KOH-Assisted Chemical Activation of Camelina Meal (Wild Flax) to Treat PFOA-Contaminated Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Feed Stock and Chemicals Employed
2.2. Chemical Activation of Camelina Meal Biochar
2.3. Design of Experiments for Optimization of Chemical Activation
2.4. Analysis of Activated Carbon Derived from Biochar
2.4.1. Proximate Analysis
2.4.2. Ultimate Analysis
2.4.3. Activated Surface Area and Porosity
2.4.4. Structural Analysis
2.4.5. Morphological Analysis by Scanning Electron Microscopy (SEM)
2.5. Adsorption of PFOA Using Activated Carbon
3. Results
3.1. Screening of Chemical Activation
3.2. Overview of Chemical Activation
3.3. Statistical Analysis of Chemical Activation Process
3.4. Influence of Process Variables on Porous Structure and Yield
3.5. Optimization of the Chemical Activation of Camelina Meal Biochar
3.6. Fourier Transform Infrared Spectral Analysis (FTIR)
3.7. Molecular Analysis by 13C Solid-State NMR
3.8. Morphological Analysis
3.9. Adsorption of PFOA
4. Discussion and Future Outlook
Past and Present Research Trends
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hsiao, C.H.; Gupta, S.; Lee, C.Y.; Tai, N.H. Effects of physical and chemical activations on the performance of biochar applied in supercapacitors. Appl. Surf. Sci. 2023, 610, 155560. [Google Scholar] [CrossRef]
- Han, L.; Sun, K.; Yang, Y.; Xia, X.; Li, F.; Yang, Z.; Xing, B. Biochar’s stability and effect on the content, composition and turnover of soil organic carbon. Geoderma 2020, 364, 114184. [Google Scholar] [CrossRef]
- Jha, S.; Pattnaik, F.; Nanda, S.; Zapata, O.; Acharya, B.; Dalai, A.K. Investigations of thermal effects during pyrolysis of agro-forestry biomass and physicochemical characterizations of biofuel products. Biocatal. Agric. Biotechnol. 2024, 61, 103379. [Google Scholar] [CrossRef]
- Bedia, J.; Peñas-Garzón, M.; Gómez-Avilés, A.; Rodriguez, J.J.; Belver, C. Review on activated carbons by chemical activation with FeCl3. C 2020, 6, 21. [Google Scholar] [CrossRef]
- Abioye, A.M.; Ani, F.N. Recent development in the production of activated carbon electrodes from agricultural waste biomass for supercapacitors: A review. Renew. Sustain. Energy Rev. 2015, 52, 1282–1293. [Google Scholar] [CrossRef]
- Serafin, J.; Dziejarski, B.; Junior, O.F.C.; Sreńscek-Nazzal, J. Design of highly microporous activated carbons based on walnut shell biomass for H2 and CO2 storage. Carbon 2023, 201, 633–647. [Google Scholar] [CrossRef]
- Trakal, L.; Šigut, R.; Šillerová, H.; Faturíková, D.; Komárek, M. Copper removal from aqueous solution using biochar: Effect of chemical activation. Arab. J. Chem. 2014, 7, 43–52. [Google Scholar] [CrossRef]
- Angın, D.; Altintig, E.; Köse, T.E. Influence of process parameters on the surface and chemical properties of activated carbon obtained from biochar by chemical activation. Bioresour. Technol. 2013, 148, 542–549. [Google Scholar] [CrossRef]
- Liu, H.; Xu, G.; Li, G. Preparation of porous biochar based on pharmaceutical sludge activated by NaOH and its application in the adsorption of tetracycline. J. Colloid Interface Sci. 2021, 587, 271–278. [Google Scholar] [CrossRef]
- Yorgun, S.; Vural, N.; Demiral, H. Preparation of high-surface area activated carbons from Paulownia wood by ZnCl2 activation. Microporous Mesoporous Mater. 2009, 122, 189–194. [Google Scholar] [CrossRef]
- Bell, E.M.; De Guise, S.; McCutcheon, J.R.; Lei, Y.; Levin, M.; Li, B.; Rusling, J.F.; Lawrence, D.A.; Cavallari, J.M.; O’Connell, C.; et al. Exposure, health effects, sensing, and remediation of the emerging PFAS contaminants–Scientific challenges and potential research directions. Sci. Total Environ. 2021, 780, 146399. [Google Scholar] [CrossRef]
- Choudhary, A.; Dong, D.; Tsianou, M.; Alexandridis, P.; Bedrov, D. Adsorption mechanism of perfluorooctanoate on cyclodextrin-based polymers: Probing the synergy of electrostatic and hydrophobic interactions with molecular dynamics simulations. ACS Mater. Lett. 2022, 4, 853–859. [Google Scholar] [CrossRef]
- Liu, Z.; Yang, F.; Zhai, T.; Yu, J.; Wang, C.; Liu, Z.; Liu, Z.; Gao, Y.; Yang, M. Removal of PFOA from water by activated carbon adsorption: Influence of pore structure. J. Environ. Chem. Eng. 2024, 12, 113923. [Google Scholar] [CrossRef]
- Sajjadi, B.; Chen, W.Y.; Egiebor, N.O. A comprehensive review on physical activation of biochar for energy and environmental applications. Rev. Chem. Eng. 2019, 35, 735–776. [Google Scholar] [CrossRef]
- Jha, S.; Nanda, S.; Acharya, B.; Dalai, A.K. Introduction to sustainability science in addressing energy security and achieving sustainable development goals. In Biomass to Bioenergy; Woodhead Publishing: Sawston, UK, 2024; pp. 1–14. [Google Scholar]
- Arora, A.; Jha, S.; Saini, V. Aspects of green-sustainable tribology and its impacts on future product development: A review. Ecol. Environ. Conserv. 2019, 25, S146–S157. [Google Scholar]
- ASTM D3175-11; Test Method for Volatile Matter in the Analysis Sample of Coal and Coke. ASTM International: West Conshohocken, PA, USA, 2011.
- ASTM E1755-01; Standard Test Method for Ash in Biomass. ASTM International: West Conshohocken, PA, USA, 2007.
- ASTM E871-82; Standard Test Method for Moisture Analysis of Particulate Wood Fuels. ASTM International: West Conshohocken, PA, USA, 2006.
- Patra, B.R.; Nanda, S.; Dalai, A.K.; Meda, V. Taguchi-based process optimization for activation of agro-food waste biochar and performance test for dye adsorption. Chemosphere 2021, 285, 131531. [Google Scholar] [CrossRef]
- Okolie, J.A.; Mukherjee, A.; Nanda, S.; Dalai, A.K.; Kozinski, J.A. Catalytic supercritical water gasification of soybean straw: Effects of catalyst supports and promoters. Ind. Eng. Chem. Res. 2021, 60, 5770–5782. [Google Scholar] [CrossRef]
- Behnami, A.; Pourakbar, M.; Ayyar AS, R.; Lee, J.W.; Gagnon, G.; Benis, K.Z. Treatment of Aqueous Per-and Poly-Fluoroalkyl Substances: A Review of Biochar Adsorbent Preparation Methods. Chemosphere 2024, 357, 142088. [Google Scholar] [CrossRef]
- Mafu, L.D. Chemical and Structural Changes of Biomass During Pyrolysis and the Influence on Gasification Reactivity in Coal-Biomass Blends. Ph.D. Dissertation, North-West University, Potchefstroom, South Africa, 2018. [Google Scholar]
- Jha, A.; Amar, V.; Tukkaraja, P. Investigation on fire potential of ventilation ducts. J. Sustain. Min. 2019, 18, 270–276. [Google Scholar] [CrossRef]
- Margellou, A.G.; Pappa, C.P.; Psochia, E.A.; Petala, M.D.; Triantafyllidis, K.S. Mild isolation and characterization of surface lignin from hydrothermally pretreated lignocellulosic forestry and agro-industrial waste biomass. Sustain. Chem. Pharm. 2023, 33, 101056. [Google Scholar] [CrossRef]
- Fu, K.; Huang, J.; Luo, F.; Fang, Z.; Yu, D.; Zhang, X.; Wang, D.; Xing, M.; Luo, J. Understanding the Selective Removal of Perfluoroalkyl and Polyfluoroalkyl Substances via Fluorine–Fluorine Interactions: A Critical Review. Environ. Sci. Technol. 2024, 58, 16669–16689. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Mishra, P.K.; Upadhyay, S.N. Pyrolysis of Saccharum munja: Optimization of process parameters using response surface methodology (RSM) and evaluation of kinetic parameters. Bioresour. Technol. Rep. 2019, 8, 100332. [Google Scholar] [CrossRef]
- Liu, L.; Liu, Y.; Gao, B.; Ji, R.; Li, C.; Wang, S. Removal of perfluorooctanoic acid (PFOA) and perfluorooctane sulfonate (PFOS) from water by carbonaceous nanomaterials: A review. Crit. Rev. Environ. Sci. Technol. 2020, 50, 2379–2414. [Google Scholar] [CrossRef]
- Schneider, E.; Czech, H.; Popovicheva, O.; Chichaeva, M.; Kobelev, V.; Kasimov, N.; Minkina, T.; Rüger, C.P.; Zimmermann, R. Mass spectrometric analysis of unprecedented high levels of carbonaceous aerosol particles long-range transported from wildfires in the Siberian Arctic. Atmos. Chem. Phys. 2024, 24, 553–576. [Google Scholar] [CrossRef]
- He, Z.; Guo, M.; Fortier, C.; Cao, X.; Schmidt-Rohr, K. Fourier Transform Infrared and Solid State 13C Nuclear Magnetic Resonance Spectroscopic Characterization of Defatted Cottonseed Meal-Based Biochars. 2020. Available online: https://papers.ssrn.com/sol3/papers.cfm?abstract_id=3598083 (accessed on 28 January 2025).
- Romero, C.M.; Redman, A.A.P.H.; Terry, S.A.; Hazendonk, P.; Hao, X.; McAllister, T.A.; Okine, E. Molecular speciation and aromaticity of biochar-manure: Insights from elemental, stable isotope and solid-state DPMAS 13C NMR analyses. J. Environ. Manag. 2021, 280, 111705. [Google Scholar] [CrossRef]
- Xiao, F. A review of biochar functionalized by thermal air oxidation. Environ. Funct. Mater. 2022, 1, 187–195. [Google Scholar] [CrossRef]
- Zhao, C.; Ge, L.; Wang, R.; Chu, H.; Mai, L.; Zha, W.; Wang, Y.; Xu, C. Effects of cellulose addition on the physicochemical properties, pore structure and iodine adsorption of lignin-based biochar. Fuel 2023, 352, 129061. [Google Scholar] [CrossRef]
- Sakhiya, A.K.; Aier, I.; Pathak, S.; Anand, A.; Jha, S.; Vijay, V.K.; Kaushal, P. Copper (II) removal from aqua solution using rice straw derived biochar. Mater. Today Proc. 2021, 43, 740–745. [Google Scholar] [CrossRef]
- Wang, L.; Ok, Y.S.; Tsang, D.C.; Alessi, D.S.; Rinklebe, J.; Mašek, O.; Bolan, N.S.; Hou, D. Biochar composites: Emerging trends, field successes and sustainability implications. Soil Use Manag. 2022, 38, 14–38. [Google Scholar] [CrossRef]
- Gao, R.; Ding, S.; Liu, Z.; Jiang, H.; Liu, G.; Fang, J. Recent advances and perspectives of biochar for livestock wastewater: Modification methods, applications, and resource recovery. J. Environ. Chem. Eng. 2024, 12, 113678. [Google Scholar] [CrossRef]
- Leung SC, E.; Shukla, P.; Chen, D.; Eftekhari, E.; An, H.; Zare, F.; Ghasemi, N.; Zhang, D.; Nguyen, N.; Li, Q. Emerging technologies for PFOS/PFOA degradation and removal: A review. Sci. Total Environ. 2022, 827, 153669. [Google Scholar] [CrossRef]
- Wu, Y.; Qi, L.; Chen, G. A mechanical investigation of perfluorooctane acid adsorption by engineered biochar. J. Clean. Prod. 2022, 340, 130742. [Google Scholar] [CrossRef]
- Hassan, M.; Liu, Y.; Naidu, R.; Du, J.; Qi, F. Adsorption of Perfluorooctane sulfonate (PFOS) onto metal oxides modified biochar. Environ. Technol. Innov. 2020, 19, 100816. [Google Scholar] [CrossRef]
- Mulabagal, V.; Baah, D.A.; Egiebor, N.O.; Sajjadi, B.; Chen, W.Y.; Viticoski, R.L.; Hayworth, J.S. Biochar from biomass: A strategy for carbon dioxide sequestration, soil amendment, power generation, CO2 utilization, and removal of perfluoroalkyl and polyfluoroalkyl substances (PFAS) in the environment. In Handbook of Climate Change Mitigation and Adaptation; Springer International Publishing: Cham, Switzerland, 2022; pp. 1023–1085.4. [Google Scholar]
- Lin, S.; Kedzior, S.A.; Zhang, J.; Yu, M.; Saini, V.; Huynh, R.P.; Shimizu GK, H.; Trifkovic, M. A superprotonic membrane derived from proton-conducting metal-organic frameworks and cellulose nanocrystals. Chem 2023, 9, 2547–2560. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, X.; Lu, R.; Tang, Y.; Qie, H.; Huang, Z.; Zhao, J.; Cui, J.; Yang, W.; Lin, A. Enhanced removal of polyfluoroalkyl substances by simple modified biochar: Adsorption performance and theoretical calculation. ACS ES&T Water 2023, 3, 817–826. [Google Scholar]
- Deng, S.; Zhang, Q.; Nie, Y.; Wei, H.; Wang, B.; Huang, J.; Yu, G.; Xing, B. Sorption mechanisms of perfluorinated compounds on carbon nanotubes. Environ. Pollut. 2012, 168, 138–144. [Google Scholar] [CrossRef]
- Trivedi, Y.; Sharma, M.; Mishra, R.K.; Sharma, A.; Joshi, J.; Gupta, A.B.; Achintya, B.; Shah, K.; Vuppaladadiyamd, A.K. Biochar potential for pollutant removal during wastewater treatment: A comprehensive review of separation mechanisms, technological integration, and process analysis. Desalination 2025, 600, 118509. [Google Scholar] [CrossRef]
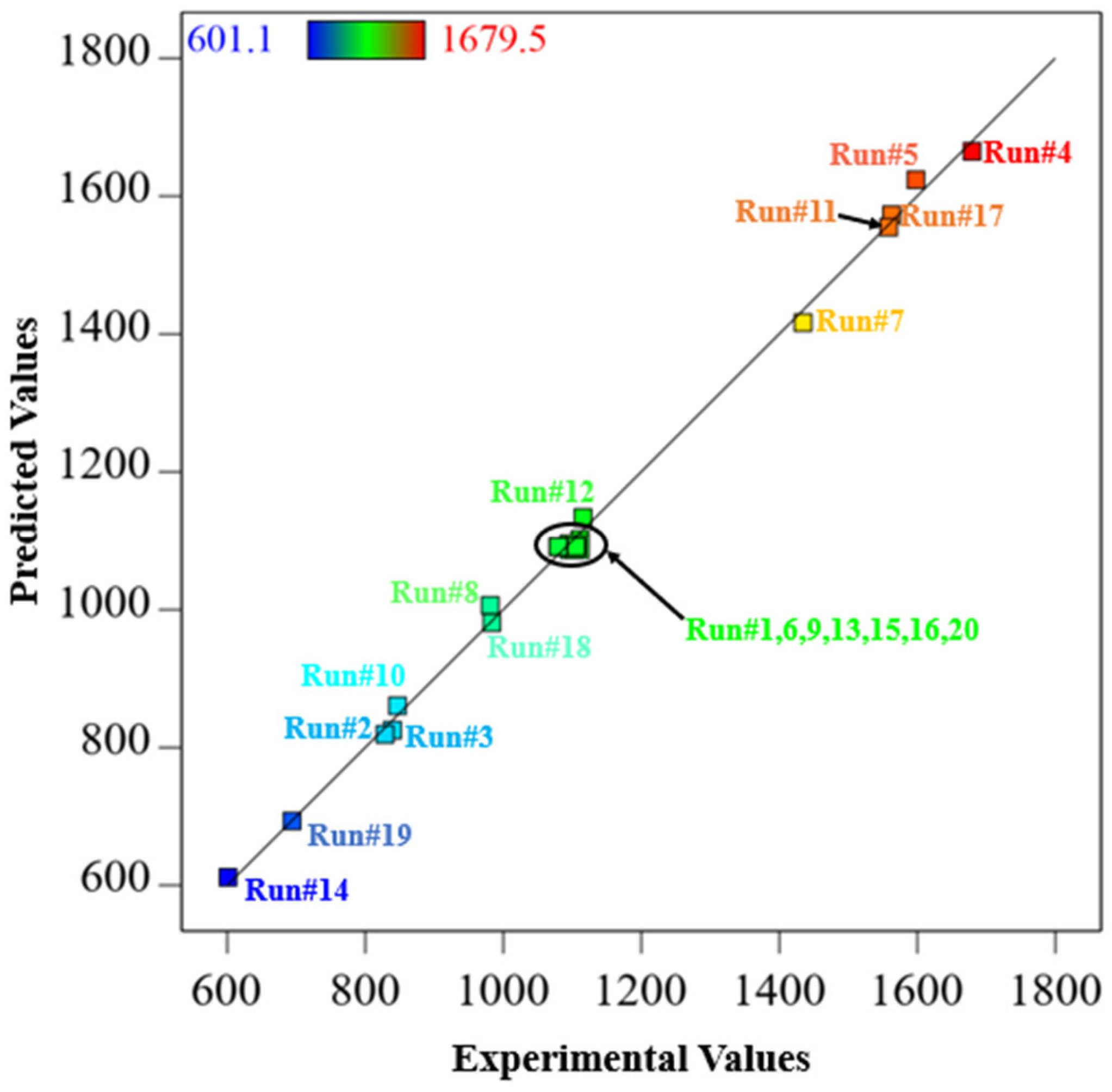

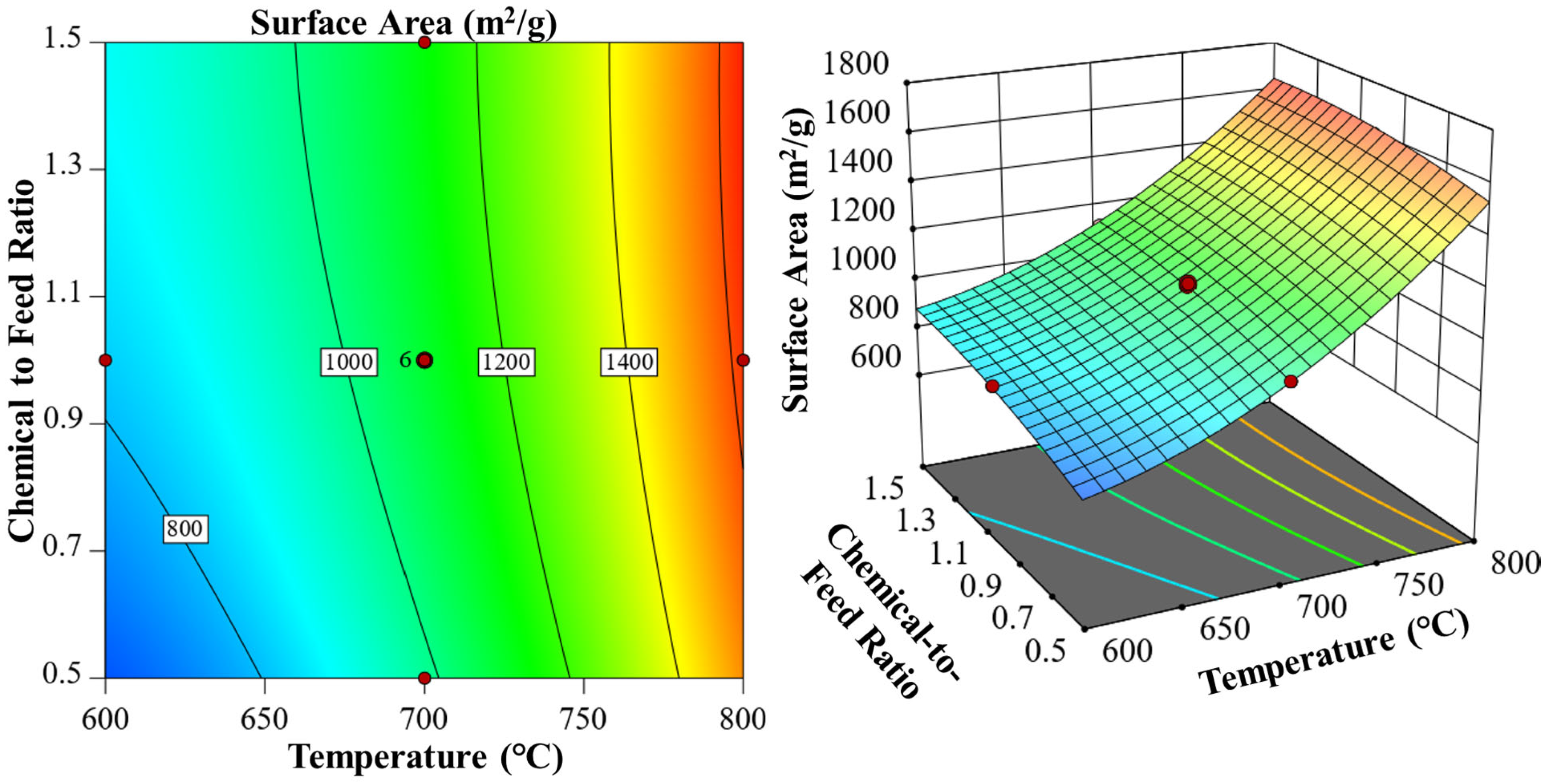
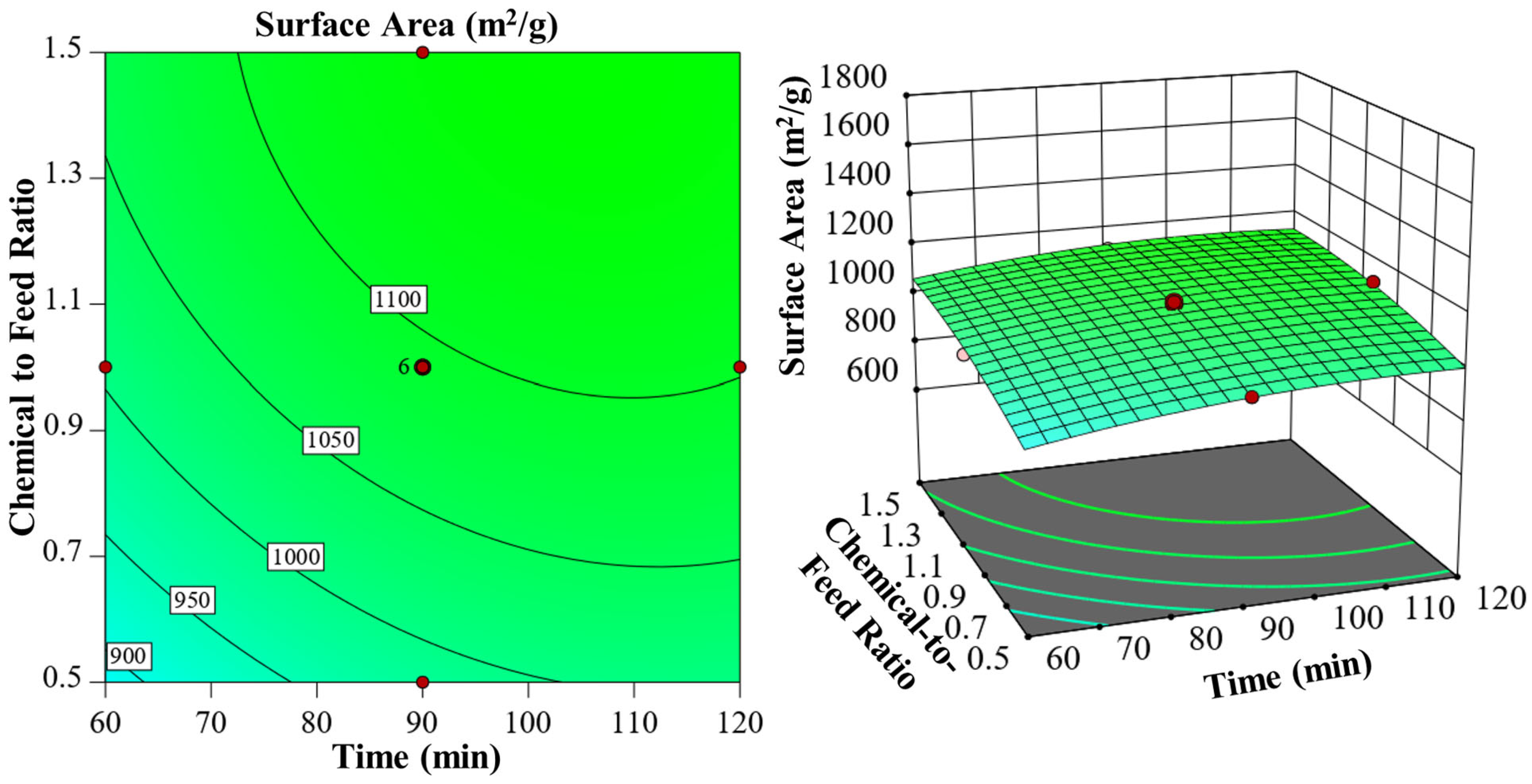


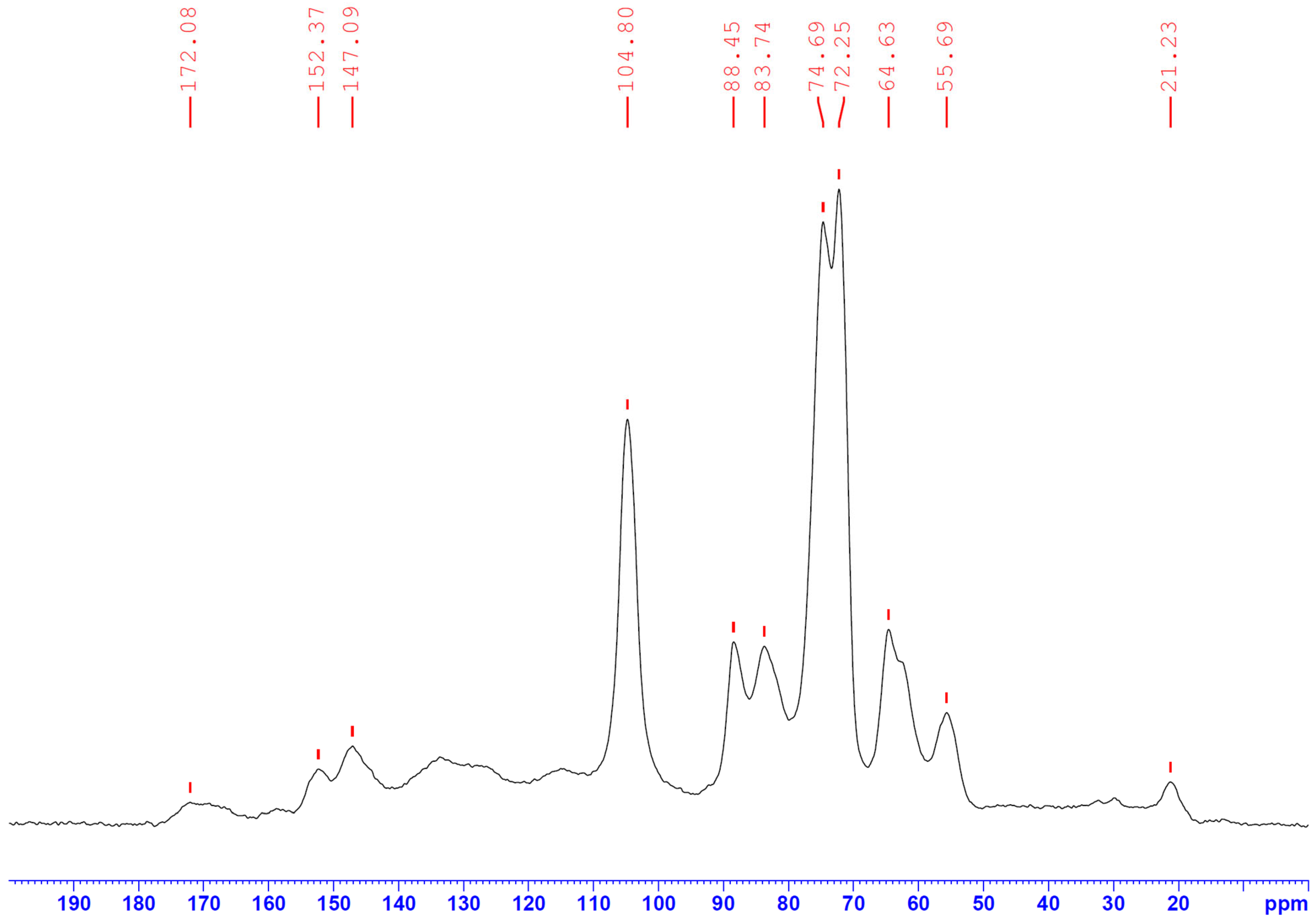




| Chemical Agents | Temperature (°C) | Time (min) | Ratio | C (wt.%) | H (wt.%) | N (wt.%) | S (wt.%) | O (wt.%) | Specific Surface Area (m2/g) | HHV |
|---|---|---|---|---|---|---|---|---|---|---|
| K2CO3 | 800 | 60 | 1 | 69.9 | 1.7 | 5.1 | 0.3 | 13.03 | 511 | 24.7 |
| KOH | 800 | 60 | 1 | 87.4 | 0.4 | 0.8 | 0.1 | 14.2 | 1493 | 28.4 |
| NaOH | 800 | 60 | 1 | 82.6 | 0.4 | 0.8 | 0.03 | 16.2 | 956 | 26.6 |
| Run | Sample Name | CCD Parameters | Yield (wt. %) | C (wt.%) | H (wt.%) | N (wt.%) | S (wt.%) | O (wt.%) | O/C | H/C | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Factor 1: Temperature (°C) | Factor 2: Time (min) | Factor 3: Chemical-to-Feed Ratio | Response: Specific Surface Area (m2/g) | ||||||||||
| 14 | CM-KOH-600-60-0.5 | 600 | 60 | 0.5 | 601 | 64.5 | 64 | 3.1 | 8.1 | 0.2 | 10.4 | 0.16 | 0.05 |
| 3 | CM-KOH-600-60-1.5 | 600 | 60 | 1.5 | 839 | 66.3 | 67.4 | 1.6 | 6.8 | 0.4 | 10.7 | 0.16 | 0.02 |
| 2 | CM-KOH-600-90-1 | 600 | 90 | 1 | 829 | 65.1 | 68.3 | 1.9 | 4.3 | 0.7 | 11.6 | 0.17 | 0.03 |
| 19 | CM-KOH-600-120-0.5 | 600 | 120 | 0.5 | 694 | 60.0 | 67.9 | 1.4 | 7.5 | 0.4 | 8.6 | 0.13 | 0.02 |
| 10 | CM-KOH-600-120-1.5 | 600 | 120 | 1.5 | 847 | 64.0 | 69.8 | 1.6 | 5.4 | 0.6 | 9.0 | 0.13 | 0.02 |
| 8 | CM-KOH-700-60-1 | 700 | 60 | 1 | 981 | 59.0 | 76.7 | 1.01 | 0.8 | 0.05 | 10.2 | 0.13 | 0.01 |
| 18 | CM-KOH-700-90-0.5 | 700 | 90 | 0.5 | 983 | 55.3 | 70.9 | 0.8 | 2.7 | 0.4 | 10.5 | 0.15 | 0.01 |
| 1 | CM-KOH-700-90-1 | 700 | 90 | 1 | 1106 | 57.7 | 75 | 0.9 | 3.8 | 0.1 | 9.0 | 0.12 | 0.01 |
| 9 | CM-KOH-700-90-1 | 700 | 90 | 1 | 1100 | 58.1 | 75.5 | 1.2 | 3.9 | 0.2 | 7.5 | 0.10 | 0.02 |
| 15 | CM-KOH-700-90-1 | 700 | 90 | 1 | 1080 | 57.2 | 74.7 | 1.5 | 1.0 | 0.1 | 9.5 | 0.13 | 0.02 |
| 16 | CM-KOH-700-90-1 | 700 | 90 | 1 | 1081 | 57.5 | 75.2 | 1.5 | 0.9 | 0.1 | 10.6 | 0.14 | 0.02 |
| 20 | CM-KOH-700-90-1 | 700 | 90 | 1 | 1109 | 56.4 | 76.3 | 1.4 | 1.3 | 0.05 | 8.2 | 0.11 | 0.02 |
| 13 | CM-KOH-700-90-1 | 700 | 90 | 1 | 1107 | 58.9 | 72.6 | 1.1 | 2.8 | 0.2 | 10.2 | 0.14 | 0.01 |
| 12 | CM-KOH-700-90-1.5 | 700 | 90 | 1.5 | 1116 | 57.5 | 74.4 | 1.03 | 3.4 | 0.1 | 9.0 | 0.12 | 0.01 |
| 6 | CM-KOH-700-120-1 | 700 | 120 | 1 | 1111 | 56.4 | 82.8 | 0.7 | 3.3 | 0.2 | 9.2 | 0.04 | 0.01 |
| 7 | CM-KOH-800-60-0.5 | 800 | 60 | 0.5 | 1435 | 49.0 | 79 | 0.6 | 3.8 | 0.4 | 10.4 | 0.07 | 0.01 |
| 11 | CM-KOH-800-60-1.5 | 800 | 60 | 1.5 | 1558 | 51.0 | 86 | 0.5 | 1.0 | 0.1 | 6.9 | 0.03 | 0.01 |
| 5 | CM-KOH-800-90-1 | 800 | 90 | 1 | 1598 | 48.6 | 72.8 | 1.2 | 3.6 | 0.1 | 9.5 | 0.13 | 0.02 |
| 17 | CM-KOH-800-120-0.5 | 800 | 120 | 0.5 | 1563 | 44.0 | 76.9 | 0.8 | 5.6 | 0.4 | 8.9 | 0.05 | 0.01 |
| 4 | CM-KOH-800-120-1.5 | 800 | 120 | 1.5 | 1680 | 48.7 | 73.9 | 1.4 | 6.01 | 0.2 | 6.1 | 0.08 | 0.02 |
| Std. Dev. | 20.16 | R2 | 0.997 |
| Mean | 1120.76 | Adjusted R2 | 0.995 |
| C.V. % | 1.80 | Predicted R2 | 0.979 |
| Adequate Precision | 73.9 |
| Source | Sum of Squares | df | Mean Square | F-Value | p-Value | Remark |
|---|---|---|---|---|---|---|
| Model | 1,756,000 | 9 | 195,100 | 480.09 | <0.0001 | Significant |
| A: Temperature | 1,619,000 | 1 | 1,619,000 | 3983.34 | <0.0001 | |
| B: Time | 22,992.02 | 1 | 22,992.02 | 56.58 | <0.0001 | |
| C: Chemical-to-Feed | 58,369.60 | 1 | 58,369.60 | 143.63 | <0.0001 | |
| AB | 2782.58 | 1 | 2782.58 | 6.85 | 0.0257 | |
| AC | 2827.52 | 1 | 2827.52 | 6.96 | 0.0248 | |
| BC | 1071.84 | 1 | 1071.84 | 2.64 | 0.1354 | |
| A2 | 46,377.55 | 1 | 46,377.55 | 114.12 | <0.0001 | |
| B2 | 3823.25 | 1 | 3823.25 | 9.41 | 0.0119 | |
| C2 | 3185.80 | 1 | 3185.80 | 7.84 | 0.0188 | |
| Residual | 4063.86 | 10 | 406.39 | |||
| Lack of Fit | 3123.04 | 5 | 624.61 | 3.32 | 0.1070 | Not significant |
| Pure Error | 940.82 | 5 | 188.16 | |||
| Corrected Total | 1,760,000 | 19 |
| Run | Sample Name | Specific Surface Area (m2/g) | Pore Volume (cm3/g) | Average Pore Size (nm) | Removal Efficiency (%) |
|---|---|---|---|---|---|
| 14 | CM-KOH-600-60-0.5 | 601 | 0.3 | 2.1 | 62.2 |
| 3 | CM-KOH-600-60-1.5 | 839 | 0.5 | 2.5 | 63.3 |
| 2 | CM-KOH-600-90-1 | 829 | 0.5 | 2.6 | 65.8 |
| 19 | CM-KOH-600-120-0.5 | 694 | 0.4 | 2.2 | 61.0 |
| 10 | CM-KOH-600-120-1.5 | 847 | 0.6 | 2.8 | 68.7 |
| 8 | CM-KOH-700-60-1 | 981 | 0.7 | 2.7 | 65.8 |
| 18 | CM-KOH- 700-90-0.5 | 983 | 0.7 | 2.8 | 69.7 |
| 1 | CM-KOH- 700-90-1 | 1106 | 0.9 | 3.3 | 79.7 |
| 9 | CM-KOH-700-90-1 | 1100 | 0.8 | 3 | 72.7 |
| 15 | CM-KOH-700-90-1 | 1080 | 0.8 | 3.1 | 75.7 |
| 16 | CM-KOH-700-90-1 | 1081 | 0.8 | 3.1 | 74.1 |
| 20 | CM-KOH-700-90-1 | 1109 | 0.7 | 2.7 | 65.1 |
| 13 | CM-KOH-700-90-1.5 | 1107 | 0.8 | 2.9 | 70.8 |
| 12 | CM-KOH-700-90-1.5 | 1116 | 0.9 | 3.2 | 77.8 |
| 6 | CM-KOH-700-120-1 | 1111 | 0.9 | 3.2 | 78.6 |
| 7 | CM-KOH-800-60-0.5 | 1435 | 1.2 | 3.4 | 84.3 |
| 11 | CM-KOH-800-60-1.5 | 1558 | 1.4 | 3.6 | 92.3 |
| 5 | CM-KOH-800-90-1 | 1598 | 1.3 | 3.4 | 85.3 |
| 17 | CM-KOH-800-120-0.5 | 1563 | 1.3 | 3.3 | 82.7 |
| 4 | CM-KOH-800-120-1.5 | 1680 | 1.4 | 3.4 | 86.1 |
| Adsorbent Dosage (mgL−1) | Removal Efficiency: CM Biochar (%) | Removal Efficiency: KOH-Activated Carbon (%) |
|---|---|---|
| 50 | 64 | 93.1 |
| 100 | 67.6 | 95 |
| 150 | 68.6 | 96.1 |
| Feedstock/Biochar Type | Activation/Engineering | Max Adsorption Capacity | Other Findings | Reference |
|---|---|---|---|---|
| Switchgrass, Water Oak, Biosolid | FeCl3 impregnation, carbon nanotubes | 52.1 µmol/g (biochar) | Fe-engineered biochar improves the porosity and surface area; carbon nanotubes increase surface functionality. | [35] |
| Sludge | 45.88 mg/g | Functional group optimization plays a critical role under acidic conditions. | [36] | |
| Red Mud and Sawdust | Metal oxide modification, pyrolysis at 600 °C | 194.6 mg/g (metal-modified biochar) | Enhanced graphitic structure and protonated metal-based functional groups boost adsorption at pH 3.1. | [37] |
| Camelina Meal | Chemical activation using KOH | 93.1 mg/g | Aimed to maximize surface area via steam flow optimization; focused on PFAS adsorption metrics. | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jha, S.; Pattnaik, F.; Zapata, O.; Acharya, B.; Dalai, A.K. KOH-Assisted Chemical Activation of Camelina Meal (Wild Flax) to Treat PFOA-Contaminated Wastewater. Sustainability 2025, 17, 2170. https://doi.org/10.3390/su17052170
Jha S, Pattnaik F, Zapata O, Acharya B, Dalai AK. KOH-Assisted Chemical Activation of Camelina Meal (Wild Flax) to Treat PFOA-Contaminated Wastewater. Sustainability. 2025; 17(5):2170. https://doi.org/10.3390/su17052170
Chicago/Turabian StyleJha, Shivangi, Falguni Pattnaik, Oscar Zapata, Bishnu Acharya, and Ajay K. Dalai. 2025. "KOH-Assisted Chemical Activation of Camelina Meal (Wild Flax) to Treat PFOA-Contaminated Wastewater" Sustainability 17, no. 5: 2170. https://doi.org/10.3390/su17052170
APA StyleJha, S., Pattnaik, F., Zapata, O., Acharya, B., & Dalai, A. K. (2025). KOH-Assisted Chemical Activation of Camelina Meal (Wild Flax) to Treat PFOA-Contaminated Wastewater. Sustainability, 17(5), 2170. https://doi.org/10.3390/su17052170









