The Effect of Newly Developed Microbial Biopreparations on the Chemical Composition of Strawberry (Fragaria × ananassa Duch.) Fruit Grown in an Organic Farming System
Abstract
:1. Introduction
2. Materials and Methods
2.1. Experimental Study
2.2. Meteorological Conditions
2.3. Statistical Analysis
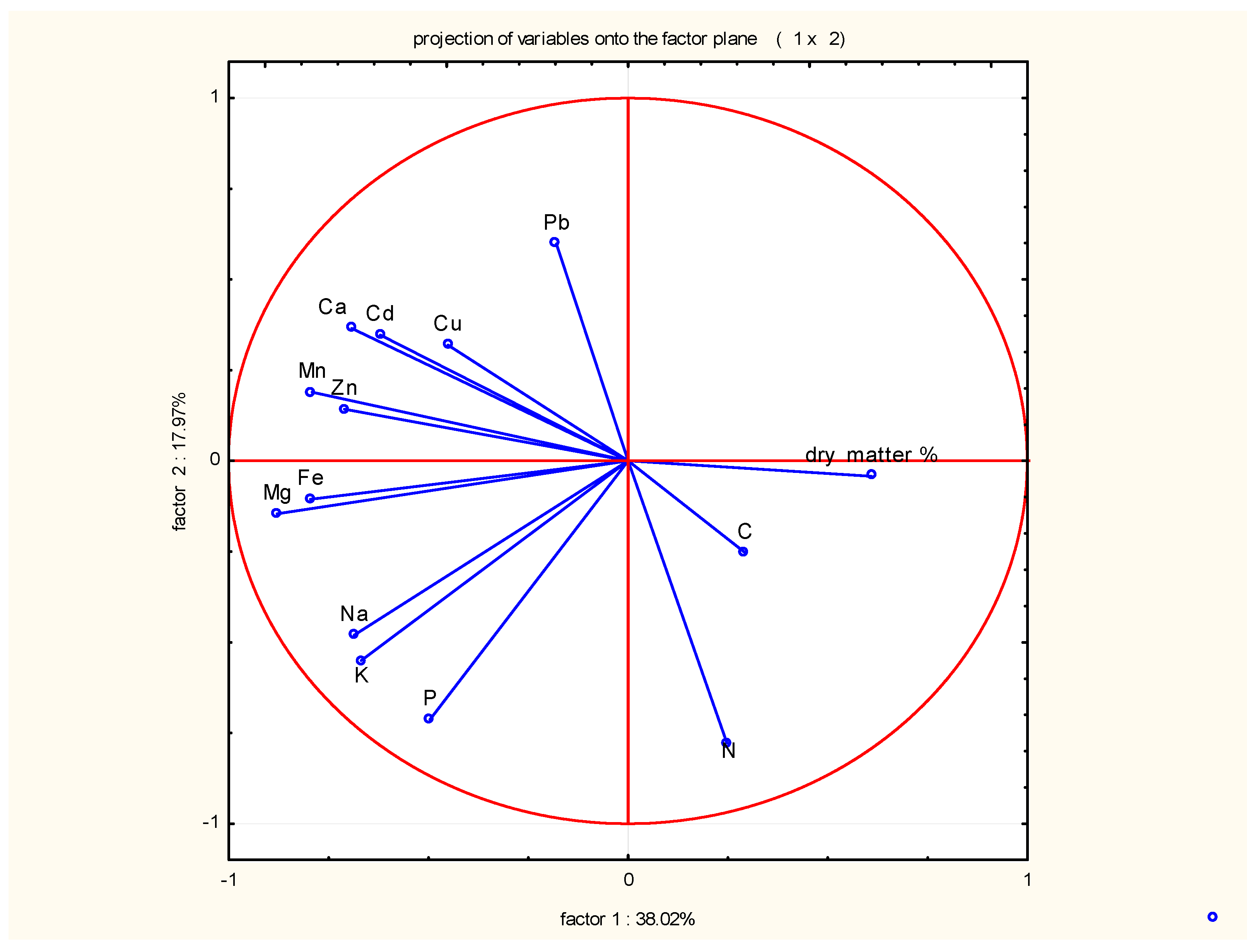
3. Results and Discussion
4. Summary and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sas-Paszt, L.; Sumorok, B.; Grzyb, Z.S.; Głuszek, S.; Sitarek, M.; Lisek, A.; Derkowska, E.; Trzciński, P.; Przybył, M.; Frąc, M.; et al. Effect of microbiologically enriched fertilizers on the yielding of strawberry plants under field conditions in the second year of plantation. J. Res. Appl. Agric. Eng. 2020, 65, 31–38. [Google Scholar]
- Derkowska, E.; Sas Paszt, L.; Trzciński, P.; Przybył, M.; Weszczak, K. Influence of biofertilizers on plant growth and rizosphere microbiology of greenhouse-grown strawberry cultivars. Acta Sci. Pol. Hortorum Cultus 2015, 14, 83–96. [Google Scholar]
- Şener, S.; Cantemur, M.H. Comparison of fruit quality criteria and heavy metal contents of strawberries grown in organic and conventional agriculture. Appl. Sci. 2023, 13, 7919. [Google Scholar] [CrossRef]
- Giampieri, F.; Forbes-Hernández, T.Y.; Gasparrini, M.; Álvarez-Suarez, J.M.; Afrin, S.; Bompadre, S.; Quiles, J.L.; Mezzetti, B.; Battino, M. Strawberry as a health promoter: An evidence based review. Food Funct. 2015, 6, 1386–1398. [Google Scholar] [CrossRef]
- Basu, A.; Nguyen, A.; Betts, N.M.; Lyons, T.J. Strawberry as a functional food: An evidence-based review. Crit. Rev. in Food Sci. and Nutr. 2014, 54, 790–806. [Google Scholar] [CrossRef]
- Kilic, N. Improvement in plant growth, yield and fruit quality with biostimulant treatment in organic strawberry cultivation. HortScience 2024, 59, 1165–1171. [Google Scholar] [CrossRef]
- de la Peña Moreno, F.; Blanch, G.P.; Flores, G.; Ruiz del Castillo, M.L. Impact of postharvest methyl jasmonate treatment on the volatile composition and flavonol content of strawberries. J. Sci. Food Agric. 2010, 90, 989–994. [Google Scholar] [CrossRef]
- Wang, X.; Wu, L.; Qui, J.; Qian, Y.; Wang, M. Comparative metabolomic analysis of the nutritional aspects from ten cultivars of the strawberry fruit. Foods 2023, 12, 1153. [Google Scholar] [CrossRef]
- Shen, J.; Shao, W.; Li, J.; Lu, H. Integrated metabolomic and transcriptomic analysis reveals factors underlying differences in fruit quality between Fragaria nilgerrensis and Fragaria pentaphylla. J. Sci. Food Agric. 2022, 102, 3287–3296. [Google Scholar] [CrossRef]
- Katel, S.; Mandal, H.R.; Kattel, S.; Yadav, S.P.S.; Lamshal, B.S. Impact of plant growth regulators in strawberry plant: A review. Heliyon 2022, 8, e11959. [Google Scholar] [CrossRef]
- Khunte, S.D.; Kumar, A.; Ansari, N.; Saravanan, S. 2020. Effect of Different Levels of PGRs with Organic Manure on Growth Characters and Economics of Strawberry (Fragaria x ananassa Duch.) cv. Chandler in Northern region. Int. J. Curr. Microbiol. App. Sci. 2020, 9, 1633–1638. [Google Scholar] [CrossRef]
- Sharma, K.; Negi, M. Effect of organic manures and inorganic fertilizers on plant growth of strawberry (Fragaria x ananassa) cv. Shimla delicious under mid-hill conditions of Uttarakhand. J. Pharmacogn. Phytochem. 2019, 8, 1440–1444. [Google Scholar]
- Kumra, R.; Saravanan, R.S.; Bakshi, P.; Kumar, A.; Singh, M.; Kumar, V. Influence of plant growth regulators on strawberry: A review. Int. J. Chem. Stud. 2018, 6, 1236–1239. [Google Scholar]
- Kilic, N. Synergistic effect of organic and biofertilizers on strawberry cultivation. Sustainability 2023, 15, 8206. [Google Scholar] [CrossRef]
- Giamperi, F.; Tulipani, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Compositon, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef]
- Wójcik- Seliga, J.; Studnicki, M.; Wójcik-Gront, E. Evaluating economic value of 23 strawberry cultivars in the climatic conditions of central Europe. Acta Sci. Pol. Hortorum Cultus 2017, 16, 11–19. [Google Scholar]
- Kobi, H.B.; Martins, M.C.; Silva, P.I.; Souza, J.L.; Carneiro, J.C.S.; Heleno, F.F.; Queiroz, M.E.L.R.; Costa, N.M.B. Organic and conventional strawberries: Nutritional quality, antioxidant characteristics and pesticide residues. Fruits 2018, 73, 39–47. [Google Scholar] [CrossRef]
- Camargo, L.K.P.; Resende, J.T.V.; Tominaga, T.T.; Kurchaidt, S.M.; Camargo, C.K.; Figueiredo, A.S.T. Postharvest quality of strawberry fruits produced in organic and conventional systems. Hortic. Bras. 2011, 29, 577–583. [Google Scholar] [CrossRef]
- Aninowski, M.; Kazimierczak, R.; Hallmann, E.; Rachtan-Janicka, J.; Fijoł-Adach, E.; Feledyn-Szewczyk, B.; Majak, I.; Leszczyńska, J. Evaluation of the potential allergenicity of strawberries in response to different farming practices. Metabolites 2020, 10, 102. [Google Scholar] [CrossRef]
- Winter, C.K. Pesticide residues in imported, organic, and “suspect” fruits and vegetables. J. Agric. Food Chem. 2012, 60, 4425–4429. [Google Scholar] [CrossRef]
- Alsanius, B.W.; Von Essen, E.; Hartmann, R.; Vagsholm, I.; Doyle, O.; Schmutz, U.; Stützel, H.; Fricke, A.; Dorais, M. The “one health”-concept and organic production of vegetables and fruits. Acta Hortic. 2019, 1242, 1–14. [Google Scholar] [CrossRef]
- Ochmian, I.; Błaszak, M.; Lachowicz, S.; Piwowarczyk, R. The impact of cultivation systems on the nutritional and phytochemical content, and microbiological contamination of highbush blueberry. Sci. Rep. 2020, 10, 16696. [Google Scholar] [CrossRef]
- Błajet-Kosicka, A.; Twarużek, M.; Kosicki, R.; Sibiorowska, E.; Grajewski, J. Co-occurrence and evaluation of mycotoxins in organic and conventional rye grain and products. Food Control. 2014, 38, 61–66. [Google Scholar] [CrossRef]
- Brodal, G.; Hofgaard, I.S.; Eriksen, G.S.; Bernhoft, A.; Sundheim, L. Mycotoxins in organically versus conventionally produced cereal grains and some other crops in temperate regions. World Mycotoxin J. 2016, 9, 755–770. [Google Scholar] [CrossRef]
- Lázaro, A.; de Lorenzo, C. Texture Analysis in Melon Landraces through Instrumental and Sensory Methods. Int. J. Food Prop. 2015, 18, 1575–1583. [Google Scholar] [CrossRef]
- Altisent, R.; Jaeger, S.R.; Johnston, J.W.; Harker, F.R. Injection of flavor essences into fruit pieces: A new approach for exploring consumer preferences for novel flavors of apple fruit. J. Sens. Stud. 2013, 28, 405–413. [Google Scholar] [CrossRef]
- Gao, Z.; Wong, S.S.; House, L.A.; Spreen, T.H. French consumer perception, preference of, and willingness to pay for fresh fruit based on country of origin. Br. Food J. 2014, 116, 805–820. [Google Scholar] [CrossRef]
- Reganold, J.P.; Andrews, P.K.; Reeve, J.R.; Carpenter-Boggs, L.; Schadt, C.W.; Alldredge, J.R.; Ross, C.F.; Davies, N.M.; Zhou, J. Fruit and Soil Quality of Organic and Conventional Strawberry Agroecosystems. PLoS ONE 2010, 5, e12346. [Google Scholar] [CrossRef]
- Jamiołkowska, A.; Hetman, B. The mechanism of action of biological preparations used in plant protection against pathogens. Ann. UMCS Sect. E Agric. 2016, 71, 13–29. [Google Scholar]
- Lipa, J.J.; Pruszyński, S. Stan wykorzystania metod biologicznych w ochronie roślin w Polsce i na świecie. Prog. Plant Prot. 2010, 50, 1033–1043. [Google Scholar]
- Babalola, O.O. Beneficial bacteria of agricultural importance. Biotechnol. Lett. 2010, 32, 1559–1570. [Google Scholar] [CrossRef]
- Mynett, K.; Podwyszyńska, M.; Derkowska, E.; Górnik, K.; Sas-Paszt, L.; Wojtania, A. Effect of biologically active TotalHumus® and Bacterbase on the growth ex vitro of strawberry, blueberry and hip rose microcuttings. Acta Sci. Pol. Hortorum Cultus 2022, 21, 7–20. [Google Scholar] [CrossRef]
- Schönbichler, A.; Diaz-Moreno, S.M.; Srivastava, V.; McKee, L.S. Exploring the potential for fungal antagonism and cell wall attack by Bacillus subtilis natto. Frontin Microbiol. 2020, 11, 521. [Google Scholar] [CrossRef]
- Grzegorczyk, M.; Szalewicz, A.; Żarowska, B.; Połomska, X.; Wątorek, W.; Wójtowicz, M. Microorganisms in biological control of phytopathogenic fungi. Acta Sci. Pol. Biotechnol. 2015, 14, 19–42. [Google Scholar]
- Rusin, M.; Domagalska, J.; Rogala, D.; Razzaghi, M.; Szymala, I. Concentration of cadmium and lead in vegetables and fruits. Sci. Rep. 2021, 11, 11913. [Google Scholar] [CrossRef]
- Grzyb, A.; Waraczewska, Z.; Niewiadomska, A.; Wolna-Maruwka, A. What are biopreparations and what is their use? Nauka Przyr. Technol. 2019, 13, 65–76. [Google Scholar] [CrossRef]
- Nakielska, M.; Berbeć, A.K.; Madej, A.; Feledyn-Szewczyk, B. Microbial fertilizing products impact on productivity and profitability of organic strawberry cultivars. Horticulturae 2024, 10, 1112. [Google Scholar] [CrossRef]
- Available online: https://agronomplants.pl/truskawki/odmiany-wczesne/sadzonki-truskawek-rumba/ (accessed on 15 January 2025).
- Available online: https://agronomplants.pl/truskawki/odmiany-wczesne/sadzonki-truskawek-vibrant/ (accessed on 15 January 2025).
- Available online: https://agronomplants.pl/truskawki/odmiany-wczesne/sadzonki-truskawek-honeoye/ (accessed on 15 January 2025).
- Drobek, M.; Cybulska, J.; Frąc, M.; Pieczywek, P.; Pertile, G.; Chibrikov, V.; Nosalewicz, A.; Feledyn-Szewczyk, B.; Sas-Paszt, L.; Zdunek, A. Microbial biostimulants affect the development of pathogenic microorganisms and the quality of fresh strawberries (Fragaria ananassa Duch.). Sci. Hortic. 2024, 327, 112793. [Google Scholar] [CrossRef]
- Ayaz, M.; Li, C.-H.; Ali, Q.; Zhao, W.; Chi, Y.-K.; Shafiq, M.; Ali, F.; Yu, X.-Y.; Yu, Q.; Zhao, J.-T.; et al. Bacterial and fungal biocontrol agents for plant disease protection: Journey from lab to field, current status, challanges, and global perspectives. Molecules 2023, 28, 6735. [Google Scholar] [CrossRef]
- Bojarska, J.E.; Zadernowski, R.; Tońska, E. Macroelements of strawberry fruits. Bromat. Chem. Toksykol. 2011, 44, 572–576. [Google Scholar]
- Çakmakçi, S.; Çakmakçi, R. Quality and nutritional parameters of food in agri-food production systems. Foods 2023, 12, 351. [Google Scholar] [CrossRef]
- Dinç, S.; Kara, M.; Takma, Ç.; Kara, Y.; Kolayli, S. Strawberries from Konya in the Central Anatolia Region of Türkiye: Phenolic profile, antioxidant potential and mineral composition. Appl. Fruit Sci. 2024, 66, 1229–1240. [Google Scholar] [CrossRef]
- Santamaria, P. Nitrate in vegetables: Toxicity, content, intake, and EC regulation. J. Sci. Food Agric. 2006, 86, 10–17. [Google Scholar] [CrossRef]
- Ahmed, M.; Rauf, M.; Mukhtar, Z.; Saeed, N.A. Excessive use of nitrogenous fertilizers: An unawareness causing serious threats to environment and human health. Environ. Sci. Pollut. Res. 2017, 24, 26983–26987. [Google Scholar] [CrossRef]
- Kopeć, M.; Gondek, K.; Mierzwa-Hersztek, M.; Zaleski, T.; Bogdał, S.; Bienisz, M.; Błaszczyk, J.; Kaczmarczyk, E.; Knaga, J.; Łapczyńska-Kordon, B.; et al. The effect of stimulating biomass growth of everbearing strawberry variety San Andreas® by the foliage application of a product containing zinc. Prog. Plant Prot. 2019, 59, 126–132. [Google Scholar] [CrossRef]
- Shrestha, B.; Tripathi, K.M.; Shrestha, A.K.; Gautam, R. Evaluation of chemical attributes of low chilling strawberry (Fragaria ananassa) varieties in Chitwan, Nepal. Nepal. Hortic. 2023, 17, 69–73. [Google Scholar] [CrossRef]
- Hattab, S.; Bougattass, I.; Hassine, R.; Dridi-Al-Mohandes, B. Metals and micronutrients in some edible crops and their cultivation soils in estern-central region of Tunisia: A comparision between organic and conventional farming. Food Chem. 2019, 270, 293–298. [Google Scholar] [CrossRef]
- Kumar, A.; Kumari, N.; Singh, A.; Kumar, D.; Kumar Yadav, D.; Varshney, A.; Sharma, N. The effect of cadmium tolerant Plant Growth Promoting Rhizobacteria on plant growth promotion and phytoremediation: A review. Curr. Microbiol. 2023, 80, 153. [Google Scholar] [CrossRef]
- Recamales, A.F.; López Medina, J.; Hernanz, D. Physicochemical characteristics and mineral content of strawberries grown in soil and soilless system. J. of Food Qual. 2007, 30, 837–853. [Google Scholar] [CrossRef]
- Commission Regulation (EU). 2023/915 of 25 April 2023 on maximum levels for certain contaminants in food and repealing Regulation (EC) No 1881/2006. Off. J. Eur. Union 2023, 119, 103–157. [Google Scholar]
- Verma, S.; Bairwa, R.K.; Rana, D.K.; Dimiri, T.; Dotaniya, C.K. Influence of different concentrations of zinc, boron and iron on yield characters of strawberry (Fragaria x ananassa Duch.) cv. Chandler under valley condition of Garhwal Himalaya. Jof Pharmacogn. and Phytochem. 2018, 7, 3881–3884. [Google Scholar]
- Bebek Markovinović, A.; Putnik, P.; Duralija, B.; Krivohlavek, A.; Ivešić, M.; Mandić Andačić, I.; Palac Bešlić, I.; Pavlić, B.; Lorenzo, J.M.; Bursać Kovačević, D. Chemometric valorization of strawberry (Fragaria x ananasa Duch.) cv. ‘Albion’ for the production of functional juice: The impact of physiochemical, toxicological, sensory and bioactive value. Foods 2022, 11, 640. [Google Scholar] [CrossRef]
- Şahin, M.; Eşitken, A.; Pirlak, L.; Altintaş, S.; Turan, M. Effect of DN1 bacterial strain applied by different methods on some morphological characteristics of strawberry cv. San Andreas (Fragaria × ananassa Duch.). AGROFOR Int. J. 2019, 4, 57–64. [Google Scholar] [CrossRef]
- Alengebawy, A.; Abdelkhalek, S.T.; Quereshi, S.R.; Wang, M.-Q. Heavy metals and pesticides toxicity in agricultural soil and plants: Ecological risk and human health implications. Toxics 2021, 9, 42. [Google Scholar] [CrossRef] [PubMed]
- Tsotetsi, T.; Nephali, L.; Malebe, M.; Tugizimana, F. Bacillus for plant growth promotion and stress resilience: What have we learned? Plants 2022, 11, 2482. [Google Scholar] [CrossRef]
- Gałązka, A. Preparaty mikrobiologiczne potrzeby, szanse i znaczenie. In Monografie i Rozprawy Naukowe: Preparaty Mikrobiologiczne w Rolnictwie i Ochronie Środowiska, 2nd ed.; Gałązka, A., Podleśny, J., Eds.; Wydawnictwo IUNG-PIB: Puławy, Poland, 2024; Volume 66, pp. 9–27. [Google Scholar]
- Furtak, K. Bakterie z rodzaju Bacillus jako składniki biopreparatów o szerokim spektrum zastosowania w rolnictwie. In Monografie i Rozprawy Naukowe: Preparaty Mikrobiologiczne w Rolnictwie i Ochronie Środowiska, 2nd ed.; Gałązka, A., Podleśny, J., Eds.; Wydawnictwo IUNG-PIB: Puławy, Poland, 2024; Volume 66, pp. 89–104. [Google Scholar]







| Abbreviation | Trade Name | Details |
|---|---|---|
| K1 | - | Control object |
| K2 | BacilRoots | Preparation containing Bacillus sp. AF75BC and Bacillus subtilis AF75AB2 on a carrier that consists of dry humic acids, mustard, rapeseed oil, and clove oil in micronised dolomite (109 CFU/plant) |
| K3 | BacilRoots + BacilExtra | Preparation containing Bacillus sp. AF75BC and Bacillus subtilis AF75AB2 on a carrier that consists of dry humic acids, mustard, rapeseed oil, and clove oil in micronised dolomite (109 CFU/plant), and Bacillus subtilis AF75AB2 and Bacillus sp. Sp115AD on a carrier that consists of plant extracts (nettle, horsetail, calendula) and humic acids in micronised dolomite (105 CFU/cm2) |
| K4 | BacilRoots + BacilHumus | Preparation containing Bacillus sp. AF75BC and Bacillus subtilis AF75AB2 on a carrier that consists of dry humic acids, mustard, rapeseed oil, and clove oil in micronised dolomite (109 CFU/plant), as well as Bacillus sp. Sp116AC*, Bacillus sp. Sp115AD, humic acids, and yeast culture effluent in micronised dolomite (105 CFU/cm2) |
| K5 | BacilRoots + BacilExtra + BacilHumus | Preparation containing Bacillus sp. AF75BC and Bacillus subtilis AF75AB2 on a carrier that consists of dry humic acids, mustard, rapeseed oil, and clove oil in micronised dolomite (109 CFU/plant), and Bacillus subtilis AF75AB2 and Bacillus sp. Sp115AD on a carrier that consists of plant extracts (nettle, horsetail, calendula) and humic acids in micronised dolomite (105 CFU/cm2), as well as Bacillus sp. Sp116AC*, Bacillus sp. Sp115AD, humic acids, and yeast culture effluent in micronised dolomite (105 CFU/cm2) |
| K6 | BacilExtra + BacilHumus | Preparation containing Bacillus subtilis AF75AB2 and Bacillus sp. Sp115AD on a carrier that consists of plant extracts (nettle, horsetail, calendula) and humic acids in micronised dolomite (105 CFU/cm2), as well as Bacillus sp. Sp116AC*, Bacillus sp. Sp115AD, humic acids, and yeast culture effluent in micronised dolomite (105 CFU/cm2) |
| Treatment Type | Product and/or Formulation | Manufacturer | Dose per Hectare | Number of Treatments |
|---|---|---|---|---|
| Fertilisation | Redarom Activstart | Biodevas Laboratoires Savigné-1’ Évêque, France | 1.5 L | 2 |
| Olibio | Biodevas Laboratoires Savigné-1’ Évêque, France | 2 L | 2 | |
| Aminosol (N) | AZELIS POLAND Sp. z o. o., Poznań, Poland | 3 L | 2 | |
| Potassium sulphate Patentkali® | K+S Polska Sp. z o.o., Poznań, Poland | 250 kg | 1 | |
| Fertilisation | Potassium salt | K+S Minerals and Agriculture GmbH, Kassel, Germany | 60 kg | 1 |
| Carbonate lime Polcalc | Polcalc Nawozy Wapniowe Sp. z o.o., Warsaw Poland | 500 kg | 1 | |
| Biopreparations (microbial fertilising products) | K2 (BacilRoots) | Bacto-Tech Sp. z o.o., Toruń, Poland | 50 kg | 3 |
| K3 (BacilRoots + BacilExtra) | Bacto-Tech Sp. z o.o., Toruń, Poland | 50 kg | 3 | |
| K4 (BacilRoots + BacilHumus) | Bacto-Tech Sp. z o.o., Toruń, Poland | 50 kg | 3 | |
| K5 (BacilRoots + BacilExtra + BacilHumus) | Bacto-Tech Sp. z o.o., Toruń, Poland | 50 kg | 3 | |
| K6 (BacilExtra + BacilHumus) | Bacto-Tech Sp. z o.o., Toruń, Poland | 50 kg | 3 |
| Month | Precipitation [mm] | Temperature [°C] | ||||
|---|---|---|---|---|---|---|
| 2021 | 2022 | Multi-Annual Average | 2021 | 2022 | Multi-Annual Average | |
| April | 51.2 | 42.4 | 42.0 | 6.4 | 6.5 | 7.5 |
| May | 49.9 | 23.3 | 53.0 | 12.5 | 14.4 | 12.4 |
| June | 70.1 * | 31.5 | 110.0 | 19.5 | 19.5 | 16.7 |
| July | 61.7 * | 80.6 | 105.0 | 21.8 | 19.1 | 17.8 |
| Varieties | Biopreparations | ||||||
|---|---|---|---|---|---|---|---|
| K1 | K2 | K3 | K4 | K5 | K6 | Average | |
| ‘Honeoye’ | 10.64 a * (±0.50) | 11.58 b (±0.56) | 11.06 ab (±0.92) | 11.44 ab (±1.38) | 10.95 ab (±0.77) | 11.24 ab (±1.04) | 11.18 C (±0.36) |
| ‘Rumba’ | 8.78 a (±0.42) | 9.04 a (±0.61) | 9.81 a (±0.62) | 10.02 a (±0.78) | 9.78 a (±1.03) | 9.83 a (±1.05) | 9.59 B (±0.32) |
| ‘Vibrant’ | 8.07 a (±0.46) | 8.93 a (±0.45) | 9.28 a (±0.47) | 8.99 a (±0.81) | 8.70 a (±0.75) | 8.31 a (±0.51) | 8.75 A (±0.24) |
| Average | 9.17 a (±0.41) | 9.85 a (±0.42) | 10.05 a (±0.42) | 10.15 a (±0.61) | 9.81 a (±0.52) | 9.79 a (±0.57) | 9.84 (±0.20) |
| Varieties | Biopreparations | ||||||
|---|---|---|---|---|---|---|---|
| K1 | K2 | K3 | K4 | K5 | K6 | Average | |
| ‘Honeoye’ | 446.6 a * (±20.0) | 455.1 a (±14.0) | 452.6 a (±13.8) | 458.0 a (±15.6) | 459.3 a (±13.4) | 436.9 a (±7.7) | 451.7 A (±5.4) |
| ‘Rumba’ | 452.4 a (±19.5) | 447.6 a (±16.3) | 462.9 a (±15.7) | 448.7 a (±12.2) | 455.6 a (±18.0) | 461.9 a (±18.5) | 455.0 A (±6.4) |
| ‘Vibrant’ | 439.9 a (±9.2) | 459.2 a (±17.5) | 449.4 a (±15.5) | 456.1 a (±14.9) | 454.7 a (±13.7) | 452.7 a (±15.6) | 452.7 A (±5.8) |
| Average | 446.3 a (±9.0) | 454.0 a (±8.7) | 454.9 a (±8.3) | 454.3 a (±7.8) | 456.5 a (±8.2) | 450.5 a (±8.3) | 453.1 (±3.4) |
| Varieties | Biopreparations | ||||||
|---|---|---|---|---|---|---|---|
| K1 | K2 | K3 | K4 | K5 | K6 | Average | |
| ‘Honeoye’ | 14.8 a * (±1.3) | 16.7 a (±2.2) | 18.3 a (±2.3) | 18.2 a (±1.7) | 17.1 a (±1.7) | 16.9 a (±1.6) | 17.1 B (±0.7) |
| ‘Rumba’ | 18.6 a (±2.4) | 17.6 a (±2.1) | 19.3 a (±1.2) | 19.2 a (±2.1) | 18.4 a (±1.5) | 19.8 a (±2.4) | 18.8 C (±0.7) |
| ‘Vibrant’ | 13.8 a (±0.7) | 15.2 ab (±1.5) | 16.3 b (±2.3) | 16.0 b (±1.6) | 16.3 b (±1.6) | 16.2 b (±1.5) | 15.7 A (±0.7) |
| Average | 15.7 a (±1.1) | 16.5 ab (±1.1) | 18.0 b (±1.1) | 17.8 ab (±1.0) | 17.3 ab (±0.9) | 17.7 ab (±1.1) | 17.2 (±0.4) |
| Varieties | Biopreparations | ||||||
|---|---|---|---|---|---|---|---|
| K1 | K2 | K3 | K4 | K5 | K6 | Average | |
| ‘Honeoye’ | 28.7 a * (±3.2) | 33.3 a (±4.3) | 30.1 a (±2.9) | 30.0 a (±3.4) | 26.3 a (±2.9) | 26.7 a (±2.3) | 29.2 A (±1.3) |
| ‘Rumba’ | 34.6 b (±2.9) | 34.0 b (±4.4) | 34.9 b (±3.5) | 37.3 b (±2.7) | 24.6 a (±1.7) | 36.5 b (±2.7) | 33.6 B (±1.4) |
| ‘Vibrant’ | 40.8 a (±4.3) | 45.2 a (±3.9) | 38.9 a (±6.1) | 44.0 a (±4.4) | 36.2 a (±2.2) | 45.3 a (±2.1) | 41.8 C (±1.7) |
| Average | 34.7 ab (±2.4) | 37.5 b (±2.6) | 34.7 ab (±2.5) | 37.1 b (±2.4) | 29.0 a (±1.8) | 36.2 b (±2.3) | 34.9 (±1.0) |
| Varieties | Biopreparations | ||||||
|---|---|---|---|---|---|---|---|
| K1 | K2 | K3 | K4 | K5 | K6 | Average | |
| ‘Honeoye’ | 32.7 a * (±3.4) | 34.1 a (±4.7) | 31.3 a (±0.4) | 34.6 a (±4.5) | 30.4 a (±1.3) | 34.7 a (±1.6) | 33.0 A (±1.2) |
| ‘Rumba’ | 38.4 b (±5.6) | 32.4 ab (±2.2) | 36.6 ab (±4.6) | 35.3 ab (±3.5) | 30.6 a (±1.5) | 37.2 ab (±1.5) | 34.9 A (±1.3) |
| ‘Vibrant’ | 46.4 b (±11.1) | 35.4 ab (±4.8) | 30.5 a (±1.6) | 35.7 ab (±2.4) | 35.3 ab (±1.2) | 34.9 ab (±2.4) | 35.8 A (±1.7) |
| Average | 39.1 b (±4.2) | 33.9 ab (±2.2) | 32.8 a (±1.7) | 35.2 ab (±1.9) | 32.1 a (±0.9) | 35.6 ab (±1.1) | 34.5 (±0.8) |
| Varieties | Biopreparations | ||||||
|---|---|---|---|---|---|---|---|
| K1 | K2 | K3 | K4 | K5 | K6 | Average | |
| ‘Honeoye’ | 13.2 a * (±2.1) | 14.2 a (±2.4) | 13.2 a (±2.1) | 18.5 a (±3.4) | 16.0 a (±1.5) | 14.9 a (±1.8) | 15.1 A (±0.9) |
| ‘Rumba’ | 33.9 a (±4.1) | 24.3 a (±2.1) | 34.0 a (±7.4) | 24.7 a (±6.2) | 24.3 a (±2.8) | 31.4 a (±5.9) | 28.5 C (±2.1) |
| ‘Vibrant’ | 31.2 a (±10.0) | 26.9 a (±4.7) | 21.3 a (±2.8) | 21.9 a (±1.8) | 21.2 a (±2.5) | 24.8 a (±4.2) | 24.2 B (±1.7) |
| Average | 26.1 a (±4.3) | 21.8 a (±2.2) | 22.9 a (±3.3) | 21.7 a (±2.3) | 20.5 a (±1.5) | 23.7 a (±2.9) | 22.6 (±1.1) |
| Varieties | Biopreparations | ||||||
|---|---|---|---|---|---|---|---|
| K1 | K2 | K3 | K4 | K5 | K6 | Average | |
| ‘Honeoye’ | 18,381 a * (±1718) | 18,828 a (±1568) | 17,686 a (±1257) | 18,322 a (±1464) | 16,107 a (±631) | 16,763 a (±795) | 17,640 A (±503) |
| ‘Rumba’ | 19,609 a (±590) | 20,326 a (±1252) | 21,953 a (±2024) | 19,974 a (±1770) | 19,509 a (±917) | 19,392 a (±689) | 20,158 B (±546) |
| ‘Vibrant’ | 20,099 ab (±816) | 21,033 b (±1531) | 17,493 a (±1420) | 17,757 ab (±923) | 18,286 ab (±964) | 20,469 ab (±1390) | 19,136 B (±532) |
| Average | 19,363 a (±639) | 20,062 a (±821) | 19,044 a (±1002) | 18,684 a (±808) | 17,968 a (±574) | 18,874 a (±664) | 18,978 (±319) |
| Varieties | Biopreparations | ||||||
|---|---|---|---|---|---|---|---|
| K1 | K2 | K3 | K4 | K5 | K6 | Average | |
| ‘Honeoye’ | 2899 a * (±142) | 2536 a (±264) | 2575 a (±263) | 3176 a (±106) | 2935 a (±222) | 2792 a (±381) | 2814 A (±105) |
| ‘Rumba’ | 3092 a (±434) | 2542 a (±182) | 2604 a (±351) | 2470 a (±261) | 2796 a (±369) | 2824 a (±109) | 2700 A (±115) |
| ‘Vibrant’ | 3792 a (±406) | 3507 a (±269) | 3014 a (±166) | 3487 a (±352) | 3861 a (±381) | 3905 a (±329) | 3583 B (±132) |
| Average | 3261 a (±217) | 2862 a (±172) | 2731 a (±155) | 3045 a (±175) | 3198 a (±213) | 3174 a (±204) | 3032 (±78) |
| Varieties | Biopreparations | ||||||
|---|---|---|---|---|---|---|---|
| K1 | K2 | K3 | K4 | K5 | K6 | Average | |
| ‘Honeoye’ | 1488 a * (±109) | 1437 a (±97) | 1432 a (±100) | 1467 a (±81) | 1422 a (±102) | 1388 a (±104) | 1436 A (±38) |
| ‘Rumba’ | 1793 a (±130) | 1613 a (±125) | 1720 a (±179) | 1695 a (±135) | 1573 a (±47) | 1632 a (±64) | 1664 B (±47) |
| ‘Vibrant’ | 1912 a (±113) | 1798 a (±148) | 1582 a (±104) | 1794 a (±158) | 1856 a (±105) | 1951 a (±111) | 1810 C (±52) |
| Average | 1731 a (±82) | 1616 a (±77) | 1578 a (±77) | 1652 a (±77) | 1617 a (±65) | 1657 a (±76) | 1637 (±31) |
| Varieties | Biopreparations | ||||||
|---|---|---|---|---|---|---|---|
| K1 | K2 | K3 | K4 | K5 | K6 | Average | |
| ‘Honeoye’ | 2074 a * (±111) | 2339 a (±340) | 2112 a (±119) | 2234 a (±221) | 2122 a (±227) | 2003 a (±123) | 2152 A (±84) |
| ‘Rumba’ | 2310 a (±331) | 2498 a (±244) | 2579 a (±277) | 2647 a (±331) | 2741 a (±349) | 2457 a (±281) | 2552 B (±117) |
| ‘Vibrant’ | 2284 ab (±336) | 2285 ab (±176) | 1905 a (±164) | 2150 ab (±193) | 2252 ab (±264) | 2518 b (±278) | 2229 A (±94) |
| Average | 2223 a (±150) | 2374 a (±144) | 2199 a (±127) | 2343 a (±148) | 2372 a (±167) | 2326 a (±141) | 2311 (±59) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakielska, M.; Feledyn-Szewczyk, B.; Berbeć, A.K.; Ukalska-Jaruga, A.; Frąc, M. The Effect of Newly Developed Microbial Biopreparations on the Chemical Composition of Strawberry (Fragaria × ananassa Duch.) Fruit Grown in an Organic Farming System. Sustainability 2025, 17, 2571. https://doi.org/10.3390/su17062571
Nakielska M, Feledyn-Szewczyk B, Berbeć AK, Ukalska-Jaruga A, Frąc M. The Effect of Newly Developed Microbial Biopreparations on the Chemical Composition of Strawberry (Fragaria × ananassa Duch.) Fruit Grown in an Organic Farming System. Sustainability. 2025; 17(6):2571. https://doi.org/10.3390/su17062571
Chicago/Turabian StyleNakielska, Małgorzata, Beata Feledyn-Szewczyk, Adam Kleofas Berbeć, Aleksandra Ukalska-Jaruga, and Magdalena Frąc. 2025. "The Effect of Newly Developed Microbial Biopreparations on the Chemical Composition of Strawberry (Fragaria × ananassa Duch.) Fruit Grown in an Organic Farming System" Sustainability 17, no. 6: 2571. https://doi.org/10.3390/su17062571
APA StyleNakielska, M., Feledyn-Szewczyk, B., Berbeć, A. K., Ukalska-Jaruga, A., & Frąc, M. (2025). The Effect of Newly Developed Microbial Biopreparations on the Chemical Composition of Strawberry (Fragaria × ananassa Duch.) Fruit Grown in an Organic Farming System. Sustainability, 17(6), 2571. https://doi.org/10.3390/su17062571









