Addressing Catfish (Clarias spp.) Supply Gap in Nigeria: A Perspective on Strategies for Sustainable Aquaculture Growth
Abstract
1. Introduction
2. Industry Overview
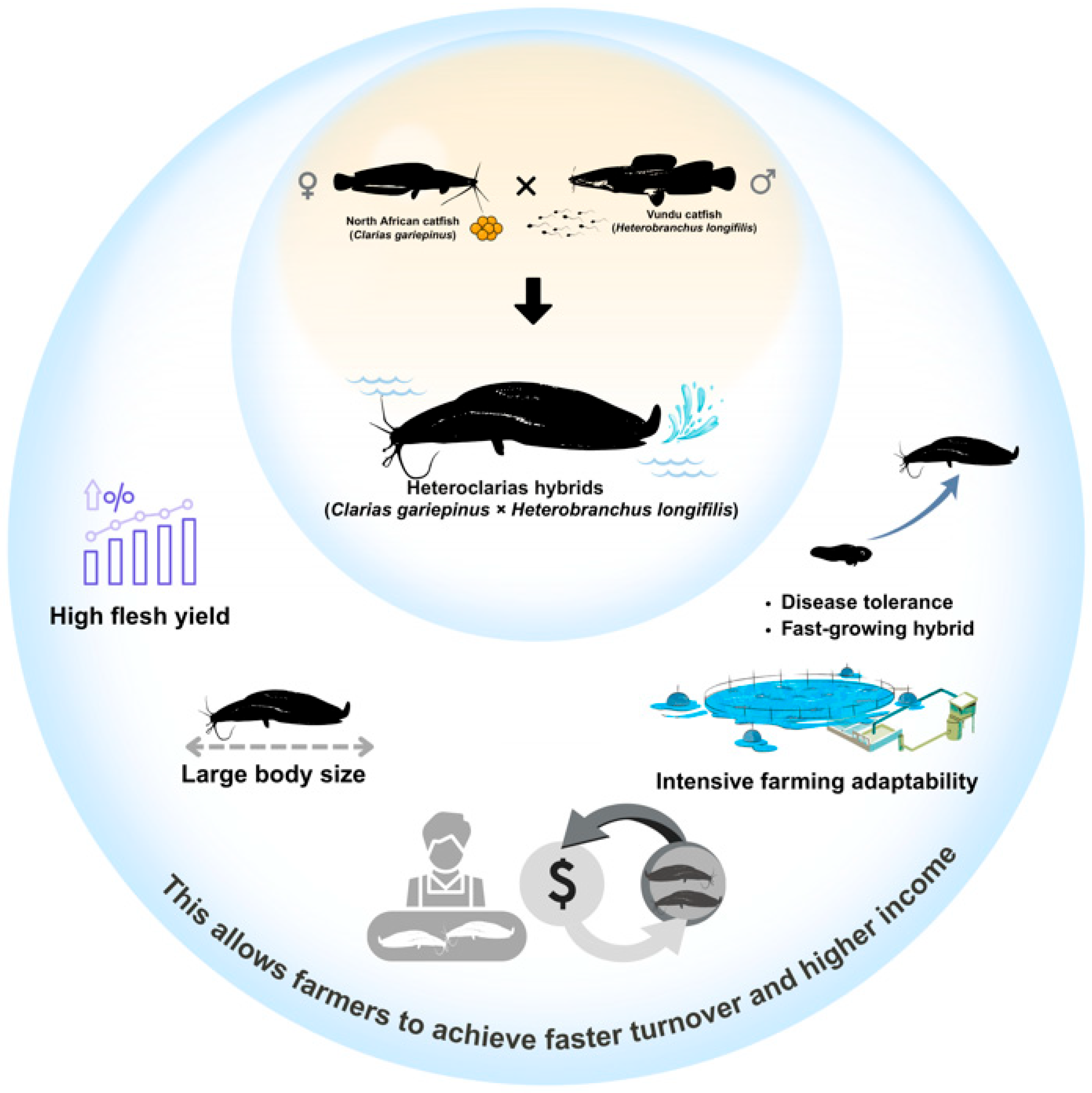
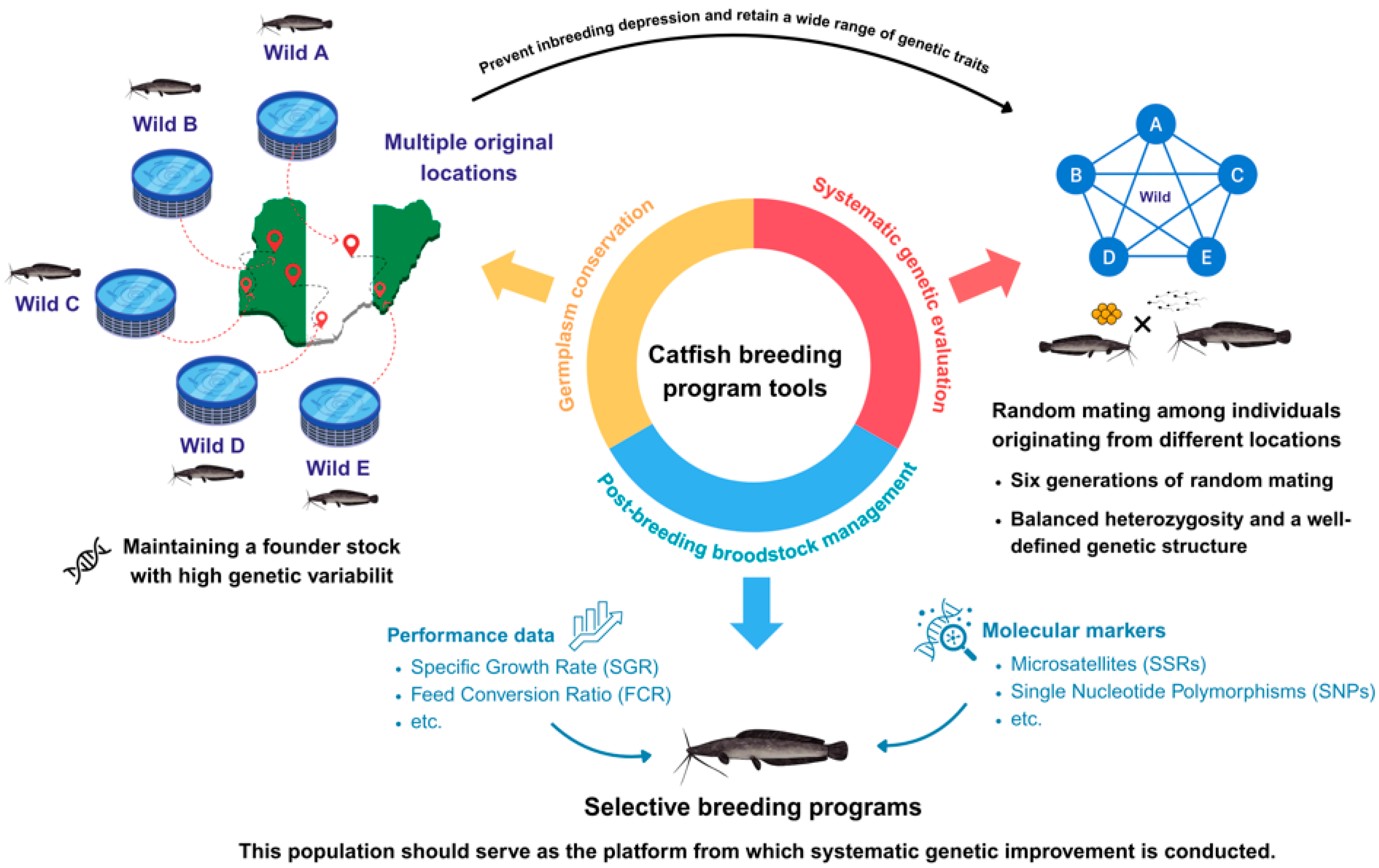
3. Developing a Complete Breeding Program in Nigeria
4. Hatchery and Farming Systems: Current Status, Challenges, and Opportunities
5. Capacity Building and Knowledge Transfer in Nigeria’s Aquaculture Sector
6. Technological Innovation and Progress in Nigeria’s Aquaculture Sector
7. Ecological and Sustainability Issues
8. Export and Market Development
9. Opportunities and Future Outlook
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rocha, C.P.; Cabral, H.N.; Marques, J.C.; Gonçalves, A.M. A global overview of aquaculture food production with a focus on the activity’s development in transitional systems—The case study of a South European Country (Portugal). J. Mar. Sci. Eng. 2022, 10, 417. [Google Scholar] [CrossRef]
- Subasinghe, R.; Siriwardena, S.N.; Byrd, K.; Chan, C.Y.; Dizyee, K.; Shikuku, K.; Tran, N.; Adegoke, A.; Adeleke, M.; Anastasiou, K.; et al. Nigeria Fish Futures: Aquaculture in Nigeria—Increasing Income, Diversifying Diets and Empowering Women; The WorldFish Center: Penang, Malaysia, 2021. [Google Scholar]
- Walakira, J.K.; Hinrichsen, E.; Tarus, V.; Langi, S.; Ibrahim, N.A.; Badmus, O.; Aziz, A.; Baumüller, H. Scaling Aquaculture for Food Security and Employment in Africa: Insights from Egypt, Kenya and Nigeria; ZEF Working Paper No. 223; Center for Development Research, University Bonn: Bonn, Germany, 2023; Available online: https://hdl.handle.net/10419/278612 (accessed on 25 August 2025).
- Bartley, D.M. World Aquaculture 2020—A Brief Overview; FAO Fisheries and Aquaculture Circular No. 1233; FAO: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Dewali, S.; Sharma, N.; Melkani, D.; Arya, M.; Kathayat, N.; Panda, A.K.; Bisht, S.S. Aquaculture: Contributions to global food security. In Emerging Solutions in Sustainable Food and Nutrition Security; Springer: Cham, Switzerland, 2023; pp. 123–139. [Google Scholar] [CrossRef]
- Atawodi, J.C.; Abovegodwin, G.O.; Ngwamah, J.S.; Ugwoke, V.E. A critical review of fish production dynamics and sustainability in Nigeria. Conflu. J. Pure Appl. Sci. 2025, 4, 69–81. [Google Scholar]
- Samuel, O.O. Involvement in Fish Farming and the Wellbeing of Youths in Southwestern Nigeria. Ph.D. Thesis, University of Ibadan, Ibadan, Nigeria, 2021. Available online: http://hdl.handle.net/123456789/1426 (accessed on 25 August 2025).
- Atkins, M.; Byrd, K.A.; Pincus, L.; Naziri, D.; Yossa, R.; Thilsted, S.H. Integrating Fish, Roots, Tubers and Bananas in Food Systems: Opportunities and Constraints; Working Paper: FISH-2020-06; CGIAR Research Program on Fish Agri-Food Systems & CGIAR Research Program on Roots, Tubers and Bananas: Penang, Malaysia, 2020. [Google Scholar]
- Ajayi, O.; Akinrinlola, A.; Usman, A.; Muhammed, A.; Van der Knaap, M. Aquaculture development in Nigeria and FAO’s role. FAO Aquac. Newsl. 2022, 65, 21–24. Available online: https://www.proquest.com/scholarly-journals/aquaculture-development-nigeria-faos-role/docview/2680352926/se-2 (accessed on 25 August 2025).
- Mapfumo, B. Regional Review on Status and Trends in Aquaculture Development in Sub-Saharan Africa–2020. 2022. Available online: https://www.google.com/books?hl=nl&lr=&id=AsNfEAAAQBAJ&oi=fnd&pg=PR3&dq=Regional+Review+on+Status+and+Trends+in+Aquaculture+Development+in+Sub-Saharan+Africa%E2%80%932020&ots=rJR1RFiLxp&sig=0z8bp6hVKPy8uWS4yXQ7MxYAj-s (accessed on 22 August 2025).
- Ogunji, J.; Wuertz, S. Aquaculture development in Nigeria: The second biggest aquaculture producer in Africa. Water 2023, 15, 4224. [Google Scholar] [CrossRef]
- FAO. The State of World Fisheries and Aquaculture 2022: Towards Blue Transformation; Food and Agriculture Organization of the United Nations: Rome, Italy, 2022. [Google Scholar] [CrossRef]
- Olagunju, O.F.; Kristófersson, D.; Tómasson, T.; Kristjánsson, T. Profitability assessment of catfish farming in the Federal Capital Territory of Nigeria. Aquaculture 2022, 555, 738192. [Google Scholar] [CrossRef]
- Akinwole, A.O.; Faturoti, E.O. Biological performance of African catfish (Clarias gariepinus) cultured in recirculating system in Ibadan. Aquac. Eng. 2007, 36, 18–23. [Google Scholar] [CrossRef]
- Isa, S.I. Development of Genetic Improvement in the African Catfish (Clarias gariepinus, Burchell, 1822). 2019. Available online: http://hdl.handle.net/1893/29986 (accessed on 18 August 2025).
- Kafumukachemilu, E. Fish Value Chain Dynamics: Livelihood Opportunities and Challenges for Small-Scale Farmers in Lusaka District. 2021. Available online: http://dspace.unza.zm/handle/123456789/7689 (accessed on 25 August 2025).
- Adeleke, B.; Robertson-Andersson, D.; Moodley, G.; Taylor, S. Aquaculture in Africa: A comparative review of Egypt, Nigeria, and Uganda vis-à-vis South Africa. Rev. Fish. Sci. Aquac. 2020, 29, 167–197. [Google Scholar] [CrossRef]
- Engle, C.; Kumar, G. Ictalurus punctatus (Channel Catfish). CABI Compendium Datasheet. 2023. Available online: https://www.cabidigitallibrary.org/doi/abs/10.1079/cabicompendium.79127 (accessed on 10 September 2025).
- Oluwagbemiga, E.A.; Alabi, J. Nigeria’s mono-cultural economy and the African catfish aquaculture industry. In Proceedings of the Covenant University Conference on e-Governance, Ota, Nigeria, 7–9 June 2017. [Google Scholar]
- Chan, C.Y.; Chu, L.; Tran, N.; Sarı, E.C.; Cheong, K.C.; Olagunju, O.; Shikuku, K.; Byrd, K.; Kanar, D.; Subasinghe, R.; et al. Future of Fish for Food and Nutrition Security in Nigeria; WorldFish Working Paper: 2024-62; WorldFish: Penang, Malaysia, 2024; Available online: https://hdl.handle.net/10568/163446 (accessed on 25 August 2025).
- Olagunju, O.F.; Kristófersson, D.; Tómasson, T.; Kristjánsson, T. Farm strategies and characteristics influencing profitability in Nigerian catfish aquaculture: Lessons on resilience during economic crisis and COVID-type shock. J. World Aquacult. Soc. 2024, 55, e13058. [Google Scholar] [CrossRef]
- FAO. Aquaculture Growth Potential in Western Africa: WAPI Factsheet to Facilitate Evidence-Based Policy Making and Sector Management in Aquaculture; FAO: Rome, Italy, 2023. [Google Scholar]
- FAO. Nigeria Catfish Value Chain Analysis; FISH4ACP Prog.; Food & Agriculture Organization: Rome, Italy, 2021. [Google Scholar]
- Areola, F.O.; Osanyinlusi, O.I.; Alatise, O.M.; Oladele, O.O. Implications of Inbreeding in Clarias Gariepinus Fish Farming: Farmers’ Perspectives in Nigeria. J. Aqua. Mar. Bio Ecol. 2024, 124, 1–5. [Google Scholar] [CrossRef]
- Fregene, B.T.; Bolorunduro, P.; Yossa, R.; Karisa, H.C.; Olaniyi, A.; Ajose, I. Extension manual on production of quality catfish seed. Gates Open Res. 2024, 8, 68. [Google Scholar] [CrossRef]
- Akintayo, I.A. Assessing the Feasibility of a Selective Breeding Programme for African Catfish in Nigeria (Final Project); GRÓ Fisheries Training Programme, UNESCO: Reykjavík, Iceland, 2022; Available online: https://www.grocentre.is/static/gro/publication/1726/document/Akintayo21prf.pdf (accessed on 25 August 2025).
- Sanda, M.K.; Metcalfe, N.B.; Mable, B.K. The potential impact of aquaculture on the genetic diversity and conservation of wild fish in sub-Saharan Africa. Aquat. Conserv. Mar. Freshwater Ecosyst. 2024, 34, e4105. [Google Scholar] [CrossRef]
- Khor, L.; Bodunde, O.A.; Wills, R.; Hanson, L.; Adeyemo, O.K.; Aina, O.O.; Chadag, V.M. Understanding aquaculture biosecurity to improve catfish disease management in Ogun and Delta states, Nigeria. Aquaculture 2024, 584, 740664. [Google Scholar] [CrossRef]
- Ogello, E.; Schindler, L.; Chan, C.Y.; Tran, N.; Obiero, K.; Outa, N.; Muthoka, M.; Kyule, D.; Atieno, V. Exploring Future Scenarios for Advancing Low-Emission Development in Kenyan Aquatic Food Systems; WorldFish Working Paper: 2024-63; WorldFish: Penang, Malaysia, 2024. [Google Scholar]
- Anetekhai, M.A. Catfish Aquaculture Industry Assessment in Nigeria (Tech. Rep.); Lagos State University: Lagos, NA, USA, 2017. [Google Scholar] [CrossRef]
- Olagunju, O.F.; Kristófersson, D.; Kristjánsson, T.; Tómasson, T. Technical efficiency of African catfish production in Nigeria: An analysis involving input quality and COVID-19 effects. Aquac. Econ. Manag. 2023, 28, 1–27. [Google Scholar] [CrossRef]
- Kaleem, O.; Sabi, A.-F.B.S. Overview of aquaculture systems in Egypt and Nigeria: Prospects, potentials, and constraints. Aquacult. Fish. 2021, 6, 535–547. [Google Scholar] [CrossRef]
- ReportLinker. Nigeria Aquaculture Market Report: Trends, Forecasts, and Statistics. 2023. Available online: https://www.reportlinker.com/ (accessed on 25 August 2025).
- FAO. Cultured Aquatic Species Information Programme. Clarias gariepinus; FAO Fisheries Aquacult. Dep.: Rome, Italy, 2024; Available online: https://www.fao.org/fishery/en/culturedspecies/clarias_gariepinus/en (accessed on 25 August 2025).
- Tan, N.D.; Tuyen, V.T.X.; Ha, H.T.N.; Dao, D.T.A. Overview: The value chain of tra catfish in Mekong Delta region, Vietnam. Vietnam J. Chem. 2023, 61, 1–14. [Google Scholar] [CrossRef]
- Cocker, L.M. Strategic Review on African Aquaculture Markets and Export Potential; Partnership for African Fisheries (PAF) Aquaculture Working Group, University of Stirling/NEPAD Report; Institute of Aquaculture, University of Stirling: Stirling, UK, 2014. [Google Scholar]
- Adeyeye, S.A.O. Traditional fish processing in Nigeria: A critical review. Nutr. Food Sci. 2016, 46, 321–335. [Google Scholar] [CrossRef]
- Clearinghouse, T.A.A.T. Aquaculture Technology Toolkit Catalogue; Clearinghouse Technical Report Series No. 012; Technologies for African Agricultural Transformation, Clearinghouse Office, IITA: Nairobi, Kenya, 2022. [Google Scholar]
- Adebayor, F.T. A survey of fish sold in Liverpool market in Lagos. Afr. J. Trop. Agric. 2014, 2, 93–97. [Google Scholar]
- Akintola, S.L.; Fakoya, K.A. Small-scale fisheries in the context of traditional post-harvest practice and the quest for food and nutritional security in Nigeria. Agric. Food Secur. 2017, 6, 34. [Google Scholar] [CrossRef]
- Abdullahi, S.A.; Shuaibu, S. Marketing of smoked fish in Kano State, Nigeria. Bayero J. Pure Appl. Sci. 2013, 6, 120–125. [Google Scholar]
- Ewumi, A.F.; Rahman, M.A.; Sarfo, I.; Darko, G.; Olowe, O.S. Catfish farming: A sustainability study at Eriwe fish farming village in southwest Nigeria. Aquacult. Int. 2021, 29, 1619–1631. [Google Scholar] [CrossRef]
- Osundare, F.; Adedeji, T. Economic analysis of market performance of fresh fish in Lagos State, Nigeria. Int. J. Environ. Agric. Biotechnol. 2018, 3, 594–599. [Google Scholar] [CrossRef]
- Sobczak, M.; Panicz, R.; Sadowski, J.; Półgęsek, M.; Żochowska-Kujawska, J. Does production of Clarias gariepinus × Heterobranchus longifilis hybrids influence quality attributes of fillets? Foods 2022, 11, 2074. [Google Scholar] [CrossRef]
- Abit, L.Y.; Mojilis, M.; Latif, K.; Al-Asif, A. Successful hybridization between Clarias microstomus ♂ and Clarias gariepinus ♀. AACL Bioflux 2023, 16, 3285–3295. [Google Scholar]
- Rahman, M.A.; Lee, S.G.; Yusoff, F.M.; Rafiquzzaman, S.M. Hybridization and its application in aquaculture. In Sex Control in Aquaculture; Wiley: Hoboken, NJ, USA, 2018; pp. 163–178. [Google Scholar] [CrossRef]
- Adewumi, A.A.; Olaleye, V.F. Catfish culture in Nigeria: Progress, prospects and problems. Afr. J. Agric. Res. 2011, 6, 1281–1285. [Google Scholar] [CrossRef]
- Ayele, T.A. Growth Performance and Survival Rate of African Catfish Larvae Clarias gariepinus (Burchell 1822) Fed on Different Types of Live and Formulated Feeds. Master’s Thesis, University of Natural Resources and Life Sciences, Vienna, Austria, 2015. [Google Scholar]
- Barasa, J.E.; Ouma, D.F. Towards sustainability in seed supply for African catfish, Clarias gariepinus (Burchell, 1822) culture in Kenya: Lessons from the Asian catfish industry. Aquac. Res. 2024, 1341858. [Google Scholar] [CrossRef]
- Uche, C.G.; Aga, T.; Ulasi, G.F. Review on status of African catfish aquaculture in Nigeria. Int. J. Latest Technol. Eng. Manag. Appl. Sci. 2023, 12, 102–111. [Google Scholar] [CrossRef]
- Oman, S.; Kapaipi, M.; Hodzic, H.; Megahed, M. The Catfish Aquaculture Value Chain in Nigeria: Analysis and Design Report; Food and Agriculture Organization of the United Nations (FAO): Rome, Italy, 2023. [Google Scholar]
- Oyebola, O.O.; Olatunde, O.M. Climate change adaptation through aquaculture: Ecological considerations and regulatory requirements for tropical Africa. In Agriculture and Ecosystem Resilience in Sub-Saharan Africa; Bamutaze, Y., Kyamanywa, S., Singh, B., Nabanoga, G., Lal, R., Eds.; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar] [CrossRef]
- Collins, R.; Dent, B. A Manual for Agribusiness Value Chain Analysis in Developing Countries; Tech. Rep. No. 20210424166; CABI: Wallingford, UK, 2021. [Google Scholar]
- Oboh, A. Diversification of farmed fish species: A means to increase aquaculture production in Nigeria. Rev. Aquacult. 2022, 14, 2089–2098. [Google Scholar] [CrossRef]
- Adesina, S.A.; Agbatan, O.D. Growth response and feed utilization in Clarias gariepinus fingerlings fed diets supplemented with processed flamboyant (Delonix regia) leaf meal. Agro-Sci. J. Trop. Agric. Food Environ. Ext. 2021, 20, 38–45. [Google Scholar] [CrossRef]
- Hasan, M.R.; New, M.B. On-Farm Feeding and Feed Management in Aquaculture. FAO Fisheries and Aquaculture Technical Paper, (583), I, III, IV, VIII, IX, X, 1, 3-67. 2013. Available online: https://www.proquest.com/scholarly-journals/on-farm-feeding-feed-management-aquaculture/docview/1496068411/se-2 (accessed on 25 August 2025).
- Sanda, M.K.; Metcalfe, N.B.; Capstick, M.; Mable, B.K. Genetic diversity, population structure and differentiation of farmed and wild African catfish (Clarias gariepinus) in Nigeria. bioRxiv. 2024. [Google Scholar] [CrossRef]
- Akin-Obasola, B.J.; Fapohunda, O.O.; Ogunlade, A.O. Comparative study of growth performance, nutritional composition and gonadal development of Heteroclarias cultured in concrete and earthen ponds. Ekiti State Univ. J. Sci. Technol. 2022, 7, 49–56. [Google Scholar]
- Ayanwale, A.V.; Mohammed, Y.M.; Adama, S.B.; Ajayi, T.R. Different stocking density levels on some growth parameters and survival of Heteroclarias fingerlings reared under laboratory conditions in Minna, Niger State. Dutse J. Pure Appl. Sci. 2020, 6, 69–78. [Google Scholar]
- Oluwashina, M.M.; Solomon, R.J. Comparative study of feed utilization and growth performance in Heteroclarias. Research 2012, 4, 45–54. [Google Scholar]
- Ajah, P.O.; Edeghe, A.I.; Enin, U.I. Growth of Clarias gariepinus reared in earthen ponds in Calabar, South-South, Nigeria under duo nutritional diet. J. Aquac. Fish. 2022, 6, 2. [Google Scholar] [CrossRef]
- Owodeinde, F.G.; Fakoya, K.; Anetekhai, M.A. Growth performance of hybrid catfish (Clarias gariepinus × Heterobranchus bidorsalis) in earthen ponds. Asian J. Biol. Sci. 2012, 5, 192–200. [Google Scholar] [CrossRef]
- FAO. Fishery and Aquaculture Country Profiles: Nigeria 2024; Country Profile Fact Sheet; Fisheries and Aquaculture Division: Rome, Italy, 2025; Available online: https://www.fao.org/fishery/en/facp/nga (accessed on 21 July 2025).
- Olutumise, A.I.; Adene, I.C.; Ajibefun, A.I.; Amos, T.T. Adoption of improved technologies and profitability of the catfish processors in Ondo State, Nigeria: A Cragg’s double-hurdle model approach. Sci. Afr. 2020, 10, e00576. [Google Scholar] [CrossRef]
- FAO. Report of the FAO Expert Workshop on the Use of Wild Fish and/or Other Aquatic Species as Feed in Aquaculture and Its Implications to Food Security and Poverty Alleviation; FAO Fish. Rep. No. 867; FAO: Rome, Italy, 2008. [Google Scholar]
- Negari, B.; Yusuf, Y.; Hundie, D.; Ameha, N.; Kebede, K.; Abrar, B.; Diba, D. A comparison of growth performance, feed intake, and feed efficiency of broiler chickens fed on commercial and farm-formulated diets. East Afr. J. Vet. Anim. Sci. 2024, 8, 27–34. [Google Scholar] [CrossRef]
- Ossai, N.I.; Eneje, V.O.; Andong, F.A.; Enyi, K.M.; Nnachetam, R.C.; Orji, E.A.; Eyo, J.E. Studies on the genetic improvement of the African catfish (Clarias gariepinus Burchell, 1822) by triploidy. Anim. Res. Int. 2024, 21, 5503–5517. [Google Scholar]
- Project Championz. Survival and growth rate of Heterobranchus longifilis induced with Ovaprim and pituitary extract of H. longifilis. 2017. Available online: https://projectchampionz.com.ng/ (accessed on 24 August 2025).
- Kwikiriza, G.; Tebitendwa, S.M.; Rwezawula, P.; Mwanja, W.W.; Abaho, I.; Meimberg, H. Enhancing African catfish (Clarias gariepinus) aquaculture in Uganda: Insights into hatchery propagation, population suitability, and broodstock management. Fishes 2025, 10, 290. [Google Scholar] [CrossRef]
- Lind, C.E.; Ponzoni, R.W.; Nguyen, N.H.; Khaw, H.L. Selective breeding in fish and conservation of genetic resources for aquaculture. Reprod. Domest. Anim. 2012, 47, 255–263. [Google Scholar] [CrossRef]
- Hilsdorf, A.W.; Hallerman, E.M. Genetic Resources of Neotropical Fishes; Springer: Cham, Switzerland, 2017. [Google Scholar] [CrossRef]
- Wachirachaikarn, A.; Na-Nakorn, U. Genetic diversity of the North African catfish, Clarias gariepinus (Burchell, 1822) hatchery stocks in Thailand. ScienceAsia 2019, 45, 301–308. [Google Scholar] [CrossRef]
- Sankaran, G.B.; Mandal, A. Genetic improvements in aquaculture. Trout J. Atatürk Univ. 2024, 2, 16–25. [Google Scholar] [CrossRef]
- Casillas, S.; Barbadilla, A. Molecular population genetics. Genetics 2017, 205, 1003–1035. [Google Scholar] [CrossRef] [PubMed]
- Myers, J.N. Analysis of Gamete Interactions, Maternal, and Paternal Effects for Improving Hybrid CATFISH Aquaculture (Order No. 30263049). 2019. Available from ProQuest One Academic. (2779137434). Available online: https://www.proquest.com/dissertations-theses/analysis-gamete-interactions-maternal-paternal/docview/2779137434/se-2 (accessed on 25 August 2025).
- Collard, B.C.; Mackill, D.J. Marker-assisted selection: An approach for precision plant breeding in the twenty-first century. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2008, 363, 557–572. [Google Scholar] [CrossRef] [PubMed]
- Yue, G.H. Recent advances of genome mapping and marker-assisted selection in aquaculture. Fish Fish. 2014, 15, 376–396. [Google Scholar] [CrossRef]
- Ponzoni, R.W.; Nguyen, N.H. (Eds.) Proceedings of a Workshop on the Development of a Genetic Improvement Program for African Catfish Clarias gariepinus; WorldFish Center Conf. Proc. No. 1889; The WorldFish Center: Penang, Malaysia, 2008; Available online: https://cabidigitallibrary.org/terms-and-conditions (accessed on 24 August 2025).
- Chattopadhyay, N.R. Induced Fish Breeding: A Practical Guide for Hatcheries; Academic Press: Cambridge, MA, USA, 2016. [Google Scholar]
- Sucipto, A.; Muhammad, R. Seed Production of Catfish in Biofloc; Akuatika Indonesia Raya: West Java, Indonesia, 2024. [Google Scholar]
- Ingram, B.A.; Nguyen, T.T.T. Broodstock management and breeding in relation to culture-based fisheries. In Proceedings of the Regional Consultation on Culture-Based Fisheries Development in Asia, Siem Reap, Cambodia, 21–23 October 2014. [Google Scholar]
- Coogan, M.P. Genetic Technologies for Growth Enhancement in Catfish. Ph.D. Thesis, Auburn University, Auburn, Alabama, 2021. [Google Scholar]
- Patta, C.; Panthum, T.; Thatukan, C.; Wongloet, W.; Chalermwong, P.; Wattanadilokchatkun, P. Questioning inbreeding: Could outbreeding affect productivity in the North African catfish in Thailand? PLoS ONE 2024, 19, e0302584. [Google Scholar] [CrossRef]
- Senanan, W.; Kapuscinski, A.R.; Na-Nakorn, U.; Miller, L.M. Genetic impacts of hybrid catfish farming (Clarias macrocephalus × C. gariepinus) on native catfish populations in central Thailand. Aquaculture 2004, 235, 167–184. [Google Scholar] [CrossRef]
- Kwikiriza, G.; Abaho, I.; Tibihika, P.D.; Izaara, A.A.; Atukwatse, F.; Omara, T.; Nattabi, J.K.; Kasozi, N.; Curto, M.; Melcher, A.; et al. Genetic Diversity and Population Differentiation of Farmed Nile Tilapia (Oreochromis niloticus Linnaeus, 1758) to Advance Selective Breeding in Uganda. Diversity 2025, 17, 128. [Google Scholar] [CrossRef]
- Bassey, H. Phenotypic and Genetic Evaluation of Farmed and Wild Clarias Gariepinus Broodstocks in Nigeria (Final Project Report). UNESCO GRÓ—Fisheries Training Programme, Iceland. 2020. Available online: https://www.grocentre.is/static/gro/publication/697/document/Happiness19prf.pdf (accessed on 23 August 2025).
- Oyebola, O.O. Assessment of Genetic Structure of Clarias gariepinus (Burchell, 1822) Population in Asejire Lake. Ph.D. Thesis, University of Ibadan, Ibadan, Nigeria, 2014. [Google Scholar]
- Ashley-Dejo, S.; Adelaja, O.A. Economics of catfish hatchery farmers and its contribution to household poverty alleviation in Nigeria. Agric. Trop. Subtrop. 2022, 55, 19–29. [Google Scholar] [CrossRef]
- Ibiwoye, Y.E.; Thorarensen, H. Assessment of broodstock management practices in Nigeria. Int. J. Innov. Res. Dev. 2018, 7, 198–205. [Google Scholar] [CrossRef]
- Teka, M. Factors of Fish Farming Productivity and Sustainability; the Case of Africa Sustainable Aquaculture bv Ethiopian Branch. Ph.D. Thesis, St. Mary’s University, San Antonio, TX, USA, 2020. Available online: http://www.repository.smuc.edu.et/bitstream/123456789/5540/1/MEKDES%20TEKA.pdf (accessed on 25 August 2025).
- Jordan, N.R.; Slotterback, C.S.; Cadieux, K.V.; Mulla, D.J.; Pitt, D.G.; Olabisi, L.S.; Kim, J.O. TMDL implementation in agricultural landscapes: A communicative and systemic approach. Environ. Manag. 2011, 48, 1–12. [Google Scholar] [CrossRef]
- Olesen, I.; Gjedrem, T.; Bentsen, H.Á.; Gjerde, B.; Rye, M. Breeding programs for sustainable aquaculture. J. Appl. Aquac. 2023, 13, 179–204. [Google Scholar] [CrossRef]
- Ansa, E.J. Challenges and Production Process of Catfish Hatcheries in the Niger Delta Region of Nigeria; Technical Report No. DAIvbg_1_WGA; Development Alternatives Inc.: Bethesda, MD, USA, 2014. [Google Scholar] [CrossRef]
- Mbokane, E.M.; Mbokane, L.M.; Motimele, S.S.; Hlophe-Ginindza, S.N. Successes and challenges of catfish farming in the small-scale industry in Southern Africa. In Catfish: Advances, Technology, Experiments; Atamanalp, M., Ed.; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Katampe, B. The contribution of aquaculture to poverty alleviation/food security among the rural poor in the Federal Capital Territory (FCT) Abuja, Nigeria. Ph.D Thesis, University of Northampton, Northampton, UK, 2023. [Google Scholar]
- Oyieng, P.E. Characterization of Smallholder Aquaculture Systems and Growth Performance of the African Catfish (Clarias gariepinus) in High Altitude Areas of Kenya. Ph.D. Thesis, Egerton University, Nakuru, Kenya, 2014. [Google Scholar]
- Assefa, A.; Abunna, F. Maintenance of fish health in aquaculture: Review of epidemiological approaches for prevention and control of infectious disease of fish. Vet. Med. Int. 2018, 2018, 5432497. [Google Scholar] [CrossRef]
- Dimelu, M.; Ifeonu, C.F.; Asaduchie, A.N.; Ayogu, B.J. Challenges of disease management in small scale fish farms in Lagos State, Nigeria. J. Agric. Ext. 2018, 22, 28–41. [Google Scholar]
- Mukaila, R.; Ukwuaba, I.C.; Umaru, I.I. Economic impact of disease on small-scale catfish farms in Nigeria. Aquaculture 2023, 575, 739773. [Google Scholar] [CrossRef]
- Akpalu, W.; Nnaemeka, C. A comparative assessment of economic, social, and environmental performance of tilapia aquaculture in Nigeria and Ghana. Aquac. Econ. Manag. 2025, 29, 159–179. [Google Scholar] [CrossRef]
- Waite, R.; Beveridge, M.; Brummett, R.; Castine, S.; Chaiyawannakarn, N.; Kaushik, S.; Mungkung, R.; Nawapakpilai, S.U.P.A.W.A.T.; Phillips, M.I.C.H.A.E.L. Improving Productivity and Environmental Performance of Aquaculture; WorldFish: Penang, Malaysia, 2014. [Google Scholar]
- Audu, R.; Yola, I.A. Contemporary issues in fisheries and aquaculture: A review on non–conventional feed ingredients for fish feed in Nigeria. Bayero J. Pure Appl. Sci. 2021, 13, 22–28. [Google Scholar] [CrossRef]
- Oguguah, N.M.; Eyo, J.E. Finfish feed technology in Nigeria. J. Res. Biosci. 2007, 3, 1–17. [Google Scholar]
- Piate, R.C.; Orok, U.N. Fish farming: Assessing the process and economic benefits of establishing subsistent and commercial farming. KING-UK Int. J. Acad. Anthol. 2024, 8, 1–18. [Google Scholar]
- Menezes, A.; Ligeon, C.; Murekezi, P.; Jolly, C. Diagnosis of Aquaculture Employment Governance in Selected African Countries; FAO Fisheries and Aquaculture Technical Paper, No. 715; FAO: Rome, Italy, 2024. [Google Scholar] [CrossRef]
- Digun-Aweto, O.; Oladele, A.H. Constraints to adoption of improved hatchery management practices among catfish farmers in Lagos State. J. Cent. Eur. Agric. 2017, 18, 841–850. [Google Scholar] [CrossRef]
- Joscelyne, M. The Influence of Aquahatch on the Growth Performance of Larval and Juvenile African Catfish (Clarias gariepinus, Burchell 1822). 2020. Available online: http://hdl.handle.net/10019.1/107968 (accessed on 25 August 2025).
- Liverpool-Tasie, L.S.O.; Wineman, A.; Amadi, M.U.; Gona, A.; Emenekwe, C.C.; Fang, M.; Belton, B. Rapid transformation in aquatic food value chains in three Nigerian states. Front. Aquacult. 2024, 3, 1302100. [Google Scholar] [CrossRef]
- Mbiuki, W.N. Relationship Between Fish Farming Enterprise Productivity Training Programme and Adoption of Inland-Based Pond Fish Farming in Meru South Sub-County, Kenya. Ph.D. Thesis, Egerton University, Njoro, Kenya, 2019. [Google Scholar]
- Aly, S.M.; Fathi, M. Advancing aquaculture biosecurity: A scientometric analysis and future outlook for disease prevention and environmental sustainability. Aquacult. Int. 2024, 32, 8763–8789. [Google Scholar] [CrossRef]
- Anikwe, S.O.; Unachukwu, L.C.; Onah, F.N. Human capacity development and sustainable growth in the blue economy: Opportunities, challenges, and strategies for Nigeria. Afr. Bank. Finance Rev. J. 2024, 15, 202–212. [Google Scholar]
- Kaminski, A.M.; Kruijssen, F.; Cole, S.M.; Beveridge, M.C.; Dawson, C.; Mohan, C.V.; Suri, S.; Karim, M.; Chen, O.L.; Phillips, M.J.; et al. A review of inclusive business models and their application in aquaculture development. Rev. Aquacult. 2020, 12, 1881–1902. [Google Scholar] [CrossRef]
- Ababouch, L.; Nguyen, K.A.T.; Castro de Souza, M.; Fernández-Polanco, J. Value chains and market access for aquaculture products. J. World Aquac. Soc. 2023, 54, 527–553. [Google Scholar] [CrossRef]
- Ateweberhan, M.; Hudson, J.; Rougier, A.; Jiddawi, N.S.; Msuya, F.E.; Stead, S.M.; Harris, A. Community-based aquaculture in the western Indian Ocean: Challenges and opportunities for developing sustainable coastal livelihoods. Ecol. Soc. 2018, 23, 34. [Google Scholar] [CrossRef]
- Hasan, M.R.; Bueno, P.B.; Corner, R.A. Strengthening, empowering and sustaining small-scale aquaculture farmers’ associations. FAO Fish. Aquacult. Tech. Pap. 2020, 655, I-181. [Google Scholar] [CrossRef]
- Lakra, W.S.; Krishnani, K.K. Circular bioeconomy for stress-resilient fisheries and aquaculture. In Biomass, Biofuels, Biochemicals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 481–516. [Google Scholar] [CrossRef]
- Critchley, A.T.; Molloy, F.J.; van Harmelen, J.D.; Mshigeni, K.E. A strategy for appropriate technology for development in Namibia–recent University of Namibia initiatives: Selected examples and call for collaboration. Pop.–Dev.–Environ. Namibia. 2000, p. 203. Available online: https://core.ac.uk/download/pdf/33897684.pdf#page=220 (accessed on 25 August 2025).
- Hoang, T. The Contribution of Tertiary Education to Sustainable Economic Development: Evidence from the Seafood Industry in Vietnam. 2023. Available online: https://researchers.cdu.edu.au/files/99475868/Thesis_CDU_Hoang_T_H.pdf (accessed on 23 August 2025).
- Brugère, C.; Aguilar-Manjarrez, J.; Beveridge, M.C.M.; Soto, D. The ecosystem approach to aquaculture 10 years on—A critical review and consideration of its future role in blue growth. Rev. Aquacult. 2019, 11, 493–514. [Google Scholar] [CrossRef]
- Jolly, C.M.; Nyandat, B.; Yang, Z.; Ridler, N.; Matias, F.; Zhang, Z.; Menezes, A. Dynamics of aquaculture governance. J. World Aquacult. Soc. 2023, 54, 427–481. [Google Scholar] [CrossRef]
- Baskaran, A.; Chandran, V.G.R.; Ng, B.K. Inclusive entrepreneurship, innovation and sustainable growth: Role of business incubators, academia and social enterprises in Asia. Sci. Technol. Soc. 2019, 24, 385–400. [Google Scholar] [CrossRef]
- Ohia, C.M.D. Aquaculture technologies and practices: Balancing innovation, environment and economy for sustainability. In Food Security, Nutrition and Sustainability Through Aquaculture Technologies; Springer: Cham, Switzerland, 2025; pp. 417–424. [Google Scholar] [CrossRef]
- Ahmed, N.; Turchini, G.M. Recirculating aquaculture systems (RAS): Environmental solution and climate change adaptation. J. Clean. Prod. 2021, 297, 126604. [Google Scholar] [CrossRef]
- Badiola, M.; Basurko, O.C.; Piedrahita, R.; Hundley, P.; Mendiola, D. Energy use in Recirculating Aquaculture Systems (RAS): A review. Aquac. Eng. 2018, 81, 57–70. [Google Scholar] [CrossRef]
- Colombo, S.M.; Roy, K.; Mraz, J.; Wan, A.H.; Davies, S.J.; Tibbetts, S.M.; Turchini, G.M. Towards achieving circularity and sustainability in feeds for farmed blue foods. Rev. Aquacult. 2023, 15, 1115–1141. [Google Scholar] [CrossRef]
- Chen, J.; Wang, Z.; Shen, X.; Chen, R.; Peng, Y.; Cai, Y.; Ying, H. Solid-state fermentation through synthetic microbiome: An effective strategy for converting Chinese distillers’ grains into functional protein feed. Int. J. Food Microbiol. 2025, 435, 111154. [Google Scholar] [CrossRef] [PubMed]
- Hoseinifar, S.H.; Faheem, M.; Liaqat, I.; Van Doan, H.; Ghosh, K.; Ringø, E. Promising probiotic candidates for sustainable aquaculture: An updated review. Animals 2024, 14, 3644. [Google Scholar] [CrossRef] [PubMed]
- Jiang, H.; Wang, J.; Jiang, W.; Wang, J.; Gu, X.; Feng, X.; Fang, J. Simultaneous detection of Vibrio parahaemolyticus and antimicrobial resistance genes using immunomagnetic separation combined with RPA-microfluidic method in seafood. Food Cont. 2024, 165, 110653. [Google Scholar] [CrossRef]
- Intarakumnerd, P.; Chairatana, P.A.; Kamondetdacha, R. Innovation system of the seafood industry in Thailand. Asian J. Technol. Innov. 2015, 23, 271–287. [Google Scholar] [CrossRef]
- Tongmee, B.; Unpaprom, Y.; Ramaraj, R.; Mengumphan, K.; Amornlerdpison, D. Sustainability innovation and circular economy of freshwater hybrid catfish oil extraction. Maejo Int. J. Energy Environ. Commun. 2023, 5, 35–40. [Google Scholar] [CrossRef]
- Arulingam, I.; Nigussie, L.; Senaratna Sellamuttu, S.; Debevec, L. Youth Participation in Small-Scale Fisheries, Aquaculture and Value Chains in Africa and the Asia-Pacific; CGIAR Research Program on Fish Agri-Food Systems: Penang, Malaysia, 2019. [Google Scholar]
- Janssen, K.; Saatkamp, H.; Komen, H. Cost-benefit analysis of aquaculture breeding programs. Genet. Sel. Evol. 2018, 50, 2. [Google Scholar] [CrossRef]
- Elisha, O.D.; Felix, M.J. Destruction of coastal ecosystems and the vicious cycle of poverty in Niger Delta region. J. Glob. Agric. Ecol. 2021, 11, 7–24. [Google Scholar]
- Emmanuel, E.I.; Asuquo, I.A.E.; Abiaobo, N.O. Threatened and endangered fish species in Nigeria, a menace to biodiversity—A review. Afr. J. Educ. Sci. Technol. 2016, 3, 12–26. [Google Scholar]
- Okonkwo, C.N.P.; Kumar, L.; Taylor, S. The Niger Delta wetland ecosystem: What threatens it and why should we protect it? Afr. J. Environ. Sci. Technol. 2015, 9, 451–463. [Google Scholar] [CrossRef]
- Popoola, O.M. Fish production and biodiversity conservation: An interplay for life sustenance. In Biodiversity in Africa: Potentials, Threats and Conservation; Chibueze Izah, S., Ed.; Springer: Berlin/Heidelberg, Germany, 2022; Volume 29. [Google Scholar] [CrossRef]
- Nyakeya, K.; Masese, F.O.; Gichana, Z.; Nyamora, J.M.; Getabu, A.; Onchieku, J.; Nyakwama, R. Cage farming in the environmental mix of Lake Victoria: An analysis of its status, potential environmental and ecological effects, and a call for sustainability. Aquat. Ecosyst. Health Manag. 2022, 25, 37–52. [Google Scholar] [CrossRef]
- Tiwari, A.K.; Pal, D.B. Nutrients contamination and eutrophication in the river ecosystem. In Ecological Significance of River Ecosystems; Elsevier: Amsterdam, The Netherlands, 2022; pp. 203–216. [Google Scholar] [CrossRef]
- Aransiola, S.A.; Zobeashia, S.L.T.; Ikhumetse, A.A.; Musa, O.I.; Abioye, O.P.; Ijah, U.J.J.; Maddela, N.R. Niger Delta mangrove ecosystem: Biodiversity, past and present pollution, threat and mitigation. Reg. Stud. Mar. Sci. 2024, 75, 103568. [Google Scholar] [CrossRef]
- Modeel, S.; Dolkar, P.; Siwach, S.; Yadav, P.; Negi, R.K. Role of science and technology for sustainable aquaculture development and aquatic ecosystem management. In Role of Science and Technology for Sustainable Future: Volume 1: Sustainable Development: A Primary Goal; Springer: Singapore, 2024; pp. 277–301. [Google Scholar]
- Choudhary, B.; Deb, S.; Chouhan, N.; Azmeera, S.; Choudhary, V. Integrated multi-trophic aquaculture (IMTA) for wastewater treatment and resource recovery: A sustainable approach. In Nature-Based Technologies for Wastewater Treatment and Bioenergy Production; IWA Publishing: London, UK, 2025; p. 77. [Google Scholar] [CrossRef]
- Ghosh, A.K.; Hasanuzzaman, A.F.M.; Islam, S.S.; Sarower, M.G.; Mistry, S.K.; Arafat, S.T.; Huq, K.A. Integrated multi-trophic aquaculture (IMTA): Enhancing growth, production, immunological responses, and environmental management in aquaculture. Aquacult. Int. 2025, 33, 336. [Google Scholar] [CrossRef]
- Akindele, E.O.; Ekwemuka, M.C.; Apeverga, P.; Amusa, T.O.; Olajuyigbe, S.; Coker, O.M.; Kolawole-Daniels, A. Assessing awareness on biodiversity conservation among Nigerians: The Aichi Biodiversity Target 1. Biodivers. Conserv. 2021, 30, 1947–1970. [Google Scholar] [CrossRef]
- Domician, C.L. Improving Traceability to Achieve Sustainable Development and Commercial Scaling-Up of Fisheries Resources in Tanzania. Ph.D. Thesis, University Reading, Reading, UK, 2024. [Google Scholar] [CrossRef]
- Saha, C.K. Emergence and evolution of aquaculture sustainability certification schemes. Mar. Policy 2022, 143, 105196. [Google Scholar] [CrossRef]
- Yeşilsu, A.F.; Alak, G.; Alp Erbay, E.; Sağdıç, O.; Özoğul, F.; Demirkesen, İ.; Aydın, İ. Sustainable horizons: A review on sustainable processing, quality enhancement, and safety assurance in aquatic food products. Food Rev. Int. 2025, 41, 1709–1737. [Google Scholar] [CrossRef]
- Kitinoja, L.; Tokala, V.Y.; Mohammed, M.; Mahajan, B.V.C. Cold chain and its importance—Current global status. In Cold Chain Management for the Fresh Produce Industry in the Developing World; CRC Press: Boca Raton, FL, USA, 2021; pp. 3–17. [Google Scholar]
- Khan, M.A.; Hossain, M.E.; Shahaab, A.; Khan, I. ShrimpChain: A blockchain-based transparent and traceable framework to enhance the export potentiality of Bangladeshi shrimp. Smart Agric. Technol. 2022, 2, 100041. [Google Scholar] [CrossRef]
- Ismail, S.; Reza, H.; Salameh, K.; Kashani Zadeh, H.; Vasefi, F. Toward an intelligent blockchain IoT-enabled fish supply chain: A review and conceptual framework. Sensors 2023, 23, 5136. [Google Scholar] [CrossRef]
- Williams, M.J.; Agbayani, R.; Bhujel, R.; Bondad-Reantaso, M.G.; Brugère, C.; Choo, P.S.; Dhont, J.; Galmiche-Tejeda, A.; Ghulam, K.; Kusakabe, K.; et al. Sustaining aquaculture by developing human capacity and enhancing opportunities for women. In Farming the waters for people and food: Proceedings of the Global Conference on Aquaculture 2010, Phuket, Thailand, 22–25 September 2010; Subasinghe, R.P., Arthur, J.R., Bartley, D.M., De Silva, S.S., Halwart, M., Hishamunda, N., Mohan, C.V., Sorgeloos, P., Eds.; FAO & NACA: Rome, Italy, 2012; pp. 785–874. [Google Scholar]
- Barabaschi, D.; Tondelli, A.; Desiderio, F.; Volante, A.; Vaccino, P.; Valè, G.; Cattivelli, L. Next generation breeding. Plant Sci. 2016, 242, 3–13. [Google Scholar] [CrossRef]
- Elmessery, W.M.; Abdallah, S.E.; Oraiath, A.A.T. A deep deterministic policy gradient approach for optimizing feeding rates and water quality management in recirculating aquaculture systems. Aquacult. Int. 2025, 33, 253. [Google Scholar] [CrossRef]
- Lal, J.; Vaishnav, A.; Deb, S.; Gautam, P.; Pavankalyan, M.; Kumari, K.; Verma, D.K. Re-circulatory aquaculture systems: A pathway to sustainable fish farming. Arch. Curr. Res. Int. 2024, 24, 799–810. [Google Scholar] [CrossRef]
- Turlybek, N.; Nurbekova, Z.; Mukhamejanova, A.; Baimurzina, B.; Kulatayeva, M.; Aubakirova, K.M.; Alikulov, Z. Sustainable aquaculture systems and their impact on fish nutritional quality. Fishes 2025, 10, 206. [Google Scholar] [CrossRef]
- Boyd, C.E.; McNevin, A.A. An early assessment of the effectiveness of aquaculture certification and standards. Roles Limit. Certif. 2012, 35, 168–202. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Srikulnath, K.; Panthum, T.; Singchat, W.; Chaiyes, A.; Prasanpan, J.; Uno, U.; Edem, U.; Obidiegwu, J.E. Addressing Catfish (Clarias spp.) Supply Gap in Nigeria: A Perspective on Strategies for Sustainable Aquaculture Growth. Sustainability 2025, 17, 9645. https://doi.org/10.3390/su17219645
Srikulnath K, Panthum T, Singchat W, Chaiyes A, Prasanpan J, Uno U, Edem U, Obidiegwu JE. Addressing Catfish (Clarias spp.) Supply Gap in Nigeria: A Perspective on Strategies for Sustainable Aquaculture Growth. Sustainability. 2025; 17(21):9645. https://doi.org/10.3390/su17219645
Chicago/Turabian StyleSrikulnath, Kornsorn, Thitipong Panthum, Worapong Singchat, Aingorn Chaiyes, Jiraboon Prasanpan, Ukam Uno, Uduak Edem, and Jude Ejikeme Obidiegwu. 2025. "Addressing Catfish (Clarias spp.) Supply Gap in Nigeria: A Perspective on Strategies for Sustainable Aquaculture Growth" Sustainability 17, no. 21: 9645. https://doi.org/10.3390/su17219645
APA StyleSrikulnath, K., Panthum, T., Singchat, W., Chaiyes, A., Prasanpan, J., Uno, U., Edem, U., & Obidiegwu, J. E. (2025). Addressing Catfish (Clarias spp.) Supply Gap in Nigeria: A Perspective on Strategies for Sustainable Aquaculture Growth. Sustainability, 17(21), 9645. https://doi.org/10.3390/su17219645





