Algicidal Bacteria: A Sustainable Proposal to Control Microalgae in the Conservation and Restoration of Stone Cultural Heritage
Abstract
1. Introduction
2. Algicidal Bacteria and Possible Applications for Heritage Sites
3. How Do Bacteria with an Algicidal Capacity Act?
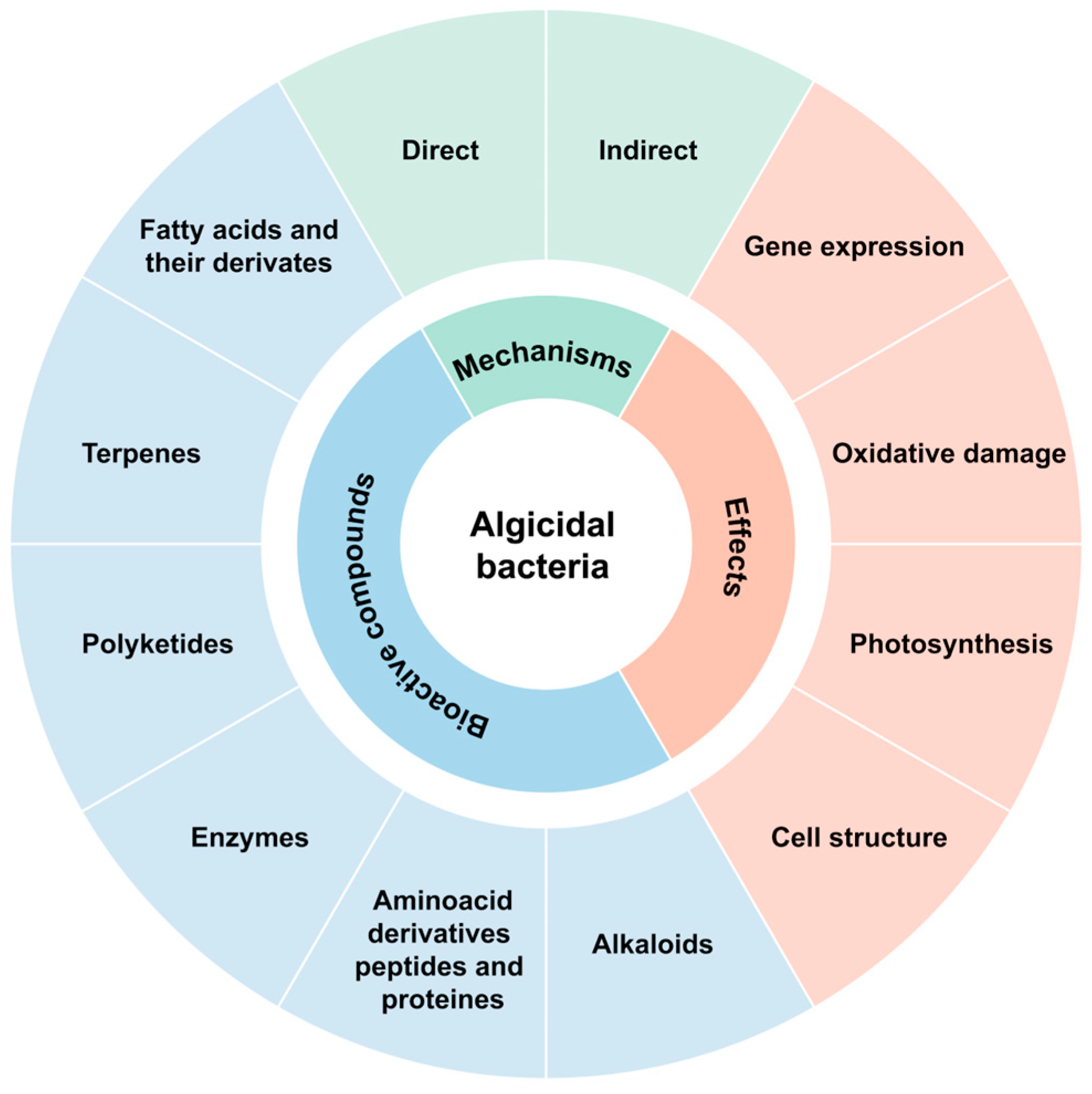
4. Main Metabolites with Algicidal Capacity Excreted by Bacteria
5. Denitrifying Algicidal Bacteria: A Combined Approach for Stone Cultural Heritage Conservation
6. Synergies Among Bacteria to Control Algal Growth
7. Methods in Practice
- 1.
- Direct application:
- 2.
- Immobilization:
- 3.
- Recruitment of bacteria in natural environments:
8. Algicidal Bacteria Versus Traditional Biocides: Advantages and Drawbacks
9. Discussion
9.1. Bacteria for Controlling the Growth of Photosynthetic Microorganisms in Stone Heritage
9.2. Selectivity in Treatments
9.3. Synergies Between Bacteria
9.4. Can Microorganisms Involved in Biodeterioration Processes Be Used for the Conservation of Cultural Assets?
9.5. The Use of Living Organisms: An Environmental Risk or a Biotechnological Opportunity?
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Vázquez-Nion, D.; Rodríguez-Castro, J.; López-Rodríguez, M.C.; Fernández-Silva, I.; Prieto, B. Subaerial Biofilms on Granitic Historic Buildings: Microbial Diversity and Development of Phototrophic Multi-Species Cultures. Biofouling 2016, 32, 657–669. [Google Scholar] [CrossRef] [PubMed]
- Bolívar-Galiano, F.; Abad-Ruiz, C.; Sánchez-Castillo, P.; Toscano, M.; Romero-Noguera, J. Frequent Microalgae in the Fountains of the Alhambra and Generalife: Identification and Creation of a Culture Collection. Appl. Sci. 2020, 10, 6603. [Google Scholar] [CrossRef]
- Bolívar-Galiano, F.; Cuzman, O.A.; Abad-Ruiz, C.; Sánchez-Castillo, P. Facing Phototrophic Microorganisms That Colonize Artistic Fountains and Other Wet Stone Surfaces: Identification Keys. Appl. Sci. 2021, 11, 8787. [Google Scholar] [CrossRef]
- Favero-Longo, S.E.; Viles, A. Heather A Review of the Nature, Role and Control of Lithobionts on Stone Cultural Heritage: Weighing-up and Managing Biodeterioration and Bioprotection. World J. Microbiol. Biotechnol. 2020, 36, 1–18. [Google Scholar] [CrossRef]
- Mohammadi, P.; Gholami-Nejad, P.; Asghari-Daryasari, R.; Asgarani, E. The Study of Microbial Communities of Rudkhan Castle. Geomicrobiol. J. 2020, 37, 119–129. [Google Scholar] [CrossRef]
- Biotechnology and Conservation of Cultural Heritage; Palla, F., Barresi, G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; ISBN 978-3-319-46166-3. [Google Scholar]
- Zhang, H.; Wang, Y.; Huang, J.; Fan, Q.; Wei, J.; Wang, F.; Jia, Z.; Xiang, W.; Liang, W. Inhibition of Microcystis aeruginosa Using Brevundimonas sp. AA06 Immobilized in Polyvinyl Alcohol-Sodium Alginate Beads. Desalination Water Treat. 2018, 111, 192–200. [Google Scholar] [CrossRef]
- Grogan, A.E.; Alves-de-Souza, C.; Cahoon, L.B.; Mallin, M.A. Harmful Algal Blooms: A Prolific Issue in Urban Stormwater Ponds. Water 2023, 15, 2436. [Google Scholar] [CrossRef]
- Negi, A.; Sarethy, I.P. Microbial Biodeterioration of Cultural Heritage: Events, Colonization, and Analyses. Microb. Ecol. 2019, 78, 1014–1029. [Google Scholar] [CrossRef]
- Golubić, S.; Pietrini, A.M.; Ricci, S. Euendolithic Activity of the Cyanobacterium Chroococcus lithophilus Erc. In Biodeterioration of the Pyramid of Caius Cestius, Rome, Italy. Int. Biodeterior. Biodegrad. 2015, 100, 7–16. [Google Scholar] [CrossRef]
- Bolívar Galiano, F.C. Diagnosis y Tratamiento del Deterioro por Microalgas en los Palacios Nazaríes de la Alhambra. Doctoral Thesis, Universidad de Granada, Granada, Spain, 1995. [Google Scholar]
- Scheerer, S.; Ortega-Morales, O.; Gaylarde, C. Chapter 5. Microbial Deterioration of Stone Monuments—An Updated Overview. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2009; Volume 66, pp. 97–139. ISBN 978-0-12-374788-4. [Google Scholar]
- Cuzman, O.A.; Ventura, S.; Sili, C.; Mascalchi, C.; Turchetti, T.; D’Acqui, L.P.; Tiano, P. Biodiversity of Phototrophic Biofilms Dwelling on Monumental Fountains. Microb. Ecol. 2010, 60, 81–95. [Google Scholar] [CrossRef]
- Peraza Zurita, Y.; Cultrone, G.; Sánchez Castillo, P.; Sebastián, E.; Bolívar, F.C. Microalgae Associated with Deteriorated Stonework of the Fountain of Bibatauín in Granada, Spain. Int. Biodeterior. Biodegrad. 2005, 55, 55–61. [Google Scholar] [CrossRef]
- Komar, M.; Nowicka-Krawczyk, P.; Ruman, T.; Nizioł, J.; Dudek, M.; Gutarowska, B. Biodeterioration Potential of Algae on Building Materials—Model Study. Int. Biodeterior. Biodegrad. 2023, 180, 105593. [Google Scholar] [CrossRef]
- Peraza Zurita, Y. Biodeterioro por Microalgas en Fuentes de Mármol: Descripción y Formas de Alteración Relacionadas: Propuesta de Material de Intervención: Interacciones Entre Microalgas y Sustrato: Estudio de Superficie. Doctoral Thesis, Editorial de la Universidad de Granada, Granada, Spain, 2005. ISBN 978-84-338-3319-8. [Google Scholar]
- Gaylarde, C. Influence of Environment on Microbial Colonization of Historic Stone Buildings with Emphasis on Cyanobacteria. Heritage 2020, 3, 1469–1482. [Google Scholar] [CrossRef]
- Ortega-Morales, O.; Montero-Muñoz, J.L.; Baptista Neto, J.A.; Beech, I.B.; Sunner, J.; Gaylarde, C. Deterioration and Microbial Colonization of Cultural Heritage Stone Buildings in Polluted and Unpolluted Tropical and Subtropical Climates: A Meta-Analysis. Int. Biodeterior. Biodegrad. 2019, 143, 104734. [Google Scholar] [CrossRef]
- Pfendler, S.; Munch, T.; Bousta, F.; Alaoui-Sosse, L.; Aleya, L.; Alaoui-Sossé, B. Bleaching of Biofilm-Forming Algae Induced by UV-C Treatment: A Preliminary Study on Chlorophyll Degradation and Its Optimization for an Application on Cultural Heritage. Environ. Sci. Pollut. Res. 2018, 25, 14097–14105. [Google Scholar] [CrossRef]
- Cappitelli, F.; Cattò, C.; Villa, F. The Control of Cultural Heritage Microbial Deterioration. Microorganisms 2020, 8, 1542. [Google Scholar] [CrossRef]
- Fidanza, M.R.; Caneva, G. Natural Biocides for the Conservation of Stone Cultural Heritage: A Review. J. Cult. Herit. 2019, 38, 271–286. [Google Scholar] [CrossRef]
- Tretiach, M.; Bertuzzi, S.; Candotto Carniel, F. Heat Shock Treatments: A New Safe Approach against Lichen Growth on Outdoor Stone Surfaces. Environ. Sci. Technol. 2012, 46, 6851–6859. [Google Scholar] [CrossRef] [PubMed]
- López, A.J.; Rivas, T.; Lamas, J.; Ramil, A.; Yáñez, A. Optimisation of Laser Removal of Biological Crusts in Granites. Appl. Phys. A 2010, 100, 733–739. [Google Scholar] [CrossRef]
- Mascalchi, M.; Orsini, C.; Pinna, D.; Salvadori, B.; Siano, S.; Riminesi, C. Assessment of Different Methods for the Removal of Biofilms and Lichens on Gravestones of the English Cemetery in Florence. Int. Biodeterior. Biodegrad. 2020, 154, 105041. [Google Scholar] [CrossRef]
- Osticioli, I.; Mascalchi, M.; Pinna, D.; Siano, S. Removal of Verrucaria nigrescens from Carrara Marble Artefacts Using Nd:YAG Lasers: Comparison among Different Pulse Durations and Wavelengths. Appl. Phys. A 2015, 118, 1517–1526. [Google Scholar] [CrossRef]
- Favero-Longo, S.E.; Matteucci, E.; Pinna, D.; Ruggiero, M.G.; Riminesi, C. Efficacy of the Environmentally Sustainable Microwave Heating Compared to Biocide Applications in the Devitalization of Phototrophic Communities Colonizing Rock Engravings of Valle Camonica, UNESCO World Heritage Site, Italy. Int. Biodeterior. Biodegrad. 2021, 165, 105327. [Google Scholar] [CrossRef]
- Sanmartín, P.; Bosch-Roig, P.; Pangallo, D.; Kraková, L.; Serrano, M. Unraveling Disparate Roles of Organisms, from Plants to Bacteria, and Viruses on Built Cultural Heritage. Appl. Microbiol. Biotechnol. 2023, 107, 2027–2037. [Google Scholar] [CrossRef]
- Pinna, D. Can We Do without Biocides to Cope with Biofilms and Lichens on Stone Heritage? Int. Biodeterior. Biodegrad. 2022, 172, 105437. [Google Scholar] [CrossRef]
- Pyzik, A.; Ciuchcinski, K.; Dziurzynski, M.; Dziewit, L. The Bad and the Good—Microorganisms in Cultural Heritage Environments—An Update on Biodeterioration and Biotreatment Approaches. Materials 2021, 14, 177. [Google Scholar] [CrossRef]
- Soffritti, I.; D’Accolti, M.; Lanzoni, L.; Volta, A.; Bisi, M.; Mazzacane, S.; Caselli, E. The Potential Use of Microorganisms as Restorative Agents: An Update. Sustainability 2019, 11, 3853. [Google Scholar] [CrossRef]
- Sanmartín, P.; Bosch-Roig, P. Biocleaning to Remove Graffiti: A Real Possibility? Advances towards a Complete Protocol of Action. Coatings 2019, 9, 104. [Google Scholar] [CrossRef]
- Le Métayer-Levrel, G.; Castanier, S.; Orial, G.; Loubière, J.-F.; Perthuisot, J.-P. Applications of Bacterial Carbonatogenesis to the Protection and Regeneration of Limestones in Buildings and Historic Patrimony. Sediment. Geol. 1999, 126, 25–34. [Google Scholar] [CrossRef]
- Sasso, S.; Miller, A.Z.; Rogerio-Candelera, M.A.; Cubero, B.; Coutinho, M.L.; Scrano, L.; Bufo, S.A. Potential of Natural Biocides for Biocontrolling Phototrophic Colonization on Limestone. Int. Biodeterior. Biodegrad. 2016, 107, 102–110. [Google Scholar] [CrossRef]
- Santos, A.C.C.; Malta, S.M.; Dantas, R.C.C.; Coelho Rocha, N.D.; Ariston De Carvalho Azevedo, V.; Ueira-Vieira, C. Antimicrobial Activity of Supernatants Produced by Bacteria Isolated from Brazilian Stingless Bee’s Larval Food. BMC Microbiol. 2022, 22, 127. [Google Scholar] [CrossRef] [PubMed]
- Jroundi, F.; Schiro, M.; Ruiz-Agudo, E.; Elert, K.; Martín-Sánchez, I.; González-Muñoz, M.T.; Rodriguez-Navarro, C. Protection and Consolidation of Stone Heritage by Self-Inoculation with Indigenous Carbonatogenic Bacterial Communities. Nat. Commun. 2017, 8, 279. [Google Scholar] [CrossRef]
- Jroundi, F.; Gonzalez-Muñoz, M.T.; Rodriguez-Navarro, C. Chapter 13. Protection and Consolidation of Stone Heritage by Bacterial Carbonatogenesis. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 281–299. ISBN 978-3-030-69411-1. [Google Scholar]
- Lyu, P.; Li, H.; Zheng, X.; Zhang, H.; Wang, C.; Qin, Y.; Xia, B.; Wang, D.; Xu, S.; Zhuang, X. Oxidative Stress of Microcystis aeruginosa Induced by Algicidal Bacterium Stenotrophomonas sp. KT48. Appl. Microbiol. Biotechnol. 2022, 106, 4329–4340. [Google Scholar] [CrossRef]
- Jia, L.; Cheng, X.; Fang, L.; Huang, X. Flocculation and Lysis of Microcystis aeruginosa by Paebubacillus sp. A9 and Inhibition of Microcystin Release. Environ. Technol. Innov. 2023, 31, 103152. [Google Scholar] [CrossRef]
- Abate, R.; Oon, Y.-L.; Oon, Y.-S.; Bi, Y.; Mi, W.; Song, G.; Gao, Y. Diverse Interactions between Bacteria and Microalgae: A Review for Enhancing Harmful Algal Bloom Mitigation and Biomass Processing Efficiency. Heliyon 2024, 10, e36503. [Google Scholar] [CrossRef]
- Shunyu, S.; Yongding, L.; Yinwu, S.; Genbao, L.; Dunhai, L. Lysis of Aphanizomenon flos-aquae (Cyanobacterium) by a Bacterium Bacillus cereus. Biol. Control 2006, 39, 345–351. [Google Scholar] [CrossRef]
- Sun, P.; Hui, C.; Wang, S.; Khan, R.A.; Zhang, Q.; Zhao, Y.-H. Enhancement of Algicidal Properties of Immobilized Bacillus methylotrophicus ZJU by Coating with Magnetic Fe3O4 Nanoparticles and Wheat Bran. J. Hazard. Mater. 2016, 301, 65–73. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-M.; Sheu, F.-S.; Sheu, S.-Y. Aquimarina Salinaria sp. Nov., a Novel Algicidal Bacterium Isolated from a Saltpan. Arch. Microbiol. 2012, 194, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Fallico, M.J.; Sambeth, J.E. Remoción biológica de Microcystis aeruginosa a partir de Achromobacter xylosoxidans, microorganismo aislado del Río de la Plata. In Transformar Diálogos de Saberes en Diálogos de Haceres: Ciencia, Comunidad y Políticas Públicas; Universidad Nacional de La Plata: La Plata, Argentina, 2020; Volume 16, pp. 320–342. ISBN 978-987-8348-66-7. [Google Scholar]
- Su, J.F.; Shao, S.C.; Huang, T.L.; Ma, F.; Lu, J.S.; Zhang, K. Algicidal Effects and Denitrification Activities of Acinetobacters. J25 against Microcystis aeruginosa. J. Environ. Chem. Eng. 2016, 4, 1002–1007. [Google Scholar] [CrossRef]
- Li, H.; Ai, H.; Kang, L.; Sun, X.; He, Q. Simultaneous Microcystis Algicidal and Microcystin Degrading Capability by a Single Acinetobacter Bacterial Strain. Environ. Sci. Technol. 2016, 50, 11903–11911. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.; Wu, L.; Pan, J.; Yang, H. The Algicidal Activity of Aeromonas sp. Strain GLY-2107 against Bloom-forming Microcystis aeruginosa Is Regulated by N-acyl Homoserine Lactone-mediated Quorum Sensing. Environ. Microbiol. 2016, 18, 3867–3883. [Google Scholar] [CrossRef]
- Ren, H.; Zhang, P.; Liu, C.; Xue, Y.; Lian, B. The Potential Use of Bacterium Strain R219 for Controlling of the Bloom-Forming Cyanobacteria in Freshwater Lake. World J. Microbiol. Biotechnol. 2010, 26, 465–472. [Google Scholar] [CrossRef]
- Weiss, G.; Kovalerchick, D.; Lieman-Hurwitz, J.; Murik, O.; De Philippis, R.; Carmeli, S.; Sukenik, A.; Kaplan, A. Increased Algicidal Activity of Aeromonas veronii in Response to Microcystis aeruginosa: Interspecies Crosstalk and Secondary Metabolites Synergism. Environ. Microbiol. 2019, 21, 1140–1150. [Google Scholar] [CrossRef]
- He, L.; Lin, Z.; Wang, Y.; He, X.; Zhou, J.; Guan, M.; Zhou, J. Facilitating Harmful Algae Removal in Fresh Water via Joint Effects of Multi-Species Algicidal Bacteria. J. Hazard. Mater. 2021, 403, 123662. [Google Scholar] [CrossRef]
- Guo, X.; Liu, X.; Pan, J.; Yang, H. Synergistic Algicidal Effect and Mechanism of Two Diketopiperazines Produced by Chryseobacterium sp. Strain GLY-1106 on the Harmful Bloom-Forming Microcystis aeruginosa. Sci. Rep. 2015, 5, 14720. [Google Scholar] [CrossRef]
- Nishu, S.D.; Kang, Y.; Han, I.; Jung, T.Y.; Lee, T.K. Nutritional Status Regulates Algicidal Activity of Aeromonas sp. L23 against Cyanobacteria and Green Algae. PLoS ONE 2019, 14, e0213370. [Google Scholar] [CrossRef]
- Zhang, H.; Yu, Z.; Huang, Q.; Xiao, X.; Wang, X.; Zhang, F.; Wang, X.; Liu, Y.; Hu, C. Isolation, Identification and Characterization of Phytoplankton-Lytic Bacterium CH-22 against Microcystis aeruginosa. Limnologica 2011, 41, 70–77. [Google Scholar] [CrossRef]
- Liu, Y.-M.; Wang, M.-H.; Jia, R.-B.; Li, L. Removal of Cyanobacteria by an Aeromonas sp. Desalination Water Treat. 2012, 47, 205–210. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Tan, J.; Lin, S.; Li, D.; Yang, H. Isolation, Identification and Characterization of an Algicidal Bacterium from Lake Taihu and Preliminary Studies on Its Algicidal Compounds. J. Environ. Sci. 2012, 24, 1823–1831. [Google Scholar] [CrossRef] [PubMed]
- Mu, R.; Fan, Z.; Pei, H.; Yuan, X.; Liu, S.; Wang, X. Isolation and Algae-Lysing Characteristics of the Algicidal Bacterium B5. J. Environ. Sci. 2007, 19, 1336–1340. [Google Scholar] [CrossRef] [PubMed]
- Park, B.S.; Park, C.-S.; Shin, Y.; Yoon, S.; Han, M.-S.; Kang, Y.-H. Different Algicidal Modes of the Two Bacteria Aeromonas bestiarum HYD0802-MK36 and Pseudomonas syringae KACC10292T against Harmful Cyanobacteria Microcystis aeruginosa. Toxins 2022, 14, 128. [Google Scholar] [CrossRef]
- Pal, M.; Yesankar, P.J.; Dwivedi, A.; Qureshi, A. Biotic Control of Harmful Algal Blooms (HABs): A Brief Review. J. Environ. Manag. 2020, 268, 110687. [Google Scholar] [CrossRef]
- Wu, L.; Wu, H.; Chen, L.; Xie, S.; Zang, H.; Borriss, R.; Gao, X. Bacilysin from Bacillus amyloliquefaciens FZB42 Has Specific Bactericidal Activity against Harmful Algal Bloom Species. Appl. Environ. Microbiol. 2014, 80, 7512–7520. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Kong, Y.; Gao, S.; Miao, L.; Zou, P.; Xu, B.; Zeng, C.; Zhang, X. Bacillus amyloliquefaciens T1 as a Potential Control Agent for Cyanobacteria. J. Appl. Phycol. 2015, 27, 1213–1221. [Google Scholar] [CrossRef]
- Xu, B.; Miao, L.; Yu, J.; Ji, L.; Lu, H.; Yang, J.; Gao, S.; Kong, Y. Isolation and Identification of Amino Acids Secreted by Bacillus amyloliquefaciens T1 with Anti-Cyanobacterial Effect against Cyanobacterium Microcystis aeruginosa. Desalination Water Treat. 2021, 231, 329–339. [Google Scholar] [CrossRef]
- Liu, J.; Yang, C.; Chi, Y.; Wu, D.; Dai, X.; Zhang, X.; Igarashi, Y.; Luo, F. Algicidal Characterization and Mechanism of Bacillus lcheniformis Sp34 against Microcystis aeruginosa in Dianchi Lake. J. Basic. Microbiol. 2019, 59, 1112–1124. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Zhu, S.; Qin, L.; Feng, P.; Xu, J.; Zhou, W.; Wang, Z. Isolation, Identification of Algicidal Bacteria and Contrastive Study on Algicidal Properties against Microcystis aeruginosa. Biochem. Eng. J. 2022, 185, 108525. [Google Scholar] [CrossRef]
- Gumbo, J.R.; Cloete, T.E. The Mechanism of Microcystis aeruginosa Death upon Exposure to Bacillus mycoides. Phys. Chem. Earth A/B/C 2011, 36, 881–886. [Google Scholar] [CrossRef]
- Font-Nájera, A.; Morón-López, J.; Glińska, S.; Balcerzak, Ł.; Grzyb, T.; Mankiewicz-Boczek, J. Algicidal Bacteria Induce a Molecular Stress Response in Microcystis aeruginosa and Aphanizomenon gracile Leading to Physiological Alterations and Cell Death. Int. Biodeterior. Biodegrad. 2024, 189, 105763. [Google Scholar] [CrossRef]
- Wu, D.; Yang, C.; Zhang, X.; Hou, X.; Zhang, S.; Dai, X.; Zhang, X.; Igarashi, Y.; Luo, F. Algicidal Effect of Tryptoline against Microcystis aeruginosa: Excess Reactive Oxygen Species Production Mediated by Photosynthesis. Sci. Total Environ. 2022, 806, 150719. [Google Scholar] [CrossRef]
- Li, Z.; Geng, M.; Yang, H. Algicidal Activity of Bacillus sp. Lzh-5 and Its Algicidal Compounds against Microcystis aeruginosa. Appl. Microbiol. Biotechnol. 2015, 99, 981–990. [Google Scholar] [CrossRef]
- Pei, H.; Hu, W. Lytic Characteristics and Identification of Two Alga-Lysing Bacterial Strains. J. Ocean. Univ. China 2006, 5, 368–374. [Google Scholar] [CrossRef]
- Alhakimi, A.A.; Alminderej, F.; Noman, E. Optimizing Microcystis aeruginosa Inactivation in Freshwater Using Algicidal Bacillus subtilis by Central Composite Design. Desalination Water Treat. 2020, 181, 228–238. [Google Scholar] [CrossRef]
- Chen, Y.; Xiong, F.; Zhu, Y.; Zhai, D.; Liu, H.; Zhang, L.; Xia, M. A Bacillus subtilis Strain with Efficient Algaecide of Microcystis aeruginosa and Degradation of Microcystins. Front. Microbiol. 2024, 15, 1430097. [Google Scholar] [CrossRef]
- Qiao, J.; Zhang, C. Identification of a Bacillus thuringiensis Q1 Compound with Algicidal Activity. Heliyon 2023, 9, e17649. [Google Scholar] [CrossRef] [PubMed]
- Rashidan, K.K.; Bird, D.F. Role of Predatory Bacteria in the Termination of a Cyanobacterial Bloom. Microb. Ecol. 2001, 41, 97–105. [Google Scholar] [CrossRef]
- Zhang, B.; Yang, Y.; Xie, W.; He, W.; Xie, J.; Liu, W. Identifying Algicides of Enterobacter hormaechei F2 for Control of the Harmful Alga Microcystis aeruginosa. Int. J. Environ. Res. Public Health 2022, 19, 7556. [Google Scholar] [CrossRef]
- Li, Y.; Liu, L.; Xu, Y.; Li, P.; Zhang, K.; Jiang, X.; Zheng, T.; Wang, H. Stress of Algicidal Substances from a Bacterium Exiguobacterium sp. H10 on Microcystis aeruginosa. Lett. Appl. Microbiol. 2017, 64, 57–65. [Google Scholar] [CrossRef]
- Zhang, D.; Ye, Q.; Zhang, F.; Shao, X.; Fan, Y.; Zhu, X.; Li, Y.; Yao, L.; Tian, Y.; Zheng, T.; et al. Flocculating Properties and Potential of Halobacillus sp. Strain H9 for the Mitigation of Microcystis aeruginosa Blooms. Chemosphere 2019, 218, 138–146. [Google Scholar] [CrossRef]
- Li, Y.; Qin, M.; Han, S.; Wang, Y.; Gao, C.; Niu, W.; Xia, X. Elimination of Microcystis aeruginosa through Leuconostoc mesenteroides DH and Its Underlying Mechanism. Sci. Total Environ. 2024, 908, 168290. [Google Scholar] [CrossRef]
- Kodani, S. Studies on Antialgal Compounds of Algicidal Bacteria. Doctoral Thesis, The University of Tokyo, Tokyo, Japan, 2002. [Google Scholar]
- Mu, R.; He, Y.; Liu, S.; Wang, X.; Fan, Z. The Algicidal Characteristics of One Algae-Lysing FDT5 Bacterium on Microcystis aeruginosa. Geomicrobiol. J. 2009, 26, 516–521. [Google Scholar] [CrossRef]
- Le, V.V.; Ko, S.-R.; Kang, M.; Lee, S.-A.; Oh, H.-M.; Ahn, C.-Y. Algicide Capacity of Paucibacter aquatile DH15 on Microcystis aeruginosa by Attachment and Non-Attachment Effects. Environ. Pollut. 2022, 302, 119079. [Google Scholar] [CrossRef]
- Yang, L.; Maeda, H.; Yoshikawa, T.; Zhou, G. Algicidal Effect of Bacterial Isolates of Pedobacter sp. against Cyanobacterium Microcystis aeruginosa. Water Sci. Eng. 2012, 5, 375–382. [Google Scholar] [CrossRef]
- Chen, S.; Haga, M.; Imai, I.; Sakai, R.; Fujita, M.J. Function of the Algicidal Bacterium Pseudomonas sp. Go58 Isolated from the Biofilm on a Water Plant, and Its Active Compounds, Pyoluteorins. Sci. Total Environ. 2023, 872, 162088. [Google Scholar] [CrossRef]
- Zhou, S.; Yin, H.; Tang, S.; Peng, H.; Yin, D.; Yang, Y.; Liu, Z.; Dang, Z. Physiological Responses of Microcystis aeruginosa against the Algicidal Bacterium Pseudomonas aeruginosa. Ecotoxicol. Environ. Saf. 2016, 127, 214–221. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Q.; Wang, Y.; Wen, X.; Liu, H.; Zhang, Y.; Zhang, Z. The Effect of Algicidal and Denitrifying Bacteria on the Vertical Distribution of Cyanobacteria and Nutrients. Water 2022, 14, 2129. [Google Scholar] [CrossRef]
- Yang, J.; Qiao, K.; Lv, J.; Liu, Q.; Nan, F.; Xie, S.; Feng, J. Isolation and Identification of Two Algae-Lysing Bacteria against Microcystis aeruginosa. Water 2020, 12, 2485. [Google Scholar] [CrossRef]
- Li, D.; Kang, X.; Chu, L.; Wang, Y.; Song, X.; Zhao, X.; Cao, X. Algicidal Mechanism of Raoultella ornithinolytica against Microcystis aeruginosa: Antioxidant Response, Photosynthetic System Damage and Microcystin Degradation. Environ. Pollut. 2021, 287, 117644. [Google Scholar] [CrossRef] [PubMed]
- Pal, M.; Pal, S.; Qureshi, A.; Sangolkar, L. Perspective of Cyanobacterial Harmful Algal Bloom (HAB) Mitigation: Microcystis Toxin Degradation by Bacterial Consortia. Indian J. Exp. Biol. 2018, 56, 511–518. [Google Scholar]
- Lee, Y.-K.; Ahn, C.-Y.; Kim, H.-S.; Oh, H.-M. Cyanobactericidal Effect of Rhodococcus sp. Isolated from Eutrophic Lake on Microcystis sp. Biotechnol. Lett. 2010, 32, 1673–1678. [Google Scholar] [CrossRef]
- Wei, J.; Xie, X.; Huang, F.; Xiang, L.; Wang, Y.; Han, T.; Massey, I.Y.; Liang, G.; Pu, Y.; Yang, F. Simultaneous Microcystis Algicidal and Microcystin Synthesis Inhibition by a Red Pigment Prodigiosin. Environ. Pollut. 2020, 256, 113444. [Google Scholar] [CrossRef]
- Yang, F.; Wei, H.Y.; Li, X.Q.; Li, Y.H.; Li, X.B.; Yin, L.H.; Pu, Y.P. Isolation and Characterization of an Algicidal Bacterium Indigenous to Lake Taihu with a Red Pigment Able to Lyse Microcystis aeruginosa. Biomed. Environ. Sci. 2013, 26, 148–154. [Google Scholar] [CrossRef]
- Darveau, R.P.; Lynch, D.L. The Antibiotic Activity of Prodigiosins against Certain Blue-Green and Green Algae. Phycologia 1977, 16, 349–351. [Google Scholar] [CrossRef]
- Ashton, P.J.; Robarts, R.D. Apparent Predation of Microcystis aeruginosa KüTZ. Emend Elenkin by a Saprospira-like Bacterium in a Hypertrophic Lake (Hartbeespoort Dam, South Africa). J. Limnol. Soc. South. Afr. 1987, 13, 44–47. [Google Scholar] [CrossRef]
- Yang, F.; Zhou, Y.; Yin, L.; Zhu, G.; Liang, G.; Pu, Y. Microcystin-Degrading Activity of an Indigenous Bacterial Strain Stenotrophomonas acidaminiphila MC-LTH2 Isolated from Lake Taihu. PLoS ONE 2014, 9, e86216. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Geng, M.; Liu, X.; Tan, J.; Yang, H. On the Control of Microcystis aeruginosa and Synechococccus Species Using an Algicidal bacterium, Stenotrophomonas F6, and Its Algicidal Compounds Cyclo-(Gly-Pro) and Hydroquinone. J. Appl. Phycol. 2016, 28, 345–355. [Google Scholar] [CrossRef]
- Zeng, Y.; Wang, J.; Yang, C.; Ding, M.; Hamilton, P.B.; Zhang, X.; Yang, C.; Zhnag, L.; Dai, X. A Streptomyces globisporus Strain Kills Microcystis aeruginosa via Cell-to-Cell Contact. Sci. Total Environ. 2021, 769, 144489. [Google Scholar] [CrossRef]
- Zhang, B.-H.; Ding, Z.-G.; Li, H.-Q.; Mou, X.-Z.; Zhang, Y.-Q.; Yang, J.-Y.; Zhou, E.-M.; Li, W.-J. Algicidal Activity of Streptomyces eurocidicus JXJ-0089 Metabolites and Their Effects on Microcystis Physiology. Appl. Environ. Microbiol. 2016, 82, 5132–5143. [Google Scholar] [CrossRef]
- Ma, B.; Li, A.; Chen, S.; Guo, H.; Li, N.; Pan, S.; Chen, K.; Liu, H.; Kosolapov, D.B.; Liu, X.; et al. Algicidal Activity Synchronized with Nitrogen Removal by Actinomycetes: Algicidal Mechanism, Stress Response of Algal Cells, Denitrification Performance, and Indigenous Bacterial Community Co-Occurrence. J. Hazard. Mater. 2024, 470, 134117. [Google Scholar] [CrossRef]
- Zhang, B.-H.; Cheng, J.; Chen, W.; Li, H.-Q.; Yang, J.-Y.; Park, D.-J.; Kim, C.-J.; Shen, R.; Duan, Y.-Q.; Li, W.-J. Streptomyces lushanensis sp. Nov., a Novel Actinomycete with Anti-Cyanobacterial Activity. J. Antibiot. 2015, 68, 5–8. [Google Scholar] [CrossRef]
- Choi, H.; Kim, B.; Kim, J.; Han, M. Streptomyces neyagawaensis as a Control for the Hazardous Biomass of Microcystis aeruginosa (Cyanobacteria) in Eutrophic Freshwaters. Biol. Control 2005, 33, 335–343. [Google Scholar] [CrossRef]
- Zhang, H.; Xie, Y.; Zhang, R.; Zhang, Z.; Hu, X.; Cheng, Y.; Geng, R.; Ma, Z.; Li, R. Discovery of a High-Efficient Algicidal Bacterium against Microcystis aeruginosa Based on Examinations toward Culture Strains and Natural Bloom Samples. Toxins 2023, 15, 220. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Cai, G.; Wang, H.; Hu, Z.; Zheng, W.; Lei, X.; Zhu, X.; Chen, Y.; Chen, Q.; Din, H.; et al. Fast-Growing Algicidal Streptomyces sp. U3 and Its Potential in Harmful Algal Bloom Controls. J. Hazard. Mater. 2018, 341, 138–149. [Google Scholar] [CrossRef]
- Kim, B.; Sang, M.; Hwang, S.; Han, M. In Situ Bacterial Mitigation of the Toxic Cyanobacterium Microcystis aeruginosa: Implications for Biological Bloom Control. Limnol. Ocean. Methods 2008, 6, 513–522. [Google Scholar] [CrossRef]
- Lu, L.; Niu, X.; Zhang, D.; Ma, J.; Zheng, X.; Xiao, H.; Huang, X.; Lin, Z.; Hu, H. The Algicidal Efficacy and the Mechanism of Enterobacter sp. EA-1 on Oscillatoria Dominating in Aquaculture System. Environ. Res. 2021, 197, 111105. [Google Scholar] [CrossRef]
- Nakamura, N.; Nakano, K.; Sugiura, N.; Matsumura, M. A Novel Control Process of Cyanobacterial Bloom Using Cyanobacteriolytic Bacteria Immobilized in Floating Biodegradable Plastic Carriers. Environ. Technol. 2003, 24, 1569–1576. [Google Scholar] [CrossRef]
- Su, J.F.; Ma, M.; Wei, L.; Ma, F.; Lu, J.S.; Shao, S.C. Algicidal and Denitrification Characterization of Acinetobacter sp. J25 against Microcystis aeruginosa and Microbial Community in Eutrophic Landscape Water. Mar. Pollut. Bull. 2016, 107, 233–239. [Google Scholar] [CrossRef]
- Fu, L.; An, X.; Li, D.; Zhou, L.; Tian, Y.; Zheng, T. Isolation and Alga-Inhibiting Characterization of Vibrio sp. BS02 against Alexandrium tamarense. World J. Microbiol. Biotechnol. 2011, 27, 2949–2956. [Google Scholar] [CrossRef]
- Zhao, S.; Pan, W.; Ma, C. Stimulation and Inhibition Effects of Algae-Lytic Products from Bacillus cereus Strain L7 on Anabaena flos-aquae. J. Appl. Phycol. 2012, 24, 1015–1021. [Google Scholar] [CrossRef]
- Wijesooriya, M.M.; Masakorala, K.; Widana Gamage, S.M.K. A Novel Cyanolytic Bacterium, Pseudomonas fluorescens BG-E as a Potential Biological Control Agent for Freshwater Bloom-forming Cyanobacteria Pseudanabaena Spp. J. Phycol. 2023, 59, 570–589. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Lu, J.; Wang, M.; Qin, W.; Chen, B.; Xu, H.; Ma, Z. Algicidal Bacteria in Phycosphere Regulate Free-Living Symbiodinium Fate via Triggering Oxidative Stress and Photosynthetic System Damage. Ecotoxicol. Environ. Saf. 2023, 263, 115369. [Google Scholar] [CrossRef]
- Alamri, S.A.; Mohamed, Z.A. Selective Inhibition of Toxic Cyanobacteria by β-Carboline-Containing Bacterium Bacillus flexus Isolated from Saudi Freshwaters. Saudi J. Biol. Sci. 2013, 20, 357–363. [Google Scholar] [CrossRef]
- Sallal, A.K. Lysis of Cyanobacteria with Flexibacter spp Isolated from Domestic Sewage. Microbios 1994, 77, 57–67. [Google Scholar]
- Hu, X.-J.; Xu, Y.; Su, H.-C.; Xu, W.-J.; Wang, L.-H.; Xu, Y.-N.; Li, Z.-J.; Cao, Y.-C.; Wen, G.-L. Algicidal Bacterium CZBC1 Inhibits the Growth of Oscillatoria chlorina, Oscillatoria tenuis, and Oscillatoria planctonica. AMB Express 2019, 9, 144. [Google Scholar] [CrossRef]
- Burnham, J.C.; Collart, S.A.; Highison, B.W. Entrapment and Lysis of the Cyanobacterium Phormidium luridum by Aqueous colonies of Myxococcus xanthus PCO2. Arch. Microbiol. 1981, 129, 285–294. [Google Scholar] [CrossRef]
- Burnham, J.C.; Collart, S.A.; Daft, M.J. Myxococcal Predation of the Cyanobacterium Phormidium luridum in Aqueous Environments. Arch. Microbiol. 1984, 137, 220–225. [Google Scholar] [CrossRef]
- Flaherty, K.W.; Walker, H.L.; Britton, C.H.; Lembi, C.A. Response of Cylindrospermopsis raciborskii and Pseudanabaena limnetica to a Potential Biological Control Agent, Bacterium SG-3 (Lysobacter Cf. brunescens). Lake Reserv. Manag. 2007, 23, 255–263. [Google Scholar] [CrossRef]
- Chen, W.M.; Sheu, F.S.; Sheu, S.Y. Novel L-Amino Acid Oxidase with Algicidal Activity against Toxic Cyanobacterium Microcystis aeruginosa Synthesized by a Bacterium Aquimarina sp. Enzym. Microb. Technol. 2011, 49, 372–379. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-H.; Kim, J.-D.; Kim, B.-H.; Kong, D.-S.; Han, M.-S. Isolation and Characterization of a Bio-Agent Antagonistic to Diatom, Stephanodiscus hantzschii. J. Appl. Microbiol. 2005, 98, 1030–1038. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Ren, C.; Hu, C.; Zhao, Z. Isolation and Algicidal Characterization of Bowmanella denitrificans S088 against Chlorella vulgaris. World J. Microbiol. Biotechnol. 2014, 30, 621–629. [Google Scholar] [CrossRef]
- Lu, Q.; Zhou, X.; Liu, R.; Shi, G.; Zheng, N.; Gao, G.; Wang, Y. Impacts of a Bacterial algicide on Metabolic Pathways in Chlorella vulgaris. Ecotoxicol. Environ. Saf. 2023, 249, 114451. [Google Scholar] [CrossRef]
- Afi, L.; Metzger, P.; Largeau, C.; Connan, J.; Berkaloff, C.; Rousseau, B. Bacterial Degradation of Green Microalgae: Incubation of Chlorella emersonii and Chlorella vulgaris with Pseudomonas oleovorans and Flavobacterium aquatile. Org. Geochem. 1996, 25, 117–130. [Google Scholar] [CrossRef]
- Chen, C.-Y.; Bai, M.-D.; Chang, J.-S. Improving Microalgal Oil Collecting Efficiency by Pretreating the Microalgal Cell Wall with Destructive Bacteria. Biochem. Eng. J. 2013, 81, 170–176. [Google Scholar] [CrossRef]
- Wang, X.; Gong, L.; Liang, S.; Han, X.; Zhu, C.; Li, Y. Algicidal Activity of Rhamnolipid Biosurfactants Produced by Pseudomonas aeruginosa. Harmful Algae 2005, 4, 433–443. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, H.; Lei, X.; Zhang, H.; Guan, C.; Chen, Z.; Zheng, W.; Xu, H.; Tian, Y.; Yu, Z.; et al. The First Evidence of Deinoxanthin from Deinococcus sp. Y35 with Strong Algicidal Effect on the Toxic Dinoflagellate Alexandrium tamarense. J. Hazard. Mater. 2015, 290, 87–95. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, J.; Stoyancheva, G.; Pouneva, I. Lysis of Antarctic Algal Strains by Bacterial Pathogen. Antonie Van Leeuwenhoek 2014, 105, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.M.; Lin, C.Y.; Sheu, S.Y. Investigating Antimicrobial Activity in Rheinheimera sp. Due to Hydrogen Peroxide Generated by l-Lysine Oxidase Activity. Enzym. Microb. Technol. 2010, 46, 487–493. [Google Scholar] [CrossRef]
- Gu, Y.; Wang, H.; Sun, M.; Ye, R.; Chen, B.; Cao, H.; An, J. Bacillus subtilis A4, a Potential Algicidal Bacterium against Spirogyra. Res. Sq. 2023, 1–21. [Google Scholar] [CrossRef]
- Bedoshvili, Y.; Bayramova, E.; Sudakov, N.; Klimenkov, I.; Kurilkina, M.; Likhoshway, Y.; Zakharova, Y. Impact of Algicidal Bacillus mycoides on Diatom Ulnaria acus from Lake Baikal. Diversity 2021, 13, 469. [Google Scholar] [CrossRef]
- Zakharova, Y.R.; Galachyants, Y.P.; Kurilkina, M.I.; Likhoshvay, A.V.; Petrova, D.P.; Shishlyannikov, S.M.; Ravin, N.V.; Mardanov, A.V.; Beletsky, A.V.; Likhoshway, Y.V. The Structure of Microbial Community and Degradation of Diatoms in the Deep Near-Bottom Layer of Lake Baikal. PLoS ONE 2013, 8, e59977. [Google Scholar] [CrossRef]
- Griffith, A.W.; Gobler, C.J. Harmful Algal Blooms: A Climate Change Co-Stressor in Marine and Freshwater Ecosystems. Harmful Algae 2020, 91, 101590. [Google Scholar] [CrossRef]
- Coyne, K.J.; Wang, Y.; Johnson, G. Algicidal Bacteria: A Review of Current Knowledge and Applications to Control Harmful Algal Blooms. Front. Microbiol. 2022, 13, 871177. [Google Scholar] [CrossRef]
- Meyer, N.; Bigalke, A.; Kaulfuß, A.; Pohnert, G. Strategies and Ecological Roles of Algicidal Bacteria. FEMS Microbiol. Rev. 2017, 41, 880–899. [Google Scholar] [CrossRef]
- Shi, X.; Liu, L.; Li, Y.; Xiao, Y.; Ding, G.; Lin, S.; Chen, J. Isolation of an Algicidal Bacterium and Its Effects against the Harmful-Algal- Bloom Dinoflagellate Prorocentrum donghaiense (Dinophyceae). Harmful Algae 2018, 80, 72–79. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, J.; Niu, Y.; Yang, W.; Liu, J.; Li, H. Systems-Level Analysis of the Metabolic Responses of the Diatom Phaeodactylum tricornutum to Phosphorus Stress. Environ. Microbiol. 2014, 16, 1793–1807. [Google Scholar] [CrossRef]
- Lenneman, E.M.; Barney, B.M. Draft Genome Sequences of the Alga-Degrading Bacteria Aeromonas hydrophila Strain AD9 and Pseudomonas pseudoalcaligenes Strain AD6. Genome Announc. 2014, 2, e00709-14. [Google Scholar] [CrossRef] [PubMed]
- Demuez, M.; González-Fernández, C.; Ballesteros, M. Algicidal Microorganisms and Secreted Algicides: New Tools to Induce Microalgal Cell Disruption. Biotechnol. Adv. 2015, 33, 1615–1625. [Google Scholar] [CrossRef] [PubMed]
- Oren, A. On Validly Published Names, Correct Names, and Changes in the Nomenclature of Phyla and Genera of Prokaryotes: A Guide for the Perplexed. npj Biofilms Microbiomes 2024, 10, 20. [Google Scholar] [CrossRef] [PubMed]
- Dungca-Santos, J.C.R.; Caspe, F.J.O.; Tablizo, F.A.; Purganan, D.J.E.; Azanza, R.V.; Onda, D.F.L. Algicidal Potential of Cultivable Bacteria from Pelagic Waters against the Toxic Dinoflagellate Pyrodinium bahamense (Dinophyceae). J. Appl. Phycol. 2019, 31, 3721–3735. [Google Scholar] [CrossRef]
- Zheng, N.; Ding, N.; Gao, P.; Han, M.; Liu, X.; Wang, J.; Sun, L.; Fu, B.; Wang, R.; Zhou, J. Diverse Algicidal Bacteria Associated with Harmful Bloom-Forming Karenia mikimotoi in Estuarine Soil and Seawater. Sci. Total Environ. 2018, 631–632, 1415–1420. [Google Scholar] [CrossRef]
- Jeong, S.-Y.; Son, H.-J. Effects of Mycosubtilin Homolog Algicides from a Marine Bacterium, Bacillus sp. SY-1, against the Harmful Algal Bloom Species Cochlodinium polykrikoides. J. Microbiol. 2021, 59, 389–400. [Google Scholar] [CrossRef]
- Silva, M.; Rosado, T.; Teixeira, D.; Candeias, A.; Caldeira, A.T. Green Mitigation Strategy for Cultural Heritage: Bacterial Potential for Biocide Production. Environ. Sci. Pollut. Res. 2017, 24, 4871–4881. [Google Scholar] [CrossRef]
- Marin, E.; Vaccaro, C.; Leis, M. Biotechnology Applied to Historic Stoneworks Conservation: Testing the Potential Harmfulness of Two Biological Biocides. Int. J. Conserv. Sci. 2016, 7, 227–238. [Google Scholar]
- Sprocati, A.R.; Alisi, C.; Migliore, G.; Marconi, P.; Tasso, F. Sustainable Restoration Through Biotechnological Processes: A Proof of Concept. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 235–261. ISBN 978-3-030-69411-1. [Google Scholar]
- Mazzoni, M.; Alisi, C.; Tasso, F.; Cecchini, A.; Marconi, P.; Sprocati, A.R. Laponite Micro-Packs for the Selective Cleaning of Multiple Coherent Deposits on Wall Paintings: The Case Study of Casina Farnese on the Palatine Hill (Rome-Italy). Int. Biodeterior. Biodegrad. 2014, 94, 1–11. [Google Scholar] [CrossRef]
- Barbabietola, N.; Tasso, F.; Alisi, C.; Marconi, P.; Perito, B.; Pasquariello, G.; Sprocati, A.R. A Safe Microbe-Based Procedure for a Gentle Removal of Aged Animal Glues from Ancient Paper. Int. Biodeterior. Biodegrad. 2016, 109, 53–60. [Google Scholar] [CrossRef]
- Jeszeová, L.; Benžová, R.; Gluštíková, M.; Šišková, A.; Kisová, Z.; Planý, M.; Kraková, L.; Bauerová-Hlinková, V.; Pangallo, D. Biocleaning of Historical Documents: The Use and Characterization of Bacterial Enzymatic Resources. Int. Biodeterior. Biodegrad. 2019, 140, 106–112. [Google Scholar] [CrossRef]
- Kooli, W.M.; Junier, T.; Shakya, M.; Monachon, M.; Davenport, K.W.; Vaideeswaran, K.; Vernudachi, A.; Marozau, I.; Monrouzeau, T.; Gleasner, C.D.; et al. Remedial Treatment of Corroded Iron Objects by Environmental Aeromonas Isolates. Appl. Environ. Microbiol. 2019, 85, e02042-18. [Google Scholar] [CrossRef] [PubMed]
- Jimenez-Lopez, C.; Rodriguez-Navarro, C.; Piñar, G.; Carrillo-Rosúa, F.J.; Rodriguez-Gallego, M.; Gonzalez-Muñoz, M.T. Consolidation of Degraded Ornamental Porous Limestone Stone by Calcium Carbonate Precipitation Induced by the Microbiota Inhabiting the Stone. Chemosphere 2007, 68, 1929–1936. [Google Scholar] [CrossRef]
- Castanier, S.; Le Métayer-Levrel, G.; Orial, G.; Loubière, J.-F.; Perthuisot, J.-P. Bacterial Carbonatogenesis and Applications to Preservation and Restoration of Historic Property. In Of Microbes and Art: The Role of Microbial Communities in the Degradation and Protection of Cultural Heritage; Ciferri, O., Tiano, P., Mastromei, G., Eds.; Springer US: Boston, MA, USA, 2000; pp. 203–218. ISBN 978-1-4615-4239-1. [Google Scholar]
- González-Muñoz, M.T.; Rodriguez-Navarro, C.; Martínez-Ruiz, F.; Arias, J.M.; Merroun, M.L.; Rodriguez-Gallego, M. Bacterial Biomineralization: New Insights from Myxococcus-Induced Mineral Precipitation. SP 2010, 336, 31–50. [Google Scholar] [CrossRef]
- Fouad ElHagrassy, A. Bio-Restoration of Mural Paintings Using Viable Cells of Pseudomonas stutzeri and Characterization of These Murals. Int. J. Archaeol. 2019, 7, 8. [Google Scholar] [CrossRef]
- Zhang, F.; Fan, Y.; Zhang, D.; Chen, S.; Bai, X.; Ma, X.; Xie, Z.; Xu, H. Effect and Mechanism of the Algicidal Bacterium Sulfitobacter Porphyrae ZFX1 on the Mitigation of Harmful Algal Blooms Caused by Prorocentrum donghaiense. Environ. Pollut. 2020, 263, 114475. [Google Scholar] [CrossRef]
- Lee, T.C.; Lam, W.; Tam, N.F.; Xu, S.J.; Chung, W.L.; Lee, F.W. Revealing the Algicidal Characteristics of Maribacter dokdonensis: An Investigation into Bacterial Strain P4 Isolated from Karenia mikimotoi Bloom Water. J. Phycol. 2024, 60, 541–553. [Google Scholar] [CrossRef]
- Ongena, M.; Jacques, P. Bacillus lipopeptides: Versatile Weapons for Plant Disease Biocontrol. Trends Microbiol. 2008, 16, 115–125. [Google Scholar] [CrossRef]
- Zhao, X.; Kuipers, O.P. Identification and Classification of Known and Putative Antimicrobial Compounds Produced by a Wide Variety of Bacillales Species. BMC Genom. 2016, 17, 882. [Google Scholar] [CrossRef]
- Zhang, C.; Massey, I.Y.; Liu, Y.; Huang, F.; Gao, R.; Ding, M.; Xiang, L.; He, C.; Wei, J.; Li, Y.; et al. Identification and Characterization of a Novel Indigenous Algicidal Bacterium Chryseobacterium Species against Microcystis aeruginosa. J. Toxicol. Environ. Health A 2019, 82, 845–853. [Google Scholar] [CrossRef]
- Chen, Y.; Luo, G.; Chen, S.; Zhang, D.; Xie, W.; Wang, Z.; Zheng, W.; Xu, H. The Potential of Prodigiosin for Control of Prorocentrum donghaiense Blooms: Algicidal Properties and Acute Toxicity to Other Marine Organisms at Various Trophic Levels. Ecotoxicol. Environ. Saf. 2021, 228, 112913. [Google Scholar] [CrossRef]
- Zhang, S.; Zheng, W.; Wang, H. Physiological Response and Morphological Changes of Heterosigma akashiwo to an Algicidal Compound Prodigiosin. J. Hazard. Mater. 2020, 385, 121530. [Google Scholar] [CrossRef]
- Yi, Y.-L.; Yu, X.-B.; Zhang, C.; Wang, G.-X. Growth Inhibition and Microcystin Degradation Effects of Acinetobacter guillouiae A2 on Microcystis aeruginosa. Res. Microbiol. 2015, 166, 93–101. [Google Scholar] [CrossRef]
- Weiss, G.; Kovalerchick, D.; Murik, O.; Sukenik, A.; Kaplan, A.; Carmeli, S. Secondary Metabolites of Aeromonas veronii Strain A134 Isolated from a Microcystis aeruginosa Bloom. Metabolites 2019, 9, 110. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Zhang, J.; Zhao, X.; Wang, P.; Tong, M.; Glibert, P.M. Allelopathic Inhibition by the Bacteria Bacillus cereus BE23 on Growth and Photosynthesis of the Macroalga Ulva prolifera. J. Mar. Sci. Eng. 2020, 8, 718. [Google Scholar] [CrossRef]
- Wu, L.; Guo, X.; Liu, X.; Yang, H. NprR-NprX Quorum-Sensing System Regulates the Algicidal Activity of Bacillus sp. Strain S51107 against Bloom-Forming Cyanobacterium Microcystis aeruginosa. Front. Microbiol. 2017, 8, 1968. [Google Scholar] [CrossRef] [PubMed]
- Azizoglu, U.; Argentel-Martínez, L.; Peñuelas-Rubio, O.; Herrera-Sepúlveda, A.; Ibal, J.C.; Sharafi, R.; Salehi Jouzani, G.; Ortiz, A.; Vaca, J.; Sansinenea, E. Natural Products Produced by the Species of Bacillus cereus Group: Recent Updates. J. Basic. Microbiol. 2025, 65, e2400666. [Google Scholar] [CrossRef]
- Su, J.F.; Shao, S.C.; Ma, F.; Lu, J.S.; Zhang, K. Bacteriological Control by Raoultella sp. R11 on Growth and Toxins Production of Microcystis aeruginosa. Chem. Eng. J. 2016, 293, 139–150. [Google Scholar] [CrossRef]
- Xie, Y.; Zhang, H.; Cui, B.; Geng, R.; Grossart, H.-P.; Xiao, P.; Zuo, J.; Zhang, H.; Wang, Z.; Wang, G.; et al. Enhanced Inhibitory Efficiency against Toxic Bloom Forming Raphidiopsis raciborskii by Streptomyces sp. HY through Triple Algicidal Modes: Direct and Indirect Attacks Combined with Bioflocculation. J. Hazard. Mater. 2024, 477, 135152. [Google Scholar] [CrossRef]
- Bosch-Roig, P.; Allegue, H.; Bosch, I. Granite Pavement Nitrate Desalination: Traditional Methods vs. Biocleaning Methods. Sustainability 2019, 11, 4227. [Google Scholar] [CrossRef]
- Bosch-Roig, P.; Lustrato, G.; Zanardini, E.; Ranalli, G. Biocleaning of Cultural Heritage Stone Surfaces and Frescoes: Which Delivery System Can Be the Most Appropriate? Ann. Microbiol. 2015, 1227–1241. [Google Scholar] [CrossRef]
- Romanelli, A.; Soto, D.X.; Matiatos, I.; Martínez, D.E.; Esquius, S. A Biological and Nitrate Isotopic Assessment Framework to Understand Eutrophication in Aquatic Ecosystems. Sci. Total Environ. 2020, 715, 136909. [Google Scholar] [CrossRef]
- Pinna, D. Chapter 1. Microbial Growth and Its Effects on Inorganic Heritage Materials. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer International Publishing: Berlin/Heidelberg, Germany, 2021; Volume 156, pp. 3–35. ISBN 978-3-030-69410-4. [Google Scholar]
- Paredes, I.; Otero, N.; Soler, A.; Green, A.J.; Soto, D.X. Agricultural and Urban Delivered Nitrate Pollution Input to Mediterranean Temporary Freshwaters. Agric. Ecosyst. Environ. 2020, 294, 106859. [Google Scholar] [CrossRef]
- Bozdağ, A.; İnce, İ.; Bozdağ, A.; Hatır, M.E.; Tosunlar, M.B.; Korkanç, M. An Assessment of Deterioration in Cultural Heritage: The Unique Case of Eflatunpınar Hittite Water Monument in Konya, Turkey. Bull. Eng. Geol. Environ. 2020, 79, 1185–1197. [Google Scholar] [CrossRef]
- Roig, P.B.; Regidor Ros, J.L.; Estellés, R.M. Biocleaning of Nitrate Alterations on Wall Paintings by Pseudomonas stutzeri. Int. Biodeterior. Biodegrad. 2013, 84, 266–274. [Google Scholar] [CrossRef]
- Ranalli, G.; Zanardini, E. The Role of Microorganisms in the Removal of Nitrates and Sulfates on Artistic Stoneworks. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 263–279. ISBN 978-3-030-69411-1. [Google Scholar]
- Bosch-Roig, P.; Ranalli, G. The Safety of Biocleaning Technologies for Cultural Heritage. Front. Microbiol. 2014, 5. [Google Scholar] [CrossRef]
- Fernando, P.U.A.I.; Kennedy, A.J.; Pokrzywinski, K.; Jernberg, J.; Thornell, T.; George, G.; Kosgei, G.K.; Wang, Y.; Coyne, K.J. Development of Alginate Beads for Precise Environmental Release Applications: A Design of Experiment Based Approach and Analysis. J. Environ. Manag. 2024, 351, 119872. [Google Scholar] [CrossRef]
- Sun, P.; Lin, H.; Wang, G.; Zhang, X.; Zhang, Q.; Zhao, Y. Wheat Bran Enhances the Cytotoxicity of Immobilized Alcaligenes aquatilis F8 against Microcystis aeruginosa. PLoS ONE 2015, 10, e0136429. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Coyne, K.J. Immobilization of Algicidal Bacterium Shewanella sp. IRI-160 and Its Application to Control Harmful Dinoflagellates. Harmful Algae 2020, 94, 101798. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-H.; Jung, S.W.; Joo, J.-H.; Han, M.-S. Use of Immobilized Algicidal Bacteria to Control Natural Freshwater Diatom Blooms. Hydrobiologia 2012, 683, 151–162. [Google Scholar] [CrossRef]
- Inaba, N.; Kodama, I.; Nagai, S.; Shiraishi, T.; Matsuno, K.; Yamaguchi, A.; Imai, I. Distribution of Harmful Algal Growth-Limiting Bacteria on Artificially Introduced Ulva and Natural Macroalgal Beds. Appl. Sci. 2020, 10, 5658. [Google Scholar] [CrossRef]
- Fuentes, J.; Garbayo, I.; Cuaresma, M.; Montero, Z.; González-del-Valle, M.; Vílchez, C. Impact of Microalgae-Bacteria Interactions on the Production of Algal Biomass and Associated Compounds. Mar. Drugs 2016, 14, 100. [Google Scholar] [CrossRef]
- Zhang, B.; Li, W.; Guo, Y.; Zhang, Z.; Shi, W.; Cui, F.; Lens, P.N.L.; Tay, J.H. Microalgal-Bacterial Consortia: From Interspecies Interactions to Biotechnological Applications. Renew. Sustain. Energy Rev. 2020, 118, 109563. [Google Scholar] [CrossRef]
- Lee, C.; Jeon, M.S.; Vo, T.-T.; Park, C.; Choi, J.-S.; Kwon, J.; Roh, S.W.; Choi, Y.-E. Establishment of a New Strategy against Microcystis Bloom Using Newly Isolated Lytic and Toxin-Degrading Bacteria. J. Appl. Phycol. 2018, 30, 1795–1806. [Google Scholar] [CrossRef]
- Sun, R.; Sun, P.; Zhang, J.; Esquivel-Elizondo, S.; Wu, Y. Microorganisms-Based Methods for Harmful Algal Blooms Control: A Review. Bioresour. Technol. 2018, 248, 12–20. [Google Scholar] [CrossRef]
- MacDonald, L.C.; Weiler, E.B.; Berger, B.W. Engineering Broad-Spectrum Digestion of Polyuronides from an Exolytic Polysaccharide Lyase. Biotechnol. Biofuels 2016, 9, 43. [Google Scholar] [CrossRef]
- Sakami, T.; Sakamoto, S.; Takagi, S.; Inaba, N.; Imai, I. Distribution of Three Algicidal Alteromonas sp. Strains in Seagrass Beds and Surrounding Areas in the Seto Inland Sea, Japan. Fish. Sci. 2017, 83, 113–121. [Google Scholar] [CrossRef]
- Morris, R.L.; Konlechner, T.M.; Ghisalberti, M.; Swearer, S.E. From Grey to Green: Efficacy of Eco-Engineering Solutions for Nature-Based Coastal Defence. Glob. Chang. Biol. 2018, 24, 1827–1842. [Google Scholar] [CrossRef]
- Imai, I.; Inaba, N.; Yamamoto, K. Harmful Algal Blooms and Environmentally Friendly Control Strategies in Japan. Fish. Sci. 2021, 87, 437–464. [Google Scholar] [CrossRef]
- Marvasi, M.; Mastromei, G.; Perito, B. Bacterial Calcium Carbonate Mineralization in Situ Strategies for Conservation of Stone Artworks: From Cell Components to Microbial Community. Front. Microbiol. 2020, 11, 1386. [Google Scholar] [CrossRef] [PubMed]
- Gauri, K.L.; Chowdhury, A.N.; Kulshreshtha, N.P.; Adinarayana, R.P. The Sulfation of Marble and the Treatment of Gypsum Crusts. Stud. Conserv. 1989, 34, 201–206. [Google Scholar] [CrossRef]
- Ranalli, G.; Zanardini, E. Chapter 10. Advanced Biocleaning System for Historical Wall Paintings. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer Nature: Berlin, Germany, 2012; pp. 51–61. ISBN 978-3-030-69410-4. [Google Scholar]
- Atlas, R.M.; Chowdhury, A.N.; Gauri, K.L. Microbial Calcification of Gypsum-Rock and Sulfated Marble. Stud. Conserv. 1988, 33, 149–153. [Google Scholar] [CrossRef]
- Ranalli, G.; Zanardini, E. Biocleaning on Cultural Heritage: New Frontiers of Microbial Biotechnologies. J. Appl. Microbiol. 2021, 131, 583–603. [Google Scholar] [CrossRef]
- Dhami, N.K.; Reddy, M.S.; Mukherjee, A. Application of Calcifying Bacteria for Remediation of Stones and Cultural Heritages. Front. Microbiol. 2014, 5, 304. [Google Scholar] [CrossRef]
- Monachon, M.; Albelda-Berenguer, M.; Pelé, C.; Cornet, E.; Guilminot, E.; Rémazeilles, C.; Joseph, E. Characterization of Model Samples Simulating Degradation Processes Induced by Iron and Sulfur Species on Waterlogged Wood. Microchem. J. 2020, 155, 104756. [Google Scholar] [CrossRef]
- Ranalli, G.; Bosch-Roig, P.; Crudele, S.; Rampazzi, L.; Corti, C.; Zanardini, E. Dry Biocleaning of Artwork: An Innovative Methodology for Cultural Heritage Recovery? Microb. Cell 2021, 8, 91–105. [Google Scholar] [CrossRef] [PubMed]
- Ayangbenro, A.S.; Olanrewaju, O.S.; Babalola, O.O. Sulfate-Reducing Bacteria as an Effective Tool for Sustainable Acid Mine Bioremediation. Front. Microbiol. 2018, 9, 1986. [Google Scholar] [CrossRef]
- Del Bosch Roig, M.P.; Regidor Ros, J.L.; Soriano Sancho, M.P.; Domenech Carbo, M.T.; Montes Estellés, R.M. Ensayos de biolimpieza con bacterias en pinturas murales. Arché 2010, 5, 117–124. [Google Scholar]
- Ranalli, G.; Chiavarini, M.; Guidetti, V.; Marsala, F.; Matteini, M.; Zanardini, E.; Sorlini, C. The Use of Microorganisms for the Removal of Sulphates on Artistic Stoneworks. Int. Biodeterior. Biodegrad. 1997, 40, 255–261. [Google Scholar] [CrossRef]
- Cappitelli, F.; Zanardini, E.; Toniolo, L.; Abbruscato, P.; Ranalli, G. Bioconservation of the Marble Base of the Pietà Rondanini by Michelangelo Buonarroti. Geophys. Res. Abstr. 2005, 7, 1–2. [Google Scholar]
- Elhagrassy, A.F.; Hakeem, A. Comparative Study of Biological Cleaning and Laser Techniques for Conservation of Weathered Stone in Failaka Island, Kuwait. Sci. Cult. 2018, 4, 43–50. [Google Scholar] [CrossRef]
- Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer International Publishing: Cham, Switzerland, 2021; ISBN 978-3-030-69410-4. [Google Scholar]
- Alfano, G.; Lustrato, G.; Belli, C.; Zanardini, E.; Cappitelli, F.; Mello, E.; Sorlini, C.; Ranalli, G. The Bioremoval of Nitrate and Sulfate Alterations on Artistic Stonework: The Case-Study of Matera Cathedral after Six Years from the Treatment. Int. Biodeterior. Biodegrad. 2011, 65, 1004–1011. [Google Scholar] [CrossRef]
- Jroundi, F.; Fernández-Vivas, A.; Rodriguez-Navarro, C.; Bedmar, E.J.; González-Muñoz, M.T. Bioconservation of Deteriorated Monumental Calcarenite Stone and Identification of Bacteria with Carbonatogenic Activity. Microb. Ecol. 2010, 60, 39–54. [Google Scholar] [CrossRef]
- Spairani-Berrio, Y.; Huesca-Tortosa, J.A.; Rodriguez-Navarro, C.; Gonzalez-Muñoz, M.T.; Jroundi, F. Bioconsolidation of Damaged Construction Calcarenites and Evaluation of the Improvement in Their Petrophysical and Mechanical Properties. Materials 2023, 16, 6043. [Google Scholar] [CrossRef]
- Jroundi, F.; Gómez-Suaga, P.; Jimenez-Lopez, C.; González-Muñoz, M.T.; Fernandez-Vivas, M.A. Stone-Isolated Carbonatogenic Bacteria as Inoculants in Bioconsolidation Treatments for Historical Limestone. Sci. Total Environ. 2012, 425, 89–98. [Google Scholar] [CrossRef]
- Ko, S.-R.; Le, V.V.; Srivastava, A.; Kang, M.; Oh, H.-M.; Ahn, C.-Y. Algicidal Activity of a Novel Bacterium, Qipengyuania sp. 3-20A1M, against Harmful Margalefidinium polykrikoides: Effects of Its Active Compound. Mar. Pollut. Bull. 2023, 186, 114397. [Google Scholar] [CrossRef]
- Pinna, D. Coping with Biological Growth on Stone Heritage Objects: Methods, Products, Applications, and Perspectives; Apple Academic Press: New York, NY, USA, 2017; ISBN 978-1-315-36551-0. [Google Scholar]
- Caldeira, A.T. Chapter 6. Green Mitigation Strategy for Cultural Heritage Using Bacterial Biocides. In Microorganisms in the Deterioration and Preservation of Cultural Heritage; Joseph, E., Ed.; Springer International Publishing: Cham, Switzerland, 2021; pp. 137–154. ISBN 978-3-030-69411-1. [Google Scholar]
- Pinna, D. Microbial Recolonization of Artificial and Natural Stone Artworks after Cleaning and Coating Treatments. J. Cult. Herit. 2023, 61, 217–228. [Google Scholar] [CrossRef]
- Li, H.; Xing, R.; Ji, X.; Liu, Y.; Chu, X.; Gu, J.; Wang, S.; Wang, G.; Zhao, S.; Cao, X. Natural Algicidal Compounds: Strategies for Controlling Harmful Algae and Application. Plant Physiol. Biochem. 2024, 215, 108981. [Google Scholar] [CrossRef]
- Qian, Y.; He, Y.; Li, H.; Yi, M.; Zhang, L.; Zhang, L.; Liu, L.; Lu, Z. Benzalkonium Chlorides (C12) Inhibits Growth but Motivates Microcystins Release of Microcystis aeruginosa Revealed by Morphological, Physiological, and iTRAQ Investigation. Environ. Pollut. 2022, 292, 118305. [Google Scholar] [CrossRef]
- Zhang, S.-J.; Du, X.-P.; Zhu, J.-M.; Meng, C.-X.; Zhou, J.; Zuo, P. The Complete Genome Sequence of the Algicidal Bacterium Bacillus subtilis Strain JA and the Use of Quorum Sensing to Evaluate Its Antialgal Ability. Biotechnol. Rep. 2020, 25, e00421. [Google Scholar] [CrossRef]
- Barresi, G.; Cammarata, M.; Palla, F. Biocide. In Biotechnology and Conservation of Cultural Heritage; Palla, F., Barresi, G., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 49–65. ISBN 978-3-319-46168-7. [Google Scholar]
- Arreche, R.; Vázquez, P. Green Biocides to Control Biodeterioration in Materials Science and the Example of Preserving World Heritage Monuments. Curr. Opin. Green. Sustain. Chem. 2020, 25, 100359. [Google Scholar] [CrossRef]
- Comensoli, L.; Maillard, J.; Albini, M.; Sandoz, F.; Junier, P.; Joseph, E. Use of Bacteria To Stabilize Archaeological Iron. Appl. Environ. Microbiol. 2017, 83, e03478-16. [Google Scholar] [CrossRef]
- Petraretti, M.; Siciliano, A.; Carraturo, F.; Cimmino, A.; De Natale, A.; Guida, M.; Pollio, A.; Evidente, A.; Masi, M. An Ecotoxicological Evaluation of Four Fungal Metabolites with Potential Application as Biocides for the Conservation of Cultural Heritage. Toxins 2022, 14, 407. [Google Scholar] [CrossRef] [PubMed]
- Xue, G.; Wang, X.; Xu, C.; Song, B.; Chen, H. Removal of Harmful Algae by Shigella sp. H3 and Alcaligenes sp. H5: Algicidal Pathways and Characteristics. Environ. Technol. 2022, 43, 4341–4353. [Google Scholar] [CrossRef] [PubMed]
- Kang, Y.-H.; Kim, B.-R.; Choi, H.J.; Seo, J.G.; Kim, B.-H.; Han, M.-S. Enhancement of Algicidal Activity by Immobilization of Algicidal Bacteria Antagonistic to Stephanodiscus hantzschii (Bacillariophyceae). J. Appl. Microbiol. 2007, 103, 1983–1994. [Google Scholar] [CrossRef] [PubMed]
- Jung, S.W.; Kang, Y.-H.; Baek, S.H.; Lim, D.; Han, M.-S. Biological Control of Stephanodiscus hantzschii (Bacillariophyceae) Blooms in a Field Mesocosm by the Immobilized Algicidal Bacterium Pseudomonas fluorescens HYK0210-SK09. J. Appl. Phycol. 2013, 25, 41–50. [Google Scholar] [CrossRef]
- Jain, S.; Fang, C.; Achal, V. A Critical Review on Microbial Carbonate Precipitation via Denitrification Process in Building Materials. Bioengineered 2021, 12, 7529–7551. [Google Scholar] [CrossRef] [PubMed]
- Gherardi, F.; Turyanska, L.; Ferrari, E.; Weston, N.; Fay, M.W.; Colston, B.J. Immobilized Enzymes on Gold Nanoparticles: From Enhanced Stability to Cleaning of Heritage Textiles. ACS Appl. Bio Mater. 2019, 2, 5136–5143. [Google Scholar] [CrossRef]
- Caselli, E.; Arnoldo, L.; Rognoni, C.; D’Accolti, M.; Soffritti, I.; Lanzoni, L.; Bisi, M.; Volta, A.; Tarricone, R.; Brusaferro, S.; et al. Impact of a Probiotic-Based Hospital Sanitation on Antimicrobial Resistance and HAI-Associated Antimicrobial Consumption and Costs: A Multicenter Study. Infect. Drug Resist. 2019, 12, 501–510. [Google Scholar] [CrossRef]
- Elsabagh, M.; Mohamed, R.; Moustafa, E.M.; Hamza, A.; Farrag, F.; Decamp, O.; Dawood, M.A.O.; Eltholth, M. Assessing the Impact of Bacillus Strains Mixture Probiotic on Water Quality, Growth Performance, Blood Profile and Intestinal Morphology of Nile Tilapia, Oreochromis Niloticus. Aquac. Nutr. 2018, 24, 1613–1622. [Google Scholar] [CrossRef]
- Zink, I.C.; Benetti, D.D.; Douillet, P.A.; Margulies, D.; Scholey, V.P. Improvement of Water Chemistry with Bacillus Probiotics Inclusion during Simulated Transport of Yellowfin Tuna Yolk Sac Larvae. North. Am. J. Aquac. 2011, 73, 42–48. [Google Scholar] [CrossRef]
- Gomes, L.C.; Brinn, R.P.; Marcon, J.L.; Dantas, L.A.; Brandão, F.R.; Sampaio de Abreu, J.; McComb, D.M.; Baldisserotto, B. Using Efinol®L during Transportation of Marbled Hatchetfish, Carnegiella Strigata (Günther). Aquac. Res. 2008, 39, 1292–1298. [Google Scholar] [CrossRef]
- George, M.; Joseph, L.; Mathew, R.; Jose, P.C. Evaluation of Clinical Outcome, Drug Utilization and Effect on Liver Enzymes of Anti-Epileptics in Epileptic Patients. Int. J. Curr. Rch. Acd. Rev. 2016, 4, 32–37. [Google Scholar] [CrossRef][Green Version]
- Hlordzi, V.; Kuebutornye, F.K.A.; Afriyie, G.; Abarike, E.D.; Lu, Y.; Chi, S.; Anokyewaa, M.A. The Use of Bacillus Species in Maintenance of Water Quality in Aquaculture: A Review. Aquac. Rep. 2020, 18, 100503. [Google Scholar] [CrossRef]
- Kim, J.-D.; Kim, J.-Y.; Park, J.-K.; Lee, C.-G. Selective Control of the Prorocentrum Minimum Harmful Algal Blooms by a Novel Algal-Lytic Bacterium Pseudoalteromonas haloplanktis AFMB-008041. Mar. Biotechnol. 2009, 11, 463–472. [Google Scholar] [CrossRef]
- Li, S.; Wang, S.; Xie, L.; Liu, Y.; Chen, H.; Feng, J.; Ouyang, L. Identification and Optimization of the Algicidal Activity of a Novel Marine Bacterium Against Akashiwo Sanguinea. Front. Mar. Sci. 2022, 9, 798544. [Google Scholar] [CrossRef]
- Wang, J.; Yin, X.; Xu, M.; Chen, Y.; Ji, N.; Gu, H.; Cai, Y.; Shen, X. Isolation and Characterization of a High-Efficiency Algicidal Bacterium Pseudoalteromonas sp. LD-B6 against the Harmful Dinoflagellate Noctiluca Scintillans. Front. Microbiol. 2022, 13, 1091561. [Google Scholar] [CrossRef]
- Kooli, W.M.; Comensoli, L.; Maillard, J.; Albini, M.; Gelb, A.; Junier, P.; Joseph, E. Bacterial Iron Reduction and Biogenic Mineral Formation for the Stabilisation of Corroded Iron Objects. Sci. Rep. 2018, 8, 764. [Google Scholar] [CrossRef]
- Cote, C.; Rosas, O.; Basseguy, R. Geobacter Sulfurreducens: An Iron Reducing Bacterium That Can Protect Carbon Steel against Corrosion? Corros. Sci. 2015, 94, 104–113. [Google Scholar] [CrossRef]
- Schütz, M.K.; Schlegel, M.L.; Libert, M.; Bildstein, O. Impact of Iron-Reducing Bacteria on the Corrosion Rate of Carbon Steel under Simulated Geological Disposal Conditions. Environ. Sci. Technol. 2015, 49, 7483–7490. [Google Scholar] [CrossRef]
- Mazzoli, R.; Giuffrida, M.G.; Pessione, E. Back to the Past: “Find the Guilty Bug—Microorganisms Involved in the Biodeterioration of Archeological and Historical Artifacts”. Appl. Microbiol. Biotechnol. 2018, 102, 6393–6407. [Google Scholar] [CrossRef] [PubMed]
- Drumond, M.M.; Tapia-Costa, A.P.; Neumann, E.; Nunes, Á.C.; Barbosa, J.W.; Kassuha, D.E.; Mancha-Agresti, P. Cell-Free Supernatant of Probiotic Bacteria Exerted Antibiofilm and Antibacterial Activities against Pseudomonas aeruginosa: A Novel Biotic Therapy. Front. Pharmacol. 2023, 14, 1152588. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.J.; Huang, L.P.; Su, J.Q.; Tian, Y.; Zheng, T.L. Algicidal Effects of a Novel Marine Actinomycete on the Toxic Dinoflagellate Alexandrium tamarense. Curr. Microbiol. 2011, 62, 1774–1781. [Google Scholar] [CrossRef] [PubMed]
- Luo, J.; Wang, Y.; Tang, S.; Liang, J.; Lin, W.; Luo, L. Isolation and Identification of Algicidal Compound from Streptomyces and Algicidal Mechanism to Microcystis aeruginosa. PLoS ONE 2013, 8, e76444. [Google Scholar] [CrossRef]
- Vanittanakom, N.; Loeffler, W.; Koch, U.; Jung, G. Fengycin—A Novel Antifungal Lipopeptide Antibiotic Produced by Bacillus subtilis F-29-3. J. Antibiot. 1986, 39, 888–901. [Google Scholar] [CrossRef]
- Besson, F.; Michel, G. Isolation and Characterization of New Iturins: Iturin D and Iturin E. J. Antibiot. 1987, 40, 437–442. [Google Scholar] [CrossRef]
- Kowall, M.; Vater, J.; Kluge, B.; Stein, T.; Franke, P.; Ziessow, D. Separation and Characterization of Surfactin Isoforms Produced by Bacillus subtilis OKB 105. J. Colloid. Interface Sci. 1998, 204, 1–8. [Google Scholar] [CrossRef] [PubMed]


| Photosynthetic Microorganism | Risks to Cultural Heritage | Algicidal Bacteria | Reference |
|---|---|---|---|
| Unicellular cyanobacteria | |||
| Microcystis sp. | 1, 2, 4, 5, 6, 7, 8, 9, 11, 12 | Against Microcystis aeruginosa: Achromobacter sp. Acinetobacter sp. Aeromonas sp. Aeromonas bestarium Aeromonas guillouiae Aeromonas veronii Agrobacterium vitis Aquimarina salinaria sp. nov Aquimarina sp. Bacillus amyloliquefaciens Bacillus cereus Bacillus fusiformis Bacillus licheniformis Bacillus methylotrophicus Bacillus mycoides Bacillus thuringiensis Bacillus siamensis Bacillus sp. Bacillus subtilis Bacillus pumilus Brevibacillus sp. Brevundimonas diminuta Chryseobacterium sp. Cytophaga sp. Enterobacter sp. Enterobacter hormaechei Exiguobacterium sp. Halobacillus sp. Leuconostoc mesenteroides Lysobacter sp. Morganella morganii Ochrobactrum sp. Paebubacillus sp. Paenibacillus polymyxa Paenibacillus alvei Paucibacter aquatile Pedobacter sp. Pseudomonas sp. Pseudomonas aeruginosa Pseudomonas putida Pseudomonas stutzeri Pseudomonas syringae Raoultella planticola Raoultella ornithinolytica Raoultella sp. Rhizobium sp. Rhodococcus sp. Serratia marcescens Saprospira albida Stenotrophomonas sp. Stenotrophomonas acidaminiphila Streptomyces globisporus Streptomyces eurocidicus Streptomyces lushanensis sp.nov. Streptomyces neyagawaensis Streptomyces sp. Xanthobacter autotrophicus Against M. viridis: Aeromonas sp. Bacillus cereus Bacillus sp. Chryseobacterium sp. Exiguobacterium sp. Pseudomonas sp. Pseudomonas putida Stenotrophomonas sp. Streptomyces lushanensis sp.nov. Against M. wesenbergii: Bacillus cereus Bacillus licheniformis Enterobacter sp. Stenotrophomonas sp. Streptomyces lushanensis sp.nov. Pseudomonas putida Against M. flos-aquae: Aeromonas sp. Streptomyces lushanensis sp.nov. | [7,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103] |
| Chroococcus sp. | 1, 2, 3, 6, 7, 9, 11, 13, 14 | Aeromonas sp. Bacillus sp. Chryseobacterium sp. Exiguobacterium sp. | [46,50,54,66,104] |
| Filamentous cyanobacteria | |||
| Anabaena sp. | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12 | Pseudomonas sp. Serratia marcescens, Streptomyces sp. Streptomyces eurocidicus Bacillus amyloliquefaciens Against Anabaena variabilis: Aeromonas sp. Lysobacter sp. Rhodococcus sp. Pseudomonas sp. Against Anabaena cylindrica: Aeromonas sp. Lysobacter sp. Pseudomonas sp. Pseudomonas putida Against Anabaena flos-aquae: Aeromonas sp. Bacillus cereus Bacillus thuringiensis Cytophaga sp. Pseudomonas putida Streptomyces globisporus Streptomyces lushanensis sp.nov. | [39,52,53,58,71,81,87,90,93,95,96,99,105] |
| Leptolyngbya sp. | 1, 2, 3, 4, 5, 6, 7, 8, 9, 11 | Pseudomonas fluorescens | [106] |
| Nostoc sp. | 1, 2, 3, 4, 5, 6, 7, 8, 9, 11, 12 | Bacillus cereus (against N. punctiforme specie) Bacillus amyloliquefaciens Exiguobacterium sp. Streptomyces lushanensis sp. nov. Pseudomonas fluorescens | [40,54,58,96,106] |
| Oscillatoria sp. | 1, 2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 15 | Aeromonas sp. Bacillus sp. Bacillus cereus Bacillus flexus Chryseobacterium sp. Enterobacter sp. Exiguobacterium sp. Flexibacter sp. Pseudomonas sp. Pseudomonas fluorescens Stenotrophomonas sp. Streptomyces globisporus Against O. tenuis: Bacillus cereus Enterobacter sp. Enterobacter asburiae Pseudomonas simiae oli Streptomyces lushanensis sp. nov. Against O. planctonica: Bacillus cereus Streptomyces lushanensis sp. nov. Exiguobacterium sp. | [41,46,50,54,66,92,93,97,101,106,107,108,109,110] |
| Phormidium sp. | 1, 2, 3, 4, 5, 7, 9 | Bacillus licheniformis Myxococcus xanthus (against P. luridum specie) Streptomyces globisporus Pseudomonas fluorescens Aquimarina sp. (against P. persicinum specie) | [61,93,106,111,112] |
| Pseudanabaena sp. | 1, 2, 3, 4, 6, 7, 8, 9, 10, 11, 12 | Streptomyces sp. Pseudomonas fluorescens Lysobacter cf. brunescens (against limnetica specie) | [98,106,113] |
| Unicellular green algae | |||
| Chlamydomonas sp. | 1, 2, 4, 7, 8, 9 | Aeromonas sp. Aquimarina sp. (against C. raudensis) Bacillus cereus (against C. reinhardtii specie) Exiguobacterium sp. Chryseobacterium sp. Stenotrophomonas sp. | [46,50,54,92,114] |
| Chlorella sp. | 1, 2, 4, 5, 6, 7, 8, 9, 11, 15 | Bacillus fusiformis Bacillus sp. Against C. vulgaris: Aeromonas sp. Aquimarina sp. Bowmanella denitrificans Enterobacter sp. Flammeovirga yaeyamensis Flavobacterium aquatile Microbacterium paraoxydans Pseudomonas oleovorans Stenotrophomonas sp. Against C. ellipsoidea: Bacillus cereus Pseudomonas putida Against C. emersonii: Flavobacterium aquatile Pseudomonas oleovorans Against C. autotrophica Streptomyces sp. Deinococcus sp. Against C. pyrenoidosa Aquimarina sp. | [40,42,51,52,55,92,99,101,115,116,117,118,119,120,121] |
| Choricystis minor | 2, 4, 5, 6, 7, 8, 9 | Microbacterium sp. | [122] |
| Scenedesmus sp. | 1, 2, 3, 4, 5, 6, 7, 11, 15 | Against S. quadricauda: Aeromonas sp. Enterobacter asburiae Pseudomonas simiae oli Against S. obliquus: Bacillus fusiformis | [49,51,55,107,123] |
| Filamentous green algae | |||
| Spyrogira gracilis | 1, 2, 4, 7, 8, 9, 11 | Bacillus subtilis | [124] |
| Diatoms | |||
| Synedra acus subsp. radians (Ulnaria) | 1, 2, 4, 5, 7, 8, 9, 11 * Although Synedra acus subsp. radians has not been reported in cultural heritage biodeterioration, species of the genus Synedra (currently included in Ulnaria) have been observed forming biofilms on stone surfaces in ornamental fountains and similar water-contact environments. | Brevundimonas bullata Sphingomonas rhizogenes Agrobacterium tumefaciens Methylobacterium adhaesivum Acinetobacter johnsonii Bacillus simplex Bacillus mycoides Deinococcus aquaticus | [125,126] |
| Algicidal Bacteria | Bioactive Compounds | Type | Reference |
|---|---|---|---|
| Aeromonas sp. | 3-methylindole and 3-benzyl-piperazine-2,5-dione | Amino acid and peptide derivatives | [46] |
| Aeromonas guillouiae | 4-hydroxyphenethylamine | Alkaloid | [156] |
| Aeromonas veronii | lumichrome, 9-chlorolumichrome, veronimide, and veronipyrazine | Flavin derivatives, nitrogen compounds, and pyrazine | [48,157] |
| Aquimarina sp. | l-amino acid oxidase (l-AAO) | Enzimes | [114] |
| Bacillus amyloliquefaciens | Bacilysin; L-lysine (Lys) and L-phenylalanine (Phe) | Amino acids | [58,59,60] |
| Bacillus cereus | N-phenethylacetamide; Ciclo (L-Pro-L-Val); Ciclo (L-Pro-L-Pro) | Derivatives of simple amides and cyclic diketopiperazine | [158] |
| Bacillus siamensis | tryptoline (C11H12N2) | Alkaloids | [65] |
| Bacillus sp. | 3-Isopropyl-hexahydropyrrolo [1, 2-a] pyrazine-1,4-dione and Hexahydropyrrolo [1,2-a] pyrazine-1, 4-dione Indole-3-carboxaldehyde, cyclo(Pro-Phe), and unidentified high-molecular-weight compound(s) (>3 kDa) | Alkaloid and diketopiperazine alkaloid, DKP, and macromolecular compound (>3 kDa) | [66,159] |
| Bacillus subtilis | Bacteriocins, sactibiotics, non-ribosomal polypeptides, and lipopeptides (such as surfactin and fengycin) with unidentified antimicrobial activity against freshwater photosynthetic microorganisms | Peptides and lipopeptides | [160] |
| Bacillus thuringiensis | Purine derivative identified as C12H15O5N5 | Derivative of nitrogenous bases | [70] |
| Chryseobacterium sp. | cyclo(4-OH-Pro-Leu), cyclo(Pro-Leu) | Diketopiperazines | [50] |
| Deinococcus sp. | Deinoxanthin | Terpenes | [121] |
| Enterobacter hormaechei | Prodigiosin | Alkaloids | [72] |
| Flammeovirga yaeyamensis | Amylases, celulases, xylanases | Enzimes | [119] |
| Leuconostoc mesenteroides | phenyl-lactic acid | Organic acids derived from amino acids | [75] |
| Lysobacter sp. | L-tyrosine | Derivated from amino acids | [113] |
| Microbacterium paraoxydans | Atrazine-desethyl | Nitrogenous organic compound | [117] |
| Raoultella sp. | dissolved microbial metabolites and humic acid) and smaller amounts of other substances (protein-like substances and fulvic acid) | Undefined microbial metabolites and humic substances | [161] |
| Raoultella ornithinolytica | D-Gluconic acid, Chlorogenic acid, L-Malic acid, 5-Hydroxy-2,4-dioxopentanoate, 2-Methyl-3-oxopropanoic acid | Low-molecular-weight organic acids (e.g., gluconic acid, malic acid) and a phenolic compound (chlorogenic acid) | [84] |
| Serratia marcescens | Prodigiosin | Alkaloids | [89] |
| Stenotrophomonas sp. | Cyclo-(Gly-Pro), Hydroquinone | Diketopiperazine and phenolic compound | [92] |
| Streptomyces sp. | Flavonoids and unidentified extracellular metabolites | Flavonoids | [162] |
| Streptomyces eurocidicus | tryptamine and tryptoline | Alkaloids | [65] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Calvo-Bayo, I.; Bolívar-Galiano, F.; Romero-Noguera, J. Algicidal Bacteria: A Sustainable Proposal to Control Microalgae in the Conservation and Restoration of Stone Cultural Heritage. Sustainability 2025, 17, 10610. https://doi.org/10.3390/su172310610
Calvo-Bayo I, Bolívar-Galiano F, Romero-Noguera J. Algicidal Bacteria: A Sustainable Proposal to Control Microalgae in the Conservation and Restoration of Stone Cultural Heritage. Sustainability. 2025; 17(23):10610. https://doi.org/10.3390/su172310610
Chicago/Turabian StyleCalvo-Bayo, Isabel, Fernando Bolívar-Galiano, and Julio Romero-Noguera. 2025. "Algicidal Bacteria: A Sustainable Proposal to Control Microalgae in the Conservation and Restoration of Stone Cultural Heritage" Sustainability 17, no. 23: 10610. https://doi.org/10.3390/su172310610
APA StyleCalvo-Bayo, I., Bolívar-Galiano, F., & Romero-Noguera, J. (2025). Algicidal Bacteria: A Sustainable Proposal to Control Microalgae in the Conservation and Restoration of Stone Cultural Heritage. Sustainability, 17(23), 10610. https://doi.org/10.3390/su172310610







