Occurrence of Linear Alkylbenzene Sulfonates Homologues in Sludge Stabilization Treatments
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
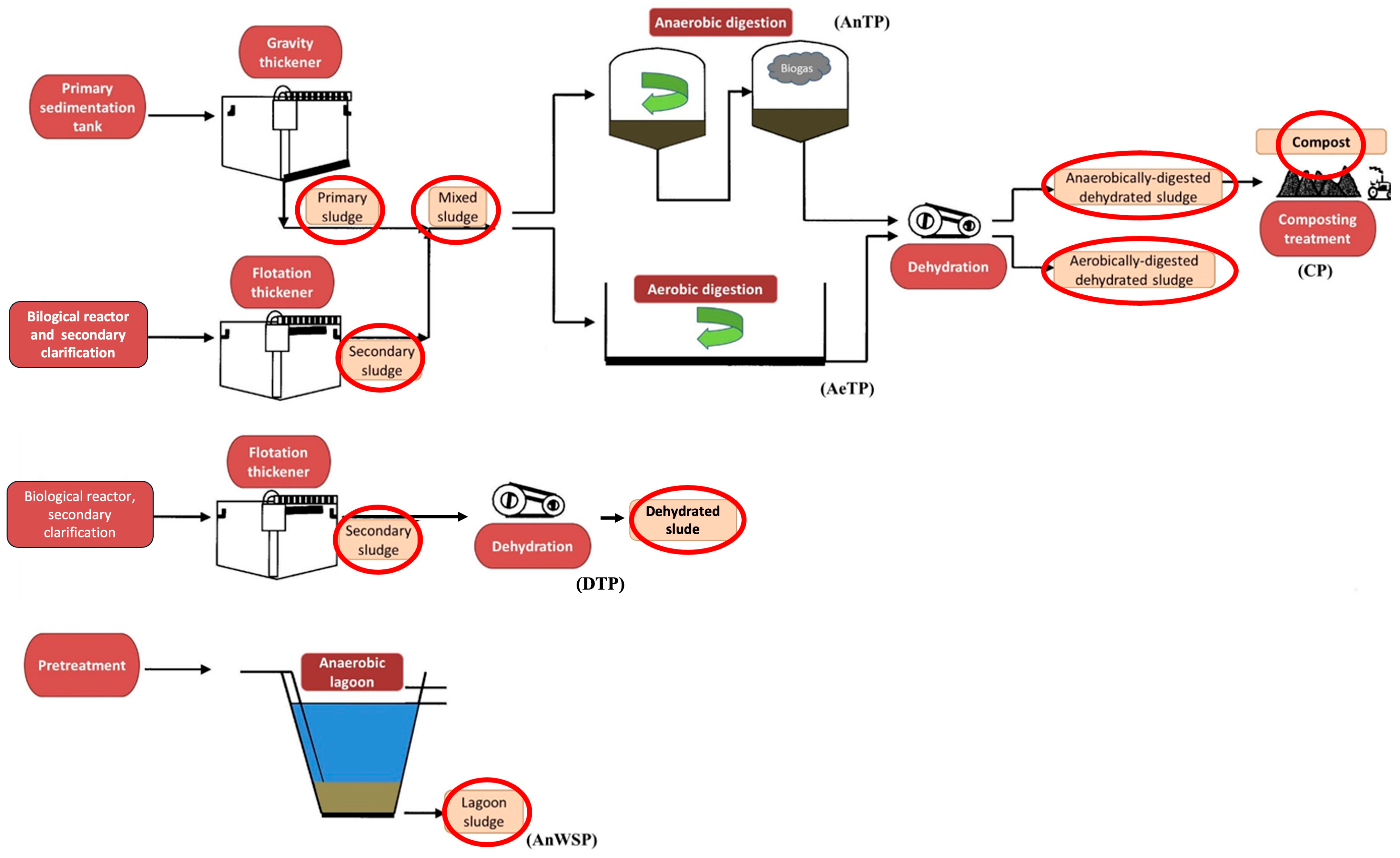
2.2. Sewage Sludge Treatment Plants and Monitoring Program
2.3. LAS Determination
3. Results and Discussion
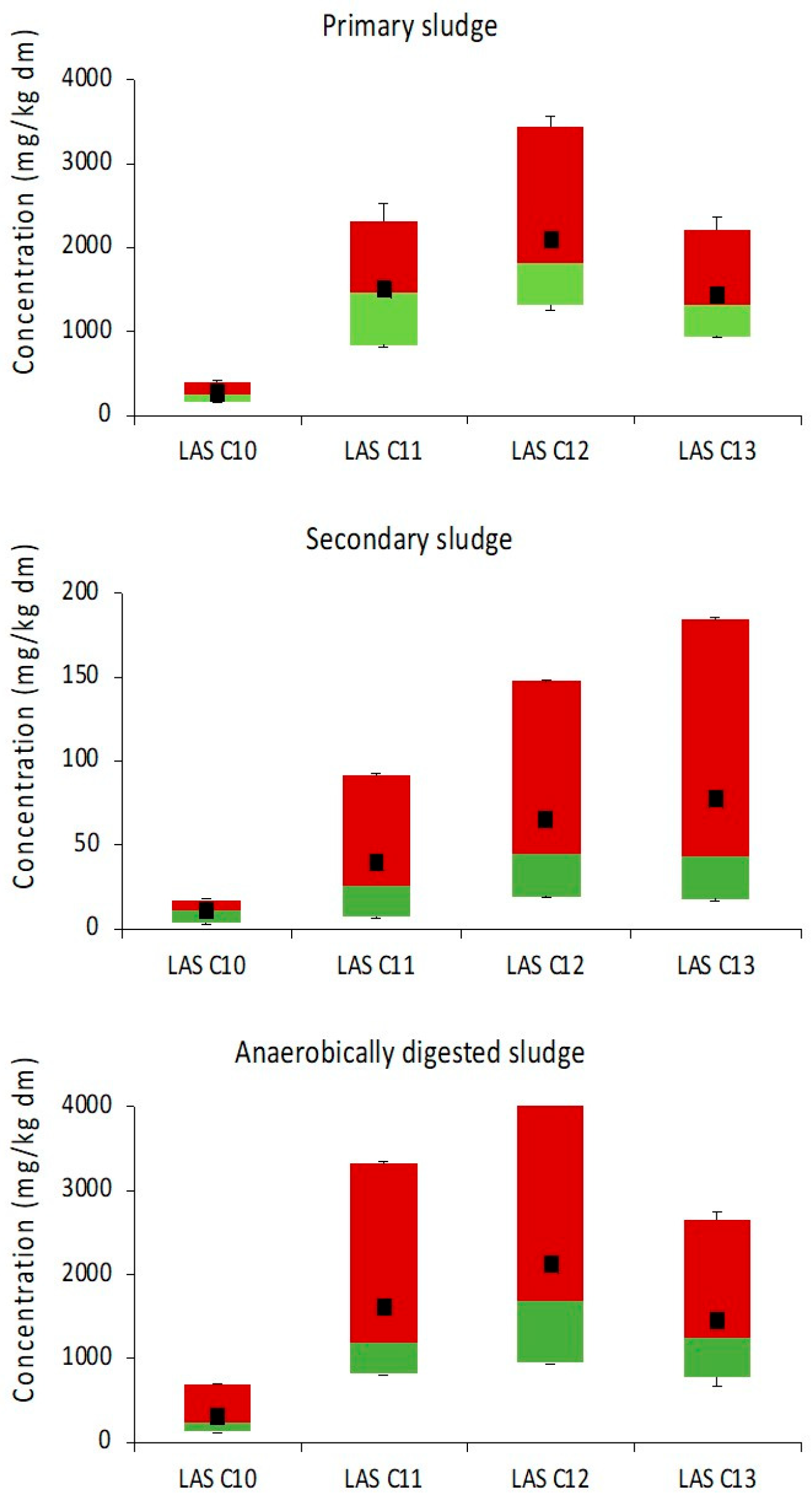
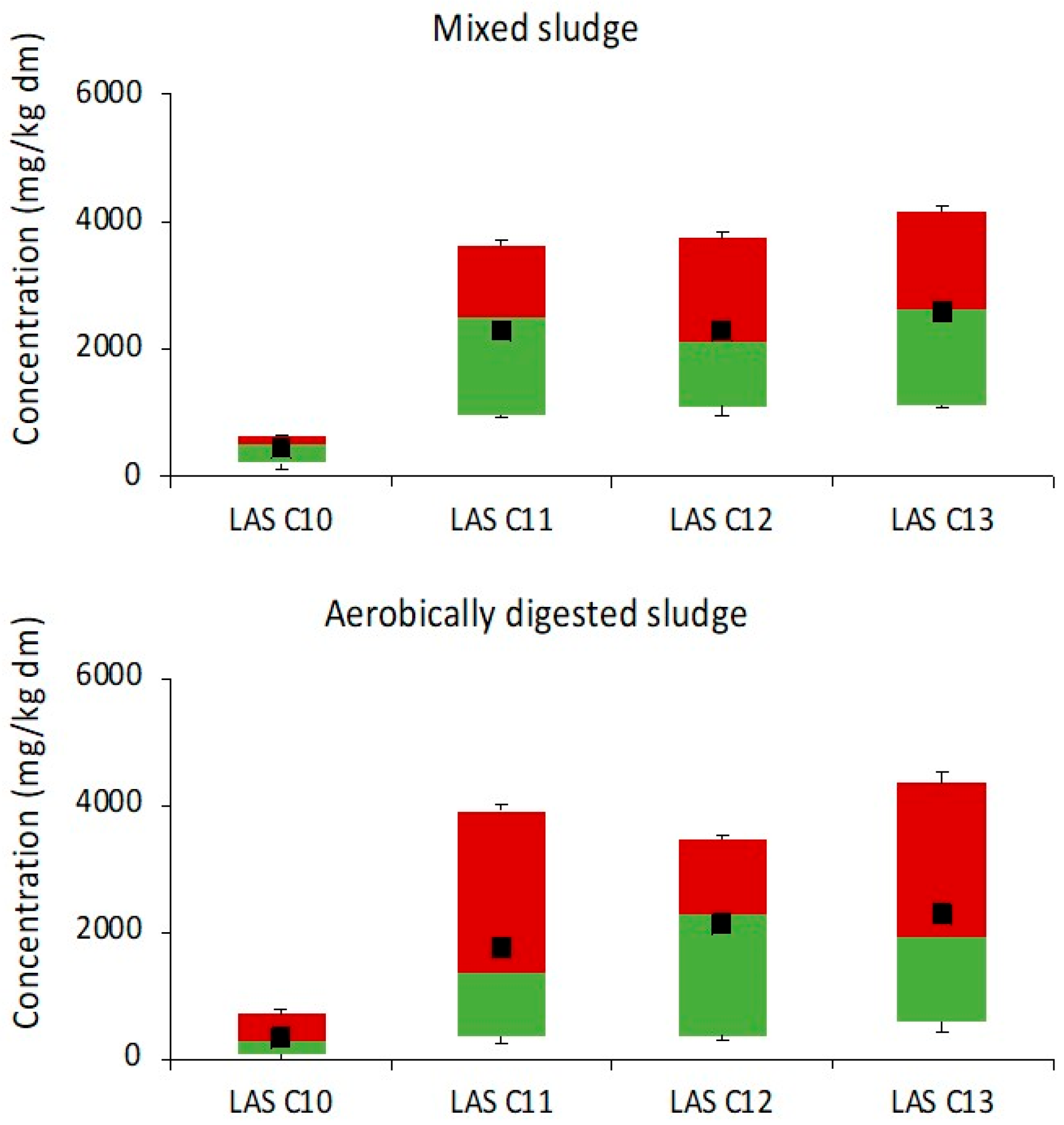
3.1. Dynamic of LAS in Sludge Stabilization Processes
3.1.1. Anaerobic Treatment Plants
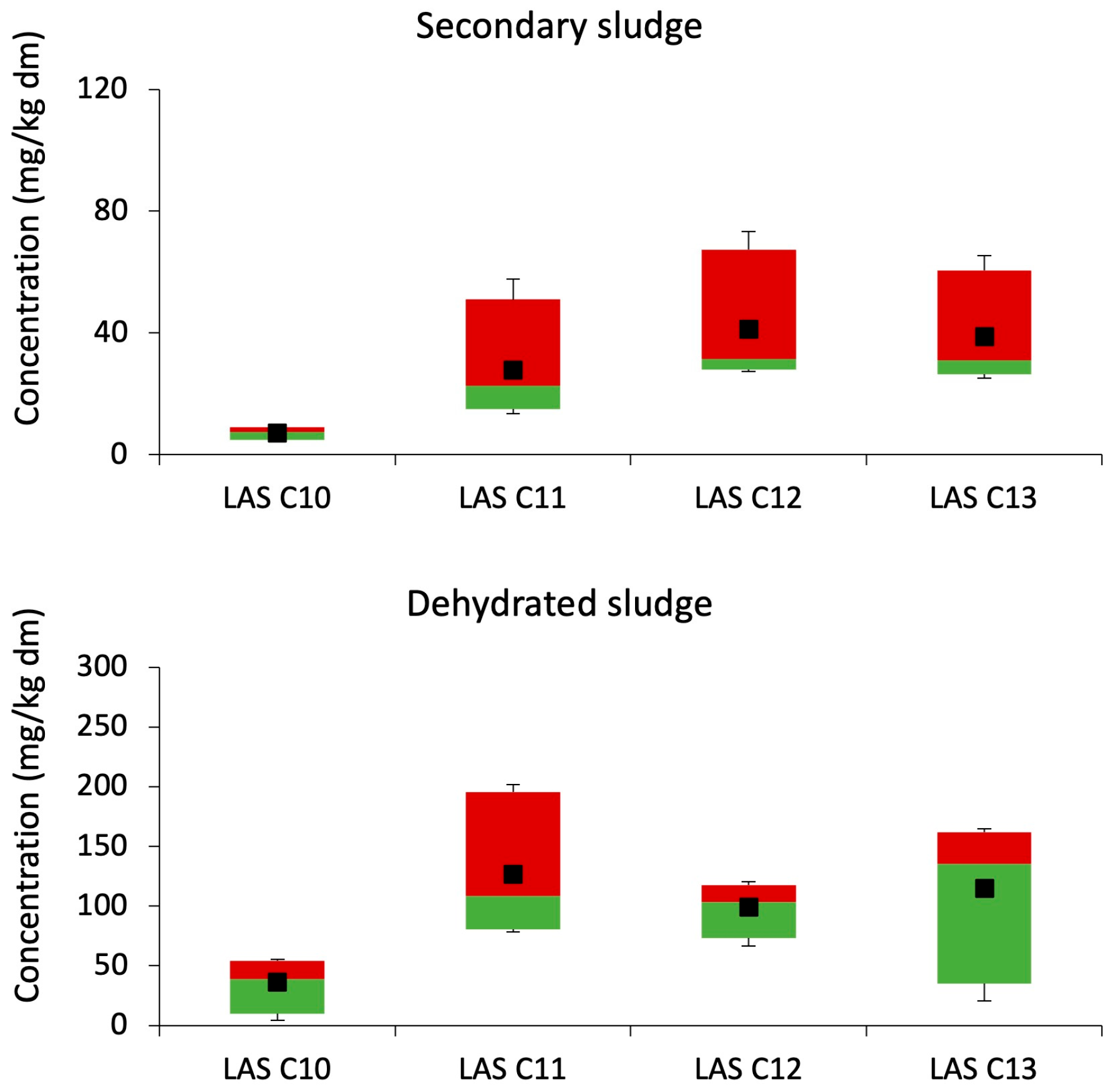
3.1.2. Aerobic Treatment Plants
3.1.3. Dehydration Treatment Plant
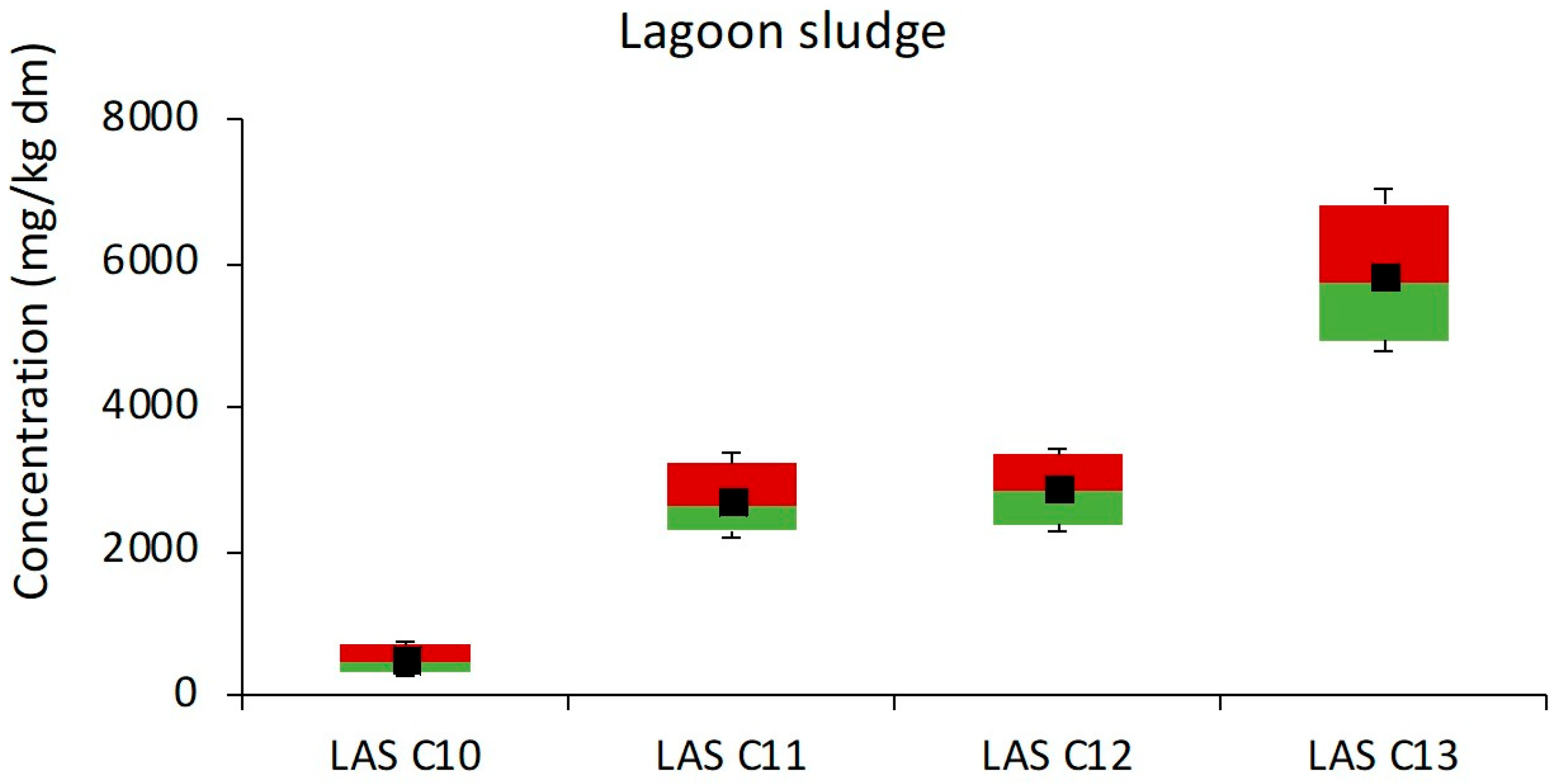
3.1.4. Anaerobic Stabilization Pond Treatments
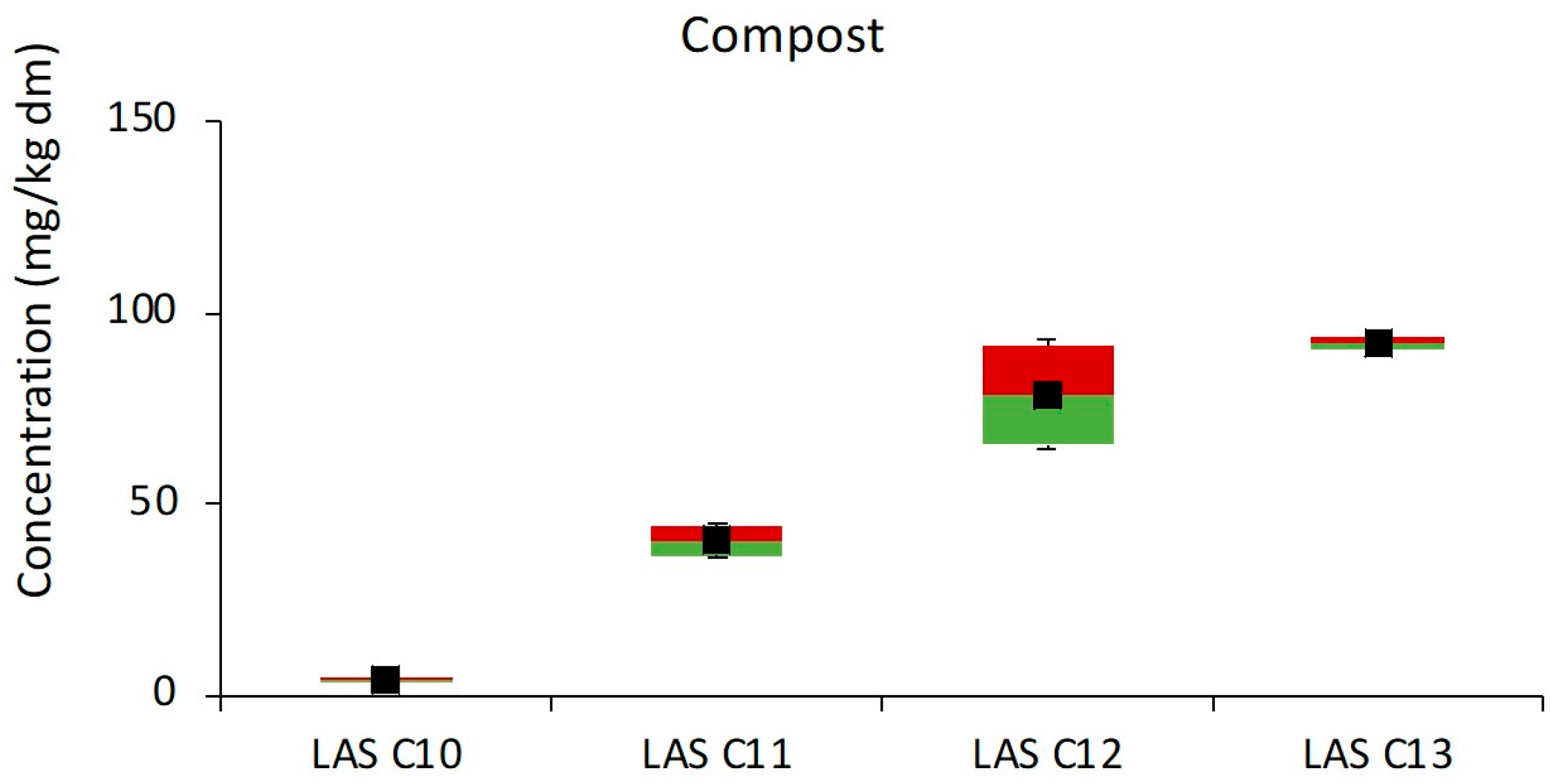
3.1.5. Composting
3.2. Implications of the EU Directive
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AeTPs | Aerobic treatment plants |
| AnTPs | Anaerobic treatment plants |
| AnWSPs | Anaerobic wastewater stabilization ponds |
| CPs | Composting plants |
| dm | Dry matter |
| DTPs | Dehydration treatment plants |
| LAS | Linear alkylbenzene sulfonates |
| WWTPs | Wastewater treatment plants |
References
- Mejías, C.; Martín, J.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of pharmaceuticals and their metabolites in sewage sludge and soil: A review on their distribution and environmental risk assessment. Trends Environ. Anal. Chem. 2021, 30, e00125. [Google Scholar] [CrossRef]
- Fijalkowski, K.; Rorat, A.; Grobelak, A.; Kacprzak, M.J. The presence of contaminations in sewage sludge: The current situation. J. Environ. Manag. 2017, 203, 1126–1136. [Google Scholar] [CrossRef]
- European Union (EU). Council directive 86/278/EEC of 12 June 1986 on the protection of the environment, and in particular of the soil, when sewage sludge is used in agriculture. Off. J. Eur. Union L Ser. 1986, 181, 6–12. [Google Scholar]
- Fytili, D.; Zabaniotou, A. Utilization of sewage sludge in EU application of old and new methods-A review. Renew. Sustain. Energy Rev. 2008, 12, 116–140. [Google Scholar] [CrossRef]
- European Commission (EC). Working Document on Sludge; 3rd Draft; DG Environment, European Commission: Brussels, Belgium, 2000. Available online: https://www.isprambiente.gov.it/it/progetti/cartella-progetti-in-corso/suolo-e-territorio-1/uso-dei-fanghi-di-depurazione-in-agricoltura-attivita-di-controllo-e-vigilanza-del-territorio/files/3rd_Draft_sludge_en.pdf (accessed on 16 September 2025).
- Hudcová, H.; Vymazal, J.; Rozkošný, M. Present restrictions of sewage sludge application in agriculture within the European Union. Soil Water Res. 2019, 14, 104–120. [Google Scholar] [CrossRef]
- Mininni, G.; Blanch, A.R.; Lucena, F.; Berselli, S. EU policy on sewage sludge utilization and perspectives on new approaches of sludge management. Environ. Sci. Pollut. Res. 2015, 22, 7361–7374. [Google Scholar] [CrossRef]
- HERA Project, Human and Environmental Risk Assessment on Ingredients of Household Cleaning Products. LAS, Linear Alkylbenezene Sulphonate (CAS No. 68411-30-3). 2013. Available online: https://www.heraproject.com/wp-content/uploads/2025/08/HERA-LAS-revised-April-2013-Final1.pdf (accessed on 5 October 2025).
- Aparicio, I.; Santos, J.L.; Alonso, E. Limitation of the concentration of organic pollutants in sewage sludge for agricultural purposes: A case study in South Spain. Waste Manag. 2009, 29, 1747–1753. [Google Scholar] [CrossRef]
- Cantarero, S.; Prieto, C.A.; López, I. Occurrence of high-tonnage anionic surfactants in Spanish sewage sludge. J. Environ. Manag. 2012, 95, S149–S153. [Google Scholar] [CrossRef]
- Krogh, P.H.; Lopez, C.V.; Cassani, G.; Jensen, J.; Holmstrup, M.; Schraepen, N.; Jørgensen, E.; Gavor, Z.; Temara, A. Risk assessment of linear alkylbenzene sulphonates, LAS, in agricultural soil revisited: Robust chronic toxicity tests for Folsomia candida (Collembola), Aporrectodea caliginosa (Oligochaeta) and Enchytraeus crypticus (Enchytraeidae). Chemosphere 2007, 69, 872–879. [Google Scholar] [CrossRef] [PubMed]
- Schowanek, D.; David, H.; Francaviglia, R.; Hall, J.; Kirchmann, H.; Krogh, P.H.; Smith, S.; Schraepen, N. Probabilistic risk assessment for linear alkylbenzene sulfonate (LAS) in sewage sludge used on agricultural soil. Regul. Toxicol. Pharmacol. 2007, 49, 245–259. [Google Scholar] [CrossRef]
- Costa, J.L.; Silva, L.G.; Veras, S.T.S.; Gavazza, S.; Florencio, L.; Motteran, F.; Kato, M.T. Use of nitrate, sulphate, and iron (III) as electron acceptors to improve the anaerobic degradation of linear alkylbenzene sulfonate: Effects on removal potential and microbiota diversification. Environ. Sci. Pollut. Res. 2025, 32, 16640–16655. [Google Scholar] [CrossRef]
- Lara-Martin, P.A.; Gomez-Parra, A.; Sanz, J.L.; González-Mazo, E. Anaerobic degradation pathway of linear alkylbenzene sulfonates (LAS) in sulfate-reducing marine sediments. Environ. Sci. Technol. 2010, 44, 1670–1676. [Google Scholar] [CrossRef]
- Kim, N.K.; Lee, S.H.; Yoon, H.; Jeong, G.; Jung, Y.J.; Hur, M.; Lee, B.H.; Park, H.D. Microbiome degrading linear alkylbenzene sulfonate in activated sludge. J. Hazard. Mater. 2021, 418, 126365. [Google Scholar] [CrossRef] [PubMed]
- Delforno, T.P.; Macedo, T.Z.; Midoux, C.; Lacerda, G.V.; Rué, O.; Mariadassou, M.; Loux, V.; Varesche, M.B.A.; Bouchez, T.; Bize, A.; et al. Comparative metatranscriptomic analysis of anaerobic digesters treating anionic surfactant contaminated wastewater. Sci. Total Environ. 2019, 649, 482–494. [Google Scholar] [CrossRef]
- Martín, J.; Mejías, C.; Arenas, M.; Santos, J.L.; Aparicio, I.; Alonso, E. Occurrence of Linear Alkylbenzene Sulfonates, Nonylphenol Ethoxylates and Di(2-ethylhexyl)phthalate in Composting Processes: Environmental Risks. Sustainability 2022, 14, 186. [Google Scholar] [CrossRef]
- Berna, J.L.; Ferrer, J.; Moreno, A.; Prats, D.; Bevia, F.R. The fate of LAS in the environment. Tenside Surfactants Deterg. 1989, 26, 101–107. [Google Scholar] [CrossRef]
- Cavalli, L.; Gellera, A.; Landone, A. LAS removal and biodegradation in a wastewater treatment plant. Environ. Toxicol. Chem. 1993, 12, 1777–1788. [Google Scholar] [CrossRef]
- García, M.T.; Campos, E.; Dalmau, M.; Ribosa, I.; Sanchez-Leal, J. Structure–activity relationships for association of linear alkyl- benzene sulfonates with activated sludge. Chemosphere 2002, 49, 279–286. [Google Scholar] [CrossRef]
- Adám, A.A.; Ziegenheim, S.; Janovák, L.; Szabados, M.; Bús, C.; Kukovecz, Á.; Kónya, Z.; Dékány, I.; Sipos, P.; Kutus, B. Binding of Ca2+ Ions to Alkylbenzene Sulfonates: Micelle Formation, Second Critical Concentration and Precipitation. Materials 2023, 16, 494. [Google Scholar] [CrossRef]
- U.S. Environmental Protection Agency (USEPA). POTW Sludge Sampling and Analysis Guidance Document. 2018. Available online: https://www.epa.gov/biosolids/potw-sludge-sampling-and-analysis-guidance-document (accessed on 16 September 2025).
- Martín, J.; Mejías, C.; Santos, J.L.; Aparicio, I.; Alonso, E.; Heinze, J. Quantification of linear alkylbenzene sulphonates in complex sludge samples: Influence of matrix effects in calibration methods. Microchem. J. 2024, 204, 111089. [Google Scholar] [CrossRef]
- World Health Organization (WHO). Programme, United Nations Environment. Linear Alkylbenzene Sulfonates & Related Compounds. In Environmental Health Criteria Series; World Health Organization: Geneva, Switzerland, 1996; ISBN 9789241571692. [Google Scholar]
- Jensen, J.; Smith, S.R.; Krogh, P.H.; Versteeg, D.J.; Temara, A. European risk assessment of LAS in agricultural soil revisited: Species sensitivity distribution and risk estimates. Chemosphere 2007, 69, 880–892. [Google Scholar] [CrossRef]
- Organisation for Economic Co-Operation and Development Screening Information Data Sets (OECD SIDS). Initial Assessment Report on LAS. 2005. Available online: https://www.cleaninginstitute.org/sites/default/files/research-pdfs/LAS_SIAR.pdf (accessed on 16 September 2025).
- Swisher, R.D. Surfactant Biodegradation, 2nd ed.; Revised and Expanded, Surfactant Science Series; CRC Press: New York, NY, USA, 1987; Volume 18, ISBN 9780824769383. [Google Scholar]
- Fauser, P.; Vikelsøe, J.; Sørensen, P.B.; Carlsen, L. Phthalates, nonylphenols and LAS in an alternately operated wastewater treatment plant—Fate modelling based on measured concentrations in wastewater and sludge. Water Res. 2003, 37, 1288–1295. [Google Scholar] [CrossRef]
- Heinze, J.E. Understanding the Biodegradation of LAS in the Absence of Oxygen. CLER Rev. 2015, 15, 20–59. [Google Scholar]
- García, M.T.; Campos, E.; Ribosa, I.; Latorre, A.; Sánchez-Leal, J. Anaerobic digestion of linear alkyl benzene sulfonates: Biodegradation kinetics and metabolite analysis. Chemosphere 2005, 60, 1636–1643. [Google Scholar] [CrossRef] [PubMed]
- Temmink, H.; Klapwijk, B. Fate of linear alkylbenzene sulfonate (LAS) in activated sludge plants. Water Res. 2004, 38, 903–912. [Google Scholar] [CrossRef] [PubMed]
- DiCorcia, A.; Samperi, R.; Bellioni, A.; Marcomini, A.; Zanette, M.; Lenr, K.; Cavalli, L. LAS Pilot study at the “Roma-Nord” sewage treatment plant and in the Tiber river. Riv. Ital. Sostanze Gr. 1994, 71, 467–475. [Google Scholar]
- Rodríguez, P.S. Contribución Al Estudio de la Degradación Anaerobia de Tensioactivos Aniónicos Alquilbencenosulfonatos lineales, Alquilsufatos Y Alcoholes Etoxilados Sulfatos. Ph.D. Thesis, University of Alicante, San Vicente del Raspeig, Alicante, Spain, 1996. [Google Scholar]
- Kuntze, K.; Shinoda, Y.; Moutakki, H.; Mcinerney, M.J.; Vogt, C.; Richnow, H.H.; Boll, M. 6-Oxocyclohex-1-ene-1-carbonyl-coenzyme A hydrolases from obligately anaerobic bacteria: Characterization and identification of its gene as a functional marker for aromatic compounds degrading anaerobes. Environ. Microbiol. 2008, 10, 1547–1556. [Google Scholar] [CrossRef]
- Mungray, A.K.; Kumar, P. Fate of Linear Alkylbenzene Sulfonates in the Environment: A Review. Int. Biodeterior. Biodegrad. 2009, 63, 981–987. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Arja, S.; Pirjo, S.A.M. Recycling sludge on cropland as fertilizer–Advantages and risks. Resour. Conserv. Recycl. 2020, 155, 105647. [Google Scholar] [CrossRef]
- Clarke, R.M.; Cummins, E. Evaluation of ‘classic’ and emerging contaminants resulting from the application of biosolids to agricultural lands: A Review. Hum. Ecol. Risk Assess. 2015, 21, 492–5134. [Google Scholar] [CrossRef]

| Sludge Treatment | Sludge | ΣLAS | ||
|---|---|---|---|---|
| Range | Mean | RSD | ||
| (mg/kg dm) | (mg/kg dm) | (%) | ||
| Anaerobic digestion | ||||
| Anaerobic digestion | Primary | 3156–8878 | 5291 | 37 |
| Secondary | 46–468 | 192 | 83 | |
| Digested | 2487–12,161 | 5521 | 60 | |
| Anaerobic wastewater stabilization ponds | ||||
| Anaerobic lagoons | Lagooning | 103,322–12,131 | 11,311 | 6 |
| Aerobic digestion | ||||
| Aerobic digestion | Mixed | 3147–11,727 | 7647 | 48 |
| Digested | 1145–12,668 | 6438 | 71 | |
| Composting | ||||
| Composting | Compost | 194–236 | 215 | 14 |
| Dehydration | ||||
| Dehydration | Secondary | 84.8–1117 | 282 | 146 |
| Dehydrated | 203–542 | 376 | 34 | |
| Sludge | ΣLAS | Hardness | ||
|---|---|---|---|---|
| Mean | Range | Mean | RSD | |
| (mg/kg dm) | (mg/kg dm) | (mg/kg dm) | (%) | |
| Primary | 5291 | 80–451 | 309 | 44 |
| Mixed | 7647 | 68–297 | 172 | 49 |
| Secondary | 282 | 120–455 | 264 | 54 |
| Lagoon | 11,862 | 257–325 | 301 | 9 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martín, J.; Mejías, C.; García-Criado, N.; Santos, J.L.; Aparicio, I.; Alonso, E.; Heinze, J. Occurrence of Linear Alkylbenzene Sulfonates Homologues in Sludge Stabilization Treatments. Sustainability 2025, 17, 10034. https://doi.org/10.3390/su172210034
Martín J, Mejías C, García-Criado N, Santos JL, Aparicio I, Alonso E, Heinze J. Occurrence of Linear Alkylbenzene Sulfonates Homologues in Sludge Stabilization Treatments. Sustainability. 2025; 17(22):10034. https://doi.org/10.3390/su172210034
Chicago/Turabian StyleMartín, Julia, Carmen Mejías, Noelia García-Criado, Juan Luis Santos, Irene Aparicio, Esteban Alonso, and John Heinze. 2025. "Occurrence of Linear Alkylbenzene Sulfonates Homologues in Sludge Stabilization Treatments" Sustainability 17, no. 22: 10034. https://doi.org/10.3390/su172210034
APA StyleMartín, J., Mejías, C., García-Criado, N., Santos, J. L., Aparicio, I., Alonso, E., & Heinze, J. (2025). Occurrence of Linear Alkylbenzene Sulfonates Homologues in Sludge Stabilization Treatments. Sustainability, 17(22), 10034. https://doi.org/10.3390/su172210034











