The Influence of Selected Herbicides on Soil Organic Matter: Determining the Sustainable Development of Agroecosystems
Abstract
1. Introduction
2. Materials and Methods
2.1. Humic Acid Extraction
2.2. Herbicides Characteristics
2.3. Saturation of Humic Acids with Herbicides
2.4. Preparation of Humic Acid Solutions for Spectroscopic Analyses
2.5. UV–Vis and Fluorescence Analyses
3. Results and Discussion
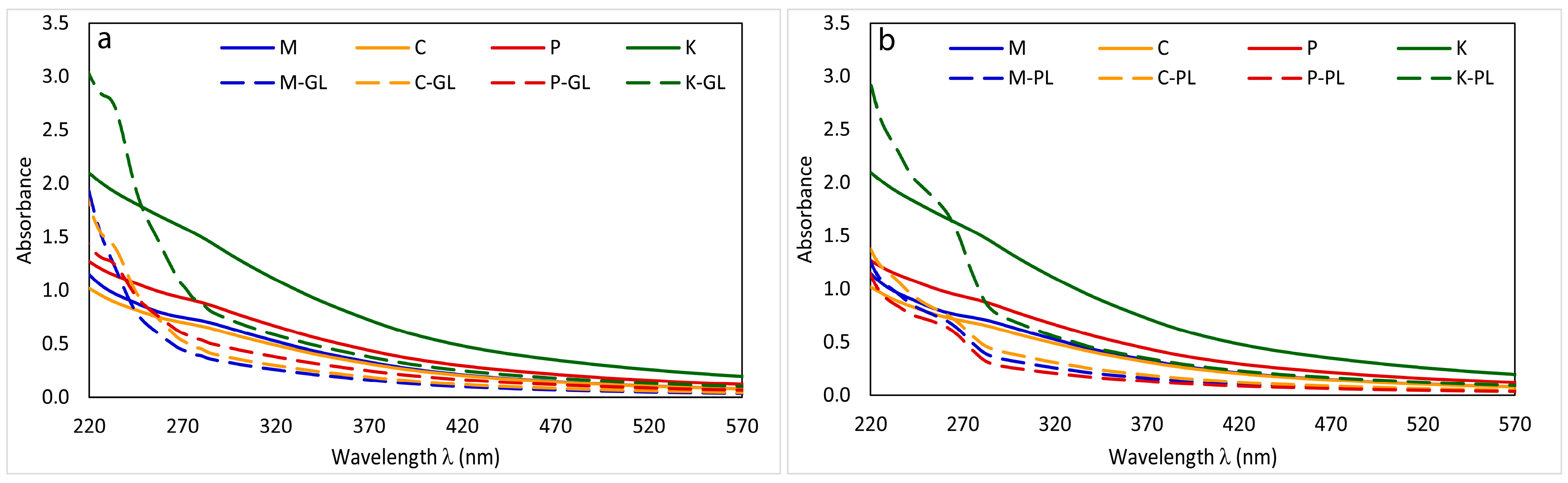
3.1. UV–Vis Spectra
3.2. Synchronous Scan Fluorescence (SSF) Spectra
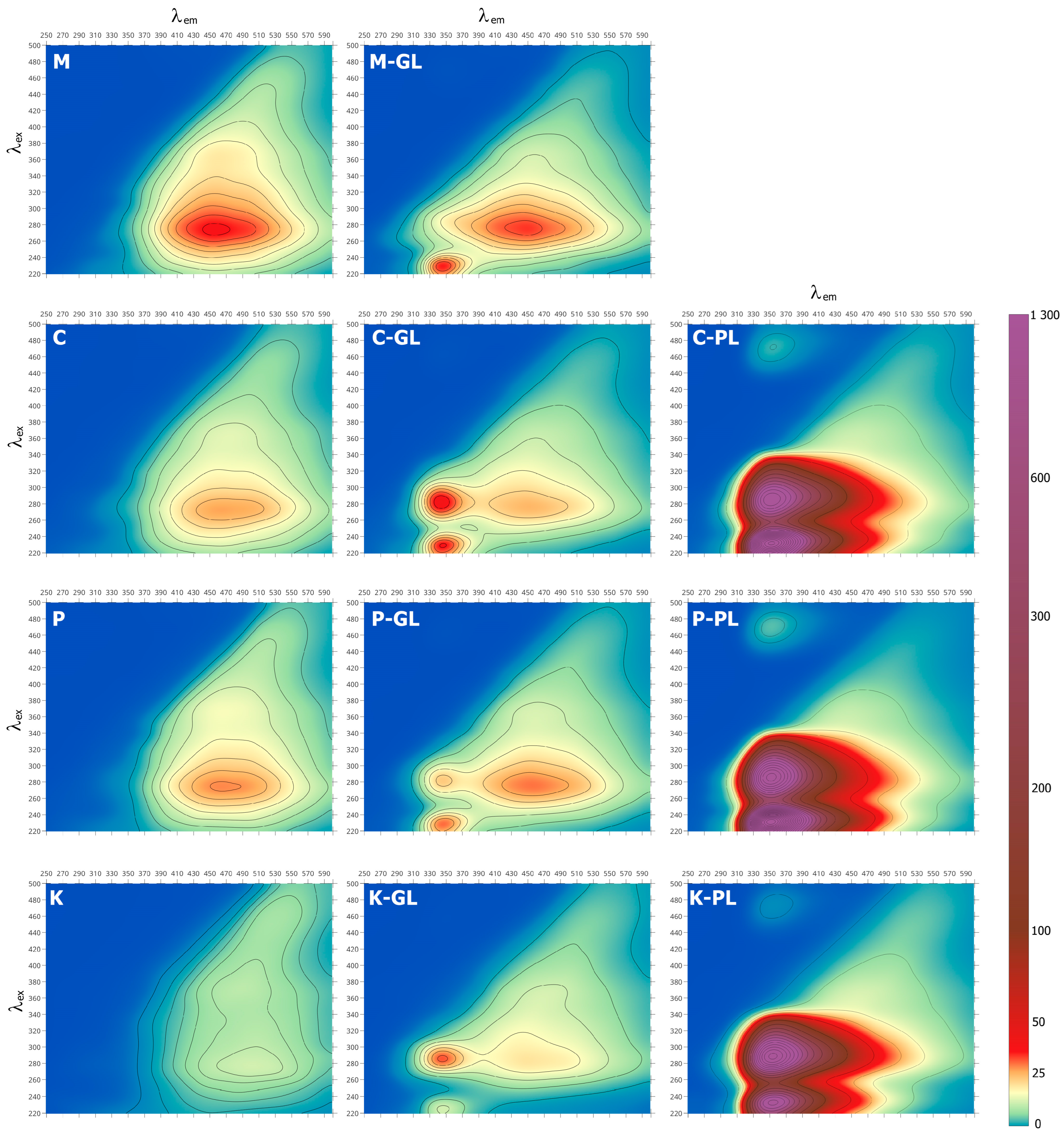
3.3. Three-Dimensional Fluorescence (EEM) Spectra
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lechenet, M.; Dessaint, F.; Py, G.; Makowski, D.; Munier-Jolain, N. Reducing pesticide use while preserving crop productivity and profitability on arable farms. Nat. Plants 2017, 3, 17008. [Google Scholar] [CrossRef]
- Mandal, A.; Sarkar, B.; Mandal, S.; Vithanage, M.; Patra, A.K.; Manna, M.C. Impact of agrochemicals on soil health. Agrochem. Detect. Treat. Remediat. Pestic. Chem. Fertil. 2020, 7, 161–187. [Google Scholar] [CrossRef]
- Gevao, B.; Semple, K.T.; Jones, K.C. Bound pesticide residues in soils: A review. Environ. Pollut. 2000, 108, 3–14. [Google Scholar] [CrossRef] [PubMed]
- Arias-Estévez, M.; López-Periago, E.; Martínez-Carballo, E.; Simal-Gándara, J.; Mejuto, J.-C.; García-Río, L. The mobility and degradation of pesticides in soils and the pollution of groundwater resources. Agric. Ecosyst. Environ. 2008, 123, 247–260. [Google Scholar] [CrossRef]
- Levine, M. Fluorescence-Based Sensing of Pesticides Using Supramolecular Chemistry. Front. Chem. 2021, 9, 616815. [Google Scholar] [CrossRef] [PubMed]
- Aktar, W.; Sengupta, D.; Chowdhury, A. Impact of pesticides use in agriculture: Their benefits and hazards. Interdiscip Toxicol. 2009, 2, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Sofo, A.; Scopa, A.; Dumontet, S.; Mazzatura, A.; Pasquale, V. Toxic effects of four sulphonylureas herbicides on soil microbial biomass. J. Environ. Sci. Health Part B 2012, 47, 653–659. [Google Scholar] [CrossRef]
- Rajmohan, K.S.; Chandrasekaran, R.; Varjani, S. A Review on Occurrence of Pesticides in Environment and Current Technologies for Their Remediation and Management. Indian J. Microbiol. 2020, 60, 125–138. [Google Scholar] [CrossRef] [PubMed]
- Karunarathna, S.C.; Ashwath, N.; Jeewon, R. Editorial: The Potential of Fungi for Enhancing Crops and Forestry Systems. Front. Microbiol. 2021, 12, 813051. [Google Scholar] [CrossRef]
- Neira-Albornoz, A.; Fuentesc, E.; Cáceres-Jensen, L. Connecting the evidence about organic pollutant sorption on soils with environmental regulation and decision-making: A scoping review. Chemosphere 2022, 308, 136164. [Google Scholar] [CrossRef] [PubMed]
- Barchańska, H.; Czaplicka, M.; Kyzioł-Komosińska, J. Interaction of selected pesticides with mineral and organic soil components. Arch. Environ. Prot. 2020, 46, 80–91. [Google Scholar] [CrossRef]
- Barriuso, E.; Benoit, P.; Dubus, I.G. Formation of pesticide nonextractable (bound) residues in soil: Magnitude, controlling factors and reversibility. Environ. Sci. Technol. 2008, 42, 1845–1854. [Google Scholar] [CrossRef] [PubMed]
- Gunasekara, A.S.; Xing, B. Sorption and Desorption of Naphthalene by Soil Organic Matter Importance of Aromatic and Aliphatic Components. J. Environ. Qual. 2003, 32, 240–246. [Google Scholar] [CrossRef]
- Ukalska-Jaruga, A.; Smreczak, B.; Siebielec, G. Assessment of Pesticide Residue Content in Polish Agricultural Soils. Molecules 2020, 25, 587. [Google Scholar] [CrossRef] [PubMed]
- Hayes, M.H.B.; Swift, R.S. Chapter One—Vindication of Humic Substances as a Key Component of Organic Matter in Soil and Water. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2020; Volume 163, pp. 1–37. [Google Scholar] [CrossRef]
- Fragouli, P.G.; Roulia, M.; Vassiliadis, A.A. Macromolecular Size and Architecture of Humic Substances Used in the Dyes’ Adsorptive Removal from Water and Soil. Agronomy 2023, 13, 2926. [Google Scholar] [CrossRef]
- Tan, K.H. Principles of Soil Chemistry, 4th ed.; Taylor and Francis Group: Boca Raton, FL, USA, 2011. [Google Scholar] [CrossRef]
- Pan, D.; Wu, X.; Chen, P.; Zhao, Z.; Fan, F.; Wang, Y.; Zhu, M.; Xue, J.; Wang, Y. New insights into the interactions between humic acid and three neonicotinoid pesticides, with multiple spectroscopy technologies, two-dimensional correlation spectroscopy analysis and density functional theory. Sci. Total Environ. 2021, 798, 149237. [Google Scholar] [CrossRef] [PubMed]
- Simpson, A.J.; Song, G.; Smith, E.; Lam, B.; Novotny, E.H.; Hayes, M.H.B. Unraveling the Structural Components of Soil Humin by Use of Solution-State Nuclear Magnetic Resonance Spectroscopy. Environ. Sci. Technol. 2007, 41, 876–883. [Google Scholar] [CrossRef] [PubMed]
- Swift, R.S. Organic matter characterization. In Methods of Soil Analysis, Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Soltanpour, P.N., Tabatabai, M.A., Sumner, M.E., Eds.; Soil Science Society of America Press: Madison, WI, USA, 1996. [Google Scholar] [CrossRef]
- Uyguner, C.S.; Bekbolet, M. Evaluation of humic acid photocatalytic degradation by UV–vis and fluorescence spectroscopy. Catal. Today 2005, 101, 267–274. [Google Scholar] [CrossRef]
- Enev, V.; Doskočil, L.; Kubíková, L.; Klučáková, M. The medium-term effect of natural compost on the spectroscopic properties of humic acids of Czech soils. J. Agric. Sci. 2018, 156, 877–887. [Google Scholar] [CrossRef]
- Mielnik, L.; Hewelke, E.; Weber, J.; Oktaba, L.; Jonczak, J.; Podlasinski, M. Changes in the soil hydrophobicity and structure of humic substances in sandy soil taken out of cultivation. Agric. Ecosyst. Environ. 2021, 319, 107554. [Google Scholar] [CrossRef]
- Korshin, G.V.; Li, C.-W.; Benjamin, M.M. Monitoring the properties of natural organic matter through UV spectroscopy: A consistent theory. Water Res. 1997, 31, 1787–1795. [Google Scholar] [CrossRef]
- Chen, J.; Gu, B.; LeBoeuf, E.J.; Pan, H.; Dai, S. Spectroscopic characterization of the structural and functional properties of natural organic matter fractions. Chemosphere 2002, 48, 59–68. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Yu, H.Q. Advances in the characterization and monitoring of natural organic matter using spectroscopic approaches. Water Res. 2021, 190, 116759. [Google Scholar] [CrossRef]
- Chen, Y.; Senesi, N.; Schnitzer, M. Information Provided on Humic Substances by E4/E6 Ratios. Soil Sci. Soc. Am. J. 1977, 41, 352–358. [Google Scholar] [CrossRef]
- Niu, H.; Yang, H.; Tong, L. Structural Characterization and Adsorption Capability of Carbonaceous Matters Extracted from Carbonaceous Gold Concentrate. Minerals 2021, 11, 23. [Google Scholar] [CrossRef]
- Sharpless, C.M.; Aeschbacher, M.; Page, S.; Wenk, J.; Sander, M.; McNeill, K. Photooxidation-Induced Changes in Optical, Electrochemical, and Photochemical Properties of Humic Substances. Environ. Sci. Technol. 2014, 48, 2688–2696. [Google Scholar] [CrossRef] [PubMed]
- Boguta, P.; Sokołowska, Z. Interactions of Zn(II) Ions with Humic Acids Isolated from Various Type of Soils. Effect of pH, Zn Concentrations and Humic Acids Chemical Properties. PLoS ONE 2016, 11, e0153626. [Google Scholar] [CrossRef]
- Mielnik, L.; Kowalczuk, P. Optical characteristic of humic acids from lake sediments by excitation-emission matrix fluorescence with PARAFAC model. J. Soil Sediments 2018, 18, 2851–2862. [Google Scholar] [CrossRef]
- Machado, W.; Franchini, J.C.; Guimarães, M.F.; Filho, J.T. Spectroscopic characterization of humic and fulvic acids in soil aggregates, Brazil. Heliyon 2020, 6, e04078. [Google Scholar] [CrossRef]
- D’Orazio, V.; Miano, T. Fluorescence properties of humic acid interaction products with s-triazine and bipyridilium herbicides and their Cu complexes: A multivariate approach. J. Soils Sediments 2018, 18, 1347–1354. [Google Scholar] [CrossRef]
- Chen, P.; Shi, M.; Liu, X.; Wang, X.; Fang, M.; Guo, Z.; Wu, X.; Wang, Y. Comparison of the binding interactions of 4-hydroxyphenylpyruvate dioxygenase inhibitor herbicides with humic acid: Insights from multispectroscopic techniques, DFT and 2D-COS-FTIR. Ecotoxicol. Environ. Saf. 2022, 239, 113699. [Google Scholar] [CrossRef]
- Mielnik, L.; Bejger, R.; Ukalska-Jaruga, A.; Ćwieląg-Piasecka, I.; Weber, J.; Jerzykiewicz, M.; Kocowicz, A.; Jamroz, J.; Dębicka, M.; Bekier, J.; et al. Sorption of pendimethalin by humin fraction isolated from Mollic horizon of Chernozems. J. Elem. 2024, 29, 37–55. [Google Scholar] [CrossRef]


| Soil | pHKCI | CaCO3 | TOC | TN | TOC/TN | Sand | Silt | Clay | WRB Textural Class |
|---|---|---|---|---|---|---|---|---|---|
| g kg−1 | % | ||||||||
| M | 7.52 | 1.53 | 21.2 | 1.06 | 13.2 | 29 | 49 | 22 | loam |
| C | 7.39 | 1.03 | 26.1 | 2.03 | 12.8 | 24 | 57 | 19 | silt loam |
| P | 7.48 | 1.54 | 24.6 | 2.12 | 11.6 | 39 | 37 | 24 | loam |
| K | 6.66 | 0.61 | 37.7 | 2.80 | 13.4 | 24 | 29 | 47 | clay |
| Herbicide | Glean 75 WG | Pleban 75 WG |
|---|---|---|
| Active substance | chlorosulfuron | tribenuron-methyl |
| Chemical formula | C12H12ClN5O4S | C15H17N5O6S |
| IUPAC name | 2-chloro-N-[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)carbamoyl]benzene-1-sulfonamide | Methyl 2-[[(4-methoxy-6-methyl-1,3,5-triazin-2-yl)-methylcarbamoyl]sulfamoyl]benzoate |
| Structure diagram | 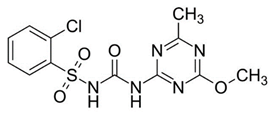 | 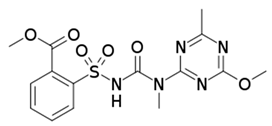 |
| Solubility in water | 12,500 mg/L (20 °C) | 2483 mg/L (20 °C) |
| Molecular mass (g/mol) | 357.78 | 395.39 |
| Appearance | White crystalline solid | White to off-white solid |
| logP | −0.99 | 0.38 |
| Acidity (pKa) | 3.40 | 4.65 |
| Sample | E235:E280 | E260:E365 | E260:E465 | E280:E665 | E465:E665 | ΔlogK |
|---|---|---|---|---|---|---|
| M | 1.34 | 2.36 | 5.16 | 16.8 | 3.58 | 0.58 |
| M-GL | 3.02 | 3.74 | 7.82 | 19.5 | 3.57 | 0.59 |
| M-PL | 2.27 | 4.50 | 9.27 | 18.6 | 3.39 | 0.56 |
| C | 1.33 | 2.33 | 4.95 | 15.5 | 3.47 | 0.56 |
| C-GL | 3.01 | 3.98 | 7.88 | 18.6 | 3.55 | 0.58 |
| C-PL | 2.18 | 4.11 | 8.68 | 19.3 | 3.50 | 0.57 |
| P | 1.28 | 2.20 | 4.39 | 12.9 | 3.22 | 0.52 |
| P-GL | 2.27 | 3.09 | 5.87 | 14.4 | 3.27 | 0.54 |
| P-PL | 2.38 | 5.05 | 10.08 | 17.6 | 3.25 | 0.54 |
| K | 1.27 | 2.28 | 4.65 | 13.8 | 3.31 | 0.53 |
| K-GL | 2.98 | 3.95 | 7.34 | 15.9 | 3.32 | 0.54 |
| K-PL | 2.41 | 5.11 | 10.24 | 18.7 | 3.36 | 0.54 |
| α | β | δ | γ | ω | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| λex/λem | IFl | λex/λem | IFl | λex/λem | IFl | λex/λem | IFl | λex/λem | IFl | |
| M | - | - | - | - | 275/455 | 41.3 | 360/462 | 23.3 | 440/515 | 13.1 |
| M-GL | 230/346 | 39.6 | 285/344 | 20.7 | 277/449 | 38.0 | 365/469 | 16.0 | 450/530 | 6.5 |
| M-PL | n.i. | n.i. | n.i. | n.i. | n.o. | n.o. | n.o. | n.o. | n.o. | n.o. |
| C | - | - | - | - | 274/464 | 30.7 | 359/473 | 17.5 | 442/521 | 10.0 |
| C-GL | 230/346 | 40.7 | 283/344 | 43.8 | 274/452 | 29.0 | 368/480 | 14.2 | 455/525 | 7.5 |
| C-PL | 233/352 | 966 | 286/352 | 545 | - | - | - | - | - | - |
| P | - | - | - | - | 276/464 | 32.6 | 365/467 | 19.4 | 441/516 | 12.92 |
| P-GL | 229/347 | 34.5 | 283/346 | 26.8 | 277/456 | 34.0 | 361/458 | 16.5 | 442/520 | 8.8 |
| P-PL | 232/351 | 1287 | 287/352 | 672 | - | - | - | - | - | - |
| K | - | - | - | - | 278/501 | 15.7 | 373/491 | 16.4 | 451/526 | 11.9 |
| K-GL | 225/346 | 15.8 | 287/345 | 36.2 | 285/449 | 23.5 | 361/471 | 13.6 | 440/518 | 9.5 |
| K-PL | 234/354 | 465.6 | 290/354 | 665.0 | - | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mielnik, L.; Bernacki, B.; Weber, J. The Influence of Selected Herbicides on Soil Organic Matter: Determining the Sustainable Development of Agroecosystems. Sustainability 2025, 17, 1376. https://doi.org/10.3390/su17041376
Mielnik L, Bernacki B, Weber J. The Influence of Selected Herbicides on Soil Organic Matter: Determining the Sustainable Development of Agroecosystems. Sustainability. 2025; 17(4):1376. https://doi.org/10.3390/su17041376
Chicago/Turabian StyleMielnik, Lilla, Brajan Bernacki, and Jerzy Weber. 2025. "The Influence of Selected Herbicides on Soil Organic Matter: Determining the Sustainable Development of Agroecosystems" Sustainability 17, no. 4: 1376. https://doi.org/10.3390/su17041376
APA StyleMielnik, L., Bernacki, B., & Weber, J. (2025). The Influence of Selected Herbicides on Soil Organic Matter: Determining the Sustainable Development of Agroecosystems. Sustainability, 17(4), 1376. https://doi.org/10.3390/su17041376





