Abstract
CoFe2O4 loaded onto red brick powder (CoFe2O4@RBP) was synthesized via coprecipitation followed by post-calcination and employed as a heterogeneous catalyst to activate peroxymonosulfate (PMS) for the degradation of methylene blue (MB), thereby valorizing red brick demolition waste within a circular economy pathway and aligning the study with sustainability-oriented resource recovery. The effects of pH, PMS concentration, catalyst dosage, and coexisting substances on MB removal were systematically investigated. Complete MB removal was achieved within 30 min, and the apparent rate constant for the CoFe2O4@RBP/PMS system was 0.22 min−1—slightly lower than that of CoFe2O4/PMS—while Co leaching was markedly reduced. The process performed well across a broad pH range (3.0–9.0). EPR and radical-quenching experiments indicate that SO4•− and HO• play a minor role, whereas the Co(II)–PMS complex is primarily responsible for MB degradation; accordingly, common coexisting species (SO42−, Cl−, NO3−, humic acid) exert negligible effects. The catalyst also maintained strong durability across numerous repetitions. These results highlight a cost-efficient route to PMS activation by coupling CoFe2O4 with construction waste-derived supports.
1. Introduction
Methylene blue (MB), one of the common azo dyes, whose chemical formula is C16H18N3ClS, has been widely employed for synthetic dyes in textile manufacturing [1]. However, MB could endanger human health and have a detrimental effect on the quality of water [2]. Traditional removal techniques such adsorption, membrane separation, and biological treatment are frequently ineffectual because of its poor biocompatibility and strong water solubility [3,4]. Thus, creating an effective treatment method for azo dye wastewater is essential.
Peroxymonosulfate (PMS)-based advanced oxidation technologies have garnered considerable interest because they can produce multiple oxidation mechanisms, such as hydroxyl radicals (•OH), sulfate radicals (SO4•−), and singlet oxygen (1O2), while minimizing interference from the water matrix [5,6]. Compared to the homogeneous reaction systems, heterogeneous catalytic systems have received extensive research due to their ability for recycling and reuse, as well as low metal dissolution [7,8]. Among these heterogeneous catalysts, CoFe2O4, as one of the most widely used catalysts, has been employed to activate PMS for the degradation of pollutants due to its high catalytic activity, magnetic properties, and chemical stability [9,10]. Unfortunately, the leaching of potentially carcinogenic Co2+/Co3+ from CoFe2O4 and the easy loss of nanomaterials makes it difficult to further apply in practice [11,12]. To address these issues, the combination of CoFe2O4 and carrier material such as Al2O3, activated carbon, and graphene is beneficial to reduce the leaching of cobalt and improve the catalytic activity [13,14,15]. You et al. [16] reported that a magnetic cobalt ferrite biochar composite was successfully prepared by pyrolysis and the sol–gel method, which was used to activate PMS for removing lomefloxacin hydrochloride, and the lower dissolution rate of Co was obtained. However, most of the material preparation processes are complex and costly, and it is difficult to achieve large-scale production for the treatment of actual wastewater. Hence, the development of low-cost carrier materials is a pressing priority.
Construction waste is the garbage produced during building construction and destruction, which usually includes a variety of materials like plaster, cement, red brick (RB), tile, wood, and glass. Construction trash is typically disposed of in an unstructured way, which depletes soil resources and seriously pollutes the environment. In order to solve these problems, we can lessen pollution in the environment and save precious resources by encouraging recycling and the reuse of construction waste. An all-purpose building material, RB is made from natural clay and is mostly made up of fused aluminum (Al2O3), quartz (SiO2), and hematite (α-Fe2O3) particles [17,18,19]; these key components could strengthen the combination between CoFe2O4 nanoparticles and RB, reducing the leaching of Co during the reaction process [12,15]. Rafique et al. [20] evaluated the fluoride adsorption properties of red pavers or brick paving blocks, which are inexpensive and readily available locally and could be used as an adsorbent for defluoridation. Inspired by this, RB as a carrier, not only can increase the adsorption of pollutants, but also can effectively fix the active components, thereby enhancing their ability to activate PMS. Nevertheless, there is no report on using red bricks as a carrier to load CoFe2O4 for PMS activation, and the related process is unclear.
In this study, red brick powder (RBP) obtained by grinding red bricks was used as the catalyst support. CoFe2O4@RBP heterogeneous catalysts were synthesized by coprecipitation followed by calcination. Physicochemical properties were characterized using multiple techniques. MB degradation in the CoFe2O4@RBP/PMS system was systematically investigated, and the dominant reactive species were identified by EPR and radical-quenching experiments. The effects of pH, PMS concentration, catalyst dosage, and coexisting substances on MB removal were evaluated. Reusability and stability were assessed over three consecutive cycles. The primary objective is to evaluate the catalytic activity and stability of CoFe2O4@RBP and to demonstrate the potential use of construction waste in wastewater treatment.
2. Materials and Methods
2.1. Materials and Reagents
Red brick was acquired from Yixing Shenyun Ceramics Co. Ltd. (Yixing, China). Fe(NO3)3•9H2O, Co(NO3)2•6H2O, citric acid, NaOH, methanol (MeOH), tertiary butanol (TBA), furfuryl alcohol (FFA), humic acid (HA), and 2,2,6,6-tetramethylpiperidine (TEMP) were purchased from Aladdin Chemistry Co. Ltd. (Shanghai, China). 5,5-dimethyl-2-pyrroline-1 N-oxide (DMPO) was supplied from Dongren Chemical Technology Co. Ltd. (Kumanoto, Japan). Analytical-grade reagents were used as received, and all solutions were prepared with ultrapure water.
2.2. Preparation of CoFe2O4@RBP
The CoFe2O4@RBP catalysts underwent fabrication via coprecipitation followed by calcination (Seen in Figure S1). Before loading CoFe2O4, the red brick was grinded into powder first. In total, 10 g of RBP was added into a 100 mL mixed solution, including 0.01 M Co(NO3)2•6H2O, 0.02 M Fe(NO3)3•9H2O, and 0.03 M citric acid, and then reacted for 1 h at 60 °C. Following this, the mixture was concentrated at 90 °C in a water bath with magnetic stirring (150 rpm). The resulting solid was dried and then calcined at 500 °C for 4 h in a muffle furnace.
2.3. Experimental Procedures
The catalytic degradation experiments were conducted in 250 mL glass bottles, and the reaction temperature was controlled at 25 ± 1 °C using a water bath. The reaction solution contained 0.1 M MB and 2.0 mM PMS, and its pH was adjusted by 0.01 M HNO3/NaOH. The CoFe2O4@RBP catalysts (2.0 g L−1) were added into the above solution to trigger the oxidation reaction. At predetermined intervals, 5.0 mL reaction aliquots were filtered through 0.22 μm membranes and immediately analyzed for MB absorbance via UV–visible spectrophotometry. For the reusability and stability of CoFe2O4@RBP, the used CoFe2O4@RBP was obtained by centrifugation after reaction, washed several times with ethanol and ultrapure water, and then dried for the next reaction.
2.4. Characterization of CoFe2O4@RBP
Morphology was examined by scanning electron microscopy (SEM; Zeiss GeminiSEM 300, Oberkochen, Germany). Crystal structure was identified by X-ray diffraction (XRD; Bruker D8 Advance, Ettlingen, Germany) using Cu Kα radiation. Textural properties—BET specific surface area, pore volume, and pore size distribution—were obtained from N2 adsorption–desorption measurements (Micromeritics, Norcross, GA, USA). Surface functional groups were analyzed by FTIR (Thermo Nicolet iS5, Thermo Fisher Scientific, Waltham, MA, USA) over 400–4000 cm−1. X-ray photoelectron spectra (XPS) were recorded on an ESCALAB 250Xi (Thermo Fisher Scientific, Waltham, MA, USA) equipped with a monochromated Al Kα source. The point zero charge (PZC) of CoFe2O4@RBP was detected using nanometer laser particle size and a Zeta potential analyzer (Brookhaven 90Plus, Nashua, NH, USA).
2.5. Analytical Methods
The MB concentration was identified at 664 nm using a UV–visible spectrophotometer (Persee T6, Beijing, China). The EPR spectral characteristics of HO•, SO4•−, and 1O2 were analyzed with DMPO and TEMP as spin-trapping agents by an electron paramagnetic resonance spectrometer (Bruker, EMX-10/12), and the EPR test conditions are an X-field scan with a width of 60 G and a central magnetic field strength of 3475 G. Total organic carbon (TOC) during MB degradation was measured using a TOC analyzer (enviro TOC, Elementar, Langenselbold, Germany). Dissolved metals were quantified by ICP–OES (Avio 200, PerkinElmer, Waltham, MA, USA).
3. Results and Discussion
3.1. Characterization of CoFe2O4@RBP Catalysts
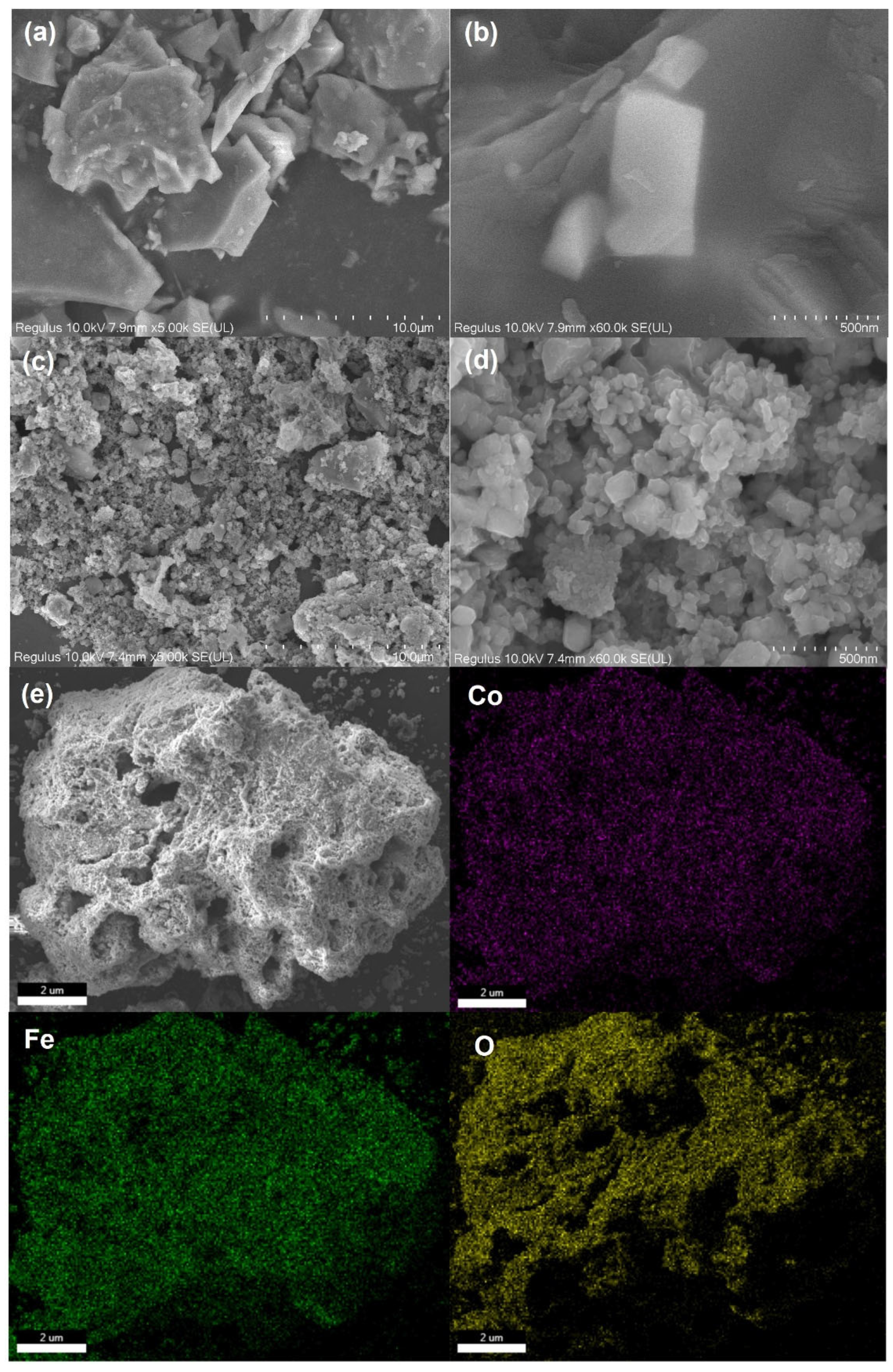
The SEM images of RBP and CoFe2O4@RBP are shown in Figure 1a–d. Obviously, the RBP exhibits an irregular shape, and its particle size range is from 1~8 μm. For the CoFe2O4@RBP catalysts, a large number of nano-sized CoFe2O4 are aggregated on the surface of RBP compared to the bare RBP, resulting in the formation of additional pore structures and an increase in active sites for PMS activation compared to the unmodified RBP. Furthermore, elemental mapping conducted through SEM-EDS (Figure 1e) displays that Co and Fe are uniformly distributed on the surface of CoFe2O4@RBP, implying that CoFe2O4 nanoparticles are successfully loaded on the surface of RBP via the coprecipitation method and post-calcination treatment.
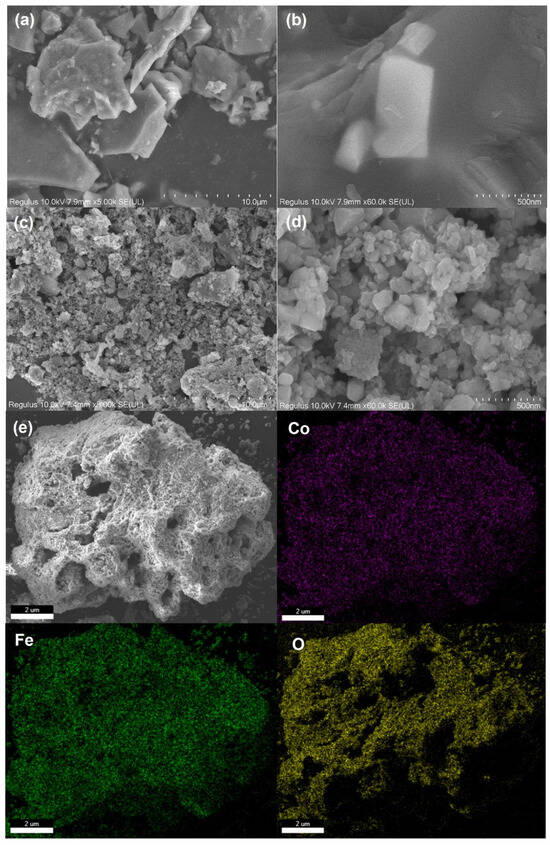
Figure 1.
SEM image of (a,b) RBP and (c,d) CoFe2O4@RBP; (e) elemental mapping of CoFe2O4@RBP by SEM-EDS analysis.
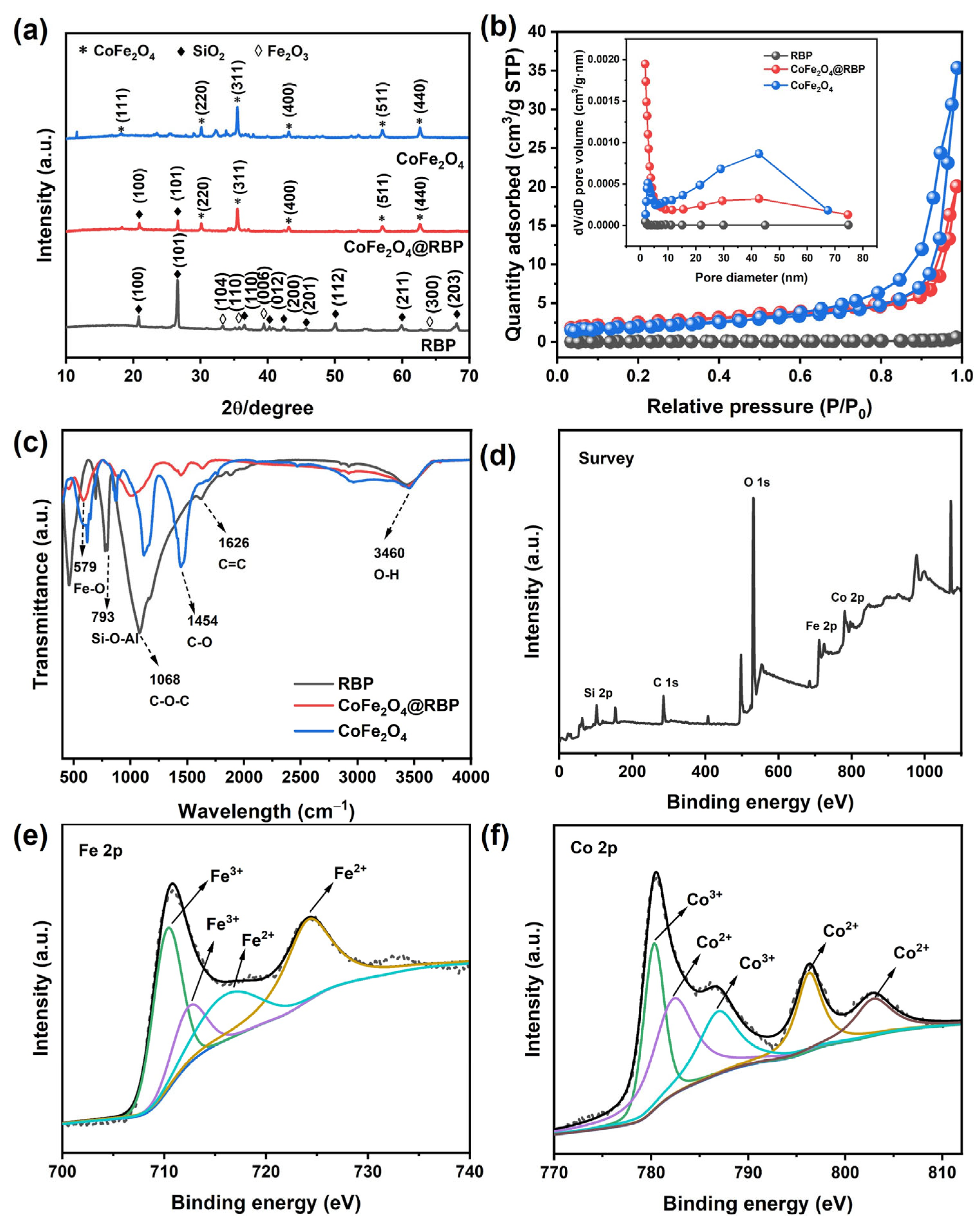
Figure 2a presents the XRD patterns of CoFe2O4, RBP, and CoFe2O4@RBP. RBP is not a pure substance, mainly composed of SiO2 and Fe2O3. The diffraction peaks of RBP at 2θ = 20.86°, 26.64°, 36.55°, 39.47°, 42.45°, 45.80°, 50.14°, 59.96°, and 68.14° are attributed to (100), (101), (110), (012), (200), (201), (112), (211), and (203) crystal planes of SiO2 according to the JCPDS card No. 46-1045, with the characteristic diffraction peaks at 2θ = 33.16°, 35.63°, 39.29°, and 63.99° corresponding to (104), (110), (006), and (300) crystal planes of Fe2O3 (JCPDS card No. 33-0664). After loading, the characteristic diffraction peaks at 2θ = 18.30°, 30.09°, 35.45°, 43.08°, 56.97°, and 62.56° attributed to (111), (220), (311), (400), (511), and (440) crystal planes of CoFe2O4 (JCPDS card No. 22-1086) were observed, and the average particle size of CoFe2O4 was 59 nm, which serves as the active site for PMS activation. In addition, some characteristic diffraction peaks of SiO2 and Fe2O3 in RBP disappeared after loading, which might have been due to the effect of calcination temperature and the content changes in these substances.
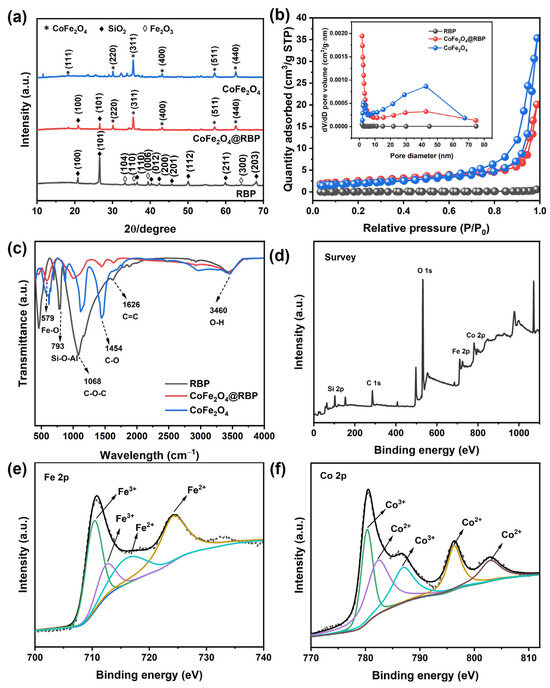
Figure 2.
(a) XRD patterns; (b) N2 adsorption–desorption isotherm and its pore size distribution (inset) and (c) FTIR spectra of different catalysts; (d) XPS survey; (e) Fe 2p and (f) Co 2p XPS of CoFe2O4@RBP.
Figure 2b presents the nitrogen adsorption–desorption isotherms for various catalysts. The CoFe2O4@RBP sample exhibits a Type IV isotherm, a telltale sign of both micropores and mesopores coexisting within its structure. Moreover, the BET surface area measurement revealed that CoFe2O4@RBP boasts 9.10 m2/g—a marked improvement over CoFe2O4 (7.21 m2/g) and RBP (0.27 m2/g) as shown in Table S1. This enhancement likely stems from CoFe2O4 nanoparticles clustering on the RBP surface, creating additional porosity that boosts the specific surface area post loading. Additionally, the pore size distribution observed in RBP does not quite line up with previous studies [17,18,20], potentially owing to variations in material composition and calcination conditions. These findings collectively demonstrate that incorporating CoFe2O4 amplifies RBP’s specific surface area, paving the way for increased active sites to facilitate PMS activation.
Figure 2c displays the FTIR spectra of CoFe2O4, RBP, and CoFe2O4@RBP. The characteristic absorption peak located at 579 cm−1 of CoFe2O4@RBP is attributed to the Fe-O bond [21]. The absorption peaks at 793 cm−1 and 1068 cm−1 represent the Si-O-Al bond and C-O-C bond [22], respectively. Furthermore, the absorption peaks at 1454 cm−1 and 1626 cm−1 of CoFe2O4 and CoFe2O4@RBP, attributed to the C-O bond and C=C bond, are observed [23]. In addition, the adsorption peak at 3460 cm−1 corresponds to the O-H bond, which might derive from surface hydroxyl species and adsorb water.
The surface composition and chemical states of CoFe2O4@RBP were analyzed by XPS, with C 1s at 284.8 eV used for calibration. As shown in Figure 2d, CoFe2O4@RBP mainly contains Fe, Co, Si, and O, and the Co:Fe molar ratio is approximately 1:2.25—Fe-richer than the stoichiometric CoFe2O4 (1:2) due to Fe originating from the RBP support (Table S1). In the Fe 2p region (Figure 2e), the Fe 2p3/2 envelope resolves into two components at 710.3 eV (Fe3+, octahedral) and 712.4 eV (Fe3+, tetrahedral); the Fe 2p1/2 peak appears at 724.1 eV, and a satellite at ~716.18 eV indicates high-spin Fe2+ in octahedral sites [24]. The Co 2p spectrum (Figure 2f) shows the 2p3/2 and 2p1/2 spin–orbit components with shake-up satellites: contributions at 780.3 eV (Co3+, octahedral) and 782.3 eV (Co2+, octahedral) within 2p3/2, and a 2p1/2 feature at 796.3 eV assigned to Co2+ in tetrahedral sites. Satellites are observed near 786.9 and 802.9 eV on the high-binding-energy side [22].
3.2. Catalytic Performance of CoFe2O4@RBP/PMS
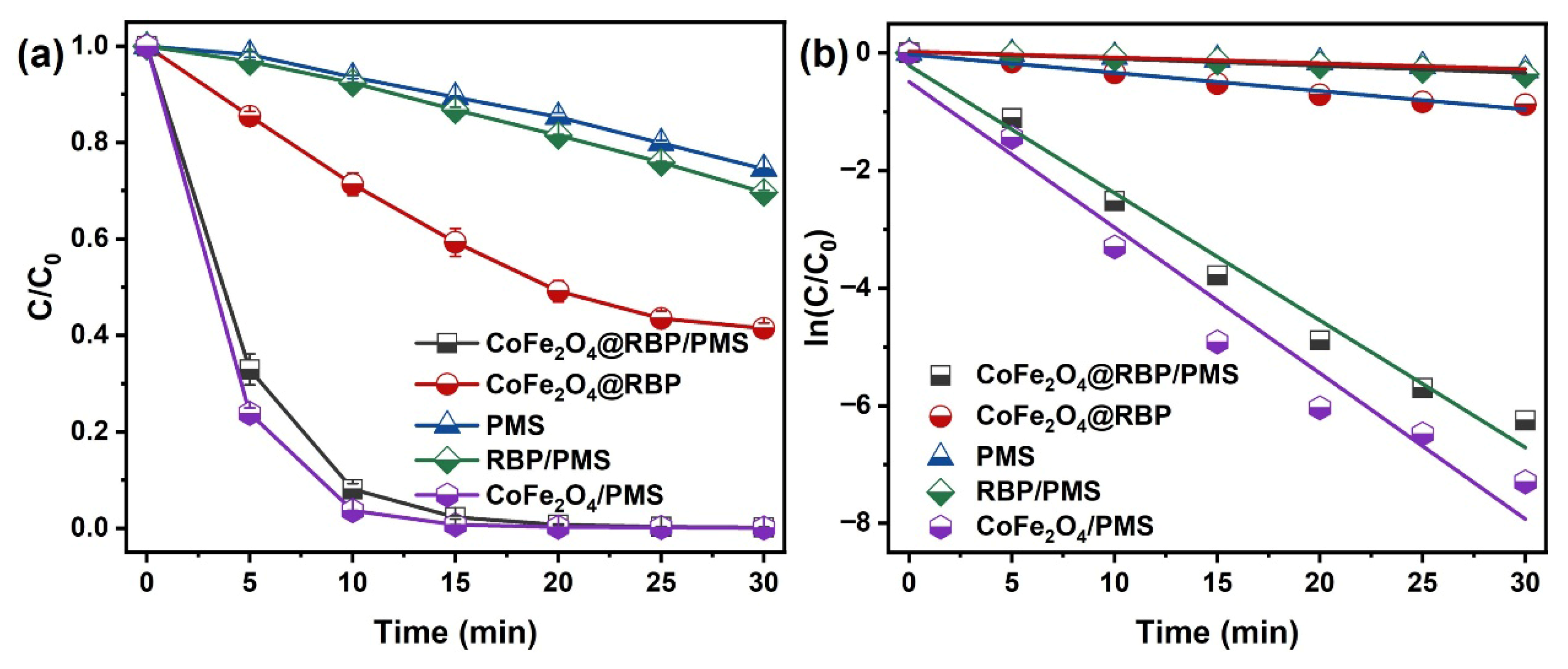
Figure 3a compares MB degradation across various process configurations. After a 30 min reaction, 58.6% of MB is adsorbed by CoFe2O4@RBP at pH = 7.0. It is worth noting that MB is completely removed in the CoFe2O4@RBP/PMS processes, with an efficiency much higher than that in the PMS (25.5%) and RBP/PMS (30.3%) processes. Correspondingly, the reaction rate constant of CoFe2O4@RBP/PMS is 0.22 min−1 (Figure 3b), which is larger than that of PMS (0.0099 min−1) and RBP/PMS (0.012 min−1) processes. However, the reaction rate constant of MB removal in the CoFe2O4@RBP/PMS process is slightly lower than in the CoFe2O4/PMS process (0.24 min−1), mainly because the Co leaching of CoFe2O4 (1.16 mg/L) is greater than that of CoFe2O4@RBP (67 μg/L) during the reaction process. The Co leaching in CoFe2O4@RBP is greatly reduced by the addition of RBP, which could be ascribed to the special pore structure of RBP and the strong interaction between CoFe2O4 nanoparticles and key components (i.e., SiO2, Fe2O3) in RBP. Meanwhile, the other CoFe2O4-based catalysts in studies [14,15,22,23,25,26,27] are compared with CoFe2O4@RBP, and the results are listed in Table S2. Obviously, CoFe2O4@RBP shows a high catalytic performance compared to these CoFe2O4-based catalysts. To distinguish the contributions of adsorption and oxidation, MB removal by methanol desorption was conducted in CoFe2O4@RBP and CoFe2O4@RBP/PMS after a 30 min reaction. As shown in Figure S2, the degradation efficiency of MB is 98.5% after the 30 min reaction, implying that the oxidation is the dominant process for the removal of MB. Moreover, 39.1% of MB is mineralized after the 30 min reaction (Figure S3), suggesting that CoFe2O4@RBP/PMS has a relatively good mineralization capacity. In addition, the degradation of MB was conducted using 67 μg/L of Co (Co leaching concentration) for PMS activation; 73.8% of MB was degraded by 67 μg/L Co2+/PMS (Figure S4), implying that the heterogeneous reaction is the major degradation pathway for the removal of MB in the CoFe2O4@RBP/PMS process. Therefore, the loading of CoFe2O4 improves the catalytic activity of RBP, as well as reducing the leaching of Co.
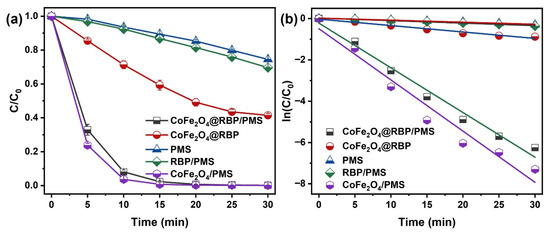
Figure 3.
(a) MB removal and (b) the plot of ln(C/C0) vs. time for MB removal across different reaction processes. Conditions: [catalyst]0 = 2.0 g L−1, [PMS]0 = 2.0 mM, [MB]0 = 0.1 mM, and initial pH = 7.0.
3.3. Reaction Mechanism of CoFe2O4@RBP/PMS
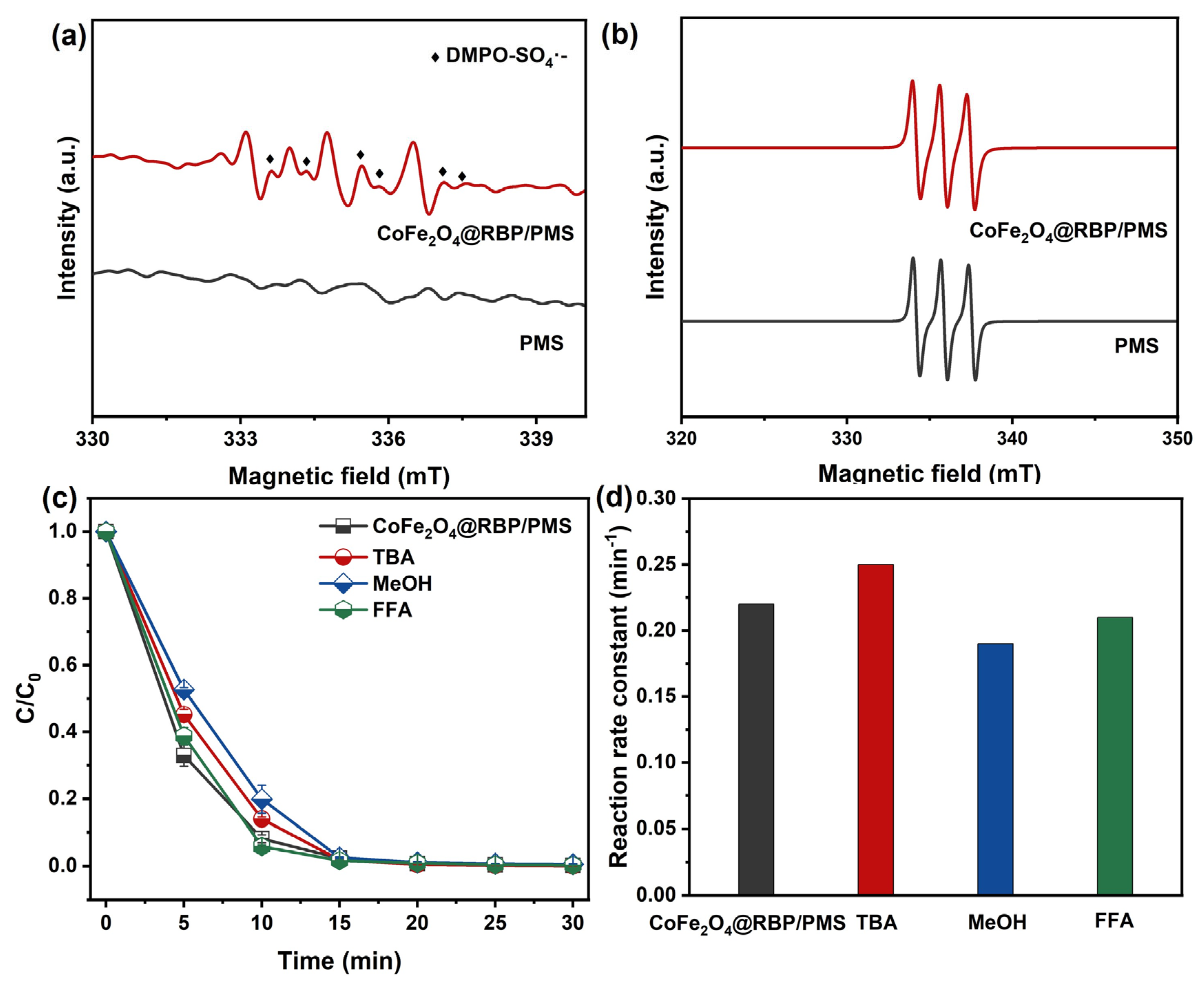
Dominant reactive species were probed by EPR in conjunction with radical-trapping assays in the CoFe2O4@RBP/PMS system. As shown in Figure 4a, no discernible DMPO adduct signals appear in PMS alone, whereas characteristic DMPO–SO4•− signals with hyperfine splitting constants (αN = 13.2 G; αH = 9.6, 1.48, and 0.78 G) are observed in the CoFe2O4@RBP/PMS system, evidencing SO4•− formation [28]. A 1:1:1 triplet due to TEMP–1O2 adducts is detected in both PMS and CoFe2O4@RBP/PMS; however, its intensity does not increase upon adding CoFe2O4@RBP (Figure 4b), indicating that the catalyst does not promote 1O2 generation. In addition, TBA, MeOH, and FFA were added into the reaction system as the quenching agents for HO•, SO4•−, and 1O2 [5,29]. The results of the radical capture experiments are displayed in Figure 4c,d, where the presence of MeOH and TBA shows a negligible effect on the removal of MB in the CoFe2O4@RBP/PMS process, implying that HO• and SO4•− play a minor role in the removal of MB. Similarly, the removal efficiency of MB and its reaction rate constant basically remain unchanged in the presence of 10 mM FFA, demonstrating that 1O2 acts as a secondary oxidizing agent in MB degradation.
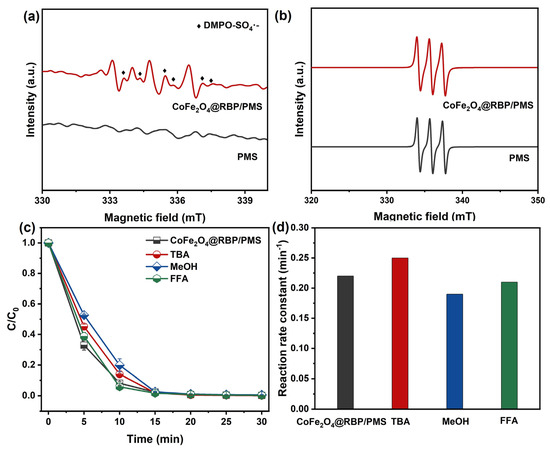
Figure 4.
EPR spectra using (a) DMPO and (b) TEMP as spin-trapping agents in different reaction processes. (c) Effect of trapping agents on the removal of MB and (d) their reaction rate constants in the CoFe2O4@RBP/PMS process. Conditions: [catalyst]0 = 2.0 g L−1, [PMS]0 = 2.0 mM, [MB]0 = 0.1 mM, [TBA]0 = [MeOH]0 = 100 mM, [FFA]0 = 10 mM, and initial pH = 7.0.
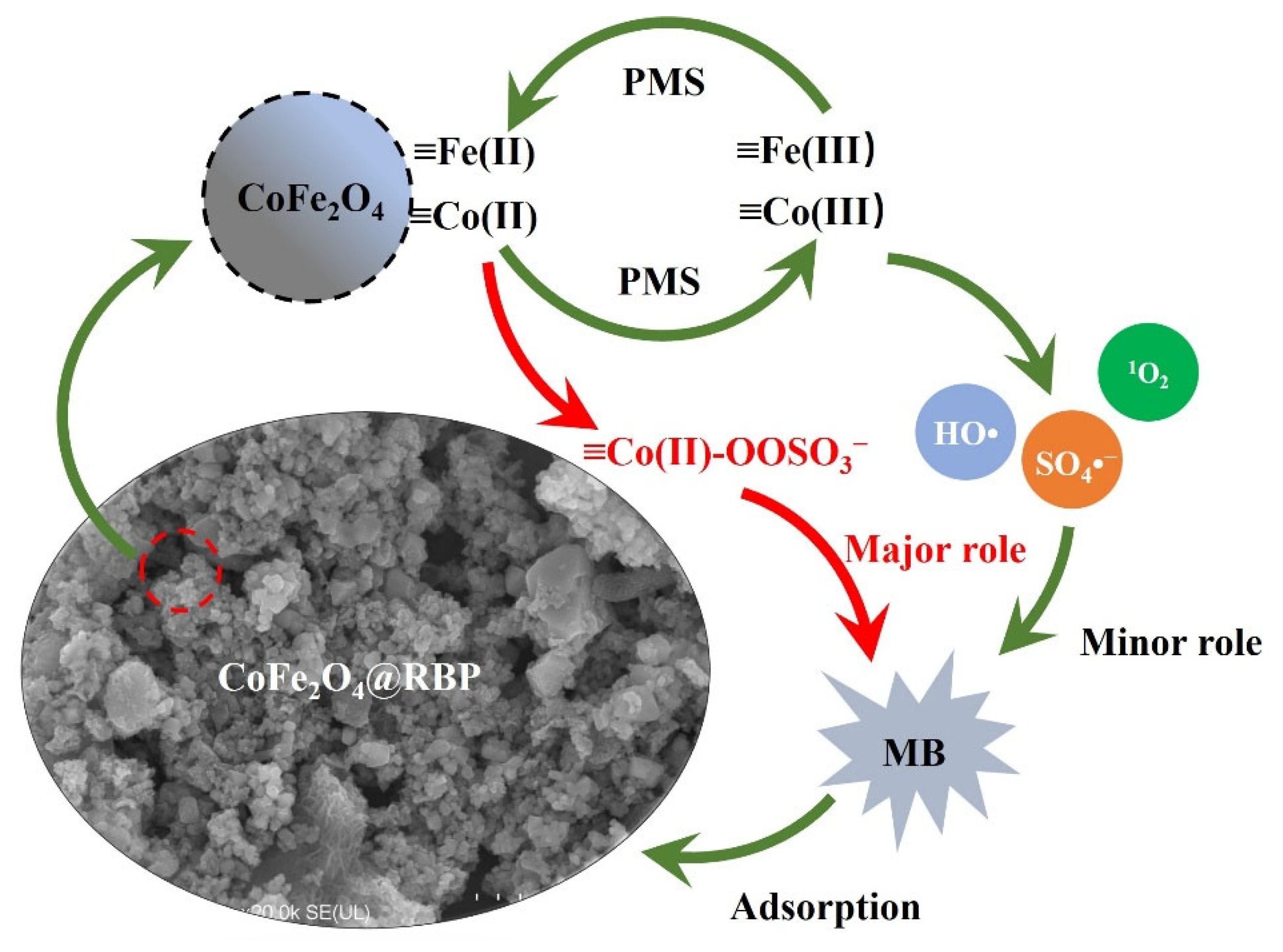
As reported in the literature [30,31], the Co(II)-PMS complex on the surface of CoFe2O4 could serve as a dominant reactive oxidizing species for the degradation of pollutants during PMS activation processes, and thus, the Co(II)-PMS complex is suggested as the dominant reactive oxidizing species in the CoFe2O4@RBP/PMS process. As shown in Figure 5, PMS first coordinates with ≡Co(II) on the surface of CoFe2O4@RBP to form the ≡Co(II)-OOSO3− complex, and then the ≡Co(II)-OOSO3− complex quickly oxidizes MB adsorbed on the surface of the CoFe2O4@RBP. Furthermore, ≡Fe(II) on the surface of CoFe2O4@RBP would react with PMS to generate SO4•− and HO•, and then the produced ≡Fe(III) could be reduced by PMS [15,32]. The redox couples of ≡Co(II)/Co(III) and ≡Fe(II)/Fe(III) participate in the PMS activation, enhancing the generation of reactive oxidizing species (i.e., HO•, SO4•− and 1O2). However, the role of HO•, SO4•−, and 1O2 could be ignored.
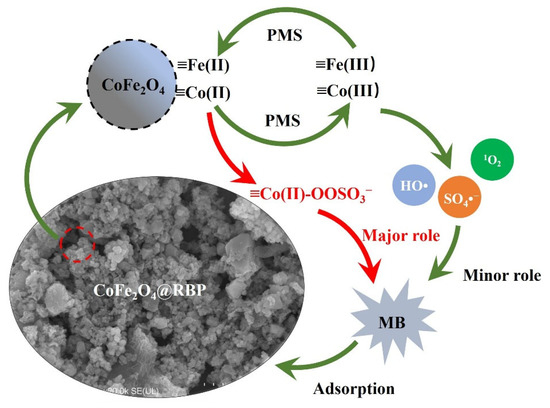
Figure 5.
Proposed mechanism for MB removal in the CoFe2O4@RBP/PMS process.
3.4. Influence of Various Parameters
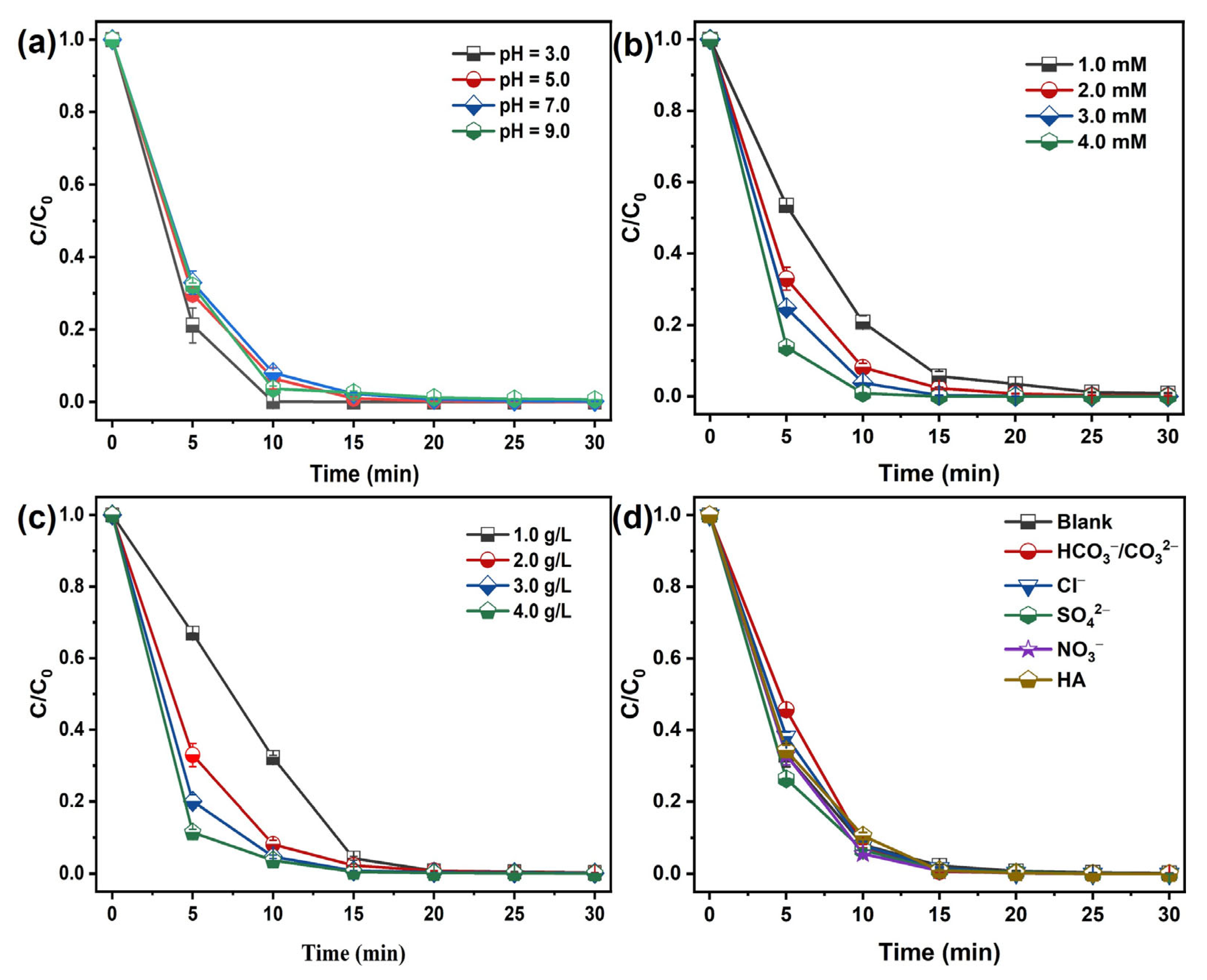
Solution pH critically governs pollutant degradation by controlling both reactive oxidant generation and organic substrate speciation. As shown in Figure 6a, after a 30 min reaction, the removal efficiency of MB is not significantly affected in the CoFe2O4@RBP/PMS process as the pH increases from 3.0 to 9.0, which might be due to the surface charge on the surface of the catalyst, which affects the adsorption of MB cationic dye. As shown in Figure S5, the PZC of CoFe2O4@RBP is 4.51, implying that when the pH is greater than 4.51, it is conducive to CoFe2O4@RBP ‘s adsorption of MB. Furthermore, a relatively minor impact on the adsorption of MB is observed at a wide pH range from 3.0 to 9.0 in the CoFe2O4@RBP process (Figure S6). Additionally, the oxidation degradation reaction mainly occurs at the solid–liquid interface, and thus pH has a relatively small impact on the removal of MB.
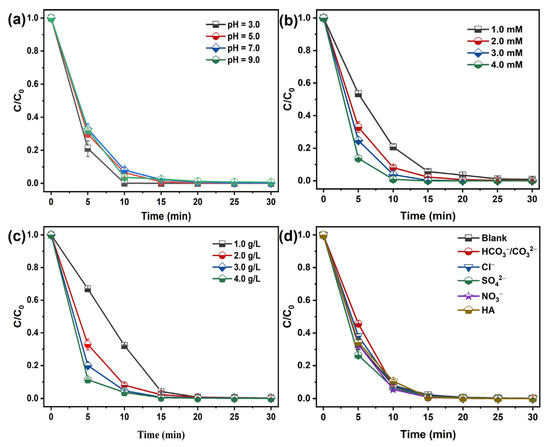
Figure 6.
Effect of (a) pH, (b) PMS concentration, (c) catalyst dosage, and (d) coexisting substances in water on the removal of MB in the CoFe2O4@RBP/PMS process. Conditions: [catalyst]0 = 2.0 g L−1, [PMS]0 = 2.0 mM, [MB]0 = 0.1 mM, and initial pH = 7.0.
Figure 6b shows that increasing PMS concentration from 1.0 to 4.0 mM accelerates MB degradation, likely due to enhanced generation of reactive oxidizing species; likewise, the catalyst dosage is positively correlated with MB removal (Figure 6c). Increasing the catalyst dosage not only increases the number of active sites, but also enhances the adsorption performance for MB, thereby facilitating the efficient removal of MB.
The coexisting substances in water including NO3−, SO42−, Cl−, HCO3−/CO32−, and HA are important factors in the removal of target pollutants. The impact of interfering materials on MB elimination is presented in Figure 6d. Obviously, the presence of SO42−, Cl−, NO3−, and HA shows negligible effect on the removal of MB in the CoFe2O4@RBP/PMS process. After 30 min of reaction, the MB removal efficiency remained unchanged. Of note is that HCO3−/CO32− shows a slight inhibition on the removal of MB in the CoFe2O4@RBP/PMS process, which could be ascribed to HCO3−/CO32− competing with reactive oxidizing species for pollutants [15]. With the addition of 10 mM HCO3−/CO32−, slower kinetics of MB removal are observed within 5 min, and MB is completely removed after a 30 min reaction.
3.5. Reusability and Stability of CoFe2O4@RBP
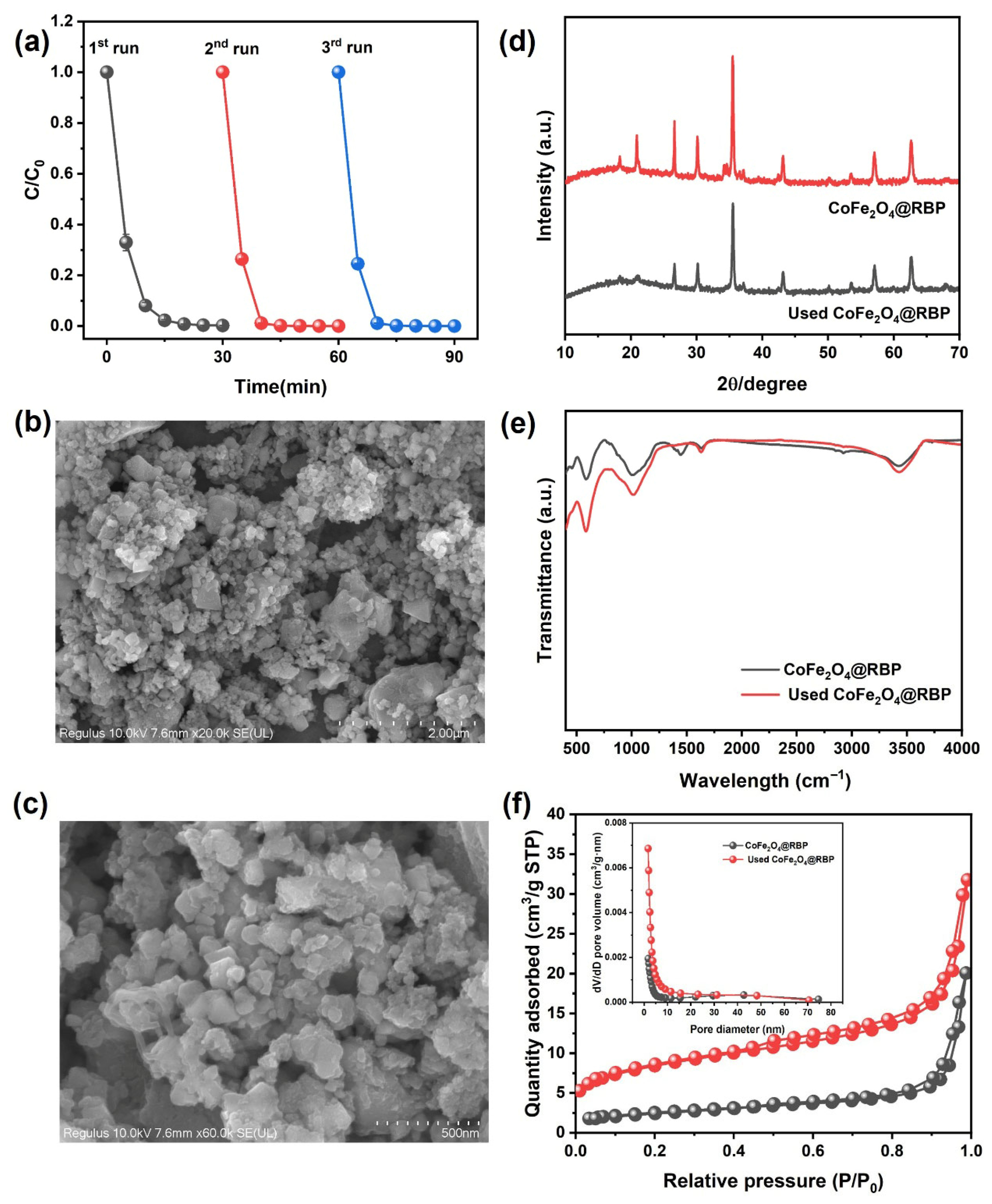
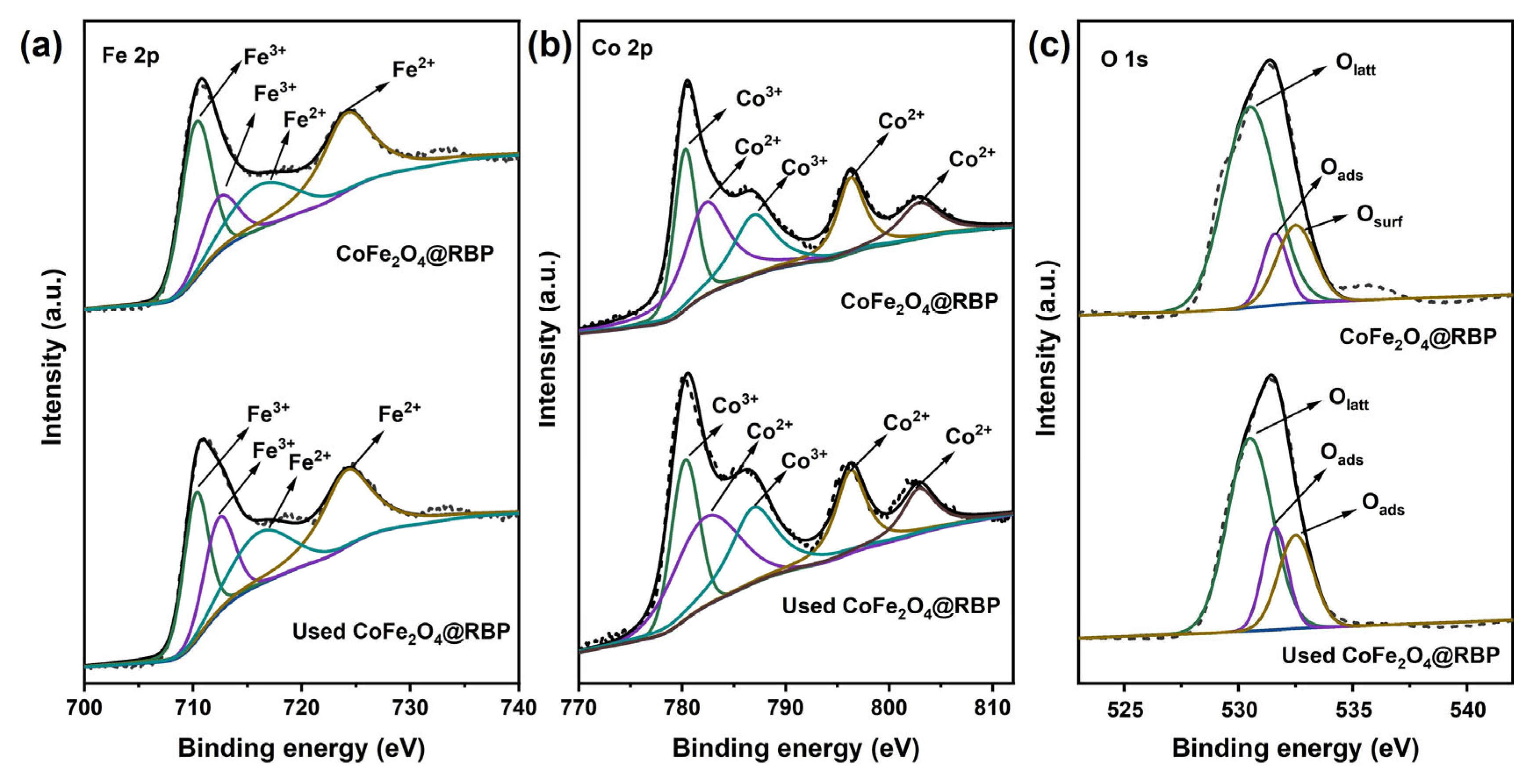
The reusability and stability of CoFe2O4@RBP on the removal of MB in the CoFe2O4@RBP/PMS process were investigated at pH = 7.0. Obviously, after a 30 min reaction, 100% of MB is removed using CoFe2O4@RBP/PMS in the third-cycle experiments (Figure 7a), indicating that CoFe2O4@RBP shows good removal performance for MB. However, after a 5 min reaction, the removal efficiency of MB increases significantly in the second- and third-cycle experiments, probably because the slightly increased specific surface area is observed after reaction (Table S1). Moreover, the Co leaching gradually decreases in the three-run experiments, and the leaching concentration of Co is 67 μg/L, 52 μg/L and 48 μg/L (Table S3), respectively. Correspondingly, the content of Co before and after reaction shows a slight decrease (Table S1), which might be due to the leaching of Co. Additionally, CoFe2O4@RBP exhibits a good catalytic stability performance compared to the cyclic stability performance of CoFe2O4@RBP with that of other materials [14,15,22,23,25,26]. To reveal the reason for the good reusability and stability of CoFe2O4@RBP, the used CoFe2O4@RBP was analyzed by multiple characteristic technologies. As shown in Figure 7b,c, a large amount of CoFe2O4 is still deposited on the surface of RBP from the SEM images, suggesting that its morphology did not change significantly during the PMS activation process. From the XRD patterns and FTIR spectra of used CoFe2O4@RBP, a slight change in the crystal and a change in functional groups are observed after reaction (Figure 7d,e), which might be due to the minor surface restructuring or deposition of reaction byproducts on the surface of CoFe2O4@RBP. Moreover, the spent CoFe2O4@RBP exhibited a BET surface area of 10.49 m2 g−1 and an average pore size of 16.52 nm, showing no significant change (Figure 7f and Table S1). The surface oxidation states of the elements were examined by XPS before and after reaction. As shown in Figure 8a and Table S4, Fe2+/Fe3+ before and after reaction accounts for 24.5% and 27.7% in CoFe2O4@RBP according to the results of Fe 2p signal deconvolution. Similarly, Co3+/Co2+ before and after reaction accounts for 46.8% and 46.6% in CoFe2O4@RBP (Figure 8b, Table S4). The findings clearly indicate that both the Co3+/Co2+ and Fe2+/Fe3+ redox pairs play a crucial role in the CoFe2O4@RBP/PMS system. When examining the O 1s XPS, three distinct sub-peaks emerge at binding energies of 530.5 eV, 531.6 eV, and 532.5 eV, which can be attributed to lattice oxygen (Olatt), physically adsorbed water (Oads), and surface hydroxyl groups (Osurf), respectively [33]. In the CoFe2O4@RBP composite, these components constitute 68.6%, 11.9%, and 19.5% of the total oxygen species present. After reaction, the Olatt, Oads, and Oads account for 59.8%, 17.8% and 22.4% (Figure 8c, Table S4), respectively. The slightly decreased Oads of used CoFe2O4@RBP is possibly because of its consumption via electron donation to reduce Co3+ to Co2+, where the gaseous O2 is formed and released [34]. The above results together clearly indicate that the CoFe2O4@RBP exhibits good catalytic activity and stability for MB removal.
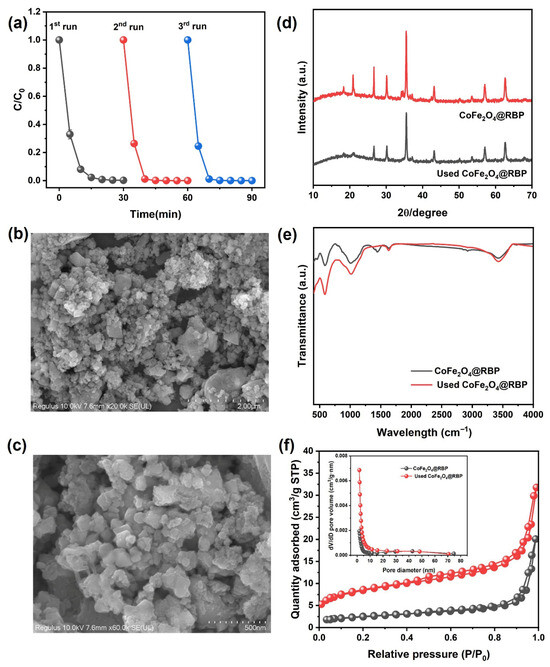
Figure 7.
(a) Cyclic repetition performance of CoFe2O4@RBP/PMS process, (b,c) SEM imagines of used CoFe2O4@RBP, (d) XRD patterns, (e) FTIR spectra, and (f) N2 adsorption–desorption isotherm and its pore size distribution (inset) of CoFe2O4@RBP and used CoFe2O4@RBP. Conditions: [catalyst]0 = 2.0 g L−1, [PMS]0 = 2.0 mM, [MB]0 = 0.1 mM, and initial pH = 7.0.
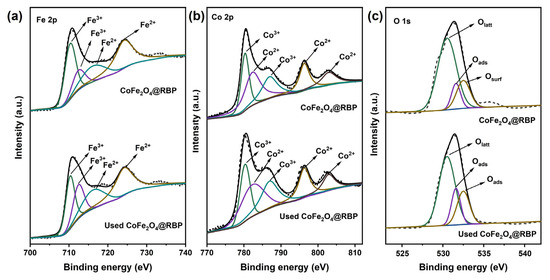
Figure 8.
(a) Fe 2p, (b) Co 2p, and (c) O 1s XPS of CoFe2O4@RBP and used CoFe2O4@RBP.
4. Conclusions
In summary, CoFe2O4@RBP was successfully synthesized and used as a heterogeneous catalyst to activate PMS for the degradation of MB. After a 30 min reaction, the CoFe2O4@RBP/PMS process exhibits good removal efficiency across a wide pH range, from 3.0 to 9.0, and 100% of MB is completely removed. The combination of CoFe2O4 and RBP not only enhances catalytic performance, but also significantly reduces the leaching of Co. The results of EPR and radical-quenching experiments suggest that the Co(II)-PMS complex is likely the primary driver of MB removal. Furthermore, the presence of SO42−, Cl−, NO3−, and HA shows negligible effect on the removal of MB in the CoFe2O4@RBP/PMS process, and HCO3−/CO32− has slight inhibition, probably because HCO3−/CO32− could compete with reactive oxidizing species for pollutants. In addition, 100% of MB is still removed in the third-cycle experiments, implying that the CoFe2O4@RBP exhibits high catalytic activity and stability for MB removal. Our work provides new insight into combining construction waste with CoFe2O4 to develop a PMS activation system for wastewater treatment with high catalytic efficiency and good reusability.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/su17219886/s1, Table S1. Basic properties of different catalysts. Table S2. Compare the catalytic performance of CoFe2O4@RBP with other CoFe2O4-based catalysts in references. Table S3. Co leaching of stability experiments in the three-cycle experiments. Table S4. The element valence state ratios of CoFe2O4@RBP and Used CoFe2O4@RBP. Figure S1. Synthesis schematic of CoFe2O4@RBP. Figure S2. MB removal by methanol desorption in the CoFe2O4@RBP and CoFe2O4@RBP/PMS after 30 min reaction. Figure S3. Mineralization efficiency in the CoFe2O4@RBP/PMS. Figure S4. MB removal in the Co2+/PMS and CoFe2O4@RBP/PMS. Figure S5. Zeta potential diagram of CoFe2O4@RBP. Figure S6. Effect of pH on the removal of MB in the CoFe2O4@RBP process. References [14,15,22,23,25,26,27] are cited in Supplementary Materials.
Author Contributions
C.S.: writing—review and editing, supervision, project administration, investigation, and conceptualization. F.C.: writing—review and editing, formal analysis, and conceptualization. S.L.: writing—review and editing, supervision, project administration, investigation, and conceptualization. C.Z.: supervision and methodology. H.Z.: supervision and methodology. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China (NSFC), grant number 52378010.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Data will be made available on request.
Conflicts of Interest
Author Fangkui Cheng was employed by the company Huaxin Design Group Co., Ltd. The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
- Benkhaya, S.; M’rabet, S.; El Harfi, A. A review on classifications, recent synthesis and applications of textile dyes. Inorg. Chem. Commun. 2020, 115, 107891. [Google Scholar] [CrossRef]
- Ramamurthy, K.; Priya, P.S.; Murugan, R.; Arockiaraj, J. Hues of risk: Investigating genotoxicity and environmental impacts of azo textile dyes. Environ. Sci. Pollut. Res. 2024, 31, 33190–33211. [Google Scholar] [CrossRef] [PubMed]
- Dhamorikar, R.S.; Lade, V.G.; Kewalramani, P.V.; Bindwal, A.B. Review on integrated advanced oxidation processes for water and wastewater treatment. J. Ind. Eng. Chem. 2024, 138, 104–122. [Google Scholar] [CrossRef]
- Moradihamedani, P. Recent advances in dye removal from wastewater by membrane technology: A review. Polym. Bull. 2021, 79, 2603–2631. [Google Scholar] [CrossRef]
- Lee, J.; Urs von Gunten, U.; Kim, J.H. Persulfate-based advanced oxidation: Critical assessment of opportunities and roadblocks. Environ. Sci. Technol. 2020, 54, 3064–3081. [Google Scholar] [CrossRef]
- Yang, Z.; Qian, J.; Shan, C.; Li, H.; Yin, Y.; Pan, B. Toward selective oxidation of contaminants in aqueous systems. Environ. Sci. Technol. 2021, 55, 14494–14514. [Google Scholar] [CrossRef]
- Oh, W.D.; Dong, Z.; Lim, T.T. Generation of sulfate radical through heterogeneous catalysis for organic contaminants removal: Current development, challenges and prospects. Appl. Catal. B Environ. 2016, 194, 169–201. [Google Scholar] [CrossRef]
- Aldulaimi, A.; Zhao, Y.X.; Abdalhuseen, R.A.; Al-Hussainy, A.F.; Abd, N.S. Designed preparation of silver doped silver oxide/cupric oxide decorated MXene nanocomposites for degradation via visible light irradiation, and antibacterial efficiency. J. Indian Chem. Soc. 2025, 102, 102035. [Google Scholar] [CrossRef]
- Zheng, X.; Niu, X.; Zhang, D.; Lv, M.; Ye, X.; Ma, J.; Lin, Z.; Fu, M. Metal-based catalysts for persulfate and peroxymonosulfate activation in heterogeneous ways: A review. Chem. Eng. J. 2022, 429, 132323. [Google Scholar] [CrossRef]
- Li, Y.; Zhu, W.; Guo, Q.; Wang, X.; Zhang, L.; Gao, X.; Luo, Y. Highly efficient degradation of sulfamethoxazole (SMX) by activating peroxymonosulfate (PMS) with CoFe2O4 in a wide pH range. Sep. Purif. Technol. 2021, 276, 119403. [Google Scholar] [CrossRef]
- Zhang, T.; Zhu, H.; Croue, J.P. Production of sulfate radical from peroxymonosulfate induced by a magnetically separable CuFe2O4 spinel in water: Efficiency, stability, and mechanism. Environ. Sci. Technol. 2013, 47, 2784–2791. [Google Scholar] [CrossRef]
- Li, Y.; Chen, Z.; Qi, J.; Kang, J.; Shen, J.; Yan, P.; Wang, W.; Bi, L.; Zhang, X.; Zhu, X. Degradation of bisphenol S by peroxymonosulfate activation through monodispersed CoFe2O4 nanoparticles anchored on natural palygorskite. Sep. Purif. Technol. 2021, 277, 119492. [Google Scholar] [CrossRef]
- Shi, P.; Dai, X.; Zheng, H.; Li, D.; Yao, W.; Hu, C. Synergistic catalysis of Co3O4 and graphene oxide on Co3O4/GO catalysts for degradation of orange II in water by advanced oxidation technology based on sulfate radicals. Chem. Eng. J. 2014, 240, 264–270. [Google Scholar] [CrossRef]
- Deng, J.; Chen, Y.J.; Lu, Y.A.; Ma, X.Y.; Feng, S.F.; Gao, N.; Li, J. Synthesis of magnetic CoFe2O4/ordered mesoporous carbon nanocomposites and application in Fenton-like oxidation of rhodamine B. Environ. Sci. Pollut. Res. 2017, 24, 14396–14408. [Google Scholar] [CrossRef]
- Wang, Q.; Shao, Y.; Gao, N.; Chu, W.; Chen, J.; Lu, X.; Zhu, Y.; An, N. Activation of peroxymonosulfate by Al2O3-based CoFe2O4 for the degradation of sulfachloropyridazine sodium: Kinetics and mechanism. Sep. Purif. Technol. 2017, 189, 176–185. [Google Scholar] [CrossRef]
- You, Y.; Shi, Z.; Li, Y.; Zhao, Z.; He, B.; Cheng, X. Magnetic cobalt ferrite biochar composite as peroxymonosulfate activator for removal of lomefloxacin hydrochloride. Sep. Purif. Technol. 2021, 272, 118889. [Google Scholar] [CrossRef]
- Bumanis, G.; Vaičiukynienė, D. Alkali activation of milled red brick waste and calcined Illite clay with silica gel addition. Materials 2022, 15, 3195. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Li, Y.; Zhang, J.; Zhao, Y.; Xu, M.; Wang, F.; Min, S. Upcycling red brick into a superior monolithic hydrogen evolution electrocatalyst. New J. Chem. 2025, 49, 12279–12288. [Google Scholar] [CrossRef]
- Song, S.; Sun, H.; Zhang, X.; He, T.; Wang, Y.; Mo, Q.; Zhuo, C. Degradation of trichloromethane by immobilized microspheres of modified red brick. Desalin. Water Treat. 2023, 306, 151–158. [Google Scholar] [CrossRef]
- Rafique, T.; Anas, M.; Chadhar, K.M.; Soomro, F.; Alvi, S.K. Defluoridation of water by wasted red brick paving blocks. Desalin. Water Treat. 2018, 108, 207–215. [Google Scholar] [CrossRef]
- Deng, J.; Shao, Y.; Gao, N.; Tan, C.; Zhou, S.; Hu, X. CoFe2O4 magnetic nanoparticles as a highly active heterogeneous catalyst of oxone for the degradation of diclofenac in water. J. Hazard. Mater. 2013, 262, 836–844. [Google Scholar] [CrossRef]
- Tan, Y.; Li, C.; Sun, Z.; Bian, R.; Dong, X.; Zhang, X.; Zheng, S. Natural diatomite mediated spherically monodispersed CoFe2O4 nanoparticles for efficient catalytic oxidation of bisphenol A through activating peroxymonosulfate. Chem. Eng. J. 2020, 388, 124386. [Google Scholar] [CrossRef]
- Li, X.; Liu, Z.; Zhu, Y.; Song, L.; Dong, Z.; Niu, S.; Lyu, C. Facile synthesis and synergistic mechanism of CoFe2O4@three-dimensional graphene aerogels towards peroxymonosulfate activation for highly efficient degradation of recalcitrant organic pollutants. Sci. Total Environ. 2020, 749, 141466. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.; Zhang, Y.; Wang, Z.; Wei, W.; Tang, W.; Shi, J.; Xiong, R. Electronic structure studies of the spinel CoFe2O4 by X-ray photoelectron spectroscopy. Appl. Surf. Sci. 2008, 254, 6972–6975. [Google Scholar] [CrossRef]
- Fan, Y.; Zhou, Z.; Feng, Y.; Zhou, Y.; Wen, L.; Shih, K. Degradation mechanisms of ofloxacin and cefazolin using peroxymonosulfate activated by reduced graphene oxide-CoFe2O4 composites. Chem. Eng. J. 2020, 383, 123056. [Google Scholar] [CrossRef]
- Chen, L.; Ding, D.; Liu, C.; Cai, H.; Qu, Y.; Yang, S.; Gao, Y.; Cai, T. Degradation of norfloxacin by CoFe2O4-GO composite coupled with peroxymonosulfate: A comparative study and mechanistic consideration. Chem. Eng. J. 2018, 334, 273–284. [Google Scholar] [CrossRef]
- Li, M.; Li, Y.; Yu, P.; Zhao, H.; Xiang, L.; Feng, N.; Li, Q.; He, K.; Luo, X.; Cai, Q.; et al. Exploring degradation mechanism of tetracycline via high-effective peroxymonosulfate catalysts of montmorillonite hybridized CoFe composites and safety assessment. Chem. Eng. J. 2022, 427, 130930. [Google Scholar] [CrossRef]
- Huang, G.X.; Wang, C.Y.; Yang, C.W.; Guo, P.C.; Yu, H.Q. Degradation of bisphenol A by peroxymonosulfate catalytically activated with Mn1.8Fe1.2O4 nanospheres: Synergism between Mn and Fe. Environ. Sci. Technol. 2017, 51, 12611–12618. [Google Scholar] [CrossRef]
- Ghanbari, F.; Moradi, M. Application of peroxymonosulfate and its activation methods for degradation of environmental organic pollutants: Review. Chem. Eng. J. 2017, 310, 41–62. [Google Scholar] [CrossRef]
- Li, X.; Zhao, Z.; Li, H.; Qian, J. Degradation of organic contaminants in the CoFe2O4/peroxymonosulfate process: The overlooked role of Co(II)-PMS complex. Chem. Eng. J. Adv. 2021, 8, 100143. [Google Scholar] [CrossRef]
- Zhu, J.; Wang, S.; Yang, Z.; Pan, B. Robust polystyrene resin-supported nano-CoFe2O4 mediated peroxymonosulfate activation for efficient oxidation of 1-hydroxyethane 1,1-diphosphonic acid. J. Hazard. Mater. 2023, 443, 130281. [Google Scholar] [CrossRef]
- Xu, M.; Li, J.; Yan, Y.; Zhao, X.; Yan, J.; Zhang, Y.; Lai, B.; Chen, X.; Song, L. Catalytic degradation of sulfamethoxazole through peroxymonosulfate activated with expanded graphite loaded CoFe2O4 particles. Chem. Eng. J. 2019, 369, 403–413. [Google Scholar] [CrossRef]
- Yang, Z.; Li, Y.; Zhang, X.; Cui, X.; He, S.; Liang, H.; Ding, A. Sludge activated carbon-based CoFe2O4-SAC nanocomposites used as heterogeneous catalysts for degrading antibiotic norfloxacin through activating peroxymonosulfate. Chem. Eng. J. 2020, 384, 123319. [Google Scholar] [CrossRef]
- Polnišer, R.; Štolcová, M.; Hronec, M.; Mikula, M. Structure and reactivity of copper iron pyrophosphate catalysts for selective oxidation of methane to formaldehyde and methanol. Appl. Catal. A Gen. 2011, 400, 122–130. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).