Alkylpolyglycosides—Based Formulations for Sustainable Remediation of Contaminated Aquifers: Lab-Scale Process Study for NAPL Solubilization Assessment
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Investigated Formulations
2.1.2. Sorbent Material
2.2. Methods
2.2.1. Critical Micelle Concentration (CMC) Determination
2.2.2. Batch Configuration Study: NAPL Adsorption Isotherms
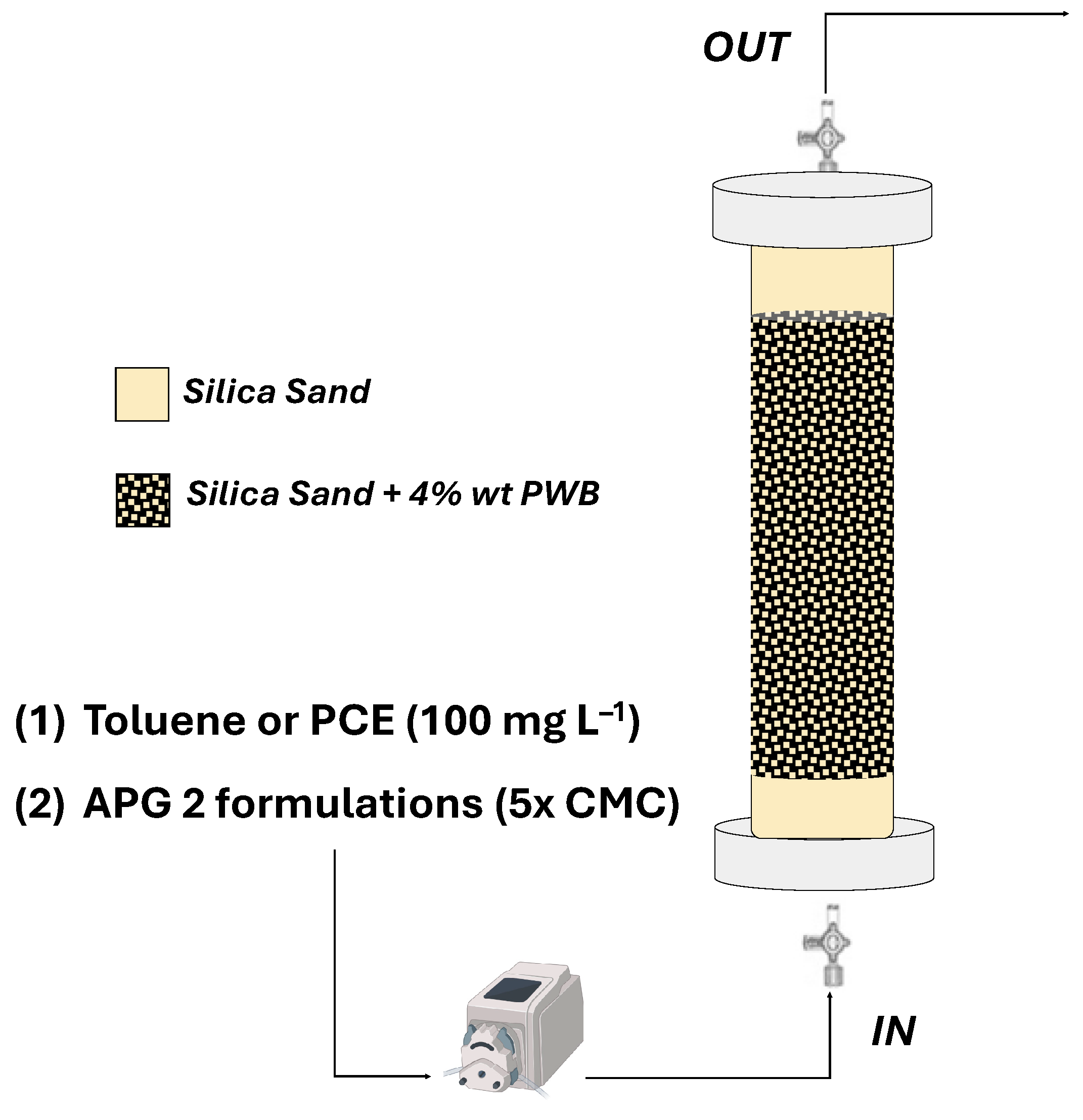
2.2.3. Continuous Configuration Column Experiments: Surfactant-Enhanced Desorption of NAPLs
2.2.4. Analytical Methods
2.3. Data Processing and Calculation
3. Results and Discussions
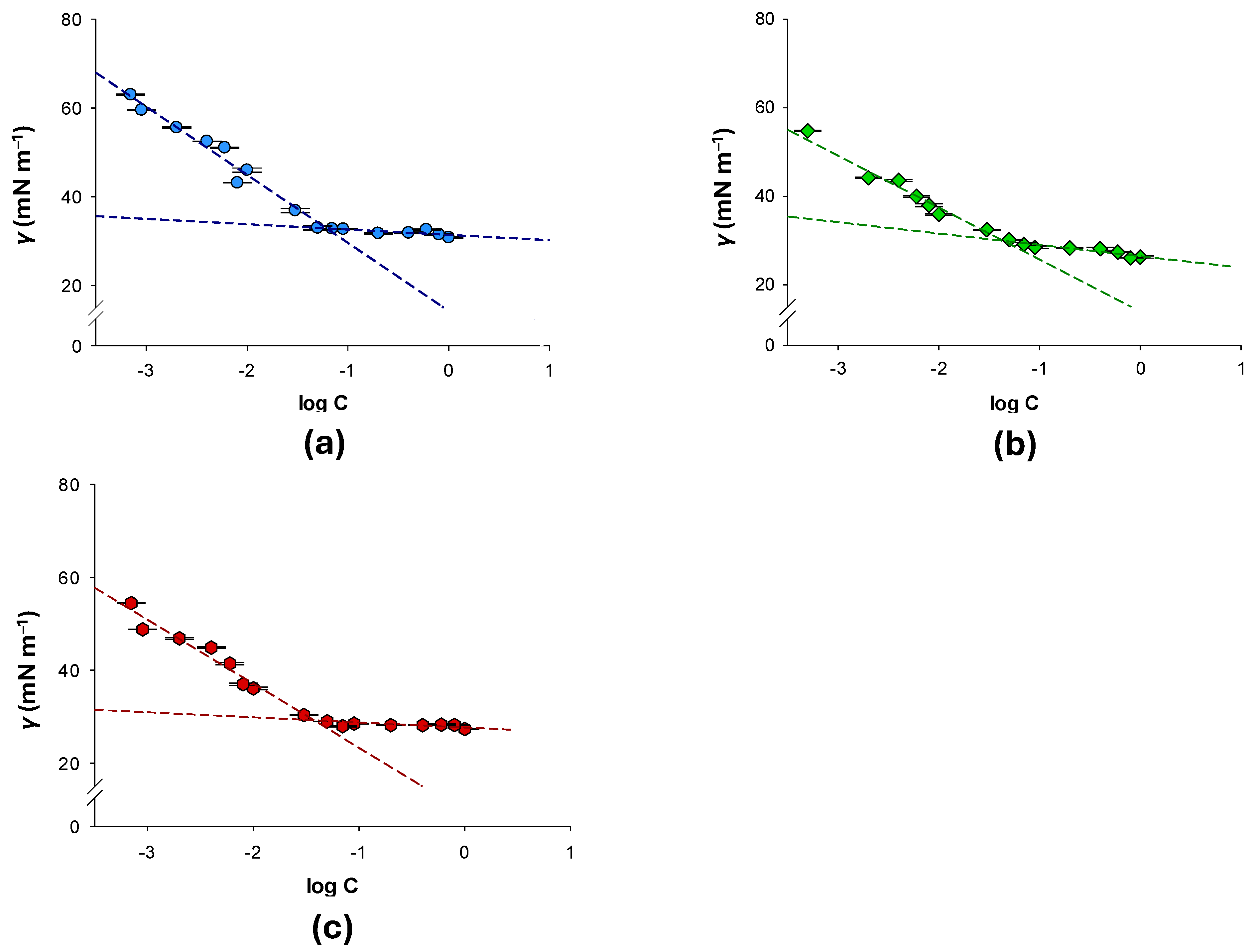
3.1. Critical Micelle Concentration (CMC) Determination
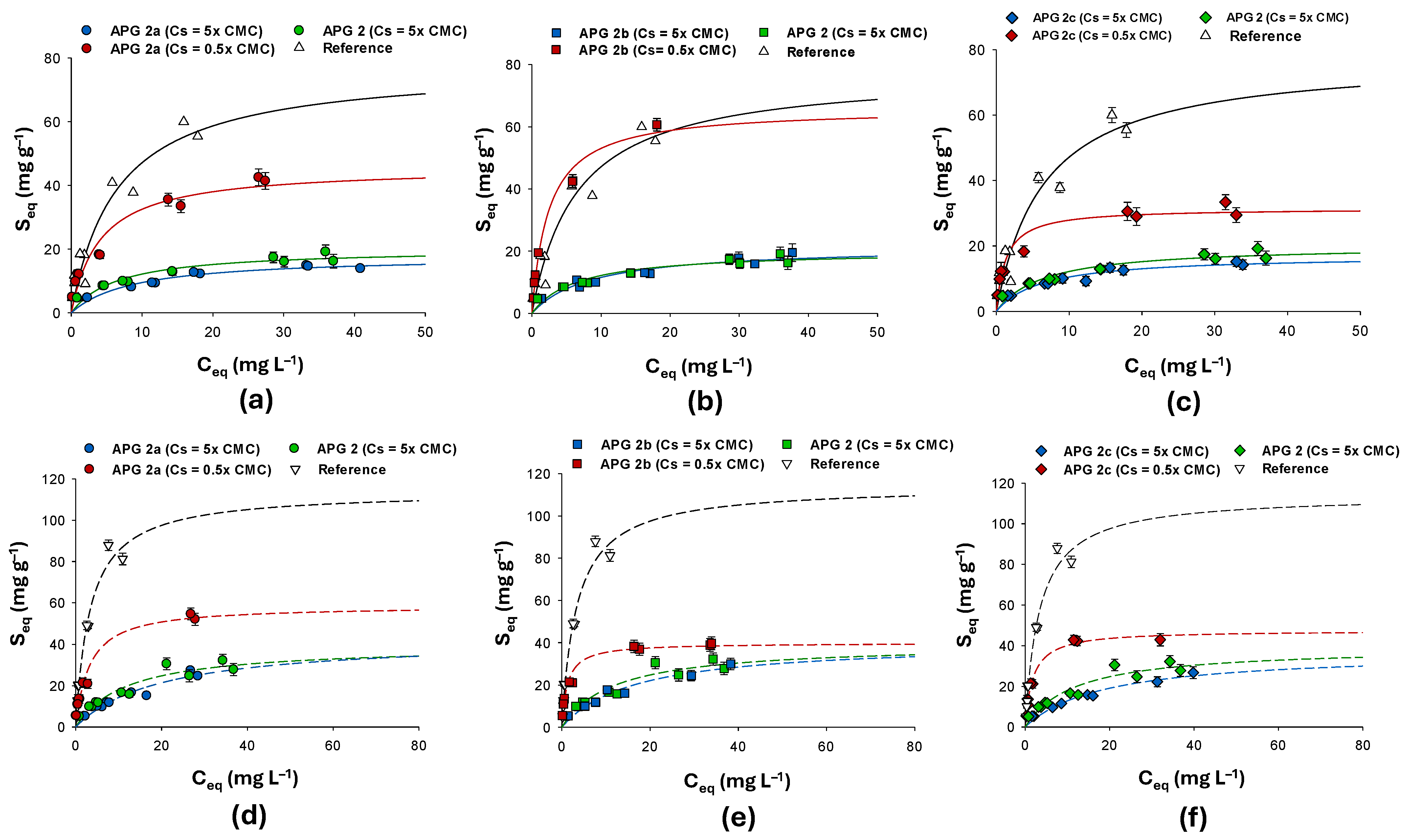
3.2. Thermodynamic Batch Study: NAPL Adsorption Isotherms
3.2.1. Adsorption Data for Toluene-Contaminated Systems
3.2.2. Adsorption Data for PCE-Contaminated Systems
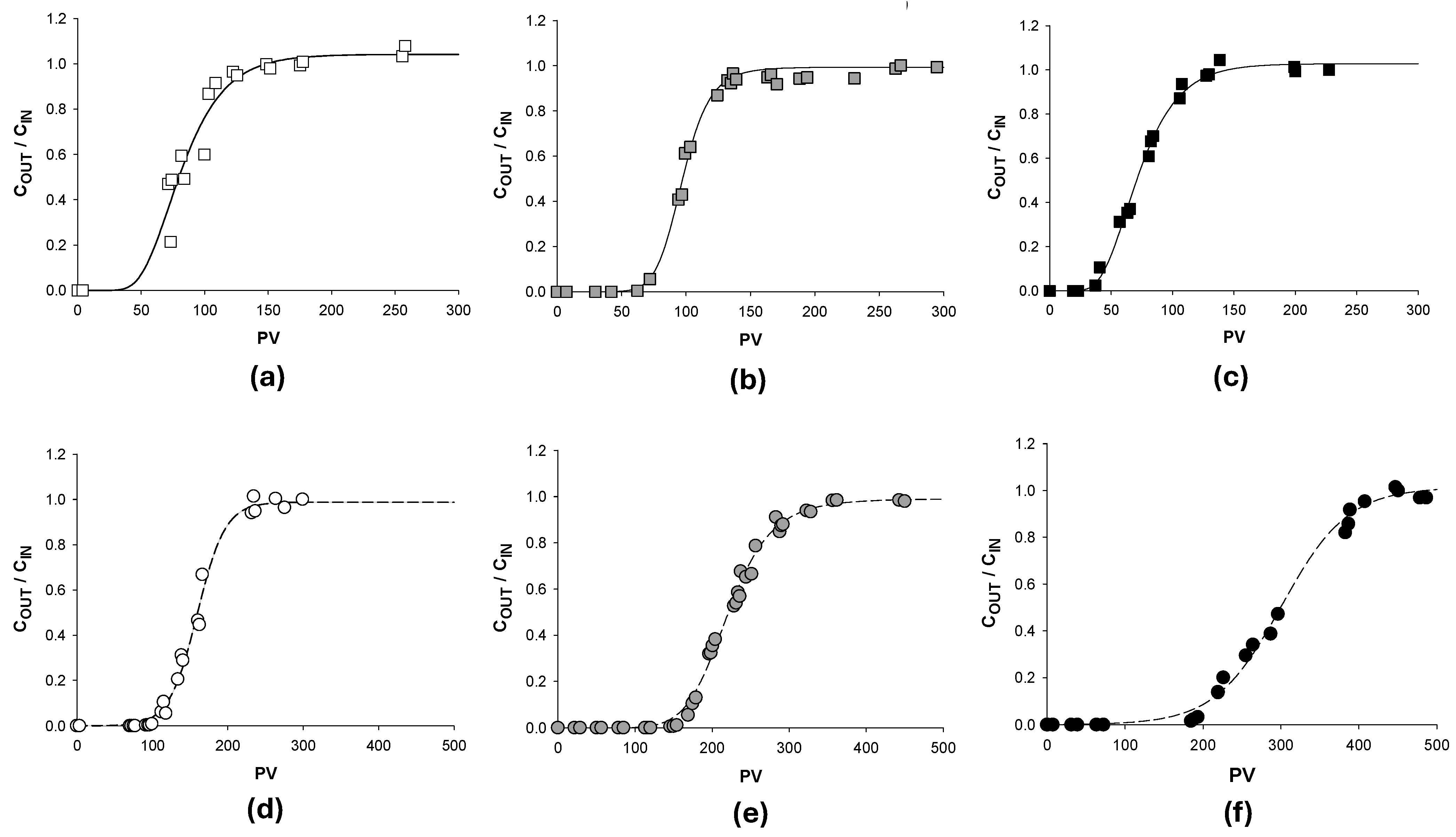
3.3. Continuous Configuration Column Experiments: Surfactant-Enhanced NAPL Desorption
3.3.1. Fluid-Dynamic Characterization of Fixed Bed Reactors
3.3.2. Contamination Phase of the Fixed Bed: Contaminant Adsorption
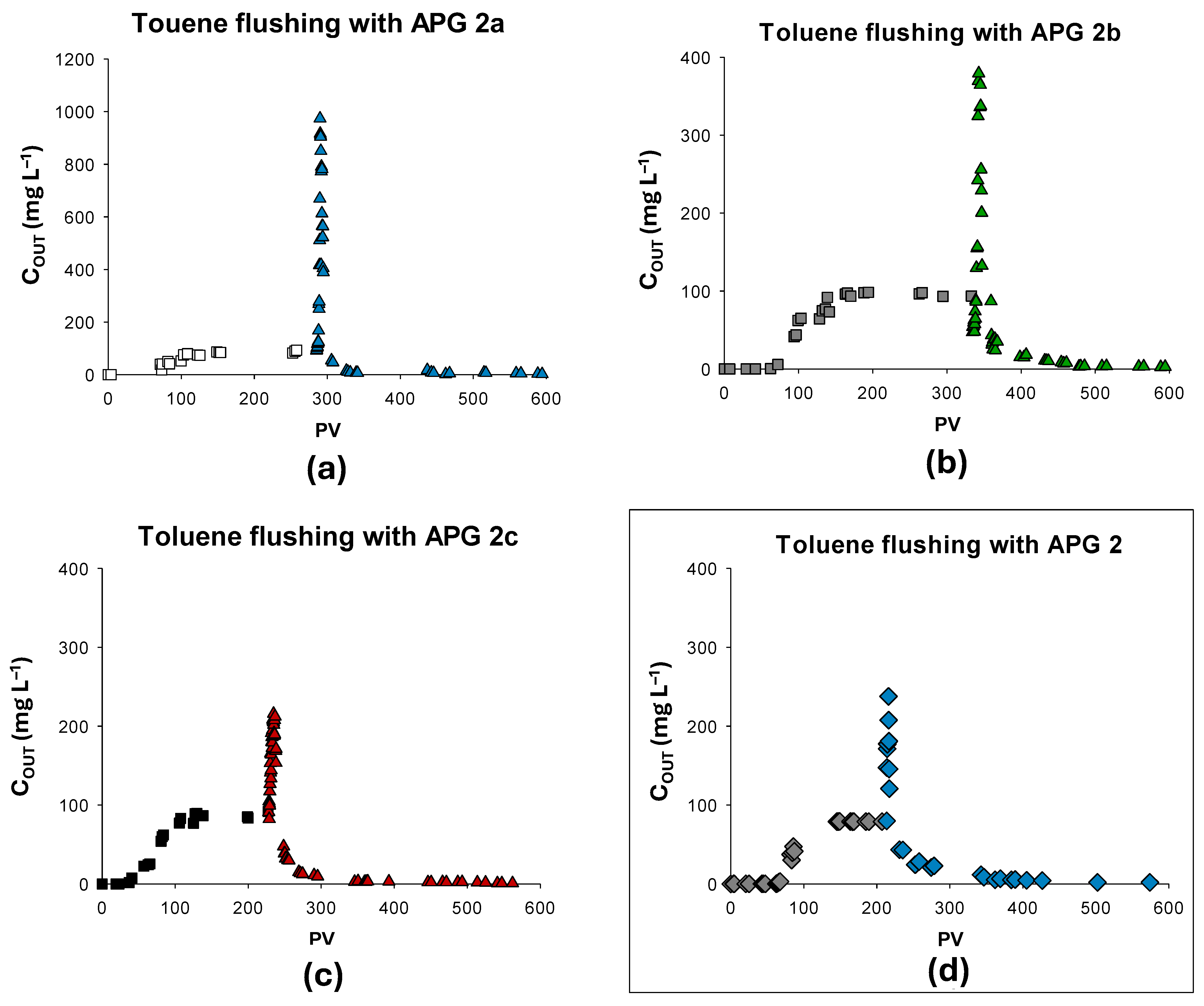
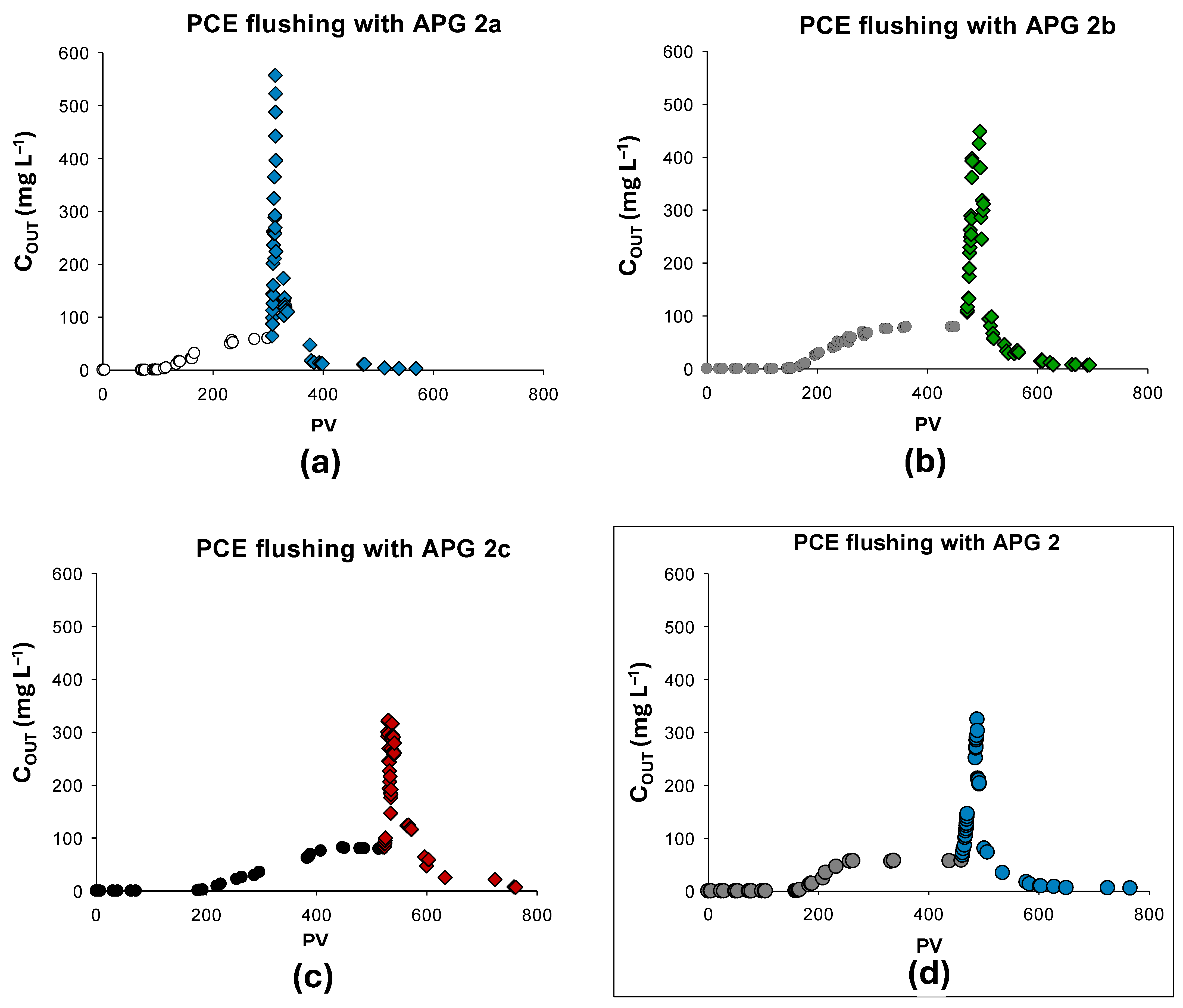
3.3.3. Flushing Phase: Contaminants Solubilization
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ciampi, P.; Felli, G.; Feriaud, D.; Esposito, C.; Papini, M.P. 3D GeoRemediation: A Digital Hydrogeophysical–Chemical Clone and Virtual Hydraulic Barrier with Groundwater Circulation Wells (GCWs) for Groundwater Remediation. Sustainability 2024, 16, 5216. [Google Scholar] [CrossRef]
- Guo, Z.; Brusseau, M.L.; Fogg, G.E. Determining the long-term operational performance of pump and treat and the possibility of closure for a large TCE plume. J. Hazard. Mater. 2019, 365, 796–803. [Google Scholar] [CrossRef]
- Javanbakht, G.; Goual, L. Mobilization and micellar solubilization of NAPL contaminants in aquifer rocks. J. Contam. Hydrol. 2016, 185–186, 61–73. [Google Scholar] [CrossRef] [PubMed]
- Javanbakht, G.; Arshadi, M.; Qin, T.; Goual, L. Micro-scale displacement of NAPL by surfactant and microemulsion in heterogeneous porous media. Adv. Water Resour. 2017, 105, 173–187. [Google Scholar] [CrossRef]
- Le Meur, M.; Cohen, G.J.V.; Laurent, M.; Höhener, P.; Atteia, O. Effect of NAPL mixture and alteration on 222Rn partitioning coefficients: Implications for NAPL subsurface contamination quantification. Sci. Total Environ. 2021, 791, 148210. [Google Scholar] [CrossRef]
- Wu, M.; Yang, Y.; Lin, J.; Wu, J.; Wu, J.; Hu, B.X. The co-effect of heterogeneity and solute concentration on representative elementary volume of DNAPL in groundwater. J. Hydrol. 2020, 585, 124795. [Google Scholar] [CrossRef]
- Liu, J.W.; Wei, K.H.; Xu, S.W.; Cui, J.; Ma, J.; Xiao, X.L.; Xi, B.D.; He, X.S. Surfactant-enhanced remediation of oil-contaminated soil and groundwater: A review. Sci. Total Environ. 2021, 756, 144142. [Google Scholar] [CrossRef]
- Papini, M.P.; Cerra, S.; Feriaud, D.; Pettiti, I.; Lorini, L.; Fratoddi, I. Biochar/Biopolymer Composites for Potential In Situ Groundwater Remediation. Materials 2024, 17, 3899. [Google Scholar] [CrossRef] [PubMed]
- Teramoto, E.H.; Pede, M.A.Z.; Chang, H.K. Impact of water table fluctuations on the seasonal effectiveness of the pump-and-treat remediation in wet–dry tropical regions. Environ. Earth Sci. 2020, 79, 435. [Google Scholar] [CrossRef]
- Carroll, K.C.; Brusseau, M.L.; Tick, G.R.; Soltanian, M.R. Rethinking pump-and-treat remediation as maximizing contaminated groundwater. Sci. Total Environ. 2024, 918, 10–13. [Google Scholar] [CrossRef]
- Casasso, A.; Tosco, T.; Bianco, C.; Bucci, A.; Sethi, R. How can we make pump and treat systems more energetically sustainable? Water 2020, 12, 67. [Google Scholar] [CrossRef]
- Alazaiza, M.Y.D.; Copty, N.K.; Abunada, Z. Experimental investigation of cosolvent flushing of DNAPL in double-porosity soil using light transmission visualization. J. Hydrol. 2020, 584, 124659. [Google Scholar] [CrossRef]
- O’Connor, D.; Hou, D.; Ok, Y.S.; Song, Y.; Sarmah, A.K.; Li, X.; Tack, F.M.G. Sustainable in situ remediation of recalcitrant organic pollutants in groundwater with controlled release materials: A review. J. Control. Release 2018, 283, 200–213. [Google Scholar] [CrossRef] [PubMed]
- Lari, K.S.; Rayner, J.L.; Davis, G.B. Toward Optimizing LNAPL Remediation. Water Resour. Res. 2019, 55, 87–96. [Google Scholar] [CrossRef]
- Kariyawasam, T.; Doran, G.S.; Howitt, J.A.; Prenzler, P.D. Polycyclic aromatic hydrocarbon contamination in soils and sediments: Sustainable approaches for extraction and remediation. Chemosphere 2022, 291, 132981. [Google Scholar] [CrossRef] [PubMed]
- Huo, L.; Liu, G.; Yang, X.; Ahmad, Z.; Zhong, H. Surfactant-enhanced aquifer remediation: Mechanisms, influences, limitations and the countermeasures. Chemosphere 2020, 252, 126620. [Google Scholar] [CrossRef]
- García-Cervilla, R.; Romero, A.; Santos, A.; Lorenzo, D. Surfactant-enhanced solubilization of chlorinated organic compounds contained in dnapl from lindane waste: Effect of surfactant type and ph. Int. J. Environ. Res. Public Health 2020, 17, 4494. [Google Scholar] [CrossRef]
- Jesus, C.F.; Alves, A.A.S.; Fiuza, S.M.; Murtinho, D.; Antunes, F.E. Mini-review: Synthetic methods for the production of cationic sugar-based surfactants. J. Mol. Liq. 2021, 342, 117389. [Google Scholar] [CrossRef]
- Wu, L.; Zhang, J.; Chen, F.; Li, J.; Wang, W.; Li, S.; Hu, L. Mechanisms, Applications, and Risk Analysis of Surfactant-Enhanced Remediation of Hydrophobic Organic Contaminated Soil. Water 2024, 16, 2093. [Google Scholar] [CrossRef]
- Alasiri, H.S.; Sultan, A.S.; Chapman, W.G. Effect of Surfactant Headgroup, Salts, and Temperature on Interfacial Properties: Dissipative Particle Dynamics and Experiment for the Water/Octane/Surfactant System. Energy Fuels 2019, 33, 6678–6688. [Google Scholar] [CrossRef]
- Malkapuram, S.T.; Sharma, V.; Gumfekar, S.P.; Sonawane, S.; Sonawane, S.; Boczkaj, G.; Seepana, M.M. A review on recent advances in the application of biosurfactants in wastewater treatment. Sustain. Energy Technol. Assess. 2021, 48, 101576. [Google Scholar] [CrossRef]
- Atteia, O.; Del Campo Estrada, E.; Bertin, H. Soil flushing: A review of the origin of efficiency variability. Rev. Environ. Sci. Bio/Technol. 2013, 12, 379–389. [Google Scholar] [CrossRef]
- Iglauer, S.; Wu, Y.; Shuler, P.; Tang, Y. Alkyl Polyglycoside/1-Naphthol Formulations: A Case Study of Surfactant Enhanced Oil Recovery. Tenside Surfactants Deterg. 2011, 48, 121–126. [Google Scholar] [CrossRef]
- Salager, J.; Marquez, R.; Bullon, J.; Forgiarini, A. Formulation in Surfactant Systems: From-Winsor-to-HLDN. Encyclopedia 2022, 2, 778–839. [Google Scholar] [CrossRef]
- Zheng, G.; Selvam, A.; Wong, J.W.C. Enhanced solubilization and desorption of organochlorine pesticides (OCPs) from soil by oil-swollen micelles formed with a nonionic surfactant. Environ. Sci. Technol. 2012, 46, 12062–12068. [Google Scholar] [CrossRef]
- Rosen, M.J.; Kunjappu, J.T. Surfactants and Interfacial Phenomena, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar] [CrossRef]
- Zdziennicka, A.; Jańczuk, B. Modification of adsorption, aggregation and wetting properties of surfactants by short chain alcohols. Adv. Colloid Interface Sci. 2020, 284, 102249. [Google Scholar] [CrossRef]
- Bozetine, I.; Zaïd, T.A.; Chitour, C.E.; Canselier, J.P. Optimization of an alkylpolyglucoside-based dishwashing detergent formulation. J. Surfactants Deterg. 2008, 11, 299–305. [Google Scholar] [CrossRef]
- Fu, Y.; Qin, C.; Gao, S.; Lv, C.; Zhang, C.; Yao, Y. Aquifer flushing using a SDS/1-butanol based in-situ microemulsion: Performance and mechanism for the remediation of nitrobenzene contamination. J. Hazard. Mater. 2022, 424, 127409. [Google Scholar] [CrossRef]
- Mao, X.; Jiang, R.; Xiao, W.; Yu, J. Use of surfactants for the remediation of contaminated soils: A review. J. Hazard. Mater. 2015, 285, 419–435. [Google Scholar] [CrossRef]
- Ji, W.; Abou, C.; Parameswarappa, M.; Zhao, L.; Boufadel, M.C. Behavior of surfactants and surfactant blends in soils during remediation: A review. Environ. Chall. 2021, 2, 100007. [Google Scholar] [CrossRef]
- Amanat, N.; Barbati, B.; Rossi, M.M.; Bellagamba, M.; Buccolini, M.; Galantini, L.; Papini, M.P. Synthetic and Natural Surfactants for Potential Application in Mobilization of Organic Contaminants: Characterization and Batch Study. Water 2022, 14, 1182. [Google Scholar] [CrossRef]
- Barbati, B.; Lorini, L.; Amanat, N.; Bellagamba, M.; Galantini, L.; Petrangeli, M. Enhanced solubilization of strongly adsorbed organic pollutants using synthetic and natural surfactants in soil flushing: Column experiment simulation. J. Environ. Chem. Eng. 2023, 11, 110758. [Google Scholar] [CrossRef]
- Rossi, M.M.; Silvani, L.; Amanat, N.; Papini, M.P. Biochar from pine wood, rice husks and iron-eupatorium shrubs for remediation applications: Surface characterization and experimental tests for trichloroethylene removal. Materials 2021, 14, 1776. [Google Scholar] [CrossRef]
- Drelich, J.; Fang, C.; White, C.L. Measurement of Interfacial Tension in Fluid-Fluid Systems. Encycl. Surf. Colloid Sci. 2002, 3, 3152–3166. [Google Scholar]
- Pal, N.; Samanta, K.; Mandal, A. A novel family of non-ionic gemini surfactants derived from sunflower oil: Synthesis, characterization and physicochemical evaluation. J. Mol. Liq. 2019, 275, 638–653. [Google Scholar] [CrossRef]
- Van Voorst Vader, F. Adsorption of detergents at the liquid-liquid interface. Part 1. Trans. Faraday Soc. 1960, 56, 1067–1077. [Google Scholar] [CrossRef]
- Steelman, C.M.; Meyer, J.R.; Wanner, P.; Swanson, B.J.; Conway-White, O.; Parker, B.L. The importance of transects for characterizing aged organic contaminant plumes in groundwater. J. Contam. Hydrol. 2020, 235, 103728. [Google Scholar] [CrossRef] [PubMed]
- Zeppilli, M.; Dell’Armi, E.; Cristiani, L.; Papini, M.P.; Majone, M. Reductive/oxidative sequential bioelectrochemical process for perchloroethylene removal. Water 2019, 11, 2579. [Google Scholar] [CrossRef]
- Kanokkarn, P.; Shiina, T.; Santikunaporn, M.; Chavadej, S. Equilibrium and dynamic surface tension in relation to diffusivity and foaming properties: Effects of surfactant type and structure. Colloids Surfaces A Physicochem. Eng. Asp. 2017, 524, 135–142. [Google Scholar] [CrossRef]
- Gaudin, T.; Rotureau, P.; Pezron, I.; Fayet, G. New QSPR models to predict the critical micelle concentration of sugar-based surfactants. Ind. Eng. Chem. Res. 2016, 55, 11716–11726. [Google Scholar] [CrossRef]
- Brito, R.O.; Silva, S.G.; Fernandes, R.M.F.; Marques, E.F.; Enrique-Borges, J.; Vale, M.L.C.D. Enhanced interfacial properties of novel amino acid-derived surfactants: Effects of headgroup chemistry and of alkyl chain length and unsaturation. Colloids Surf. B Biointerfaces 2011, 86, 65–70. [Google Scholar] [CrossRef] [PubMed]
- Lv, M.; Zhou, Y.; Wang, S.; Han, F.; Xu, B. Effects of the Polypropylene Oxide Number on the Surface Properties of a Type of Extended Surfactant. J. Surfactants Deterg. 2018, 21, 335–341. [Google Scholar] [CrossRef]
- Gaudin, T.; Lu, H.; Fayet, G.; Berthauld-Drelich, A.; Rotureau, P.; Pourceau, G.; Wadouachi, A.; Van Hecke, E.; Nesterenko, A.; Pezron, I. Impact of the chemical structure on amphiphilic properties of sugar-based surfactants: A literature overview. Adv. Colloid Interface Sci. 2019, 270, 87–100. [Google Scholar] [CrossRef]
- Bodratti, A.M.; Cheng, J.; Kong, S.M.; Chow, M.R.; Tsianou, M. Self-Assembly of Polyethylene Glycol Ether Surfactants in Aqueous Solutions: The Effect of Linker between Alkyl and Ethoxylate. J. Surfactants Deterg. 2019, 22, 1147–1161. [Google Scholar] [CrossRef]
- Chen, W.; Schechter, D.S. Surfactant selection for enhanced oil recovery based on surfactant molecular structure in unconventional liquid reservoirs. J. Pet. Sci. Eng. 2021, 196, 107702. [Google Scholar] [CrossRef]
- Ko, S.-O.; Schlautman, M.A.; Carraway, E.R. Effects of Surfactant Sorption on the Equilibrium Distribution of Organic Pollutants in Contaminated Subsurface Environments. In Physicochemical Groundwater Remediation; Springer: Boston, MA, USA, 2005; pp. 187–216. [Google Scholar] [CrossRef]
- Guo, Y.P.; Hu, Y.Y.; Lin, H.; Ou, X.L. Sorption and desorption of 17A-ethinylestradiol onto sediments affected by rhamnolipidic biosurfactants. J. Hazard. Mater. 2018, 344, 707–715. [Google Scholar] [CrossRef]
- Ballesteros-Gómez, A.; Rubio, S. Hemimicelles of alkyl carboxylates chemisorbed onto magnetic nanoparticles: Study and application to the extraction of carcinogenic polycyclic aromatic hydrocarbons in environmental water samples. Anal. Chem. 2009, 81, 9012–9020. [Google Scholar] [CrossRef]
- Gaudin, T.; Rotureau, P.; Pezron, I.; Fayet, G. Estimating the Adsorption Efficiency of Sugar-Based Surfactants From QSPR Models. Int. J. Quant. Struct. Relatsh. 2019, 4, 28–51. [Google Scholar] [CrossRef]
| Formulation | Composition | Additive Chemical Structure |
|---|---|---|
| APG 2a | APG 2 + C6 branched alcohol (15:1) | 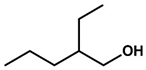 |
| APG 2b | APG 2 + C 10 POE-CS (15:1) | 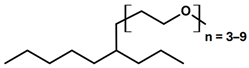 |
| APG 2c | APG 2 + C 10 POE-P-CS (15:1) | 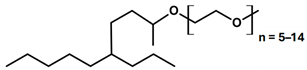 |
| g of PWB (Reactive Zone) | Contaminant | Flushing Agent | |
|---|---|---|---|
| PWB-TOL-a | 3.00 | Toluene (LNAPL) | APG 2a |
| PWB-TOL-b | 2.53 | Toluene (LNAPL) | APG 2b |
| PWB-TOL-c | 2.83 | Toluene (LNAPL) | APG 2c |
| PWB-PCE-a | 2.62 | Perchloroethylene (DNAPL) | APG 2a |
| PWB-PCE-b | 2.88 | Perchloroethylene (DNAPL) | APG 2b |
| PWB-PCE-c | 2.89 | Perchloroethylene (DNAPL) | APG 2c |
| Material | CMC (% wt) | γCMC (mN m−1) | πCMC (mN m−1) | Γm (mol cm−2) | pC20 | CMC/C20 |
|---|---|---|---|---|---|---|
| APG 2a | 6.13 × 10−2 ± 7.8 × 10−3 | 29.15 ± 0.05 | 43.65 ± 0.05 | 2.69 × 1010 ± 7.9 × 108 | 2.06 ± 0.02 | 7.08 ± 0.65 |
| APG 2b | 3.33 × 10−2 ± 1.8 × 10−3 | 30.97 ± 0.32 | 41.83 ± 0.32 | 2.42 × 1010 ± 6.4 × 108 | 2.27 ± 0.02 | 6.24 ± 0.38 |
| APG 2c | 3.74 × 10−2 ± 3.6 × 10−3 | 32.84 ± 0.25 | 39.86 ± 0.25 | 2.10 × 1010 ± 2.4 × 108 | 2.37 ± 0.05 | 8.74 ± 0.41 |
| APG 2 [32] | 7.10 × 10−3 ± 5.6 × 10−4 | 32.95 ± 0.13 | 39.95 ± 0.13 | 4.21 × 1010 ± 3.3 × 108 | 2.98 ± 0.03 | 6.70 ± 0.32 |
| Formulates’ Concentration | |||
|---|---|---|---|
| Formulate | Langmuir Parameters | 5× CMC | 0.5× CMC |
| APG 2a | qmax (mg g−1) | 17.08 ± 1.1 | 45.78 ± 2.3 |
| KL (L mg−1) | 11.4 × 10−2 ± 36 × 10−3 | 24.6 × 10−2 ± 25 × 10−3 | |
| R2 | 0.95 | 0.94 | |
| APG 2b | qmax (mg g−1) | 20.56 ± 1.5 | 65.97 ± 1.5 |
| KL (L mg−1) | 12.5 × 10−2 ± 8 × 10−3 | 41.1 × 10−2 ± 28 × 10−3 | |
| R2 | 0.90 | 0.95 | |
| APG 2c | qmax (mg g−1) | 17.86 ± 1.1 | 31.46 ± 1.8 |
| KL (L mg−1) | 13.9 × 10−2 ± 16 × 10−3 | 37.2 × 10−2 ± 25 × 10−3 | |
| R2 | 0.92 | 0.98 | |
| APG 2 (5× CMC) [32] | qmax (mg g−1) | 20.14 ± 1.4 | |
| KL (L mg−1) | 15.3 × 10−2 ± 36 × 10−3 | ||
| R2 | 0.91 | ||
| Reference PWB-TOL [32] | qmax (mg g−1) | 77.71 ± 2.5 | |
| KL (L mg−1) | 15.4 × 10−2 ± 55 × 10−3 | ||
| R2 | 0.97 | ||
| Formulates’ Concentration | |||
|---|---|---|---|
| Formulate | Langmuir Parameters | 5× CMC | 0.5× CMC |
| APG 2a | qmax (mg g−1) | 37.05 ± 3.5 | 43.68 ± 4.9 |
| KL (L mg−1) | 49.9 × 10−3 ± 12 × 10−3 | 31.83 ×10−2 ± 17 × 10−3 | |
| R2 | 0.93 | 0.96 | |
| APG 2b | qmax (mg g−1) | 40.31 ± 5.1 | 58.65 ± 6.7 |
| KL (L mg−1) | 60.6 ×10−3 ± 24 × 10−3 | 79.28 ×10−2 ± 28 × 10−3 | |
| R2 | 0.95 | 0.97 | |
| APG 2c | qmax (mg g−1) | 38.64 ± 4.1 | 47.39 ± 5.2 |
| KL (L mg−1) | 53.3 ×10−3 ± 21 × 10−3 | 55.4 ×10−2 ± 18 × 10−3 | |
| R2 | 0.96 | 0.98 | |
| APG 2 (5× CMC) [32] | qmax (mg g−1) | 39.42 ± 4.7 | |
| KL (L mg−1) | 85 × 10−3 ± 25 × 10−3 | ||
| R2 | 0.91 | ||
| Reference PWB-PCE [32] | qmax (mg g−1) | 114.12 ± 7.8 | |
| KL (L mg−1) | 29 × 10−2 ± 48 × 10−3 | ||
| R2 | 0.98 | ||
| Column | HRT (min) | VP (cm3) | ε (%) |
|---|---|---|---|
| PWB-TOL-a | 71.0 | 42.6 | 62.1 |
| PWB-TOL-b | 46.3 | 29.3 | 42.5 |
| PWB-TOL-c | 70.2 | 40.7 | 59.0 |
| PWB-PCE-a | 46.0 | 29.1 | 42.2 |
| PWB-PCE-b | 45.8 | 28.7 | 41.6 |
| PWB-PCE-c | 50.4 | 30.2 | 44.1 |
| Contamination Phase | Flushing Phase | ||||||
|---|---|---|---|---|---|---|---|
| Column | Adsorbed Contaminant (mg) | Adsorbed Contaminant (mg g−1) | PV * | Flushing Agent | PV ** | Solubilized Contaminant (mg) | Removal (%) |
| PWB-TOL-a | 349.2 | 116.3 | 148.6 | APG 2a | 51 | 334.3 | 95.7 |
| PWB-TOL-b | 241.6 | 95.6 | 194.1 | APG 2b | 102 | 209.5 | 86.7 |
| PWB-TOL-c | 306.7 | 108.4 | 141.7 | APG 2c | 169 | 179.1 | 58.4 |
| PWB-TOL-APG 2 [33] | 296.3 | 98.6 | 145 | APG 2 | 131 | 223.4 | 75.4 |
| PWB-TOL-H2O [33] | 292.2 | 97 | 202 | H2O | 250 | 145.4 | 49.7 |
| PWB-PCE-a | 382.9 | 146.3 | 234.5 | APG 2a | 94 | 380.4 | 99.3 |
| PWB-PCE-b | 450.1 | 156.1 | 356.6 | APG 2b | 118 | 427.7 | 95.0 |
| PWB-PCE-c | 527.7 | 132.2 | 414.3 | APG 2c | 152 | 314.1 | 59.5 |
| PWB-PCE-APG 2 [33] | 399.8 | 132.9 | 339 | APG 2 | 123 | 367 | 91.8 |
| PWB-PCE-H2O [33] | 427.3 | 144 | 349 | H2O | 380 | 217.8 | 50.9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barbati, B.; Lorini, L.; Bellagamba, M.; Petrangeli Papini, M. Alkylpolyglycosides—Based Formulations for Sustainable Remediation of Contaminated Aquifers: Lab-Scale Process Study for NAPL Solubilization Assessment. Sustainability 2025, 17, 1939. https://doi.org/10.3390/su17051939
Barbati B, Lorini L, Bellagamba M, Petrangeli Papini M. Alkylpolyglycosides—Based Formulations for Sustainable Remediation of Contaminated Aquifers: Lab-Scale Process Study for NAPL Solubilization Assessment. Sustainability. 2025; 17(5):1939. https://doi.org/10.3390/su17051939
Chicago/Turabian StyleBarbati, Berardino, Laura Lorini, Marco Bellagamba, and Marco Petrangeli Papini. 2025. "Alkylpolyglycosides—Based Formulations for Sustainable Remediation of Contaminated Aquifers: Lab-Scale Process Study for NAPL Solubilization Assessment" Sustainability 17, no. 5: 1939. https://doi.org/10.3390/su17051939
APA StyleBarbati, B., Lorini, L., Bellagamba, M., & Petrangeli Papini, M. (2025). Alkylpolyglycosides—Based Formulations for Sustainable Remediation of Contaminated Aquifers: Lab-Scale Process Study for NAPL Solubilization Assessment. Sustainability, 17(5), 1939. https://doi.org/10.3390/su17051939








