Fisheries Sustainability Eroded by Lost Catch Proportionality in a Coral Reef Seascape
Abstract
1. Introduction
2. Materials and Methods
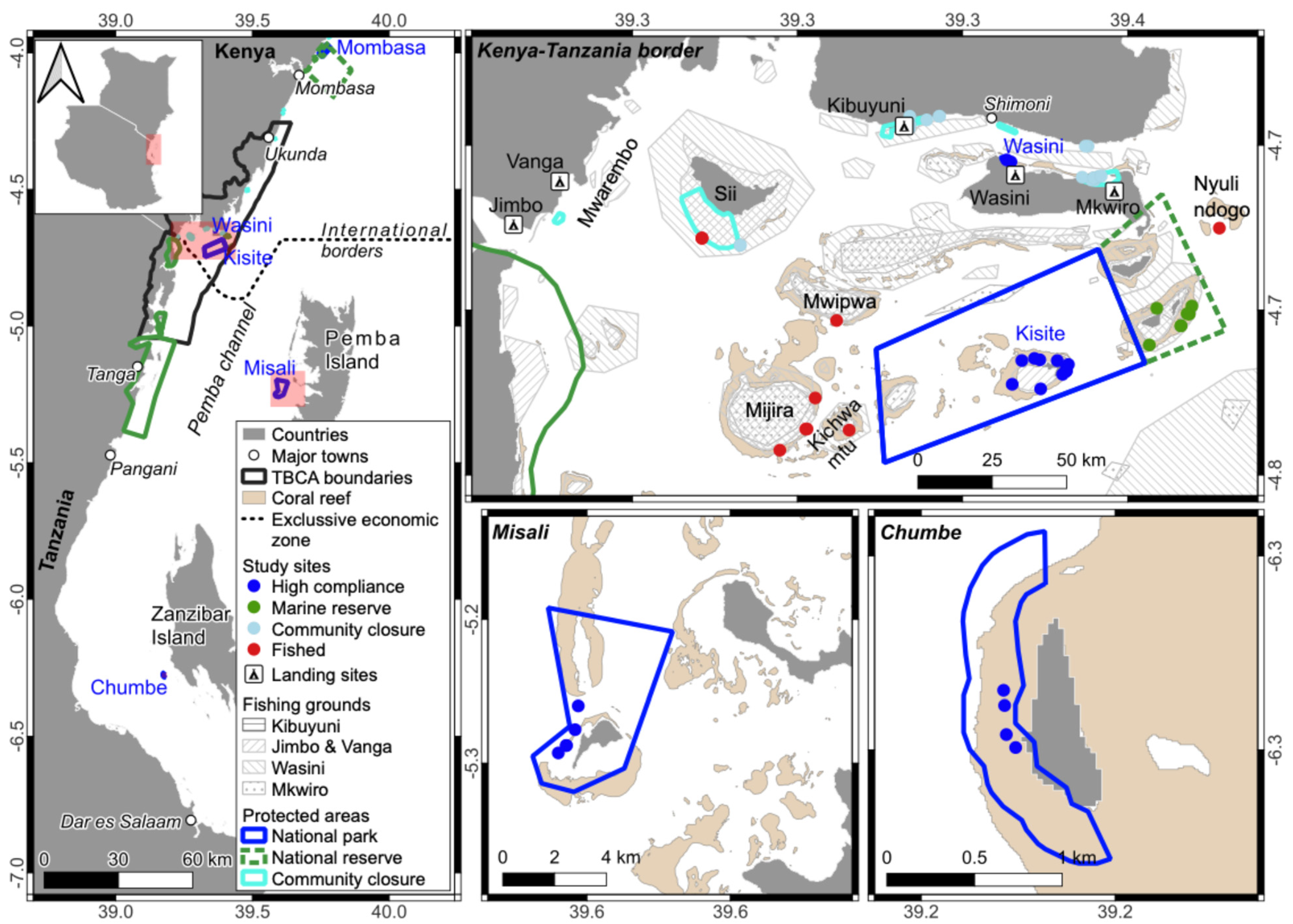
2.1. Study Sites
2.2. Field Methods
2.2.1. Fish Production Estimates from Marine Reserves
2.2.2. Estimates of Proportional Taxonomic Composition and Linear Production
2.2.3. Fisheries Catch Production Rates
2.2.4. Fish Length Estimates of Sustainability
2.3. Data Analyses
2.3.1. Production Estimates from Marine Reserves
2.3.2. Proportional Taxonomic Composition and Production
2.3.3. Evaluation of Fisheries Catch Production Rates
2.3.4. Length-Based Catch Indicators
3. Results
3.1. Production of Fish
3.1.1. Recovery Rate and Production Estimates from Marine Reserves
3.1.2. Family/Functional-Level Annual Production
3.1.3. Estimated Fisheries Catch
3.1.4. Numbers of Fish Species
3.2. Balanced Harvest Analyses
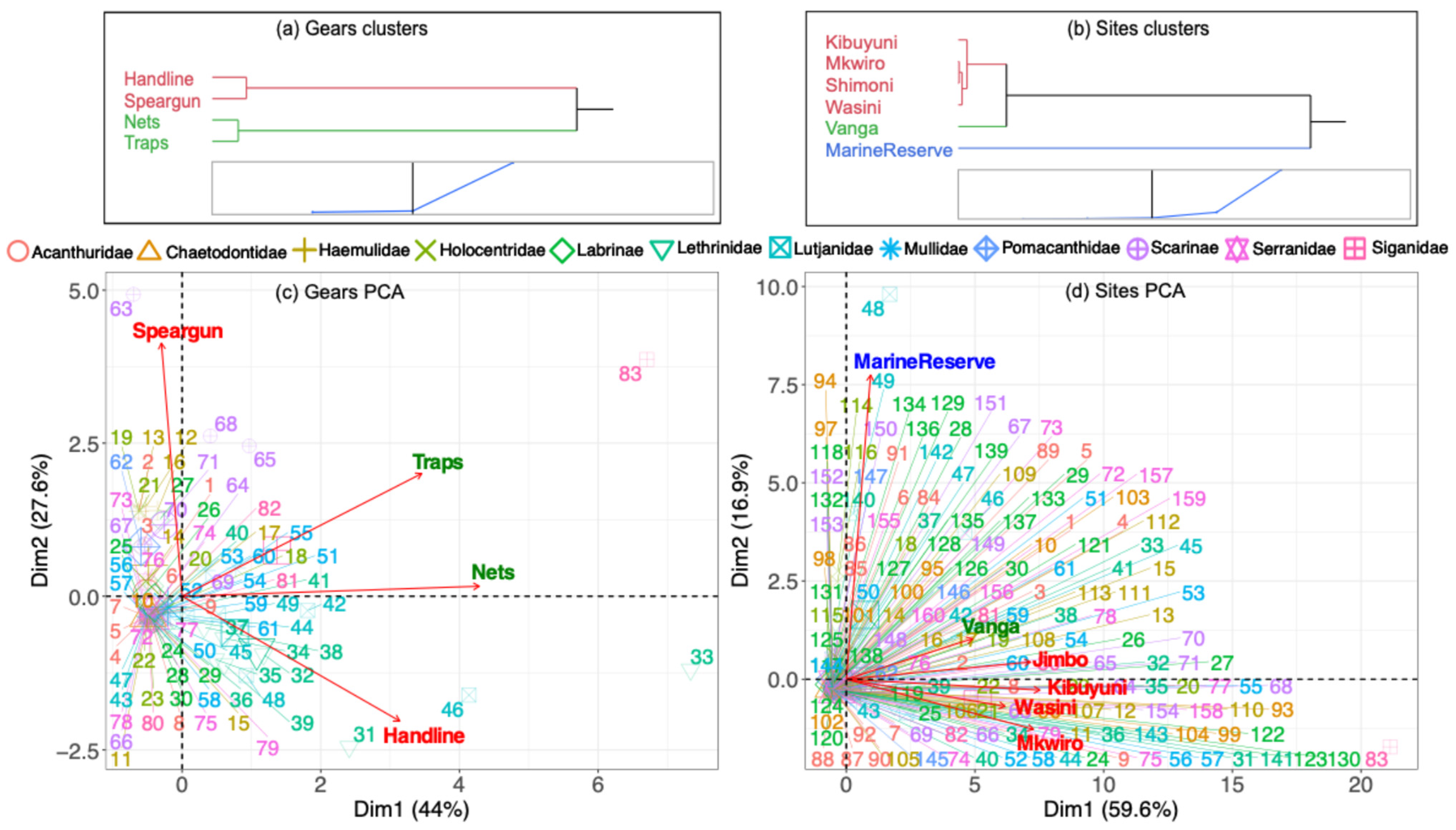
3.2.1. Balanced Gear Analyses
3.2.2. Balanced Taxonomic Composition Analyses
3.2.3. Balanced Life History Analyses
3.2.4. Catch Production and Weighted Assemblage Composition
3.3. Length-Based Catch Analyses
4. Discussion
4.1. Recovery Rates and Fisheries Production
4.2. Proportionality of the Taxa and Production
4.2.1. Fishing Gear as Capture Niches
4.2.2. Balanced Harvesting Considerations
4.3. Body Size Limitations for Estimating Sustainability
4.4. Caveats and Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cooke, S.J.; Fulton, E.A.; Sauer, W.H.H.; Lynch, A.J.; Link, J.S.; Koning, A.A.; Jena, J.; Silva, L.G.M.; King, A.J.; Kelly, R.; et al. Towards vibrant fish populations and sustainable fisheries that benefit all: Learning from the last 30 years to inform the next 30 years. Rev. Fish Biol. Fish. 2023, 33, 317–347. [Google Scholar] [CrossRef]
- Worm, B.; Hilborn, R.; Baum, J.K.; Branch, T.A.; Collie, J.S.; Costello, C.; Fogarty, M.J.; Fulton, E.A.; Hutchings, J.A.; Jennings, S.; et al. Rebuilding global fisheries. Science 2009, 325, 578–585. [Google Scholar] [CrossRef] [PubMed]
- Dalzell, P. Catch rates, selectivity and yields of reef fishing. In Polunin NVC, 1st ed.; Roberts, C.M., Ed.; Chapman & Hall: London, UK, 1996; pp. 161–192. [Google Scholar]
- Samoilys, M.A.; Osuka, K.; Maina, G.W.; Obura, D.O. Artisanal fisheries on Kenya’s coral reefs: Decadal trends reveal management needs. Fish. Res. 2017, 186, 177–191. [Google Scholar] [CrossRef]
- Prince, J.; Hordyk, A. What to do when you have almost nothing: A simple quantitative prescription for managing extremely data-poor fisheries. Fish Fish. 2019, 20, 224–238. [Google Scholar] [CrossRef]
- Hilborn, R.; Amoroso, R.O.; Anderson, C.M.; Baum, J.K.; Branch, T.A.; Costello, C.; de Moor, C.L.; Faraj, A.; Hively, D.; Jensen, O.P.; et al. Effective fisheries management instrumental in improving fish stock status. Proc. Natl. Acad. Sci. USA 2020, 117, 2218–2224. [Google Scholar] [CrossRef]
- Pauly, D.; Hilborn, R.; Branch, T.A. Fisheries: Does catch reflect abundance? Nature 2013, 494, 303–306. [Google Scholar] [CrossRef]
- Collie, J.S.; Gislason, H. Biological reference points for fish stocks in a multispecies context. Can. J. Fish. Aquat. Sci. 2001, 58, 2167–2176. [Google Scholar] [CrossRef]
- Medeiros-Leal, W.; Santos, R.; Peixoto, U.I.; Casal-Ribeiro, M.; Novoa-Pabon, A.; Sigler, M.F.; Pinho, M. Performance of length-based assessment in predicting small-scale multispecies fishery sustainability. Rev. Fish Biol. Fish. 2023, 33, 819–852. [Google Scholar] [CrossRef]
- Jacobsen, N.S.; Gislason, H.; Andersen, K.H. The consequences of balanced harvesting of fish communities. Proc. R. Soc. B Biol. Sci. 2014, 281, 20132701. [Google Scholar] [CrossRef]
- Zhou, S.; Kolding, J.; Garcia, S.M.; Plank, M.J.; Bundy, A.; Charles, A.; Hansen, C.; Heino, M.; Howell, D.; Jacobsen, N.S.; et al. Balanced harvest: Concept, policies, evidence, and management implications. Rev. Fish Biol. Fish. 2019, 29, 711–733. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Mangi, S.C. Gear-based management of a tropical artisanal fishery based on species selectivity and capture size. Fish. Manag. Ecol. 2004, 11, 51–60. [Google Scholar] [CrossRef]
- Morais, R.A.; Bellwood, D.R. Global drivers of reef fish growth. Fish Fish. 2018, 19, 874–889. [Google Scholar] [CrossRef]
- McClanahan, T.R. Coral community life histories and population dynamics driven by seascape bathymetry and temperature variability. Adv. Mar. Biol. 2020, 87, 291–330. [Google Scholar]
- McClanahan, T.R.; Graham, N.A.J.; MacNeil, M.A.; Cinner, J.E. Biomass-based targets and the management of multispecies coral reef fisheries. Conserv. Biol. 2015, 29, 409–417. [Google Scholar] [CrossRef]
- Zeller, D.; Vianna, G.; Ansell, M.; Coulter, A.; Derrick, B.; Greer, K.; Noël, S.-L.; Palomares, M.L.D.; Zhu, A.; Pauly, D. Fishing effort and associated catch per unit effort for small-scale fisheries in the Mozambique Channel region: 1950–2016. Front. Mar. Sci. 2021, 8, 707999. [Google Scholar] [CrossRef]
- Kent, P.E. The Geology and Geophysics of Coastal Tanzania; Institute of Geological Sciences, Geophysical Papers: London, UK, 1971; Volume 6, pp. 1–101. [Google Scholar]
- Emslie, M.J.; Cheal, A.J. Visual Census of Reef Fish; Australian Institute of Marine Science: Townsville, Australia, 2018. [Google Scholar]
- McClanahan, T.R.; Oddenyo, R.M.; Kosgei, J.K. Challenges to managing fisheries with high inter-community variability on the Kenya-Tanzania border. Curr. Res. Environ. Sustain. 2024, 7, 100244. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Humphries, A.T. Differential and slow life-history responses of fishes to coral reef closures. Mar. Ecol. Prog. Ser. 2012, 469, 121–131. [Google Scholar] [CrossRef]
- Lappalainen, A.; Saks, L.; Šuštar, M.; Heikinheimo, O.; Jürgens, K.; Kokkonen, E.; Kurkilahti, M.; Verliin, A.; Vetemaa, M. Length at maturity as a potential indicator of fishing pressure effects on coastal pikeperch (Sander lucioperca) stocks in the northern Baltic Sea. Fish. Res. 2016, 174, 47–57. [Google Scholar] [CrossRef]
- Froese, R.; Winker, H.; Coro, G.; Demirel, N.; Tsikliras, A.C.; Dimarchopoulou, D.; Scarcella, G.; Probst, W.N.; Dureuil, M.; Pauly, D. A new approach for estimating stock status from length frequency data. ICES J. Mar. Sci. 2018, 75, 2004–2015. [Google Scholar] [CrossRef]
- Froese, R.; Binohlan, C. Empirical relationships to estimate asymptotic length, length at first maturity and length at maximum yield per recruit in fishes, with a simple method to evaluate length frequency data. J. Fish Biol. 2000, 56, 758–773. [Google Scholar] [CrossRef]
- Goodyear, C.P. Spawning stock biomass per recruit in fisheries management: Foundation and current use. In Risk Evaluation and Biological Reference Points for Fisheries Management; Smith, S.J., Hunt, J.J., Rivard, D., Eds.; Department of Fisheries and Oceans, National Research Council: Ottawa, ON, Canada, 1993; pp. 67–82. [Google Scholar]
- Cousido-Rocha, M.; Cerviño, S.; Alonso-Fernández, A.; Gil, J.; Herraiz, I.G.; Rincón, M.M.; Ramos, F.; Rodríguez-Cabello, C.; Sampedro, P.; Vila, Y.; et al. Applying length-based assessment methods to fishery resources in the Bay of Biscay and Iberian Coast ecoregion: Stock status and parameter sensitivity. Fish. Res. 2022, 248, 106197. [Google Scholar] [CrossRef]
- Froese, R.; Pauly, D. Comment on “Metabolic scaling is the product of life-history optimization”. Science 2023, 380, eade6084. [Google Scholar] [CrossRef]
- Thorson, J.T.; Munch, S.B.; Cope, J.M.; Gao, J. Predicting life history parameters for all fishes worldwide. Ecol. Appl. 2017, 27, 2262–2276. [Google Scholar] [CrossRef] [PubMed]
- Thorson, J.T. Predicting recruitment density dependence and intrinsic growth rate for all fishes worldwide using a data–integrated life–history model. Fish Fish. 2020, 21, 237–251. [Google Scholar] [CrossRef]
- Prince, J.; Victor, S.; Kloulchad, V.; Hordyk, A. Length based SPR assessment of eleven Indo-Pacific coral reef fish populations in Palau. Fish. Res. 2015, 171, 42–58. [Google Scholar] [CrossRef]
- Kirkwood, G. Simple models for multispecies fisheries. In Theory and Management of Tropical Fisheries; The WorldFish Center: Monographs, Malaysia, 1982; pp. 83–98. [Google Scholar]
- Ralston, S.; Polovina, J.J. A multispecies analysis of the commercial deep-sea handline fishery in Hawaii. Fish. Bull. 1982, 80, 435. [Google Scholar]
- McClanahan, T.R.; Azali, M.K. Improving sustainable yield estimates for tropical reef fisheries. Fish Fish. 2020, 21, 683–699. [Google Scholar] [CrossRef]
- Lorenzen, K.; Almeida, O.; Arthur, R.; Garaway, C.; Khoa, S.N. Aggregated yield and fishing effort in multispecies fisheries: An empirical analysis. Can. J. Fish. Aquat. Sci. 2006, 63, 1334–1343. [Google Scholar] [CrossRef]
- Morais, R.A.; Depczynski, M.; Fulton, C.; Marnane, M.; Narvaez, P.; Huertas, V.; Brandl, S.J.; Bellwood, D.R. Severe coral loss shifts energetic dynamics on a coral reef. Funct. Ecol. 2020, 34, 1507–1518. [Google Scholar] [CrossRef]
- Morais, R.A.; Smallhorn-West, P.; Connolly, S.R.; Ngaluafe, P.F.; Malimali, S.; Halafihi, T.; Bellwood, D.R. Sustained productivity and the persistence of coral reef fisheries. Nat. Sustain. 2023, 6, 1199–1209. [Google Scholar] [CrossRef]
- Zamborain-Mason, J.; Cinner, J.E.; MacNeil, M.A.; Graham, N.A.J.; Hoey, A.S.; Beger, M.; Brooks, A.J.; Booth, D.J.; Edgar, G.J.; Feary, D.A.; et al. Sustainable reference points for multispecies coral reef fisheries. Nat. Commun. 2023, 14, 5368. [Google Scholar] [CrossRef]
- Dassow, C.J.; Ross, A.J.; Jensen, O.P.; Sass, G.G.; van Poorten, B.T.; Solomon, C.T.; Jones, S.E. Experimental demonstration of catch hyperstability from habitat aggregation, not effort sorting, in a recreational fishery. Can. J. Fish. Aquat. Sci. 2020, 77, 762–769. [Google Scholar] [CrossRef]
- Galligan, B.P.; McClanahan, T.R. Nutrition contributions of coral reef fisheries not enhanced by capture of small fish. Ocean. Coast. Manag. 2024, 249, 107011. [Google Scholar] [CrossRef]
- Robinson, J.P.W.; Robinson, J.; Gerry, C.; Govinden, R.; Freshwater, C.; Graham, N.A.J. Diversification insulates fisher catch and revenue in heavily exploited tropical fisheries. Sci. Adv. 2020, 6, eaaz0587. [Google Scholar] [CrossRef]
- Omukoto, J.O.; Graham, N.A.; Hicks, C.C. Fish markets facilitate nutrition security in coastal Kenya: Empirical evidence for policy leveraging. Mar. Policy 2024, 164, 106179. [Google Scholar] [CrossRef]
- Ontomwa, M.B.; Fulanda, B.M.; Kimani, E.N.; Okemwa, G.M. Hook size selectivity in the artisanal handline fishery of Shimoni fishing area, south coast. West. Indian Ocean. J. Mar. Sci. 2019, 18, 29–46. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Kosgei, J.K. Redistribution of benefits but not detection in a fisheries bycatch-reduction management initiative. Conserv. Biol. 2018, 32, 159–170. [Google Scholar] [CrossRef]
- Rochet, M.J.; Benoit, E. Fishing destabilizes the biomass flow in the marine size spectrum. Proc. R. Soc. B Biol. Sci. 2012, 279, 284–292. [Google Scholar] [CrossRef]
- Kolding, J.; van Zwieten, P.A.M. Sustainable fishing of inland waters. J. Limnol. 2014, 73, 132–148. [Google Scholar] [CrossRef]
- Law, R.; Plank, M.J.; Kolding, J. Balanced exploitation and coexistence of interacting, size-structured, fish species. Fish Fish. 2016, 17, 281–302. [Google Scholar] [CrossRef]
- Garcia, S.M.; Kolding, J.; Rice, J.; Rochet, M.J.; Zhou, S.; Arimoto, T.; Beyer, J.E.; Borges, L.; Bundy, A.; Dunn, D.; et al. Reconsidering the consequences of selective fisheries. Science 2012, 335, 1045–1047. [Google Scholar] [CrossRef] [PubMed]
- Graham, N.A.J.; Dulvy, N.K.; Jennings, S.; Polunin, N.V.C. Size-spectra as indicators of the effects of fishing on coral reef fish assemblages. Coral Reefs 2005, 24, 118–124. [Google Scholar] [CrossRef]
- Carvalho, P.G.; Setiawan, F.; Fahlevy, K.; Subhan, B.; Madduppa, H.; Zhu, G.; Humphries, A.T. Fishing and habitat condition differentially affect size spectra slopes of coral reef fishes. Ecol. Appl. 2021, 31, e02345. [Google Scholar] [CrossRef]
- McClanahan, T.R. Multicriteria estimate of coral reef fishery sustainability. Fish Fish. 2018, 19, 807–820. [Google Scholar] [CrossRef]
- Karr, K.A.; Fujita, R.; Halpern, B.S.; Kappel, C.V.; Crowder, L.; Selkoe, K.A.; Alcolado, P.M.; Rader, D. Thresholds in Caribbean coral reefs: Implications for ecosystem-based fishery management. J. Appl. Ecol. 2015, 52, 402–412. [Google Scholar] [CrossRef]
- Pauly, D.; Froese, R.; Holt, S.J. Balanced harvesting: The institutional incompatibilities. Mar. Policy 2016, 69, 121–123. [Google Scholar] [CrossRef]
- Fulton, E.A. Opportunities to improve ecosystem-based fisheries management by recognizing and overcoming path dependency and cognitive bias. Fish Fish. 2021, 22, 428–448. [Google Scholar] [CrossRef]
- MacNeil, M.A.; Graham, N.A.; Cinner, J.E.; Wilson, S.K.; Williams, I.D.; Maina, J.; Newman, S.; Friedlander, A.M.; Jupiter, S.; Polunin, N.V.C.; et al. Recovery potential of the world’s coral reef fishes. Nature 2015, 520, 341–344. [Google Scholar] [CrossRef]
- Babcock, E.A.; Tewfik, A.; Burns-Perez, V. Fish community and single-species indicators provide evidence of unsustainable practices in a multi-gear reef fishery. Fish. Res. 2018, 208, 70–85. [Google Scholar] [CrossRef]
- Pitcher, T.J. Fisheries managed to rebuild ecosystems? Reconstructing the past to salvage the future. Ecol Appl. 2001, 11, 601–617. [Google Scholar] [CrossRef]
- McClanahan, T.R.; Friedlander, A.M.; Wantiez, L.; Graham, N.A.; Bruggemann, J.H.; Chabanet, P.; Oddenyo, R.M. Best-practice fisheries management associated with reduced stocks and changes in life histories. Fish Fish. 2022, 23, 422–444. [Google Scholar] [CrossRef]
- Edgar, G.J.; Bates, A.E.; Krueck, N.C.; Baker, S.C.; Stuart-Smith, R.D.; Brown, C.J. Stock assessment models overstate sustainability of the world’s fisheries. Science 2024, 385, 860–865. [Google Scholar] [CrossRef]
- Edgar, G.J.; Stuart-Smith, R.D.; Willis, T.J.; Kininmonth, S.; Baker, S.C.; Banks, S.; Barrett, N.S.; Becerro, M.A.; Bernard, A.T.F.; Berkhout, J.; et al. Global conservation outcomes depend on marine protected areas with five key features. Nature 2014, 506, 216–220. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.C.; Winther-Janson, M.; Bainbridge, J.M.; Aitken, J.; Hawkins, J.P.; Roberts, C.M. Effective coverage targets for ocean protection. Conserv. Lett. 2016, 9, 398–404. [Google Scholar] [CrossRef]




| Category | Mkwiro | Wasini | Kibuyuni | Vanga | Jimbo | ChiSq; Prob > ChiSq | All Sites | |
|---|---|---|---|---|---|---|---|---|
| (a) Sampling | Days sampled/month | 13.6 ± 0.9 a | 11.5 ± 0.7 b | 11.9 ± 0.7 b | 11.7 ± 0.6 b | 10.0 ± 0.6 c | 17.1; 0.002 | 11.7 ± 0.3 |
| Sample size (n) | 366 | 320 | 345 | 318 | 286 | 1635 | ||
| Effort (Fishers/km2/day) | 2.06 ± 0.16 a | 1.85 ± 0.1 a | 2.56 ± 0.28 b | 1.29 ± 0.06 c | 1.15 ± 0.08 c | 63.6; <0.0001 | 1.77 ± 0.08 | |
| CPUE (kg/fisher/day) | 5.58 ± 0.28 a | 3.46 ± 0.24 b | 4.11 ± 0.2 b | 2.4 ± 0.08 c | 3.51 ± 0.21 b | 75.0; <0.0001 | 3.79 ± 0.13 | |
| Income (KES/fisher/day) | 1214.76 ± 56.06 a | 920.43 ± 66.62 b | 841.12 ± 40.59 b | 374.14 ± 17.58 c | 548.89 ± 37.03 c | 94.7; <0.0001 | 772.27 ± 32.03 | |
| Yield (kg/km2/day) | 11.95 ± 1.35 a | 6.96 ± 0.69 b | 9.99 ± 0.91 b | 2.9 ± 0.17 c | 3.6 ± 0.19 c | 90.2; <0.0001 | 6.99 ± 0.45 | |
| Yield (tons/km2/y) | 2.53 ± 0.29 a | 1.48 ± 0.15 b | 2.12 ± 0.19 b | 0.62 ± 0.04 | 0.76 ± 0.04 c | 90.2; <0.0001 | 1.48 ± 0.10 | |
| (b) Fish groups | ||||||||
| CPUE | Goatfish | 0.13 ± 0.01 a | 0.06 ± 0.01 b | 0.11 ± 0.02 b | 0.04 ± 0.01 c | 0.01 ± 0.004 c | 70.4; <0.0001 | 0.07 ± 0.01 a |
| Mixed catch | 0.43 ± 0.04 a | 0.25 ± 0.02 c | 0.19 ± 0.01 c | 0.60 ± 0.07 a | 0.76 ± 0.09 b | 68.7; <0.0001 | 0.44 ± 0.03 b | |
| Octopus | 0.21 ± 0.03 a | 0.22 ± 0.03 a | 0.39 ± 0.03 a | 0.30 ± 0.03 a | 0.97 ± 0.09 b | 59.4; <0.0001 | 0.41 ± 0.03 b | |
| Parrotfish | 0.28 ± 0.02 a | 0.20 ± 0.03 b | 0.17 ± 0.02 b | 0.15 ± 0.03 b | 0.10 ± 0.02 b | 26.8; <0.0001 | 0.18 ± 0.01 c | |
| Pelagics | 0.20 ± 0.03 a | 0.26 ± 0.04 a | 0.17 ± 0.03 a | 0.60 ± 0.08 b | 0.46 ± 0.08 b | 42.3; <0.0001 | 0.34 ± 0.03 d | |
| Rabbitfish | 0.58 ± 0.07 a | 0.40 ± 0.04 b | 0.33 ± 0.02 c | 0.23 ± 0.04 c | 0.33 ± 0.08 c | 32.7; <0.0001 | 0.37 ± 0.03 b | |
| Scavengers | 0.50 ± 0.03 a | 0.33 ± 0.03 a | 0.22 ± 0.02 b | 0.97 ± 0.11 c | 0.61 ± 0.09 a | 51.3; <0.0001 | 0.52 ± 0.04 e | |
| Yield | Goatfish | 0.11 ± 0.02 a | 0.03 ± 0.01 b | 0.06 ± 0.01 b | 0.01 ± 0.002 c | 0.003 ± 0.002 c | 88.5; <0.0001 | 0.04 ± 0.01 a |
| Mixed catch | 0.42 ± 0.07 a | 0.16 ± 0.02 b | 0.17 ± 0.03 b | 0.32 ± 0.04 c | 0.25 ± 0.03 c | 22.0; 0.0002 | 0.27 ± 0.02 b | |
| Octopus | 0.07 ± 0.01 a | 0.10 ± 0.02 a | 0.38 ± 0.07 b | 0.17 ± 0.02 a | 0.47 ± 0.05 b | 62.9; <0.0001 | 0.24 ± 0.02 b | |
| Parrotfish | 0.25 ± 0.04 a | 0.11 ± 0.02 b | 0.15 ± 0.02 b | 0.05 ± 0.01 c | 0.04 ± 0.01 c | 55.5; <0.0001 | 0.12 ± 0.01 c | |
| Pelagics | 0.09 ± 0.02 a | 0.22 ± 0.05 a | 0.13 ± 0.04 a | 0.34 ± 0.02 b | 0.23 ± 0.04 a | 42.2; <0.0001 | 0.20 ± 0.02 d | |
| Rabbitfish | 0.60 ± 0.09 a | 0.45 ± 0.07 a | 0.54 ± 0.06 a | 0.05 ± 0.01 b | 0.08 ± 0.02 b | 85.3; <0.0001 | 0.34 ± 0.03 e | |
| Scavengers | 0.52 ± 0.04 a | 0.32 ± 0.03 a | 0.31 ± 0.06 a | 0.12 ± 0.02 b | 0.17 ± 0.02 b | 50.2; <0.0001 | 0.29 ± 0.02 b | |
| Income | Goatfish | 29.66 ± 3.00 a | 17.57 ± 2.80 a | 22.75 ± 2.90 a | 6.98 ± 2.34 b | 1.95 ± 0.81 b | 74.1; <0.0001 | 15.79 ± 1.41 a |
| Mixed catch | 71.87 ± 7.16 a | 51.47 ± 4.83 a | 26.65 ± 2.05 b | 68.33 ± 7.22 a | 81.17 ± 8.89 c | 46.9; <0.0001 | 59.53 ± 3.28 b | |
| Octopus | 49.35 ± 6.65 a | 61.73 ± 8.09 b | 97.15 ± 7.76 c | 72.59 ± 7.82 b | 184.25 ± 20.38 d | 49.3; <0.0001 | 92.30 ± 6.37 c | |
| Parrotfish | 49.89 ± 4.77 a | 47.00 ± 6.02 a | 25.25 ± 2.49 b | 23.81 ± 4.90 c | 11.45 ± 2.74 c | 46.9; <0.0001 | 31.35 ± 2.30 a | |
| Pelagics | 47.64 ± 7.07 a | 69.25 ± 10.44 a | 39.00 ± 6.28 a | 85.86 ± 12.96 b | 77.94 ± 14.69 a | 14.4; 0.006 | 63.93 ± 4.99 b | |
| Rabbitfish | 149.99 ± 19.75 a | 114.39 ± 10.77 a | 70.18 ± 4.69 b | 51.49 ± 10.02 b | 64.34 ± 16.48 b | 45.1; <0.0001 | 88.61 ± 6.57 c | |
| Scavengers | 118.19 ± 8.67 a | 92.50 ± 8.79 a | 44.39 ± 4.87 b | 168.2 ± 17.56 c | 87.20 ± 12.90 a | 48.2; <0.0001 | 100.84 ± 6.04 c | |
| Category | Mkwiro (n) | Wasini (n) | Kibuyuni (n) | Vanga (n) | Jimbo (n) | ChiSq; Prob > ChiSq | All Sites (n) | |
|---|---|---|---|---|---|---|---|---|
| (a) Number of species | Traps | 30 | 16 | 28 | 32 | 22 | 66 | |
| Spearguns | 0 | 5 | 0 | 7 | 20 | 27 | ||
| Handlines | 8 | 0 | 15 | 9 | 9 | 29 | ||
| Nets | 0 | 2 | 6 | 88 | 5 | 93 | ||
| All gear types | 34 | 21 | 30 | 104 | 43 | |||
| (b) Fish length | Traps | 28.0 ± 0.5 (151) a | 25.0 ± 0.8 (63) b | 27.0 ± 0.4 (178) b | 22.0 ± 0.4 (226) c | 28.0 ± 0.8 (100) b | 120.6; <0.0001 | 26.0 ± 0.3 (718) b |
| Spearguns | 24.0 ± 1.1 (15) a | 23.0 ± 1.0 (11) a | 26.0 ± 1.5 (33) a | NS | 25.0 ± 0.9 (59) b | |||
| Handlines | 19.0 ± 0.8 (15) a | 34.0 ± 1.0 (6) b | 20.0 ± 1.0 (58) a | 20.0 ± 0.4 (37) a | 22.0 ± 1.1 (23) b | 18.8; 0.0009 | 21.0 ± 0.5 (139) a | |
| Nets | 19.0 ± 0.2 (20) a | 26.0 ± 0.7 (33) b | 19.0 ± 0.2 (840) a | 19.0 ± 0.9 (10) a | 27.6; <0.0001 | 19.0 ± 0.2 (903) a | ||
| All gear types | 28.0 ± 0.5 (166) a | 24.0 ± 0.6 (104) b | 25.0 ± 0.4 (269) a | 20.0 ± 0.2 (1114) c | 26.0 ± 0.6 166) a | 292.5; <0.0001 | 22.0 ± 0.2 (1819) | |
| (c) CPUE | Traps | 5.45 ± 0.33 a | 3.41 ± 0.27 b | 4.47 ± 0.23 a | 6.94 ± 0.51 c | 4.85 ± 0.37 a | 46.1; <0.0001 | 5.05 ± 0.19 a |
| Spearguns | 3.93 ± 0.37 a | 3.28 ± 0.23 a | 3.34 ± 0.18 a | 3.41 ± 0.23 a | 3.73 ± 0.21 a | NS | 3.51 ± 0.11 b | |
| Handlines | 5.1 ± 0.31 a | 2.82 ± 0.27 a | 3.48 ± 0.24 a | 3.38 ± 0.34 a | 2.84 ± 0.17 b | 35.9; <0.0001 | 3.52 ± 0.14 b | |
| Nets | 3.25 ± 0.46 a | 4.08 ± 0.3 b | 4.16 ± 0.4 c | 3.18 ± 0.24 b | 2.88 ± 0.26 c | 16.9; 0.002 | 3.48 ± 0.14 c | |
| All gear types | 4.64 ± 0.2 a | 3.61 ± 0.17 b | 3.82 ± 0.15 b | 3.8 ± 0.19 b | 3.37 ± 0.15 b | 33.7; <0.0001 | 3.77 ± 0.08 | |
| (d) Yield | Traps | 6.93 ± 1.07 a | 3.35 ± 0.43 b | 4.92 ± 0.49 c | 0.67 ± 0.05 d | 1.09 ± 0.14 d | 87.6; <0.0001 | 3.39 ± 0.32 a |
| Spearguns | 1.05 ± 0.1 a | 0.99 ± 0.09 a | 2.74 ± 0.33 b | 1.37 ± 0.13 a | 1.5 ± 0.15 a | 26.5; <0.0001 | 1.59 ± 0.1 c | |
| Handlines | 3.71 ± 0.44 a | 1.17 ± 0.15 b | 2.2 ± 0.39 c | 0.29 ± 0.04 d | 0.62 ± 0.06 d | 69.6; <0.0001 | 1.68 ± 0.17 b | |
| Nets | 1.08 ± 0.13 a | 2.66 ± 0.23 b | 3.25 ± 0.48 b | 1.08 ± 0.06 a | 1.55 ± 0.14 a | 51.7; <0.0001 | 1.87 ± 0.1 c | |
| All gear types | 3.52 ± 0.42 a | 2.16 ± 0.15 b | 3.21 ± 0.22 a | 0.99 ± 0.04 c | 1.3 ± 0.08 c | 130.6; <0.0001 | 2.04 ± 0.08 | |
| (e) Income | Traps | 1215.04 ± 68.75 a | 916.15 ± 77.57 b | 873.11 ± 46.77 b | 1030.59 ± 68.24 b | 715.65 ± 64.23 c | 29.1; <0.0001 | 955.8 ± 32.25 a |
| Spearguns | 924.93 ± 88.34 a | 892.86 ± 66.6 a | 718.03 ± 36.75 a | 746.13 ± 45.79 a | 692.56 ± 47.84 a | NS | 790.39 ± 26.45 c | |
| Handlines | 1093.29 ± 63.82 a | 736.76 ± 72.45 b | 674.35 ± 47.23 b | 605.34 ± 90.25 b | 366.21 ± 20.29 c | 58.9; <0.0001 | 697.55 ± 33.7 b | |
| Nets | 686.99 ± 109.17 a | 1056.54 ± 80.74 b | 822.24 ± 85.34 b | 491.23 ± 44.39 a | 436.81 ± 49.46 a | 70.8; <0.0001 | 678.73 ± 34.34 d | |
| All gear types | 1033.58 ± 43.59 a | 950.76 ± 44.63 a | 769.32 ± 29.64 b | 622.49 ± 33.58 c | 529.5 ± 28.53 c | 126.5; <0.0001 | 757.29 ± 17.91 |
| Simpson Index | Shannon Index | Evenness | |
|---|---|---|---|
| (a) Gear | |||
| Speargun | 0.93 | 2.86 | 1.0 |
| Traps | 0.82 | 2.67 | 0.93 |
| Nets | 0.88 | 2.61 | 0.91 |
| Handline | 0.90 | 2.55 | 0.89 |
| (b) Site | |||
| Marine reserve | 0.92 | 3.27 | 1.0 |
| Jimbo | 0.94 | 3.15 | 0.97 |
| Vanga | 0.88 | 2.77 | 0.85 |
| Mkwiro | 0.81 | 2.47 | 0.76 |
| Wasini | 0.87 | 2.44 | 0.75 |
| Kibuyuni | 0.79 | 2.30 | 0.70 |
| Fish Landing Sampling | Field Sampling (DGS) | ||||||
|---|---|---|---|---|---|---|---|
| Numbers/Fishing Group/Day ± SD (n) | Percentage of Total | COV, % | Numbers/500 m2 ± SD (n) | Percentage of Total | COV, % | Differential Abundance, % | |
| (a) Family | |||||||
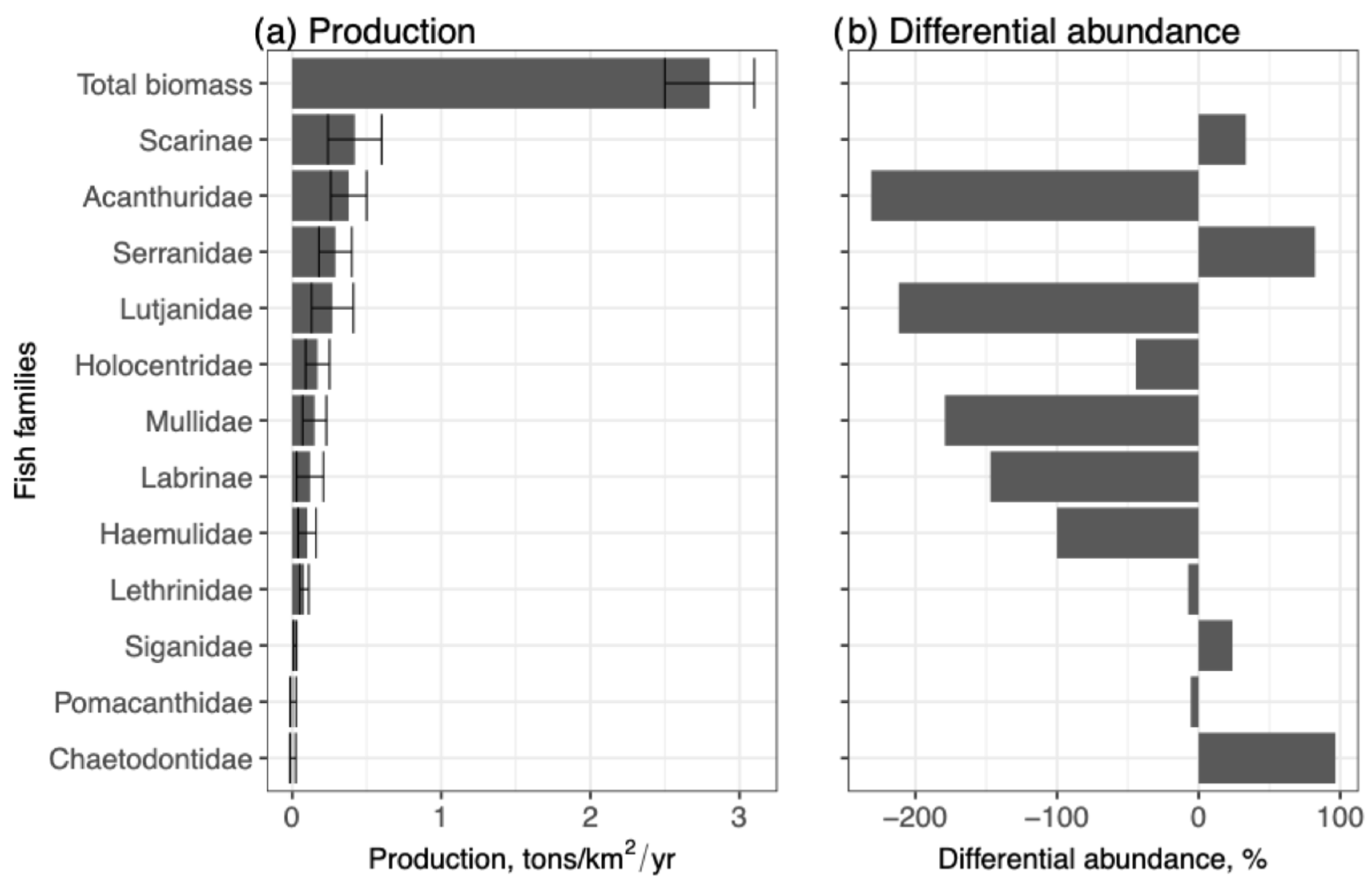
| Acanthuridae | 0.01 ± 0.11 (12) | 1.6 | 1928.8 | 3.38 ± 7.62 (152) | 5.3 | 225.7 | −231.3 |
| Lutjanidae | 0.03 ± 0.40 (13) | 1.7 | 1277.2 | 17.11 ± 19.59 (154) | 5.3 | 114.5 | −211.8 |
| Mullidae | 0.09 ± 0.65 (192) | 25.5 | 699.9 | 45.56 ± 133.93 (2050) | 71.2 | 294 | −179.2 |
| Labrinae | 0.01 ± 0.13 (13) | 1.7 | 1622 | 3.33 ± 12.41 (120) | 4.2 | 372.4 | −147.1 |
| Haemulidae | 0.005 ± 0.10 (2) | 0.3 | 2032.2 | 2.0 ± 1.8 (18) | 0.6 | 90.1 | −100 |
| Holocentridae | 0.01 ± 0.13 (7) | 0.9 | 1588 | 2.11 ± 3.63 (38) | 1.3 | 171.9 | −44.4 |
| Lethrinidae | 0.01 ± 0.10 (20) | 2.7 | 1419.3 | 1.33 ± 2.37 (84) | 2.9 | 177.7 | −7.4 |
| Pomacanthidae | 0.02 ± 0.25 (27) | 3.6 | 1169.5 | 4.07 ± 7.98 (110) | 3.8 | 195.8 | −5.6 |
| Siganidae | 0.01 ± 0.01 (16) | 2.1 | 100 | 1.0 ± 1.57 (45) | 1.6 | 156.7 | 23.8 |
| Scarinae | 0.005 ± 0.07 (2) | 0.3 | 1435.3 | 0.56 ± 0.73 (5) | 0.2 | 130.8 | 33.3 |
| Serranidae | 0.04 ± 0.37 (89) | 11.8 | 865.4 | 1.33 ± 1.82 (60) | 2.1 | 136.6 | 82.2 |
| Chaetodontidae | 0.87 ± 2.04 (360) | 47.8 | 234.3 | 5.0 ± 11.63 (45) | 1.6 | 232.6 | 96.7 |
| Simpson index | 0.69 | 0.48 | |||||
| Shannon index | 1.53 | 1.16 | |||||
| Evenness | 1 | 0.76 | |||||
| (b) Species | |||||||
| Gomphosus caeruleus | 0.049 ± 0.002 (1) | 0.1 | 4.9 | 5.6 ± 3.5 (50) | 1.74 | 63.7 | −1640 |
| Lutjanus kasmira | 0.46 ± 0.05 (20) | 2.7 | 10.5 | 124.2 ± 250.7 (1118) | 38.81 | 201.8 | −1337.4 |
| Lutjanus gibbus | 0.049 ± 0.002 (1) | 0.1 | 4.9 | 4.3 ± 8.9 (39) | 1.35 | 205.1 | −1250 |
| Naso annulatus | 0.098 ± 0.005 (2) | 0.3 | 4.9 | 12.4 ± 13.8 (112) | 3.89 | 110.6 | −1196.7 |
| Myripristis berndti | 0.139 ± 0.01 (4) | 0.5 | 7 | 11.7 ± 23.8 (105) | 3.64 | 203.8 | −628 |
| Mulloidichthys vanicolensis | 0.148 ± 0.01 (3) | 0.4 | 4.9 | 6.8 ± 13.3 (61) | 2.12 | 195.7 | −430 |
| Ctenochaetus striatus | 0.049 ± 0.002 (1) | 0.1 | 4.9 | 1.4 ± 1.7 (13) | 0.45 | 120.5 | −350 |
| Aethaloperca rogaa | 0.049 ± 0.002 (1) | 0.1 | 4.9 | 1.2 ± 1.4 (11) | 0.38 | 114.1 | −280 |
| Lutjanus lutjanus | 0.462 ± 0.08 (35) | 4.6 | 18.3 | 53.3 ± 138.9 (480) | 16.66 | 260.5 | −262.2 |
| Cephalopholis argus | 0.098 ± 0.005 (2) | 0.3 | 4.9 | 3.2 ± 1.8 (29) | 1.01 | 55.5 | −236.7 |
| Lethrinus obsoletus | 0.402 ± 0.03 (13) | 1.7 | 7.8 | 17.1 ± 19.6 (154) | 5.35 | 114.5 | −214.7 |
| Scarus frenatus | 0.07 ± 0.005 (2) | 0.3 | 7 | 2.4 ± 2.0 (22) | 0.76 | 82.1 | −153.3 |
| Plectorhinchus gaterinus | 0.12 ± 0.01 (4) | 0.5 | 8.1 | 4.0 ± 4.4 (36) | 1.25 | 111.1 | −150 |
| Chaetodon auriga | 0.098 ± 0 (2) | 0.3 | 4.9 | 2.0 ± 1.8 (18) | 0.62 | 90.1 | −106.7 |
| Thalassoma lunare | 0.049 ± 0 (1) | 0.1 | 4.9 | 0.6 ± 0.7 (5) | 0.17 | 130.8 | −70 |
| Halichoeres hortulanus | 0.085 ± 0.01 (3) | 0.4 | 8.5 | 2.1 ± 1.7 (19) | 0.66 | 80.1 | −65 |
| Acanthurus leucosternon | 0.148 ± 0.01 (3) | 0.4 | 4.9 | 1.9 ± 2.9 (17) | 0.59 | 155.4 | −47.5 |
| Parupeneus barberinus | 0.322 ± 0.03 (13) | 1.7 | 9.8 | 5.3 ± 1.7 (48) | 1.67 | 32.5 | 1.8 |
| Lutjanus fulviflamma | 0.824 ± 0.28 (116) | 15.4 | 34.1 | 45.8 ± 71.9 (412) | 14.3 | 157.1 | 7.1 |
| Acanthurus nigricauda | 0.07 ± 0.005 (2) | 0.3 | 7 | 0.8 ± 1.0 (7) | 0.24 | 124.9 | 20 |
| Sargocentron caudimaculatum | 0.098 ± 0.005 (2) | 0.3 | 4.9 | 0.8 ± 1.6 (7) | 0.24 | 201 | 20 |
| Scarus psittacus | 0.13 ± 0.01 (5) | 0.7 | 9.3 | 1.7 ± 1.8 (15) | 0.52 | 108.2 | 25.7 |
| Pomacanthus imperator | 0.07 ± 0.005 (2) | 0.3 | 7 | 0.6 ± 0.7 (5) | 0.17 | 130.8 | 43.3 |
| Myripristis murdjan | 0.148 ± 0.01 (3) | 0.4 | 4.9 | 0.4 ± 1.0 (4) | 0.14 | 228.1 | 65 |
| Calotomus carolinus | 0.36 ± 0.04 (16) | 2.1 | 10.8 | 2.1 ± 2.2 (19) | 0.66 | 104.4 | 68.6 |
| Anampses caeruleopunctatus | 0.049 ± 0.002 (1) | 0.1 | 4.9 | 0.1 ± 0.3 (1) | 0.03 | 300 | 70 |
| Bodianus bilunulatus | 0.049 ± 0.002 (1) | 0.1 | 4.9 | 0.1 ± 0.3 (1) | 0.03 | 300 | 70 |
| Epinephelus spilotoceps | 0.049 ± 0.002 (1) | 0.1 | 4.9 | 0.1 ± 0.3 (1) | 0.03 | 300 | 70 |
| Cheilinus trilobatus | 0.098 ± 0.01 (4) | 0.5 | 9.9 | 0.4 ± 0.5 (4) | 0.14 | 118.6 | 72 |
| Sargocentron diadema | 0.12 ± 0.01 (4) | 0.5 | 8.1 | 0.4 ± 1.0 (4) | 0.14 | 228.1 | 72 |
| Acanthurus dussumieri | 0.155 ± 0.01 (4) | 0.5 | 6.2 | 0.3 ± 0.7 (3) | 0.1 | 212.1 | 80 |
| Plectorhinchus flavomaculatus | 0.148 ± 0.01 (3) | 0.4 | 4.9 | 0.2 ± 0.4 (2) | 0.07 | 198.4 | 82.5 |
| Cheilio inermis | 0.202 ± 0.02 (9) | 1.2 | 10.8 | 0.4 ± 0.7 (4) | 0.14 | 163.5 | 88.3 |
| Epinephelus merra | 0.07 ± 0.005 (2) | 0.3 | 7 | 0.1 ± 0.3 (1) | 0.03 | 300 | 90 |
| Cephalopholis boenak | 0.26 ± 0.02 (10) | 1.3 | 9.3 | 0.3 ± 0.5 (3) | 0.1 | 150 | 92.3 |
| Scarus rubroviolaceus | 0.109 ± 0.01 (5) | 0.7 | 11.1 | 0.1 ± 0.3 (1) | 0.03 | 300 | 95.7 |
| Siganus sutor | 2.042 ± 0.87 (360) | 47.8 | 42.7 | 5.0 ± 11.6 (45) | 1.56 | 232.6 | 96.7 |
| Parupeneus cyclostomus | 0.264 ± 0.03 (11) | 1.5 | 10.1 | 0.1 ± 0.3 (1) | 0.03 | 300 | 98 |
| Scarus ghobban | 0.721 ± 0.15 (61) | 8.1 | 20.5 | 0.3 ± 1.0 (3) | 0.1 | 300 | 98.8 |
| Lutjanus argentimaculatus | 0.984 ± 0.05 (20) | 2.7 | 4.9 | 0.1 ± 0.3 (1) | 0.03 | 300 | 98.9 |
| Simpson index | 0.74 | 0.79 | |||||
| Shannon index | 2.09 | 2.16 | |||||
| Evenness | 0.96 | 1.0 | |||||
| Differential Category | Number of Species | Trophic Level | Growth Rate (K/Year) | Natural Mortality (M) | Life Span | Generation Time | Age at First Maturity™ | Length at Maturity (Lmat) | Length at MSY (Lopt) | Maximum Length (Lmax) | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| (a) Sampling group | |||||||||||
| Catch | Negative | 32 | 3.6 | 0.6 | 0.4 | 7.6 | 2.3 | 1.8 | 25.0 | 28.1 | 55.1 |
| Positive | 11 | 2.3 | 1.1 | 1.2 | 5.9 | 1.7 | 1.4 | 21.8 | 23.4 | 39.7 | |
| Marine reserve | Negative | 45 | 2.3 | 0.5 | 0.8 | 7.8 | 2.4 | 2.0 | 16.0 | 16.5 | 27.5 |
| Positive | 24 | 2.9 | 1.0 | 1.2 | 4.3 | 1.5 | 1.2 | 15.2 | 13.5 | 29.3 | |
| Species shared | Negative | 28 | 3.8 | 0.4 | 0.6 | 9.2 | 2.7 | 2.3 | 21.2 | 23 | 42.8 |
| Positive | 12 | 2.5 | 0.6 | 1.0 | 5.6 | 1.7 | 1.4 | 27.1 | 30.6 | 48.8 | |
| All species | Negative | 105 | 3.2 | 0.5 | 0.6 | 8.2 | 2.5 | 2 | 20.7 | 22.5 | 41.8 |
| Positive | 47 | 1.7 | 0.7 | 0.8 | 3.4 | 1.1 | 0.9 | 12.4 | 12.3 | 23 | |
| (b) Fishing gear type | Nets | 90 | 3.1 | 0.7 | 1.1 | 5.8 | 1.8 | 1.4 | 25.9 | 29.8 | 54.7 |
| Traps | 65 | 2.8 | 0.6 | 0.8 | 6.7 | 2.0 | 1.6 | 25.2 | 28.1 | 48.0 | |
| Handlines | 29 | 3.7 | 0.5 | 0.5 | 8.0 | 2.3 | 1.9 | 22.7 | 25.2 | 49.6 | |
| Spearguns | 27 | 2.6 | 0.4 | 0.7 | 8.4 | 2.5 | 2.0 | 28.1 | 32.0 | 52.3 | |
| All gear types | 211 | 3.05 | 0.55 | 0.78 | 7.3 | 2.15 | 1.73 | 25.48 | 28.78 | 51.2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
McClanahan, T.R.; Kosgei, J.K.; Humphries, A.T. Fisheries Sustainability Eroded by Lost Catch Proportionality in a Coral Reef Seascape. Sustainability 2025, 17, 2671. https://doi.org/10.3390/su17062671
McClanahan TR, Kosgei JK, Humphries AT. Fisheries Sustainability Eroded by Lost Catch Proportionality in a Coral Reef Seascape. Sustainability. 2025; 17(6):2671. https://doi.org/10.3390/su17062671
Chicago/Turabian StyleMcClanahan, Timothy Rice, Jesse Kiprono Kosgei, and Austin Turner Humphries. 2025. "Fisheries Sustainability Eroded by Lost Catch Proportionality in a Coral Reef Seascape" Sustainability 17, no. 6: 2671. https://doi.org/10.3390/su17062671
APA StyleMcClanahan, T. R., Kosgei, J. K., & Humphries, A. T. (2025). Fisheries Sustainability Eroded by Lost Catch Proportionality in a Coral Reef Seascape. Sustainability, 17(6), 2671. https://doi.org/10.3390/su17062671






