Advances in Adsorptive Atmospheric Water Harvesting Technology: Materials, Desorption, and Systems
Abstract
1. Introduction
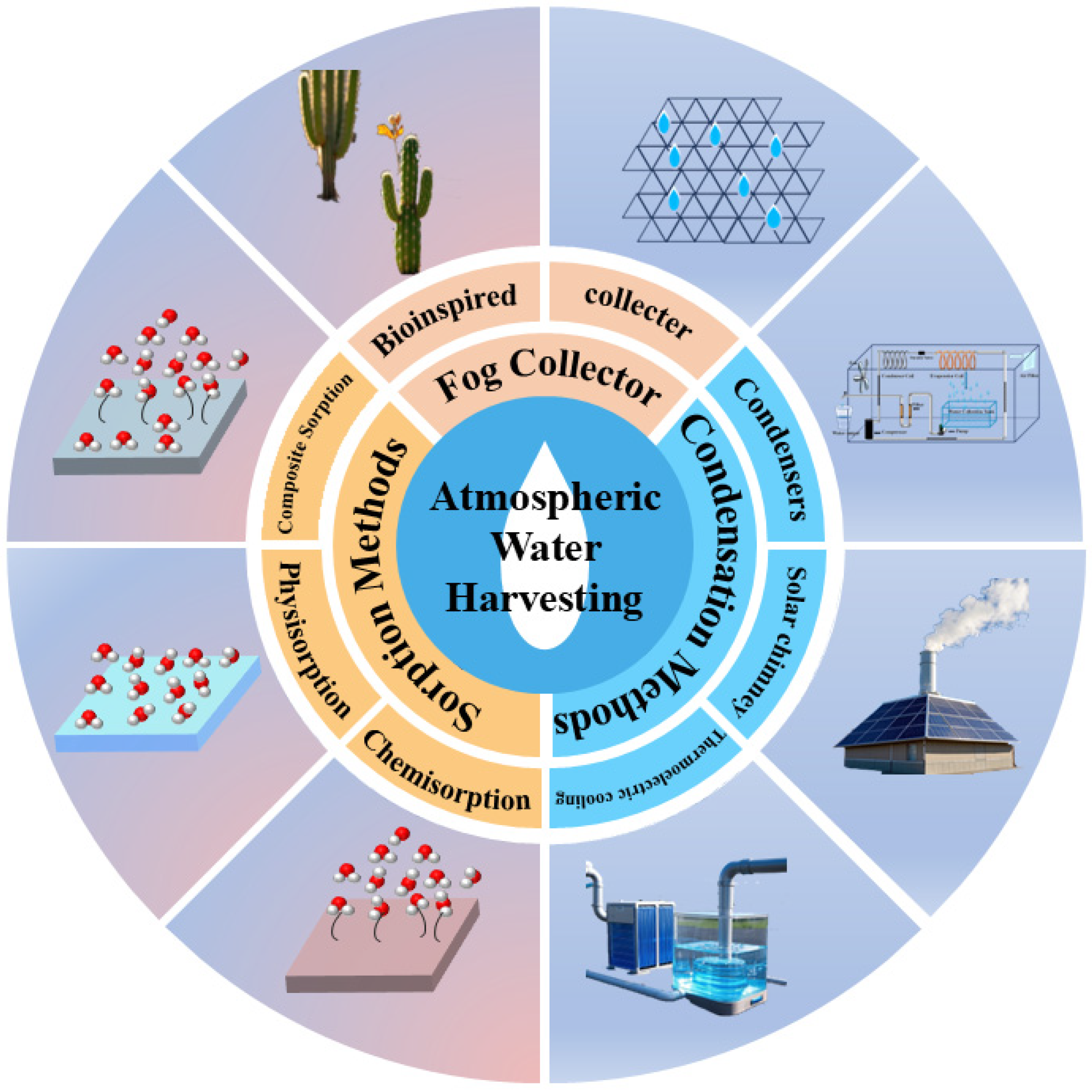
2. Atmospheric Water Harvesting Technology Framework and Challenges
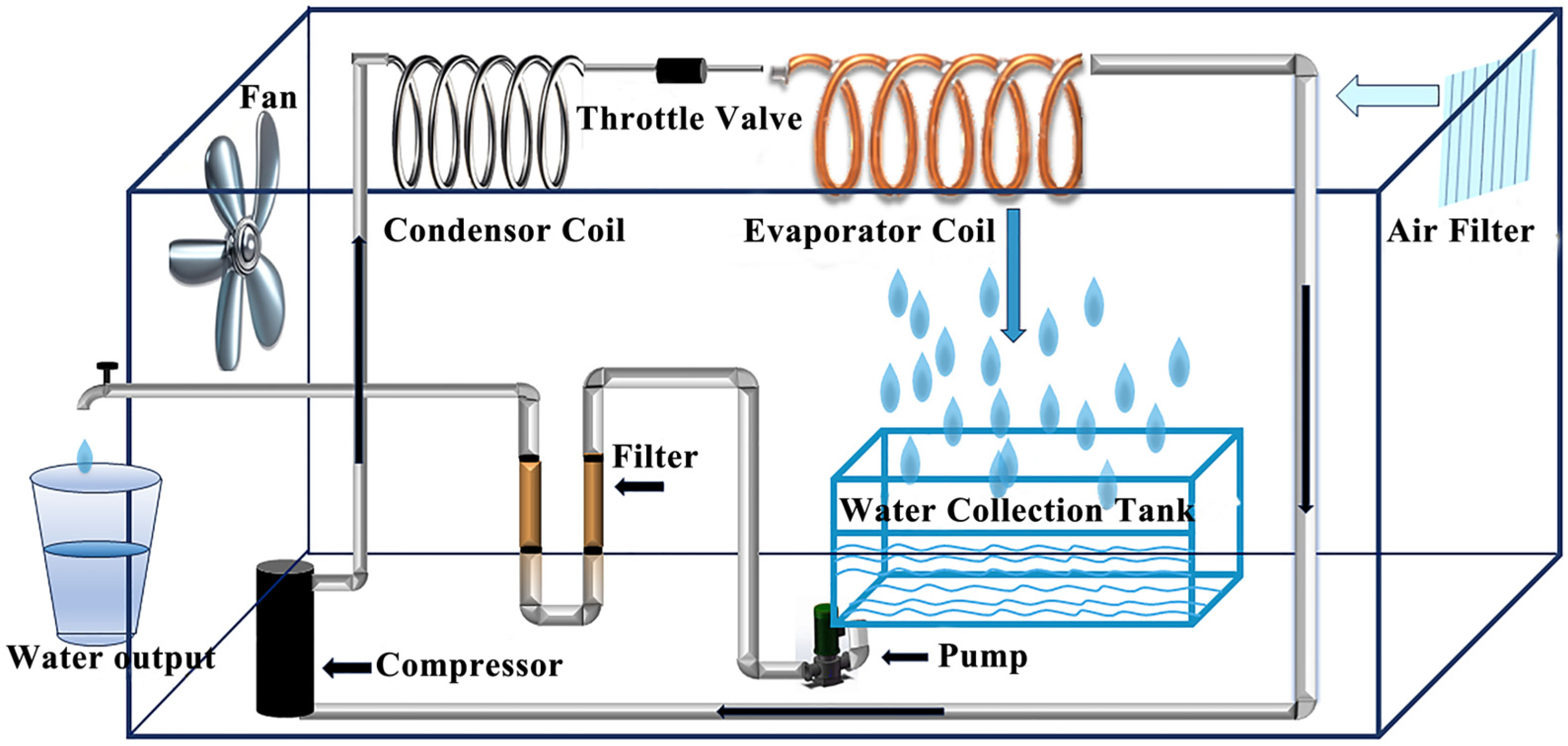
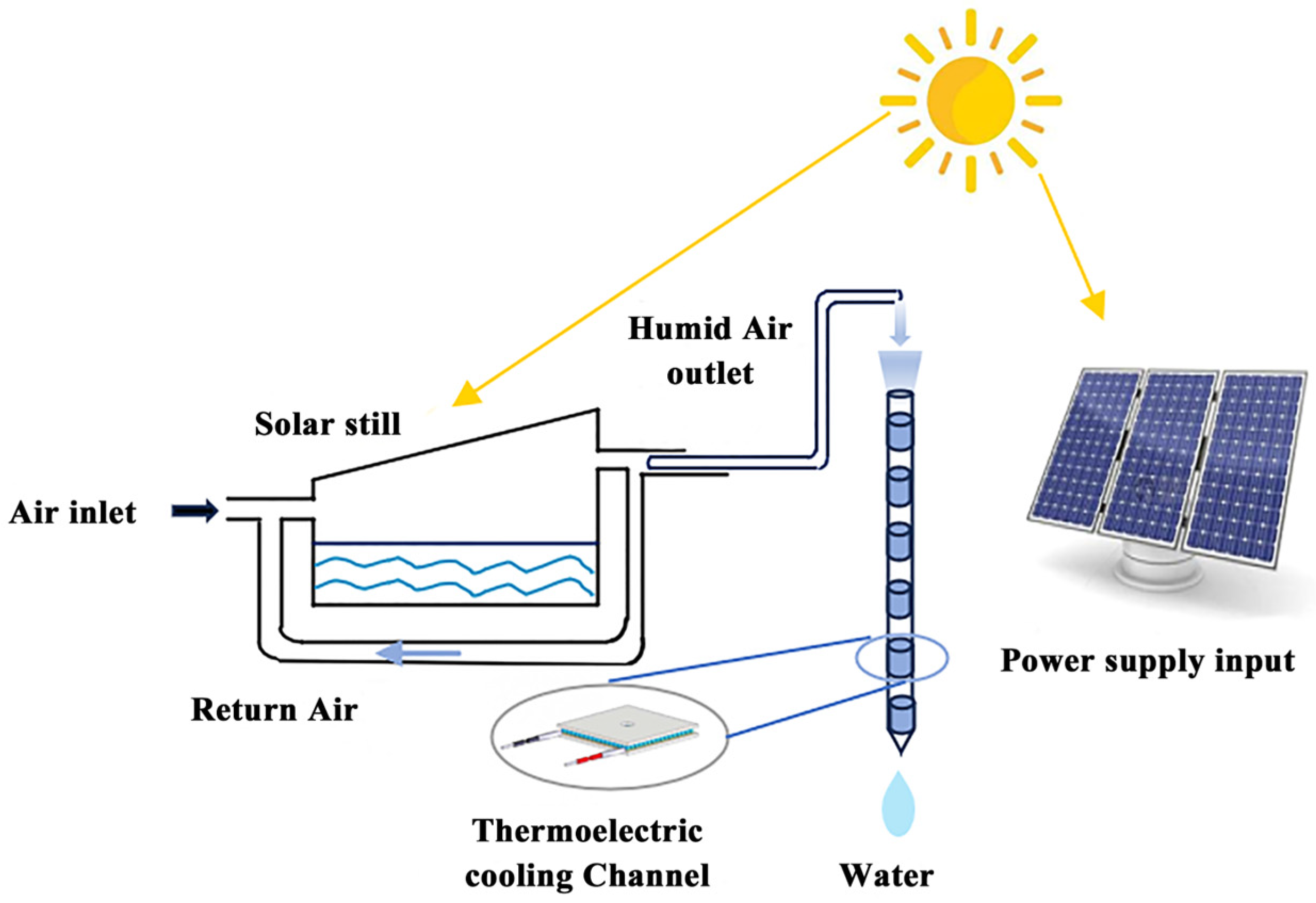
2.1. Condensing
2.2. Fog Collection
2.3. Adsorption
2.3.1. Intermittent SAWH (Day–Night Cycle)
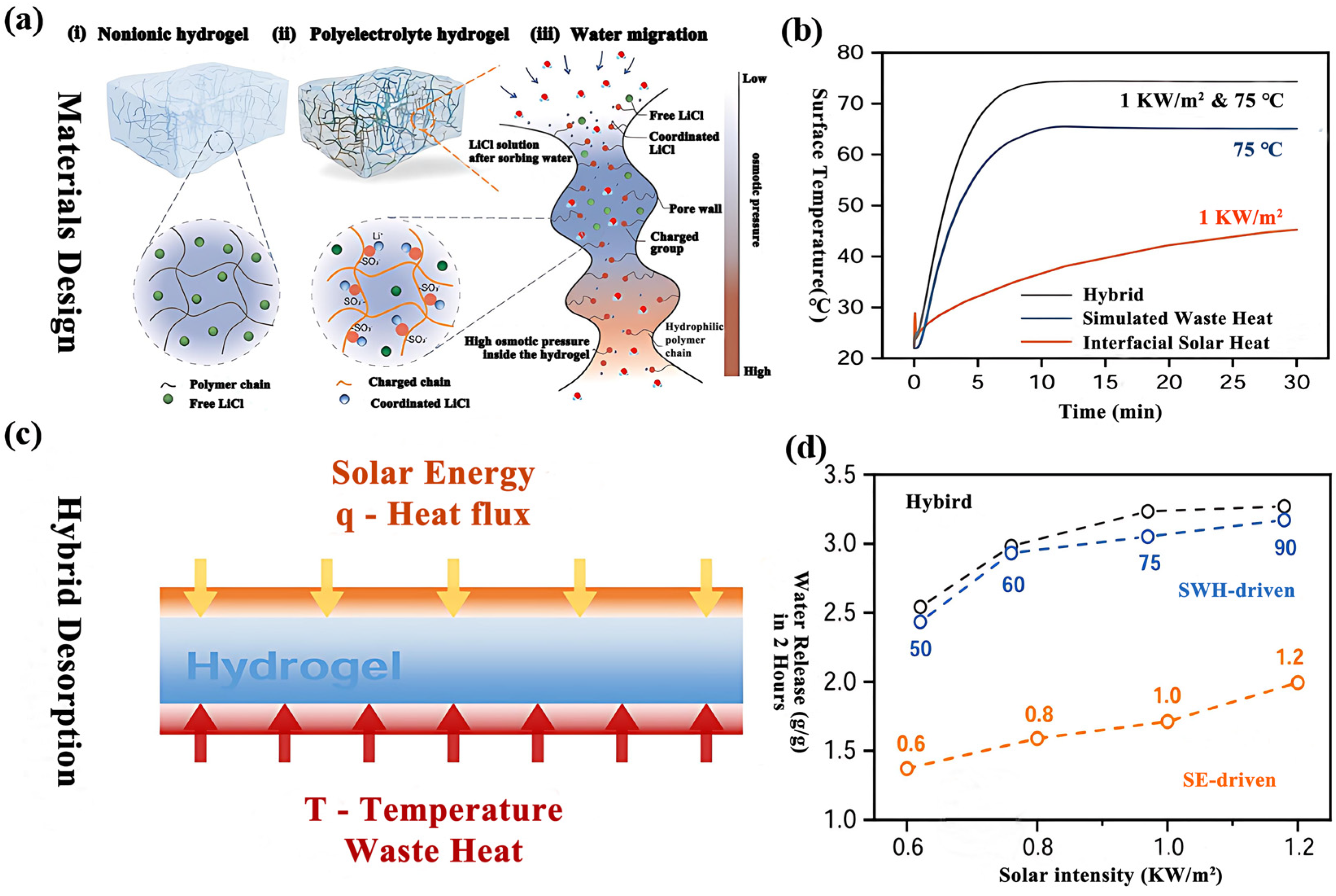
2.3.2. Continuous/Hybrid SAWH (24 h Operation)
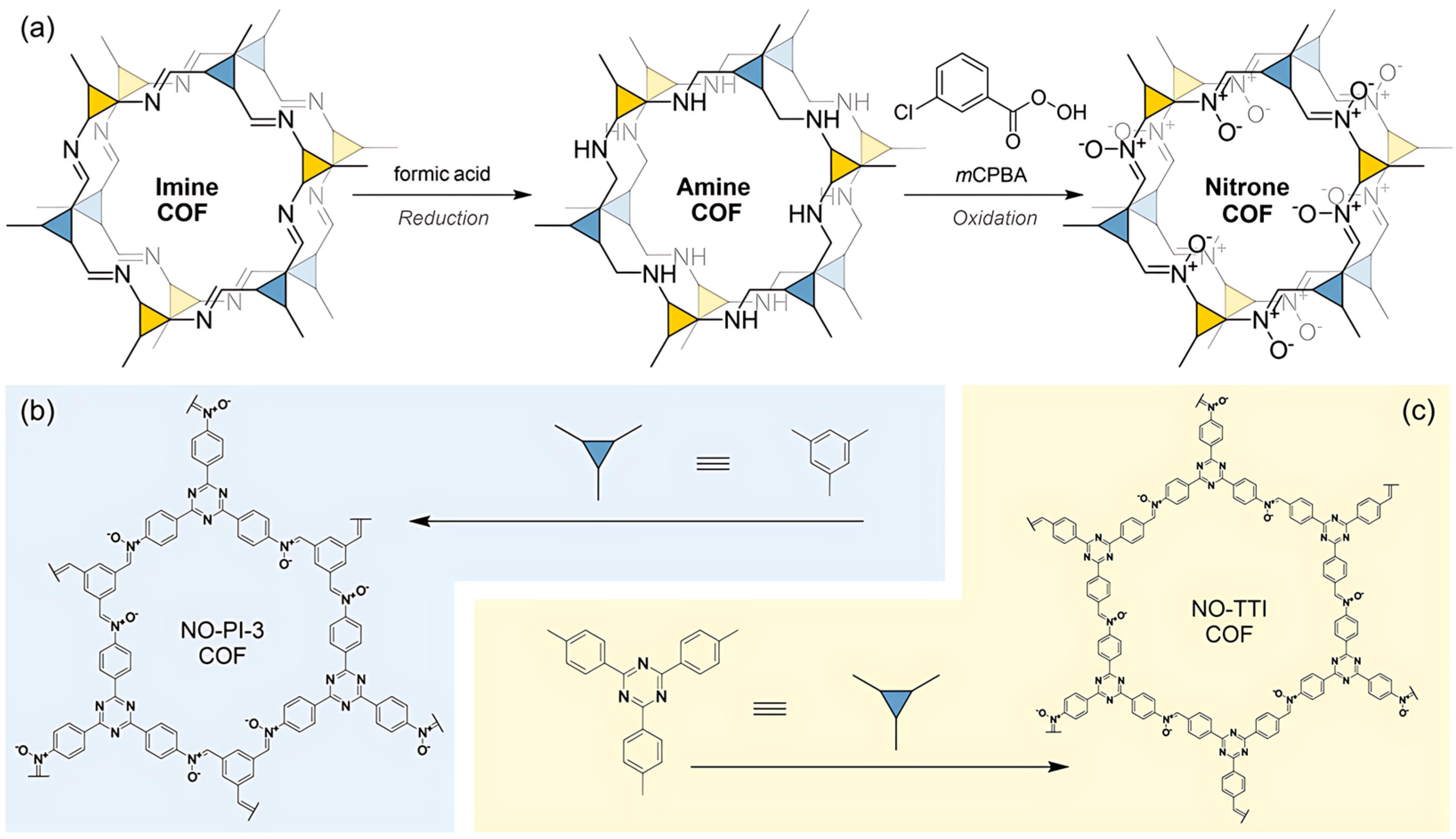
3. Mechanism and Modification Strategy of Adsorbent Materials
3.1. Physical Adsorption and Modification
3.1.1. Microporous Adsorption
3.1.2. Mesoporous–Macroporous Adsorption
3.1.3. Surface Functionalization Modification
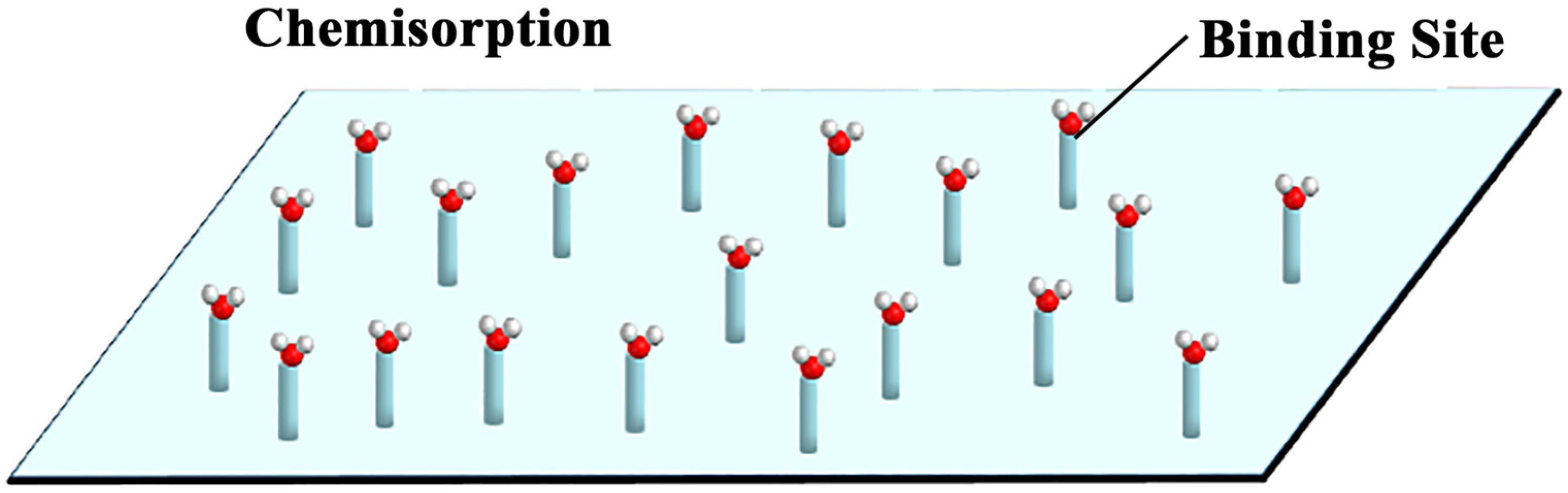
3.2. Bonding Regulation of Chemisorption
3.2.1. Cation Coordination Adsorption
3.2.2. Acid–Base Reaction Adsorption
3.3. Biomass-Based Composite Adsorbent Materials
3.3.1. Cellulose-Based Multi-Level Pore Design
3.3.2. Lignin-Based Photothermal Synergy
3.3.3. Life Cycle Assessment of Modified Biomass-Based Materials
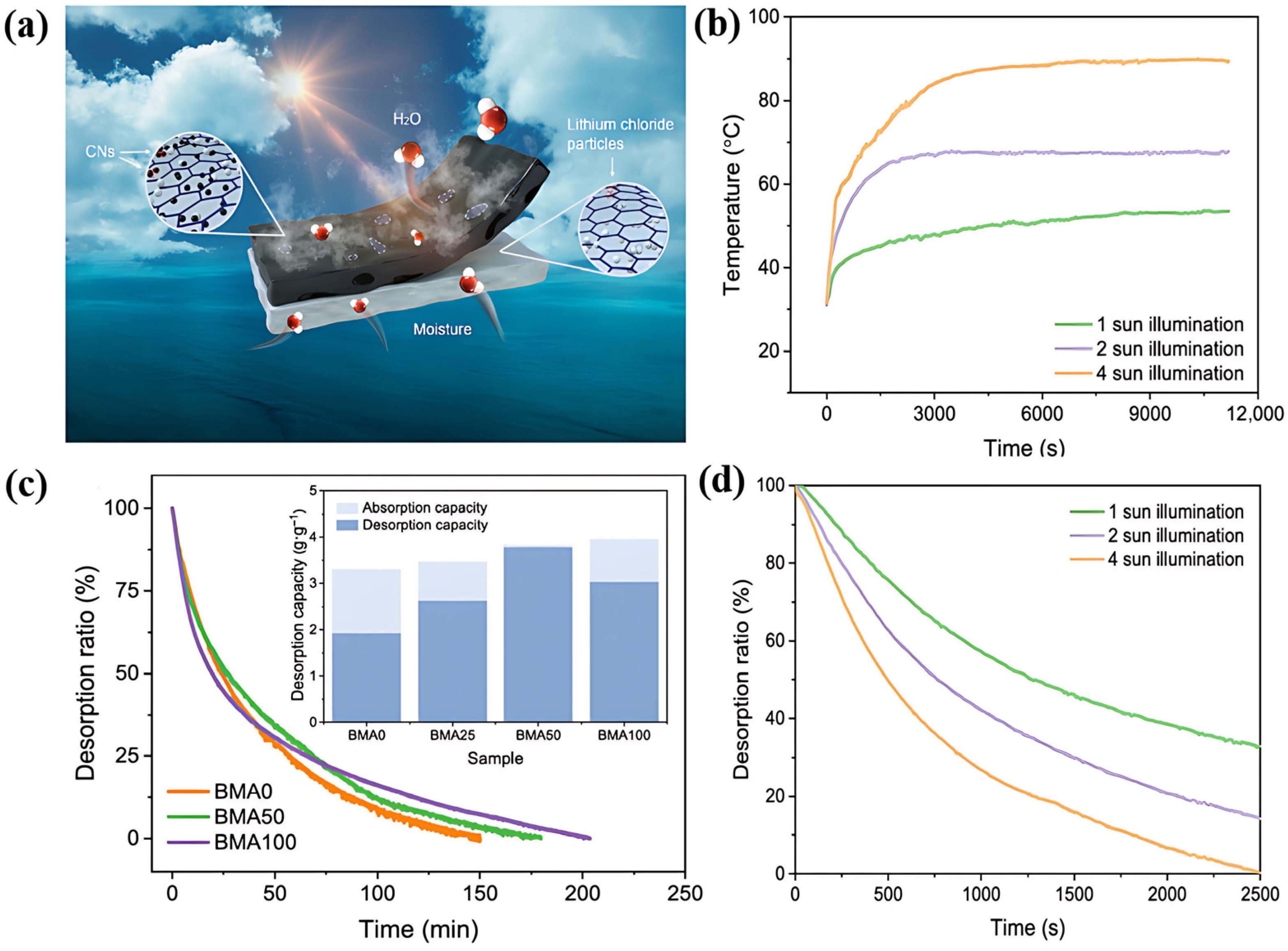
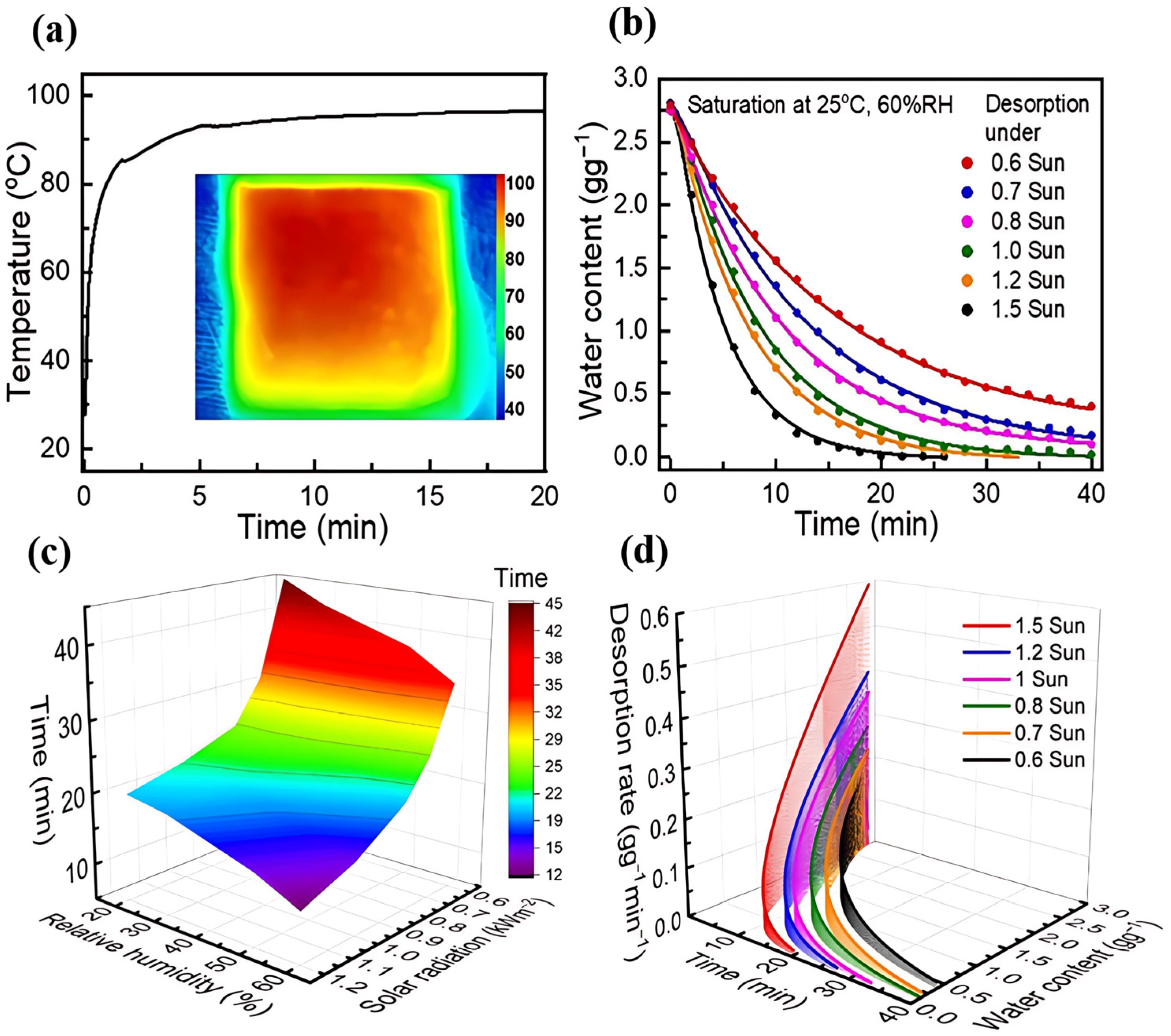
4. Enhancement Mechanism for Desorption Process
4.1. Thermally Driven Enhanced Desorption
4.2. Regulation of Desorption Enthalpy
5. Optimization of Condensing System Energy Efficiency
6. Conclusions and Outlook
- (1)
- Lignin, a wood-based material, is renewable, biocompatible, and degradable, and its intramolecular conjugated structure enhances light absorption through the π-π stacking effect, while the hydrogen bonding network formed by the natural hydroxyl functional groups and water molecules significantly improves the low humidity adsorption capacity, which can be utilized to dynamically adjust the lignin photothermal properties, coupled with the cellulose hydrogel network and the temperature-sensitive polymers (e.g., PNIPAM), to construct a humidity response system. Adsorption affinity can be dynamically adjusted to enhance solar energy utilization to break through the bottleneck of low humidity water harvesting.
- (2)
- Drawing on the multistage pore structure of the wood conduit, the aerogel is designed with gradient porosity to enhance the directional transport of water vapor; and the surface grafting of amphiphilic ionic groups is used to construct the “water slide” effect, which reduces the resistance to condensate desorption and improves the efficiency of the phase change.
- (3)
- To address the problem of wind and sand erosion in arid/semi-arid regions, we can have developed a composite protective coating of TiO2 nanoparticles and lignosulfonate, which combines photocatalytic self-cleaning and anti-wear properties, and built an intelligent monitoring system with the Internet of Things (IoT), which realizes the multi-modal dynamic regulation and control of solar energy and waste heat; this primarily addresses the issue of large-scale water supply in semi-arid regions. Given the complexity and cost of the system, remote areas should still consider simple decentralized adsorption water collection methods.
- (4)
- Explore sustainable pretreatment technologies for biomass materials, selectively separating cellulose and lignin and retaining the natural porous structure through ionic liquid or deep eutectic solvent pretreatment, so as to realize sustainable manufacturing; develop 3D-printed structured adsorbent material preparation technology, accurately controlling the pore network to reduce the resistance to water vapor mass transfer, and promote the application of SAWH technology on a large scale. Improve through controllable mass transfer kinetics and further promote the large-scale application of SAWH technology.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Yan, S.; Zeng, M.; Wang, X.; Shi, P.; Fei, M.; Zhu, J. Hierarchical Engineering of Sorption-Based Atmospheric Water Harvesters. Adv. Mater. 2024, 36, 12. [Google Scholar] [CrossRef]
- Salehi, M. Global water shortage and potable water safety; Today’s concern and tomorrow’s crisis. Environ. Int. 2021, 158, 106936. [Google Scholar] [CrossRef]
- Meran, G.; Siehlow, M.; von Hirschhausen, C. Water Availability: A Hydrological View. In The Economics of Water; Springer: Cham, Switzerland, 2021; pp. 9–21. [Google Scholar] [CrossRef]
- Potyka, J.; Günter Tovar, A.D. Energetic analysis and economic viability of active atmospheric water generation technologies. Discov. Appl. Sci. 2024, 6, 153. [Google Scholar] [CrossRef]
- Menin, B. Innovative Technologies for Large-Scale Water Production in Arid Regions: Strategies for Sustainable Development. J. Appl. Math. Phys. 2024, 12, 2506–2558. [Google Scholar] [CrossRef]
- Cattani, L.; Cattani, P.; Vadivel, D.; Magrini, A.; Figoni, R.; Dondi, D. Suitability and Energy Sustainability of Atmospheric Water Generation Technology for Green Hydrogen Production. Energies 2023, 16, 6440. [Google Scholar] [CrossRef]
- Tashtoush, B.; Atmospheric, A. water harvesting: A review of techniques, performance, renewable energy solutions, and feasibility. Energy 2023, 280, 128186. [Google Scholar] [CrossRef]
- He, C.; Liu, Z.; Wu, J.; Pan, X.; Fang, Z.; Li, J.; Bryan, B. Future global urban water scarcity and potential solutions. Nat. Commun. 2021, 12, 4667. [Google Scholar] [CrossRef]
- Thomas, T.M.; Mahapatra, P.S.; Ganguly, R.; Tiwari, M. Preferred Mode of Atmospheric Water Vapor Condensation on Nanoengineered Surfaces: Dropwise or Filmwise? Langmuir 2023, 39, 5396–5407. [Google Scholar] [CrossRef]
- Kwana, T.H.; Yuan, S.; Shen, Y.; Pei, G. Comparative meta-analysis of desalination and atmospheric water harvesting technologies based on the minimum energy of separation. Energy Rep. 2022, 8, 10072–10087. [Google Scholar] [CrossRef]
- Kardon-Dryer, K.; Huang, Y.-W.; Cziczo, D.J. Laboratory studies of collection efficiency of sub-micrometer aerosol particles by cloud droplets on a single-droplet basis. Atmos. Chem. Phys. 2015, 15, 9159–9171. [Google Scholar] [CrossRef]
- Bilal, M.; Sultan, M.; Morosuk, T.; Den, W.; Sajjad, U.; Aslam, M.M.A.; Shahzad, M.W.; Farooq, M. Adsorption-based atmospheric water harvesting: A review of adsorbents and systems. Int. Commun. Heat Mass Transf. 2022, 133, 105961. [Google Scholar] [CrossRef]
- Kim, H.; Yang, S.; Rao, S.R.; Narayanan, S.; Kapustin, E.A.; Furukawa, H.; Umans, A.S.; Yaghi, O.M.; Wang, E.N. Water harvesting from air with metal-organic frameworks powered by natural sunlight. Science 2017, 356, 430–434. [Google Scholar] [CrossRef]
- Hu, Y.; Wang, Y.; Fang, Z.; Wan, X.; Dong, M.; Ye, Z.; Peng, X. MOF supraparticles for atmosphere water harvesting at low humidity. J. Mater. Chem. A 2022, 10, 15116–15126. [Google Scholar] [CrossRef]
- Zhang, L.; Li, R.; Zheng, S.; Zhu, H.; Cao, M.; Li, M.; Hu, Y.; Long, L.; Feng, H.; Tang, C.Y. Hydrogel-embedded vertically aligned metal-organic framework nanosheet membrane for efficient water harvesting. Nat. Commun. 2024, 15, 9738. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Guan, W.; Lei, C.; Lu, H.; Shi, W.; Yu, G. Scalable super hygroscopic polymer films for sustainable moisture harvesting in arid environments. Nat. Commun. 2022, 13, 2761. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Liu, Y.; Xu, B.; Ganesan, M.; Tan, B.; Tan, Y.; Luo, F.; Liang, X.; Wang, S.; Gao, X.; et al. A Functionally Asymmetric Janus Hygro-Photothermal Hybrid for Atmospheric Water Harvesting in Arid Regions. Small 2024, 20, 2306521. [Google Scholar] [CrossRef]
- Goswami, A.; Pillai, S.C.; McGranaghan, G. Surface modifications to enhance dropwise condensation. Surf. Interfaces 2021, 25, 101143. [Google Scholar] [CrossRef]
- Hou, Y.; Yu, M.; Chen, X.; Wang, Z.; Yao, S. Recurrent Filmwise and Dropwise Condensation on a Beetle Mimetic Surface. ACS Nano 2014, 9, 71–81. [Google Scholar] [CrossRef]
- Ju, J.; Bai, H.; Zheng, Y.; Zhao, T.; Fang, R.; Jiang, L. A multi-structural and multi-functional integrated fog collection system in cactus. Nat. Commun. 2012, 3, 1247. [Google Scholar] [CrossRef]
- Yang, X.; Song, J.; Liu, J.; Liu, X.; Jin, Z. A Twice Electrochemical-Etching Method to Fabricate Superhydrophobic-Superhydrophilic Patterns for Biomimetic Fog Harvest. Sci. Rep. 2017, 7, 8816. [Google Scholar] [CrossRef]
- Ascione, F.; Colonna, P.; De Servi, C.M. Integrated design optimization method for novel vapour-compression-cycle-based environmental control systems. Appl. Therm. Eng. 2023, 236, 121261. [Google Scholar] [CrossRef]
- Eslami, M.; Tajeddini, F.; Etaati, N. Thermal analysis and optimization of a system for water harvesting from humid air using thermoelectric coolers. Energy Convers. Manag. 2018, 174, 417–429. [Google Scholar] [CrossRef]
- Ibrahim, N.I.; Al-Sulaiman, F.A.; Saidur, R. Performance assessment of water production from solar cooling system in humid climate. Energy Convers. Manag. 2016, 127, 647–655. [Google Scholar] [CrossRef]
- Magrini, A.; Cattani, L.; Cartesegna, M.; Magnani, L. Integrated systems for air conditioning and production of drinking water–Preliminary considerations. Energy Procedia 2015, 75, 1659–1665. [Google Scholar] [CrossRef]
- Groendijk, L.; De Vries, H. Development of a mobile water maker, a sustainable way to produce safe drinking water in developing countries. Desalination 2009, 248, 106–113. [Google Scholar] [CrossRef]
- Zolfagharkhani, S.; Zamen, M.; Shahmardan, M.M. Thermodynamic analysis and evaluation of a gas compression refrigeration cycle for fresh water production from atmospheric air. Energy Convers. Manag. 2018, 170, 97–107. [Google Scholar] [CrossRef]
- Joshi, V.; Joshi, V.; Kothari, H.; Mahajan, M.; Chaudhari, M.; Sant, K. Experimental investigations on a portable fresh water generator using a thermoelectric cooler. Energy Procedia 2017, 109, 161–166. [Google Scholar] [CrossRef]
- Muñoz-García, M.A.; Moreda, G.; Raga-Arroyo, M.P.; Marín-González, O. Water harvesting for young trees using Peltier plates powered by photovoltaic solar energy. Comput. Electron. Agric. 2012, 93, 60–67. [Google Scholar] [CrossRef]
- Atta, R.M. Solar water condensation using thermoelectric coolers. Int. J. Water Resour. Arid. Environ. 2011, 1, 142. [Google Scholar] [CrossRef]
- Liu, S.; He, W.; Hu, D.; Lv, S.; Chen, D.; Wu, X.; Xu, F.; Li, S. Experimental analysis of a portable atmospheric water generator by thermoelectric cooling method. Energy Procedia 2017, 142, 1609–1614. [Google Scholar] [CrossRef]
- Salehi, A.A.; Ghannadi-Maragheh, M.; Torab-Mostaedi, M.; Torkaman, R.; Asadollahzadeh, M. A review on the water-energy nexus for drinking water production from humid air. Renew. Sustain. Energy Rev. 2020, 120, 109627. [Google Scholar] [CrossRef]
- Verbrugghe, N.; Khan, A. Water harvesting through fog collectors: A review of conceptual, experimental and operational aspects. Int. J. Low-Carbon Technol. 2023, 18, 392–403. [Google Scholar] [CrossRef]
- Parisi, G.; Szewczyk, P.K.; Narayan, S.; Ura, D.P.; Knapczyk-Korczak, J.; Stachewicz, U. Multifunctional Piezoelectric Yarns and Meshes for Efficient Fog Water Collection, Energy Harvesting, and Sensing. Small Sci. 2024, 4, 7. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Xie, S.; Fu, Y.; Tian, G.; Zheng, J.; Wang, B.; Guo, Z. 3D Bionic Water Harvesting System for Efficient Fog Capturing and Transporting. Adv. Funct. Mater. 2024, 34, 2408522. [Google Scholar] [CrossRef]
- Zhang, S.; Chi, M.; Mo, J.; Liu, T.; Liu, Y.; Fu, Q.; Wang, J.; Luo, B.; Qin, Y.; Wang, S. Bioinspired asymmetric amphiphilic surface for triboelectric enhanced efficient water harvesting. Nat. Commun. 2022, 13, 4168. [Google Scholar] [CrossRef]
- Jung, W.; Park, S.; Lee, K.S.; Jeon, J.-D.; Lee, H.K.; Kim, J.-H.; Lee, J.S. Rapid thermal swing adsorption process in multi-beds scale with sensible heat recovery for continuous energy-efficient CO2 capture. Chem. Eng. J. 2020, 392, 123656. [Google Scholar] [CrossRef]
- Ejeian, M.; Wang, R. Adsorption-based atmospheric water harvesting. Joule 2021, 5, 1678–1703. [Google Scholar] [CrossRef]
- Attalla, M.; Sadek, S.; Abd El-Fadeel, W. Adsorption characteristics and heat of adsorption measurements of R-134a on granular activated carbon. Int. J. Air-Cond. Refrig. 2014, 22, 1450014. [Google Scholar] [CrossRef]
- Thakur, A.K.; Sathyamurthy, R.; Sharshir, S.W.; Elkadeem, M.; Ma, Z.; Manokar, A.M.; Arıcı, M.; Pandey, A.; Saidur, R. Performance analysis of a modified solar still using reduced graphene oxide coated absorber plate with activated carbon pellet. Sustain. Energy Technol. Assess. 2021, 45, 101046. [Google Scholar] [CrossRef]
- Paul, D.; Noori, M.; Rajesh, P.; Ghangrekar, M.; Mitra, A. Modification of carbon felt anode with graphene oxide-zeolite composite for enhancing the performance of microbial fuel cell. Sustain. Energy Technol. Assess. 2018, 26, 77–82. [Google Scholar] [CrossRef]
- Hu, G.; Yang, J.; Duan, X.; Farnood, R.; Yang, C.; Yang, J.; Liu, W.; Liu, Q. Recent developments and challenges in zeolite-based composite photocatalysts for environmental applications. Chem. Eng. J. 2021, 417, 129209. [Google Scholar] [CrossRef]
- Askalany, A.A.; Ali, E.S. A new approach integration of ejector within adsorption desalination cycle reaching COP higher than one. Sustain. Energy Technol. Assess. 2020, 41, 100766. [Google Scholar] [CrossRef]
- Kallenberger, P.A.; Fröba, M. Water harvesting from air with a hygroscopic salt in a hydrogel–derived matrix. Commun. Chem. 2018, 1, 28. [Google Scholar] [CrossRef]
- Kandeal, A.; Joseph, A.; Elsharkawy, M.; Elkadeem, M.; Hamada, M.A.; Khalil, A.; Moustapha, M.E.; Sharshir, S.W. Research progress on recent technologies of water harvesting from atmospheric air: A detailed review. Sustain. Energy Technol. Assess. 2022, 52, 102000. [Google Scholar] [CrossRef]
- Zhao, R.; Wang, Q.; Zhao, L.; Deng, S.; Bian, X.; Liu, L. Comparative study on energy efficiency of moving-bed adsorption for carbon dioxide capture by two evaluation methods. Sustain. Energy Technol. Assess. 2021, 44, 101042. [Google Scholar] [CrossRef]
- Shafeian, N.; Ranjbar, A.; Gorji, T.B. Progress in atmospheric water generation systems: A review. Renew. Sustain. Energy Rev. 2022, 161, 112325. [Google Scholar] [CrossRef]
- Shao, Z.; Wang, Z.-S.; Lv, H.; Tang, Y.-C.; Wang, H.; Du, S.; Sun, R.; Feng, X.; Poredoš, P.; Zhou, D.-D. Modular all-day continuous thermal-driven atmospheric water harvester with rotating adsorption strategy. Appl. Phys. Rev. 2023, 10, 4. [Google Scholar] [CrossRef]
- Zhu, R.; Yu, Q.; Li, M.; Li, A.; Zhan, D.; Li, Y.; Mo, Z.; Sun, S.; Zhang, Y. Green synthesis of natural nanocomposite with synergistically tunable sorption/desorption for solar-driven all-weather moisture harvesting. Nano Energy 2024, 124, 109471. [Google Scholar] [CrossRef]
- Li, Y.; Deng, J.; Li, H. Enhancing solar-driven atmospheric water harvesting by a bilayer macroporous hydrogel. Appl. Therm. Eng. 2024, 247, 123045. [Google Scholar] [CrossRef]
- Xu, J.; Huo, X.; Yan, T.; Wang, P.; Bai, Z.; Chao, J.; Yang, R.; Wang, R.; Li, T. All-in-one hybrid atmospheric water harvesting for all-day water production by natural sunlight and radiative cooling. Energy Environ. Sci. 2024, 17, 4988–5001. [Google Scholar] [CrossRef]
- Shan, H.; Poredoš, P.; Ye, Z.; Qu, H.; Zhang, Y.; Zhou, M.; Wang, R.; Tan, S.C. All-Day Multicyclic Atmospheric Water Harvesting Enabled by Polyelectrolyte Hydrogel with Hybrid Desorption Mode. Adv. Mater. 2023, 35, 2302038. [Google Scholar] [CrossRef]
- Wu, Q.; Zeng, L.; Liu, Z.; Xu, K.; Li, M.; Li, Z.; Gong, Y.; Qu, Y.; Liu, G.; Li, L. Design of Adsorption Air Catchment Device Based on Semiconductor Refrigeration. Adv. Energy Power Eng. 2021, 9, 199–210. [Google Scholar] [CrossRef]
- Manoj Kumar, M.K.; Avadhesh Yadav, A.Y. Comparative study of solar-powered water production from atmospheric air using different desiccant materials. Int. J. Sustain. Eng. 2016, 9, 390–400. [Google Scholar] [CrossRef]
- Fathieh, F.; Kalmutzki, M.J.; Kapustin, E.A.; Waller, P.J.; Yang, J.; Yaghi, O.M. Practical water production from desert air. Sci. Adv. 2018, 4, eaat3198. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Yao, X.; Wong, M.Y.; Xu, Q.; Li, H.; Lin, K.; Zhou, Y.; Ho, T.C.; Pan, A.; Chen, J.; et al. Enhancement of Water Productivity and Energy Efficiency in Sorption-based Atmospheric Water Harvesting Systems: From Material, Component to System Level. ACS Nano 2024, 18, 31597–31631. [Google Scholar] [CrossRef]
- Zhang, S.; Fu, J.; Xing, G.; Zhu, W.; Ben, T. Recent advances in porous adsorbent assisted atmospheric water harvesting: A review of adsorbent materials. Chem. Synth. 2023, 3, 10. [Google Scholar] [CrossRef]
- Srivastava, N.; Eames, I. A review of adsorbents and adsorbates in solid–vapour adsorption heat pump systems. Appl. Therm. Eng. 1998, 18, 707–714. [Google Scholar] [CrossRef]
- Xiangyan, H.; Jiaxing, X.; Taisen, Y.; Ruzhu, W.; Tingxian, L. Research status of physical sorbents for sorption-based atmospheric water harvesting. Sci. Bull. 2023, 68, 1392–1405. [Google Scholar] [CrossRef]
- Huang, X.; Qin, Q.; Ma, Q.; Wang, B. Atmospheric water harvesting with metal-organic frameworks and their composites: From materials to devices. Water 2022, 14, 3487. [Google Scholar] [CrossRef]
- Xiao, C.; Shi, P.; Yan, W.; Chen, L.; Qian, L.; Kim, S.H. Thickness and structure of adsorbed water layer and effects on adhesion and friction at nanoasperity contact. Colloids Interfaces 2019, 3, 55. [Google Scholar] [CrossRef]
- Rieth, A.J.; Yang, S.; Wang, E.N.; Dincă, M. Record atmospheric fresh water capture and heat transfer with a material operating at the water uptake reversibility limit. ACS Cent. Sci. 2017, 3, 668–672. [Google Scholar] [CrossRef]
- De Lange, M.F.; Verouden, K.J.; Vlugt, T.J.; Gascon, J.; Kapteijn, F. Adsorption-driven heat pumps: The potential of metal–organic frameworks. Chem. Rev. 2015, 115, 12205–12250. [Google Scholar] [CrossRef]
- Stoeckli, F. Dubinin’s theory and its contribution to adsorption science. Russ. Chem. Bull. 2001, 50, 2265–2272. [Google Scholar] [CrossRef]
- Dawoud, B.; Aristov, Y. Experimental study on the kinetics of water vapor sorption on selective water sorbents, silica gel and alumina under typical operating conditions of sorption heat pumps. Int. J. Heat Mass Transf. 2003, 46, 273–281. [Google Scholar] [CrossRef]
- Essa, F.; Elsheikh, A.H.; Sathyamurthy, R.; Manokar, A.M.; Kandeal, A.; Shanmugan, S.; Kabeel, A.; Sharshir, S.W.; Panchal, H.; Younes, M. Extracting water content from the ambient air in a double-slope half-cylindrical basin solar still using silica gel under Egyptian conditions. Sustain. Energy Technol. Assess. 2020, 39, 100712. [Google Scholar] [CrossRef]
- Sögütoglu, L.-C.; Steiger, M.; Houben, J.; Biemans, D.; Fischer, H.R.; Donkers, P.; Huinink, H.; Adan, O.C. Understanding the hydration process of salts: The impact of a nucleation barrier. Cryst. Growth Des. 2019, 19, 2279–2288. [Google Scholar] [CrossRef]
- Srivastava, S.; Yadav, A. Water generation from atmospheric air by using composite desiccant material through fixed focus concentrating solar thermal power. Sol. Energy 2018, 169, 302–315. [Google Scholar] [CrossRef]
- Vainio, E.; DeMartini, N.; Hupa, L.; Åmand, L.-E.; Richards, T.; Hupa, M. Hygroscopic properties of calcium chloride and its role on cold-end corrosion in biomass combustion. Energy Fuels 2019, 33, 11913–11922. [Google Scholar] [CrossRef]
- Elashmawy, M.; Alshammari, F. Atmospheric water harvesting from low humid regions using tubular solar still powered by a parabolic concentrator system. J. Clean. Prod. 2020, 256, 120329. [Google Scholar] [CrossRef]
- Gibson, E.R.; Hudson, P.K.; Grassian, V.H. Aerosol chemistry and climate: Laboratory studies of the carbonate component of mineral dust and its reaction products. Geophys. Res. Lett. 2006, 33, 13. [Google Scholar] [CrossRef]
- Guo, L.; Gu, W.; Peng, C.; Wang, W.; Li, Y.J.; Zong, T.; Tang, Y.; Wu, Z.; Lin, Q.; Ge, M. A comprehensive study of hygroscopic properties of calcium-and magnesium-containing salts: Implication for hygroscopicity of mineral dust and sea salt aerosols. Atmos. Chem. Phys. 2019, 19, 2115–2133. [Google Scholar] [CrossRef]
- Zhang, H.; Gu, W.; Li, Y.J.; Tang, M. Hygroscopic properties of sodium and potassium salts as related to saline mineral dusts and sea salt aerosols. J. Environ. Sci. 2020, 95, 65–72. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shi, Y.; Shi, L.; Alsaedi, M.; Wang, P. Harvesting water from air: Using anhydrous salt with sunlight. Environ. Sci. Technol. 2018, 52, 5398–5406. [Google Scholar] [CrossRef]
- Yuan, Y.; Zhang, H.; Yang, F.; Zhang, N.; Cao, X. Inorganic composite sorbents for water vapor sorption: A research progress. Renew. Sustain. Energy Rev. 2016, 54, 761–776. [Google Scholar] [CrossRef]
- Mulchandani, A.; Westerhoff, P. Geospatial climatic factors influence water production of solar desiccant driven atmospheric water capture devices. Environ. Sci. Technol. 2020, 54, 8310–8322. [Google Scholar] [CrossRef]
- Trapani, F.; Polyzoidis, A.; Loebbecke, S.; Piscopo, C. On the general water harvesting capability of metal-organic frameworks under well-defined climatic conditions. Microporous Mesoporous Mater. 2016, 230, 20–24. [Google Scholar] [CrossRef]
- Stach, H.; Mugele, J.; Jänchen, J.; Weiler, E. Influence of cycle temperatures on the thermochemical heat storage densities in the systems water/microporous and water/mesoporous adsorbents. Adsorption 2005, 11, 393–404. [Google Scholar] [CrossRef]
- Yan, T.; Li, T.; Xu, J.; Wang, R. Water sorption properties, diffusion and kinetics of zeolite NaX modified by ion-exchange and salt impregnation. Int. J. Heat Mass Transf. 2019, 139, 990–999. [Google Scholar] [CrossRef]
- Teo, H.W.B.; Chakraborty, A.; Fan, W. Improved adsorption characteristics data for AQSOA types zeolites and water systems under static and dynamic conditions. Microporous Mesoporous Mater. 2017, 242, 109–117. [Google Scholar] [CrossRef]
- Ng, E.-P.; Mintova, S. Nanoporous materials with enhanced hydrophilicity and high water sorption capacity. Microporous Mesoporous Mater. 2008, 114, 1–26. [Google Scholar] [CrossRef]
- Sleiti, A.K.; Al-Khawaja, H.; Al-Khawaja, H.; Al-Ali, M. Harvesting water from air using adsorption material–Prototype and experimental results. Sep. Purif. Technol. 2021, 257, 117921. [Google Scholar] [CrossRef]
- Batten, S.R.; Champness, N.R.; Chen, X.-M.; Garcia-Martinez, J.; Kitagawa, S.; Öhrström, L.; O’keeffe, M.; Paik Suh, M.; Reedijk, J. Terminology of metal–organic frameworks and coordination polymers (IUPAC Recommendations 2013). Pure Appl. Chem. 2013, 85, 1715–1724. [Google Scholar] [CrossRef]
- Furukawa, H.; Gándara, F.; Zhang, Y.-B.; Jiang, J.; Queen, W.L.; Hudson, M.R.; Yaghi, O.M. Water adsorption in porous metal–organic frameworks and related materials. J. Am. Chem. Soc. 2014, 136, 4369–4381. [Google Scholar] [CrossRef] [PubMed]
- Cong, S.; Yuan, Y.; Wang, J.; Wang, Z.; Kapteijn, F.; Liu, X. Highly water-permeable metal–organic framework MOF-303 membranes for desalination. J. Am. Chem. Soc. 2021, 143, 20055–20058. [Google Scholar] [CrossRef]
- Li, Z.-Q.; Yang, J.-C.; Sui, K.-W.; Yin, N. Facile synthesis of metal-organic framework MOF-808 for arsenic removal. Mater. Lett. 2015, 160, 412–414. [Google Scholar] [CrossRef]
- Logan, M.W.; Langevin, S.; Xia, Z. Reversible atmospheric water harvesting using metal-organic frameworks. Sci. Rep. 2020, 10, 1492. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, H.L. Covalent organic frameworks for atmospheric water harvesting. Adv. Mater. 2023, 35, 2300018. [Google Scholar] [CrossRef]
- Kalmutzki, M.J.; Diercks, C.S.; Yaghi, O.M. Metal–organic frameworks for water harvesting from air. Adv. Mater. 2018, 30, 1704304. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of gases, with special reference to the evaluation of surface area and pore size distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Yang, K.; Pan, T.; Lei, Q.; Dong, X.; Cheng, Q.; Han, Y. A roadmap to sorption-based atmospheric water harvesting: From molecular sorption mechanism to sorbent design and system optimization. Environ. Sci. Technol. 2021, 55, 6542–6560. [Google Scholar] [CrossRef]
- Grunenberg, L.; Savasci, G.K.; Emmerling, S.T.; Heck, F.; Bette, S.; Cima Bergesch, A.; Ochsenfeld, C.; Lotsch, B.V. Postsynthetic transformation of imine-into nitrone-linked covalent organic frameworks for atmospheric water harvesting at decreased humidity. J. Am. Chem. Soc. 2023, 145, 13241–13248. [Google Scholar] [CrossRef]
- Zhao, H.; Li, Q.; Wang, Z.; Wu, T.; Zhang, M. Synthesis of MIL-101 (Cr) and its water adsorption performance. Microporous Mesoporous Mater. 2020, 297, 110044. [Google Scholar] [CrossRef]
- Celeste, A.; Paolone, A.; Itié, J.-P.; Borondics, F.; Joseph, B.; Grad, O.; Blanita, G.; Zlotea, C.; Capitani, F. Mesoporous metal–organic framework MIL-101 at high pressure. J. Am. Chem. Soc. 2020, 142, 15012–15019. [Google Scholar] [CrossRef] [PubMed]
- Hodgson, A.; Haq, S. Water adsorption and the wetting of metal surfaces. Surf. Sci. Rep. 2009, 64, 381–451. [Google Scholar] [CrossRef]
- Coasne, B.; Galarneau, A.; Pellenq, R.J.; Di Renzo, F. Adsorption, intrusion and freezing in porous silica: The view from the nanoscale. Chem. Soc. Rev. 2013, 42, 4141–4171. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, H.; Zhao, F.; Yu, G. Atmospheric water harvesting: A review of material and structural designs. ACS Mater. Lett. 2020, 2, 671–684. [Google Scholar] [CrossRef]
- Shi, L.; Kirlikovali, K.O.; Chen, Z.; Farha, O.K. Metal-organic frameworks for water vapor adsorption. Chem 2024, 10, 484–503. [Google Scholar] [CrossRef]
- Xu, J.; Li, T.; Chao, J.; Wu, S.; Yan, T.; Li, W.; Cao, B.; Wang, R. Efficient solar-driven water harvesting from arid air with metal–organic frameworks modified by hygroscopic salt. Angew. Chem. Int. Ed. 2020, 59, 5202–5210. [Google Scholar] [CrossRef]
- Yang, K.; Pan, T.; Pinnau, I.; Shi, Z.; Han, Y. Simultaneous generation of atmospheric water and electricity using a hygroscopic aerogel with fast sorption kinetics. Nano Energy 2020, 78, 105326. [Google Scholar] [CrossRef]
- Zhang, Y.; Nandakumar, D.K.; Tan, S.C. Digestion of ambient humidity for energy generation. Joule 2020, 4, 2532–2536. [Google Scholar] [CrossRef]
- Zhang, M.; Liu, R.; Li, Y. Diversifying Water Sources with Atmospheric Water Harvesting to Enhance Water Supply Resilience. Sustainability 2022, 14, 7783. [Google Scholar] [CrossRef]
- Ming, Y.; Kumar, N.; Siegel, D.J. Water Adsorption and Insertion in MOF-5. ACS Omega 2017, 2, 4921–4928. [Google Scholar] [CrossRef]
- Henninger, S.K.; Schmidt, F.P.; Henning, H.M. Water adsorption characteristics of novel materials for heat transformation applications. Appl. Therm. Eng. 2010, 30, 1692–1702. [Google Scholar] [CrossRef]
- Tahraoui, Z.N.; Nouali, H.; Marichal, C.; Forler, P.; Klein, J.; Daou, T.J. Influence of the Compensating Cation Nature on the Water Adsorption Properties of Zeolites. Molecules 2020, 25, 944. [Google Scholar] [CrossRef]
- Bonaccorsi, L.; Bruzzaniti, P.; Calabrese, L.; Proverbio, E. Organosilanes functionalization of alumino-silica zeolites for water adsorption applications. Microporous Mesoporous Mater. 2016, 234, 113–119. [Google Scholar] [CrossRef]
- Cai, C.; Chen, Y.; Cheng, F.; Wei, Z.; Zhou, W.; Fu, Y. Biomimetic Dual Absorption–Adsorption Networked MXene Aerogel-Pump for Integrated Water Harvesting and Power Generation System. ACS Nano 2024, 18, 4376–4387. [Google Scholar] [CrossRef]
- Zhu, P.; Yu, Z.; Sun, H.; Zheng, D.; Zheng, Y.; Qian, Y.; Wei, Y.; Lee, J.; Srebnik, S.; Chen, W. 3D Printed Cellulose Nanofiber Aerogel Scaffold with Hierarchical Porous Structures for Fast Solar-Driven Atmospheric Water Harvesting. Adv. Mater. 2024, 36, 2306653. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Shi, Y.; Alsaedi, M.; Wu, M.; Shi, L.; Wang, P. Hybrid hydrogel with high water vapor harvesting capacity for deployable solar-driven atmospheric water generator. Environ. Sci. Technol. 2018, 52, 11367–11377. [Google Scholar] [CrossRef]
- Aleid, S.; Wu, M.; Li, R.; Wang, W.; Zhang, C.; Zhang, L.; Wang, P. Salting-in effect of zwitterionic polymer hydrogel facilitates atmospheric water harvesting. ACS Mater. Lett. 2022, 4, 511–520. [Google Scholar] [CrossRef]
- Entezari, A.; Ejeian, M.; Wang, R. Super atmospheric water harvesting hydrogel with alginate chains modified with binary salts. ACS Mater. Lett. 2020, 2, 471–477. [Google Scholar] [CrossRef]
- Zhao, F.; Zhou, X.; Liu, Y.; Shi, Y.; Dai, Y.; Yu, G. Super moisture-absorbent gels for all-weather atmospheric water harvesting. Adv. Mater. 2019, 31, 1806446. [Google Scholar] [CrossRef]
- Wang, M.; Sun, T.; Wan, D.; Dai, M.; Ling, S.; Wang, J.; Liu, Y.; Fang, Y.; Xu, S.; Yeo, J. Solar-powered nanostructured biopolymer hygroscopic aerogels for atmospheric water harvesting. Nano Energy 2021, 80, 105569. [Google Scholar] [CrossRef]
- Ni, F.; Qiu, N.; Xiao, P.; Zhang, C.; Jian, Y.; Liang, Y.; Xie, W.; Yan, L.; Chen, T. Tillandsia-inspired hygroscopic photothermal organogels for efficient atmospheric water harvesting. Angew. Chem. Int. Ed. 2020, 59, 19237–19246. [Google Scholar] [CrossRef]
- Zhou, H.; Yan, L.; Tang, D.; Xu, T.; Dai, L.; Li, C.; Chen, W.; Si, C. Solar-Driven Drum-Type Atmospheric Water Harvester Based on Bio-Based Gels with Fast Adsorption/Desorption Kinetics. Adv. Mater. 2024, 36, 2403876. [Google Scholar] [CrossRef]
- Liang, C.; Xia, H.; Yin, L.; Du, C.; Wu, X.; Wang, J.; Li, S.; Xu, J.; Zhang, X.; Wang, Y. Carbon foam directly synthesized from industrial lignin powder as featured material for high efficiency solar evaporation. Chem. Eng. J. 2024, 481, 148375. [Google Scholar] [CrossRef]
- Gu, Y.; Wu, W.; Zhang, C.; Li, X.; Guo, X.; Wang, Y.; Yuan, Y.; Jiang, B.; Jin, Y. Multi-Solvent-Induced Gradient Aggregation Rendered Superstrong, Tough, Stretchable, and Fatigue-Resistant Lignin-Based Supramolecular Hydrogels. Adv. Funct. Mater. 2024, 35, 2417206. [Google Scholar] [CrossRef]
- Caravella, A. Fick’s Laws. In Encyclopedia of Membranes; Drioli, E., Giorno, L., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 772–773. [Google Scholar] [CrossRef]
- Song, Y.; Xu, N.; Liu, G.; Qi, H.; Zhao, W.; Zhu, B.; Zhou, L.; Zhu, J. High-yield solar-driven atmospheric water harvesting of metal–organic-framework-derived nanoporous carbon with fast-diffusion water channels. Nat. Nanotechnol. 2022, 17, 857–863. [Google Scholar] [CrossRef]
- Xiang, C.; Yang, X.; Deng, F.; Chen, Z.; Wang, R. Daytime air–water harvesting based on super hygroscopic porous gels with simultaneous adsorption–desorption. Appl. Phys. Rev. 2023, 10, 041413. [Google Scholar] [CrossRef]
- Lu, H.; Shi, W.; Zhang, J.H.; Chen, A.C.; Guan, W.; Lei, C.; Greer, J.R.; Boriskina, S.V.; Yu, G. Tailoring the Desorption Behavior of Hygroscopic Gels for Atmospheric Water Harvesting in Arid Climates. Adv. Mater. 2022, 34, 37. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Li, T.; Yan, T.; Wu, S.; Wu, M.; Chao, J.; Huo, X.; Wang, P.; Wang, R. Ultrahigh solar-driven atmospheric water production enabled by scalable rapid-cycling water harvester with vertically aligned nanocomposite sorbent. Energy Environ. Sci. 2021, 14, 5979–5997. [Google Scholar] [CrossRef]
- Lei, C.; Guo, Y.; Guan, W.; Lu, H.; Shi, W.; Yu, G. Polyzwitterionic Hydrogels for Efficient Atmospheric Water Harvesting. Angew. Chem. Int. Ed. 2022, 61, e202200271. [Google Scholar] [CrossRef]
- Wang, J.; Wang, R.; Tu, Y.; Wang, L. Universal scalable sorption-based atmosphere water harvesting. Energy 2018, 165, 387–395. [Google Scholar] [CrossRef]
- Wang, W.; Xie, S.; Pan, Q.; Dai, Y.; Wang, R.; Ge, T. Air-cooled adsorption-based device for harvesting water from island air. Renew. Sustain. Energy Rev. 2021, 141, 110802. [Google Scholar] [CrossRef]
- Li, R.; Shi, Y.; Wu, M.; Hong, S.; Wang, P. Improving atmospheric water production yield: Enabling multiple water harvesting cycles with nano sorbent. Nano Energy 2020, 67, 104255. [Google Scholar] [CrossRef]
- Yang, X.; Chen, Z.; Xiang, C.; Shan, H.; Wang, R. Enhanced continuous atmospheric water harvesting with scalable hygroscopic gel driven by natural sunlight and wind. Nat. Commun. 2024, 15, 7678. [Google Scholar] [CrossRef] [PubMed]
- Jiayun, W.; Wenjun, Y.; Bowen, L.; Chunfeng, L.; Chaohe, D.; Hua, Z.; Shige, W.; Ruzhu, W. Tillandsia-Inspired Ultra-Efficient Thermo-Responsive Hygroscopic Nanofibers for Solar-Driven Atmospheric Water Harvesting. Adv. Mater. 2024, 37, 3. [Google Scholar] [CrossRef]
- Li, R.; Wang, P. Sorbents, processes and applications beyond water production in sorption-based atmospheric water harvesting. Nat. Water 2023, 1, 573–586. [Google Scholar] [CrossRef]









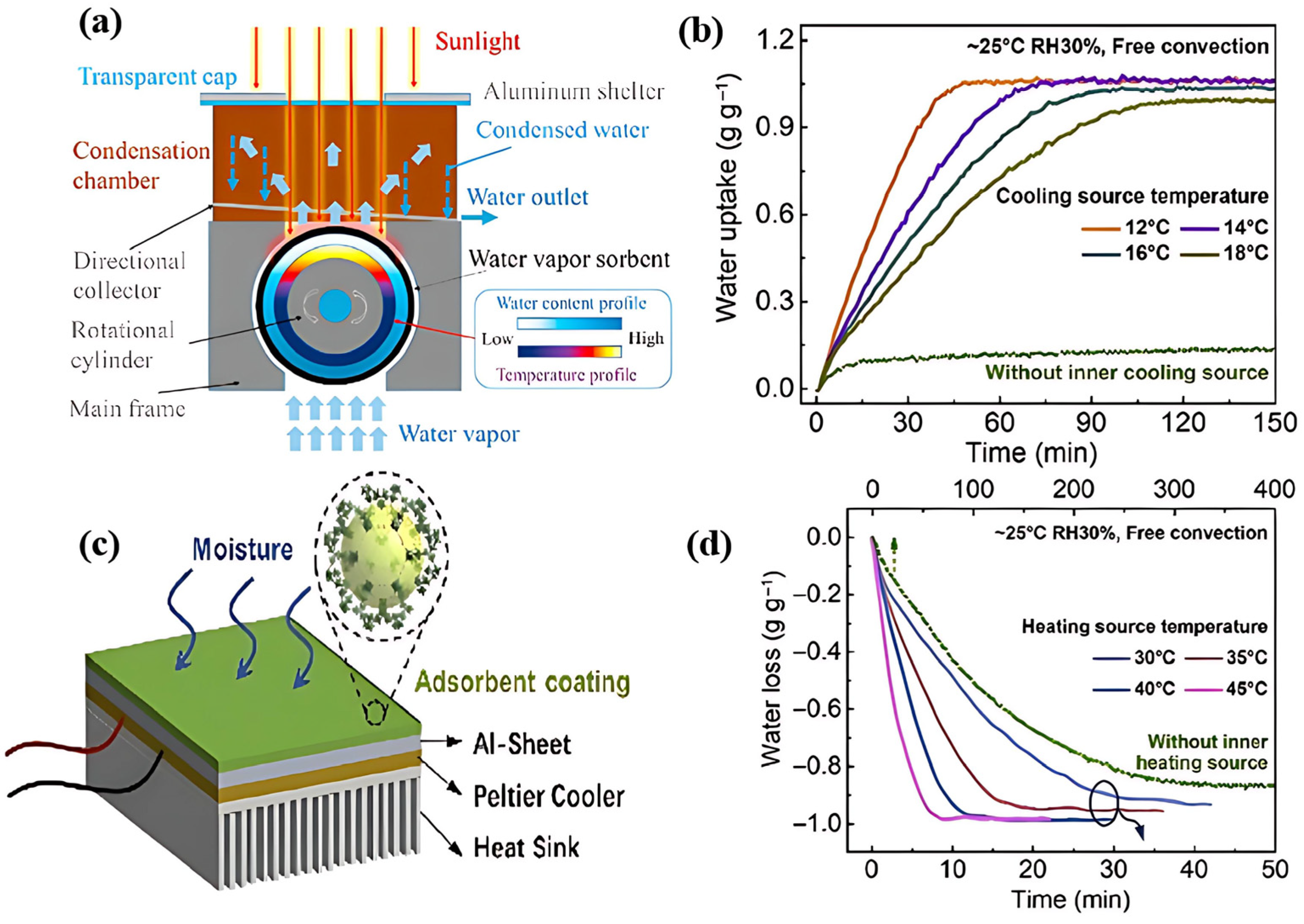
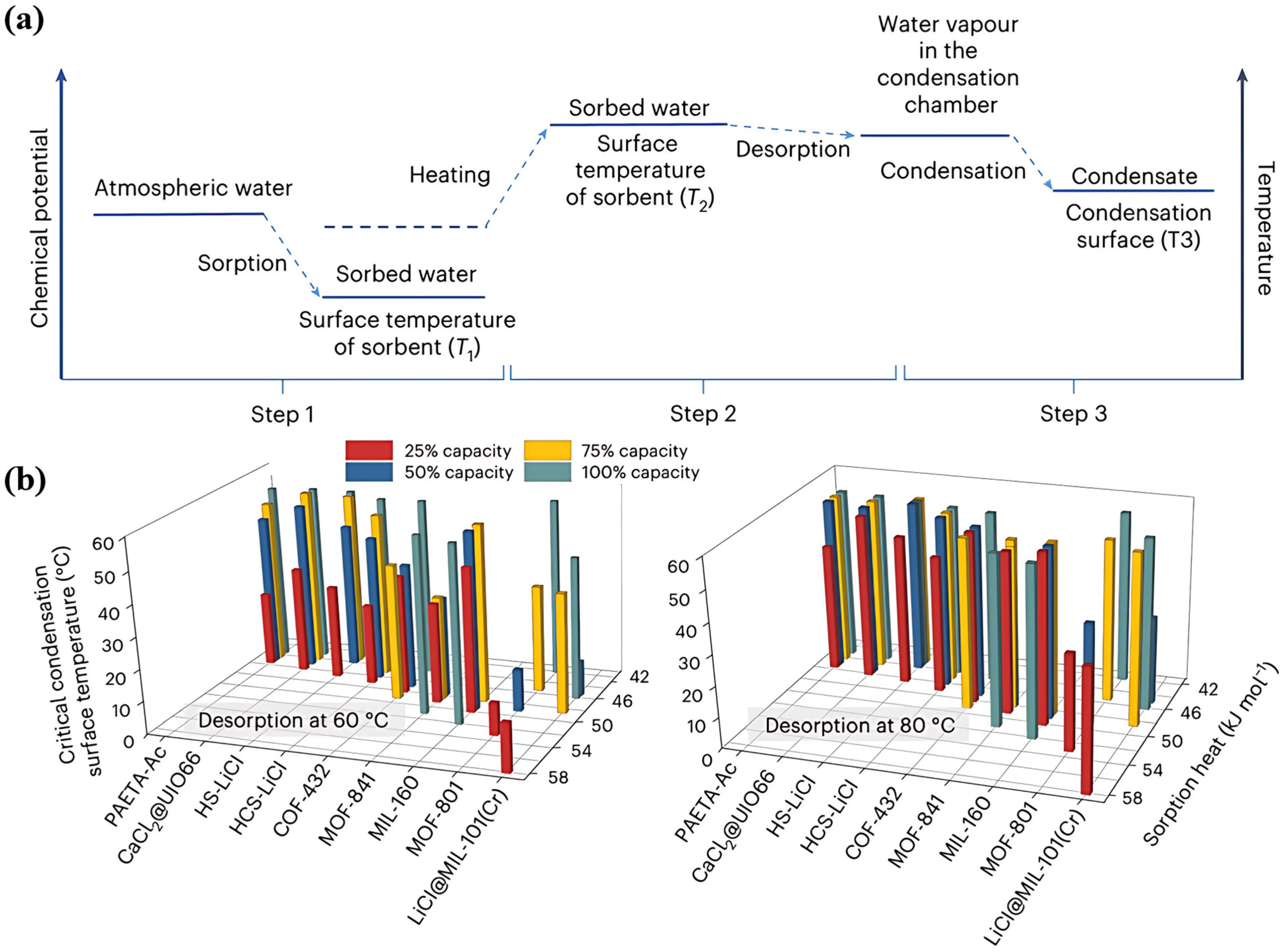
| System Classification | System | Unit Power Consumption (UPC) (kWh/L) | Test Humidity Range | References |
|---|---|---|---|---|
| Condensing system | VCC | 0.69 (specific conditions); 0.6–1.5 (normal conditions) | >40% | [10,26] |
| TEC | 2.1 | >20% | [28] | |
| Intermittent adsorption system | Intermittent SAWH MOF substrate (MOF-801) | 0.2–0.3 | 15–20% | [13,48] |
| Intermittent SAWH silicone-based | 0.2–0.3 | 20–80% | [54] | |
| Continuous/hybrid adsorption system | Continuous/hybrid system MOF base (MOF-303) | 0.3–0.45 | 10–90% | [55,56] |
| Continuous/hybrid system polyelectrolyte hydrogel | 0.35–0.5 | 10–95% | [52] |
| Name | Temperature | Relative Humidity | Adsorption Capacity | Material Strengths/Weaknesses | References |
|---|---|---|---|---|---|
| Zeolite A3 | 0–100 °C | 20–60% | 0.3–0.5 kg/kg | High-temperature resistant and reusable; High desorption temperature | [77] |
| Na(NO3), KNO3 and other salts | 15–35 °C 20–40 °C | 40–85% 35–80% | 0.5–1.5 kg/kg | Chemically stable; Limited adsorption efficiency | [69] |
| [poly-NIPAM] hydrogel | 10–40 °C | 40–85% | 0.5–2.0 kg/kg | Temperature responsive, adjustable adsorption; High humidity adsorption improvement is not obvious | [112] |
| Nano-cellulose MXene aerogel | 20–30 °C | 40–90% | 0.66–4.14 kg/kg | Lightweight, high porosity; High cost | [107] |
| Lignin-based foam carbon | 0–50 °C | 30–90% | 0.8–2.5 kg/kg | Low cost and good adsorption; Slightly lower than MOFs, etc. | [116] |
| Silica gel | 20–80 °C | 20–80% | 0.8–1.0 kg/kg | Chemically stable; Slow desorption after saturation | [54] |
| MOF-801 | 5–40 °C | 30–90% | 1.0–3.0 kg/kg | High specific surface area and porosity; Complex and costly to synthesize | [87] |
| CAL-gel | 20–30 °C | 45–95% | 1.0–3.0 kg/kg | Stable exposure to water vapor; Limited adsorption | [115] |
| NBHA aerogel | 5–35 °C | 40–90% | 1–1.5 kg/kg | High porosity hydrophilicity; Low mechanical strength | [113] |
| Ca(NO3)2 | 20–35 °C | 50–90% | 1.0–2.0 kg/kg | No strong corrosive substances; Low adsorption | [71] |
| UIO-66 | 15–45 °C | 30–90% | 1.2–3.0 kg/kg | Good adsorption; Special and costly reaction conditions | [86] |
| Nano-cellulose/LiCl- CNT | 10–40 °C | 35–90% | 1.5–4.0 kg/kg | Good porosity and high mechanical strength; Higher cost | [108] |
| MOF-808 | 10–40 °C | 25–90% | 1.5–3.0 kg/kg | Large specific surface area and high efficiency; Complex synthesis, high cost | [86] |
| MOF-303 | 0–40 °C | 40–90% | 1.5–3.5 kg/kg | Good adsorption; Special and costly reaction conditions | [55] |
| MIL-101 | 10–50 °C | 25–95% | 2.0–4.0 kg/kg | Good adsorption; Special and costly reaction conditions | [98] |
| PAM-CNT-CaCl2 hydrogel | 20–30 °C | 40–95% | 2.0–6.0 kg/kg | Excellent moisture absorption and high strength; Possible leakage of impregnating solution | [109] |
| LiCl | 0–50 °C | 40–90% | 3–4 kg/kg | Strong moisture absorption, cheap; Corrosive, easily deliquescent | [68] |
| [PDMAPs/CNT/LiCl]-hydrogels | 15–35 °C | 35–90% | 4.5–6 kg/kg | Good conductivity and moisture absorption; High cost | [110] |
| CaCl2 | 0–40 °C | 30–95% | 6–10 kg/kg | Good moisture absorption, wide range; Easy to deliquesce, high energy consumption for desorption | [70] |
| POG organic gel | 20–30 °C | 40–90% | 6–16 kg/kg | High moisture absorption; High cost | [114] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, W.; Cheng, L.; Wang, X.; Zhang, J.; Wang, X.; Wang, Z. Advances in Adsorptive Atmospheric Water Harvesting Technology: Materials, Desorption, and Systems. Sustainability 2025, 17, 10309. https://doi.org/10.3390/su172210309
Zhang W, Cheng L, Wang X, Zhang J, Wang X, Wang Z. Advances in Adsorptive Atmospheric Water Harvesting Technology: Materials, Desorption, and Systems. Sustainability. 2025; 17(22):10309. https://doi.org/10.3390/su172210309
Chicago/Turabian StyleZhang, Weitao, Lingyun Cheng, Xiangkai Wang, Jianying Zhang, Ximing Wang, and Zhe Wang. 2025. "Advances in Adsorptive Atmospheric Water Harvesting Technology: Materials, Desorption, and Systems" Sustainability 17, no. 22: 10309. https://doi.org/10.3390/su172210309
APA StyleZhang, W., Cheng, L., Wang, X., Zhang, J., Wang, X., & Wang, Z. (2025). Advances in Adsorptive Atmospheric Water Harvesting Technology: Materials, Desorption, and Systems. Sustainability, 17(22), 10309. https://doi.org/10.3390/su172210309






