1. Introduction
Historically, fishing has been a major source of livelihood for coastal and inland fishing communities, as well as a source of healthy food for humanity at large. Aquaculture has emerged as a potential key contributor to global food production, particularly in Asia, which accounts for more than 90% of the world’s aquaculture production [
1]. The growth in aquaculture has been significant, outpacing red meat growth and playing a critical role in addressing food insecurity and improving a healthier human lifestyle [
2]. Given the stagnation in growth of capture fisheries and the increasing demand for seafood, global aquaculture production must strive to reach 106 million tons by 2030, which is an ambitious target [
3]. Facing increasing demand for animal protein and climate change, aquaculture is considered to have rich potential to enhance the resilience of the food system [
2]. Aquaculture’s significant contribution to achieving the Sustainable Development Goals is widely acknowledged [
4].
In recent decades, aquaculture in Saudi Arabia has experienced a significant transformation. Motivated by the ambitious goals laid out in Saudi Vision 2030, the sector has observed significant progress and is well on its way to achieving the government’s target of producing 600,000 tons of seafood yearly [
5]. This progress is mostly due to supportive government strategies, vital infrastructure growth, and significant investments in infrastructure and informative programs. This study seeks to assess the role of credit in the performance of the fisheries sector in Saudi Arabia by analyzing the key determinants that influence total fish production, both in the short and long run.
Despite the Kingdom of Saudi Arabia’s unique location, which includes a vast coastline of roughly 2640 km on the Arabian Gulf to the east and the Red Sea to the west (nearly 2060 km on the Red Sea and 580 km on the Arabian Gulf), its domestic fish production falls short of individual needs, resulting in a reliance on imports to fill the nutritional gap. The average period for this reliance was estimated to be around 112.7 thousand tons (2020–2022). Furthermore, Saudi people consume an average of 8.0 kg of fish annually per person, while the global average for the same period (2020–2022) was 20.0 kg [
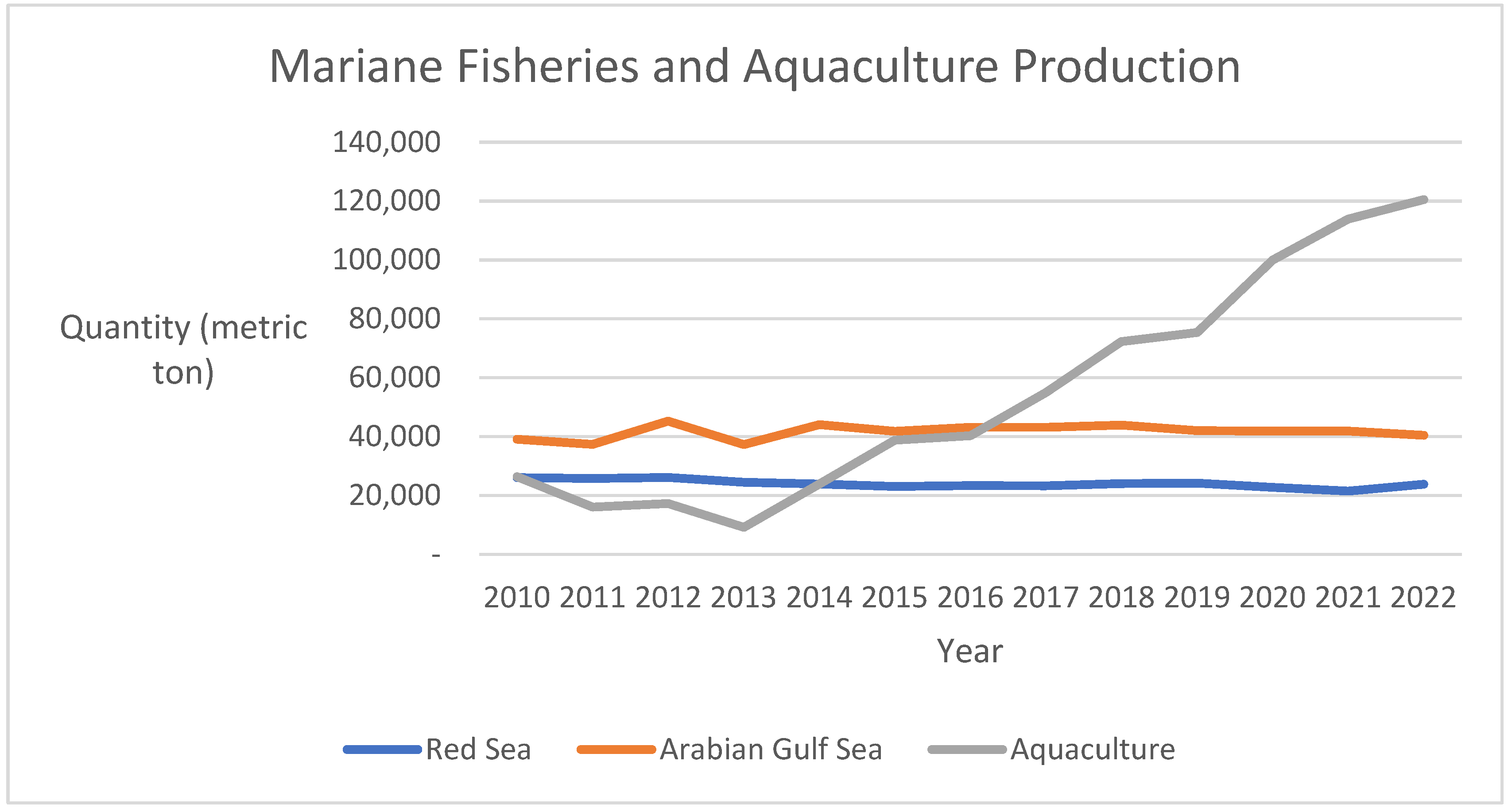
6]. As a result, the kingdom’s government is working hard to create policies and initiatives that can satisfy the growing demand. It is also guiding plans for investment and development in fish farming projects in a way that maximizes and sustainably uses the available agricultural resources. Only by paying attention to this important sector and supporting its development and sustainability through easily accessible loans and the necessary credit facilities will this be possible. This will encourage and motivate investors, fish producers, and the technical staff working in this sector to invest in and establish productive projects and to move toward fish farming projects that will result in a qualitative and quantitative leap for this vital sector in the kingdom. Through its emphasis on training initiatives and the granting of essential soft loans, the kingdom has recently aimed to motivate all aquaculture workers, producers, and investors to adhere to the highest standards of care and comprehend appropriate nutrition practices. Additionally, the kingdom has aimed to strengthen its ability to detect fish diseases and enhance the methods used for their treatment and management. With an annual growth rate of roughly 5.1%, fish production rose from about 61,335 tons in 2001 to nearly 184,759 tons in 2022. About 40.4% of the entire fish production, which averages about 161.7 thousand tons over the years 2018–2022, comes from marine fisheries, while the remaining 59.6% comes from aquaculture. The Arabian Gulf accounts for around 26.0% of the production of marine fisheries, followed by the Red Sea at 14.4% and fishing in international seas at a negligible percentage [
7]. On average, during the period in question, freshwater aquaculture accounted for 12.0% of aquaculture production, while saltwater aquaculture accounted for 47.6% [
6]. The number of fishing boats has dropped from approximately 12,113 boats in 2005 to approximately 10,737 boats in 2022, despite the Kingdom of Saudi Arabia’s interest in expanding fish production and diversifying its sources. The most significant of these measures include the creation of fishery regulations, the issuance of more fishing licenses, the establishment of the General Directorate of Fish Wealth at the Ministry of Agriculture in 1988 to oversee fishing activities, and the establishment of centers for fishery research along the kingdom’s coasts. Furthermore, aquaculture presents promising investment opportunities in the kingdom, as demonstrated by a shift in the sector’s labor dynamics. The number of fishermen declined from approximately 9207 in 2005 to about 8746 in 2022, while the number of fishery workers increased from around 27,986 to 30,099 over the same period [
6]. Government policies have strongly influenced the geographic distribution of aquaculture growth, as well as the types of species, technology, management practices, and infrastructure adopted in different locations.
Saudi Arabia’s arid climate and limited freshwater availability pose substantial challenges to domestic aquaculture growth. Moreover, extreme salinity levels in some coastal and groundwater sources further limit the range of feasible aquaculture practices. These environmental restrictions are combined with the effects of climate change, including increasing sea surface temperatures, which threaten the sustainability of marine habitats and fish stocks. Some studies have stated indicators of high fishing pressure in Saudi Arabia, which resulted in low stock availability of some species, changes in behavior, and the decrease or removal of some predators [
8,
9,
10,
11,
12]. Fishermen also face challenges in market access and price volatility. Moreover, the quantity demanded for fish in Saudi Arabia is insensitive to changes in price and elastic with respect to consumers’ income [
13].
Several studies have explored the economic, technical, and social dimensions of fisheries and aquaculture across different regions. In the reviewed literature, several investigations considered the economics of the fisheries sector in terms of various segments, such as lending, fish production trends, the environment, credit, food security, and marketing, using different statistical and econometric approaches [
14,
15,
16,
17,
18,
19]. A study conducted an integrated assessment of the United Kingdom’s aquatic food system by synthesizing data across the fisheries, aquaculture, trade, health, welfare, and environmental sectors. In an evaluation of the system’s ability to deliver aquatic food that is sufficient, safe, sustainable, resilient to shocks, and ethically sound, the results confirmed that a unified, ongoing assessment framework is essential for understanding and enhancing aquatic food security in the face of social, economic, and environmental changes [
20]. Another study employed cluster analysis to examine data from 26 United States–based mitigation banks that generate credit for freshwater species and ecosystems. The analysis identified two main credit generation approaches: (1) barrier removal and (2) whole-community targeting. The findings revealed that while both strategies address critical freshwater conservation objectives, each carries significant risks [
21].
Global cross-sectional studies revealed a broad spectrum of under- to overregulated aquaculture systems that correspond, respectively, to high- and low-growth areas for aquaculture [
2]. Credit is needed not only for investment in fishing craft and gear, fishponds, fish handling, processing, and marketing facilities and services, but also, or even more, for the smooth day-to-day capture, culture, handling, processing, and distribution of fish [
22]. Financial inclusion can help reduce the different vulnerabilities of poor fishing households and rural communities and lead to improved economic resilience [
23].
Several studies have been conducted in the African continent concerning the economics of fisheries and the aquaculture sector. Inoni [
24] investigated how effectively resources are used in pond fish farming in Delta State, Nigeria. Data were collected from 72 farms (about 31% of the state’s estimated 232 fish farms) through interviews. Using descriptive and regression analyses, the researchers found that labor negatively impacts fish production—that is, more labor leads to lower yields. The study also revealed inefficiencies in how fish farmers allocate productive resources. Njagi et al. [
25] aimed to analyze the factors affecting the profitability of fish farming under Kenya’s Economic Stimulus Program in East Tigania. A descriptive analytical approach with data from 132 fish farmers focused on the roles of marketing, advisory services, cultural practices, and pond management in influencing aquaculture profitability. The results revealed that marketing has a positive impact on fish farming profitability. Wetengere and Kihongo [
26] assessed the constraints hindering access to credit facilities among fish farmers in rural Morogoro, Tanzania. After using descriptive statistical methods, the results revealed several key barriers to credit access, including limited access to information, unfavorable lending terms, inadequate support services, and low levels of financial literacy.
In the Asian continent, Keshavanath et al. [
27] conducted experimental and analytical research on artificial substrates for periphyton growth in Indian freshwater ponds by assessing fish production in varying substrate types and fish densities. The results included the survival, growth, and production rates of fish, which revealed that during the fish harvest, the mortality rate associated with the treatment of sugarcane pulp reaches 100%. Goswami [
28] used a purposive stratified random sampling approach involving 120 farmers to investigate the attitudes of fish farmers in West Bengal toward scientific aquaculture, focusing on the socioeconomic and psychological factors influencing these attitudes. The study emphasized the importance of risk orientation and economic motivation in shaping farmers’ acceptance of new aquaculture technologies. The research revealed that risk orientation has a positive and statistically significant correlation with farmers’ attitudes toward scientific aquaculture. Moreover, a study was conducted to understand how well fish farmers grasp critical aquaculture practices and to identify gaps in technology adoption to inform development efforts in the fisheries sector. A descriptive analytical method was applied, with a binary questionnaire distributed among 90 fish farmers in Jabalpur, India. The results showed that 64% of the farmers exhibited a low attitude toward fish farming, suggesting the need for awareness and training to improve technology adoption [
29]. Several studies investigated the role of credit in fish production, with one investigating the impact of collateral-free microcredit provided by nongovernmental organizations on the household food expenditure of credit-constrained, poor fish farmers in Bangladesh. The empirical approach revealed that while credit access alone has a limited effect, household assets significantly influence the capacity of fish farmers to increase their food expenditure [
30]. The link between credit access, food security, and dietary diversity was examined in Bangladesh by applying both descriptive and econometric methods to data from the Household Income and Expenditure Survey. The findings indicated that access to financial resources positively influences household food stability and contributes to more varied and balanced diets [
31]. A study analyzed the impact of credit constraints on aquaculture productivity in Bangladesh using an endogenous switching regression model. The results established that farmers without credit constraints exhibit significantly higher productivity levels [
32]. Another study examined the effect of access to credit on the food security of small fishermen in East Java, Indonesia, using ordinary least squares and an ordered probit model. The outcome of this study showed that fishermen’s food security is included in the borderline category [
33].
The literature concerning fisheries production and consumption in Egypt is rich. Kassem and Meglla [
34] conducted a mixed-method economic and technical assessment of mechanical fishing boats in the Alexandria governorate, Egypt, and identified key productivity factors and challenges based on field data analysis. The study revealed that the primary factors affecting fish production for mechanical boats include the crew’s efficiency level, the distance to fishing areas measured in kilometers, the quantity of fishing nets utilized in kilograms, the frequency of net repairs throughout the season, and the total variable costs. Abu Alainin [
35] highlighted the critical role of lending in advancing Egypt’s fishing economy and the limitations of safe borrowing. The study found that the current financial support was insufficient and emphasized the success of proactive cooperative boards in securing funding through the Social Development Fund. It recommended strategies to improve credit access, reduce interest rates, and enhance institutional engagement with the fishing sector. Albasyouni and Maglad [
36] performed an economic analysis using time-series fishery data from Egypt to examine fish production trends and seasonal consumption patterns. The results indicated that the overall pattern of seasonal fluctuations in total fish production from three fisheries combined is largely consistent with that of freshwater fisheries, followed by lake fisheries. Salim [
37] utilized descriptive economic analysis to evaluate fish production sources and the contribution of different species in Egypt, emphasizing the growing role of aquaculture. Among the most significant fish species harvested from Egyptian fisheries, the study identified tilapia as the leading species, followed by catfish, sardines, and mullet. Barrania [
38] used a qualitative analytical method and emphasized the role of cooperative organizations in Arab fisheries, calling for a redefinition of roles and stronger member engagement supported by financial tools. The study revealed that cooperatives have untapped potential in advancing fishery development and highlighted the importance of member participation in governance, rulemaking, and economic planning.
Few economic studies have examined the productivity of the fisheries and aquaculture sector in Saudi Arabia. Alhindi and Aldwis [
39] used maximum sustainable yield modeling and econometric analysis to assess Saudi Arabia’s fishery resources. They found declining Red Sea yields and increased contributions from the Arabian Gulf, fish farms, and international waters. They showed that more fishing days improve yield, while more trips reduce efficiency. Optimal sustainable production was estimated at 53,000 tons, supporting better resource use policies. Alnafissa et al. [
40] also estimated the maximum sustainable yield of fish in the Red Sea and Arabian Gulf to be 24,646.9 and 48,610.8 tons, respectively. Furthermore, Alnaim and Shehata [
41] employed descriptive and statistical analysis to assess marine fish production in Saudi Arabia and identified species trends, productivity shifts, and regional variations in labor and boat use. They evaluated various aspects of marine fish production in Saudi Arabia to support policymaking aimed at improving fishery efficiency, boosting production, reducing food supply gaps, and promoting exports. The researchers covered fisheries in the Arabian Gulf and the Red Sea, aquaculture, and international waters and highlighted production trends and influencing factors across regions and fishing methods. Their results indicated that Arabian Gulf fisheries focused on kan’ad (threadfin), hamour (crustaceans), and sha’ri (redfish), while Red Sea fisheries targeted
Trachurus indicus, rubian (shrimp), khanaq (squid), and beyadh (white mullet). Elhendy and Alzoom [
42] collected a cross-sectional sample representing 23 tilapia farms in the central region of Saudi Arabia. Their results showed that fish feed cost accounts for the largest proportion of the total production cost. Moreover, the estimated cost elasticity was less than one, indicating that tilapia farms are achieving economies of scale.
The reviewed studies underscore the importance of farmer education, resource optimization, technology adoption, and institutional support for advancing sustainable and profitable aquaculture systems. Building on this foundation, the present study contributes a dissimilar perspective by employing a Cobb–Douglas production function to quantitatively assess the impact of credit on the fisheries sector by examining the factors that affect fish production in Saudi Arabia. Unlike earlier research focused on credit access constraints or group-based productivity comparisons, this approach captures the studied variables as being cointegrated in the long run and short run alongside other variables. It offers practical insights for optimizing the factors and informing the design of targeted credit programs in the aquaculture sector.
4. Discussion
After confirming the existence of a long-run relationship, we moved on to estimate model (3), as shown in
Table 4. The standard errors reported in parentheses in
Table 4 are heteroscedasticity-autocorrelation robust standard errors. The adjusted R-squared value showed that the model explained 96% of the variation in the dependent variable. The F-statistic allowed us to reject the null hypothesis, which states that all the parameters are equal to zero. We accept the alternative hypothesis, as at least one variable was significantly different from zero.
The error correction term (ECM) was significant at the 5% level and had the expected negative sign. The ECM showed that the speed of adjustment back to equilibrium was 37% annually. Although the number of fishing boats positively affected fish production, their impact was statistically insignificant. This could be attributed to several reasons, such as boats aging, GPS dysfunction, and an obligation to use boats with gasoline engines rather than diesel engine, which incurs larger cost per fishing trip compared to diesel engine [
56]. Other factors that affect fishing boats efficiency include the crew’s experience level, the distance to fishing areas, the quantity and quality of fishing nets, and periodical maintenance [
34]. Fishermen positively affected fish production in both the short and long run. This result was consistent with [
40], who found a positive relationship between the number of fishermen and fish production. This outcome was expected because fishermen rely on fish catch as their main source of income [
57]. Fish from the sea have the largest positive impact in the short run generally and in the long run specifically. In the long term, a 1% increase in fisheries production from the sea increased Saudi fish sector production by 1.91%. However, marine fisheries in Saudi Arabia face key challenges, including but not limited to wind speed, environmental pollution, and overfishing [
40,
58,
59,
60]. The quantity produced by aquaculture farms affects the Saudi fisheries sector positively; nonetheless, its impact is not as strong as marine production because it only contributes approximately 10% to the kingdom’s average annual fish production [
61]. In line with Saudi Vision 2030, aquaculture production in Saudi Arabia is increasing annually and has exceeded sea production starting from 2018. The quantity produced by aquaculture farms in 2023 reached 139,949 tons, while the quantity produced from traditional marine fisheries reached 74,700 tons [
7]. Although the quantity from aquaculture farms has surpassed that from the sea in recent years, some species are still only producible through traditional marine fishing due to the economic costs of production and biological factors.
Table A1,
Table A2,
Table A3 and
Table A4 show that the species produced by marine fisheries are varied, while the species produced by aquatic farms are limited. The Saudi aquaculture sector also faces several challenges that may hinder its growth, such as environmental and climate challenges, water stress, and water scarcity [
62,
63]. Credit facilities granted to fisheries have proven to have a positive impact on the sector’s output. Indeed, a 1% increase in credit facilities for the fisheries sector can increase fish production by 0.03% in the long run. Providing unconstrained credit to fish farmers not only impacts productivity but also increases fish output [
32,
64]. The linear trend coefficient was positive and statistically significant at the 1% level, indicating technical progress in the Saudi fisheries sector.
To judge the performance of the Saudi fishing sector, testing the existence of economies of scale in the sector is important.
Table 4 illustrates that we tested the hypothesis of constant returns to scale in the Saudi fishing sector by assessing whether all the coefficients were equal to one. The results show that we rejected the null hypothesis of constant returns to scale at the 5% level, which implicitly confirms that the Saudi fisheries sector operates under economies of scale, consistent with [
42]. Evidence of increasing returns to scale in the fisheries and aquaculture sectors was also found in Nigeria and Ghana [
65,
66].