Global Research Trends and Hotspots in White Clover (Trifolium repens L.) Responses to Drought Stress (1990–2024)
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources
2.2. Data Analysis
3. Results
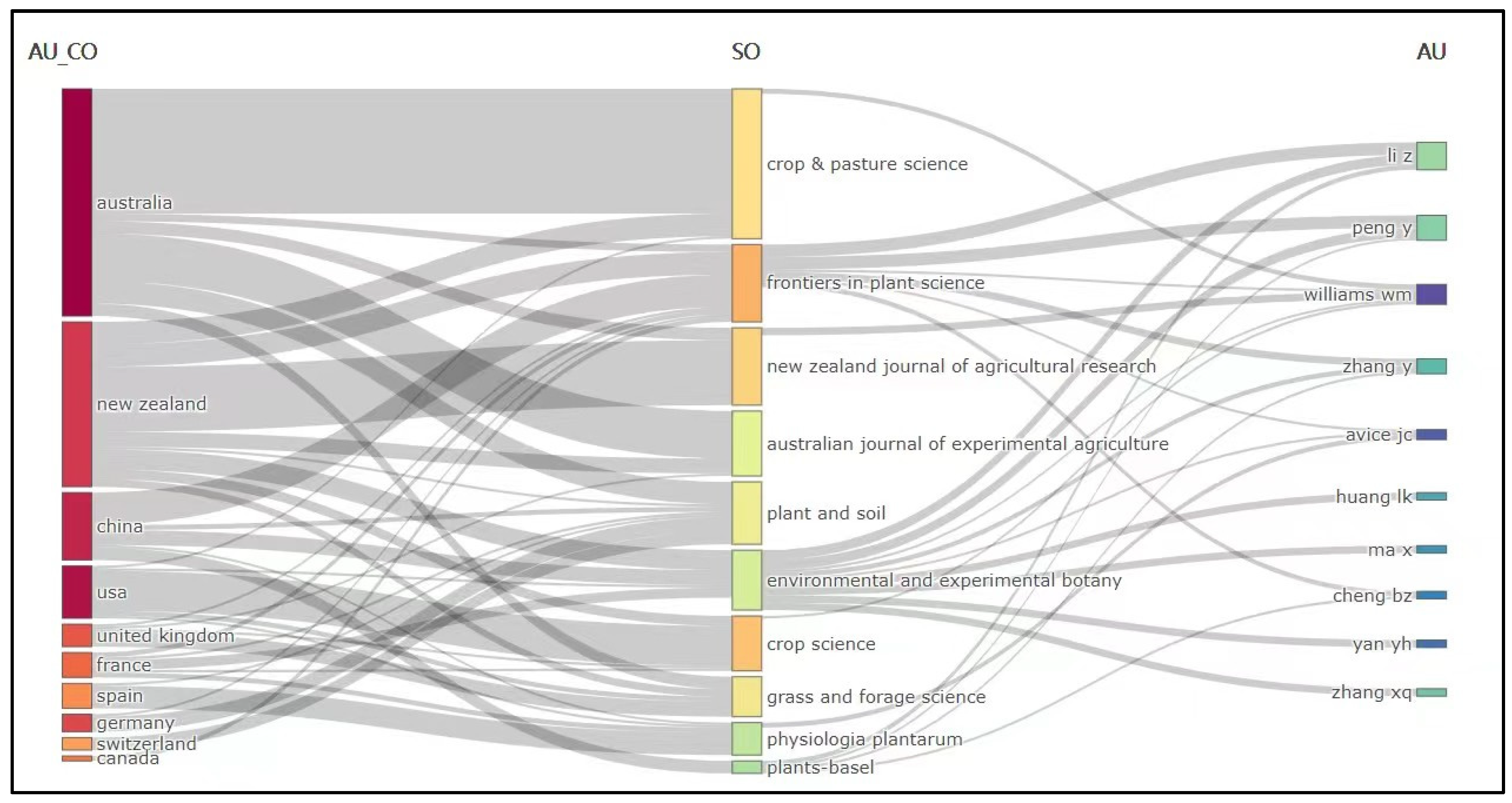
3.1. Analysis of the Number of Publications and Citations
3.2. Distribution of Countries and Institutions
3.3. Main Journals and Most Impacting Papers
3.4. Research Hotspots and Evolution Trend Analysis
3.4.1. Topic Keyword Mapping and Hotspot Evolutionary Trends
3.4.2. Identification of Research Frontiers
4. Discussion
4.1. Response Mechanism of White Clover to Drought Stress
4.1.1. Effects of Drought Stress on Yield
4.1.2. Effects of Drought Stress on the Physiology and Biochemistry
4.1.3. Effects of Drought Stress on the Molecular
4.2. Drought-Resistant Technologies of White Clover
4.3. Research Gaps and Challenge
4.4. Perspectives on Drought Tolerance in White Clover
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Christian, J.I.; Basara, J.B. Global distribution, trends, and drivers of flash drought occurrence. Nat. Commun. 2021, 12, 6330. [Google Scholar] [CrossRef] [PubMed]
- Cavin, L.; Mountford, E.P. Extreme drought alters competitive dominance within and between tree species in a mixed forest stand. Funct. Ecol. 2013, 27, 1424–1435. [Google Scholar] [CrossRef]
- Anjum, S.A.; Ashraf, U.; Zohaib, A. Growth and developmental responses of crop plants under drought stress: A review. Zemdirbyste 2017, 104, 267–276. [Google Scholar] [CrossRef]
- Fahad, S.; Bajwa, A.A.; Nazir, U.; Anjum, S.A.; Farooq, A.; Zohaib, A.; Sadia, S.; Nasim, W. Crop Production under drought and heat stress: Plant responses and management options. Front. Plant Sci. 2017, 8, 1147. [Google Scholar] [CrossRef] [PubMed]
- Murray, V.; Ebi, K.L. IPCC special report on managing the risks of extreme events and disasters to advance climate change adaptation (SREX). J. Epidemiol. Commun. Health 2012, 66, 759–760. [Google Scholar] [CrossRef]
- Zhao, L.L.; Xie, W.H.; Huang, L.; Long, S.S.; Wang, P.C. Characterization of the gibberellic oxidase gene SdGA20ox1 in Sophora davidii (Franch.) Skeels and interaction protein screening. Front. Plant Sci. 2024, 15, 1478854. [Google Scholar] [CrossRef]
- Zhou, Z.J.; Li, J.Q.; Yang, Y.T.; Gao, Y.; Wang, X.T.; Zhao, L.L.; Wang, R.; Huang, H.Y.; Wang, P.C. Effects of karst environmental stresses on seed germination and seedling growth of alfalfa (Medicago sativa L.). Front. Sustain. Food Syst. 2024, 8, 1510596. [Google Scholar] [CrossRef]
- Huang, H.Y.; Yang, Y.T.; Li, J.Q.; Gao, Y.; Wang, X.T.; Wang, R.; Zhou, Z.J.; Wang, P.C.; Zhao, L.L. Effects of rocky desertification stress on oat seed germination and seedling growth in the karst areas of southwest China. Plants 2024, 13, 3260. [Google Scholar] [CrossRef]
- Lilley, J.M.; Fukai, S. Effect of timing and severity of water-deficit on 4 diverse rice cultivars. 2. physiological-responses to soil-water deficit. Field Crop Res. 1994, 37, 215–223. [Google Scholar] [CrossRef]
- Xu, H.L.; Yamagishi, T. Effects of water deficit on photosynthesis in wheat plants. 1. effects of a water deficit treatment on photosynthesis and transpiration in various parts of plant. Jpn. J. Crop Sci. 1987, 56, 455–460. [Google Scholar] [CrossRef]
- Mouradi, M.; Farissi, M. Effects of water deficit on growth, nodulation and physiological and biochemical processes in Medicago sativa-rhizobia symbiotic association. Arid Land Res. Manag. 2016, 30, 193–208. [Google Scholar] [CrossRef]
- Mhamdi, A.; van Breusegem, F. Reactive oxygen species in plant development. Development 2018, 145, dev164376. [Google Scholar] [CrossRef] [PubMed]
- Liao, W.B.; Huang, G.B. At the cellular level, oxidative stress intensifies, as reactive oxygen species (ROS), including superoxide anions and hydrogen peroxide, accumulate in plant tissues. Plant Physiol. Bioch. 2012, 58, 6–15. [Google Scholar] [CrossRef]
- Samanta, S.; Seth, C.S. The molecular paradigm of reactive oxygen species (ROS) and reactive nitrogen species (RNS) with different phytohormone signaling pathways during drought stress in plants. Plant Physiol. Bioch. 2024, 206, 108259. [Google Scholar] [CrossRef] [PubMed]
- Hussain, M.S.; Farooq, W.; Hasan, S.; Ul-Allah, M.; Tanveer, M. Drought stress in sunflower: Physiological effects and its management through breeding and agronomic alternatives. Agric. Water Manag. 2018, 201, 152–167. [Google Scholar] [CrossRef]
- Jaleel, C.A.; Manivannan, P. Induction of drought stress tolerance by ketoconazole in Catharanthus roseus is mediated by enhanced antioxidant potentials and secondary metabolite accumulation. Colloid. Surface B 2007, 60, 201–206. [Google Scholar] [CrossRef]
- El-Beltagi, H.S.; Mohamed, H.I. Role of ascorbic acid, glutathione and proline applied as singly or in sequence combination in improving chickpea plant through physiological change and antioxidant defense under different levels of irrigation intervals. Molecules 2020, 25, 1702. [Google Scholar] [CrossRef]
- Anjum, S.A.; Xie, X.Y. Morphological, physiological and biochemical responses of plants to drought stress. Afr. J. Agric. Res. 2011, 6, 2026–2032. [Google Scholar]
- Toscano, S.; Ferrante, A. Response of mediterranean ornamental plants to drought stress. Horticulturae 2019, 5, 6. [Google Scholar] [CrossRef]
- Deguchi, S.; Uozumi, S.; Kaneko, M.; Touno, E. Organic cultivation system of corn–triticale rotation using white clover living mulch. Grassl. Sci. 2015, 61, 188–194. [Google Scholar] [CrossRef]
- Ballizany, W.L.; Hofmann, R.W. Multivariate associations of flavonoid and biomass accumulation in white clover (Trifolium repens) under drought. Funct. Plant Biol. 2012, 39, 167–177. [Google Scholar] [CrossRef] [PubMed]
- Bidar, G.; Verdin, A.; Garçon, G.; Pruvot, C.; Laruelle, F.; Grandmougin-Ferjani, A.; Douay, F.; Shirali, P. Changes in fatty acid composition and content of two plants (Lolium perenne and Trifolium repens) grown during 6 and 18 months in a metal (Pb, Cd, Zn) contaminated field. Water Air. Soil Poll. 2008, 192, 281–291. [Google Scholar] [CrossRef]
- Abberton, M.T.; Marshall, A.H. Progress in breeding perennial clovers for temperate agriculture: Centenary review. J. Agric. Sci. 2005, 143, 117–135. [Google Scholar] [CrossRef]
- Zhao, L.L.; Wang, L.T.; Chen, K.K.; Sun, H.; Wang, P.C. Effects of arbuscular mycorrhizal fungi on the growth and physiological performance of Sophora davidii seedling under low-phosphorus stress. J. Plant Growth Regul. 2024, 43, 2383–2395. [Google Scholar] [CrossRef]
- Caradus, J.R.; Woodfield, D.R. Genetic control of adaptive root characteristics in white clover. Plant Soil 1998, 200, 63–69. [Google Scholar] [CrossRef]
- Nichols, S.N.; Hofmann, R.W.; Williams, W.M. Physiological drought resistance and accumulation of leaf phenolics in white clover interspecific hybrids. Environ. Exp. Bot. 2015, 119, 40–47. [Google Scholar] [CrossRef]
- Peng, D.; Wang, X.; Li, Z.; Zhang, Y.; Peng, Y.; Li, Y.; He, X.; Zhang, X.; Ma, X.; Huang, L. NO is involved in spermidine-induced drought tolerance in white clover via activation of antioxidant enzymes and genes. Protoplasma 2015, 253, 1243–1254. [Google Scholar] [CrossRef]
- Yan, Z.; Zhou, L.; Li, Y.P. Chitosan and spermine enhance drought resistance in white clover, associated with changes in endogenous phytohormones and polyamines, and antioxidant metabolisml. Funct. Plant Biol. 2018, 12, 1205–1222. [Google Scholar]
- Li, Z.; Peng, Y.; Zhang, X.Q. Exogenous spermidine improves seed germination of white clover stress via involvementin starch metabolism, antioxidant defenses and relevant gene expression. Molecules 2014, 19, 18003–18024. [Google Scholar] [CrossRef]
- Vera-Baceta, M.A.; Thelwall, M.; Kousha, K. Web of Science and Scopus language coverage. Scientometrics 2019, 121, 1803–1813. [Google Scholar] [CrossRef]
- Dobrescu, A.I.; Nussbaumer-Streit, B.; Klerings, I.; Wagner, G.; Persad, E.; Sommer, I.; Herkner, H.; Gartlehner, G. Restricting evidence syntheses of interventions to english-language publications is a viable methodological shortcut for most medical topics: A systematic review. J. Clin. Epidemiol. 2021, 137, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Liang, B.; Cao, T. A bibliometric analysis of speech and language impairments in parkinson’s disease based on web of science. Front. Psychol. 2024, 15, 1374924. [Google Scholar] [CrossRef]
- Craene, V.D.; Koelemaij, J.; Zee, E.V.D.; Meeteren, M.V. A world beyond web of science: Agora magazine’s 35 years in dutch language human geography. Tijdschr. Voor Econ. En Soc. Geogr. 2021, 112, 456–473. [Google Scholar] [CrossRef]
- Liu, F. Retrieval strategy and possible explanations for the abnormal growth of research publications: Re-evaluating a bibliometric analysis of climate change. Scientometrics 2023, 128, 853–859. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Li, J.Q.; Gao, Y.; Wang, X.; Wang, R.; Huang, H.; Zhang, Y.; Zhao, L.; Wang, P. Research on drought stress in Medicago sativa L. from 1998 to 2023: A bibliometric analysis. Front. Plant Sci. 2024, 15, 1406256. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, J.Q.; Wang, X.T.; Yang, Y.; Zhou, Z.; Deng, X.; Gao, Y.; Wang, P.C. A bibliometric analysis review of the Pennisetum (1970–2023). Front. Sustain. Food Syst. 2024, 8, 1405684. [Google Scholar] [CrossRef]
- Aria, M.; Cuccurullo, C. Bibliometrix: An R-tool for comprehensive science mapping analysis. J. Informetr. 2018, 11, 959–975. [Google Scholar] [CrossRef]
- Van Eck, N.J.; Waltman, L. VOSviewer: A computer program for bibliometric mapping. Erim. Rep. Ser. Res. Manag. 2009, 2, 886–897. [Google Scholar]
- Eck, N.J.V.; Waltman, L. Software survey: VOSviewer, a computer program for bibliometric mapping. Scientometrics 2010, 84, 523–538. [Google Scholar]
- Lee, B.R.; Kim, K.Y.; Jung, W.J.; Avice, J.C.; Ourry, A.; Kim, T.H. Peroxidases and lignification in relation to the intensity of water-deficit stress in white clover (Trifolium repens L.). J. Exp. Bot. 2007, 58, 1271–1279. [Google Scholar] [CrossRef]
- Hofmann, R.W.; Campbell, B.D.; Bloor, S.J. Responses to UV-B radiation in Trifolium repens L.—Physiological links to plant productivity and water availability. Plant Cell Environ. 2003, 26, 603–612. [Google Scholar] [CrossRef]
- Lee, B.R.; Jin, Y.L.; Jung, W.J.; Avice, J.C.; Morvan-Bertrand, A.; Ourry, A.; Park, C.W.; Kim, T.H. Water-deficit accumulates sugars by starch degradation—Not by de novo synthesis—In white clover leaves (Trifolium repens). Physiol. Plant. 2008, 08, 134. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, Y.; Zhang, X.; Merewitz, E.; Peng, Y.; Ma, X. Metabolic pathways regulated by chitosan contributing to drought resistance in white clover. J. Proteome Res. 2017, 7, 00334. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Y.; Zhang, X.Q.; Peng, Y.; Merewitz, E.; Ma, X.; Huang, L.K.; Yan, Y.H. The alterations of endogenous polyamines and phytohormones induced by exogenous application of spermidine regulate antioxidant metabolism, metallothionein and relevant genes conferring drought tolerance in white clover. Environ. Exp. Bot. 2016, 124, 22–38. [Google Scholar] [CrossRef]
- Bin, Y.; Huan, X.; Zhou, L. Exogenous application of GABA improves PEG-induced drought tolerance positively associated with GABA-Shunt, polyamines, and proline metabolism in white clover. Fronti. Physiol. 2017, 8, 1107. [Google Scholar]
- Zhang, Y.; Li, Y.; Hassan, M.J. Indole-3-acetic acid improves drought tolerance of white clover via activating auxin, abscisic acid and jasmonic acid related genes and inhibiting senescence genes. BMC Plant Biol. 2020, 20, s12870. [Google Scholar] [CrossRef]
- Wang, X.T.; Zhang, C.; Zhao, N.N.; Sun, X.R.; Hou, S.; Wang, P.C. Physiological nitrogen uptake and utilisation responses in two native plants from the Qinghai-Tibet plateau under different water and fertiliser conditions. Agronomy 2024, 14, 440. [Google Scholar] [CrossRef]
- Ealing, P.M.; Hancock, K.R.; White, D.W.R. Expression of the pea albumin 1 gene in transgenic white clover and tobacco. Transgenic Res. 1994, 3, 344–354. [Google Scholar] [CrossRef]
- Panter, S.; Mouradov, A. Development and validation of protocols for product stewardship in transgenic white clover (Trifolium repens L.): Detection of the AMV CP and npt2 transgenes in seeds, herbage and hay. Crop Pasture Sci. 2015, 66, 1039–1048. [Google Scholar] [CrossRef]
- Islam, A.; Leung, S. Kunitz proteinase inhibitors limit water stress responses in white clover (Trifolium repens L.). Front. Plant Sci. 2017, 8, 1683. [Google Scholar] [CrossRef]
- Jahufer, M.Z.Z.; Cooper, M. Pattern analysis of the diversity of morphological plant attributes and herbage yield in a world collection of white clover (Trifolium repens L.) germplasm characterised in a summer moisture stress environment of Australia. Genet Resour Crop Evol. 1997, 44, 289–300. [Google Scholar] [CrossRef]
- Lucero, D.W.; Grieu, P. Effects of water deficit and plant interaction on morphological growth parameters and yield of white clover (Trifolium repens L.) and ryegrass (Lolium perenne L.) mixtures. Eur. J. Agron. 1999, 1, 167–177. [Google Scholar] [CrossRef]
- Hogh-Jensen, H.; Schjoerring, J.K. Interactions between white clover and ryegrass under contrasting nitrogen availability: N2 fixation, N fertilizer recovery, N transfer and water use efficiency. Plant Soil. 1997, 197, 187–199. [Google Scholar] [CrossRef]
- Annicchiarico, P.; Piano, E. Indirect selection for root development of white clover and implications for drought tolerance. J. Agron. Crop Sci. 2004, 190, 28–34. [Google Scholar] [CrossRef]
- Sanderson, M.A.; Byers, R.A. Growth and complexity of white clover stolons in response to biotic and abiotic stress. Crop Sci. 2003, 43, 2197–2205. [Google Scholar] [CrossRef]
- Thomas, H. Effects of drought on growth and competitive ability of perennial ryegrass and white clover. Ecol. Appl. 1984, 21, 591–602. [Google Scholar] [CrossRef]
- Wang, X.T.; Yang, J.; Gao, Y.; Li, J.; Yang, Y.; Wang, P.C. Allocation, morphology, physiology: Multiple aspects of above- and below-ground responses to water table stress, duration of drainage in alpine wetland plants (Carex muliensis). Plant Soil 2024, 505, 703–718. [Google Scholar] [CrossRef]
- Wang, P.C.; Xie, W.H.; Ding, L.L.; Zhou, Y.P.; Gao, Y.; Zhao, L.L. Effects of maize-crop rotation on soil physicochemical properties, enzyme activities, microbial biomass and microbial community structure in southwest China. Microorganisms 2023, 11, 2621. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Z.; Peng, Y. Clones of FESOD, MDHAR, DHAR genes from white clover and gene expression analysis of ros-scavenging enzymes during abiotic stress and hormone treatments. Molecules 2015, 20, 20939–20954. [Google Scholar] [CrossRef]
- Corpas, F.J.; Barroso, J.B. Peroxisomes as a source of reactive oxygen species and nitric oxide signal molecules in plant cells. Trends Plant Sci. 2001, 6, 145–150. [Google Scholar] [CrossRef]
- Blokhina, O. Antioxidants, oxidative damage and oxygen deprivation stress: A Review. Ann. Bot. 2003, 91, 179–194. [Google Scholar] [CrossRef] [PubMed]
- Apel, K.; Hirt, H. Reactive oxygen species: Metabolism, oxidative stress, and signal transduction. Annu. Rev. Plant Biol. 2004, 55, 373–399. [Google Scholar] [CrossRef] [PubMed]
- Asada, K. Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol. 2006, 141, 391–396. [Google Scholar] [CrossRef] [PubMed]
- Smirnoff, N.; Cumbes, Q. Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 1989, 28, 1057–1060. [Google Scholar] [CrossRef]
- Upadhyaya, H.; Panda, S.; Dutta, B. Variation of physiological and antioxidative responses in tea cultivars subjected to elevated water stress followed by rehydration recovery. Acta Physiol. Plant. 2008, 30, 457–468. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative stress, antioxidants, and stress tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Li, Z.; Peng, Y. Different response on drought tolerance and post-drought recovery between the small-leafed and the large-leafed white clover (Trifolium repens L.) associated with antioxidative enzyme protection and lignin metabolism. Acta Physiol. Plant. 2013, 35, 213–222. [Google Scholar] [CrossRef]
- Wang, C.Q.; Li, R.C. Enhancement of superoxide dismutase activity in the leaves of white clover (Trifolium repens L.) in response to polyethylene glycol-induced water stress. Acta Physiol. Plant. 2008, 30, 841–847. [Google Scholar]
- HoghJensen, H.; Schjoerring, J.K. Effects of drought and inorganic N form on nitrogen fixation and carbon isotope discrimination in Trifolium repens. Plant Physiol. Bioch. 1997, 35, 55–62. [Google Scholar]
- Song, Z.H.; Yang, Q. Melatonin enhances stress tolerance in pigeon pea by promoting flavonoid enrichment, particularly luteolin in response to salt stress. J. Exp. Bot. 2022, 73, 5992–6008. [Google Scholar] [CrossRef]
- Li, L.J.; Gu, W.R. Exogenously applied spermidine alleviates photosynthetic inhibition under drought stress in maize (Zea mays L.) seedlings associated with changes in endogenous polyamines and phytohormones. Plant Physiol. Bioch. 2018, 129, 35–55. [Google Scholar] [CrossRef] [PubMed]
- Du, H.Y.; Liu, G.T. Significance of putrescine conversion in filling grain embryos of wheat plants subjected to drought stress. Plant Soil. 2023, 448, 58–610. [Google Scholar] [CrossRef]
- Quiroga, G.; Erice, G. Radial water transport in arbuscular mycorrhizal maize plants under drought stress conditions is affected by indole-acetic acid (IAA) application. J. Plant Physiol. 2020, 246, 153115. [Google Scholar] [CrossRef] [PubMed]
- Defez, R.; Andreozzi, A. Improved drought stress response in alfalfa plants nodulated by an IAA over-producing rhizobium strain. Front. Microbiol. 2017, 8, 02466. [Google Scholar] [CrossRef]
- Lim, C.W.; Baek, W. Arabidopsis pyl8 plays an important role for ABA signaling and drought stress responses. Plant Pathol. J. 2013, 29, 471–476. [Google Scholar] [CrossRef]
- Soma, F.; Takahashi, F.; Tamura, T.; Hara, K.; Yamaguchi, Y. Cellular phosphorylation signaling and gene expression in drought stress responses: ABA-dependent and ABA-independent regulatory systems. Plants 2021, 10, 756. [Google Scholar] [CrossRef]
- Mukherjee, A.; Dwivedi, S. Integration of light and ABA signaling pathways to combat drought stress in plants. Plant Cell Rep. 2023, 42, 829–841. [Google Scholar] [CrossRef]
- Chaves, M.M.; Flexas, J. Photosynthesis under drought and salt stress: Regulation mechanisms from whole plant to cell. Ann. Bot. 2009, 103, 551–560. [Google Scholar] [CrossRef]
- Widodo, W.; Boote, K.J. Elevated growth CO2 delays drought stress and accelerates recovery of rice leaf photosynthesis. Aenviron. Exp. Bot. 2003, 49, 259–272. [Google Scholar] [CrossRef]
- Grieu, P.; Robin, C. Effect of drought on photosynthesis in Trifolium repens: Maintenance of photosystem II efficiency photosynthesis. Plant Physiol. Bioch. 1995, 33, 19–24. [Google Scholar]
- Mafakheri, A.; Siosemardeh, A. Effect of drought stress on yield, proline and chlorophyll contents in three chickpea cultivars. Aust. J. Crop Sci. 2010, 4, 580–585. [Google Scholar]
- Rao, X.; Zhang, Y.J.; Gao, Y.; Zhao, L.L.; Wang, P.C. Influence of exogenous abscisic acid on germination and physiological traits of Sophora viciifolia seedlings under drought conditions. Appl. Sci. 2024, 14, 4359. [Google Scholar] [CrossRef]
- Silva, E.N.; Ribeiro, R.V. Coordinate changes in photosynthesis, sugar accumulation and antioxidative enzymes improve the performance of Jatropha curcas plants under drought stress. Biomass Bioenerg. 2012, 45, 270–279. [Google Scholar] [CrossRef]
- Wingler, A.; Quick, W.P. The role of photorespiration during drought stress: An analysis utilizing barley mutants with reduced activities of photorespiratory enzymes. Plant Cell Environ. 1999, 22, 361–373. [Google Scholar] [CrossRef]
- Zhou, L.; Zhang, Y. The inhibition of polyamine biosynthesis weakens the drought tolerance in white clover (Trifolium repens) associated with the alteration of extensive proteins. Protoplasma Inter. J. Cell Biol. 2018, 255, 803–817. [Google Scholar]
- Bisaga, M.; Lowe, M. Deep sequencing of suppression subtractive hybridisation drought and recovery libraries of the non-model crop Trifolium repens L. Front Plant Sci. 2017, 8, 1–18. [Google Scholar] [CrossRef]
- Demirevska, K.; Simova-Stoilova, L. Rubisco and some chaperone protein responses to water stress and rewatering at early seedling growth of drought sensitive and tolerant wheat varieties. Plant Growth Regul. 2008, 56, 97–106. [Google Scholar] [CrossRef]
- Xu, C.P.; Huang, B.R. Comparative analysis of drought responsive proteins in kentucky bluegrass cultivars contrasting in drought tolerance. Crop Sci. 2010, 50, 2543–2552. [Google Scholar] [CrossRef]
- Jiang, Q.; Zhang, J.Y.; Guo, X. Improvement of drought tolerance in white clover (Trifolium repens) by transgenic expression of a transcription factor gene WXP1. Funct. Plant Biol. 2010, 37, 157–165. [Google Scholar] [CrossRef]
- Li, Z.; Shi, P. Improved drought tolerance through drought preconditioning associated with changes in antioxidant enzyme activities, gene expression and osmoregulatory solutes accumulation in White clover (Trifolium repens L.). Plant Omics 2014, 6, 481–489. [Google Scholar]
- Lei, J.L.; Wang, D.; Cao, H. Transformation of a novel drought-response transcription factor gene PeDREB2b into white clover via soaking seeds with Agrobacterium tumefaciens. Acta Hortic. 2012, 929, 343–350. [Google Scholar] [CrossRef]
- Vaseva, I.; Akiscan, Y. Drought stress tolerance of red and white clover-comparative analysis of some chaperonins and dehydrins. Sci. Hortic. 2011, 130, 653–659. [Google Scholar] [CrossRef]
- Hassan, M.J.; Geng, W. Diethyl Aminoethyl Hexanoate Priming ameliorates seed germination via involvement in hormonal changes, osmotic adjustment, and dehydrins accumulation in white clover under drought stress. Front. Plant Sci. 2021, 12, 709187. [Google Scholar] [CrossRef] [PubMed]
- Gerhards, R. Weed Suppression Ability and Yield Impact of Living Mulch in Cereal Crops. Agriculture 2018, 8, 39. [Google Scholar] [CrossRef]
- Ehret, M.; Grass, R. The effect of shade and shade material on white clover/perennial ryegrass mixtures for temperate agroforestry systems. Agroforest Syst. 2015, 89, 557–570. [Google Scholar] [CrossRef]
- Stutz, R.S.; Culvenor, R.A. Legume options for summer-active pastures in a temperate rainfall environment of south-eastern Australia. Crop Pasture Sci. 2023, 74, CP22406. [Google Scholar] [CrossRef]
- Zhao, X.Y.; Tian, Z.; Cheng, L. Comparative Study on the Morpho-Physiological Responses of White Clover Cultivars with Different Leaf Types to Water Deficiency. Agronomy 2023, 13, 1859. [Google Scholar] [CrossRef]
- The effect of drought and interspecific interactions on depth of water uptake in deep- and shallow-rooting grassland species as determined by δ~(18)O natural abundance. Biogeosciences 2014, 11, 4493–4506. [CrossRef]
- Norton, M.R.; Li, G. Differences in dehydration tolerance affect survival of white clover (Trifolium repens) and lucerne (Medicago sativa) during a drying cycle. Crop Pasture Sci. 2021, 72, 723–730. [Google Scholar] [CrossRef]
- Staniak, M.; Bojarszczuk, J.; Kraska, P. Prolonged drought stress induced changes in yield and physiological processes of Trifolium repens and Festulolium braunii. Biol. Plant. 2020, 64, 701–709. [Google Scholar] [CrossRef]
- Williams, W.M.; Verry, I.M.; Ansari, H.A.; Hussain, S.W.; Ullah, I.; Ellison, N.W. 4xTrifolium ambiguum and 2xT. occidentale hybridise despite wide geographic separation and polyploidisation: Implications for clover breeding. Theor. Appl. Genet. 2019, 132, 2899–2912. [Google Scholar] [CrossRef] [PubMed]
- Ma, S.N.; Hu, R.C. Integrative analysis of the metabolome and transcriptome provides insights into the mechanisms of anthocyanins and proanthocyanidins biosynthesis in Trifolium repens. Ind. Crop. Prod. 2022, 187, 115529. [Google Scholar] [CrossRef]
- Kuo, W.H.; Wright, S.; Small, L.; Olsen, K. De novo genome assembly of white clover (Trifolium repens L.) reveals the role of Copy Number Variation in rapid environmental adaptation. BMC Biol. 2024, 22, 165. [Google Scholar] [CrossRef] [PubMed]
- Goh, K.M.; Bruce, G.E. Comparison of biomass production and biological nitrogen fixation of multi-species pastures (mixed herb leys) with perennial ryegrass-white clover pasture with and without irrigation in Canterbury, New Zealand. Agric. Ecosyst. Environ. 2005, 110, 230–240. [Google Scholar] [CrossRef]
- Li, Z.; Li, Y.P. Indole-3-acetic acid modulates phytohormones and polyamines metabolism associated with the tolerance to water stress in white clover. Plant Physiol. Biochem. 2018, 129, 251–263. [Google Scholar] [CrossRef]
- Pu, Q.; Li, Z. Selection and validation of reference genes for quantitative real-time PCR in white clover (Trifolium repens L.) Involved in Five Abiotic Stresses. Plants 2020, 9, 996. [Google Scholar] [CrossRef]
- Ma, S.; Han, C.Y. Fingerprint identification of white clover cultivars based on SSR molecular markers. Mol. Biol. Rep. 2020, 47, 8513–8521. [Google Scholar] [CrossRef]
- Jia, T.; Tang, T. Development of two protocols for agrobacterium-mediated transformation of white clover (Trifolium repens) via the callus system. 3 Biotech 2023, 13, 150. [Google Scholar] [CrossRef]
- Riesinger, P.; Herzon, I. Variability of herbage production in mixed leys as related to ley age and environmental factors: A farm survey. Agric. Food Sci. 2008, 17, 394–412. [Google Scholar] [CrossRef]
- Kumar, V.M.; Daud, K.; Mayank, Y. Scientometric assessment of funded scientometrics and bibliometrics research (2011–2021). Scientometr. Int. J. All Quant. Asp. Sci. Sci. Policy 2023, 128, 4305. [Google Scholar]
- El-Ouahi, J. Research funding in the middle east and north africa: Analyses of acknowledgments in scientific publications indexed in the web of science (2008–2021). Scientometrics 2024, 129, 2933–2968. [Google Scholar] [CrossRef]
- Abalkina, A. Challenges posed by hijacked journals in scopus. JASIST 2024, 75, 395–422. [Google Scholar] [CrossRef]
- Martin-Martin, A.; Orduna-Malea, E.; Thelwall, M.; Delgado Lopez-Cozar, E. Google scholar, web of science, and scopus: A systematic comparison of citations in 252 subject categories. J. Informetr. 2018, 12, 1160–1177. [Google Scholar] [CrossRef]
- Marina, T.; Sterligov, I. Prevalence of potentially predatory publishing in scopus on the country level. Scientometrics 2021, 126, 5079. [Google Scholar] [CrossRef]
- Marshall, A.; Rascle, C.; Abberton, M.; Michaelson-Yeates, T.; Rhodes, I. Introgression as a route to improved drought tolerance in white clover (Trifolium repens L.). J. Agron. Crop Sci. 2001, 187, 11–18. [Google Scholar] [CrossRef]
- Abberton, M.T.; Thomas, I. Genetic resources in Trifolium and their utilization in plant breeding. Plant Genet. Resour. Charact. Util. 2011, 9, 38–44. [Google Scholar] [CrossRef]
- Abberton, M.T.; MacDuff, J.H.; Vagg, S.; Marshall, A.H.; Michaelson-Yeates, T.P.T. Nitrogen fixation in hybrids of white clover and caucasian clover. J. Agron. Crop Sci. 2000, 185, 241–247. [Google Scholar] [CrossRef]
- Hussain, S.; Shah, M.N. Organic amendments mitigate drought stress-induced oxidative changes in synthetic cultivars of maize. Pak. J. Bot. 2023, 55, 429–436. [Google Scholar] [CrossRef]








| Country | TC | AAC | NP |
|---|---|---|---|
| China | 1923 | 23.45 | 82 |
| New Zealand | 1285 | 22.95 | 56 |
| Australia | 1130 | 22.16 | 51 |
| USA | 2019 | 41.2 | 49 |
| France | 1460 | 45.63 | 32 |
| United Kingdom | 843 | 32.42 | 26 |
| Germany | 712 | 29.67 | 24 |
| Switzerland | 902 | 45.1 | 20 |
| Spain | 739 | 41.06 | 18 |
| Canada | 309 | 25.75 | 12 |
| Institution | Country | NP | TC | CPP |
|---|---|---|---|---|
| Sichuan Agriculture University | China | 43 | 1142 | 26.5581 |
| Massey University | New Zealand | 17 | 404 | 23.7647 |
| Lincoln University | New Zealand | 15 | 302 | 20.1333 |
| Agresearch | New Zealand | 12 | 529 | 44.0833 |
| Chonnam national University | South Korea | 10 | 530 | 53 |
| Agricultural and Food Research Council | United Kingdom | 9 | 227 | 25.2222 |
| Institute National de la Recherche Agronomique | France | 8 | 488 | 61 |
| Grassland Environment Research Institute | China | 8 | 345 | 43.125 |
| La Trobe University | Australia | 7 | 272 | 38.8571 |
| Université de Caen Normandie | French | 7 | 439 | 62.7143 |
| Rank | Title | Journal | Year | IF | TC | TC per Year |
|---|---|---|---|---|---|---|
| 1 | Peroxide and lignification in relation to the intensity of water-deficit stress in white clover (Trifolium repens L.) | Journal of Experimental Botany | 2007 | 6.9 | 196 | 11.53 |
| 2 | Progress in Breeding Perennial Clovers for Temperate Agriculture | Journal of Agricultural Science | 2005 | 2.0 | 98 | 5.16 |
| 3 | Responses to Uv-B radiation in Trifolium repens L.—physiological links to plant productivity and water availability | Plant Cell and Environment | 2003 | 7.3 | 95 | 4.52 |
| 4 | Exogenous spermidine improves seed germination of white clover under water stress via involvement in starch metabolism, antioxidant defenses and relevant gene expression | Molecules | 2014 | 4.6 | 85 | 8.5 |
| 5 | Metabolic pathways regulated by chitosan contributing to drought resistance in white clover | Journal of Proteome Research | 2017 | 11.6 | 74 | 10.57 |
| 6 | The alterations of endogenous polyamines and phytohormones induced by exogenous application of spermidine regulate antioxidant metabolism, metallothionein and relevant genes conferring drought tolerance in white clover | Environmental and Experimental Botany | 2016 | 5.7 | 73 | 9.13 |
| 7 | Exogenous application of gaba improves peg-induced drought tolerance positively associated with gaba-shunt, polyamines, and proline metabolism in white clover | Frontiers in Physiology | 2017 | 4.0 | 69 | 9.86 |
| 8 | Indole-3-acetic acid improves drought tolerance of white clover via activating auxin, abscisic acid and jasmonic acid related genes and inhibiting senescence genes | Bmc Plant Biology | 2020 | 5.3 | 63 | 15.75 |
| 9 | Water-deficit accumulates sugars by starch degradation-not by de novo synthesis-in white clover leaves (Trifolium repens) | Physiologia Plantarum | 2008 | 6.4 | 61 | 3.81 |
| 10 | Stress-induced memory alters growth of clonal offspring of white clover (Trifolium repens) | American Journal of Botany | 2016 | 3 | 58 | 7.25 |
| Keyword Plus | Cluster | Occurrences | Links | Total Connection Strength |
|---|---|---|---|---|
| drought stress | 1 | 239 | 82 | 773 |
| white clover | 2 | 205 | 87 | 599 |
| growth | 2 | 115 | 68 | 376 |
| yield | 2 | 110 | 68 | 333 |
| nitrogen | 2 | 43 | 40 | 142 |
| responses | 3 | 32 | 35 | 114 |
| elevated CO2 | 4 | 26 | 24 | 85 |
| soil | 4 | 23 | 29 | 89 |
| leaves | 1 | 22 | 31 | 82 |
| photosynthesis | 4 | 21 | 30 | 84 |
| salinity | 1 | 21 | 38 | 93 |
| abscisic acid | 1 | 20 | 34 | 96 |
| fungi | 5 | 20 | 18 | 67 |
| hydrogen-peroxide | 1 | 20 | 31 | 95 |
| Arabidopsis | 1 | 19 | 27 | 76 |
| root | 5 | 16 | 20 | 54 |
| lipid-peroxidation | 1 | 15 | 27 | 73 |
| osmotic adjustment | 1 | 15 | 28 | 69 |
| leaf senescence | 1 | 14 | 26 | 61 |
| proline | 1 | 14 | 31 | 61 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Deng, X.; Wang, X.; Yang, Y.; Li, J.; Gao, Y.; Huang, H.; Zhang, Y.; Du, J.; Wang, P. Global Research Trends and Hotspots in White Clover (Trifolium repens L.) Responses to Drought Stress (1990–2024). Sustainability 2025, 17, 1883. https://doi.org/10.3390/su17051883
Deng X, Wang X, Yang Y, Li J, Gao Y, Huang H, Zhang Y, Du J, Wang P. Global Research Trends and Hotspots in White Clover (Trifolium repens L.) Responses to Drought Stress (1990–2024). Sustainability. 2025; 17(5):1883. https://doi.org/10.3390/su17051883
Chicago/Turabian StyleDeng, Xiaolin, Xiangtao Wang, Yuting Yang, Junqin Li, Yang Gao, Haiyan Huang, Yu Zhang, Jing Du, and Puchang Wang. 2025. "Global Research Trends and Hotspots in White Clover (Trifolium repens L.) Responses to Drought Stress (1990–2024)" Sustainability 17, no. 5: 1883. https://doi.org/10.3390/su17051883
APA StyleDeng, X., Wang, X., Yang, Y., Li, J., Gao, Y., Huang, H., Zhang, Y., Du, J., & Wang, P. (2025). Global Research Trends and Hotspots in White Clover (Trifolium repens L.) Responses to Drought Stress (1990–2024). Sustainability, 17(5), 1883. https://doi.org/10.3390/su17051883






