Impacts of Fertilizers with Varying Nitrogen Contents on Millet Yield and Rhizosphere Soil Microbial Communities: Implications for Sustainable Agricultural Development
Abstract
1. Introduction
2. Materials and Methods
2.1. DNA Isolation, Library Preparation, and Sequencing
2.2. Bioinformatics Analysis
2.3. Statistical Analysis
3. Results
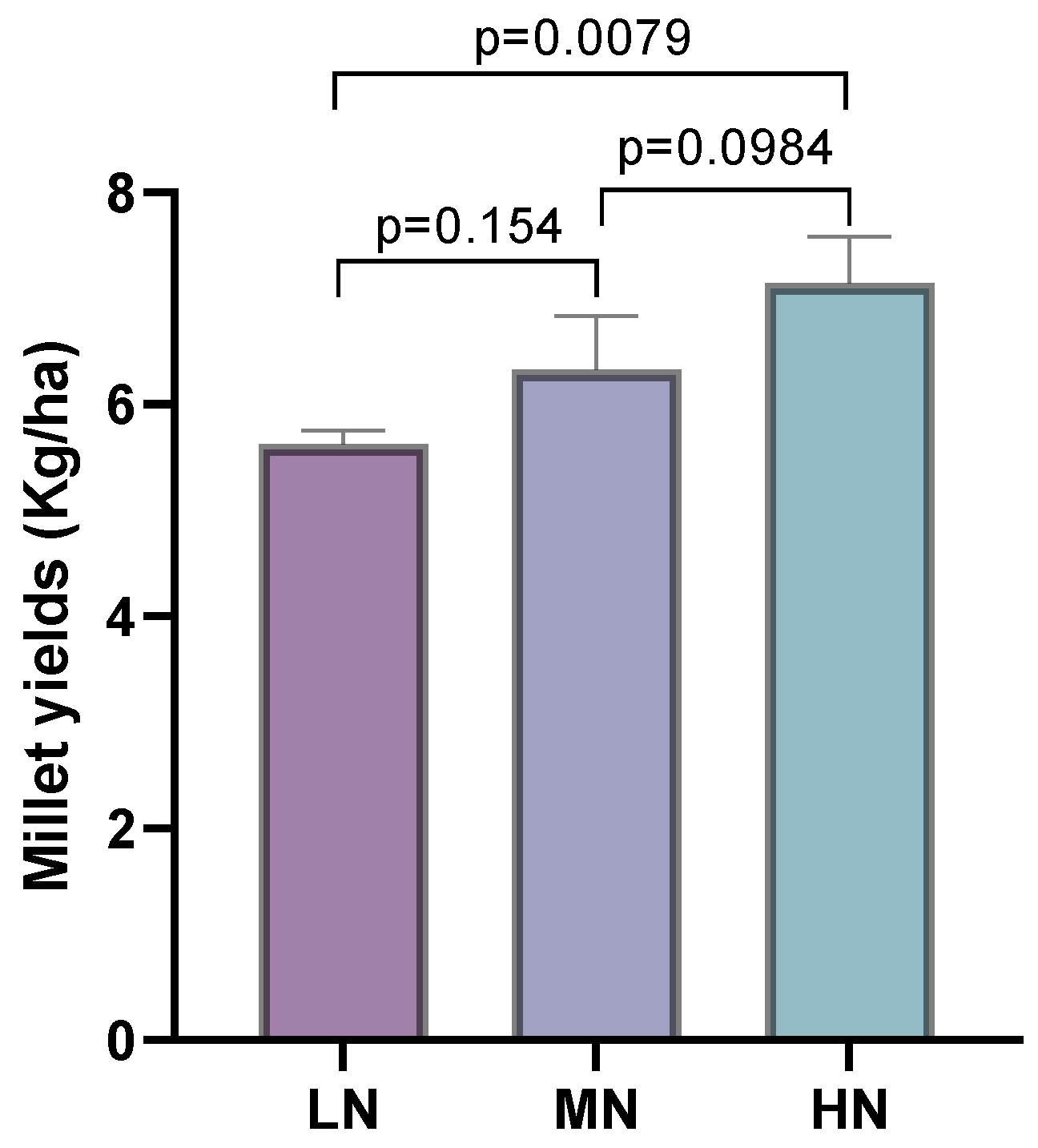
3.1. Effects of Different Fertilization Rates on Millet Yield
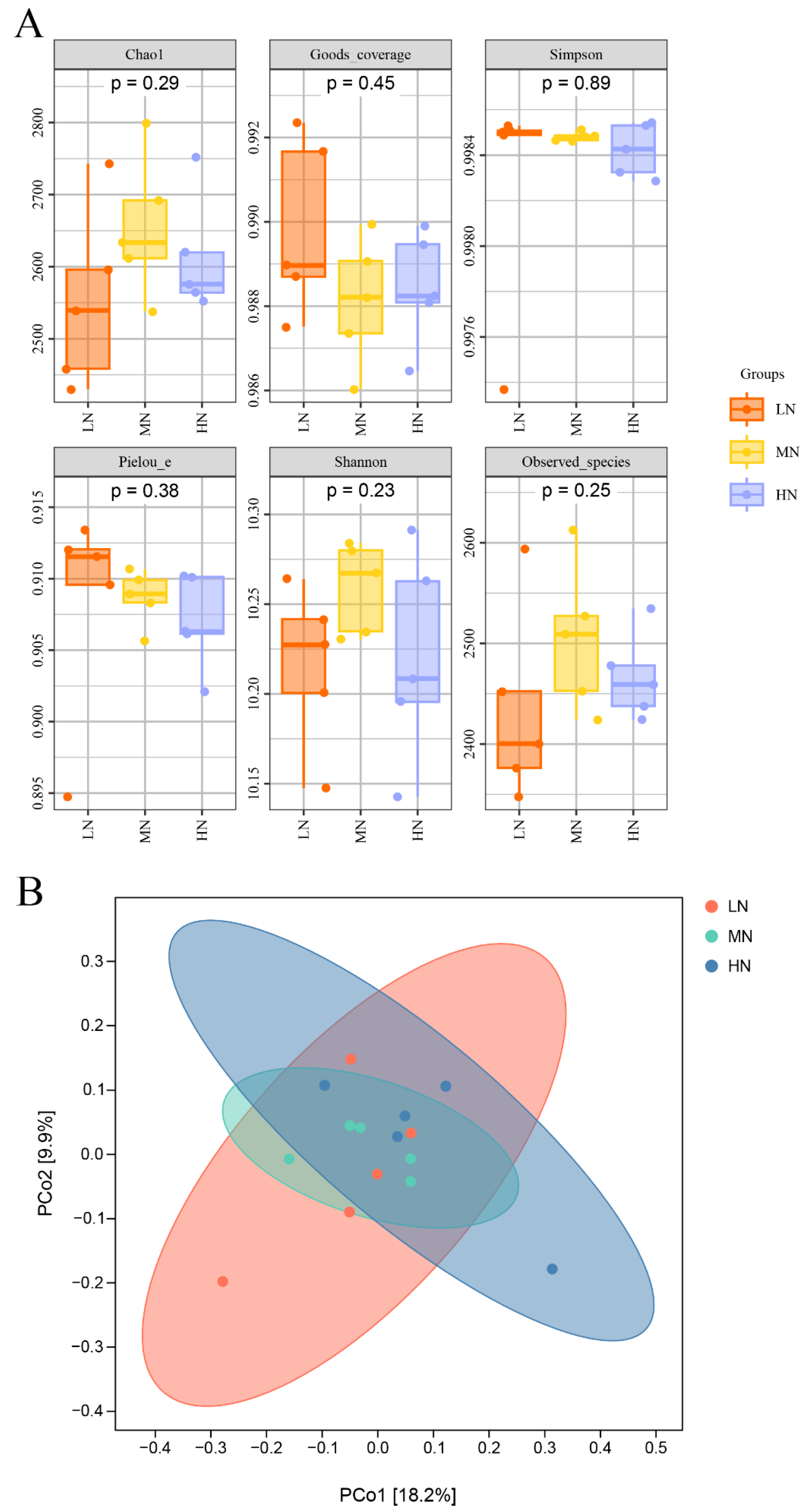
3.2. Bacterial Diversity
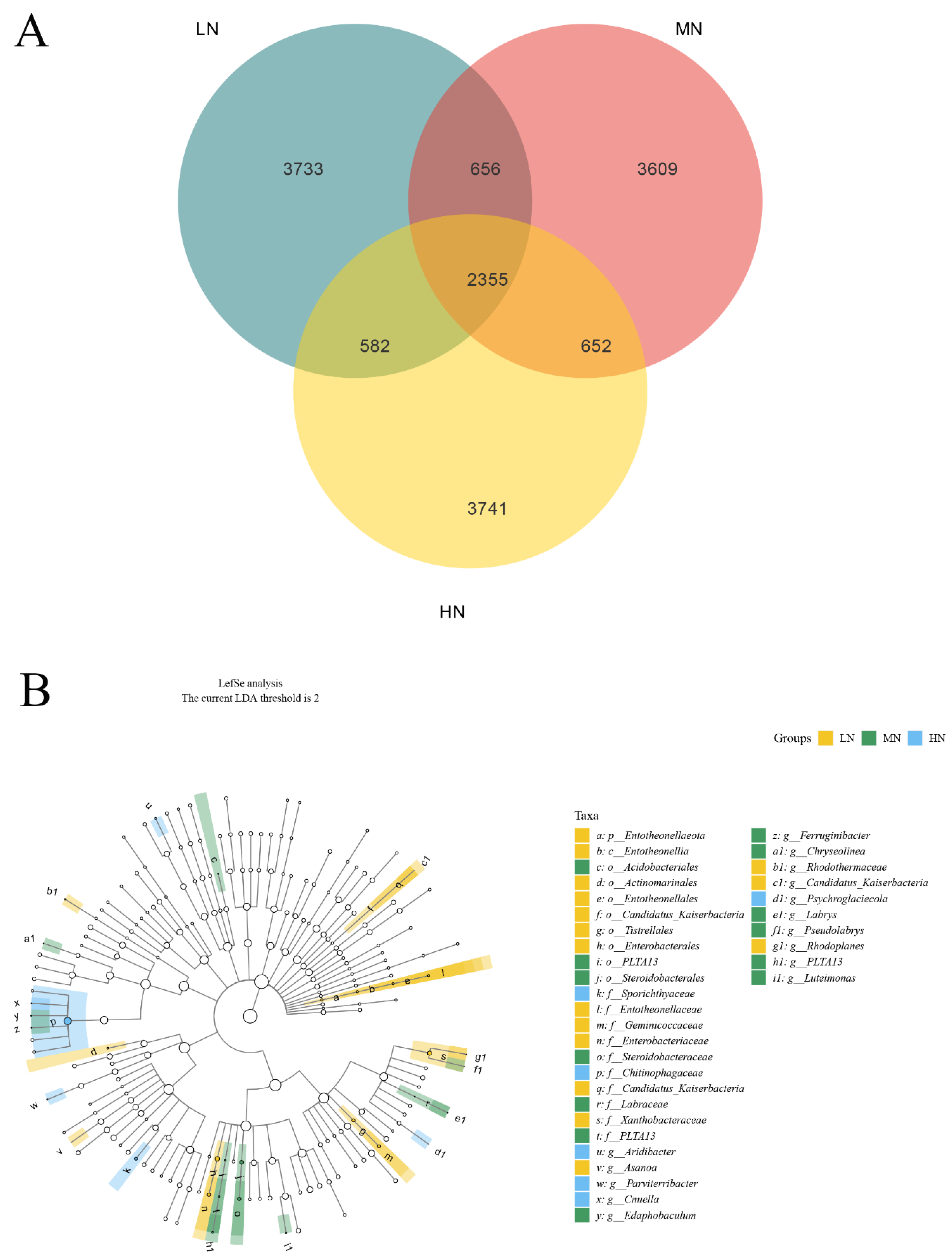
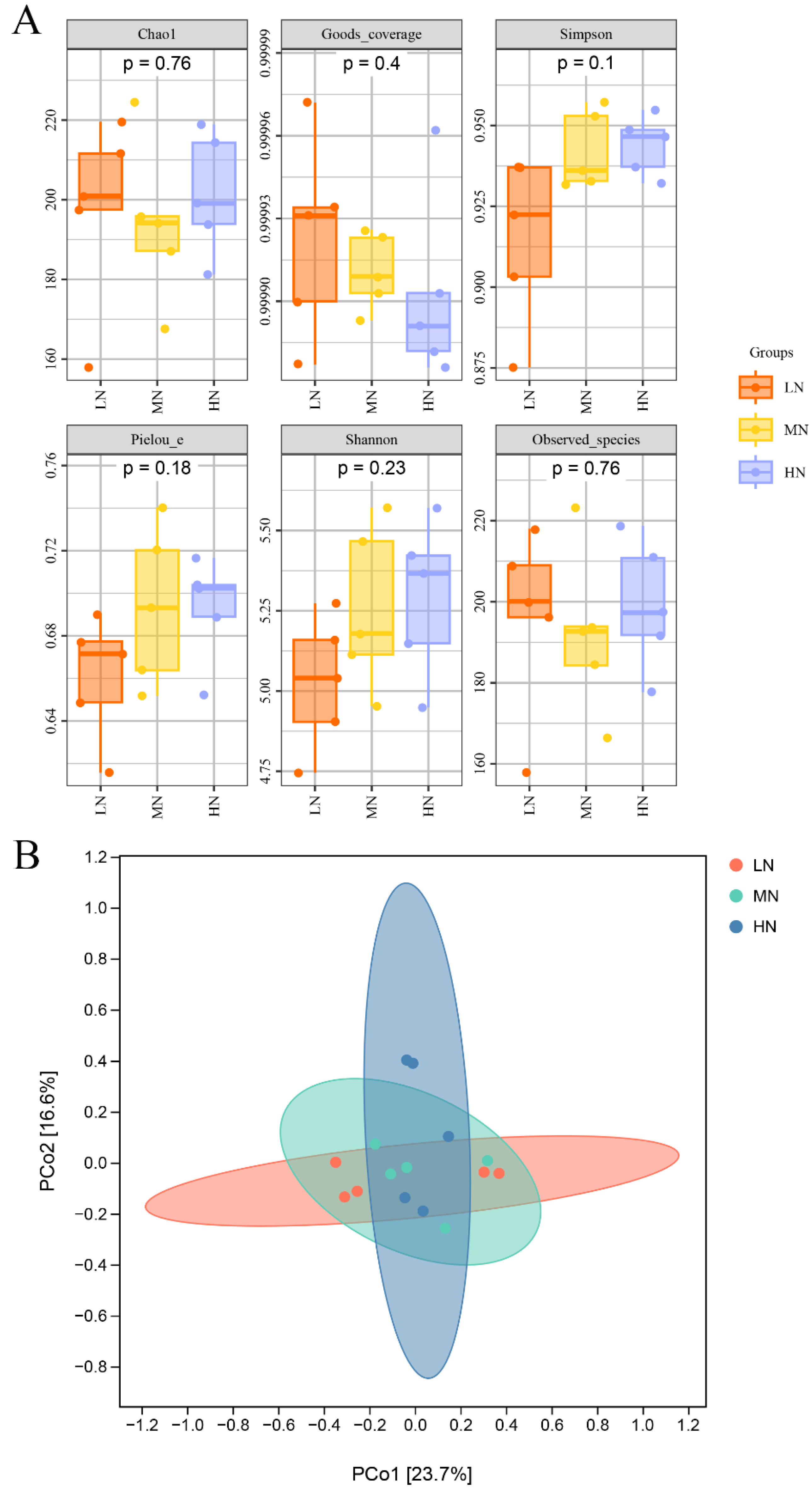
3.3. Bacterial Community Structure
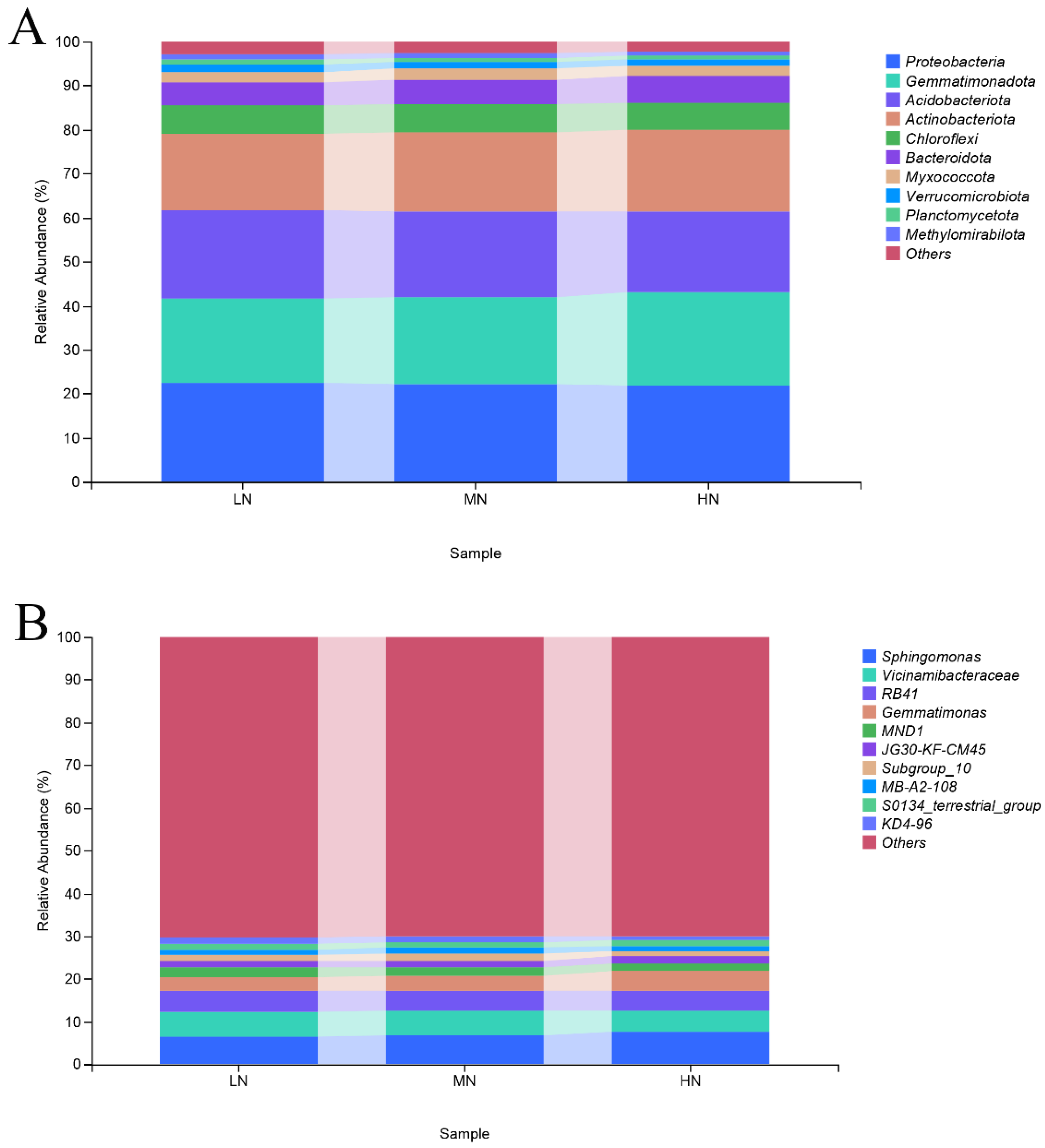
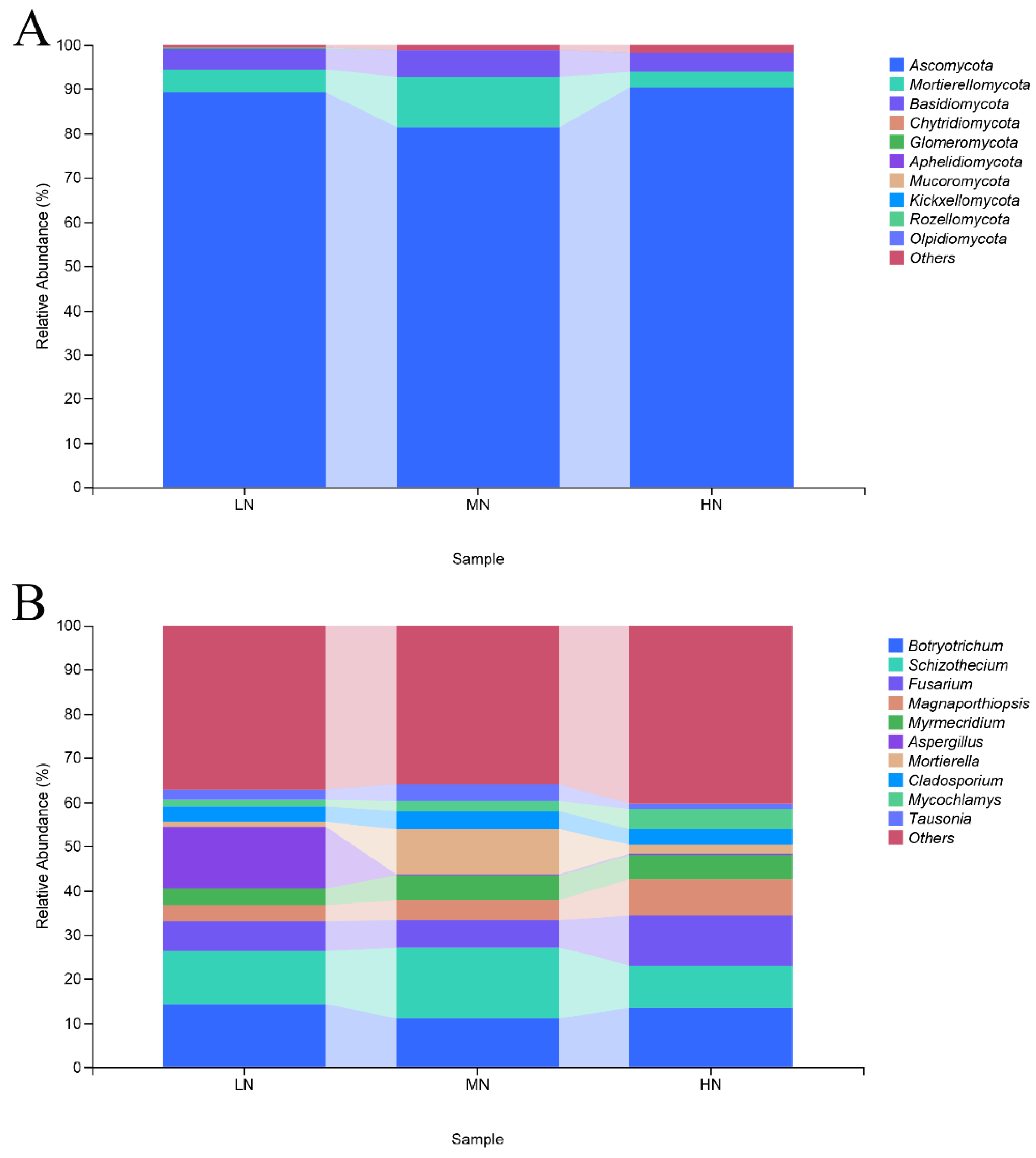
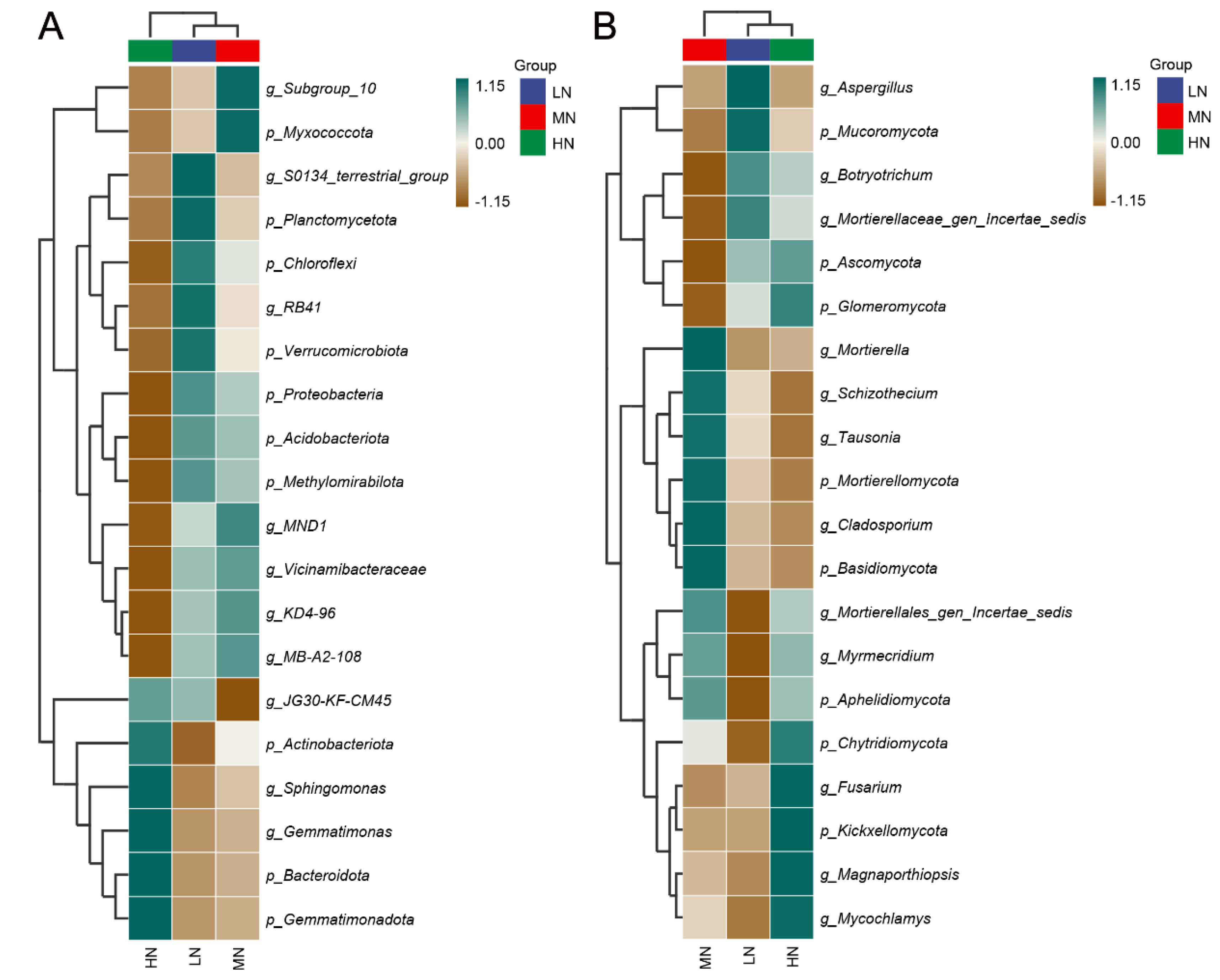
3.4. Taxonomic Composition of Bacteria
3.5. Fungal Diversity
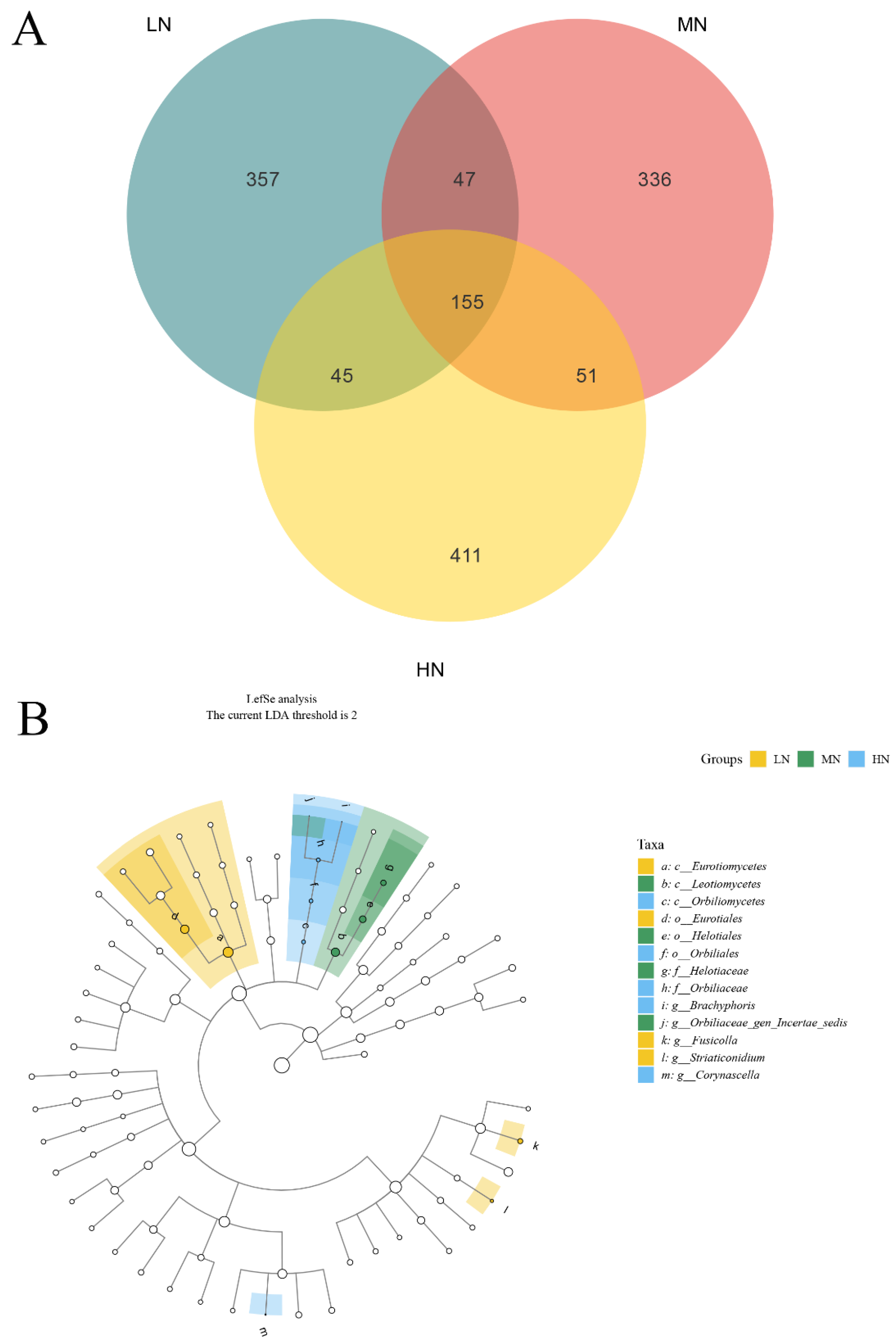
3.6. Fungal Community Structure
3.7. Taxonomic Composition of Fungal
3.8. The Correlation Between Millet Yield and the Composition of Bacterial and Fungal Communities
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yao, X.; Wang, L.; Guo, Y.; Yang, Y.; Wu, F.; Jia, X.; Li, C.; Hao, L. Effects of different fertilization methods on agronomic traits and yield of Zhangzagu 11. Bull. Agric. Sci. Technol. 2017, 161–164. [Google Scholar]
- Amadou, I. Millet Based Functional Foods: Bio-Chemical and Bio-Functional Properties. In Functional Foods; Wiley: Hoboken, NJ, USA, 2022; pp. 303–329. [Google Scholar] [CrossRef]
- Chauhan, M.; Sonawane, S.K.; Arya, S. Nutritional and nutraceutical properties of millets: A review. Clin. J. Nutr. Diet. 2018, 1, 1–10. [Google Scholar]
- Muthamilarasan, M.; Dhaka, A.; Yadav, R.; Prasad, M. Exploration of millet models for developing nutrient rich graminaceous crops. Plant Sci. 2016, 242, 89–97. [Google Scholar] [CrossRef]
- VP, A.A.; Joshi, A.; Mudey, A.; Choudhari, S.; Raut, J.; Ahmed, S. Unlocking the Potential: Millets and Their Impact on Diabetes Management. Cureus 2024, 16, e59283. [Google Scholar] [CrossRef]
- Rotela, S.; Borkar, S.; Borah, A. Health benefits of millets and their significance as functional food: A review. J. Pharma Innov. 2021, 10, 158–162. [Google Scholar] [CrossRef]
- Raut, D.; Sudeepthi, B.; Gawande, K.N.; Reddy, G.; Vamsi, S.; Padhan, S.R.; Panigrahi, C.K. Millet’s Role as a Climate Resilient Staple for Future Food Security: A Review. Int. J. Environ. Clim. Chang. 2023, 13, 4542–4552. [Google Scholar] [CrossRef]
- Arinarayanasamy, T.I.; Premnath, A.; Balakrishnan, N.; Jeyaprakash, P.; Manickam, S.; Chockalingam, V.; Muthurajan, R. Climate resilient millets: Emerging paradigms for the rising paradox. Genet. Resour. Crop Evol. 2024. [Google Scholar] [CrossRef]
- Lu, H.; Zhang, J.; Liu, K.-b.; Wu, N.; Li, Y.; Zhou, K.; Ye, M.; Zhang, T.; Zhang, H.; Yang, X. Earliest domestication of common millet (Panicum miliaceum) in East Asia extended to 10,000 years ago. Proc. Natl. Acad. Sci. USA 2009, 106, 7367–7372. [Google Scholar] [CrossRef] [PubMed]
- Maitra, S. Intercropping of small millets for agricultural sustainability in drylands: A review. Crop Res. 2020, 55, 162–171. [Google Scholar] [CrossRef]
- Sahu, N.; Vasu, D.; Sahu, A.; Lal, N.; Singh, S.K. Strength of Microbes in Nutrient Cycling: A Key to Soil Health. In Agriculturally Important Microbes for Sustainable Agriculture: Volume I: Plant-Soil-Microbe Nexus; Meena, V.S., Mishra, P.K., Bisht, J.K., Pattanayak, A., Eds.; Springer: Singapore, 2017; pp. 69–86. [Google Scholar]
- Van Der Heijden, M.G.; Bardgett, R.D.; Van Straalen, N.M. The unseen majority: Soil microbes as drivers of plant diversity and productivity in terrestrial ecosystems. Ecol. Lett. 2008, 11, 296–310. [Google Scholar] [CrossRef] [PubMed]
- Meena, M.; Swapnil, P.; Zehra, A.; Aamir, M.; Dubey, M.K.; Goutam, J.; Upadhyay, R.S. Beneficial Microbes for Disease Suppression and Plant Growth Promotion. In Plant-Microbe Interactions in Agro-Ecological Perspectives: Volume 2: Microbial Interactions and Agro-Ecological Impacts; Singh, D.P., Singh, H.B., Prabha, R., Eds.; Springer: Singapore, 2017; pp. 395–432. [Google Scholar]
- Nabi, M. Chapter eleven—Role of microorganisms in plant nutrition and soil health. In Sustainable Plant Nutrition; Aftab, T., Hakeem, K.R., Eds.; Academic Press: Cambridge, MA, USA, 2023; pp. 263–282. [Google Scholar]
- Zhang, F.-F.; Gao, S.; Zhao, Y.-Y.; Zhao, X.-L.; Liu, X.-M.; Xiao, K. Growth traits and nitrogen assimilation-associated physiological parameters of wheat (Triticum aestivum L.) under low and high N conditions. J. Integr. Agric. 2015, 14, 1295–1308. [Google Scholar] [CrossRef]
- Zhao, B.; Duan, A.; Ata-Ul-Karim, S.T.; Liu, Z.; Chen, Z.; Gong, Z.; Zhang, J.; Xiao, J.; Liu, Z.; Qin, A.; et al. Exploring new spectral bands and vegetation indices for estimating nitrogen nutrition index of summer maize. Eur. J. Agron. 2018, 93, 113–125. [Google Scholar] [CrossRef]
- Uppal, R.K.; Wani, S.P.; Garg, K.K.; Alagarswamy, G. Balanced nutrition increases yield of pearl millet under drought. Field Crops Res. 2015, 177, 86–97. [Google Scholar] [CrossRef]
- Leghari, S.J.; Wahocho, N.A.; Laghari, G.M.; HafeezLaghari, A.; MustafaBhabhan, G.; HussainTalpur, K.; Bhutto, T.A.; Wahocho, S.A.; Lashari, A.A. Role of nitrogen for plant growth and development: A review. Adv. Environ. Biol. 2016, 10, 209–219. [Google Scholar]
- Pahalvi, H.N.; Rafiya, L.; Rashid, S.; Nisar, B.; Kamili, A.N. Chemical Fertilizers and Their Impact on Soil Health. In Microbiota and Biofertilizers, Vol 2: Ecofriendly Tools for Reclamation of Degraded Soil Environs; Dar, G.H., Bhat, R.A., Mehmood, M.A., Hakeem, K.R., Eds.; Springer International Publishing: Cham, Switzerland, 2021; pp. 1–20. [Google Scholar]
- Ahmed, M.; Rauf, M.; Akhtar, M.; Mukhtar, Z.; Saeed, N.A. Hazards of nitrogen fertilizers and ways to reduce nitrate accumulation in crop plants. Environ. Sci. Pollut. Res. 2020, 27, 17661–17670. [Google Scholar] [CrossRef]
- Lawlor, D.W.; Lemaire, G.; Gastal, F. Nitrogen, Plant Growth and Crop Yield. In Plant Nitrogen; Lea, P.J., Morot-Gaudry, J.-F., Eds.; Springer Berlin Heidelberg: Berlin/Heidelberg, Germany, 2001; pp. 343–367. [Google Scholar]
- Mu, X.; Chen, Y. The physiological response of photosynthesis to nitrogen deficiency. Plant Physiol. Biochem. 2021, 158, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Cordovez, V.; Dini-Andreote, F.; Carrión, V.J.; Raaijmakers, J.M. Ecology and Evolution of Plant Microbiomes. Annu. Rev. Microbiol. 2019, 73, 69–88. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Li, X.; Zhu, A.; Zhang, X.; Zhang, H.; Liang, W. Effects of tillage and residue management on soil microbial communities in North China. Plant Soil Environ. 2012, 58, 28–33. [Google Scholar] [CrossRef]
- Dincă, L.C.; Grenni, P.; Onet, C.; Onet, A. Fertilization and soil microbial community: A review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Mei, N.; Zhang, X.; Wang, X.; Peng, C.; Gao, H.; Zhu, P.; Gu, Y. Effects of 40 years applications of inorganic and organic fertilization on soil bacterial community in a maize agroecosystem in northeast China. Eur. J. Agron. 2021, 130, 126332. [Google Scholar] [CrossRef]
- Ullah, S.; Ai, C.; Ding, W.; Jiang, R.; Zhao, S.; Zhang, J.; Zhou, W.; Hou, Y.; He, P. The response of soil fungal diversity and community composition to long-term fertilization. Appl. Soil Ecol. 2019, 140, 35–41. [Google Scholar] [CrossRef]
- Zhou, J.; Jiang, X.; Zhou, B.; Zhao, B.; Ma, M.; Guan, D.; Li, J.; Chen, S.; Cao, F.; Shen, D. Thirty four years of nitrogen fertilization decreases fungal diversity and alters fungal community composition in black soil in northeast China. Soil Biol. Biochem. 2016, 95, 135–143. [Google Scholar] [CrossRef]
- Li, C.; Yan, K.; Tang, L.; Jia, Z.; Li, Y. Change in deep soil microbial communities due to long-term fertilization. Soil Biol. Biochem. 2014, 75, 264–272. [Google Scholar] [CrossRef]
- Wang, Y.; Zhu, G.; Song, L.; Wang, S.; Yin, C. Manure fertilization alters the population of ammonia-oxidizing bacteria rather than ammonia-oxidizing archaea in a paddy soil. J. Basic Microbiol. 2014, 54, 190–197. [Google Scholar] [CrossRef] [PubMed]
- Gu, Y.; Wang, Y.; Lu, S.; Xiang, Q.; Yu, X.; Zhao, K.; Zou, L.; Chen, Q.; Tu, S.; Zhang, X. Long-term Fertilization Structures Bacterial and Archaeal Communities along Soil Depth Gradient in a Paddy Soil. Front. Microbiol. 2017, 8, 1516. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Meng, J.; Jiang, L.; Yang, X.; Lan, Y.; Cheng, X.; Chen, W. Rice husk biochar impacts soil phosphorous availability, phosphatase activities and bacterial community characteristics in three different soil types. Appl. Soil Ecol. 2017, 116, 12–22. [Google Scholar] [CrossRef]
- DeForest, J.L.; Zak, D.R.; Pregitzer, K.S.; Burton, A.J. Atmospheric nitrate deposition, microbial community composition, and enzyme activity in northern hardwood forests. Soil Sci. Soc. Am. J. 2004, 68, 132–138. [Google Scholar] [CrossRef]
- Ai, C.; Liang, G.; Sun, J.; Wang, X.; Zhou, W. Responses of extracellular enzyme activities and microbial community in both the rhizosphere and bulk soil to long-term fertilization practices in a fluvo-aquic soil. Geoderma 2012, 173, 330–338. [Google Scholar] [CrossRef]
- Caporaso, J.G.; Kuczynski, J.; Stombaugh, J.; Bittinger, K.; Bushman, F.D.; Costello, E.K.; Fierer, N.; Peña, A.G.; Goodrich, J.K.; Gordon, J.I.; et al. QIIME allows analysis of high-throughput community sequencing data. Nat. Methods 2010, 7, 335–336. [Google Scholar] [CrossRef]
- Magoč, T.; Salzberg, S.L. FLASH: Fast length adjustment of short reads to improve genome assemblies. Bioinformatics 2011, 27, 2957–2963. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. Search and clustering orders of magnitude faster than BLAST. Bioinformatics 2010, 26, 2460–2461. [Google Scholar] [CrossRef]
- Abarenkov, K.; Henrik Nilsson, R.; Larsson, K.H.; Alexander, I.J.; Eberhardt, U.; Erland, S.; Høiland, K.; Kjøller, R.; Larsson, E.; Pennanen, T.; et al. The UNITE database for molecular identification of fungi--recent updates and future perspectives. New Phytol. 2010, 186, 281–285. [Google Scholar] [CrossRef] [PubMed]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.K.; Muthamilarasan, M.; Prasad, M. Foxtail Millet: An Introduction. In The Foxtail Millet Genome; Prasad, M., Ed.; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–9. [Google Scholar]
- Fischer, H.W.; Reddy, N.N.; Rao, M.S. Can more drought resistant crops promote more climate secure agriculture? Prospects and challenges of millet cultivation in Ananthapur, Andhra Pradesh. World Dev. Perspect. 2016, 2, 5–10. [Google Scholar] [CrossRef]
- Kathpalia, R.; Bhatla, S.C. Plant Mineral Nutrition. In Plant Physiology, Development and Metabolism; Bhatla, S.C., Lal, M.A., Eds.; Springer Nature: Singapore, 2018; pp. 37–81. [Google Scholar]
- Ludwig, B.; Geisseler, D.; Michel, K.; Joergensen, R.G.; Schulz, E.; Merbach, I.; Raupp, J.; Rauber, R.; Hu, K.; Niu, L. Effects of fertilization and soil management on crop yields and carbon stabilization in soils. A review. Agron. Sustain. Dev. 2011, 31, 361–372. [Google Scholar] [CrossRef]
- Thomas, C.L.; Acquah, G.E.; Whitmore, A.P.; McGrath, S.P.; Haefele, S.M. The effect of different organic fertilizers on yield and soil and crop nutrient concentrations. Agronomy 2019, 9, 776. [Google Scholar] [CrossRef]
- Kumari, G.; Thakur, S.; Kumar, V.; Kumar, N.; Singh, S. Long term effect of fertilizer, farm-yard manure and lime on yield sustainability and soil organic carbon pools under soybean (Glysine max)–wheat (Triticum aestivum) cropping system in Alfisol. J. Soil Water Conserv. 2019, 18, 196–204. [Google Scholar] [CrossRef]
- Qingfeng, M.; Shanchao, Y.; Peng, H.; Zhenling, C.; Xinping, C. Improving yield and nitrogen use efficiency simultaneously for maize and wheat in China: A review. Pedosphere 2016, 26, 137–147. [Google Scholar] [CrossRef]
- Liu, Q.; Xu, H.; Mu, X.; Zhao, G.; Gao, P.; Sun, W. Effects of different fertilization regimes on crop yield and soil water use efficiency of millet and soybean. Sustainability 2020, 12, 4125. [Google Scholar] [CrossRef]
- Zhao, B.; Chen, J.; Zhang, J.; Xin, X.; Hao, X. How different long-term fertilization strategies influence crop yield and soil properties in a maize field in the North China Plain. J. Plant Nutr. Soil Sci. 2013, 176, 99–109. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Srivastava, D.S.; Duffy, J.E.; Wright, J.P.; Downing, A.L.; Sankaran, M.; Jouseau, C. Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 2006, 443, 989–992. [Google Scholar] [CrossRef]
- Wang, J.; Xue, C.; Song, Y.; Wang, L.; Huang, Q.; Shen, Q. Wheat and Rice Growth Stages and Fertilization Regimes Alter Soil Bacterial Community Structure, But Not Diversity. Front. Microbiol. 2016, 7, 1207. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Tu, P.; Yang, Y.; Xue, X.; Feng, Z.; Dan, C.; Cheng, F.; Yang, Y.; Deng, L. Diversity of rice rhizosphere microorganisms under different fertilization modes of slow-release fertilizer. Sci. Rep. 2022, 12, 2694. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Zhang, P.; Riggins, C.W.; Zabaloy, M.C.; Rodríguez-Zas, S.; Villamil, M.B. Long-term N fertilization decreased diversity and altered the composition of soil bacterial and archaeal communities. Agronomy 2019, 9, 574. [Google Scholar] [CrossRef]
- Liu, X.; Meng, M.; Zhang, Y.; Li, C.; Ma, S.; Li, Q.; Ren, Q.; Zhang, Y.; Zhang, J. Effects of sulfuric, nitric, and mixed acid rain on the decomposition of fine root litter in Southern China. Ecol. Process. 2021, 10, 65. [Google Scholar] [CrossRef]
- Shi, Y.; Delgado-Baquerizo, M.; Li, Y.; Yang, Y.; Zhu, Y.G.; Peñuelas, J.; Chu, H. Abundance of kinless hubs within soil microbial networks are associated with high functional potential in agricultural ecosystems. Environ. Int. 2020, 142, 105869. [Google Scholar] [CrossRef]
- Lu, T.; Xu, N.; Lei, C.; Zhang, Q.; Zhang, Z.; Sun, L.; He, F.; Zhou, N.-Y.; Peñuelas, J.; Zhu, Y.-G. Bacterial biogeography in China and its association to land use and soil organic carbon. Soil Ecol. Lett. 2023, 5, 230172. [Google Scholar] [CrossRef]
- Fierer, N.; Lauber, C.L.; Ramirez, K.S.; Zaneveld, J.; Bradford, M.A.; Knight, R. Comparative metagenomic, phylogenetic and physiological analyses of soil microbial communities across nitrogen gradients. ISME J. 2012, 6, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Mujakić, I.; Piwosz, K.; Koblížek, M. Phylum Gemmatimonadota and Its Role in the Environment. Microorganisms 2022, 10, 151. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Shang, S.; Shi, D.; Xu, H.; Wang, J. Composition and structural characteristics of rhizosphere microorganisms of Polygonum sibiricum (Laxm.) Tzvelev in the Yellow River Delta. Diversity 2022, 14, 965. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Y.; Wei, H.; Zhang, J. Nitric Acid Rain Decreases Soil Bacterial Diversity and Alters Bacterial Community Structure in Farmland Soils. Agronomy 2024, 14, 971. [Google Scholar] [CrossRef]
- Li, Y.; Wang, C.; Wang, T.; Liu, Y.; Jia, S.; Gao, Y.; Liu, S. Effects of different fertilizer treatments on rhizosphere soil microbiome composition and functions. Land 2020, 9, 329. [Google Scholar] [CrossRef]
- Ji, L.; Xu, X.; Zhang, F.; Si, H.; Li, L.; Mao, G. The preliminary research on shifts in maize rhizosphere soil microbial communities and symbiotic networks under different fertilizer sources. Agronomy 2023, 13, 2111. [Google Scholar] [CrossRef]
- Boubekri, K.; Soumare, A.; Mardad, I.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. The Screening of Potassium- and Phosphate-Solubilizing Actinobacteria and the Assessment of Their Ability to Promote Wheat Growth Parameters. Microorganisms 2021, 9, 470. [Google Scholar] [CrossRef]
- Soumare, A.; Boubekri, K.; Lyamlouli, K.; Hafidi, M.; Ouhdouch, Y.; Kouisni, L. From Isolation of Phosphate Solubilizing Microbes to Their Formulation and Use as Biofertilizers: Status and Needs. Front. Bioeng. Biotechnol. 2019, 7, 425. [Google Scholar] [CrossRef]
- Dai, Z.; Su, W.; Chen, H.; Barberán, A.; Zhao, H.; Yu, M.; Yu, L.; Brookes, P.C.; Schadt, C.W.; Chang, S.X. Long-term nitrogen fertilization decreases bacterial diversity and favors the growth of Actinobacteria and Proteobacteria in agro-ecosystems across the globe. Glob. Change Biol. 2018, 24, 3452–3461. [Google Scholar] [CrossRef]
- Ullah, S.; He, P.; Ai, C.; Zhao, S.; Ding, W.; Song, D.; Zhang, J.; Huang, S.; Abbas, T.; Zhou, W. How do soil bacterial diversity and community composition respond under recommended and conventional nitrogen fertilization regimes? Microorganisms 2020, 8, 1193. [Google Scholar] [CrossRef]
- Wolna-Maruwka, A.; Piechota, T.; Niewiadomska, A.; Kamiński, A.; Kayzer, D.; Grzyb, A.; Pilarska, A.A. The effect of biochar-based organic amendments on the structure of soil bacterial community and yield of maize (Zea mays L.). Agronomy 2021, 11, 1286. [Google Scholar] [CrossRef]
- Wang, Y.; Xu, X.; Liu, T.; Wang, H.; Yang, Y.; Chen, X.; Zhu, S. Analysis of bacterial and fungal communities in continuous-cropping ramie (Boehmeria nivea L. Gaud) fields in different areas in China. Sci. Rep. 2020, 10, 3264. [Google Scholar] [CrossRef]
- Gatheru Waigi, M.; Sun, K.; Gao, Y. Sphingomonads in Microbe-Assisted Phytoremediation: Tackling Soil Pollution. Trends Biotechnol. 2017, 35, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Bai, Z.; Jin, T.; Jin, D.; Pan, J.; Yu, X.; Cernava, T. Arthrobacter is a universal responder to di-n-butyl phthalate (DBP) contamination in soils from various geographical locations. J. Hazard Mater. 2022, 422, 126914. [Google Scholar] [CrossRef] [PubMed]
- Huber, K.J.; Overmann, J. Vicinamibacteraceae fam. nov., the first described family within the subdivision 6 Acidobacteria. Int. J. Syst. Evol. Microbiol. 2018, 68, 2331–2334. [Google Scholar] [CrossRef]
- Huang, B.; Chen, Y.; Pei, Z.; Jiang, L.; Zhang, Y.; Wang, J.; Wang, J. Application of microbial organic fertilizers promotes the utilization of nutrients and restoration of microbial community structure and function in rhizosphere soils after dazomet fumigation. Front. Microbiol. 2022, 13, 1122611. [Google Scholar] [CrossRef]
- Yi, M.; Zhang, L.; Qin, C.; Lu, P.; Bai, H.; Han, X.; Yuan, S. Temporal changes of microbial community structure and nitrogen cycling processes during the aerobic degradation of phenanthrene. Chemosphere 2022, 286, 131709. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Li, J.; Hu, B.; Chu, G.; Tao, R. Response of abundance, diversity, and network of rhizosphere fungal community to monoculture of cut chrysanthemum. Appl. Microbiol. Biotechnol. 2023, 107, 3673–3685. [Google Scholar] [CrossRef]
- Zhang, Y.; Bo, G.; Shen, M.; Shen, G.; Yang, J.; Dong, S.; Shu, Z.; Wang, Z. Differences in microbial diversity and environmental factors in ploughing-treated tobacco soil. Front. Microbiol. 2022, 13, 924137. [Google Scholar] [CrossRef]
- Li, W.; Hou, Y.; Long, M.; Wen, X.; Han, J.; Liao, Y. Long-term effects of biochar application on rhizobacteria community and winter wheat growth on the Loess Plateau in China. Geoderma 2023, 429, 116250. [Google Scholar] [CrossRef]
- Kruczyńska, A.; Kuźniar, A.; Podlewski, J.; Słomczewski, A.; Grządziel, J.; Marzec-Grządziel, A.; Gałązka, A.; Wolińska, A. Bacteroidota structure in the face of varying agricultural practices as an important indicator of soil quality—A culture independent approach. Agric. Ecosyst. Environ. 2023, 342, 108252. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Liang, B.; Li, J. Changes in the abundance and structure of bacterial communities in the greenhouse tomato cultivation system under long-term fertilization treatments. Appl. Soil Ecol. 2017, 121, 82–89. [Google Scholar] [CrossRef]
- Liu, H.; Li, J.; Hu, Y.; Guo, J.; Lou, T.; Luo, G.; Chen, S.; Wang, W.; Ruan, H.; Sun, Z.; et al. Association Between Tranexamic Acid Use and Heterotopic Ossification Prevalence After Elbow Trauma Surgery A Propensity-Score-Matched Cohort Study. J. Bone Jt. Surg.-Am. Vol. 2023, 105, 1093–1100. [Google Scholar] [CrossRef]
- Trivedi, P.; Anderson, I.C.; Singh, B.K. Microbial modulators of soil carbon storage: Integrating genomic and metabolic knowledge for global prediction. Trends Microbiol 2013, 21, 641–651. [Google Scholar] [CrossRef]
- Fan, F.; Yin, C.; Tang, Y.; Li, Z.; Song, A.; Wakelin, S.A.; Zou, J.; Liang, Y. Probing potential microbial coupling of carbon and nitrogen cycling during decomposition of maize residue by 13C-DNA-SIP. Soil Biol. Biochem. 2014, 70, 12–21. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, R.; Ren, Y.; Ren, G.; Zhang, S.; Feng, J. Impacts of Fertilizers with Varying Nitrogen Contents on Millet Yield and Rhizosphere Soil Microbial Communities: Implications for Sustainable Agricultural Development. Sustainability 2025, 17, 1557. https://doi.org/10.3390/su17041557
Guo R, Ren Y, Ren G, Zhang S, Feng J. Impacts of Fertilizers with Varying Nitrogen Contents on Millet Yield and Rhizosphere Soil Microbial Communities: Implications for Sustainable Agricultural Development. Sustainability. 2025; 17(4):1557. https://doi.org/10.3390/su17041557
Chicago/Turabian StyleGuo, Ruifeng, Yuemei Ren, Guangbing Ren, Shou Zhang, and Jing Feng. 2025. "Impacts of Fertilizers with Varying Nitrogen Contents on Millet Yield and Rhizosphere Soil Microbial Communities: Implications for Sustainable Agricultural Development" Sustainability 17, no. 4: 1557. https://doi.org/10.3390/su17041557
APA StyleGuo, R., Ren, Y., Ren, G., Zhang, S., & Feng, J. (2025). Impacts of Fertilizers with Varying Nitrogen Contents on Millet Yield and Rhizosphere Soil Microbial Communities: Implications for Sustainable Agricultural Development. Sustainability, 17(4), 1557. https://doi.org/10.3390/su17041557




