1. Introduction
Globally, agri-food residues account for approximately 5 billion metric tons of organic waste generated annually [
1]. Managing this vast quantity of residue presents significant economic, environmental, logistical, and health challenges [
2,
3]. However, these residues are often rich in nutrients, including carbohydrates, proteins, lipids, and phytochemicals, which can be recovered to produce other valuable products, such as feed, bioenergy, and fertilizer. Therefore, residue valorization has become a crucial strategy for enhancing the environmental sustainability of agri-food production.
Although conventional waste management technologies, such as landfilling, anaerobic digestion, and composting, help recover energy and nutrients, they face certain health and environmental limitations that hinder their effectiveness in addressing agri-food residue management. These limitations include a higher land requirement, greenhouse gas emissions, leachate contamination, and the need for strict process control conditions to process residues (such as composting and anaerobic digestion) [
4,
5]. To mitigate some of these challenges, using the black soldier fly larvae (
Hermetia illucens Linnaeus) for organic waste management has been recognized as a promising sustainable alternative within a circular bioeconomy framework [
6,
7,
8,
9]. This approach strongly aligns with current policy, as the EC Waste Framework Directive and the European Green Deal emphasize the valorization of residue from agri-food production as a key strategy for achieving circularity [
10,
11].
Black soldier fly larvae (BSFL) can rapidly convert low-quality organic substrates into high-quality protein and fat-rich biomass, and their versatility in feeding on various organic materials makes them a good option for waste management [
12,
13]. Under optimal conditions, BSFL can significantly reduce the volume of organic waste within two weeks, offering economic benefits in terms of logistics and potential applications for residual frass as a soil amendment [
14]. BSFL production requires minimal water and land resources. Moreover, BSFL can help address food security challenges by recovering and upcycling nutrients from organic residues into valuable biomass, which can serve as a source of protein and fat. This makes BSFL a promising strategy for enhancing the sustainability of agri-food production. It poses a low risk of zoonotic disease [
15,
16]. With the EU lifting the ban on Processed Animal Protein (PAP) for non-ruminants in 2021, BSFL are now recognized as a valuable source of PAP (Regulation EU 2021/1372), thereby broadening their market potential for animal feed [
17]. Beyond protein production, the BSFL value chain offers additional economic opportunities, including bioenergy (e.g., biodiesel derived from accumulated lipids), biopolymers (such as chitin and chitosan), organic fertilizers, bioplastics, and bioactive compounds [
18,
19,
20]. These products could replace conventional resources, contributing to a more circular approach to biomass utilization.
Although the potential benefits of BSFL production are apparent, industrial-scale rearing, processing, and valorization come with some environmental and socio-economic challenges [
21]. Large-scale BSFL production, particularly during value-added processes such as protein and lipid extraction, can be resource and energy intensive, potentially offsetting some of its advantages [
22,
23]. Factors such as substrate material sourcing, energy consumption, infrastructure, and post-harvest processing are critical in determining the overall environmental performance of BSFL production systems. Moreover, substrate quality, breeding facility conditions, and potential emissions of greenhouse gases and ammonia can also impact the environmental sustainability of BSFL bioconversion [
24,
25].
To promote the sustainable utilization of BSFL, a comprehensive Life Cycle Assessment (LCA) is essential for accurately assessing the environmental impacts of its bioconversion activities and associated post-processing. LCA is a decision-making tool that comprehensively quantifies the environmental impacts associated with each component (input) of the product (output), from raw material extraction to end-of-life disposal [
26]. As a valuable tool in promoting environmental sustainability, LCA helps identify areas for improvement and informs decision-making at various stages of the product life cycle [
27,
28]. Within the agri-food sector, LCA has gained widespread popularity for assessing the environmental impacts of food products at different stages of their production and consumption [
29]. To this end, there is a growing interest in applying LCA to evaluate the environmental sustainability of BSFL production and its associated bioconversion activities [
24].
However, the environmental sustainability of BSFL production is highly context-specific and influenced by various factors at different stages of the value chain. Previous studies have explored multiple objectives of BSFL production, including biowaste management [
30,
31,
32,
33,
34,
35,
36], larvae biomass production [
20,
37,
38,
39,
40,
41], biodiesel production [
42], and bioplastic production [
22]. Therefore, a comprehensive evaluation of its life cycle is essential to support the sustainable commercialization of BSFL and maximize its environmental and socio-economic benefits. This study aims to assess the environmental performance of BSFL production, focusing on diets based on agro-industrial residues and liquid digestate in a medium-scale mass rearing system. The goal is to quantify environmental burdens, identify critical hotspots, and provide insights into strategies for improving the environmental sustainability of BSFL-based bioconversion systems.
2. Materials and Methods
To evaluate the potential environmental impacts of the studied BSFL production system, the guidelines provided in the ISO 14040 and 14044 standards [
43,
44] and the International Reference Life Cycle Data System (ILCD) Handbook [
45] were followed to conduct the LCA study. The LCA software SimaPro PhD version 9.5 was used. Background LCI data were based on the attributional Ecoinvent database version 3.9 (allocation, cut-off by classification processes) [
46].
2.1. Goal and Scope Definition
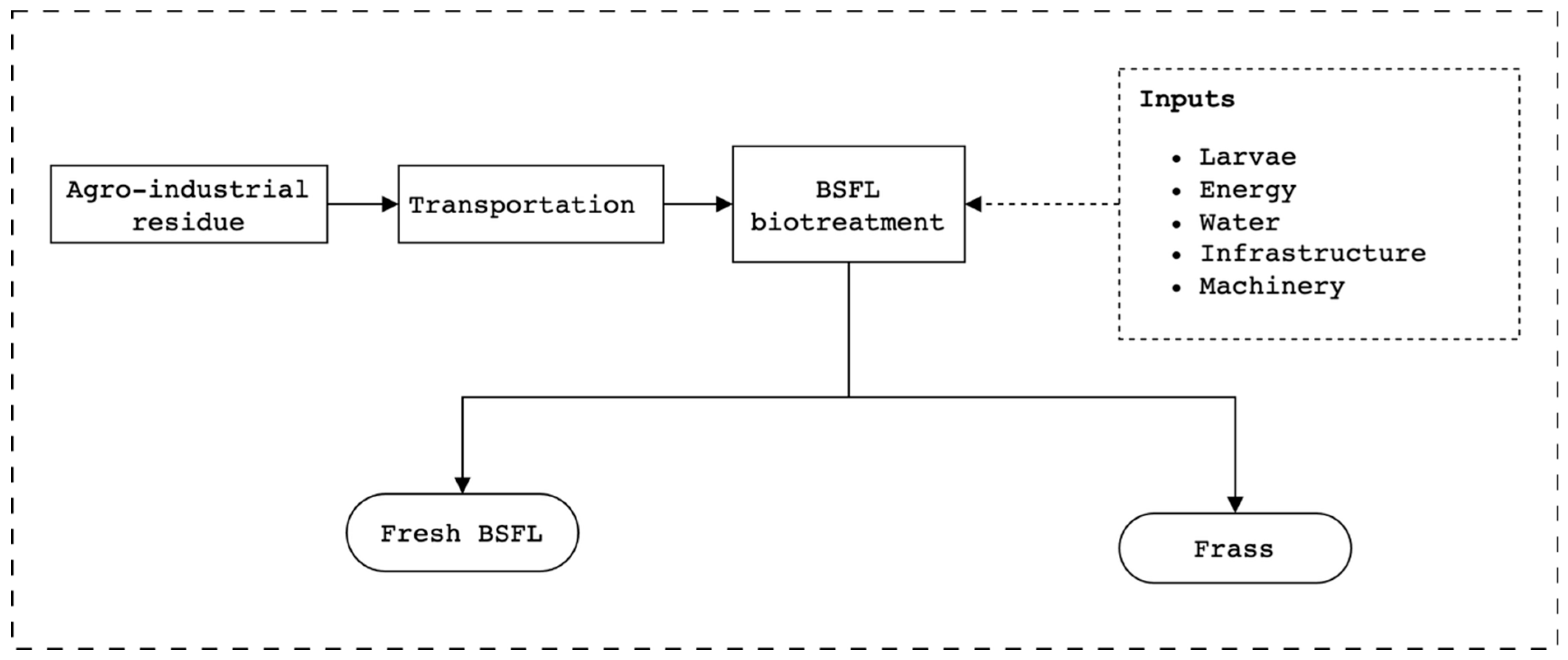
The goal of the study is to evaluate the environmental impacts of BSFL biotreatment of agri-food industrial residue intended to improve the environmental sustainability of frozen horticultural products. Specifically, the study’s objectives are to compare the environmental performance of BSFL on different agri-food industrial residues and identify key environmental hotspots for improvement. Given the multifunctionality of the BSFL bioconversion system, several functional units were chosen;
The production of 1 kg fresh BSFL biomass in a medium-scale prototype in 2024
The provision of 1 kg protein derived from BSFL in feed
The provision of 1 MJ energy from BSFL lipids
These functional units were selected to reflect the main valorization pathways of the system: biomass generation, protein recovery, and lipid-derived energy use, thereby enabling a comprehensive assessment of environmental performance across multiple functions. The mass-based FU is essential for internal benchmarking and process optimization, providing a direct measure of the physical production’s environmental burden within the prototype. The protein-based unit enables nutritional equivalence comparisons with conventional feed proteins such as soybean meal or fishmeal, while the energy-based unit facilitates evaluation of the environmental performance of BSFL-derived fat relative to other bioenergy sources, particularly when considering the downstream use of the extracted fat.
This attributional LCA study, with substitution, focuses on the biotreatment of agro-industrial residues using BSFL (
Figure 1). This study aligns with “Situation A: Micro-level decision support” as defined in the ILCD Handbook [
45]. The objective was to assess the environmental impacts of utilizing BSFL to valorize agri-food industrial residues while simultaneously producing fresh BSFL biomass. This micro-level focus implies that the study aims to inform decision-making at the processing level within the horticultural supply chain, without necessitating significant structural changes at the broader supply chain level. The findings of this study aim to support decision-making by various stakeholders, including horticultural processors, waste management companies, farmers, agricultural experts, and policymakers. The insights from this research could be utilized to develop strategies for reducing the environmental impact of the horticultural supply chain by promoting the sustainable management of agri-food industrial residues through BSFL bioconversion.
Given that the primary products of BSFL biotreatment are larvae (a protein and lipid source) and frass (a fertilizer), the system was expanded to account for potential substitutions with conventional products serving similar functions. This allowed for calculating the environmental credits associated with the avoided production of these alternative products. Primary data for this study were obtained from a mass-scale rearing system located in Osimo, Marche region (Italy), near a biogas plant (C.O.Val.M. Biogas S.r.l., Osimo, Italy now owned by SGR Biomethane S.r.l., Rimini, Italy). The proximity of the BSFL prototype rearing system to the biogas plant facilitated access to fresh residues and the use of energy from the biogas plant. The reference year for the BSFL biotreatment data is 2024.
2.2. Description of the BSFL Mass-Scale Rearing System
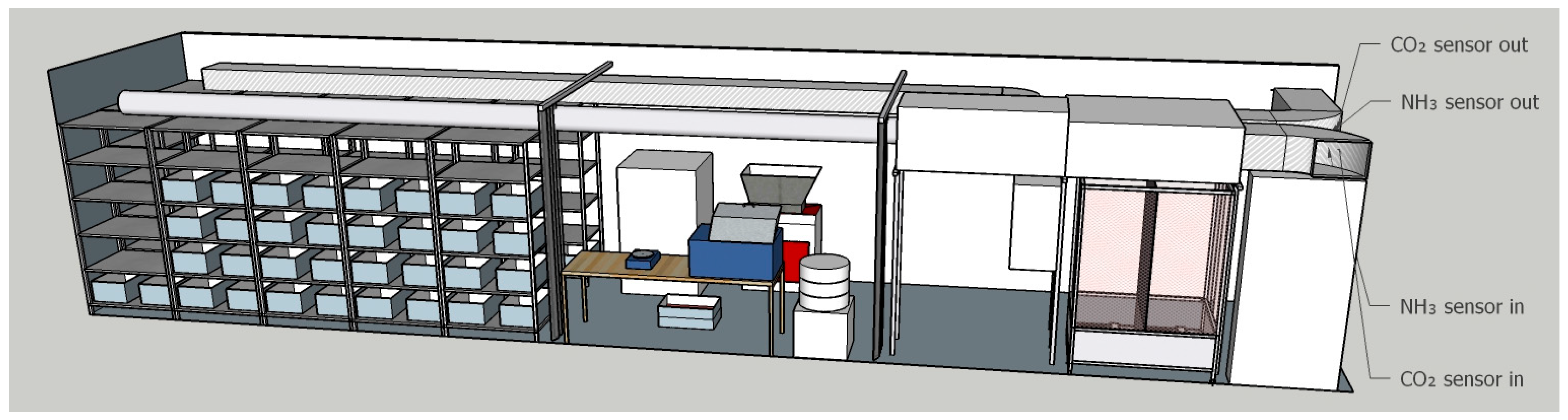
The insect biotreatment was carried out in a newly constructed and dedicated pilot plant. The plant was a modified shipping reefer trailer with standard dimensions of approximately 13.5 m in length, 2.5 m in width, and 2.7 m in height, and a total useful volume of roughly 89 m
3, which can treat about 1.58 t of residues per cycle.
Figure 2 shows the plant’s basic rendering and layout. The plant is divided into three sections: the bioconversion area, the area for feed and larvae processing, and the mating and reproduction area.
Figure S1 shows other images of the plant.
Inside the rearing facility, the residue is pre-treated by milling to reduce the particle size. The substrate is then weighed into a tray of dimensions 60 cm × 40 cm × 25 cm. To maintain optimal growing conditions for the BSFL, a climate control system has been implemented. A 4 kW heat pump, powered by renewable energy from the biogas plant, provides heating. Two 0.5 kW fans facilitate rapid heat distribution within the rearing chamber. Humidity levels are regulated using a humidifier, while a recuperator ensures adequate air exchange. The air exchange rate within the rearing chamber is maintained at 8 volumes/hour or 712 m3/h, providing a well-ventilated environment for optimal larval development. The air delivery system features a micro-perforated channel (Ø250 mm) with a length of approximately 8 m, ensuring gentle and even air distribution. The air velocity within the rearing chamber remains low (≤0.2 m/s at a distance ≥1.2 m from the duct), allowing for flexible positioning of the adult fly aviary within the container. Both the larval and adult phases require temperatures ranging from 27 °C to over 30 °C, with an optimum of 30 °C for adults. The optimal relative humidity is also around 70–80%, and this parameter is more limited for adults than for larvae. Other equipment installed in the plant for processing includes a mill (Tiger Shark S100, FULLTECH INSTRUMENTS S.R.L., Rome, Italy), a sieve (IG/1/S 500, Guiliani Tech., Turin, Italy), a thermostatic bath (PID system M408-BM, MPM Instruments S.r.l., Monza Brianza, Italy), and an oven (PID system M400VF, MPM Instruments S.r.l., Monza Brianza, Italy).
2.3. Diets and Bioconversion Trials Setup
Experimental data were obtained from six trials conducted between May and August 2024. The residues evaluated in the study were categorized into two groups: fresh residues and dry residues. Fresh residues are characterized by high moisture content and short shelf life. This group included peas, onions, and tomatoes. Additionally, liquid digestate was tested in this category. In contrast, dry residues have very low moisture content and are relatively stable. This group included dry chickpeas from sorting and wheat storage residues. The diets were formulated to achieve an optimal moisture content of approximately 70%, the ideal for BSFL growth and bioconversion efficiency [
47,
48]. Fresh and dry residues were analyzed and combined in appropriate proportions to reach the target moisture level, ensuring consistency across trials and alignment with locally available feedstocks at the biogas plant during each trial. A proximate analysis was carried out to verify the suitability of the diet in supporting BSFL growth (
Table S1). The substrates used to formulate the diets were agri-food industrial residues sourced from a consortium of companies in the Marche region. These companies are involved in the cultivation and processing of horticultural products, as well as the transformation and valorization of residues through energy generation via anaerobic digestion (C.O.Val.M.—O.R.T.O. Verde S.c.a.p.a., Senigallia, Italy, and C.O.Val.M. Biogas S.r.l.). However, the chickpea residue was provided by a seed company also based in the Marche region.
Table 1 presents the composition ratios of the substrates for each diet.
The residues were milled and mixed in the trays. Five-day-old BSFL obtained from Smart Bug S.r.l. (Ponzano Veneto, Italy) were added to the substrate. Each tray contained 12 kg of residue and 12,500 larvae, with a larva density of 5 larvae/cm
2. The feeding was divided into two batches, 6 kg on day 1 and another 6 kg on day 5. A total of 12 trays were used in each trial, corresponding to 144 kg of treated residue. The bioconversion process took approximately 10 days for the larvae to mature (5th instar stage). Afterward, harvesting was performed using a sieve to separate the larvae from the frass (
Figure S2).
Calculations for larval growth and substrate reduction.
Larvae growth and substrate reduction performances of insects were computed through the following indices:
- i.
Bioconversion rate (BCR%) = (final larvae mass (g)/total substrate mass (g)) × 100
- ii.
Assimilated feed (g DM) = total substrate dry mass (g) − total frass dry mass (g)
- iii.
Feed conversion rate (FCR) = (assimilated feed (g))/(final larvae dry mass (g))
- iv.
Substrate reduction (%) = (assimilated feed (g)/substrate dry mass(g)) × 100
- v.
Larval survival (%) = (final larval number/initial larval number) × 100
The initial larval mass was assumed to be zero.
2.4. Life Cycle Inventory of BSFL Breeding
The experimental data obtained were scaled up to an industrial level for the LCA model construction. The pilot plant, capable of managing 132 trays/cycle, can treat approximately 1.58 t of residue per cycle. Assuming a 10-day bioconversion period and 36 cycles/yr (continuous operation), approximately 57 t of residue can be treated annually, yielding 7.13 t of fresh BSFL biomass (considering 1.5 kg larvae/tray based on the average yield from the trials).
The breeding process comprises three main stages: love-cage and egg hatchery management, bioconversion management, and post-harvest management. The process begins with mating BSF flies and laying the eggs in a love-cage. This cage, of dimensions 140 cm × 130 cm × 100 cm, is constructed of aluminum poles, polycarbonate boards, a polypropylene net, and rubber. It can accommodate 12,500 adult flies per cycle, with half being females. Each female is estimated to lay 300 eggs, with a 50% hatching rate, producing 937,500 larvae/cycle. For this study, the infrastructure of the love-cage was considered, and the lifespan was assumed to be 30 yr. Unfortunately, due to elevated temperatures caused by a malfunctioning humidifier, the pilot plant could not produce the required number of larvae. As a result, larvae were sourced from an external supplier—Smart Bug S.r.l. (Ponzano Veneto, Italy).
The transportation of residues and larvae to the mass-rearing system was also considered. The fresh residue came from the processing of horticultural products by C.O.VAL.M.—O.R.T.O. Verde S.c.a.p.a. The wheat residue was sourced from a wheat milling company (Italcer Soc. Coop. Agr., Osimo, Italy), while the chickpeas were obtained from a seed company (Società Produttori Sementi S.p.a., San Severino, Italy). A 100% load capacity was assumed for residue transportation.
Table S2 provides details on the transportation distances and modes.
Regarding infrastructure, the shipping container itself was not considered, as it was assumed to be at the end of its life and would be reused. The materials for internal structures were included, primarily stainless steel support poles, the ventilation system (stainless steel and polyurethane), the scaffold for holding trays (stainless steel), and the polypropylene plastic trays. Regarding equipment, the materials for the milling machine and multidimensional sieve were considered, with lifespans of 10 and 15 years, respectively. Data for the BSFL production at the breeding facility are summarized in
Table 2.
2.5. Allocation and Substitutions
Currently, there is no standardized market for BSFL, making it challenging to establish an economic allocation between the larvae and the frass. The frass can be regarded as a substitute for manure, which generally has very low economic value or even a negative value when producers must pay for its disposal. As a result, all environmental impacts were allocated to the fresh BSFL biomass.
For the potential use of BSFL as an alternative protein source in animal feed, BSFL was assumed to replace conventional protein sources such as soybean meal, fishmeal, or pea protein. The selected processes for soybean meal, fishmeal, and pea protein in the Ecoinvent database were adjusted to reflect their respective protein contents, ensuring an accurate comparison with the total protein content of BSFL. The protein content and the comparison with conventional protein sources are detailed in
Table S3 Additionally, BSFL fat was evaluated as a potential feedstock for biodiesel production, potentially replacing vegetable oils like rapeseed oil, palm oil, soybean oil, and sunflower oil. The total lipid content and higher heating value (HHV) of BSFL lipids were compared with those of the selected vegetable oils (
Table S3). To ensure fair comparison and reduce uncertainty, the biodiesel production process was excluded from the system boundaries, focusing only on the phases up to seed production.
2.6. Impact Assessment
The life cycle impact assessment of BSFL products, per the selected functional units were evaluated in terms of acidification (A), climate change (CC) estimated over a 100-year horizon, ecotoxicity freshwater (ETF), particulate matter (PM), eutrophication marine (MEU), eutrophication freshwater (FEU), eutrophication terrestrial (TEU), human toxicity, cancer (HTC), human toxicity, non-carcinogenic (HTNC), ionizing radiation (IR), land use (LU), ozone depletion (OD), photochemical ozone formation (POF), water use (WU), resource use, fossils (RUF), and resource use, minerals and metals (RUMM), using the Environmental Footprint (EF) 3.1 life cycle impact assessment method [
50].
2.7. Interpretation
The interpretation of the LCA results includes the midpoint impact scores based on the EF 3.1 method, a contribution analysis to identify primary impact drivers, and several sensitivity analyses (scenarios) to assess the influence of varying relevant parameters, as discussed in
Section 3.
3. Results and Discussion
3.1. Growth and Bioconversion of BSFL on Different Diets
Table 3 and
Table 4 present data and results on the larval and substrate characteristics, as well as the larval growth and substrate reduction performance across various diets. The average fresh larval weight per tray was approximately 1.5 kg, with Diet 5 showing the highest performance, having an average yield of 1.75 kg per tray. In contrast, Diet 6 showed the poorest performance, attributed to elevated temperatures caused by a malfunctioning humidifier. This issue resulted in significant larval mortality and escape, which contributed to the low yield obtained. Once the environmental conditions were stabilized by deactivating the humidifier, Diet 5 demonstrated better results. These findings emphasize the importance of maintaining optimal temperature control for successful larval development. Additionally, substrates with lower lignin and lignocellulose content were easier to process and separate after harvest, further contributing to improved efficiency in the rearing system.
Regarding Diet 5, the disposal of liquid digestate poses significant challenges for biogas producers due to strict regulations on application amounts and seasonal limitations. The instability of liquid digestate, primarily due to its ammonia content, necessitates large storage tanks or lakes, which are costly and environmentally problematic. Open storage often releases methane, ammonia, and nitrous oxide emissions [
51,
52]. Therefore, using liquid digestate as a substrate for BSFL production offers the dual benefit of reducing storage and emission issues while alleviating competition between agri-food residues for biogas production. BSFL biotreatment also offers complementary advantages to anaerobic digestion, making the two processes highly compatible and promoting greater resource circularity. Analysis of the frass derived from BSFL treatment revealed a biogas potential of approximately 542.6 Nm
3/ton vs. and a biomethane potential of 315.4 Nm
3/ton VS, indicating substantial energy recovery potential. These results are consistent with previous findings. For example, Czekała et al. [
53] reported biogas and methane yields of 709.3 Nm
3/ton vs. and 455.9 Nm
3/ton VS, respectively, from BSFL reared on food waste. Therefore, both the larvae and the resulting frass represent valuable feedstocks for biogas production, contributing to improved resource circularity.
The dry matter content of the BSFL was approximately 33%, consistent with other studies [
54,
55]. The bioconversion rate ranged from 12% to 16%, aligning with previous findings on BSFL reared on various substrates. Similarly, the feed conversion ratio was between 4 and 6, which was also comparable to results reported in other studies [
25,
56]. The substrate reduction ranged between 73% and 81% within 10 days of treatment, which is slightly higher than the values reported for fruit and vegetable waste diets in other studies [
57,
58]. However, Lalander et al. [
59] reported lower substrate reduction capacities of (50–60%) for fruits and vegetables, which may be attributed to the higher lignin content of these materials, as lignin-rich substrates are generally less efficiently broken down in the rapid BSFL composting process.
Although several studies have highlighted the influence of substrate composition on BSFL development, growth, and waste reduction efficiency [
59,
60,
61,
62,
63], further detailed analysis of the diets, larvae, and frass was not feasible due to several constraints. However, this study demonstrates the suitability of agri-food industrial residues from frozen vegetable production and liquid digestate as substrates for BSFL. Nonetheless, careful consideration of microbial contamination risks is essential for ensuring safe BSFL products.
3.2. Environmental Impacts of BSFL Production from Different Diets
Table 5 presents the absolute impact scores for assessed midpoint impact categories. The yield of larvae mainly influenced the environmental performance of the diets. The results indicate no substantial difference in environmental performance among the assessed diets formulated from the agri-food industrial residues. However, diet 5 (liquid digestate + wheat) showed the best performance, primarily due to the feed substrate and higher larvae yield. Diet 5 did not require milling, as both liquid digestate and milled wheat residue were used without further processing. Moreover, the on-site availability of liquid digestate eliminated the need for residue transportation. However, it is important to note that Diet 6 was excluded from the analysis due to challenges with the humidifier during the breeding process, which compromised the data quality.
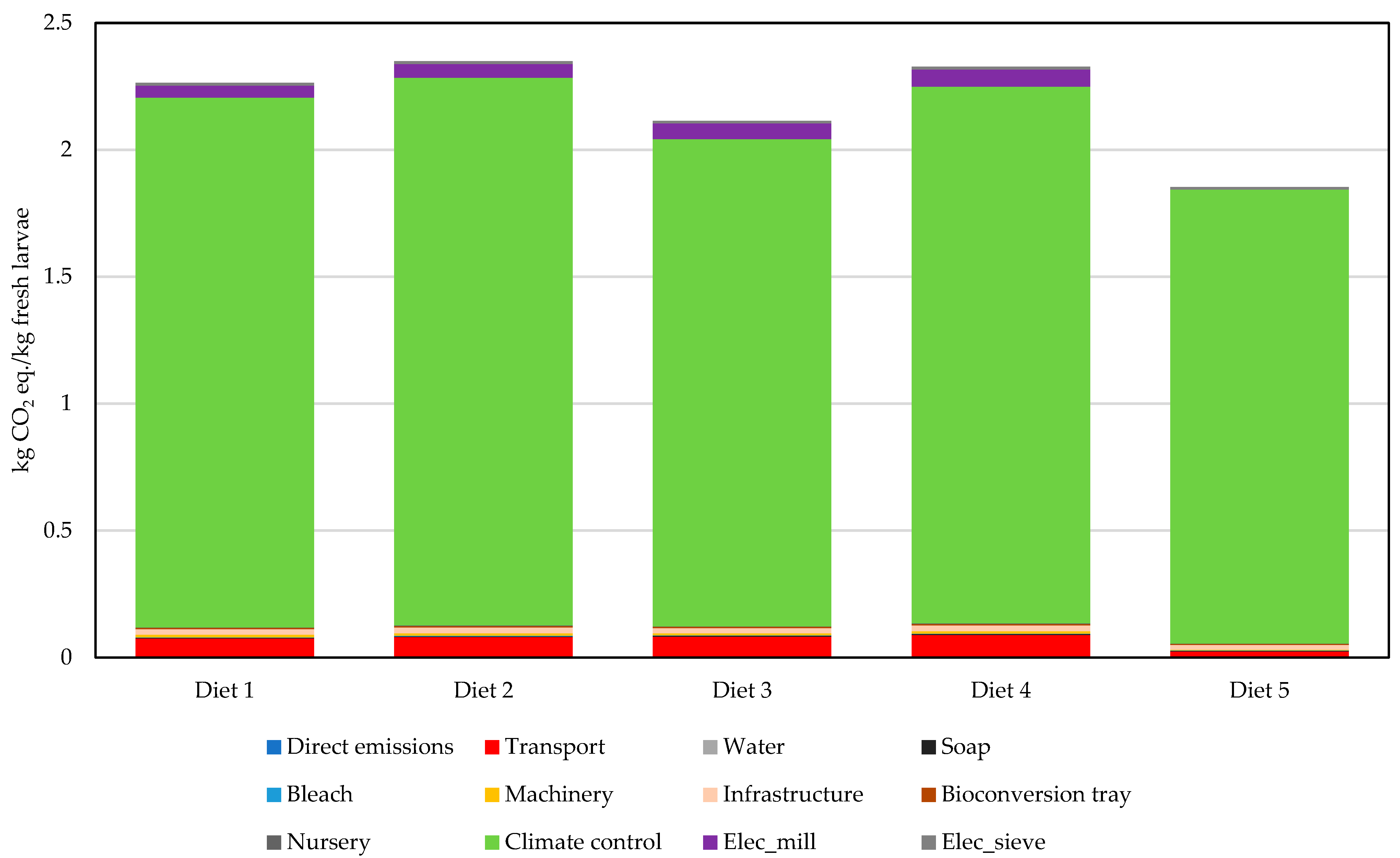
For brevity, only the contribution of climate is presented; the complete results are available in the
Supplementary Material (Figure S3). According to the contribution analysis of climate change (
Figure 3), electricity consumption for climate control was the primary driver of environmental impact, accounting for approximately 85% of the total impact across all assessed categories. Within the climate control system, electric fans contributed approximately 10% of the total impacts, whereas the heat pump accounted for about 75–85%. Similarly, for the other impact categories, climate control was the main environmental hotspot, accounting for more than 80% of the total impacts (
Figure S3).
It should be noted that the initial plan was to utilize residual heat from the biogas plant for climate control in the prototype. However, technical and authorization challenges prevented its implementation. As an alternative, an electric heat pump was installed for climate control. The results show that the electric heat pump is not a suitable option for the prototype. Opting for an air conditioning system with lower energy requirements could potentially improve energy efficiency and reduce the environmental impact associated with climate control.
Similar studies have identified energy use as a major hotspot in BSFL biotreatment or production [
35,
38,
64,
65]. While climate control is crucial in colder climates, ambient conditions may be sufficient to ensure BSFL productivity in warmer regions. In temperate zones, heat pumps could be switched off during warmer months to conserve energy. The impact on electricity production is also country-specific, as countries with a greater share of renewable energy in the average country mix will have a lower impact on some impact categories, like CC. Therefore, the geographical location and seasonality of BSFL production can significantly influence the overall environmental performance.
Other inputs had relatively lower impacts, contributing on average less than 5% across all impact categories. For instance, milling accounted for approximately 2% of the total impacts across all assessed categories, while transportation contributed less than 4%, except PM, where it accounted for 10%. The minimal impact of transportation highlights the importance of local sourcing of raw materials combined with an efficient logistics system. This approach not only provides environmental benefits but also offers significant economic advantages [
66].
3.3. BSFL vs. Conventional Protein Sources
BSFL can potentially replace common feedstuff components, such as fishmeal, soybean meal, and pea protein. To assess their environmental performance, a FU of 1 kg of protein was used to compare BSFL-based diets with these conventional protein sources (
Table 6). The results indicate that the BSFL diets were comparable to soybean meal but were outperformed by fishmeal and pea protein in most impact categories. However, in terms of water use, pea protein had a significantly higher impact score compared to BSFL. Similarly, for land use, both pea protein and soybean meal showed substantially higher impacts than BSFL.
The significant environmental impact of BSFL production was primarily attributed to electricity consumption. Thus, optimizing energy efficiency or adopting alternative, cleaner energy sources could significantly improve the overall environmental performance of BSFL production and make it more comparable to conventional animal feed protein sources.
Fishmeal had the lowest environmental burden among the protein sources assessed. However, its production can have significant environmental impacts, including biodiversity loss, species depletion, and microplastic pollution, which are not fully captured presently in LCAs [
67,
68]. Additionally, increasing soybean and pea protein production to meet growing demand would require the expansion and intensification of crop cultivation, leading to increased resource use (including land, water, and agrochemicals) and emissions, as well as their related negative environmental impacts. Thus, BSFL presents an environmentally sustainable alternative to these conventional protein sources for animal feed.
3.4. BSFL vs. Conventional Vegetable Oil Sources for Producing Biodiesel
BSFL fat has the potential to serve as a feedstock for biodiesel production, providing an alternative to traditional sources such as rapeseed, palm, soybeans, and sunflowers [
69,
70]. Based on fat content and higher heating value (HHV), the environmental impacts of producing 1 MJ of biodiesel from BSFL-derived fat were compared to conventional sources (
Table 7). The results show that the environmental burden of BSFL as a biodiesel source was higher than that of vegetable oils, except soybean, across all impact categories except for LU. This finding aligns with the results observed for using BSFL protein in feed production. The lower LU impact of BSFL can be attributed to its efficient conversion of organic waste into biomass, with less land requirement.
Although biodiesel produced from vegetable oils can be more expensive than petroleum diesel and may compete with food production, BSFL fat, primarily derived from organic waste streams such as liquid digestate or manure, offers a more sustainable and potentially cost-effective alternative [
70,
71]. Currently, the use of BSFL fed on digestate as livestock feed is prohibited. However, given the promising growth performance of BSFL on diet 5, exploring it for biodiesel production could be an environmentally sustainable option. The extensive production of bioenergy from dedicated crops could potentially lead to changes in land use, both direct and indirect, with associated economic, social, and environmental implications [
72,
73]. However, by addressing the energy consumption challenges associated with BSFL production, this approach can contribute to a more environmentally sustainable bioenergy future, reducing the need for land-intensive bioenergy crops.
The CC impacts for 1 kg BSFL protein (4.63 to 5.66 kg CO
2 eq.) assessed in this study were generally comparable to findings reported in the literature. Pahmeyer et al. [
64] reported a value of 6.96 kg CO
2 eq./kg BSFL protein, while Smetana et al. [
39] presented a wider range of 1.36–15.1 kg CO
2 eq./kg insect protein meal. However, the CC results from this study were higher than those reported by Salomone et al. [
30], who found a score of 2.1 kg CO
2 eq./kg protein for dried BSFL. Similarly, Komakech et al. [
74] reported a lower CC score of 1.24 kg CO
2 eq./kg BSFL protein meal. In contrast, Ramzy et al. [
75] had a much higher CC score of 49.4 kg CO
2 eq./kg protein. For lipids, Salomone et al. [
30] also reported a CC impact of 2.9 kg CO
2 eq./kg BSFL lipid, which is lower than the findings of this study (5.8–7.3 kg CO
2 eq./kg BSFL lipid on a dry matter basis).
The differences in results can be attributed to several factors, including differences in system boundaries, methodological choices, assumptions made, and technology and scale of production in the various studies. The variation in CC impact findings across studies emphasizes the importance of standardized methodologies and transparent reporting in LCA studies. These studies demonstrate that BSFL production can achieve a lower environmental impact under optimized conditions and compete with conventional products.
3.5. Sensitivity Analysis (Scenario)
3.5.1. Energy with Climate Control
The results showed that electrical energy use was a significant concern, as it contributed to all the impacts assessed. Electricity was needed for heating during insect breeding and processing activities such as milling and sieving. Switching to other energy sources is likely to change the impact results. To test this hypothesis, two scenarios regarding energy for climate control were tested, considering the following changes:
- (a)
Scenario 1—utilization of residual heat from the biogas plant for heating.
- (b)
Scenario 2—alternative source of energy (photovoltaic system).
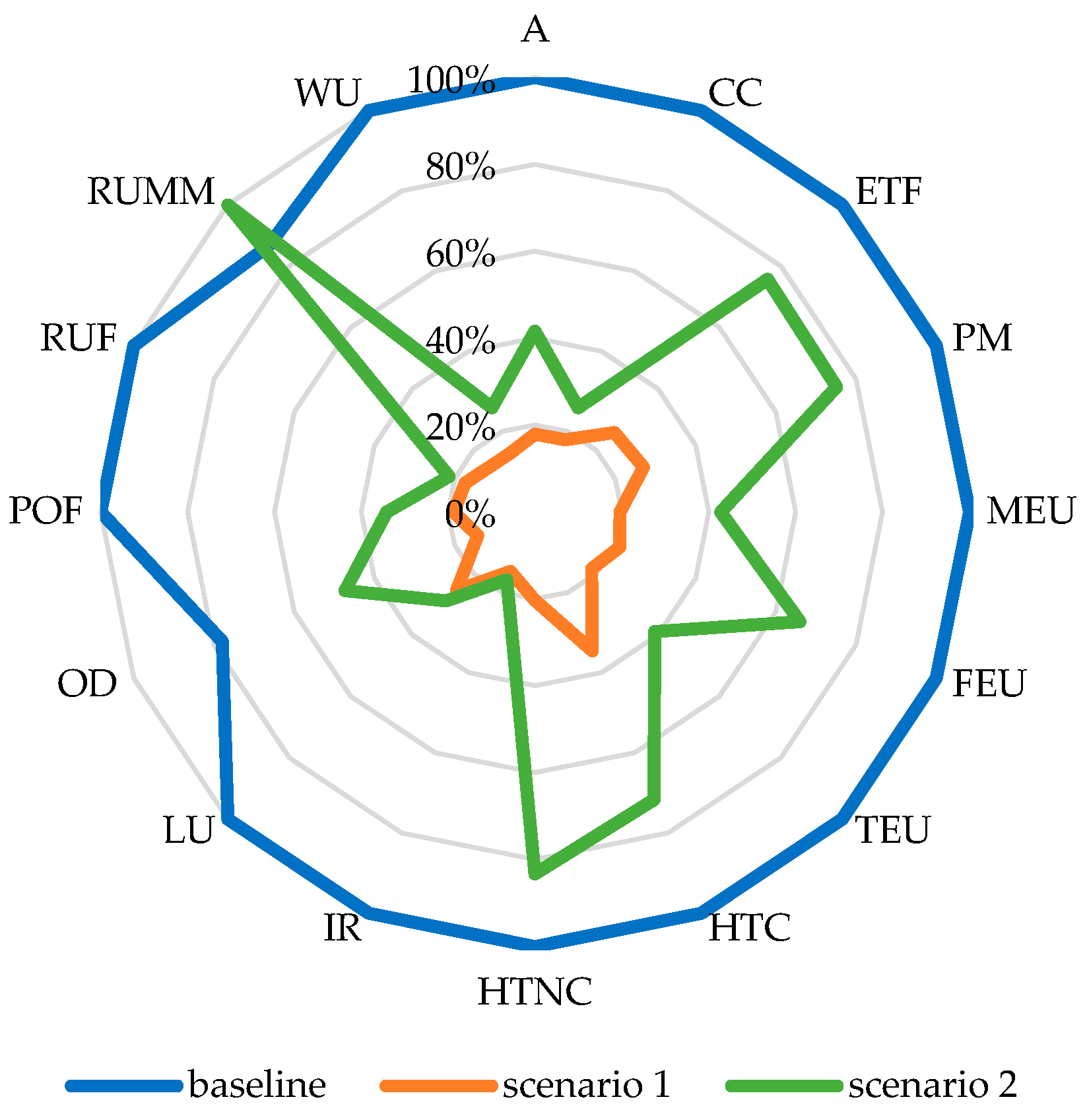
The results showed that the two scenarios had net reductions in environmental impacts relative to the baseline scenario across all categories, except RUMM in scenario 2 (
Figure 4). Scenario 1 achieved the highest reductions (70–85%) in environmental impacts. The heat from biogas plants is often underutilized due to their remote locations, the inefficiency of long-distance heat transport, and the need for significant infrastructure investments [
71,
72]. However, BSFL production facilities near biogas plants can use this waste heat, leveraging proximity for easy access to residues and heat. Both activities align with waste management objectives, further enhancing their synergy. The CC impact for 1 kg BSFL protein ranged between 3.3–3.6 kg CO
2 eq. for Diet 1–4 and 2.1 kg CO
2 eq. for Diet 5 when residual heat is used for climate control.
Replacing grid electricity with electricity from a 3 kWp (scaled-up according to the energy requirement of the rearing system) building-integrated photovoltaic system (PV) in Scenario 2 resulted in substantial reductions in all impact categories, except RUMM. The CC impact decreased by 74% (4.47–4.91 kg CO
2 eq. for Diet 1–4 and 3.59 kg CO
2 eq. for Diet 5). However, RUMM increased by 13% due to material depletion associated with the production of PV panels. While solar energy significantly improves environmental performance, challenges include the upfront investment cost, seasonal variability (especially in winter), and end-of-life waste management for PV systems [
68,
69,
70].
3.5.2. Scaling-Up Considerations (Prospective LCA)
Given that the data from the mass-scale prototype is based on limited trials and has not yet been fully commercialized, the environmental impacts of BSFL production are expected to change as the system scales. As production expands, changes in efficiency, resource utilization, and operational dynamics can significantly impact the sustainability of the process [
13].
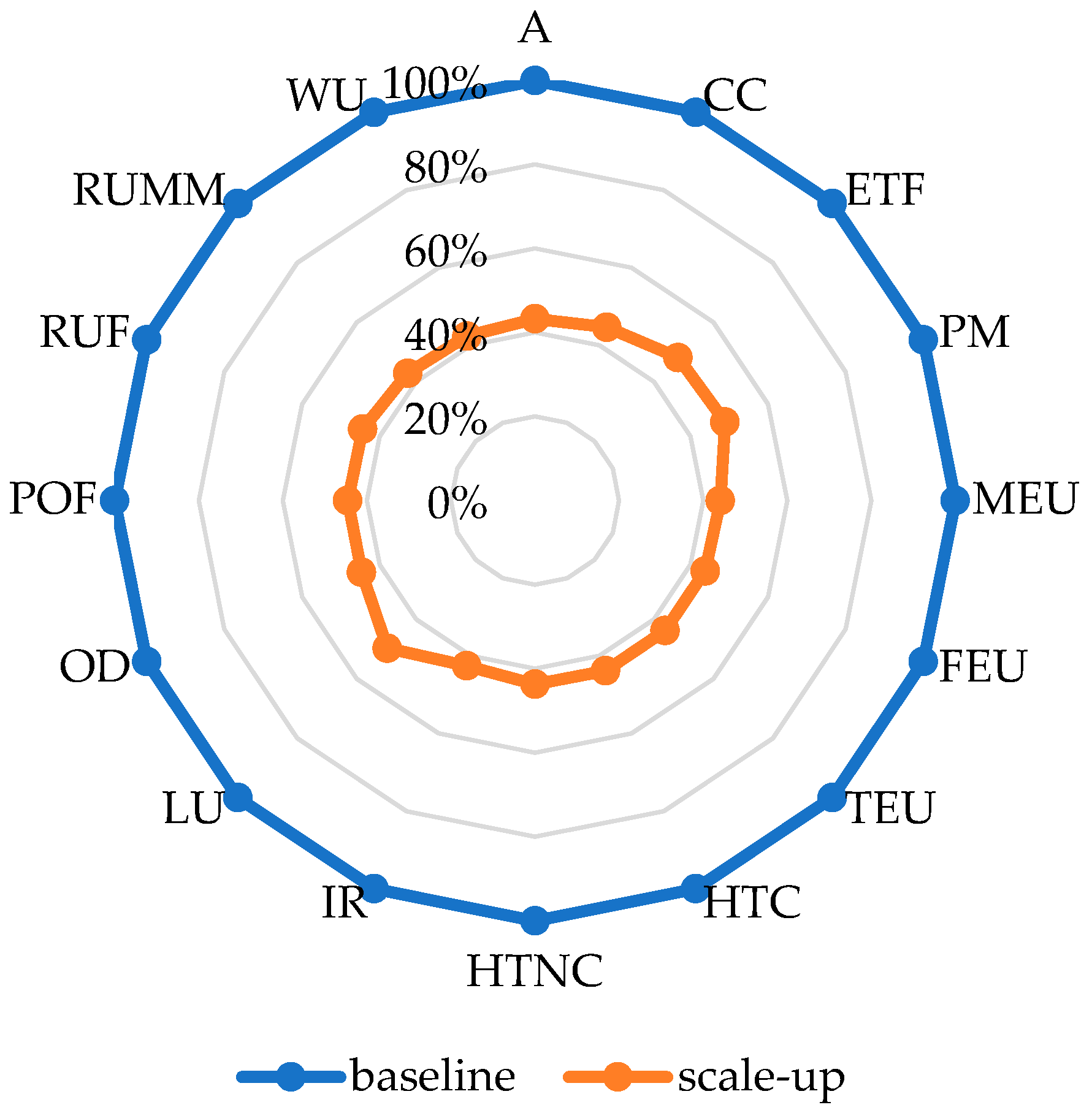
A key factor in scaling up BSFL production is increasing production yield per tray. Currently, each tray yields about 1.5 kg of fresh larvae. However, by optimizing tray utilization, this could potentially increase to 2.5 kg per tray. Currently, only about half of the tray volume is utilized, with 12 kg of substrate per tray; however, this could be expanded to 20 kg. Moreover, the number of trays per production unit can be expanded. The current mass-scale prototype operates with 132 trays, occupying about a quarter of the available space. By optimizing the spatial arrangement, the total number of trays can be increased to 200, effectively maximizing production capacity within the same footprint.
A scenario analysis was performed based on these possible adjustments. The results show a 50–60% reduction in impacts (
Figure 5). The CC score reduced to 1.01 kg CO
2 eq./kg fresh larvae. With potential improvement in energy use for climate control, this could be significantly lower. These results highlight that medium-scale mass production of BSFL could have a similar environmental performance to conventional products. A four-year full-scale BSFL bioconversion study in Hangzhou, China, treating 15 tonnes of daily domestic biodegradable waste, demonstrated significant performance improvements through continuous technical optimization. The average yield of fresh larvae increased from 8.5% in 2017 to 15.3% in 2020. These improvements resulted in enhanced overall process efficiency and impact reduction [
76]. From a life cycle perspective, scaling up BSFL production could bring both environmental benefits and trade-offs. Increased production efficiency reduces the impacts associated with energy consumption, machinery, and infrastructure. However, higher production volumes may also lead to greater resource use, necessitating careful management of inputs such as fuel for substrate transportation and other consumables. Therefore, as BSFL production scales, continuous environmental monitoring and assessment are necessary to ensure that increased efficiency does not result in higher overall environmental burdens.
Beyond physical expansion, automation and process optimization are critical for improving efficiency and environmental sustainability. Implementing automated feeding, monitoring, and harvesting systems could reduce labor intensity and improve consistency in production. Future research could focus on developing optimized production models and automated systems to further enhance the environmental sustainability of large-scale BSFL production.
3.6. Limitations of the Study
Despite its contributions, this study has limitations that may influence the interpretation of its results and should be taken into consideration. Firstly, the BSFL bioconversion system evaluated in this study is currently at a prototype or pilot-scale stage, lacking the operational maturity and optimization characteristics typically found in fully industrialized systems. As such, it does not yet achieve optimal levels of efficiency, productivity, or process stability. This is evident in the use of electricity for climate control instead of the intended residual heat from the biogas plant. Consequently, the performance metrics and environmental impacts reported here may not fully reflect those of a scaled-up, commercial-scale facility, where improvements in resource utilization, process integration, and automation are expected to enhance overall system performance. Uncertainty also exists due to the natural variability in moisture and nutritional composition of agri-food residues, which were sourced seasonally. This variability impacts bioconversion efficiency and, consequently, the environmental performance of the studied system. Future studies should therefore account for scale-up dynamics, including changes in substrate composition, energy use, and emissions, which can significantly influence the system’s environmental sustainability profile at an industrial scale.
Another limitation was the inability to accurately measure real-time gas emissions using low-cost sensors due to their limited detection thresholds and interference from the nearby biogas plant. This limitation hindered the precise quantification and monitoring of emissions, which is essential for comprehensive environmental assessments. Investing in high-precision, robust, and affordable sensors specifically designed for insect-rearing facilities would significantly enhance data reliability.
Furthermore, analytical constraints limited the comprehensive characterization of larvae and frass biomass. A comprehensive understanding of these characteristics is essential for optimizing feed formulations and maximizing the value of BSFL-derived products. Such data are also important for the accurate modelling of substitutable products (e.g., protein meals, fertilizers) in impact assessments, enabling more precise attribution of environmental burdens and benefits in LCA studies.
The environmental impact data for conventional protein and fat sources were sourced from the Ecoinvent v3.9 database, as no site-specific data were available. Although Ecoinvent provides reliable and widely accepted datasets, they may not fully represent current technologies or regional conditions in Italy, which could potentially affect the accuracy of comparative results. Additionally, the BSFL bioconversion system was modeled using secondary data for direct emissions, which may introduce some uncertainty in the results.
Finally, economic feasibility remains a critical knowledge gap in this study, as the financial viability of BSFL production is fundamental to its large-scale adoption. However, the current market for BSFL in Europe is still emerging, making it difficult to assess its competitiveness with conventional products. Therefore, a detailed techno-economic assessment is recommended to evaluate potential cost-reduction strategies and optimized production processes.
4. Conclusions
This study assessed the environmental performance of BSFL production on various agri-food industrial residues, focusing on its potential for feed and energy production. An attributional LCA was conducted using primary data from a pilot BSFL rearing system.
All tested diets supported BSFL growth, but the diet comprising liquid digestate and wheat residue demonstrated slightly better results in both larvae growth performance and environmental impact than diets consisting solely of agri-food industrial residues. The primary environmental hotspot identified was electricity consumption for climate control, which contributed over 75% of the total impacts across all categories. For protein production, BSFL was comparable to soybean meal but had relatively higher impacts than pea protein and fishmeal. Similarly, BSFL production had higher environmental impacts than conventional bioenergy sources, such as rapeseed, palm fruit, and sunflower, but was comparable to soybean oil. Although the results suggest that BSFL production faces challenges in achieving environmental competitiveness, it remains a promising avenue for producing protein for animal feed and biodiesel for energy. Addressing specific limitations, such as energy use for climate control, could enhance its viability. Utilizing residual heat energy from biogas plants and scaling up can reduce impacts by more than 80%.
In Italy, industrial-scale BSFL production lags behind other insect-rearing sectors, such as crickets and mealworms. Regulatory challenges hinder development, including prohibitions on using BSFL reared on waste or manure due to safety concerns. However, given the high growth performance of BSFL on liquid digestate, this approach could offer a synergistic relationship with anaerobic digestion. Using liquid digestate as a substrate for BSFL not only produces larvae but also generates frass with high biomethane potential. This synergy can enhance resource circularity and reduce environmental impacts by displacing traditional, resource-intensive feedstocks like maize silage. This study highlights the potential of BSFL production to enhance resource circularity when implemented on a commercial scale. Thus, integrating BSFL bioconversion into the horticultural production chain could improve the environmental sustainability of the sector.