Identifying AMF-Rich Tir Wheat Rhizospheres to Foster Microbial Inoculants Useful in Sustainable Agriculture: Evidence from the Van Lake Basin
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Soil and Host Plant Root Sampling
2.3. Determination of Soil Physicochemical Parameters
2.3.1. Electrical Conductivity and pH
2.3.2. Available Phosphorus
2.3.3. Soil Structure
2.3.4. Organic Matter Contents
2.4. Determination of AMF Spore Numbers
2.5. Measurement of Mycorrhizal Colonization Indices in Host Plant Roots
2.6. Statistical Analyses
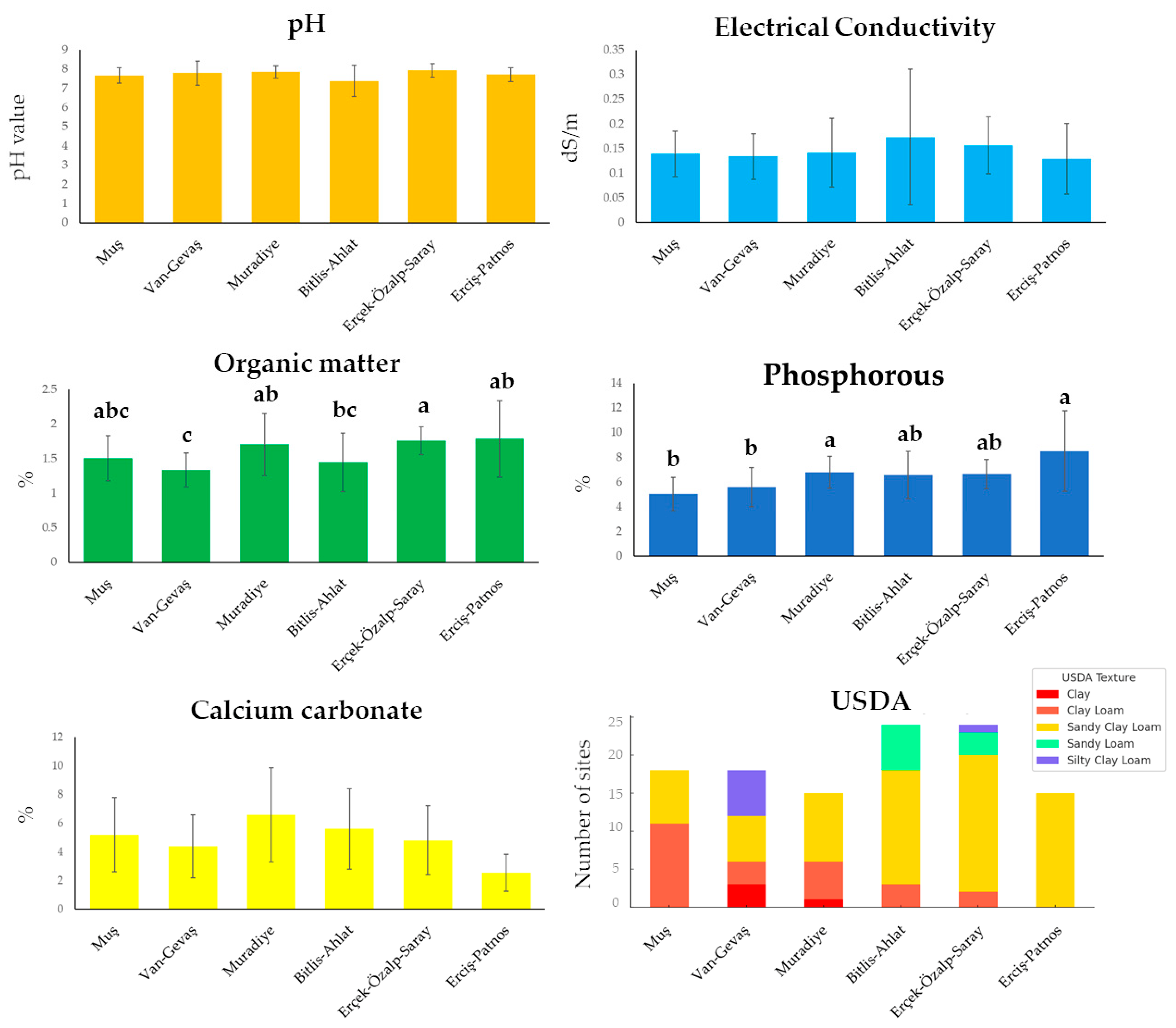
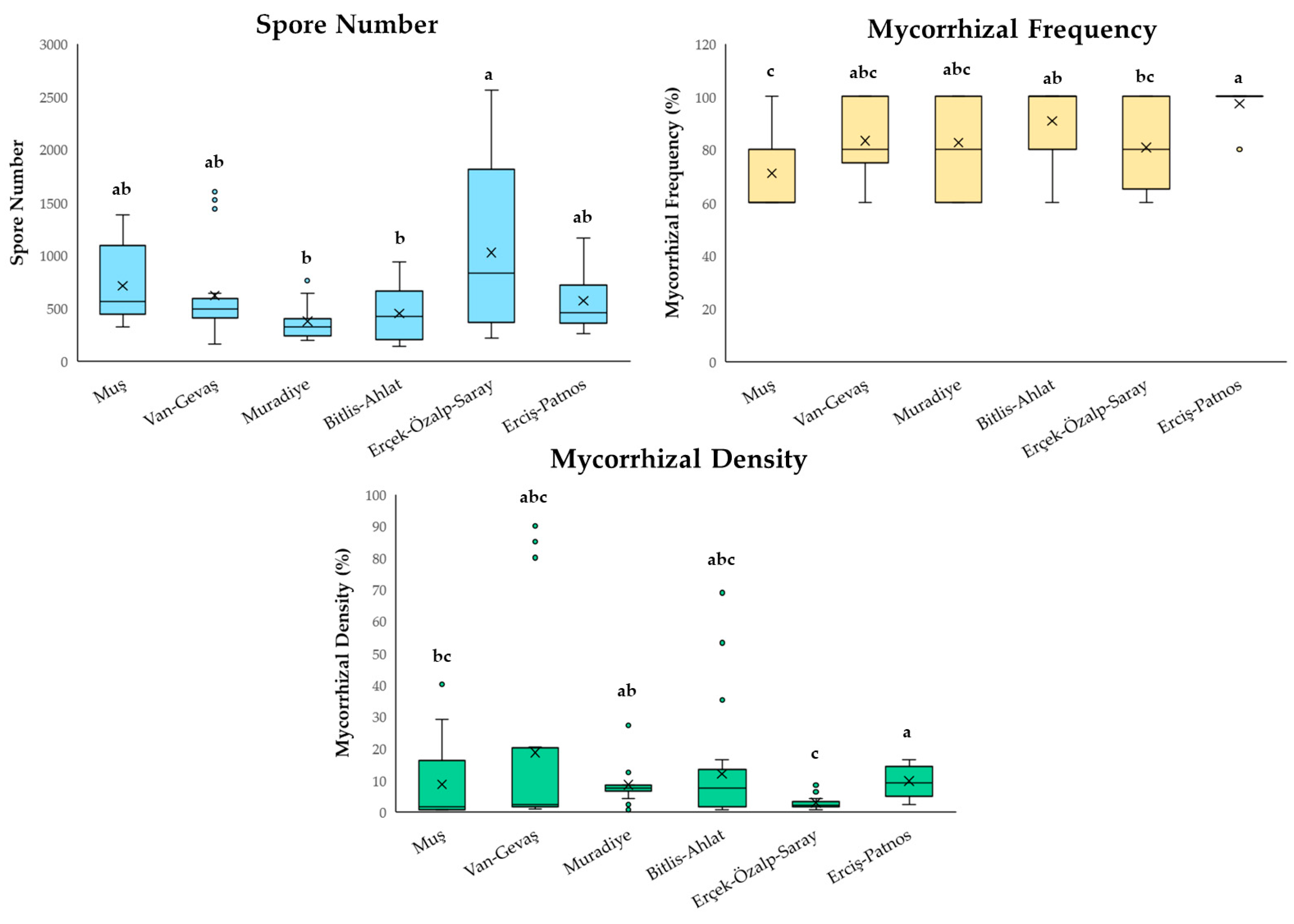
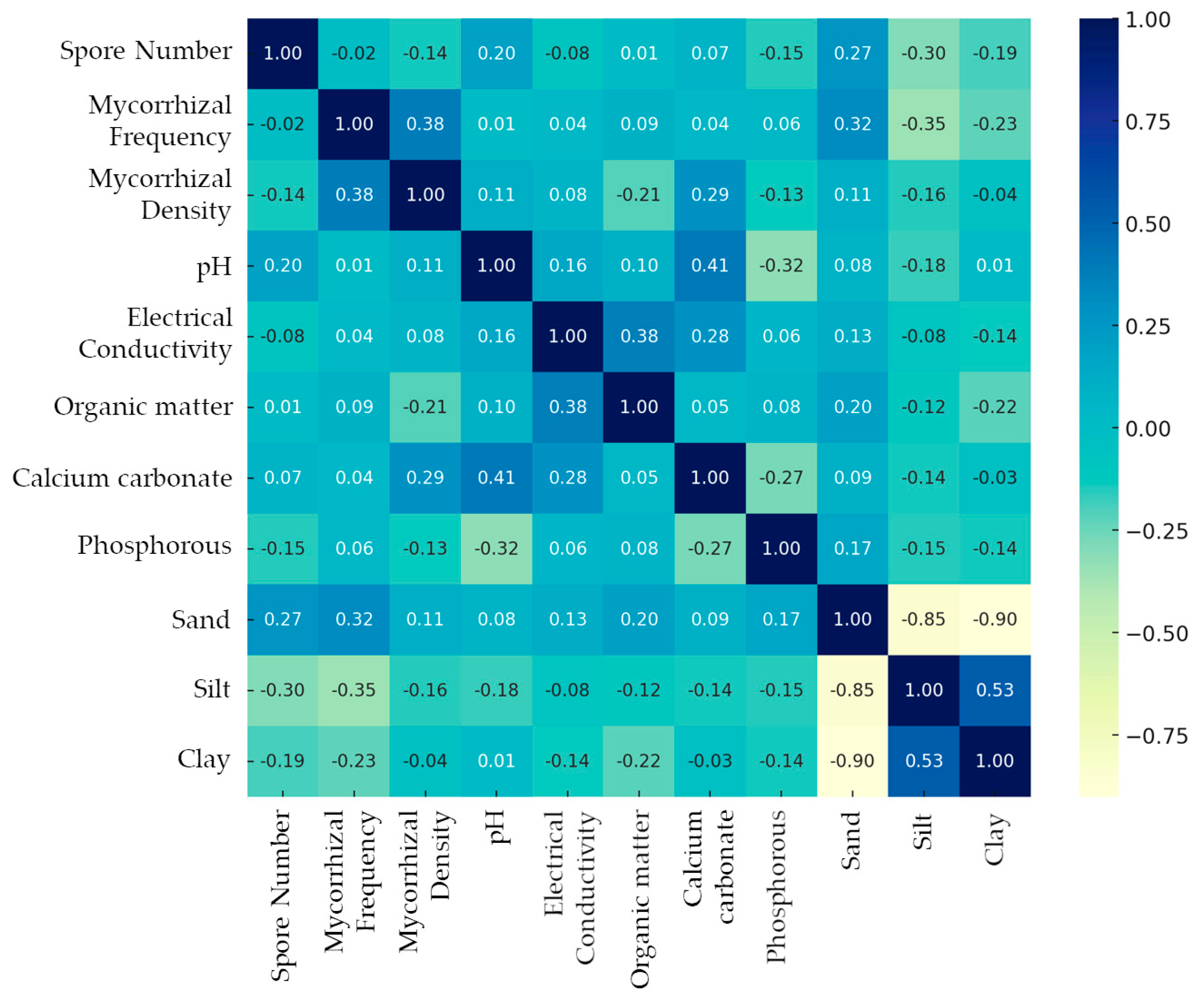
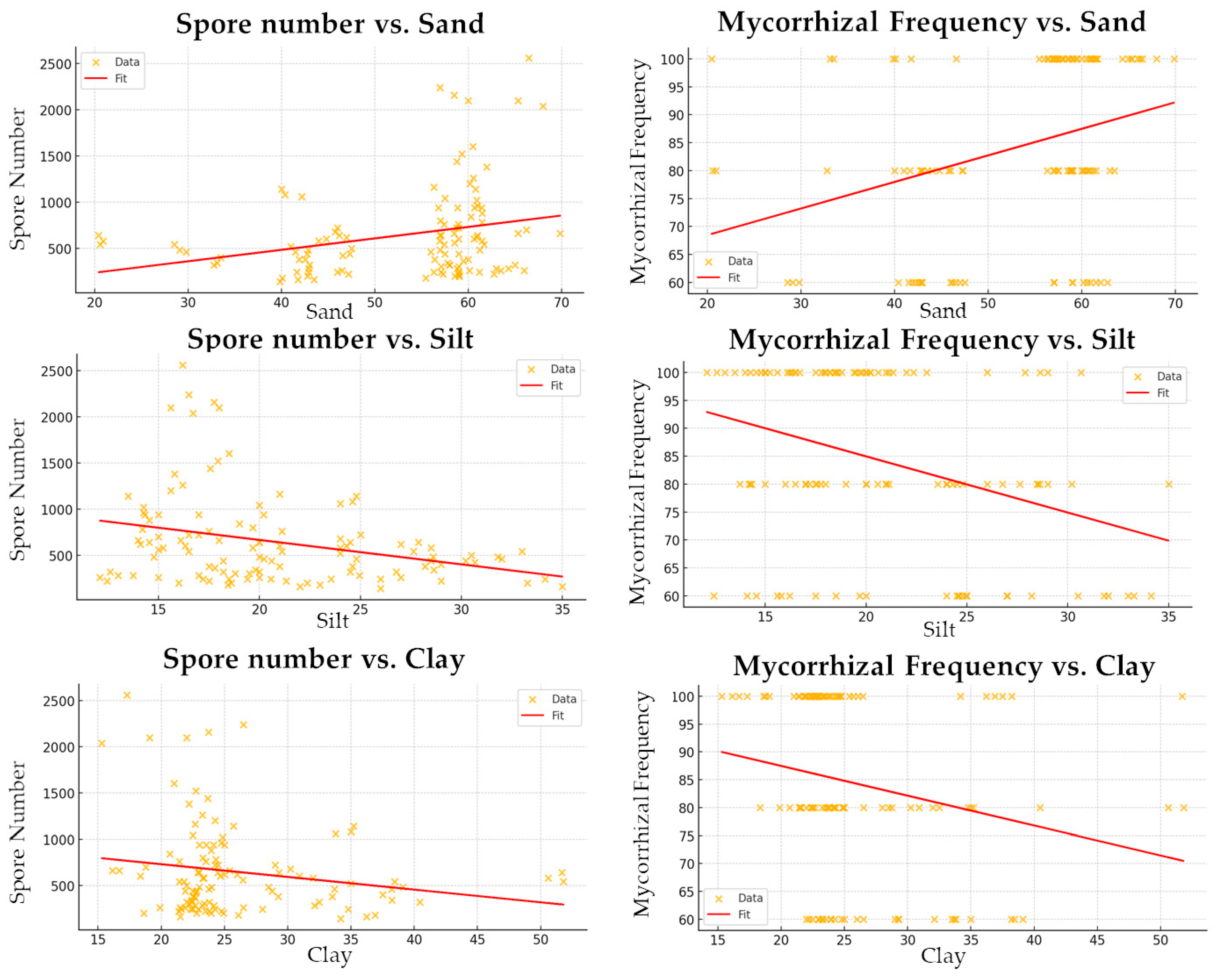
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lal, R. Food Security in a Changing Climate. Ecohydrol. Hydrobiol. 2013, 13, 8–21. [Google Scholar] [CrossRef]
- Flors, V.; Kyndt, T.; Mauch-Mani, B.; Pozo, M.J.; Ryu, C.-M.; Ton, J. Enabling Sustainable Crop Protection with Induced Resistance in Plants. Front. Sci. 2024, 2, 1407410. [Google Scholar] [CrossRef]
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops That Feed the World 10. Past Successes and Future Challenges to the Role Played by Wheat in Global Food Security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- Chan, C.Y.; Tran, N.; Pethiyagoda, S.; Crissman, C.C.; Sulser, T.B.; Phillips, M.J. Prospects and Challenges of Fish for Food Security in Africa. Glob. Food Secur. 2019, 20, 17–25. [Google Scholar] [CrossRef]
- Lohani, N.; Singh, M.B.; Bhalla, P.L. Biological Parts for Engineering Abiotic Stress Tolerance in Plants. BioDesign Res. 2022, 2022, 9819314. [Google Scholar] [CrossRef] [PubMed]
- Yesilsoy, M.S.; Ersahin, S. Soil, Water and Crop Management Practices in the Dryland Farming Regions of Turkey. Am. J. Altern. Agric. 1997, 12, 130–132. [Google Scholar] [CrossRef]
- Aktas-Akyildiz, E.; Masatcioglu, M.T.; Köksel, H. Effect of Extrusion Treatment on Enzymatic Hydrolysis of Wheat Bran. J. Cereal Sci. 2020, 93, 102941. [Google Scholar] [CrossRef]
- Kashiwagi, J.; Krishnamurthy, L.; Purushothaman, R.; Upadhyaya, H.D.; Gaur, P.M.; Gowda, C.L.L.; Ito, O.; Varshney, R.K. Scope for Improvement of Yield under Drought through the Root Traits in Chickpea (Cicer arietinum L.). Field Crops Res. 2015, 170, 47–54. [Google Scholar] [CrossRef]
- Cairns, J.E.; Prasanna, B. Developing and Deploying Climate-Resilient Maize Varieties in the Developing World. Curr. Opin. Plant Biol. 2018, 45, 226–230. [Google Scholar] [CrossRef] [PubMed]
- Delaeter, M.; Magnin-Robert, M.; Randoux, B.; Lounès-Hadj Sahraoui, A. Arbuscular Mycorrhizal Fungi as Biostimulant and Biocontrol Agents: A Review. Microorganisms 2024, 12, 1281. [Google Scholar] [CrossRef] [PubMed]
- Brar, B.; Bala, K.; Saharan, B.S.; Sadh, P.K.; Duhan, J.S. Bio-Boosting Agriculture: Harnessing the Potential of Fungi-Bacteria-Plant Synergies for Crop Improvement. Discov. Plants 2024, 1, 21. [Google Scholar] [CrossRef]
- Rosling, A.; Eshghi Sahraei, S.; Kalsoom Khan, F.; Desirò, A.; Bryson, A.E.; Mondo, S.J.; Grigoriev, I.V.; Bonito, G.; Sánchez-García, M. Evolutionary History of Arbuscular Mycorrhizal Fungi and Genomic Signatures of Obligate Symbiosis. BMC Genom. 2024, 25, 529. [Google Scholar] [CrossRef] [PubMed]
- Strullu-Derrien, C.; Strullu, D.-G. Mycorrhization of Fossil and Living Plants. Comptes Rendus Palevol 2007, 6, 483–494. [Google Scholar] [CrossRef]
- Ortaş, I.; Varma, A. Field trials of bioinoculants. In Advanced Techniques in Soil Microbiology; Springer: Berlin/Heidelberg, Germany, 2007; pp. 397–413. [Google Scholar]
- Martin, F.M.; van der Heijden, M.G.A. The Mycorrhizal Symbiosis: Research Frontiers in Genomics, Ecology, and Agricultural Application. New Phytol. 2024, 242, 1486–1506. [Google Scholar] [CrossRef] [PubMed]
- Wahab, A.; Muhammad, M.; Ullah, S.; Abdi, G.; Shah, G.M.; Zaman, W.; Ayaz, A. Agriculture and Environmental Management through Nanotechnology: Eco-Friendly Nanomaterial Synthesis for Soil-Plant Systems, Food Safety, and Sustainability. Sci. Total Environ. 2024, 926, 171862. [Google Scholar] [CrossRef]
- Guo, S.; Xia, L.; Xia, D.; Li, M.; Xu, W.; Liu, L. Enhancing Plant Resilience: Arbuscular Mycorrhizal Fungi’s Role in Alleviating Drought Stress in Vegetation Concrete. Front. Plant Sci. 2024, 15, 1401050. [Google Scholar] [CrossRef] [PubMed]
- Selvakumar, G.; Krishnamoorthy, R.; Kim, K.; Sa, T. Propagation Technique of Arbuscular Mycorrhizal Fungi Isolated from Coastal Reclamation Land. Eur. J. Soil. Biol. 2016, 74, 39–44. [Google Scholar] [CrossRef]
- Olgun, M.; Yildirim, T.; Turan, M. Adaptation of Wheat Genotypes (Triticum aestivum L.) to Cold Climate. Acta Agric. Scand. B Soil. Plant Sci. 2005, 55, 9–15. [Google Scholar] [CrossRef]
- Ortas, I. Comparative Analyses of Turkey Agricultural Soils: Potential Communities of Indigenous and Exotic Mycorrhiza Species’ Effect on Maize (Zea mays L.) Growth and Nutrient Uptakes. Eur. J. Soil. Biol. 2015, 69, 79–87. [Google Scholar] [CrossRef]
- Kaya, A.; Uzun, Y.; Karacan, İ. Macrofungi of Göksun (Kahramanmaraş) District. Turk. J. Bot. 2009, 33, 8. [Google Scholar] [CrossRef]
- Yücel, C.; Özkan, H.; Ortaş, İ.; Yağbasanlar, T. Screening of Wild Emmer Wheat Accessions (Triticum turgidum Subsp. Dicoccoides) for Mycorrhizal Dependency. Turk. J. Agric. For. 2009, 33, 513–523. [Google Scholar] [CrossRef]
- Yağmur, A.; Demir, S.; Canpolat, S.; Rezaee Danesh, Y.; Farda, B.; Djebaili, R.; Pace, L.; Pellegrini, M. Onion Fusarium Basal Rot Disease Control by Arbuscular Mycorrhizal Fungi and Trichoderma harzianum. Plants 2024, 13, 386. [Google Scholar] [CrossRef] [PubMed]
- Demir, S.; Şensoy, S.; Ocak, E.; Tüfenkçi, Ş.; Demirer Durak, E.; Erdinç, Ç.; Ünsal, H. Effects of Arbuscular Mycorrhizal Fungus, Humic Acid, and Whey on Wilt Diseasecaused by Verticillium Dahliae Kleb. in Three Solanaceous Crops. Turk. J. Agric. For. 2015, 39, 300–309. [Google Scholar] [CrossRef]
- Demir, S.; Durak, E.D.; Güneş, H.; Boyno, G.; Mulet, J.M.; Rezaee Danesh, Y.; Porcel, R. Biological Control of Three Fungal Diseases in Strawberry (Fragaria × Ananassa) with Arbuscular Mycorrhizal Fungi. Agronomy 2023, 13, 2439. [Google Scholar] [CrossRef]
- Boyno, G.; Demir, S.; Danesh, Y.R. Effects of Some Biological Agents on the Growth and Biochemical Parameters of Tomato Plants Infected with Alternaria solani (Ellis & Martin) Sorauer. Eur. J. Plant Pathol. 2022, 162, 19–29. [Google Scholar]
- Boyno, G.; Rezaee Danesh, Y.; Demir, S.; Teniz, N.; Mulet, J.M.; Porcel, R. The Complex Interplay between Arbuscular Mycorrhizal Fungi and Strigolactone: Mechanisms, Sinergies, Applications and Future Directions. Int. J. Mol. Sci. 2023, 24, 16774. [Google Scholar] [CrossRef]
- Demir, S.; Boyno, G.; Rezaee Danesh, Y.; Teniz, N.; Calayır, O.; Çevik, R.; Farda, B.; Sabbi, E.; Djebaili, R.; Ercole, C.; et al. Preliminary Insights into Sustainable Control of Solanum Lycopersicum Early Blight: Harnessing Arbuscular Mycorrhizal Fungi and Reducing Fungicide Dose. Agronomy 2024, 14, 2521. [Google Scholar] [CrossRef]
- Jackson, M. Soil Chemical Analysis Prentice Hall; Cliffs, N.J., Ed.; Prentice Hall Inc.: Englewood, NJ, USA, 1958. [Google Scholar]
- Bayraktar, H.; Dolar, F.S. Molecular Identification and Genetic Diversity of Fusarium Species Associated with Onion Fields in Turkey. J. Phytopathol. 2011, 159, 28–34. [Google Scholar] [CrossRef]
- Olsen, S.R.; Sommers, L.E. Phosphorus. In Methods of Soil Analysis Part 2 Chemical and Microbiological Properties; Page, A.L., Ed.; American Society of Agronomy, Soil Science Society of America: Madison, WI, USA, 1982; pp. 403–430. [Google Scholar]
- Bouyoucos, G.J. A Recalibration of the Hydrometer Method for Making Mechanical Analysis of Soils. Agron. J. 1951, 43, 434–438. [Google Scholar] [CrossRef]
- Hızalan, E.; Ünal, E. Topraklarda Önemli Analizler. Ank. Üniversitesi Ziraat Fakültesi Yayınları 1966, 278, 5–7. [Google Scholar]
- Boyno, G.; Demir, S.; Rezaee Danesh, Y.; Durak, E.D.; Çevik, R.; Farda, B.; Djebaili, R.; Pellegrini, M. A New Technique for the Extraction of Arbuscular Mycorrhizae Fungal Spores from Rhizosphere. J. Fungi 2023, 9, 845. [Google Scholar] [CrossRef] [PubMed]
- Phillips, J.M.; Hayman, D.S. Improved Procedures for Clearing Roots and Staining Parasitic and Vesicular-Arbuscular Mycorrhizal Fungi for Rapid Assessment of Infection. Trans. Br. Mycol. Soc. 1970, 55, 158–161, IN16–IN18. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure du Taux de Mycorhization VA d’un Système Radiculaire Recherche de Methods D’estimation Ayant une Signification Fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA Publications: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Tianyi, X.; Hongya, W. Application of Lake (Reservoir) Sediment Analysis in Soil Erosion Research. Prog. Geogr. 2018, 37, 890–900. [Google Scholar] [CrossRef]
- Karaca, S.; Gülser, F.; Selçuk, R. Relationships between Soil Properties, Topography and Land Use in the Van Lake Basin, Turkey. Eurasian J. Soil Sci. (EJSS) 2018, 7, 115–120. [Google Scholar] [CrossRef]
- Pittarello, M.; Dal Ferro, N.; Chiarini, F.; Morari, F.; Carletti, P. Influence of Tillage and Crop Rotations in Organic and Conventional Farming Systems on Soil Organic Matter, Bulk Density and Enzymatic Activities in a Short-Term Field Experiment. Agronomy 2021, 11, 724. [Google Scholar] [CrossRef]
- Rahman, M.A.; Didenko, N.O.; Sundermeier, A.P.; Islam, K.R. Agricultural Management Systems Impact on Soil Phosphorous Partition and Stratification. Water Air Soil. Pollut. 2021, 232, 248. [Google Scholar] [CrossRef]
- Phogat, V.; Tomar, V.; Dahiya, R.I.T.A. Soil physical properties. In Science: An Introduction; Rattan, R.K., Katyal, J.C., Dwivedi, B.S., Sarkar, A.K., Tapan, B., Tarafdar, J.C., Kukal, S.S., Eds.; Indian Society of Soil Science: Delhi, India, 2015; pp. 135–171. [Google Scholar]
- Dambrine, É. Soil Acidity and Acidification. In Soils as a Key Component of the Critical Zone 5; Wiley: Hoboken, NJ, USA, 2018; pp. 83–95. [Google Scholar]
- Barrow, N.J.; Hartemink, A.E. The Effects of PH on Nutrient Availability Depend on Both Soils and Plants. Plant Soil. 2023, 487, 21–37. [Google Scholar] [CrossRef]
- Povar, I.; Spinu, O.; Visnevschi, A. Quantifying the Buffering Action of Soil Minerals as Natural Defenders. Earth Syst. Environ. 2024, 1–16. [Google Scholar] [CrossRef]
- Akter, S.; Kamruzzaman, M.d.; Sarder, M.d.P.; Amin, M.d.S.; Joardar, J.C.; Islam, M.d.S.; Nasrin, S.; Islam, M.U.; Islam, F.; Rabbi, S.; et al. Mycorrhizal Fungi Increase Plant Nutrient Uptake, Aggregate Stability and Microbial Biomass in the Clay Soil. Symbiosis 2024, 93, 163–176. [Google Scholar] [CrossRef]
- Sivakumar, N. Effect of Edaphic Factors and Seasonal Variation on Spore Density and Root Colonization of Arbuscular Mycorrhizal Fungi in Sugarcane Fields. Ann. Microbiol. 2013, 63, 151–160. [Google Scholar] [CrossRef]
- Hamilton, R.L.; Trimmer, M.; Bradley, C.; Pinay, G. Deforestation for Oil Palm Alters the Fundamental Balance of the Soil N Cycle. Soil. Biol. Biochem. 2016, 95, 223–232. [Google Scholar] [CrossRef]
- Ericson, L.; Müller, W.J.; Burdon, J.J. 28-year Temporal Sequence of Epidemic Dynamics in a Natural Rust–Host Plant Metapopulation. J. Ecol. 2017, 105, 701–713. [Google Scholar] [CrossRef]
- Tamang, B.G.; Magliozzi, J.O.; Maroof, M.A.S.; Fukao, T. Physiological and Transcriptomic Characterization of Submergence and Reoxygenation Responses in Soybean Seedlings. Plant Cell Environ. 2014, 37, 2350–2365. [Google Scholar] [CrossRef] [PubMed]
- Qian, X.; Gu, J.; Sun, W.; Li, Y.-D.; Fu, Q.-X.; Wang, X.-J.; Gao, H. Changes in the Soil Nutrient Levels, Enzyme Activities, Microbial Community Function, and Structure during Apple Orchard Maturation. Appl. Soil Ecol. 2014, 77, 18–25. [Google Scholar] [CrossRef]
- Basiru, S.; Hijri, M. The Potential Applications of Commercial Arbuscular Mycorrhizal Fungal Inoculants and Their Ecological Consequences. Microorganisms 2022, 10, 1897. [Google Scholar] [CrossRef]
- Gryndler, M.; Hršelová, H.; Cajthaml, T.; Havránková, M.; Řezáčová, V.; Gryndlerová, H.; Larsen, J. Influence of Soil Organic Matter Decomposition on Arbuscular Mycorrhizal Fungi in Terms of Asymbiotic Hyphal Growth and Root Colonization. Mycorrhiza 2009, 19, 255–266. [Google Scholar] [CrossRef]
- Verzeaux, J.; Nivelle, E.; Roger, D.; Hirel, B.; Dubois, F.; Tetu, T. Spore Density of Arbuscular Mycorrhizal Fungi Is Fostered by Six Years of a No-Till System and Is Correlated with Environmental Parameters in a Silty Loam Soil. Agronomy 2017, 7, 38. [Google Scholar] [CrossRef]
- Oehl, F.; Laczko, E.; Bogenrieder, A.; Stahr, K.; Bösch, R.; van der Heijden, M.; Sieverding, E. Soil Type and Land Use Intensity Determine the Composition of Arbuscular Mycorrhizal Fungal Communities. Soil Biol. Biochem. 2010, 42, 724–738. [Google Scholar] [CrossRef]
- Cuesta, C.; Rodríguez, A.; Centeno, M.L.; Ordás, R.J.; Fernández, B. Caulogenic Induction in Cotyledons of Stone Pine (Pinus Pinea): Relationship between Organogenic Response and Benzyladenine Trends in Selected Families. J. Plant Physiol. 2009, 166, 1162–1171. [Google Scholar] [CrossRef]
- Qiu, L.; Wei, X.; Gao, J.; Zhang, X. Dynamics of Soil Aggregate-Associated Organic Carbon along an Afforestation Chronosequence. Plant Soil. 2015, 391, 237–251. [Google Scholar] [CrossRef]
- Kaur, R.; Callaway, R.M. Inderjit Soils and the Conditional Allelopathic Effects of a Tropical Invader. Soil Biol. Biochem. 2014, 78, 316–325. [Google Scholar] [CrossRef]
- Demurtas, C.E.; Seddaiu, G.; Ledda, L.; Cappai, C.; Doro, L.; Carletti, A.; Roggero, P.P. Replacing Organic with Mineral N Fertilization Does Not Reduce Nitrate Leaching in Double Crop Forage Systems under Mediterranean Conditions. Agric. Ecosyst. Environ. 2016, 219, 83–92. [Google Scholar] [CrossRef]
- Islam, M.A.; Sharma, S.S.; Sinha, P.; Negi, M.S.; Neog, B.; Tripathi, S.B. Variability in Capsaicinoid Content in Different Landraces of Capsicum Cultivated in North-Eastern India. Sci. Hortic. 2015, 183, 66–71. [Google Scholar] [CrossRef]
- Pellegrino, E.; Öpik, M.; Bonari, E.; Ercoli, L. Responses of Wheat to Arbuscular Mycorrhizal Fungi: A Meta-Analysis of Field Studies from 1975 to 2013. Soil Biol. Biochem. 2015, 84, 210–217. [Google Scholar] [CrossRef]
- Kalamulla, R.; Karunarathna, S.C.; Tibpromma, S.; Galappaththi, M.C.A.; Suwannarach, N.; Stephenson, S.L.; Asad, S.; Salem, Z.S.; Yapa, N. Arbuscular Mycorrhizal Fungi in Sustainable Agriculture. Sustainability 2022, 14, 12250. [Google Scholar] [CrossRef]
- Futa, B.; Gmitrowicz-Iwan, J.; Skersienė, A.; Šlepetienė, A.; Parašotas, I. Innovative Soil Management Strategies for Sustainable Agriculture. Sustainability 2024, 16, 9481. [Google Scholar] [CrossRef]
- Bach, E.M.; Ramirez, K.S.; Fraser, T.D.; Wall, D.H. Soil Biodiversity Integrates Solutions for a Sustainable Future. Sustainability 2020, 12, 2662. [Google Scholar] [CrossRef]
- Tiwari, P.; Park, K.-I. Advanced Fungal Biotechnologies in Accomplishing Sustainable Development Goals (SDGs): What Do We Know and What Comes Next? J. Fungi 2024, 10, 506. [Google Scholar] [CrossRef]
- Schoen, C.; Montibeler, M.; Costa, M.D.; Antunes, P.M.; Stürmer, S.L. Inter and Intra-Specific Variability in Arbuscular Mycorrhizal Fungi Affects Hosts and Soil Health. Symbiosis 2021, 85, 273–289. [Google Scholar] [CrossRef]
- Ma, X.; Xu, X.; Geng, Q.; Luo, Y.; Ju, C.; Li, Q.; Zhou, Y. Global Arbuscular Mycorrhizal Fungal Diversity and Abundance Decreases with Soil Available Phosphorus. Glob. Ecol. Biogeogr. 2023, 32, 1423–1434. [Google Scholar] [CrossRef]
- Kumar, D.; Kour, S.; Ali, M.; Sharma, R.; Parkirti; Vikram; Changotra, H.; Manhas, R.K.; Ohri, P. Interactions Between Arbuscular Mycorrhizal Fungi and Other Microorganisms in the Rhizosphere and Hyphosphere. In Arbuscular Mycorrhizal Fungi and Higher Plants; Springer: Singapore, 2024; pp. 37–66. [Google Scholar]
- Högy, P.; Kottmann, L.; Schmid, I.; Fangmeier, A. Heat, Wheat and CO2: The Relevance of Timing and the Mode of Temperature Stress on Biomass and Yield. J. Agron. Crop Sci. 2019, 205, 608–615. [Google Scholar] [CrossRef]
- Kozjek, K.; Kundel, D.; Kushwaha, S.K.; Olsson, P.A.; Ahrén, D.; Fliessbach, A.; Birkhofer, K.; Hedlund, K. Long-Term Agricultural Management Impacts Arbuscular Mycorrhizal Fungi More than Short-Term Experimental Drought. Appl. Soil. Ecol. 2021, 168, 104140. [Google Scholar] [CrossRef]
| Cluster | Sites | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 | Ahlat-1 | Erciş-4 | Erçek-1 | Erçek-4 | Muş-6 | Van-24 | Özalp-7 | ||
| 1 | Ahlat-3 | Ahlat-5 | Bitlis 10 | Bitlis-1 | Bitlis-17 | Bitlis-6 | Bitlis-9 | Erciş-10 | Erciş-2 |
| Gevaş-1 | Muradiye-1 | Muradiye-9 | Muş-19 | Patnos-1 | Patnos-3 | Saray-1 | Saray-3 | Özalp-8 | |
| 2 | Muradiye-12 | Muradiye-2 | Muradiye-6 | Muş-12 | Muş-14 | Muş-15 | Muş-3 | Van-17 | Van-21 |
| Van-3 | Van-5 | Özalp-1 | Özalp-2 | ||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Najafi, S.; Ülker, M.; Rezaee Danesh, Y.; Demir, S.; Oral, E.; Altuner, F.; Karaca, S.; Balci, M.; Özdemir, B.; Sargin, B.; et al. Identifying AMF-Rich Tir Wheat Rhizospheres to Foster Microbial Inoculants Useful in Sustainable Agriculture: Evidence from the Van Lake Basin. Sustainability 2025, 17, 1676. https://doi.org/10.3390/su17041676
Najafi S, Ülker M, Rezaee Danesh Y, Demir S, Oral E, Altuner F, Karaca S, Balci M, Özdemir B, Sargin B, et al. Identifying AMF-Rich Tir Wheat Rhizospheres to Foster Microbial Inoculants Useful in Sustainable Agriculture: Evidence from the Van Lake Basin. Sustainability. 2025; 17(4):1676. https://doi.org/10.3390/su17041676
Chicago/Turabian StyleNajafi, Solmaz, Mehmet Ülker, Younes Rezaee Danesh, Semra Demir, Erol Oral, Fevzi Altuner, Siyami Karaca, Meriç Balci, Burak Özdemir, Bulut Sargin, and et al. 2025. "Identifying AMF-Rich Tir Wheat Rhizospheres to Foster Microbial Inoculants Useful in Sustainable Agriculture: Evidence from the Van Lake Basin" Sustainability 17, no. 4: 1676. https://doi.org/10.3390/su17041676
APA StyleNajafi, S., Ülker, M., Rezaee Danesh, Y., Demir, S., Oral, E., Altuner, F., Karaca, S., Balci, M., Özdemir, B., Sargin, B., Dilsiz, A., Sagun, Ç., Selem, E., Salih, S. J., Najafi, M., Farda, B., & Pellegrini, M. (2025). Identifying AMF-Rich Tir Wheat Rhizospheres to Foster Microbial Inoculants Useful in Sustainable Agriculture: Evidence from the Van Lake Basin. Sustainability, 17(4), 1676. https://doi.org/10.3390/su17041676









