600-2 | 65.34 | 0.52 | 10.77 | 0.09 | 0.12 | 22.63 b | |
| Corn straw | |||||||
| CS | 44.53 | 5.31 | 41.18 | 1.42 | 0.69 | 19.80 b | |
| CS300 | 61.2 | 3.68 | 17.39 | 0.72 | 0.21 | 23.20 b | |
| CS400 | 63.36 | 1.96 | 16.46 | 0.37 | 0.20 | 22.27 b | |
| CS500 | 65.08 | 0.77 | 11.36 | 0.14 | 0.13 | 21.51 b | |
| CS600 | 67.48 | 0.18 | 8.98 | 0.03 | 0.10 | 16.34 b | |
| Rape straw | 44.63 | 4.89 | 42.34 | 1.31 | 0.71 | 21.91 b | |
| RP | |||||||
| RP300 | 61.8 | 3.54 | 17.95 | 0.68 | 0.22 | 23.66 b | |
| RP400 | 63.74 | 1.91 | 13.48 | 0.36 | 0.16 | 22.61 b | |
| RP500 | 66.96 | 0.87 | 9.46 | 0.15 | 0.11 | 22.45 b | |
| RP600 | 67.85 | 0.18 | 7.89 | 0.03 | 0.09 | 22.99 b | |
| Rice straw | |||||||
| RS | 42.12 | 4.16 | 41.22 | 1.18 | 0.73 | 17.30 b | |
| RS300 | 56.49 | 2.95 | 17.73 | 0.62 | 0.24 | 21.01 b | |
| RS400 | 56.42 | 1.35 | 13.71 | 0.29 | 0.18 | 19.55 b | |
| RS500 | 59.59 | 0.47 | 8.27 | 0.09 | 0.1 | 19.75 b | |
| RS600 | 61.3 | 0.12 | 5.71 | 0.02 | 0.07 | 20.71 b | |
| Brewers’ spent grain | [41] | ||||||
| BSG | 44.72 | 6.86 | 44.66 | 1.83 | 0.75 | 17.06 a/17.87 b | |
| BSG300 | 63.28 | 5.03 | 31.2 | 0.95 | 0.37 | ~24 a/23.55 b | |
| BSG500 | 71.67 | 4.11 | 23.84 | 0.68 | 0.25 | ~27 a/26.19 b | |
| BSG700 | 83.17 | 3.66 | 12.62 | 0.52 | 0.11 | 31.23 a/31.13 b | |
| Grape seeds | [72] | ||||||
| GSs | 45.00 | 6.99 | 44.40 | 0.16 | 0.99 | 18.18 b | |
| GSs300_3h | 51.00 | 5.57 | 39.20 | 0.11 | 0.77 | 18.97 b | |
| GSs300_24h | 61.70 | 3.10 | 29.80 | 0.05 | 0.48 | 20.49 b | |
| Defatted grape seeds | |||||||
| DGSs | 47.2 | 6.72 | 38.9 | 0.14 | 0.82 | 19.38 b | |
| DGSs300_3h | 57.8 | 5.08 | 28.8 | 0.09 | 0.5 | 22.16 b | |
| DGSs300_24h | 63.1 | 3.62 | 23.4 | 0.06 | 0.37 | 22.69 b | |
| WSs300_3h | 51.00 | 5.57 | 39.20 | 0.11 | 0.77 | 18.97 | |
| Wood stems | |||||||
| WGSs | 51.5 | 6.01 | 38.1 | 0.12 | 0.77 | 19.94 b | |
| WSs300_3h | 63.9 | 5.98 | 24.7 | 0.09 | 0.52 | 26.11 b | |
| WSs300_24h | 68.8 | 5.62 | 19.5 | 0.06 | 0.39 | 28.04 b | |
| Whole grape seeds | |||||||
| DGSs | 51.2 | 6.08 | 39.2 | 0.12 | 0.77 | 19.77 b | |
| DSs300_3h | 58.9 | 5.28 | 30.6 | 0.09 | 0.52 | 22.53 b | |
| DSs300_24h | 64.9 | 3.72 | 25.00 | 0.06 | 0.39 | 23.19 b | |
| Oil palm trunk | [73] | ||||||
| OPT | 45.79 | 6.15 | 46.33 | 0.13 | 1.01 | 16.96 b | |
| OPT500 | 77.53 | 3.45 | 18.63 | 0.04 | 0.24 | 28.01 b | |
| OPT550 | 79.35 | 1.87 | 16.65 | 0.02 | 0.21 | 26.68 b | |
| OPT600 | 82.02 | 1.59 | 14.22 | 0.02 | 0.17 | 27.55 b | |
| Oil palm fronds | |||||||
| OPF | 44.95 | 5.89 | 48.71 | 0.13 | 1.08 | 15.94 b | |
| OPF500 | 75.07 | 2.41 | 22.00 | 0.03 | 0.29 | 25.19 b | |
| OPF550 | 76.41 | 1.8 | 21.31 | 0.02 | 0.28 | 24.88 b | |
| OPF600 | 78.34 | 1.91 | 19.19 | 0.02 | 0.24 | 26.01 b | |
| Rubberwood awdust | |||||||
| RWS | 47.55 | 6.22 | 45.91 | 0.13 | 0.97 | 17.71 b | |
| RWS500 | 76.65 | 2.71 | 20.21 | 0.04 | 0.26 | 26.42 b | |
| RWS550 | 78.59 | 3.3 | 17.63 | 0.04 | 0.22 | 28.31 b | |
| RWS600 | 80.19 | 2.09 | 17.35 | 0.03 | 0.22 | 27.17 b |
3.3. Calorific Value
3.4. Spectroscopic Analysis
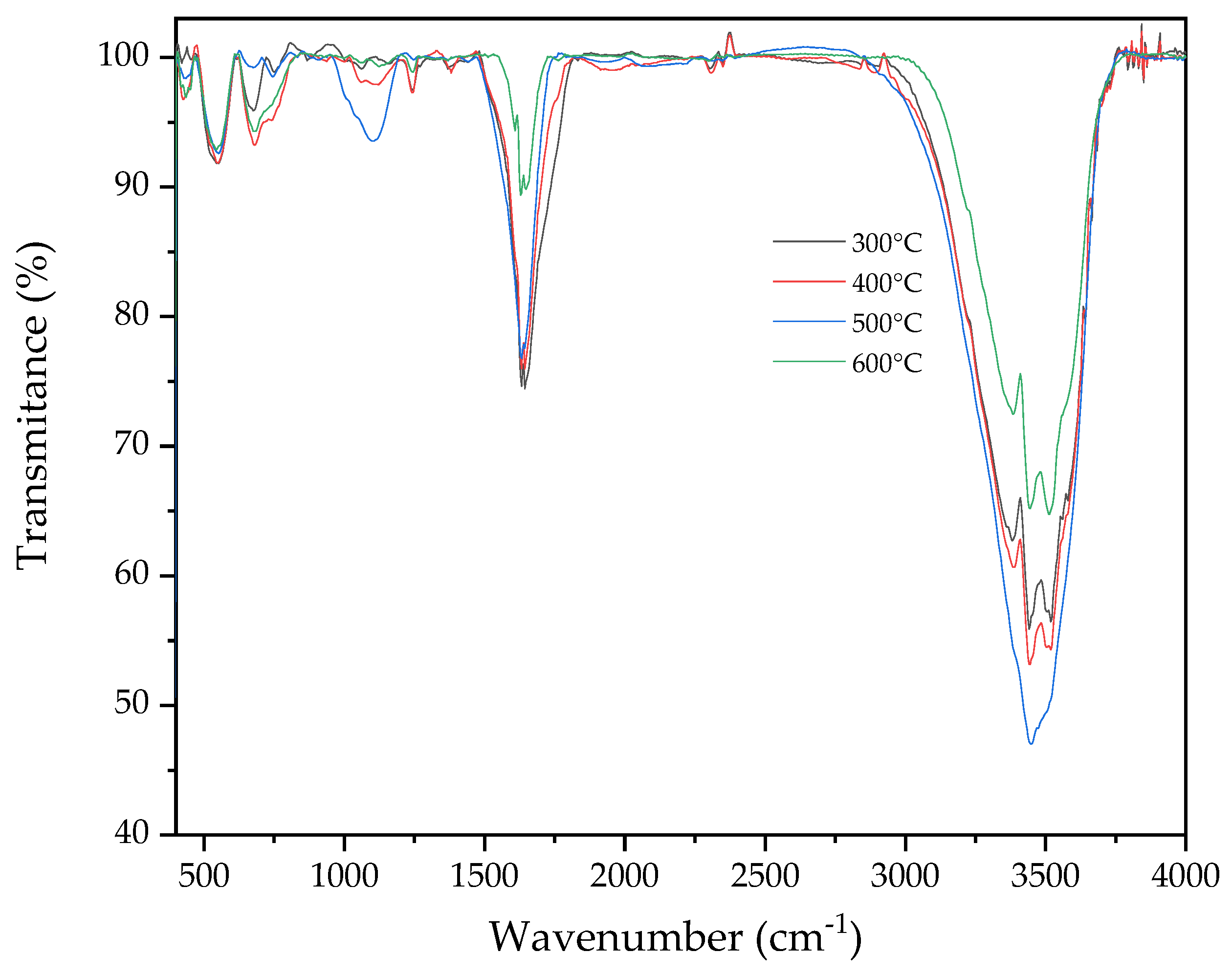
3.4.1. Fourier Transform Infrared (FTIR) Spectroscopy
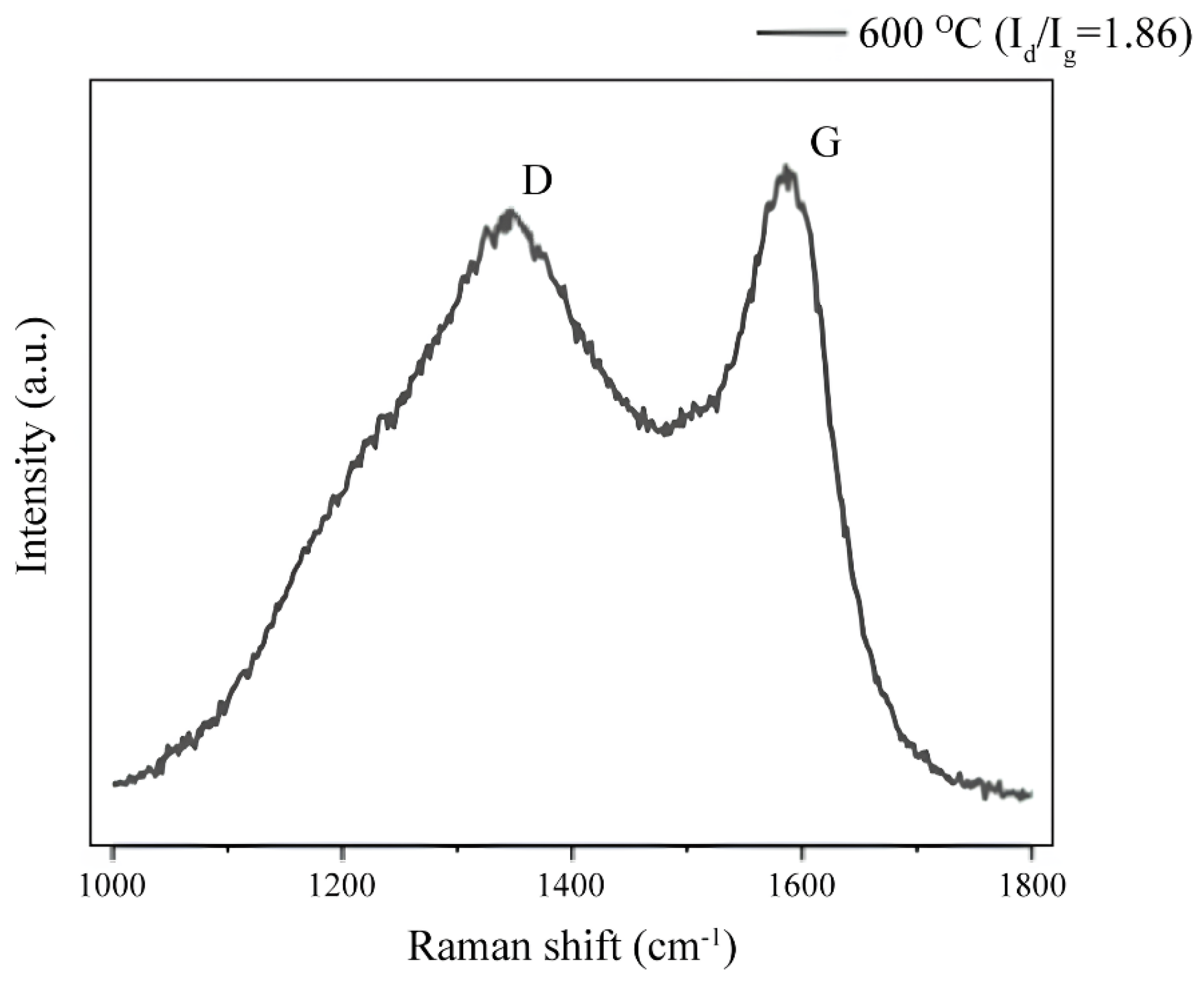
3.4.2. Raman Spectroscopy
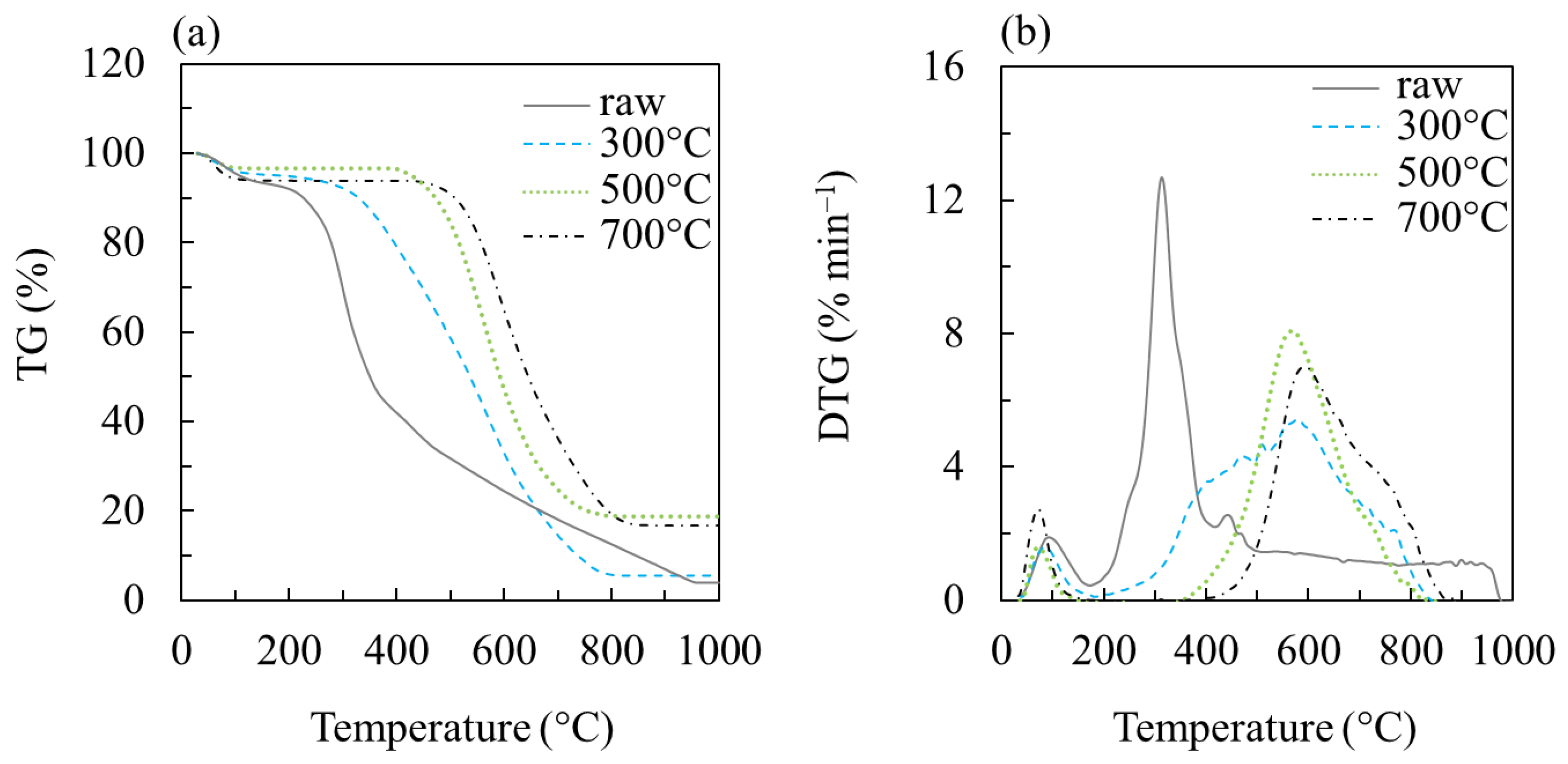
3.5. Thermogravimetric Analysis (TGA)
3.6. Scanning Electron Microscopy (SEM)
3.7. Böehm Titration—Functional Group Identification and Acidity Analysis
3.8. Solid-State Nuclear Magnetic Resonance (ssNMR)
3.9. X-Ray Photoelectron Spectroscopy (XPS)
3.10. X-Ray Diffraction (XRD)
4. Biochar Standard Application
4.1. Soil Amendment
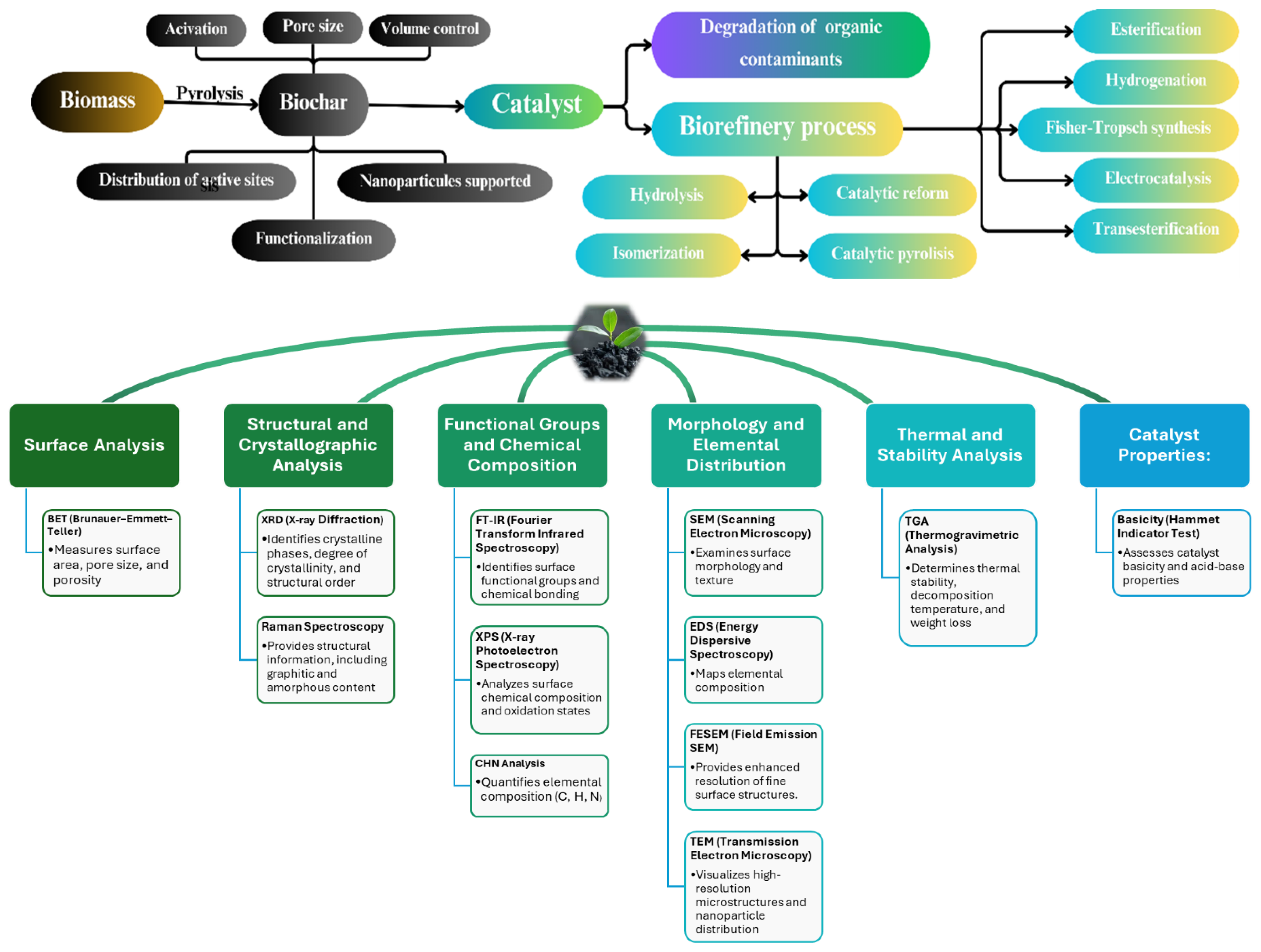
4.2. Catalysis
4.3. Biofuel
4.4. Wastewater Treatment
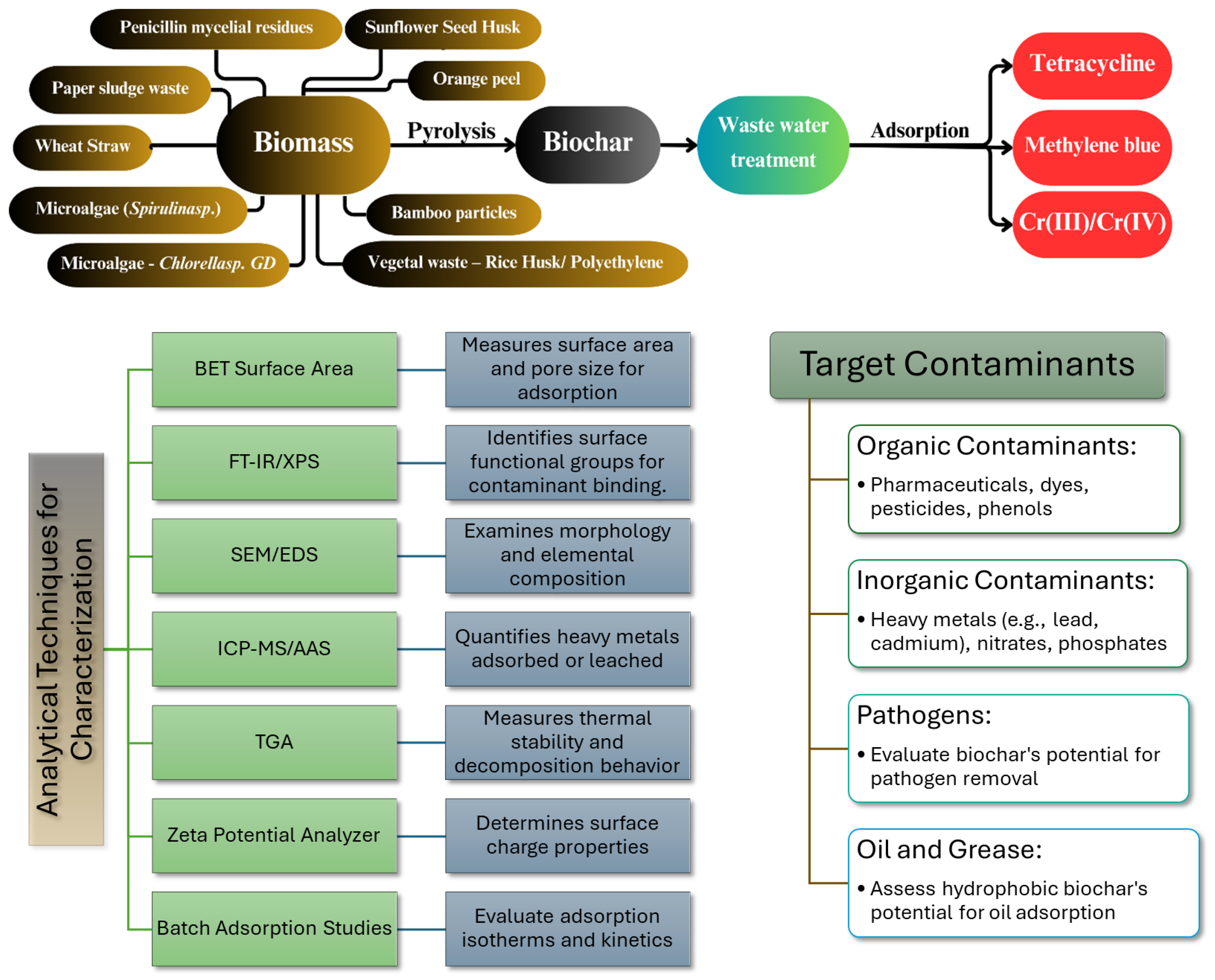
| Biomass | Process | Contam. | Biochar Properties and Adsorption Data | Ref. |
|---|---|---|---|---|
| Microalgae (Spirulina sp.) | Pyrolysis Atmosphere: N2 Temp.: 750 °C Time: 120 min | Tetracycline (TC) | O/C—0.138; H/C—1.38; SSA—2.63 m2 g−1; desorption efficiency—61%; highest TG adsorption at 147.9 mg g−1 (TC 100 mg L−1; dosage 0,1 g L−1; pH 6). | [148] |
| Wheat straw | Pyrolysis Atmosphere: N2 Temp.: 500 °C Time: 120 min Activation: KMnO4/KOH | O/C—0.225; H/C—0.007; SSA—1524.6 m2 g−1; pore volume—0.85 cm3 g−1; Raman ID/IG before adsorption–2.58; desorption efficiency (NaOH solution)—7%; highest TG adsorption at 584.19 mg g−1 at 318 K (TC 10–200 mg L−1; pH 3–10); no co-existing ion effect | [149] | |
| Sunflower seed husk | Pyrolysis Atmosphere: N2 Temp.: 700 °C Time: 120 min Activation: KMnO4/KOH/ZnCl2 | O/C—0.1; H/C—0.014; SSA—1578.3 m2 g−1; pore volume—1.138 cm3 g−1; Raman ID/IG before adsorption–0.585; desorption efficiency–97.61%; highest TG adsorption at 673.0 mg g−1 at 298 K for 24 h (TC 1–20 mg L−1; pH 3.0–11.0); highest TG adsorption with ions at 583.1 (K+), 539.8 (Mg2+) and 555.9 (Ca2+). | [150] | |
| Microalgae—Chlorella sp. GD | Wet Torrefaction (water vapor) and Microwave Torref. (2450 MHz, 800 W) Temp.: 160–170 °C Time: 5–10 min | Methylene Blue (MB) | O/C between (0.462–0.506); SSA—2.66 m2 g−1; pore volume 0.00043 cm3 g−1; maximum removal of 85.47% MB; highest MB adsorption at 113 mg g−1 (optimum pH 2–8). | [151,152] |
| Penicillin mycelial residues | Torrefaction Atmosphere: N2 Temp.: 260 °C HR.: 5 °C min−1 Time: 45 min Impregnation: KOH | O/C—0.08; H/C—0.021; SSA—1809.74 m2 g−1; pore volume 1.02 cm3 g−1; Raman ID/IG before adsorption—1.21; highest MB adsorption at 620 mg g−1. | [153] | |
| Bamboo particles | Pyrolysis Atmosphere: N2 Temp.: 700 °C HR.: 10 °C min−1 Time: 120 min Impregnation: KHCO3/Urea | O/C—0.081; SSA—1693 m2 g−1; biochar total pore volume 0.90 cm3 g−1; Raman ID/IG before adsorption—1.10; highest MB adsorption at 499 mg g−1. | [154] | |
| Orange peel | Pyrolysis Atmosphere: N2 Temp.: 400 °C HR.: 5 °C min−1 Time: 180 min Activation: KOH | Cr(III)/Cr(IV) | Cr(IV) synthetic solution; O/C—0.100; SSA—998 m2 g−1; biochar total pore volume—1.24 cm3 g−1; Raman ID/IG before adsorption–1.03; highest Cr(IV) adsorption at 285.5 mg g−1 (pH—2, dosage 0.2 g L−1, C0 100 mg L−1, T = 25 °C, contact time = 40 h). | [155] |
| Vegetal waste—rice husk/polyethylene | Co-pyrolysis Atmosphere: N2 Temp.: 390 °C Time: 35 min | Cr(III) synthetic solution; O/C 0.089; Biochar SSA < 5.0 m2 g−1; adsorption capacity—9.23 mg g−1 (final pH 4–5). | [156] | |
| Algae—Potamogeton crispus | Pyrolysis Atmosphere: N2 Temp.: 300 °C HR.: 5 °C min−1 Time: 120 min | Cr(IV) synthetic solution; O/C—0.53; H/C—0.09; SSA—0.42 m2 g−1; Pore volume—0.002 cm3 g−1; Raman ID/IG before adsorption—1.83; highest Cr(IV) adsorption at 34.37 mg g−1 (pH–2, dosage 2 g L−1, C0 100 mg L−1 and T = 25 °C.). | [157] | |
| Paper sludge waste | Pyrolysis Atmosphere: N2 Temp.: 350–550 °C HR.: 5 °C min−1 Time: 120 min Activation (KOH) Temp.: 105 °C Time: 150 min/ Pyrolysis Atmosphere: N2 Temp.: 800 °C Time: 120 min Impregnation: NH4Cl | Cr(IV) synthetic solution; O/C—0.383; H/C—0.059; SSA—3336.7 m2 g−1; pore volume—2.10 cm3 g−1; Raman ID/IG before adsorption—0.95; highest Cr(IV) adsorption at 356.25 mg g−1 (99% removal under 30 min). | [158] |
4.5. Application Trends of Biochar
4.5.1. Carbon Sequestration
4.5.2. Surface Coatings
4.5.3. Oil–Water Separation
4.5.4. Other Applications
5. Environmental Impacts of Biochar Production and Applications
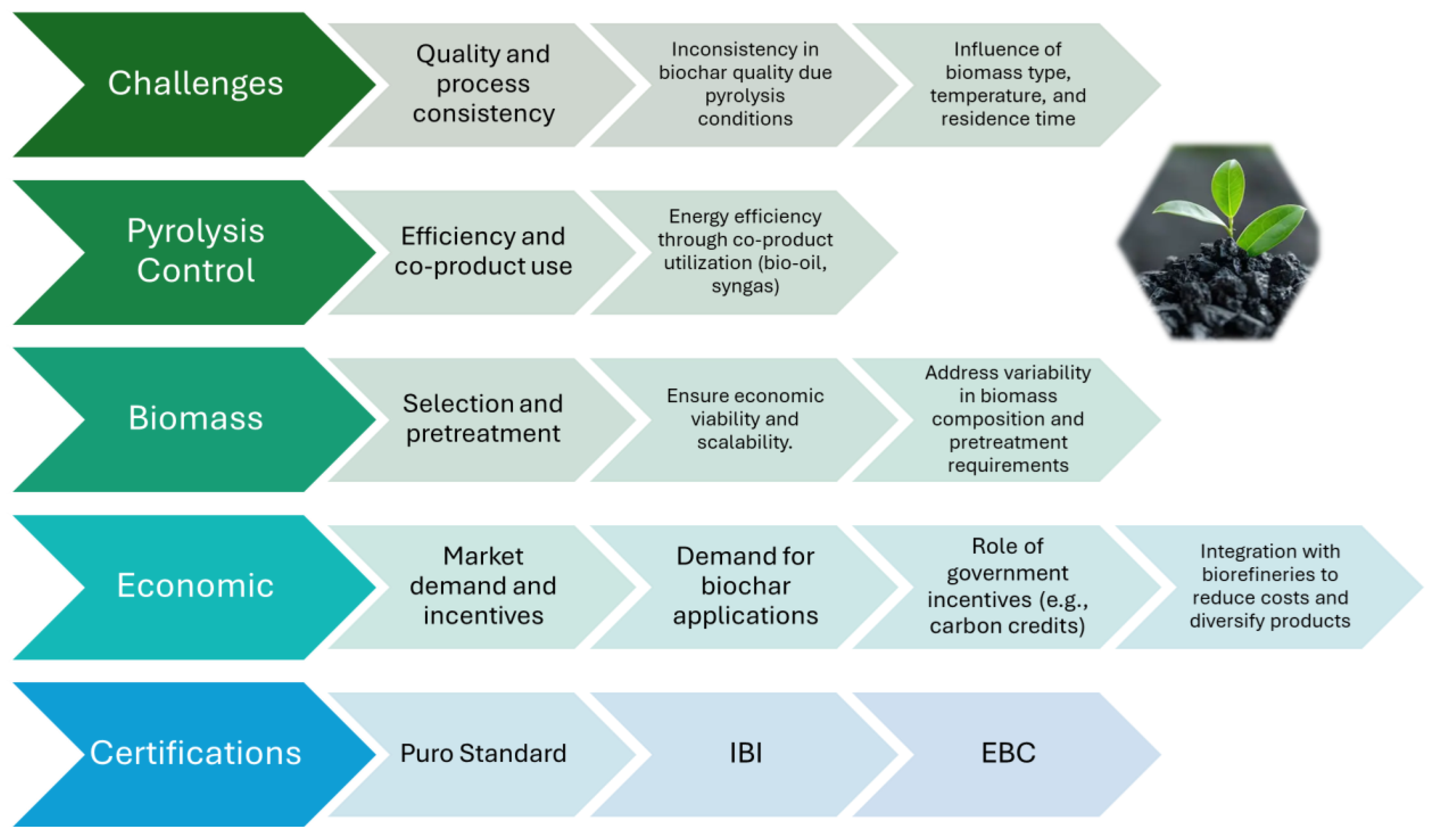
6. Scalability and Practical Large-Scale Implementation of Biochar
6.1. Control of Pyrolysis Conditions, Energy Efficiency, and Co-Product Utilization
6.2. Biomass Optimization
6.3. Economic Viability and Incentives
6.4. Companies Producing Biochar and Certifications Used
6.4.1. NetZero
6.4.2. Biochar Works
6.4.3. Carbon Gold
7. Multi-Criteria Decision Analysis
MCDM Criteria Selection, Analytical Technique, and Optimization
8. Conclusions and Perspectives
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sieradzka, M.; Kirczuk, C.; Kalemba-Rec, I.; Mlonka-Mędrala, A.; Magdziarz, A. Pyrolysis of Biomass Wastes into Carbon Materials. Energies 2022, 15, 1941. [Google Scholar] [CrossRef]
- Menezes, L.N.B.; Silveira, E.A.; Mazzoni, J.V.S.; Evaristo, R.B.W.; Rodrigues, J.S.; Lamas, G.C.; Suarez, P.A.Z.; Ghesti, G.F. Alternative Valuation Pathways for Primary, Secondary, and Tertiary Sewage Sludge: Biochar and Bio-Oil Production for Sustainable Energy. Biomass Convers. Biorefinery 2022, 12, 1–12. [Google Scholar] [CrossRef]
- Glushkov, D.; Nyashina, G.; Shvets, A.; Pereira, A.; Ramanathan, A. Current Status of the Pyrolysis and Gasification Mechanism of Biomass. Energies 2021, 14, 7541. [Google Scholar] [CrossRef]
- Xie, Y.; Wang, L.; Li, H.; Westholm, L.J.; Carvalho, L.; Thorin, E.; Yu, Z.; Yu, X.; Skreiberg, Ø. A Critical Review on Production, Modification and Utilization of Biochar. J. Anal. Appl. Pyrolysis 2022, 161, 105405. [Google Scholar] [CrossRef]
- Al-Rumaihi, A.; Shahbaz, M.; Mckay, G.; Mackey, H.; Al-Ansari, T. A Review of Pyrolysis Technologies and Feedstock: A Blending Approach for Plastic and Biomass towards Optimum Biochar Yield. Renew. Sustain. Energy Rev. 2022, 167, 112715. [Google Scholar] [CrossRef]
- Silveira, E.A.; Macedo, L.A.; Candelier, K.; Rousset, P.; Commandré, J.-M. Assessment of Catalytic Torrefaction Promoted by Biomass Potassium Impregnation through Performance Indexes. Fuel 2021, 304, 121353. [Google Scholar] [CrossRef]
- Silveira, E.A.; Luz, S.; Candelier, K.; Macedo, L.A.; Rousset, P. An Assessment of Biomass Torrefaction Severity Indexes. Fuel 2021, 288, 119631. [Google Scholar] [CrossRef]
- Thengane, S.K.; Kung, K.S.; Gomez-Barea, A.; Ghoniem, A.F. Advances in Biomass Torrefaction: Parameters, Models, Reactors, Applications, Deployment, and Market. Prog. Energy Combust. Sci. 2022, 93, 101040. [Google Scholar] [CrossRef]
- Chen, W.-H.H.; Lin, B.-J.J.; Lin, Y.-Y.Y.; Chu, Y.-S.S.; Ubando, A.T.; Show, P.L.; Ong, H.C.; Chang, J.-S.S.; Ho, S.-H.H.; Culaba, A.B.; et al. Progress in Biomass Torrefaction: Principles, Applications and Challenges. Prog. Energy Combust. Sci. 2021, 82, 100887. [Google Scholar] [CrossRef]
- Muzyka, R.; Misztal, E.; Hrabak, J.; Banks, S.W.; Sajdak, M. Various Biomass Pyrolysis Conditions Influence the Porosity and Pore Size Distribution of Biochar. Energy 2023, 263, 126128. [Google Scholar] [CrossRef]
- Premchand, P.; Demichelis, F.; Chiaramonti, D.; Bensaid, S.; Fino, D. Biochar Production from Slow Pyrolysis of Biomass under CO2 Atmosphere: A Review on the Effect of CO2 Medium on Biochar Production, Characterisation, and Environmental Applications. J. Environ. Chem. Eng. 2023, 11, 110009. [Google Scholar] [CrossRef]
- Brown, T.R.; Wright, M.M.; Brown, R.C. Estimating Profitability of Two Biochar Production Scenarios: Slow Pyrolysis vs Fast Pyrolysis. Biofuels Bioprod. Biorefining 2011, 5, 54–68. [Google Scholar] [CrossRef]
- Lamas, G.C.; Chaves, B.S.; Oliveira, P.P.; Barbosa, T.; Gonzales, T.d.S.; Ghesti, G.F.; Rousset, P.; Silveira, E.A. Effect of Torrefaction on Steam-Enhanced Co-Gasification of an Urban Forest and Landfill Waste Blend: H2 Production and CO2 Emissions Mitigation. Int. J. Hydrogen Energy 2023, 48, 27151–27169. [Google Scholar] [CrossRef]
- Ghesti, G.F.; Silveira, E.A.; Guimarães, M.G.; Evaristo, R.B.W.W.; Costa, M. Towards a Sustainable Waste-to-Energy Pathway to Pequi Biomass Residues: Biochar, Syngas, and Biodiesel Analysis. Waste Manag. 2022, 143, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, M.I.; Silveira, E.A. Visualização Da Dinâmica de Liberação de Voláteis Durante o Tratamento Térmico de Materiais Lignocelulósicos. In Proceedings of the 27o Congresso de Iniciação Científica da UnB e 18o Congresso de Iniciação Científica do DF, Online, 27 September–1 October 2021. [Google Scholar]
- Rodrigues, J.P.; Ghesti, G.F.; Silveira, E.A.; Lamas, G.C.; Ferreira, R.; Costa, M. Waste-to-Hydrogen via CO2/Steam-Enhanced Gasification of Spent Coffee Ground. Clean. Chem. Eng. 2022, 4, 100082. [Google Scholar] [CrossRef]
- Collard, F.-X.X.; Blin, J. A Review on Pyrolysis of Biomass Constituents: Mechanisms and Composition of the Products Obtained from the Conversion of Cellulose, Hemicelluloses and Lignin. Renew. Sustain. Energy Rev. 2014, 38, 594–608. [Google Scholar] [CrossRef]
- Tran, H.-T.; Bolan, N.S.; Lin, C.; Binh, Q.A.; Nguyen, M.-K.; Luu, T.A.; Le, V.-G.; Pham, C.Q.; Hoang, H.-G.; Vo, D.-V.N. Succession of Biochar Addition for Soil Amendment and Contaminants Remediation during Co-Composting: A State of Art Review. J. Environ. Manag. 2023, 342, 118191. [Google Scholar] [CrossRef]
- Mosa, A.; Mansour, M.M.; Soliman, E.; El-Ghamry, A.; El Alfy, M.; El Kenawy, A.M. Biochar as a Soil Amendment for Restraining Greenhouse Gases Emission and Improving Soil Carbon Sink: Current Situation and Ways Forward. Sustainability 2023, 15, 1206. [Google Scholar] [CrossRef]
- Zhou, S.; Jiang, Z.; Shen, J.; Yao, Q.; Yang, X.; Li, X.; Awasthi, M.K.; Zhang, Z. Biochar-Amended Compost as a Promising Soil Amendment for Enhancing Plant Productivity: A Meta-Analysis Study. Sci. Total Environ. 2023, 879, 163067. [Google Scholar] [CrossRef]
- Conte, P.; Schmidt, H.-P.; Cimò, G. Research and Application of Biochar in Europe. In Agricultural and Envioronmental Applications of Biochar: Advances and Barrirers; Soil Science Society of America, Inc.: Madison, WI, USA, 2016; pp. 409–422. [Google Scholar]
- Sato, M.K.; de Lima, H.V.; Costa, A.N.; Rodrigues, S.; Pedroso, A.J.S.; de Freitas Maia, C.M.B. Biochar from Acai Agroindustry Waste: Study of Pyrolysis Conditions. Waste Manag. 2019, 96, 158–167. [Google Scholar] [CrossRef]
- Mbugua Nyambura, S.; Li, C.; Li, H.; Xu, J.; Wang, J.; Zhu, X.; Feng, X.; Li, X.; Bertrand, G.V.; Ndiithi Ndumia, J.; et al. Microwave Co-Pyrolysis of Kitchen Food Waste and Rice Straw: Effects of Susceptor on Thermal, Surface, and Fuel Properties of Biochar. Fuel 2023, 352, 129093. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, M.; Chen, W.-H.H.; Zhang, Y.; Pétrissans, A.; Pétrissans, M.; Ho, S.-H.H. Superhydrophobic and Superlipophilic Biochar Produced from Microalga Torrefaction and Modification for Upgrading Fuel Properties. Biochar 2023, 5, 18. [Google Scholar] [CrossRef]
- Cueva Zepeda, L.; Griffin, G.; Shah, K.; Al-Waili, I.; Parthasarathy, R. Energy Potential, Flow Characteristics and Stability of Water and Alcohol-Based Rice-Straw Biochar Slurry Fuel. Renew. Energy 2023, 207, 60–72. [Google Scholar] [CrossRef]
- Su, G.; Ong, H.C.; Mohd Zulkifli, N.W.; Ibrahim, S.; Chen, W.H.; Chong, C.T.; Ok, Y.S. Valorization of Animal Manure via Pyrolysis for Bioenergy: A Review. J. Clean. Prod. 2022, 343, 130965. [Google Scholar] [CrossRef]
- Li, Y.; Gupta, R.; Zhang, Q.; You, S. Review of Biochar Production via Crop Residue Pyrolysis: Development and Perspectives. Bioresour. Technol. 2023, 369, 128423. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, H.; Wu, M. Advances in Metal/Biochar Catalysts for Biomass Hydro-Upgrading: A Review. J. Clean. Prod. 2021, 303, 126825. [Google Scholar] [CrossRef]
- Ramos, R.; Abdelkader-Fernández, V.K.; Matos, R.; Peixoto, A.F.; Fernandes, D.M. Metal-Supported Biochar Catalysts for Sustainable Biorefinery, Electrocatalysis, and Energy Storage Applications: A Review. Catalysts 2022, 12, 207. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, X.; Ren, H.; Hu, X.; Yang, X.; Liu, H. Co-Production of Spirosiloxane and Biochar Adsorbent from Wheat Straw by a Low-Cost and Environment-Friendly Method. J. Environ. Manag. 2023, 338, 117851. [Google Scholar] [CrossRef]
- Liang, H.; Wang, W.; Liu, H.; Deng, X.; Zhang, D.; Zou, Y.; Ruan, X. Porous MgO-Modified Biochar Adsorbents Fabricated by the Activation of Mg(NO3)2 for Phosphate Removal: Synergistic Enhancement of Porosity and Active Sites. Chemosphere 2023, 324, 138320. [Google Scholar] [CrossRef]
- Li, H.; Tang, M.; Huang, X.; Wang, L.; Liu, Q.; Lu, S. An Efficient Biochar Adsorbent for CO2 Capture: Combined Experimental and Theoretical Study on the Promotion Mechanism of N-Doping. Chem. Eng. J. 2023, 466, 143095. [Google Scholar] [CrossRef]
- Ajala, O.A.; Akinnawo, S.O.; Bamisaye, A.; Adedipe, D.T.; Adesina, M.O.; Okon-Akan, O.A.; Adebusuyi, T.A.; Ojedokun, A.T.; Adegoke, K.A.; Bello, O.S. Adsorptive Removal of Antibiotic Pollutants from Wastewater Using Biomass/Biochar-Based Adsorbents. RSC Adv. 2023, 13, 4678–4712. [Google Scholar] [CrossRef] [PubMed]
- Rangabhashiyam, S.; Lins, P.V.d.S.; Oliveira, L.M.T.d.M.; Sepulveda, P.; Ighalo, J.O.; Rajapaksha, A.U.; Meili, L. Sewage Sludge-Derived Biochar for the Adsorptive Removal of Wastewater Pollutants: A Critical Review. Environ. Pollut. 2022, 293, 118581. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.; Cheng, S.; Xing, B.; Qu, X.; Shi, C.; Meng, W.; Zhang, C.; Xia, H. Preparation of Pyrolysis Products by Catalytic Pyrolysis of Poplar: Application of Biochar in Antibiotic Wastewater Treatment. Chemosphere 2023, 338, 139519. [Google Scholar] [CrossRef]
- Fseha, Y.H.; Shaheen, J.; Sizirici, B. Phenol Contaminated Municipal Wastewater Treatment Using Date Palm Frond Biochar: Optimization Using Response Surface Methodology. Emerg. Contam. 2023, 9, 100202. [Google Scholar] [CrossRef]
- Ajibade, F.O.; Yin, W.-X.; Guadie, A.; Ajibade, T.F.; Liu, Y.; Kumwimba, M.N.; Liu, W.-Z.; Han, J.-L.; Wang, H.-C.; Wang, A.-J. Impact of Biochar Amendment on Antibiotic Removal and ARGs Accumulation in Constructed Wetlands for Low C/N Wastewater Treatment. Chem. Eng. J. 2023, 459, 141541. [Google Scholar] [CrossRef]
- Wijitkosum, S. Biochar Derived from Agricultural Wastes and Wood Residues for Sustainable Agricultural and Environmental Applications. Int. Soil Water Conserv. Res. 2022, 10, 335–341. [Google Scholar] [CrossRef]
- Duan, H.; Lyu, H.; Shen, B.; Tian, J.; Pu, X.; Wang, F.; Wang, X. Superhydrophobic-Superoleophilic Biochar-Based Foam for High-Efficiency and Repeatable Oil-Water Separation. Sci. Total Environ. 2021, 780, 146517. [Google Scholar] [CrossRef]
- Spanu, D.; Binda, G.; Dossi, C.; Monticelli, D. Biochar as an alternative sustainable platform for sensing applications: A review. Microchem. J. 2020, 159, 105506. [Google Scholar] [CrossRef]
- Evaristo, R.B.W.; Ferreira, R.; Petrocchi Rodrigues, J.; Sabino Rodrigues, J.; Ghesti, G.F.; Silveira, E.A.; Costa, M. Multiparameter-Analysis of CO2/Steam-Enhanced Gasification and Pyrolysis for Syngas and Biochar Production from Low-Cost Feedstock. Energy Convers. Manag. X 2021, 12, 100138. [Google Scholar] [CrossRef]
- Silveira, E.A.; Barcelo, R.; Cruz Lamas, G.; Paulo de Oliveira Rodrigues, P.; Santana Chaves, B.; de Paula Protásio, T.; Rousset, P.; Ghesti, G. Biofuel from Agro-Industrial Residues as Sustainable Strategy for CO2 Mitigation: Statistical Optimization of Pequi Seeds Torrefaction. Energy Convers. Manag. 2024, 304, 118222. [Google Scholar] [CrossRef]
- Quintella, C.M. A revista cadernos de prospecção e os níveis de maturidade de tecnologias (TRL). Cad. Prospecção 2017, 10, 1. [Google Scholar] [CrossRef]
- NASA Technology Readiness Level. Available online: www.nasa.gov/wp-content/uploads/2017/12/458490main_trl_definitions.pdf (accessed on 1 November 2024).
- Zhang, X.; Luo, Y.; Müller, K.; Chen, J.; Lin, Q.; Xu, J.; Tian, Y.; Cong, H.; Wang, H. Research and Application of Biochar in China. In Agricultural and Envioronmental Applications of Biochar: Advances and Barrirers; Soil Science Society of America, Inc.: Madison, WI, USA, 2016; pp. 377–407. [Google Scholar]
- He, Z.; Uchimiya, S.M.; Guo, M. Production and Characterization of Biochar from Agricultural By-Products: Overview and Use of Cotton Biomass Residues. In Agricultural and Envioronmental Applications of Biochar: Advances and Barrirers; Soil Science Society of America, Inc.: Madison, WI, USA, 2016; pp. 63–86. [Google Scholar]
- Zheng, W.; Holm, N.; Spokas, K.A. Research and Application of Biochar in North America. In Agricultural and Envioronmental Applications of Biochar: Advances and Barrirers; Soil Science Society of America, Inc.: Madison, WI, USA, 2016; pp. 475–494. [Google Scholar]
- Lee, J.; Kim, K.-H.; Kwon, E.E. Biochar as a Catalyst. Renew. Sustain. Energy Rev. 2017, 77, 70–79. [Google Scholar] [CrossRef]
- Karakaya, F.; Kobu, B. New Product Development Process: An Investigation of Success and Failure in High-Technology and Non-High-Technology Firms. J. Bus. Ventur. 1994, 9, 49–66. [Google Scholar] [CrossRef]
- NNovotny, E.H.; de Freitas Maia, C.M.B.; de Melo Carvalho, M.T.; Madari, B.E. Biochar: Pyrogenic Carbon for Agricultural Use—A Critical Review. Rev. Bras. Ciência Solo 2015, 39, 321–344. [Google Scholar] [CrossRef]
- Keiluweit, M.; Nico, P.S.; Johnson, M.G.; Kleber, M. Dynamic Molecular Structure of Plant Biomass-Derived Black Carbon (Biochar). Environ. Sci. Technol. 2010, 44, 1247–1253. [Google Scholar] [CrossRef] [PubMed]
- Srinivasan, P.; Sarmah, A.K.; Smernik, R.; Das, O.; Farid, M.; Gao, W. A Feasibility Study of Agricultural and Sewage Biomass as Biochar, Bioenergy and Biocomposite Feedstock: Production, Characterization and Potential Applications. Sci. Total Environ. 2015, 512–513, 495–505. [Google Scholar] [CrossRef]
- Kambo, H.S.; Dutta, A. A Comparative Review of Biochar and Hydrochar in Terms of Production, Physico-Chemical Properties and Applications. Renew. Sustain. Energy Rev. 2015, 45, 359–378. [Google Scholar] [CrossRef]
- Rawat, J.; Saxena, J.; Sanwal, P. Biochar: A Sustainable Approach for Improving Plant Growth and Soil Properties. In Biochar—An Imperative Amendment for Soil and the Environment; IntechOpen: London, UK, 2019. [Google Scholar]
- Van de Velden, M.; Baeyens, J.; Brems, A.; Janssens, B.; Dewil, R. Fundamentals, Kinetics and Endothermicity of the Biomass Pyrolysis Reaction. Renew. Energy 2010, 35, 232–242. [Google Scholar] [CrossRef]
- Ghesti, G.F.; Rodrigues, J.P.; de Mendonça Brasil, A.C.; Guimarães, M.G.; Evaristo, R.B.W. Caracterização e Aplicação de Biomassa Em Tecnologias de Conversão Termoquímica; Editora UnB: Brasília, DF, Brazil, 2021. [Google Scholar]
- Steiner, C. Considerations in Biochar Characterization. In Agricultural and Envioronmental Applications of Biochar: Advances and Barrirers; Soil Science Society of America, Inc.: Madison, WI, USA, 2016; pp. 87–100. [Google Scholar]
- Ronsse, F.; van Hecke, S.; Dickinson, D.; Prins, W. Production and Characterization of Slow Pyrolysis Biochar: Influence of Feedstock Type and Pyrolysis Conditions. GCB Bioenergy 2013, 5, 104–115. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, P.; Yuan, X.; Li, Y.; Han, L. Effect of Pyrolysis Temperature and Correlation Analysis on the Yield and Physicochemical Properties of Crop Residue Biochar. Bioresour. Technol. 2020, 296, 122318. [Google Scholar] [CrossRef]
- Maia, C.M.B.F.; Madari, B.E.; Novotny, E.H. Advances in Biochar Research in Brazil. Dyn. Soil Dyn. Plant 2011, 5, 53–58. [Google Scholar]
- NREL. National Renewable Energy Laboratory—Biomass Compositional Analysis Laboratory Procedures; NREL: Golden, CO, USA, 2020. [Google Scholar]
- EBC. European Biochar Certificate—Comparison of European Biochar Certificate Verson 4.8 and IBI Biochar Standards Version 2.0; EBC: Arbaz, Switzerland, 2014. [Google Scholar]
- ASTM D1762-84; Standard Test Mehod for Chemical Analysis of Wood Charcoal. ASTM International: West Conshohocken, PA, USA, 2013.
- Igalavithana, A.D.; Mandal, S.; Niazi, N.K.; Vithanage, M.; Parikh, S.J.; Mukome, F.N.D.D.; Rizwan, M.; Oleszczuk, P.; Al-Wabel, M.; Bolan, N.; et al. Advances and Future Directions of Biochar Characterization Methods and Applications. Crit. Rev. Environ. Sci. Technol. 2017, 47, 2275–2330. [Google Scholar] [CrossRef]
- Klasson, K.T. Biochar Characterization and a Method for Estimating Biochar Quality from Proximate Analysis Results. Biomass Bioenergy 2017, 96, 50–58. [Google Scholar] [CrossRef]
- Xiao, X.; Chen, Z.; Chen, B. H/C Atomic Ratio as a Smart Linkage between Pyrolytic Temperatures, Aromatic Clusters and Sorption Properties of Biochars Derived from Diverse Precursory Materials. Sci. Rep. 2016, 6, 22644. [Google Scholar] [CrossRef] [PubMed]
- Spokas, K.A. Review of the Stability of Biochar in Soils: Predictability of O:C Molar Ratios. Carbon Manag. 2010, 1, 289–303. [Google Scholar] [CrossRef]
- Silveira, E.A.; Santanna Chaves, B.; Macedo, L.; Ghesti, G.F.; Evaristo, R.B.W.; Cruz Lamas, G.; Luz, S.M.; Protásio, T.d.P.; Rousset, P. A Hybrid Optimization Approach towards Energy Recovery from Torrefied Waste Blends. Renew. Energy 2023, 212, 151–165. [Google Scholar] [CrossRef]
- Ivanovski, M.; Goricanec, D.; Krope, J.; Urbancl, D. Torrefaction Pretreatment of Lignocellulosic Biomass for Sustainable Solid Biofuel Production. Energy 2022, 240, 122483. [Google Scholar] [CrossRef]
- McKendry, P. Energy Production from Biomass (Part 1): Overview of Biomass. Bioresour. Technol. 2002, 83, 37–46. [Google Scholar] [CrossRef] [PubMed]
- González Martínez, M.; Anca Couce, A.; Dupont, C.; da Silva Perez, D.; Thiéry, S.; Meyer, X.; Gourdon, C. Torrefaction of Cellulose, Hemicelluloses and Lignin Extracted from Woody and Agricultural Biomass in TGA-GC/MS: Linking Production Profiles of Volatile Species to Biomass Type and Macromolecular Composition. Ind. Crops Prod. 2022, 176, 114350. [Google Scholar] [CrossRef]
- D’Eusanio, V.; Lezza, A.; Anderlini, B.; Malferrari, D.; Romagnoli, M.; Roncaglia, F. Technological Prospects of Biochar Derived from Viticulture Waste: Characterization and Application Perspectives. Energies 2024, 17, 3421. [Google Scholar] [CrossRef]
- Shrivastava, P.; Kumar, A.; Tekasakul, P.; Lam, S.S.; Palamanit, A. Comparative Investigation of Yield and Quality of Bio-Oil and Biochar from Pyrolysis of Woody and Non-Woody Biomasses. Energies 2021, 14, 1092. [Google Scholar] [CrossRef]
- Nanda, S.; Mohanty, P.; Pant, K.K.; Naik, S.; Kozinski, J.A.; Dalai, A.K. Characterization of North American Lignocellulosic Biomass and Biochars in Terms of Their Candidacy for Alternate Renewable Fuels. BioEnergy Res. 2013, 6, 663–677. [Google Scholar] [CrossRef]
- Worasuwannarak, N.; Sonobe, T.; Tanthapanichakoon, W. Pyrolysis Behaviors of Rice Straw, Rice Husk, and Corncob by TG-MS Technique. J. Anal. Appl. Pyrolysis 2007, 78, 265–271. [Google Scholar] [CrossRef]
- Biagini, E.; Barontini, F.; Tognotti, L. Devolatilization of Biomass Fuels and Biomass Components Studied by TG/FTIR Technique. Ind. Eng. Chem. Res. 2006, 45, 4486–4493. [Google Scholar] [CrossRef]
- Padilla, E.R.D.; Santos, L.R.O.; Silva, D.A.d.; Barros, J.L.d.; Belini, G.B.; Yamaji, F.M.; Souza, T.M.d.; Campos, C.I.d. Eucalyptus Bark Charcoal: The Influence of Carbonization Temperature in Thermal Behavior. Mater. Res. 2019, 22, e20190371. [Google Scholar] [CrossRef]
- Kim, P.; Johnson, A.; Edmunds, C.W.; Radosevich, M.; Vogt, F.; Rials, T.G.; Labbé, N. Surface Functionality and Carbon Structures in Lignocellulosic-Derived Biochars Produced by Fast Pyrolysis. Energy Fuels 2011, 25, 4693–4703. [Google Scholar] [CrossRef]
- Berthomieu, C.; Hienerwadel, R. Fourier Transform Infrared (FTIR) Spectroscopy. Photosynth. Res. 2009, 101, 157–170. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Fang, Y.; Johnston, C.T. A Fourier-Transform Infrared Study of Biochar Aging in Soils. Soil Sci. Soc. Am. J. 2016, 80, 613–622. [Google Scholar] [CrossRef]
- Ray, A. Characterization of Biochars from Various Agricultural By-Products Using FTIR Spectroscopy, SEM Focused with Image Processing. Int. J. Agric. Environ. Biotechnol. 2020, 13, 423–430. [Google Scholar] [CrossRef]
- Janu, R.; Mrlik, V.; Ribitsch, D.; Hofman, J.; Sedláček, P.; Bielská, L.; Soja, G. Biochar Surface Functional Groups as Affected by Biomass Feedstock, Biochar Composition and Pyrolysis Temperature. Carbon Resour. Convers. 2021, 4, 36–46. [Google Scholar] [CrossRef]
- Huang, J.; Glæsner, N.; Triolo, J.M.; Bekiaris, G.; Bruun, S.; Liu, F. Application of Fourier Transform Mid-Infrared Photoacoustic Spectroscopy for Rapid Assessment of Phosphorus Availability in Digestates and Digestate-Amended Soils. Sci. Total Environ. 2022, 832, 155040. [Google Scholar] [CrossRef]
- Mukome, F.N.D.; Kilcoyne, A.L.D.; Parikh, S.J. Alteration of Biochar Carbon Chemistry during Soil Incubations: SR-FTIR and NEXAFS Investigation. Soil Sci. Soc. Am. J. 2014, 78, 1632–1640. [Google Scholar] [CrossRef]
- Orlando, A.; Franceschini, F.; Muscas, C.; Pidkova, S.; Bartoli, M.; Rovere, M.; Tagliaferro, A. A Comprehensive Review on Raman Spectroscopy Applications. Chemosensors 2021, 9, 262. [Google Scholar] [CrossRef]
- Wu, H.; Yip, K.; Tian, F.; Xie, Z.; Li, C.-Z. Evolution of Char Structure during the Steam Gasification of Biochars Produced from the Pyrolysis of Various Mallee Biomass Components. Ind. Eng. Chem. Res. 2009, 48, 10431–10438. [Google Scholar] [CrossRef]
- Bokobza, L.; Bruneel, J.-L.; Couzi, M. Raman Spectra of Carbon-Based Materials (from Graphite to Carbon Black) and of Some Silicone Composites. C 2015, 1, 77–94. [Google Scholar] [CrossRef]
- Ferrari, A.C.; Robertson, J. Interpretation of Raman Spectra of Disordered and Amorphous Carbon. Phys. Rev. B 2000, 61, 14095–14107. [Google Scholar] [CrossRef]
- Elkhalifa, S.; Parthasarathy, P.; Mackey, H.R.; Al-Ansari, T.; Elhassan, O.; Mansour, S.; McKay, G. Biochar Development from Thermal TGA Studies of Individual Food Waste Vegetables and Their Blended Systems. Biomass Convers. Biorefinery 2022, 1–18. [Google Scholar] [CrossRef]
- Zaefferer, S. A Critical Review of Orientation Microscopy in SEM and TEM. Cryst. Res. Technol. 2011, 46, 607–628. [Google Scholar] [CrossRef]
- Dinh, V.-P.; Nguyen, D.-K.; Luu, T.-T.; Nguyen, Q.-H.; Tuyen, L.A.; Phong, D.D.; Kiet, H.A.T.; Ho, T.-H.; Nguyen, T.T.P.; Xuan, T.D.; et al. Adsorption of Pb(II) from Aqueous Solution by Pomelo Fruit Peel-Derived Biochar. Mater. Chem. Phys. 2022, 285, 126105. [Google Scholar] [CrossRef]
- Anas, A.K.; Mutiara, R.; Musawwa, M.M.; Taftazani, A. Influence of Mixing Time and Mass Ratio of Precursor on Preparation of Magnetic Biochar Derived from Cassava Peel (Manihot Utilissima). EKSAKTA J. Sci. Data Anal. 2022, 10–16. [Google Scholar] [CrossRef]
- Boehm, H.P. Some Aspects of the Surface Chemistry of Carbon Blacks and Other Carbons. Carbon N. Y. 1994, 32, 759–769. [Google Scholar] [CrossRef]
- Goertzen, S.L.; Thériault, K.D.; Oickle, A.M.; Tarasuk, A.C.; Andreas, H.A. Standardization of the Boehm Titration. Part I. CO2 Expulsion and Endpoint Determination. Carbon N. Y. 2010, 48, 1252–1261. [Google Scholar] [CrossRef]
- Zhao, W.; Fernando, L.D.; Kirui, A.; Deligey, F.; Wang, T. Solid-State NMR of Plant and Fungal Cell Walls: A Critical Review. Solid State Nucl. Magn. Reson. 2020, 107, 101660. [Google Scholar] [CrossRef]
- Yang, M.; Liu, Z.; Zhang, J.; Zhu, X.; Xie, W.; Lan, H.; Huang, Y.; Ye, X.; Yang, J. Simultaneous Quantification of Cellulose and Pectin in Tobacco Using a Robust Solid-State NMR Method. Carbohydr. Res. 2022, 521, 108676. [Google Scholar] [CrossRef] [PubMed]
- Morgan, D.J. Comments on the XPS Analysis of Carbon Materials. C 2021, 7, 51. [Google Scholar] [CrossRef]
- Bagus, P.S.; Ilton, E.S.; Nelin, C.J. The Interpretation of XPS Spectra: Insights into Materials Properties. Surf. Sci. Rep. 2013, 68, 273–304. [Google Scholar] [CrossRef]
- Chen, J.; Wang, P.; Ding, L.; Yu, T.; Leng, S.; Chen, J.; Fan, L.; Li, J.; Wei, L.; Li, J.; et al. The Comparison Study of Multiple Biochar Stability Assessment Methods. J. Anal. Appl. Pyrolysis 2021, 156, 105070. [Google Scholar] [CrossRef]
- Din, S.U.; Awan, J.M.; Imran, M.; Zain-Ul-Abdin; Haq, S.; Hafeez, M.; Hussain, S.; Khan, M.S. Novel Nanocomposite of Biochar-zerovalent Copper for Lead Adsorption. Microsc. Res. Tech. 2021, 84, 2598–2606. [Google Scholar] [CrossRef]
- Dar, A.; Hafeez, M.; Sarwar, F.; Ain, N.u.; Yaseen, G. Iron-Doped Biochar, an Agricultural and Environmentally Beneficial Fertilizer. Environ. Monit. Assess. 2024, 196, 524. [Google Scholar] [CrossRef]
- Mujtaba, G.; Hayat, R.; Hussain, Q.; Ahmed, M. Physio-Chemical Characterization of Biochar, Compost and Co-Composted Biochar Derived from Green Waste. Sustainability 2021, 13, 4628. [Google Scholar] [CrossRef]
- Pasumarthi, R.; Sawargaonkar, G.; Kale, S.; Kumar, N.V.; Choudhari, P.L.; Singh, R.; Davala, M.S.; Rani, C.S.; Mutnuri, S.; Jat, M.L. Innovative Bio-Pyrolytic Method for Efficient Biochar Production from Maize and Pigeonpea Stalks and Their Characterization. J. Clean. Prod. 2024, 448, 141573. [Google Scholar] [CrossRef]
- Phuong, D.T.M.; Loc, N.X. Rice Straw Biochar and Magnetic Rice Straw Biochar for Safranin O Adsorption from Aqueous Solution. Water 2022, 14, 186. [Google Scholar] [CrossRef]
- Asghari, H.R.; Bochmann, G.; Tabari, Z.T. Effectiveness of Biochar and Zeolite Soil Amendments in Reducing Pollution of Municipal Wastewater from Nitrogen and Coliforms. Sustainability 2022, 14, 8880. [Google Scholar] [CrossRef]
- Yan, Q.; Wan, C.; Liu, J.; Gao, J.; Yu, F.; Zhang, J.; Cai, Z. Iron Nanoparticles in Situ Encapsulated in Biochar-Based Carbon as an Effective Catalyst for the Conversion of Biomass-Derived Syngas to Liquid Hydrocarbons. Green Chem. 2013, 15, 1631. [Google Scholar] [CrossRef]
- Dehkhoda, A.M.; West, A.H.; Ellis, N. Biochar Based Solid Acid Catalyst for Biodiesel Production. Appl. Catal. A Gen. 2010, 382, 197–204. [Google Scholar] [CrossRef]
- Yusuff, A.S.; Thompson-Yusuff, K.A.; Porwal, J. Sulfonated Biochar Catalyst Derived from Eucalyptus Tree Shed Bark: Synthesis, Characterization and Its Evaluation in Oleic Acid Esterification. RSC Adv. 2022, 12, 10237–10248. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yao, Z.; Zhao, L.; Li, Z.; Yi, W.; Kang, K.; Jia, J. Synthesis and Characterization of Different Activated Biochar Catalysts for Removal of Biomass Pyrolysis Tar. Energy 2021, 232, 120927. [Google Scholar] [CrossRef]
- Taghavi, S.; Norouzi, O.; Tavasoli, A.; Di Maria, F.; Signoretto, M.; Menegazzo, F.; Di Michele, A. Catalytic Conversion of Venice Lagoon Brown Marine Algae for Producing Hydrogen-Rich Gas and Valuable Biochemical Using Algal Biochar and Ni/SBA-15 Catalyst. Int. J. Hydrogen Energy 2018, 43, 19918–19929. [Google Scholar] [CrossRef]
- Zhao, C.; Shao, B.; Yan, M.; Liu, Z.; Liang, Q.; He, Q.; Wu, T.; Liu, Y.; Pan, Y.; Huang, J.; et al. Activation of Peroxymonosulfate by Biochar-Based Catalysts and Applications in the Degradation of Organic Contaminants: A Review. Chem. Eng. J. 2021, 416, 128829. [Google Scholar] [CrossRef]
- Cao, M.; Lu, M.; Yin, H.; Zhu, Q.; Xing, K.; Ji, J. Effect of Hemicellulose Extraction Pretreatment on Sulfonated Corncob Biochar for Catalytic Biodiesel Production. J. Environ. Chem. Eng. 2023, 11, 109058. [Google Scholar] [CrossRef]
- da Luz Corrêa, A.P.; da Silva, P.M.M.; Gonçalves, M.A.; Bastos, R.R.C.; da Rocha Filho, G.N.; da Conceição, L.R.V. Study of the Activity and Stability of Sulfonated Carbon Catalyst from Agroindustrial Waste in Biodiesel Production: Influence of Pyrolysis Temperature on Functionalization. Arab. J. Chem. 2023, 16, 104964. [Google Scholar] [CrossRef]
- Azman, N.S.; Khairuddin, N.; Tengku Azmi, T.S.M.; Seenivasagam, S.; Hassan, M.A. Application of Biochar from Woodchip as Catalyst Support for Biodiesel Production. Catalysts 2023, 13, 489. [Google Scholar] [CrossRef]
- Daimary, N.; Eldiehy, K.S.H.; Boruah, P.; Deka, D.; Bora, U.; Kakati, B.K. Potato Peels as a Sustainable Source for Biochar, Bio-Oil and a Green Heterogeneous Catalyst for Biodiesel Production. J. Environ. Chem. Eng. 2022, 10, 107108. [Google Scholar] [CrossRef]
- Singh, M.; Salaudeen, S.A.; Norouzi, O.; Al-Salem, S.M.; Gilroyed, B.H.; Dutta, A. Co-Pyrolysis of Biomass and Tires Using Commercial Zeolite and Biochar-Based Catalyst. Chem. Eng. Process.-Process Intensif. 2023, 187, 109356. [Google Scholar] [CrossRef]
- Derbe, T.; Zereffa, E.A.; Sani, T.; Girma, T. Synthesis of Green Heterogeneous Bifunctional Zeolite-A/Biochar Catalyst for the Production of Biodiesel from Waste Cooking Oil. Catal. Lett. 2024, 154, 5530–5545. [Google Scholar] [CrossRef]
- Ghimiș, S.B.; Oancea, F.; Raduly, M.F.; Mîrț, A.L.; Trică, B.; Cîlțea-Udrescu, M.; Vasilievici, G. Direct Hydrothermal Synthesis and Characterization of Zr–Ce-Incorporated SBA-15 Catalysts for the Pyrolysis Reaction of Algal Biomass. Energies 2024, 17, 3765. [Google Scholar] [CrossRef]
- Macedo, L.A.; Silveira, E.A.; Rousset, P.; Valette, J.; Commandré, J.-M. Synergistic Effect of Biomass Potassium Content and Oxidative Atmosphere: Impact on Torrefaction Severity and Released Condensables. Energy 2022, 254, 124472. [Google Scholar] [CrossRef]
- Silveira, E.A.; Macedo, L.A.; Rousset, P.; Candelier, K.; Galvão, L.G.O.; Chaves, B.S.; Commandré, J.-M. A Potassium Responsive Numerical Path to Model Catalytic Torrefaction Kinetics. Energy 2022, 239, 122208. [Google Scholar] [CrossRef]
- Su, G.; Mohd Zulkifli, N.W.; Ong, H.C.; Ibrahim, S.; Bu, Q.; Zhu, R. Pyrolysis of Oil Palm Wastes for Bioenergy in Malaysia: A Review. Renew. Sustain. Energy Rev. 2022, 164, 112554. [Google Scholar] [CrossRef]
- Sun, J.; Norouzi, O.; Mašek, O. A State-of-the-Art Review on Algae Pyrolysis for Bioenergy and Biochar Production. Bioresour. Technol. 2022, 346, 126258. [Google Scholar] [CrossRef]
- Bataillou, G.; Lee, C.; Monnier, V.; Gerges, T.; Sabac, A.; Vollaire, C.; Haddour, N. Cedar Wood-Based Biochar: Properties, Characterization, and Applications as Anodes in Microbial Fuel Cell. Appl. Biochem. Biotechnol. 2022, 194, 4169–4186. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Tong, X.; Yan, X.; Li, H.; Li, Y.; Gao, X.; Guo, Y.; Wu, W.; Fu, D.; Huang, X.; et al. Direct Carbon Solid Oxide Fuel Cells Powered by Rice Husk Biochar. Int. J. Energy Res. 2022, 46, 4965–4974. [Google Scholar] [CrossRef]
- Osman, A.I.; Fawzy, S.; Farghali, M.; El-Azazy, M.; Elgarahy, A.M.; Fahim, R.A.; Maksoud, M.I.A.A.; Ajlan, A.A.; Yousry, M.; Saleem, Y.; et al. Biochar for Agronomy, Animal Farming, Anaerobic Digestion, Composting, Water Treatment, Soil Remediation, Construction, Energy Storage, and Carbon Sequestration: A Review. Environ. Chem. Lett. 2022, 20, 2385–2485. [Google Scholar] [CrossRef] [PubMed]
- Selvarajoo, A.; Wong, Y.L.; Khoo, K.S.; Chen, W.-H.; Show, P.L. Biochar Production via Pyrolysis of Citrus Peel Fruit Waste as a Potential Usage as Solid Biofuel. Chemosphere 2022, 294, 133671. [Google Scholar] [CrossRef]
- de Almeida, S.G.C.; Tarelho, L.A.C.; Hauschild, T.; Costa, M.A.M.; Dussán, K.J. Biochar Production from Sugarcane Biomass Using Slow Pyrolysis: Characterization of the Solid Fraction. Chem. Eng. Process.-Process Intensif. 2022, 179, 109054. [Google Scholar] [CrossRef]
- Selvarajoo, A.; Oochit, D. Effect of Pyrolysis Temperature on Product Yields of Palm Fibre and Its Biochar Characteristics. Mater. Sci. Energy Technol. 2020, 3, 575–583. [Google Scholar] [CrossRef]
- Lee, K.-T.; Cheng, C.-L.; Lee, D.-S.; Chen, W.-H.; Vo, D.-V.N.; Ding, L.; Lam, S.S. Spent Coffee Grounds Biochar from Torrefaction as a Potential Adsorbent for Spilled Diesel Oil Recovery and as an Alternative Fuel. Energy 2022, 239, 122467. [Google Scholar] [CrossRef]
- Ning, X.; Liang, W.; Wang, G.; Xu, R.; Wang, P.; Zhang, J.; Guo, X.; Jiang, C.; Li, J.; Wang, C. Effect of Pyrolysis Temperature on Blast Furnace Injection Performance of Biochar. Fuel 2022, 313, 122648. [Google Scholar] [CrossRef]
- Khairy, M.; Amer, M.; Ibrahim, M.; Ookawara, S.; Sekiguchi, H.; Elwardany, A. The Influence of Torrefaction on the Biochar Characteristics Produced from Sesame Stalks and Bean Husk. Biomass Convers. Biorefinery 2023, 14, 17127–17148. [Google Scholar] [CrossRef]
- Sharma, A.K.; Ghodke, P.; Sharma, P.K.; Manna, S.; Pugazhendhi, A.; Matsakas, L.; Patel, A. Holistic Utilization of Chlorella Pyrenoidosa Microalgae for Extraction of Renewable Fuels and Value-Added Biochar through in Situ Transesterification and Pyrolysis Reaction Process. Biomass Convers. Biorefinery 2024, 14, 5261–5274. [Google Scholar] [CrossRef]
- Tu, R.; Sun, Y.; Wu, Y.; Fan, X.; Cheng, S.; Jiang, E.; Xu, X. The Fuel Properties and Adsorption Capacities of Torrefied Camellia Shell Obtained via Different Steam-Torrefaction Reactors. Energy 2022, 238, 121969. [Google Scholar] [CrossRef]
- Qiu, B.; Shao, Q.; Shi, J.; Yang, C.; Chu, H. Application of Biochar for the Adsorption of Organic Pollutants from Wastewater: Modification Strategies, Mechanisms and Challenges. Sep. Purif. Technol. 2022, 300, 121925. [Google Scholar] [CrossRef]
- Sutar, S.; Patil, P.; Jadhav, J. Recent Advances in Biochar Technology for Textile Dyes Wastewater Remediation: A Review. Environ. Res. 2022, 209, 112841. [Google Scholar] [CrossRef] [PubMed]
- Kasera, N.; Kolar, P.; Hall, S.G. Nitrogen-Doped Biochars as Adsorbents for Mitigation of Heavy Metals and Organics from Water: A Review. Biochar 2022, 4, 17. [Google Scholar] [CrossRef]
- Devi, M.S.; Thangadurai, T.D.; Shanmugaraju, S.; Selvan, C.P.; Lee, Y.I. Biomass Waste from Walnut Shell for Pollutants Removal and Energy Storage: A Review on Waste to Wealth Transformation. Adsorption 2024, 30, 891–913. [Google Scholar] [CrossRef]
- Simões dos Reis, G.; Mayandi Subramaniyam, C.; Cárdenas, A.D.; Larsson, S.H.; Thyrel, M.; Lassi, U.; García-Alvarado, F. Facile Synthesis of Sustainable Activated Biochars with Different Pore Structures as Efficient Additive-Carbon-Free Anodes for Lithium- and Sodium-Ion Batteries. ACS Omega 2022, 7, 42570–42581. [Google Scholar] [CrossRef]
- Pereira Lopes, R.; Astruc, D. Biochar as a Support for Nanocatalysts and Other Reagents: Recent Advances and Applications. Coord. Chem. Rev. 2021, 426, 213585. [Google Scholar] [CrossRef]
- Zhong, Y.; Zhang, P.; Zhu, X.; Li, H.; Deng, Q.; Wang, J.; Zeng, Z.; Zou, J.-J.; Deng, S. Highly Efficient Alkylation Using Hydrophobic Sulfonic Acid-Functionalized Biochar as a Catalyst for Synthesis of High-Density Biofuels. ACS Sustain. Chem. Eng. 2019, 7, 14973–14981. [Google Scholar] [CrossRef]
- Chin, L.H.; Abdullah, A.Z.; Hameed, B.H. Sugar Cane Bagasse as Solid Catalyst for Synthesis of Methyl Esters from Palm Fatty Acid Distillate. Chem. Eng. J. 2012, 183, 104–107. [Google Scholar] [CrossRef]
- Niju, S.; Ajieth Kanna, S.K.; Ramalingam, V.; Satheesh Kumar, M.; Balajii, M. Sugarcane Bagasse Derived Biochar—A Potential Heterogeneous Catalyst for Transesterification Process. Energy Sources Part A Recover. Util. Environ. Eff. 2023, 45, 9815–9826. [Google Scholar] [CrossRef]
- Foong, S.Y.; Chan, Y.H.; Chin, B.L.F.; Lock, S.S.M.; Yee, C.Y.; Yiin, C.L.; Peng, W.; Lam, S.S. Production of Biochar from Rice Straw and Its Application for Wastewater Remediation—An Overview. Bioresour. Technol. 2022, 360, 127588. [Google Scholar] [CrossRef] [PubMed]
- Alsawy, T.; Rashad, E.; El-Qelish, M.; Mohammed, R.H. A Comprehensive Review on the Chemical Regeneration of Biochar Adsorbent for Sustainable Wastewater Treatment. npj Clean Water 2022, 5, 29. [Google Scholar] [CrossRef]
- Zeghioud, H.; Fryda, L.; Djelal, H.; Assadi, A.; Kane, A. A Comprehensive Review of Biochar in Removal of Organic Pollutants from Wastewater: Characterization, Toxicity, Activation/Functionalization and Influencing Treatment Factors. J. Water Process Eng. 2022, 47, 102801. [Google Scholar] [CrossRef]
- Li, R.; Wang, B.; Niu, A.; Cheng, N.; Chen, M.; Zhang, X.; Yu, Z.; Wang, S. Application of Biochar Immobilized Microorganisms for Pollutants Removal from Wastewater: A Review. Sci. Total Environ. 2022, 837, 155563. [Google Scholar] [CrossRef]
- Calderón-Franco, D.; Apoorva, S.; Medema, G.; van Loosdrecht, M.C.M.; Weissbrodt, D.G. Upgrading Residues from Wastewater and Drinking Water Treatment Plants as Low-Cost Adsorbents to Remove Extracellular DNA and Microorganisms Carrying Antibiotic Resistance Genes from Treated Effluents. Sci. Total Environ. 2021, 778, 146364. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.-K.; Choi, T.-R.; Gurav, R.; Bhatia, S.K.; Park, Y.-L.; Kim, H.J.; Kan, E.; Yang, Y.-H. Adsorption Behavior of Tetracycline onto Spirulina Sp. (Microalgae)-Derived Biochars Produced at Different Temperatures. Sci. Total Environ. 2020, 710, 136282. [Google Scholar] [CrossRef]
- Xu, J.; Zhang, Y.; Li, B.; Fan, S.; Xu, H.; Guan, D.-X. Improved Adsorption Properties of Tetracycline on KOH/KMnO4 Modified Biochar Derived from Wheat Straw. Chemosphere 2022, 296, 133981. [Google Scholar] [CrossRef]
- Nguyen, T.-B.; Nguyen, T.-K.-T.; Chen, W.-H.; Chen, C.-W.; Bui, X.-T.; Patel, A.K.; Dong, C.-D. Hydrothermal and Pyrolytic Conversion of Sunflower Seed Husk into Novel Porous Biochar for Efficient Adsorption of Tetracycline. Bioresour. Technol. 2023, 373, 128711. [Google Scholar] [CrossRef]
- Yu, K.L.; Lee, X.J.; Ong, H.C.; Chen, W.-H.; Chang, J.-S.; Lin, C.-S.; Show, P.L.; Ling, T.C. Adsorptive Removal of Cationic Methylene Blue and Anionic Congo Red Dyes Using Wet-Torrefied Microalgal Biochar: Equilibrium, Kinetic and Mechanism Modeling. Environ. Pollut. 2021, 272, 115986. [Google Scholar] [CrossRef]
- Yu, K.L.; Chen, W.-H.; Sheen, H.-K.; Chang, J.-S.; Lin, C.-S.; Ong, H.C.; Show, P.L.; Ng, E.-P.; Ling, T.C. Production of Microalgal Biochar and Reducing Sugar Using Wet Torrefaction with Microwave-Assisted Heating and Acid Hydrolysis Pretreatment. Renew. Energy 2020, 156, 349–360. [Google Scholar] [CrossRef]
- Wei, X.; Huang, S.; Yang, J.; Liu, P.; Li, X.; Xue, R.; Wang, Y.; Chen, L.; Chen, X.; Wu, Y.; et al. Adsorption of Methylene Blue on Activated Carbons Prepared from Penicillin Mycelial Residues via Torrefaction and Hydrothermal Pretreatment. Biomass Convers. Biorefinery 2024, 14, 28933–28945. [Google Scholar] [CrossRef]
- Li, Z.; Xing, B.; Ding, Y.; Li, Y.; Wang, S. A High-Performance Biochar Produced from Bamboo Pyrolysis with in-Situ Nitrogen Doping and Activation for Adsorption of Phenol and Methylene Blue. Chin. J. Chem. Eng. 2020, 28, 2872–2880. [Google Scholar] [CrossRef]
- Zhengfeng, S.; Ming, C.; Geming, W.; Quanrong, D.; Shenggao, W.; Yuan, G. Synthesis, Characterization and Removal Performance of Cr (VI) by Orange Peel-Based Activated Porous Biochar from Water. Chem. Eng. Res. Des. 2023, 193, 1–12. [Google Scholar] [CrossRef]
- Chen, W.-H.; Hoang, A.T.; Nižetić, S.; Pandey, A.; Cheng, C.K.; Luque, R.; Ong, H.C.; Thomas, S.; Nguyen, X.P. Biomass-Derived Biochar: From Production to Application in Removing Heavy Metal-Contaminated Water. Process Saf. Environ. Prot. 2022, 160, 704–733. [Google Scholar] [CrossRef]
- Xu, D.; Sun, T.; Jia, H.; Sun, Y.; Zhu, X. The Performance and Mechanism of Cr(VI) Adsorption by Biochar Derived from Potamogeton Crispus at Different Pyrolysis Temperatures. J. Anal. Appl. Pyrolysis 2022, 167, 105662. [Google Scholar] [CrossRef]
- Zhu, Q.; Gao, H.; Sun, Y.; Xiang, Y.; Liang, X.; Ivanets, A.; Li, X.; Su, X.; Lin, Z. Highly Efficient Adsorption of Chromium on N, S-Codoped Porous Carbon Materials Derived from Paper Sludge. Sci. Total Environ. 2022, 834, 155312. [Google Scholar] [CrossRef] [PubMed]
- Fawzy, S.; Osman, A.I.; Yang, H.; Doran, J.; Rooney, D.W. Industrial Biochar Systems for Atmospheric Carbon Removal: A Review. Environ. Chem. Lett. 2021, 19, 3023–3055. [Google Scholar] [CrossRef]
- Patra, B.R.; Mukherjee, A.; Nanda, S.; Dalai, A.K. Biochar Production, Activation and Adsorptive Applications: A Review. Environ. Chem. Lett. 2021, 19, 2237–2259. [Google Scholar] [CrossRef]
- Jing, F.; Sun, Y.; Liu, Y.; Wan, Z.; Chen, J.; Tsang, D.C.W. Interactions between Biochar and Clay Minerals in Changing Biochar Carbon Stability. Sci. Total Environ. 2022, 809, 151124. [Google Scholar] [CrossRef]
- Mohamed, M.E.; Adel, O.; Khamis, E. Fabrication of Biochar-Based Superhydrophobic Coating on Steel Substrate and Its UV Resistance, Anti-Scaling, and Corrosion Resistance Performance. Sci. Rep. 2023, 13, 9453. [Google Scholar] [CrossRef]
- Amirchand, K.D.; Kaur, K.; Singh, V. Biochar Based Self Cleaning Superhydrophobic Surface with Aqueous DESphobic Properties. J. Mol. Liq. 2023, 380, 121736. [Google Scholar] [CrossRef]
- Hwang, J.-J.; Hu, F.-H.; Li, M.-X.; Luo, K.-H.; Liu, Y.-H.; Lin, S.-R.; Yeh, J.-M. Superhydrophobic Surface of Biomass Carbon-Based PANI Composite Coatings with the Biomimetic Structure of Goose Feather for Anticorrosion/Antibiofilm Applications. Surf. Coat. Technol. 2024, 482, 130700. [Google Scholar] [CrossRef]
- Pan, X.; Kuang, S.; Wang, X. Effective Strategy for Alleviation of Oil Contamination in Marine via the Superhydrophobic Biochar: Performances, Mechanisms and Environmental Recalcitrance. J. Clean. Prod. 2024, 462, 142695. [Google Scholar] [CrossRef]
- Kane, S.; Storer, A.; Xu, W.; Ryan, C.; Stadie, N.P. Biochar as a Renewable Substitute for Carbon Black in Lithium-Ion Battery Electrodes. ACS Sustain. Chem. Eng. 2022, 10, 12226–12233. [Google Scholar] [CrossRef]
- Seroka, N.S.; Luo, H.; Khotseng, L. Biochar-Derived Anode Materials for Lithium-Ion Batteries: A Review. Batteries 2024, 10, 144. [Google Scholar] [CrossRef]
- Shimabuku, K.K.; Kearns, J.P.; Martinez, J.E.; Mahoney, R.B.; Moreno-Vasquez, L.; Summers, R.S. Biochar Sorbents for Sulfamethoxazole Removal from Surface Water, Stormwater, and Wastewater Effluent. Water Res. 2016, 96, 236–245. [Google Scholar] [CrossRef] [PubMed]
- Sakhiya, A.K.; Anand, A.; Kaushal, P. Production, Activation, and Applications of Biochar in Recent Times. Biochar 2020, 2, 253–285. [Google Scholar] [CrossRef]
- Gallego-Ramírez, C.; Chica, E.; Rubio-Clemente, A. Life Cycle Assessment of Raw and Fe-Modified Biochars: Contributing to Circular Economy. Materials 2023, 16, 6059. [Google Scholar] [CrossRef]
- Peters, J.F.; Iribarren, D.; Dufour, J. Biomass Pyrolysis for Biochar or Energy Applications? A Life Cycle Assessment. Environ. Sci. Technol. 2015, 49, 5195–5202. [Google Scholar] [CrossRef]
- Nguyen, M.H.; Zbair, M.; Dutournié, P.; Limousy, L.; Bennici, S. Corn Cobs’ Biochar as Green Host of Salt Hydrates for Enhancing the Water Sorption Kinetics in Thermochemical Heat Storage Systems. Molecules 2023, 28, 5381. [Google Scholar] [CrossRef]
- Ismail, I.S.; Othman, M.F.H.; Rashidi, N.A.; Yusup, S. Recent Progress on Production Technologies of Food Waste–Based Biochar and Its Fabrication Method as Electrode Materials in Energy Storage Application. Biomass Convers. Biorefinery 2023, 13, 14341–14357. [Google Scholar] [CrossRef]
- Vakros, J. Biochars and Their Use as Transesterification Catalysts for Biodiesel Production: A Short Review. Catalysts 2018, 8, 562. [Google Scholar] [CrossRef]
- Alhelal, A.; Mohammed, Z.; Jeelani, S.; Rangari, V.K. 3D Printing of Spent Coffee Ground Derived Biochar Reinforced Epoxy Composites. J. Compos. Mater. 2021, 55, 3651–3660. [Google Scholar] [CrossRef]
- Agyekum, E.B.; Nutakor, C. Recent Advancement in Biochar Production and Utilization – A Combination of Traditional and Bibliometric Review. Int. J. Hydrogen Energy 2024, 54, 1137–1153. [Google Scholar] [CrossRef]
- Moreira, M.T.; Noya, I.; Feijoo, G. The Prospective Use of Biochar as Adsorption Matrix – A Review from a Lifecycle Perspective. Bioresour. Technol. 2017, 246, 135–141. [Google Scholar] [CrossRef]
- Ravenni, G.; Thomsen, T.P.; Smith, A.M.; Ambye-Jensen, M.; Rohde-Nielsen, K.T.; Henriksen, U.B. Integration of a Drying and Pyrolysis System in a Green Biorefinery: Biochar Product Quality and Impacts on the Overall Energy Balance and Climate Footprint. Biomass Convers. Biorefinery 2024, 14, 25143–25159. [Google Scholar] [CrossRef]
- Puro Earth. Puro Standard General Rules. 2022; pp. 52–53. Available online: https://puro.earth/document-library (accessed on 1 November 2024).
- Puro Earth. Puro Standard Biochar Methodology. Available online: https://puro.earth/document-library?tab=methodologies#nav_tabs_content_1715928855009_50 (accessed on 1 November 2024).
- Etter, H.; Vera, A.; Aggarwal, C.; Delaney, M.; Manley, S. Methodology for Biochar Utilization in Soil and Non-Soil Applications; Verified Carbon Standard: Washington, DC, USA, 2021; pp. 1–53. [Google Scholar]
- Bednik, M.; Medyńska-Juraszek, A.; Ćwieląg-Piasecka, I. Effect of Six Different Feedstocks on Biochar’s Properties and Expected Stability. Agronomy 2022, 12, 1525. [Google Scholar] [CrossRef]
- Vlachokostas, C.; Michailidou, A.V.; Achillas, C. Multi-Criteria Decision Analysis towards Promoting Waste-to-Energy Management Strategies: A Critical Review. Renew. Sustain. Energy Rev. 2021, 138, 110563. [Google Scholar] [CrossRef]
- Khan, I.; Kabir, Z. Waste-to-Energy Generation Technologies and the Developing Economies: A Multi-Criteria Analysis for Sustainability Assessment. Renew. Energy 2020, 150, 320–333. [Google Scholar] [CrossRef]
- Cutz, L.; Haro, P.; Santana, D.; Johnsson, F. Assessment of Biomass Energy Sources and Technologies: The Case of Central America. Renew. Sustain. Energy Rev. 2016, 58, 1411–1431. [Google Scholar] [CrossRef]
- Almanaseer, N.; Dunlop, C.; Friesen, K.; Nestico-Semianiw, E.; Abbassi, B. Multi-Criteria Analysis of Waste-to-Energy Technologies in Developed and Developing Countries. Environ. Res. Eng. Manag. 2020, 76, 32–43. [Google Scholar] [CrossRef]
- Mpanang’ombe, W.; Tilley, E.; Zabaleta, I.; Zurbrügg, C. A Biowaste Treatment Technology Assessment in Malawi. Recycling 2018, 3, 55. [Google Scholar] [CrossRef]
- Thengane, S.K. Assessment of Different Technologies for Managing Yard Waste Using Analytic Hierarchy Process. Process Integr. Optim. Sustain. 2019, 3, 255–272. [Google Scholar] [CrossRef]
- Ferreira, A.F.; Ribau, J.P.; Costa, M. A Decision Support Method for Biochars Characterization from Carbonization of Grape Pomace. Biomass Bioenergy 2021, 145, 105946. [Google Scholar] [CrossRef]
- Silveira, E.A.; Santanna, M.S.; Barbosa Souto, N.P.; Lamas, G.C.; Galvão, L.G.O.; Luz, S.M.; Caldeira-Pires, A. Urban Lignocellulosic Waste as Biofuel: Thermal Improvement and Torrefaction Kinetics. J. Therm. Anal. Calorim. 2023, 148, 197–212. [Google Scholar] [CrossRef]
- Petrova, T.; Naydenova, I.; Ribau, J.; Ferreira, A.F. Biochar from Agro-Forest Residue: Application Perspective Based on Decision Support Analysis. Appl. Sci. 2023, 13, 3240. [Google Scholar] [CrossRef]
- Carlsen, L.; Bruggemann, R. The 17 United Nations’ Sustainable Development Goals: A Status by 2020. Int. J. Sustain. Dev. World Ecol. 2022, 29, 219–229. [Google Scholar] [CrossRef]

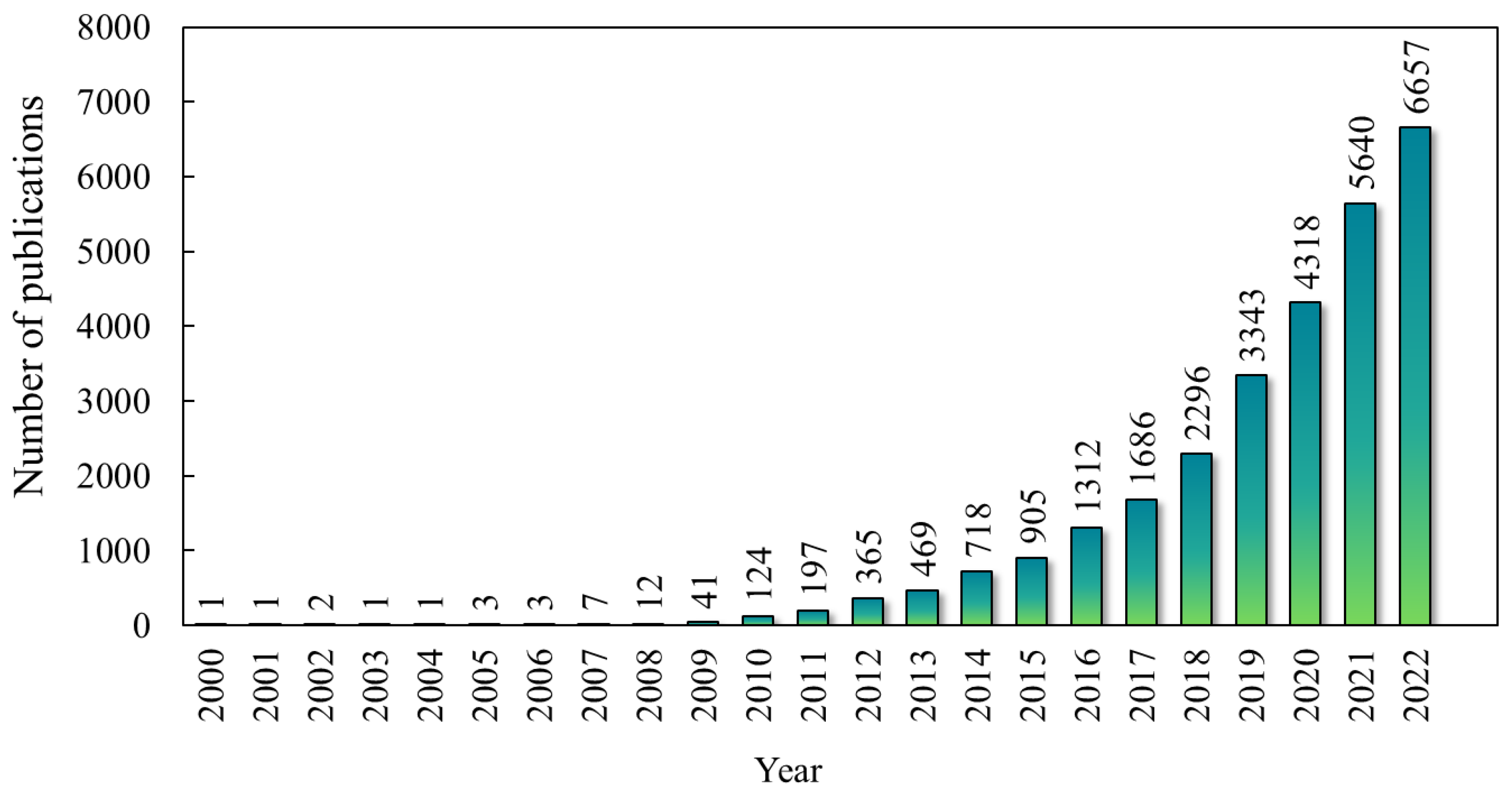
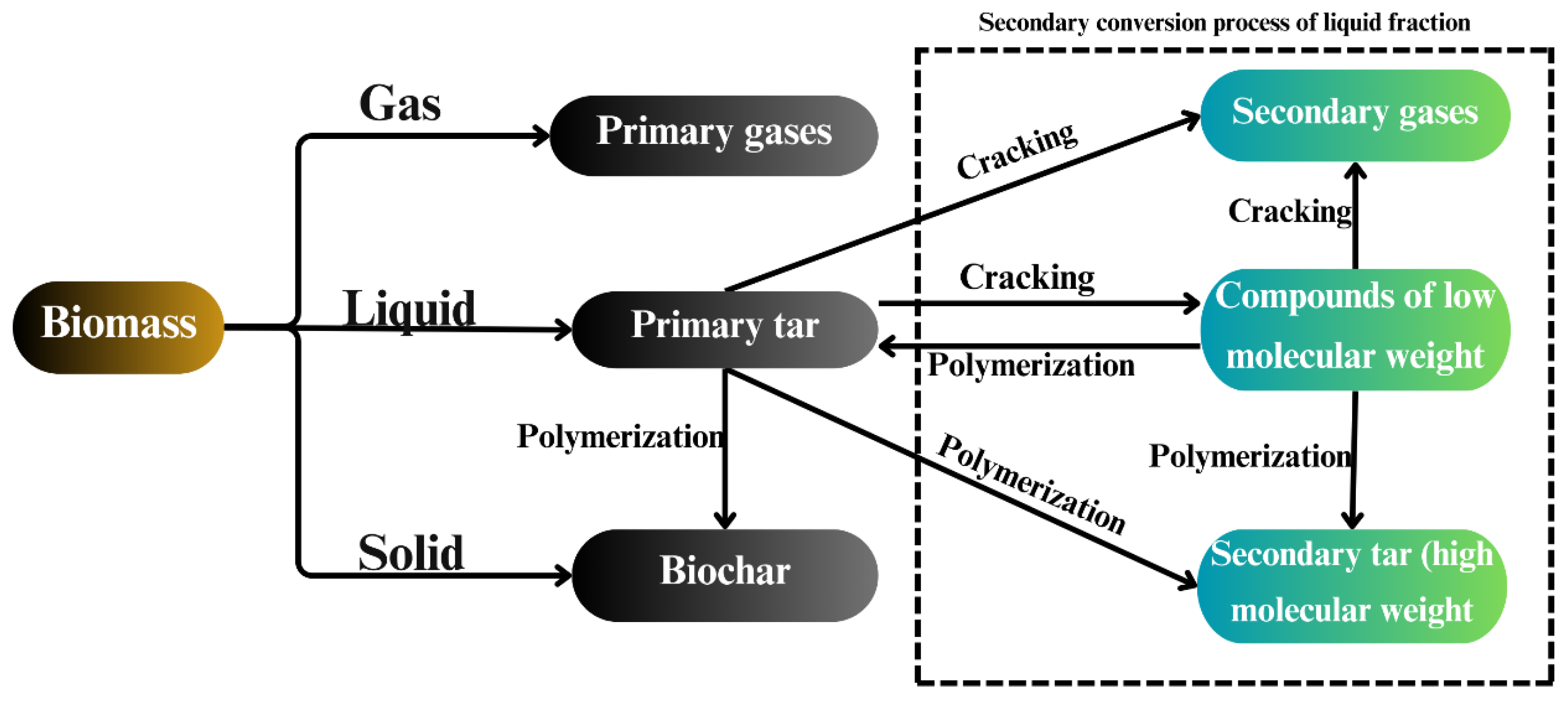



| Thermal Process | Temperature (°C) | Product |
|---|---|---|
| Torrefaction | 200–300 | Low-temperature biochar |
| Pyrolysis | 300–800 | Biochar + Bio-oil + Biogas |
| Gasification | 600–1200 | Synthesis gas + Biochar |
| Search | Keywords | Boolean Operator a | Keywords | Boolean Operator | Keywords | Results |
|---|---|---|---|---|---|---|
| 1 | biochar | AND | - | - | - | 29.645 |
| 2 | biochar | AND | pyrolys * | - | - | 11.417 |
| 3 | biochar | AND | gasificat * | - | - | 1.570 |
| 4 | biochar | AND | Soil | - | - | 13.954 |
| 5 | biochar | AND | catalys * | - | - | 2.504 |
| 6 | biochar | AND | Water | - | - | 12.264 |
| 7 * | biochar | AND | Water | AND | treatment | 4.783 |
| 8 | biochar | AND | wastewater | - | - | 3.486 |
| 9 | #7 | OR | #8 | - | - | 6.901 |
| Properties | Implications |
|---|---|
| Specific surface area (SSA) | High values of SSA are indicators of adsorption capabilities |
| Porosity | High values of porosity are indicators of adsorption capabilities |
| Pore volume/distribution | Pore volume and distribution are significant criteria for the adsorption/desorption capability and selectivity |
| Surface functional group | The superficial composition of biochar delimitates the application and adsorption capabilities |
| Water-holding capability | High water-holding capability values indicate water retention and delimit the applications of plant stress remediation |
| Ion exchange capability | Indicative of efficiency in remediation of soil nutrient leaching |
| Elemental composition | The elemental composition is an indicator of stability and possible remediation applications |
| High heating value (HHV) | Indication of combustion energy production. The greater the value, the better |
| Band Position (cm−1) | Component | Ref. |
|---|---|---|
| 480, 592, 652 | Aromatic deforming rings, C-C stretching | [74] |
| 782, 840, 885 | C-H, aromatic hydrogen | |
| 1097 | C-O-C symmetric stretching | |
| 1618 | Aromatic C-C ring stretching | |
| 1709 | Phenyl ring substitution overtones | |
| 2950 | Alkyl/aliphatic C-H stretching | |
| 3544 | -OH stretching | |
| 3642 | -OH stretching, alcohols, phenols |
| Source Biomass | Biochar Preparation Conditions | Optimum Biochar Characteristics and Combustibility Properties | Ref. |
|---|---|---|---|
| Orange peel | Pyrolysis Atmosphere: N2 Temp.: 500 °C HR: 5 °C min−1 Time: 60 min | Biochar O/C—0.12; biochar H/C—0.66; HHV—25.73 MJ kg−1; energy yield—47.52%; fuel ratio—5.87; thermal stability—0.85. | [126] |
| Sugarcane bagasse | Pyrolysis Atmosphere: N2 Temp.: 600 °C HR: 10 °C min−1 Time: 30 min | Biochar O/C—0.3; H/C—0.2; HHV—29.99 MJ kg−1; biochar yield—21.75%; fuel ratio—3.21; energy yield—36.32%. | [127] |
| Palm fiber | Pyrolysis Atmosphere: N2 Temp.: 700 °C HR: 5 °C min−1 Time: 120 min | Biochar O/C—0.23; biochar SSA—0.272 m2 g−1; HHV—26.77 MJ kg−1; biochar yield—28.37%; energy yield—45.72%. | [128] |
| Spent coffee grounds | Torrefaction Atmosphere: N2 Temp.: 300 °C Time: 30 min | Biochar O/C—0.39; biochar H/C—0.10; biochar SSA—0.524 m2 g−1; HHV—30.32 MJ kg−1; biochar yield—62%. | [129] |
| Poplar wood | Pyrolysis Atmosphere: N2 Temp.: 600 °C HR: 5–20 °C min−1 | Biochar O/C—0.06; biochar H/C—0.025; HHV—32.73 MJ kg−1; biochar yield—24.3%; fuel ratio—6.86; combustibility index—1.9 s−1 °C−2; combustion characteristic index—3.3 s−2 °C−3 | [130] |
| Sewage sludge | Pyrolysis Atmosphere: N2 Temp.: 450 °C HR: 10 °C min−1 Time: 30 min | Biochar O/C—0.19; biochar H/C—0.74; HHV—13.58 MJ kg−1; biochar yield—57.90%; fuel ratio—0.86; energy yield—42.04%; combustion index—0.59 106%−2 s−2 °C−3. | [2] |
| Sesame stalks | Torrefaction Atmosphere: N2 Temp.: 275 °C Time: 30 min | Biochar O/C—0.64; biochar H/C—1.25; HHV—20.5 MJ kg−1; biochar yield—76.25%; fuel ratio—0.52; energy yield—86.16%; bulk density—290.01 kg m−3. | [131] |
| Microalgae—Chlorella pyrenoidosa | Pyrolysis Atmosphere: N2 Temp.: 400–600 °C HR: 10 °C min−1 Time: 30 min | Biochar O/C—0.23; biochar H/C—0.055; HHV—17.15 MJ kg−1; biochar yield—51.23%; fuel ratio—1.72. | [132] |
| Camellia shell | Steam-torrefaction Atmosphere: N2 Temp.: 280 °C Time: 30 min | Biochar O/C—0.36; biochar H/C—0.07; biochar SSA—28.66 m2 g−1; HHV—24.76 MJ kg−1; biochar yield—50.45%; fuel ratio—1.04. | [133] |
| App. | Biomass | Properties/Criteria | Analytical Technique | MCDM | Ref. |
|---|---|---|---|---|---|
| Biofuel | Grape pomace | Biochar yield, carbon (%), HHV, kinetics combustion parameters (Ea) | Proximate analysis Calorific analysis Ultimate analysis TG/DTG | Pareto dominance analysis/ metric distance based on compromise programming | [189] |
| Grape pomace, cherry stones, peach stones, colza, sunflower husks, and softwood | Moisture %, ash, HHV, kinetics combustion parameters (Ea) | Proximate analysis Calorific analysis TG/DTG | Pareto dominance analysis/ metric distance based on compromise programming | [191] | |
| Fruit seeds | Carbon enhancement index (CEI), HHV and its enhancement factor (EF) and energy-mass coefficient index (EMCI) | Calorific analysis Ultimate analysis | Pareto dominance analysis/ metric distance based on compromise programming | [14] | |
| Spent coffee ground Brew spent grains | O/C and H/C, HHV and its enhancement factor (EF), energy yield (EY) | Calorific analysis Ultimate analysis | Pareto dominance analysis/ metric distance based on compromise programming | [41] | |
| Pruning trees | FC, ash, HHV, O/C and H/C | Proximate analysis Calorific analysis Ultimate analysis | Pareto dominance analysis/ metric distance based on compromise programming | [68] | |
| Catalyst | Grape pomace, cherry stones, peach stones, colza, sunflower husks, and softwood | K, Ca, P (%), carbon (%), specific surface area (m2 g−1) | EDS Ultimate analysis Brunauer– Emmett–Teller (BET) method | Pareto dominance analysis/ metric distance based on compromise programming | [191] |
| Soil amendment | Fruit seeds | O/C and H/C, carbon enhancement index (CEI), K, N, P (%) | EDS Ultimate analysis | Pareto dominance analysis/ metric distance based on compromise programming | [14] |
| Grape pomace | biochar yield, carbon content, O/C and H/C, N, P, Mg, K | EDS Ultimate analysis | Pareto dominance analysis/ metric distance based on compromise programming | [189] | |
| Soil amendment/ CO2 sequestration/ Supercapacitor development | Grape pomace, cherry stones, peach stones, colza, sunflower husks, and softwood | K, Ca, P (%)/ carbon (%)/ specific surface area, bulk density, electric conductivity, pH | EDS Ultimate analysis Brunauer– Emmett–Teller (BET) method Biochar pH, electrical conductivity and liming potential | Pareto dominance analysis/ metric distance based on compromise programming | [191] |
| Wastewater treatment | Spirulina sp. Wheat straw Sunflower seed husk Chlorella sp. Penicillin mycelia Bamboo particles Orange peel Rice husk Paper sludge | O/C SSA Pore volume Raman ID/IG Surface composition Adsorption capability | Ultimate analysis Physicochemical analysis Surface analysis Molecular/structural analysis | - | [64,148,149,150,151,153,154,155,156,157,158] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santos, D.C.B.D.; Evaristo, R.B.W.; Dutra, R.C.; Suarez, P.A.Z.; Silveira, E.A.; Ghesti, G.F. Advancing Biochar Applications: A Review of Production Processes, Analytical Methods, Decision Criteria, and Pathways for Scalability and Certification. Sustainability 2025, 17, 2685. https://doi.org/10.3390/su17062685
Santos DCBD, Evaristo RBW, Dutra RC, Suarez PAZ, Silveira EA, Ghesti GF. Advancing Biochar Applications: A Review of Production Processes, Analytical Methods, Decision Criteria, and Pathways for Scalability and Certification. Sustainability. 2025; 17(6):2685. https://doi.org/10.3390/su17062685
Chicago/Turabian StyleSantos, Diego C. B. D., Rafael B. W. Evaristo, Romulo C. Dutra, Paulo A. Z. Suarez, Edgar A. Silveira, and Grace F. Ghesti. 2025. "Advancing Biochar Applications: A Review of Production Processes, Analytical Methods, Decision Criteria, and Pathways for Scalability and Certification" Sustainability 17, no. 6: 2685. https://doi.org/10.3390/su17062685
APA StyleSantos, D. C. B. D., Evaristo, R. B. W., Dutra, R. C., Suarez, P. A. Z., Silveira, E. A., & Ghesti, G. F. (2025). Advancing Biochar Applications: A Review of Production Processes, Analytical Methods, Decision Criteria, and Pathways for Scalability and Certification. Sustainability, 17(6), 2685. https://doi.org/10.3390/su17062685









