3.1. Hydrogen Production by Natural Gas Reforming
Natural gas reforming hydrogen production includes three stages: natural gas extraction, natural gas transportation and natural gas reforming hydrogen production. In the hydrogen production reaction stage, the main chemical reactions are the reforming reaction and the CO conversion reaction:
The life cycle input and output data for hydrogen production by natural gas reforming are listed in the
Table 2.
In order to obtain 1 kg of H
2 (20 Mpa, 25 °C) exergy, a natural gas extraction company in China needs to extract 4 kg of natural gas [
15] (containing 207.5 MJ of chemical reaction exergy) from a pristine gas field. This process consumes 5.1 MJ of diesel chemical reaction exergy, 2.8 MJ of methanol (for natural gas hydrate inhibitor) chemical reaction exergy, and 0.2 MJ of electric power exergy. The exergic efficiency of this stage is 84.7%, and the exergic loss is 37.7 MJ.
The 4 kg of natural gas obtained from extraction is transported through a pipeline to a 2 km natural gas to hydrogen plant, ignoring the natural gas that escapes during the process [
16]. This stage contains 2.1 MJ of electricity in addition to the chemical reaction exergy carried by the natural gas itself, the exergic efficiency of this stage is 99% and the exergic loss is 2.1 MJ.
Upon arrival at the natural gas-to-hydrogen plant, natural gas is first injected as feedstock gas into a centrifugal compressor to pressurize to 2.5 MPa, consuming 2.1 MJ of electricity [
18], and the exergic efficiency of this unit is 99.9%, with an exergic loss of 0.1 MJ. In the presence of hydrocarbon fuels, it is preheated in the reformer furnace to 360–380 °C, consuming 1.7 MJ of hydrocarbon fuel. The chemical reaction produces a physical exergy of 2.3 MJ for the natural gas addition, and a thermal exergy of 1.4 MJ for the steam at 360–380 °C. The reactor temperature is heated to 850 °C using a hydrocarbon fuel containing 12.7 MJ of chemically reactive exergy, and natural gas is mixed with water vapor to undergo a reforming reaction that produces a mixture containing 152.1 MJ of a mixture of (CH
4 (5%), H
2 (70%), CO (9%), CO
2 (13%), and N
2 (3%)) [
17] of chemically reactive exergy as well as a mixture containing 20.7 MJ of thermal exergy in steam at 850 °C. The exergic efficiency of this unit is 71% and the exergic loss is 51.63 MJ.
The gas from the reforming reaction is cooled to 350 °C by a heat exchanger, and the reforming reaction occurs in the reformer furnace and produces a mixture containing 152.1 MJ of chemically reacted exergy (H2 (73%), CH4 (4%), CO (4%), CO2 (17%), and N2 (2%)), as well as the cooling water produced by heating the cooling water to 350 °C to produce 20.7 MJ of thermal exergy. The exergic efficiency of this unit is 98.1% and the exergic loss is 3.2 MJ.
The reacted gas was passed through a pressure-variable adsorption process (PSA) to obtain high-purity hydrogen, consuming 11.2 MJ of electrical exergy and generating 117.7 MJ of exergy contained in H2 at 0.1 MPa and 25 °C. The exergic efficiency of this unit is 65.1%, and the exergic loss is 63.1 MJ.
Finally, 22.6 MJ of electric exergy is applied to drive the hydrogen compressor to obtain 1 kg of H2 (20 MPa, 25 °C) containing 118.2 MJ of exergy, and the exergic efficiency of this unit is 84.2% with an exergic loss of 22.1 MJ.
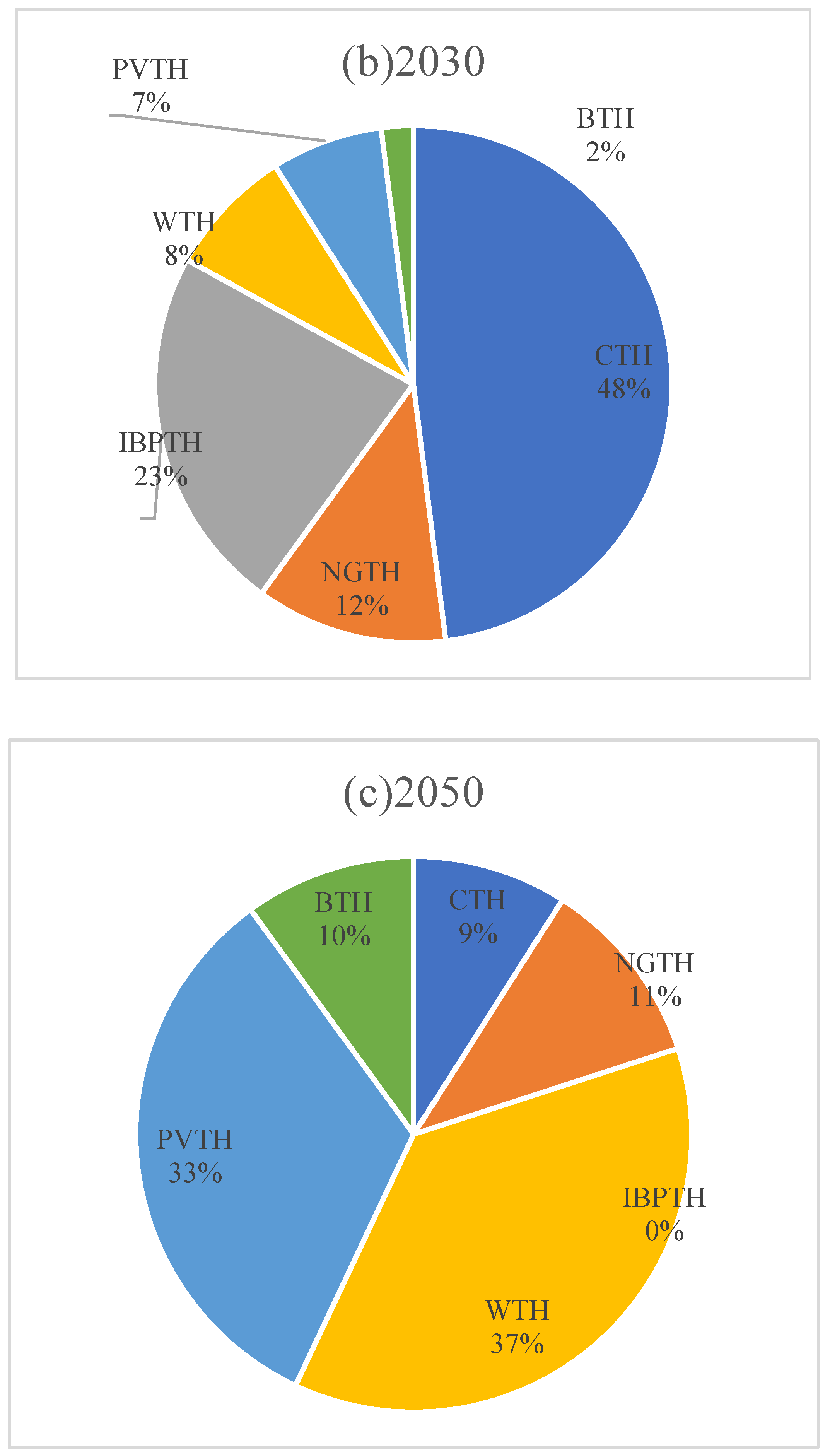
The life cycle exergic efficiency and exergic loss distribution of hydrogen production from natural gas is shown in
Figure 2.
As shown in
Figure 3, the distribution of exergic losses for the life cycle of hydrogen production from natural gas by stages is shown. It can be seen that the exergic losses generated by the variable pressure adsorption unit have the largest share of 35%. This is due to the fact that the variable pressure adsorption process is the most irreversible due to its high power and is limited by the power of the piston compressor itself. Next is the steam reforming unit, where exergic losses account for 29% of the total. The cause of exergic losses in this section is mainly due to the natural gas gasification reaction and the heat transfer effect resulting from the reactor needing to be heated up to 850 °C. The steam reforming unit is the second most important part of the steam reforming unit. Next, exergic losses due to the natural gas extraction unit account for 21% of the total, and this stage requires the passage of key equipment such as turbine pumps and the consumption of large quantities of diesel fuel, with the efficiency of the equipment itself leading to a certain irreversibility of the process. The exergic losses of the hydrogen compression unit accounted for 12% of the overall, due to the smaller power compared to the compressor of the variable pressure adsorption unit, the electrical work consumed is smaller, the exergic losses are less, and the exergic losses of the other units are negligible.
In order to reduce the exergic losses in natural gas hydrogen production, the PSA process and the hydrogen compression process should be optimized to improve the compressor efficiency and reduce the power of the equipment to reduce the use of electric power. At the same time, the natural gas steam reforming unit should be improved to lower the reaction temperature and reduce the exergic losses due to the heat transfer process, and the exergic losses due to the chemical reaction of natural gas reforming should be reduced by adopting the natural gas partial oxidation, natural gas dry reforming and natural gas autothermal reforming technologies.
By optimizing the use of catalysts and accelerating the chemical reaction rate in natural gas reforming hydrogen production technology, the heat and power required for the chemical reaction external to the reforming hydrogen production can be effectively reduced, and the product conversion efficiency of natural gas hydrogen production can be improved. In the conventional natural gas-to-hydrogen process, nickel is the predominant catalyst in the reforming hydrogen technology, but the activity of the nickel catalyst decreases (i.e., catalyst poisoning occurs) with the long-term operation of the natural gas-to-hydrogen process. The electron cloud density of Ni catalysts can be increased, and the carbon buildup of the catalysts can be suppressed by using basic metals such as Na, Mg, Ca, K, rare earth metals, and transition metals such as M
oO
3 [
19].
In order to improve the utilization of thermal hydropower, according to Wei-Hsin (2010), by improving the heating system for natural gas hydrogen production, such as preheating natural gas by using thermal cycling technology [
20], it is possible to both increase the natural gas reaction temperature, reduce the reaction temperature difference, and reduce the hydropower loss due to the heat transfer process.
3.2. Propane Dehydrogenation
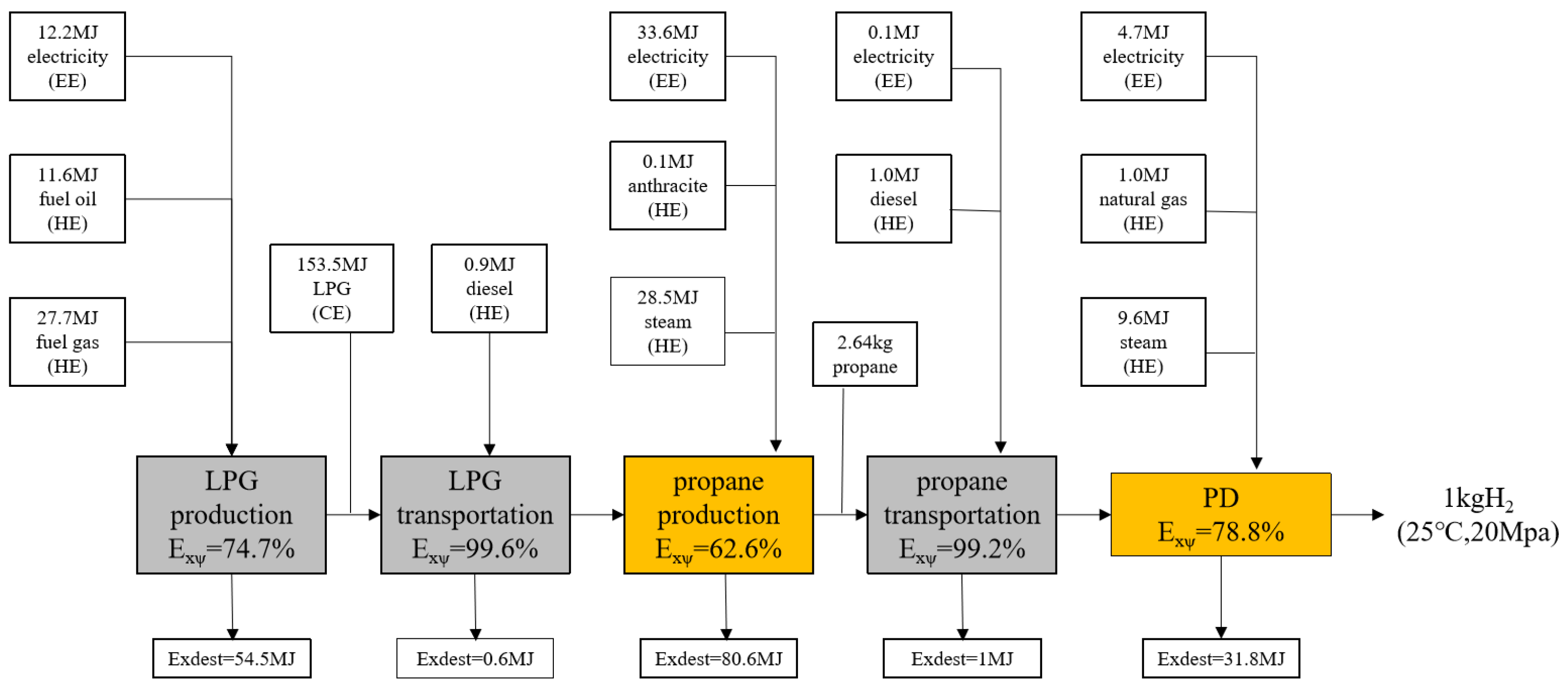
The life cycle process of propane dehydrogenation consists of several parts: LPG production and transportation, propane production and transportation, and the hydrogen production reaction. The main reaction is the Oleflex reaction [
21]:
A life-cycle inventory of input and emission data for propane dehydrogenation is shown in
Table 3.
In order to obtain 1 kg H
2 (20 Mpa, 25 °C) of exergy, a refinery in China processed the feedstock with a ratio of “crude oil: fuel oil = 6:20” into a finished product, and extracted LPG from the finished product (LPG constitutes 5.2% of the finished product [
26]). In this stage it is necessary to consume fuel oil containing 11.6 MJ exergy, fuel gas containing 27.7 MJ exergy, and electricity containing 12.24 MJ exergy, and to obtain LPG containing 150.6 MJ exergy. The exergic efficiency of this stage is 74.5%, and the exergic loss is 54.5 MJ.
The 3 kg of LPG obtained from the production is sent to the propane production plant 100 km away by means of a transportation tanker, consuming electricity containing 0.04 MJ of exergy and diesel fuel containing 0.6 MJ of exergy, with an exergic efficiency of 99.58% and an exergic loss of 0.6 MJ at this stage. The LPG is condensed to below 0–5 °C, and the propane in the LPG is extracted by variable pressure adsorption [
27], which consumes steam containing 28.5 MJ exergy, electricity containing 33.6 MJ exergy, and anthracite coal containing 0.1 MJ exergy at this stage, which has an exergic efficiency of 62.6%. The exergic loss is 80.6 MJ.
The produced propane is transported to the propane dehydrogenation plant at the 100 km location using trucks, and diesel fuel containing 1.0 MJ exergy is consumed in the process, with an exergic efficiency of 99.2% and an exergic loss of 1 MJ at this stage.
The propane was pretreated in a depropane tower to separate the non-propane hydrocarbons [
28], and then the propane was sent to a propane dehydrogenation unit, where the Oleflex reaction took place at 600–700 °C and 1 kg of H
2 was obtained by adsorption and by pressurization techniques (20 MPa, 25 °C).
In this process, propane containing 135.1 MJ exergy, steam containing 9.6 MJ exergy, electricity containing 4.7 MJ exergy, and natural gas containing 1.0 MJ exergy are input to obtain 1 kg H2 (20 MPa, 25 °C) containing 118.2 MJ exergy. The exergic efficiency of this unit was 78.8% and the exergic loss was 31.8 MJ.
The life cycle exergic efficiency and exergic loss distribution for the life cycle of propane dehydrogenation to hydrogen is shown in
Figure 4.
As shown in
Figure 5, the largest proportion of total exergic losses in the whole life cycle of propane dehydrogenation is in the “propane production” stage, accounting for 49%, which requires the use of refrigeration devices to keep the condensing temperature below 0–5 °C and drive the variable pressure adsorption equipment (pressure environment of 3.0 Mpa [
17]), which utilizes the pressure difference to obtain LPG from crude oil. Obtaining LPG from crude oil results in exergic losses due to temperature differences as well as pressure differences. Next is the LPG production stage with a share of 31%, where the LPG production stage needs to maintain the reaction temperature (450 °C) and pressure environment (0.5–1.0 Mpa) [
29]. The percentage of exergic loss in propane dehydrogenation reaction is 19%, and the Oleflex reaction requires reaction conditions of 600 °C to 700 °C, and the temperature difference effect leads to exergic loss.
3.3. Hydrogen Production from Green Electricity
In hydrogen production from green power, hydrogen production from water electrolysis is a common stage in hydrogen production from photovoltaics and wind power. In order to generate renewable electricity, the upstream of hydrogen production from wind power involves the manufacturing and transportation of wind power equipment, while the upstream of hydrogen production from photovoltaics involves the production of industrial silicon, the refining of polysilicon and the manufacturing and transportation of photovoltaic modules.
A list of life-cycle input and emission data for hydrogen production from wind power is shown in
Table 4.
To obtain 1 kg H
2 (20 Mpa, 25 °C) of exergy, a component manufacturing plant for a wind turbine in China completed the production of materials for nacelles, rotors, towers and tower bases with component manufacturing using electricity containing 4.5 MJ of exergy [
30], and the exergic efficiency at this stage was 97.7%, with an exergic loss of 4.8 MJ.
Using diesel fuel containing 0.03 MJ exergy, the wind power equipment is transported to the wind power plant 700 km away by 30% freight and 70% rail, the exergic efficiency at this stage is 99.99% and the exergic loss is 0.03 MJ.
Using electricity containing 0.72 MJ exergy to drive the wind engine to produce wind power containing 198 MJ exergy, the exergic efficiency at this stage is 99.64% and the exergic loss is 0.72 MJ.
In off-grid mode, wind power containing 198 MJ is fed into the electrolyzer to produce hydrogen, which undergoes a second stage of cooling [
31] and undergoes a pressurization system to 20 MPa to obtain 1 kg of H
2 (20 MPa, 25 °C) containing 118.2 MJ of exergy, which at this stage has an exergic efficiency of 59.7% and an exergic loss of 79.8 MJ.
A list of life cycle input and emission data for PV hydrogen production is shown in the
Table 5.
To obtain 144 MJ of exergy for 1 kg H2 (20 Mpa, 25 °C), a Chinese industrial silicon producer used electricity containing 4.4 MJ of exergy to drive the chemical reaction of silica with a carbon reductant in an electric arc furnace to obtain reduced silica, and the exergic efficiency at this stage is 97.8%, with an exergic loss of 4.4 MJ.
The modified Siemens method of industrial silicon purification is used to obtain 99.9999% purity of polysilicon [
32], then it will go through the melting and fixing process to get polysilicon ingots, through the cutting square, flat grinding chamfering, slicing and other industries to get polycrystalline silicon solar cells. They then go through the cell testing, welding, stringing, laying, laminating and other processes to make photovoltaic modules, this stage using contains 86 MJ exergy of electricity, diesel fuel containing 0.65 MJ exergy, and natural gas containing 0.006 MJ exergy, with an exergic efficiency of 70% and an exergic loss of 86.7 MJ.
The manufactured PV modules were transported to a PV power plant 100 km away using a truck, a process that used diesel fuel containing 0.39 MJ exergy, with an exergic efficiency of 99.8% and an exergic loss of 0.39 MJ.
To run the photovoltaic power generation facility, this process requires the consumption of coal containing 3.1 MJ exergy and diesel fuel containing 0.16 MJ exergy and obtains photovoltaic power containing 198 MJ exergy, with an exergic efficiency of 98.43% and an exergic loss of 3.15 MJ at this stage.
In the off-grid condition, hydrogen is produced by electrolysis of water in an alkaline electrolyzer using PV power containing 198 MJ exergy, and the resulting hydrogen is compressed to 20 MPa by secondary cooling to obtain 1 kg of H2 (20 MPa, 25 °C), containing 118.2 MJ exergy. The exergic efficiency at this stage is 59.7%, with an exergic loss of 79.8 MJ.
The distribution of exergic efficiency and exergic loss over the life cycle of green power hydrogen production is shown in
Figure 6.
The distribution of exergic losses in the staged hydrogen production from green electricity is shown in
Figure 7.
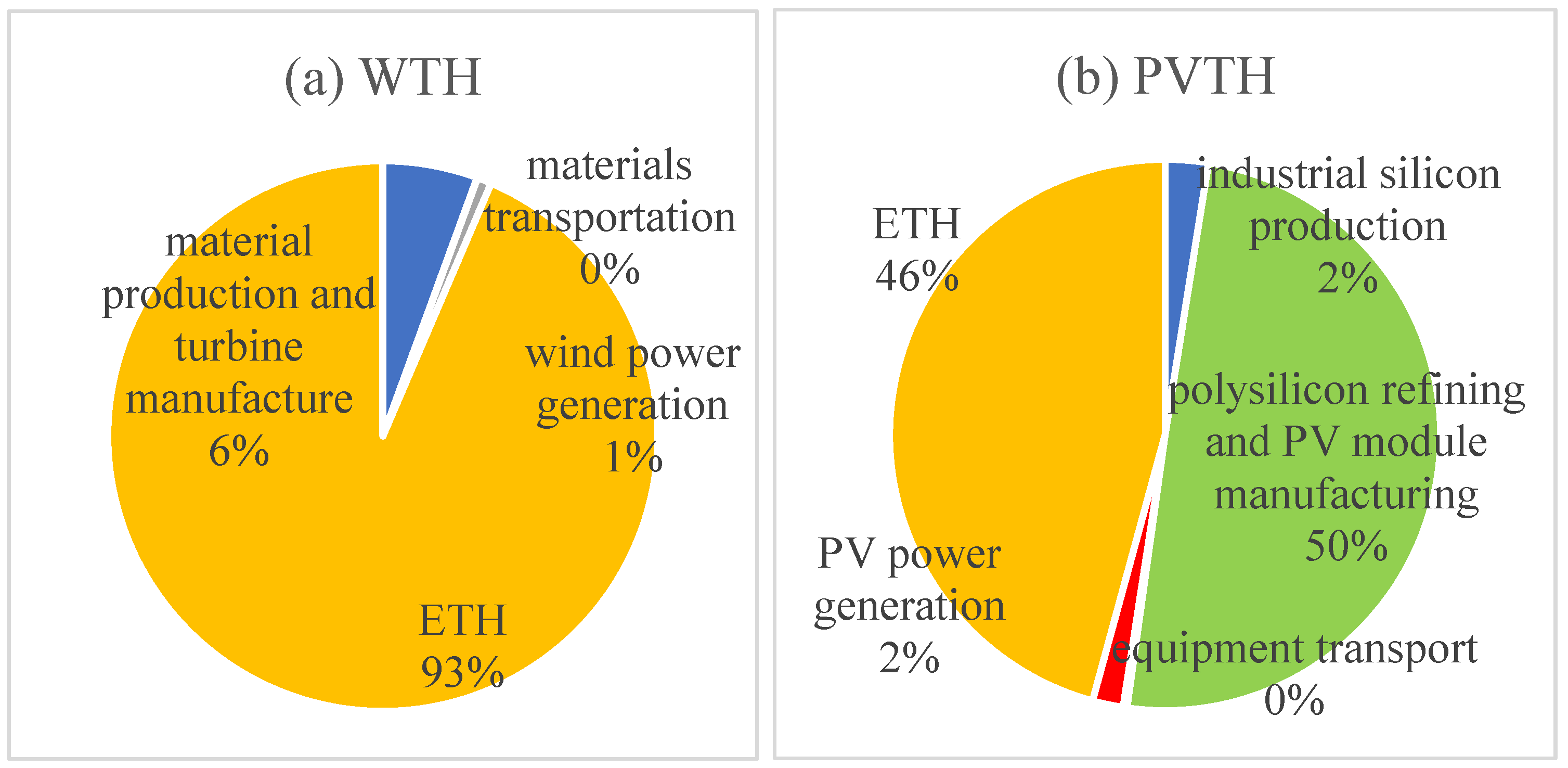
As shown in
Figure 7a, the largest exergic loss in the life cycle of wind power hydrogen production is in the “electrolysis of water for hydrogen production”, which occupies 93% of the overall proportion. In the electrolyzer system, the exergic losses caused by the oxygen and hydrogen compressors are negligible (the impact of exergic losses is about 1%). The exergic losses in hydrogen production from water electrolysis depend on the energy efficiency of the electrolyzer. Therefore, in order to reduce the exergic losses in hydrogen production from wind power, it is necessary to improve the energy utilization efficiency of the PEM electrolyzer system.
As shown in
Figure 7b, for PV hydrogen production, the largest exergic loss occurs in the “polysilicon refining and PV module manufacturing” part, which occupies 50% of the whole. The reason is that the production of polysilicon needs to be carried out in a high-temperature environment at about 1100 °C using the modified Siemens method [
32]; the temperature difference factor is the main reason for the large exergic losses in this stage. The next stage is hydrogen production from electrolyzed water, which accounts for 46% of the hydropower losses, mainly due to the same reasons as the exergic losses in the electrolyzed water stage of wind power hydrogen production.