Abstract
Trials were carried out to investigate the effects of light and temperature on C. rotundus seeds and tubers under two conditions: (i) in vitro and (ii) after sowing in soil. In the latter, seedling emergence was evaluated after sowing at increasing depths in different soil textures. While dormancy was evident in over 50% of the seeds, which also required light for germination, in contrast, tubers showed a significantly shorter period of dormancy that was independent of light. Seed burial strongly hindered seedling emergence, showing an “active” seed bank only in the shallowest soil layer (few mm). In contrast, tubers showed a marked ability to emerge from a depth exceeding 40 cm. Emergence capacity was found to be proportional to the size of the tubers, attributable to the greater energy reserves needed during autotrophic pre-emergence growth. Seedling emergence from both seeds and tubers, sown at increasing depths, was inhibited to a greater extent in a clay soil texture. A lower inhibitory effect was reported for sandy soils. Tuber vitality was significantly reduced or eliminated within a few days from progressive drying following exposure to solar rays during summer periods. Finally, the data were discussed within the context of planning the agronomic management of C. rotundus, in terms of soil tillage modalities, to ensure sustainable control of this strongly invasive and persistent weed.
1. Introduction
Cyperus rotundus has long been considered one of the most problematic weeds worldwide [1]. In various countries, this species has been extensively cited as a serious, principal or common weed of agricultural and horticultural cropping systems in tropical, subtropical and temperate areas of the world. It has been reported to cause 20–90% yield losses in various crops across the world [2]. C. rotundus is a perennial species of the botanical family Cyperaceae belonging to the order Graminoids. Its aggressiveness arises from the ability to form tubers with energy-rich substances capable of giving rise to a rapid and cyclical re-colonization of the agroecosystem [3]. These tubers, in addition to being highly vigorous, are characterized by a dense vertical arrangement (about 0–40 cm soil depth), with a strong capacity for the rapid development of a horizontal arrangement through tuber–rhizome chains [4]. Consequently, they are capable of colonizing the surrounding ecological spaces [5]. The survival strategy of this plant is based on the partitioning of about 50% of its dry weight in tuber formation [6]. Moreover, the root exudates and volatile compounds released from the shoots and tubers of C. rotundus have been shown to significantly reduce crop seedling growth via strong allelopathic activity [7]. Unfortunately, the increasing needs of conservation agriculture limit soil tillage, thereby favouring the survival dynamics of both C. rotundus and other perennial weeds [8]. Not to be overlooked, in addition to the above mentioned “tuber bank”, there is often a consistent “seed bank” [9], contributing further to the increased annual rate of seedling emergence. Regrettably, conservative cropping systems tend to promote the accumulation of the seedbank close to the soil surface where it becomes highly “active” [10]. This occurs due to the lack of soil disturbance which would otherwise ensure the deposition of seeds at greater depths causing germination inhibition. Moreover, C. rotundus infestations occur in almost all spring-–summer industrial [11] and horticultural crops [12], including submerged rice [13] due to the flooding tolerance of the tubers [14].
Some herbicides such as imazethapyr, glyphosate [15], halosulfuron [16] and other sulfonylureas [17], were found to be effective in reducing the number and vigour of tubers. However, this reduction can only be considered as partially effective as the herbicides were unsuccessful at eliminating the weeds. Other integrated control strategies are therefore necessary. These strategies, although considered ineffective alone, may be effective in eliminating C. rotundus when used in combination. Integrated control strategies include mechanical control [18], crop rotation [19], phytophagous insects [20], pathogens [21], mulching [22] and cover crops [23].
The well-known efficacy of stale seedbed preparations in reducing the weed seed bank [24] is significantly less effective with C. rotundus due to the high level of energy reserves in the tubers that permit emergence from extended soil depths [25]. Consequently, it is very important to verify to what extent seeds and tuber size give rise to seedling emergence in increasing soil depths. Moreover, the role of soil texture in the emergence dynamics of seeds and tubers is not known. An additional gap in our knowledge is the potential vulnerability of tubers to drying out after summer soil tillage operations.
The aim of the present study was to analyze the following aspects of the biology and agroecology of C. rotundus: (i) to explore the germination/sprouting ecology of seeds and tubers under different light and temperature conditions, (ii) to verify the seedling emergence capacity of seeds and tubers (of different sizes) as a function of sowing depth and soil texture and (iii) identify the extent of dehydration required (after exposure to solar desiccation) to eliminate them.
2. Materials and Methods
2.1. Plant Material
Preliminary observations identified an agronomic environment heavily infested by Cyperus rotundus. It was selected for the germplasm collection of both seeds and tubers used for this experiment (Figure 1). This agro-environment, located near Pisa (Pontasserchio, 43°77′ N, 10°39′ E, Italy), cultivated with maize (continuous cropping over 10 years), was characterized by a sandy loam alluvial soil. During September 2021, before the maize harvest, seeds were collected from >100 mature plants located in the inter-rows of senescing maize. During the next spring (late March 2022) tubers were then collected from the same area. Seeds and tubers, immediately after collection, were cleaned and placed in climate-controlled cabinets (darkness at 5 °C) to be stored until use. In March 2022, an in vitro laboratory test was conducted followed by an open-air trial in May 2022.

Figure 1.
The maize crop heavily infested by Cyperus rotundus. Both the seeds and tubers were collected from this site and used in the experiments.
2.2. Laboratory Experiments
Fifty seeds of C. rotundus (1000 seed weight 0.39 g) were placed in 9 cm Petri dishes with filter paper (Whatman no. 3) moistened with distilled water (5 mL each) to optimize seed imbibition. Incubation was performed in a climate chamber at a constant temperature (20, 30 or 40 °C) for three weeks either under light (Philips THL 20 W/33 fluorescent neon; 100 μmol m−2 s−1, Philips, Eindhoven, The Netherlands) or in darkness (by wrapping the seeds in a double layer of aluminum foil). Three replicates were performed for each incubation condition (temperature and light) for the seed treatments. Seeds were considered germinated (daily count) after radicle protrusion (1–2 mm).
The same incubation parameters (including duration (three weeks) were performed to investigate tuber sprouting. Larger Petri dishes (15 cm in diameter) were used. Tubers (selected weight range 1–2 g) were inserted (Figure 2) between two discs of filter paper (Whatman no. 3) to allow for uniform humidity (thirty tubers per Petri dish, 10 mL of distilled water each). After weighing each tuber, the filter paper was moistened with distilled water, covered with additional filter paper on top and placed in the climate chamber set to 30 °C under the same light conditions as those reported for the seeds. Similarly, three replicates were performed under each incubation condition for the tubers.

Figure 2.
An example of a tuber of Cyperus rotundus, extracted from the soil (above) with a related cross section (below), that was used in both laboratory and outdoor experiments.
2.3. Outdoor Experiments
The outdoor experiments were carried out at the Department of Agriculture, Food and Agro-environment (43°70′ N, 10°43′ E) of the University of Pisa, Italy. In the first ten days of May 2022 and 2023, seedling emergence from the seeds and tubers, respectively, was observed. The experiments were conducted in large containers (1 × 1 m, 1.2 height) filled with sandy- (sand 79.8%, loam 14.6%, clay 5.6, pH 7.2 and organic matter 1.1%), loam (sand 58.4%, silt 23.3%, clay 18.3, pH 7.4 and organic matter 2.0%) or clay (sand 34.4%, silt 30.3%, clay 35.3, pH 6.9 and organic matter 2.3%) textured soils, respectively. The soil characteristics were obtained through standard soil analysis procedures. For the seeds, the sowing depths were from 0 to 40 mm (5 mm intervals). Instead, for the tubers, the burial depths were from 0 to 50 cm (5 cm intervals). In this last case, two categories of tubers were used, namely large and small. For the large tubers, only those within the range of 2.0 and 2.5 g were used, whilst for the small tubers, only those within the range of 0.5–1.0 g were used. All tubers of an intermediate weight (outside these ranges) were discarded in order to highlight the “energy reserves” factor. The tuber fresh weight was obtained using a precision scale (Kern AXE/AXS, Kern & Sohn, Balingen, Germany).
Emerged seedlings from either the seeds or tubers (Figure 3) were counted weekly for two months after sowing (May–June 2022 and 2023). The average temperatures of the two-year period in which the emergence experiments were performed were 15–22 °C and 18–25 °C for May and June, respectively.

Figure 3.
Large pots used for the emergence test (seeds and/or tubers) at increased sowing depths.
The large pots were only irrigated immediately after sowing (20 mm) as the subsequent rainfall was considered sufficient to maintain an adequate level of humidity in the substrates.
2.4. Tuber Viability After Sun Exposure
During the month of July in both 2022 and 2023, the tubers of C. rotundus plants grown in the large experimental pots were extracted from the soil. After having cleaned the soil residues from the tubers, the fresh weight was measured using the aforementioned precision scale. The tubers were then placed in Petri dishes (15 cm diameter, equipped with filter paper, twenty in each) and arranged (uncovered and without water) on the soil surface of the same large pots from which they were extracted. During the following four days, under full sun and high temperatures (approximately 22/33 °C, day/night, respectively), the tubers’ drying dynamics were analyzed. For this purpose, tubers were weighed every 12 h to measure the degree of desiccation and to calculate the water content dynamics. After each drying period the tubers were placed on moistened filter paper (distilled water) and covered with additional filter paper on top. Petri dishes were incubated in the climate chamber set to 30 °C the under light conditions reported previously. The percentage of tubers producing sprouts, at each 12 h period of drying, was measured to evaluate the residual tuber vitality.
2.5. Statistical Analyses
All the experiments used a randomized complete block design and were performed with four replicates. After the homogeneity test of variance, the arcsine transformation of the data was performed and expressed as a percentage to normalize their distribution [26]. All data (transformed percentages and untransformed not percentage data) were subjected to the analysis of variance (ANOVA) using Duncan’s Multiple Range Test (p < 0.05 and/or p < 0.01) for mean separation (least-significant difference, LSD). Emergence data were pooled over the two experimental years as there was no significant interaction (means analyzed by one-way ANOVA, p < 0.05) between 2021 and 2022. For each statistical analysis, CoHort (Minneapolis, MN, USA, Version 6.45) was used.
3. Results
3.1. Laboratory Experiments
The seeds of C. rotundus showed a high thermal requirement for germination. The germination rate almost doubled when the temperature was increased from 20 °C to 30 °C and 40 °C (Table 1). However, the seeds of this species demonstrated a marked dormancy since, even under optimal conditions, germination did not occur in 50% of the seeds incubated in Petri dishes. The temperature-dependent increase in germination was statistically significant (p < 0.05) between 20 and 30 °C, with no further statistically significant increases occurring beyond 30 °C. C. rotundus seeds showed marked photosensitivity. At each of the tested temperatures, germination under light conditions nearly doubled (statistical significance, p < 0.01) the seed germination percentage. In contrast, the tubers showed low dormancy (almost 90% sprouting at non-limiting temperatures) and showed no dependence on light conditions during incubation. Although less markedly than the seeds, the tubers also demonstrated high thermal requirements. At 20 °C, 75% of the tubers were shown to sprout compared to almost 100% after incubation at 30 or 40 °C, respectively.

Table 1.
Seed germination and tuber sprouting after incubation at different temperatures (constant 20, 30 or 40 °C) and light conditions (light or dark). Means are expressed with ± standard error. Two asterisks indicate significant differences (p > 0.01; n.s. = not significant) according to Duncan’s Multiple Range Test. Means with different letters within a row (temperature effect) indicate significant differences (p < 0.05) according to the above-cited statistical test.
3.2. Emergence Experiments
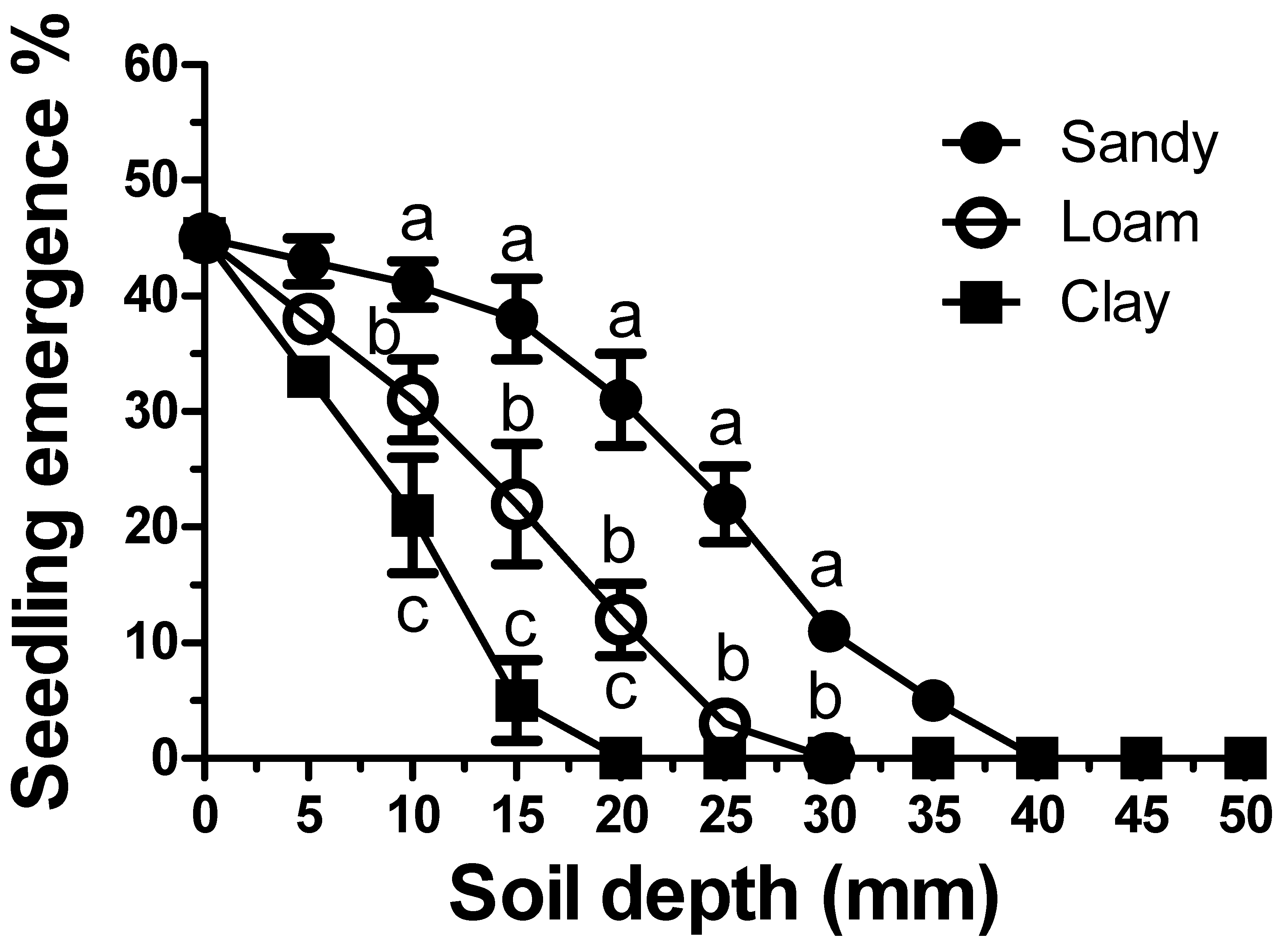
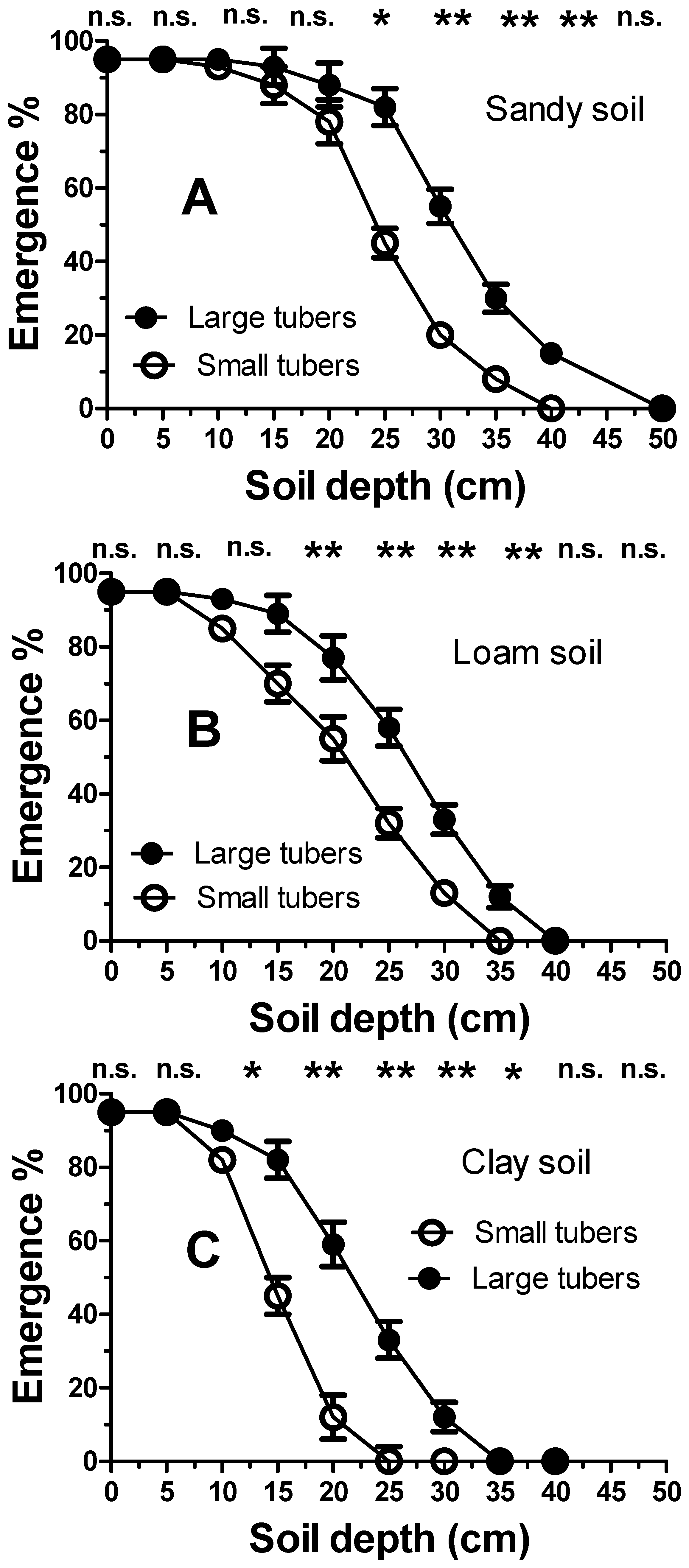
As expected, increasing the sowing depth significantly reduced the emergence dynamics of C. rotundus seedlings (Figure 4). However, this inhibition was also found to be strongly influenced by soil texture. The clay soil showed the highest degree of inhibition, with an emergence rate of approximately 5% at a depth of 15 mm. A seeding depth of 25 mm in clay soil was shown to completely inhibit seedling emergence. Less inhibition was shown in loam soil, with a 22% emergence occurring at a depth of 15 mm. Significantly less emergence inhibition was evident in the sandy soil. A significantly lower soil-depth inhibition was shown by the tubers. (Figure 5). The tubers showed much less inhibition by burial depth. For the tubers, the inhibition scale is reported in cm rather than in mm (Figure 5). However, soil texture influenced the inhibition of seedling emergence from tuber sprouts in the same manner as that reported for the seeds (Figure 4). Inhibition was higher in clay (Figure 5C), followed by loamy soils (Figure 5B) and sandy soils (Figure 5A), respectively. However, the extent of inhibition was not only linked to the depth and soil texture, but also to tuber size. For example, at a depth of 25 cm, in all soil textures, the larger tubers were significantly (p < 0.01) less susceptible to depth-mediated inhibition. At a depth of 35 cm in clay soil, seedling establishment was inhibited in all tubers. Conversely, in the sandy soil, this depth resulted in a seedling emergence of approximately 15%. Complete inhibition occurred only at 40 cm in sandy soils.
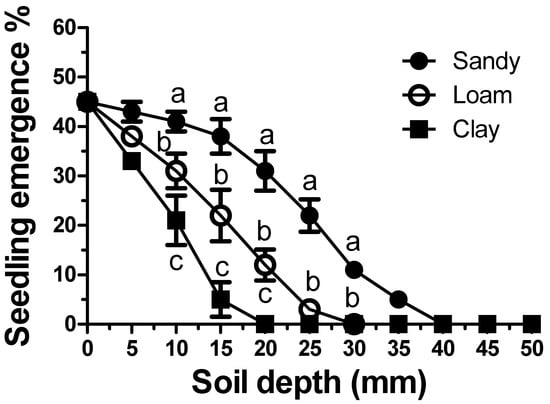
Figure 4.
Emergence of Cyperus rotundus seedlings from seeds sown at increasing depths according to soil texture. Vertical bars indicate the ±standard error of the means. Means with different letters within a depth indicate significant differences (p < 0.05) according to Duncan’s Multiple Range Test.
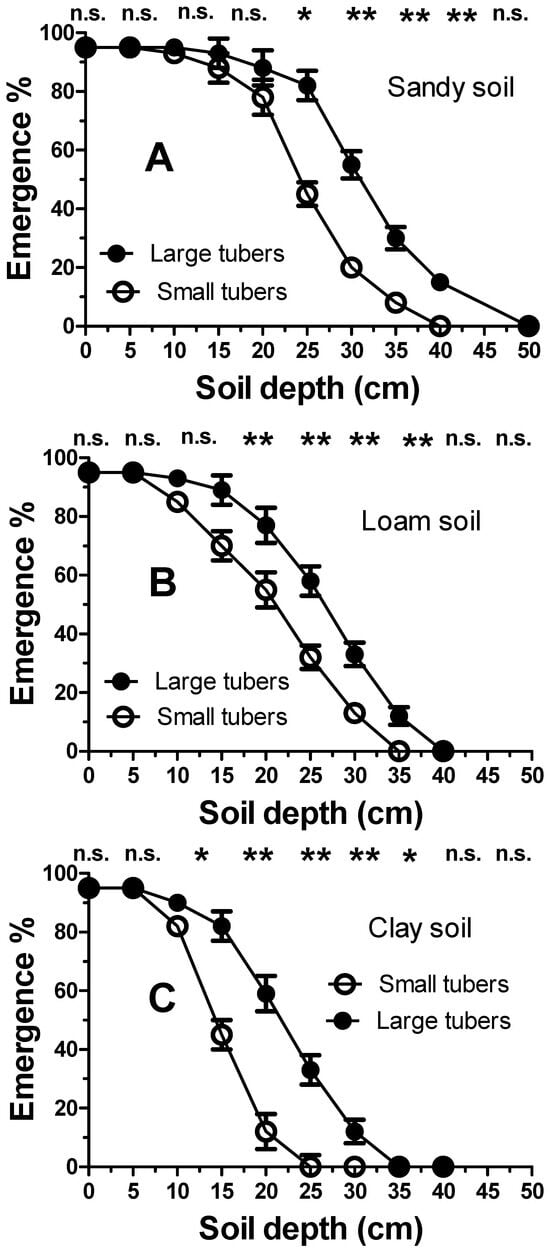
Figure 5.
Emergence of Cyperus rotundus seedlings from large and small tubers sown at increasing soil depths for different textures: (A) (sandy), (B) (loam) and (C) (clay). Vertical bars indicate the ±standard error of the means. Single (*) or double asterisks (**) indicate statistical differences (p < 0.05 or p < 0.01, respectively), according to Duncan’s LSD test (n.s.= not significant).
3.3. Tuber Sprouting After Drying
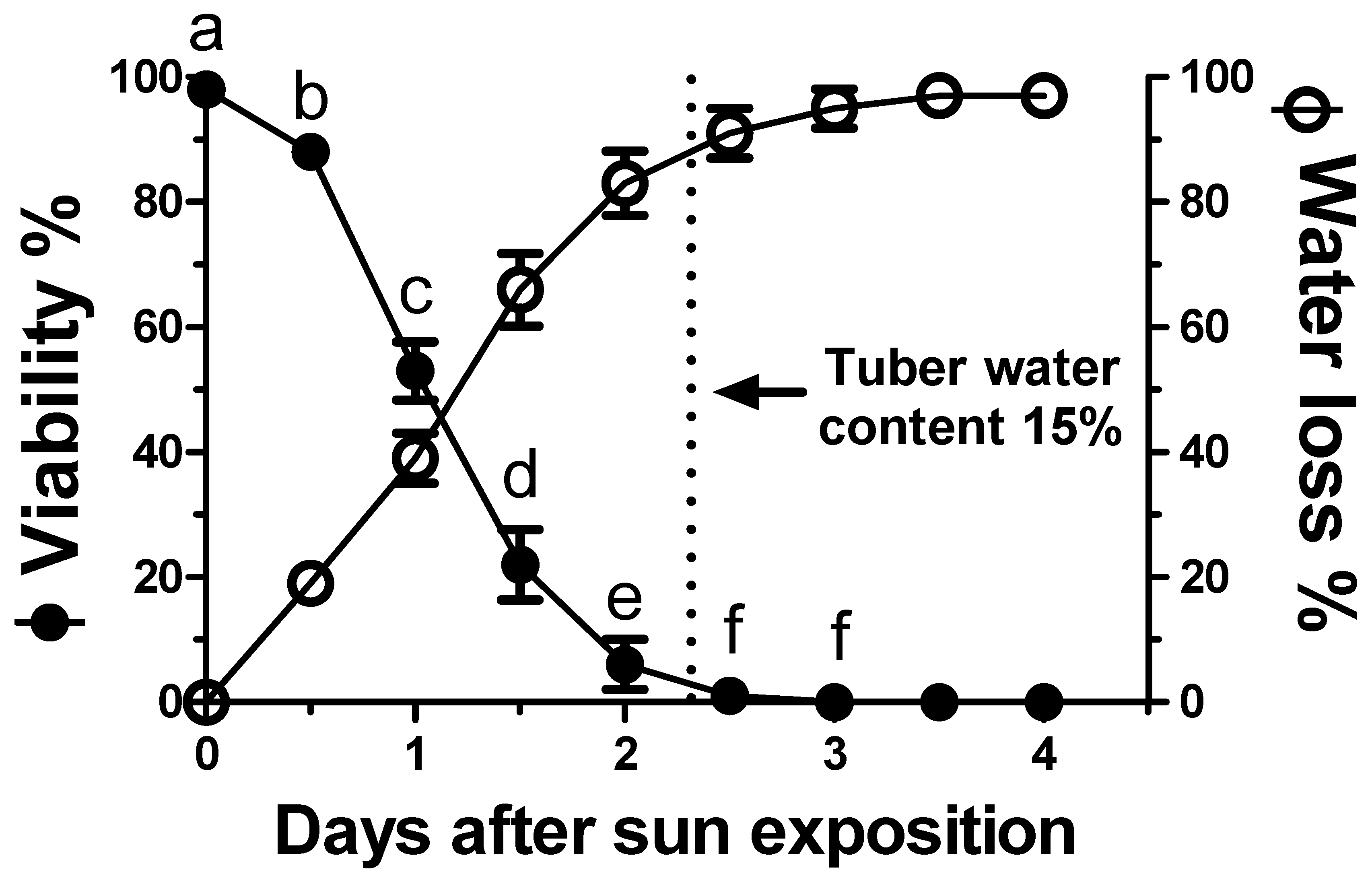
After only 12 h of sun drying, C. rotundus tubers showed a loss of vitality of approximately 15% (Figure 6). After a further 12 h (one day of sun exposure), tuber vitality declined to 50%. This loss of vitality was inversely associated with increased water loss with statistically significant differences (p < 0.05) for every additional 12 h of drying (Figure 6).
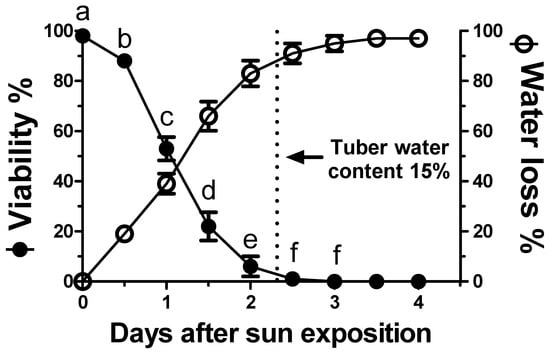
Figure 6.
Viability of Cyperus rotundus tubers as a function of the duration of sun exposure. The relative water loss percentages (initial water content was 47.7%) are reported. The dashed vertical line shows the residual tuber water content (found to be “critical” for viability) to be 15%. Vertical bars indicate the ±standard error of the means. Means with different letters within the duration of sun exposure indicate significant differences (p < 0.05) according to Duncan’s Multiple Range Test.
The loss of vitality coincided with a water loss amounting to 85% (compared to the initial 47.7% water content) after 2.5 days of drying when the residual water content of the tubers was below 15%. At this point, the tubers lacked vitality and no significant differences (p < 0.05) were shown to occur over the remaining duration of sun exposure.
4. Discussion
C. rotundus seeds showed three important features in germination ecology: (i) high thermal requirements, (ii) consistent dormancy and (iii) germination photo-dependence even under optimal light and temperature conditions (Table 1). Regarding the high thermal requirements, this experiment corroborated previous findings for this species [27] showing peak germination (about 55%) at alternate temperatures 25/35 °C. The lower level of germination in the present study (45.2%) be attributable to the absence of the well-known stimulating effect of temperature fluctuations [28]. Apart from this detail on the effect of thermal fluctuation, it can be noted that despite the optimal thermal conditions, germination inhibition exceeded 50%. This result highlights the strong dormancy of seeds. However, the persistence of this species is based on seed dormancy [29] which allows for the accumulation of significant quantities of seed banks, sometimes exceeding 500,000 seeds per square metre [30]. This seed dormancy appears to be of a physiological nature since other studies [31] have shown that various species of Cyperaceae break dormancy after treatment with oxidant chemicals (sodium hypochlorite) and/or cold stratification. In addition to high thermal requirements, the seeds of C. rotundus also possess specific light requirements to trigger germination. It is important to note that C. rotundus seeds showed a consistent photo-dependence, with germination percentages doubling those of seeds in dark germination. This result confirmed that small-sized seeds (1000 seed weight of C. rotundus only 0.38 g) have higher light requirements for germination. This germination photo-dependence, typically mediated by phytochrome [32], could have the ecological role of sensing the proximity of the seed to the soil surface. Indeed, small seeds are typically vulnerable due to limited energy reserves during pre-emergence growth. Hence, even dim light, able to reach the first mm depth [33], could have an ecological role in promoting the germination of small seeds [34], including this species.
Contrary to what was shown for the seeds, tuber sprouting showed no photo-dependence and a low dormancy. The extent of low tuber dormancy can vary [35], and is strongly ecotype-dependent [36], and by extension, also dependent on the environment [37] and the period of harvest. Indeed, tubers reach the greatest dormancy level at the end of the growing season in which they were formed (late summer) and are less dormant at the beginning of the next growing season [38]. Other experiments have confirmed the favourable effect of low winter temperatures on reducing tuber dormancy [39]. The only similarity between seed and tuber ecology is the marked thermal requirement, resulting in the sprouting of almost all tubers at both 30 °C and 40 °C. Approximately 75% of the tubers were shown to sprout in the sub-optimal thermal conditions (20 °C), suggesting a low annual accumulation of non-sprouted tubers. In other words, while tubers appear to be the ideal plant organ for a high annual emergence, seeds by contrast, appear to possess a survival role by ensuring the potential for the re-infestation of the various crops over time. It was reported that the beginning of the emergence and infestation dynamics of the crop strongly coincided with increased spring temperatures [40]. However, these hypotheses of the complementary ecological role of seeds and tubers need to be verified in the real conditions of soil burial. Consequently, the next experiments were also aimed at testing seed germination and tuber sprouting at different depths in different soil textures.
Similar to what has been observed in other weed species [41], soil texture was found to be an important factor in seedling emergence dynamics (Figure 4).
The typical soil depth-related inhibition [42] was shown to be more marked in clay soil, followed by loamy soil and sandy soil, respectively. Due to the small size of the seeds, soil depth across all textures was found to exert a strong inhibition on seedling establishment [43]. A depth of only 30 mm completely inhibited germination in both clay and loam soils, whereas only approximately 10% of seeds germinated in sandy soils. At a depth of 40 mm, seedling emergence was completely inhibited. It is important to underline that, in all soil textures, the lack of seedling emergence was not due to “suicide” germination (germination not followed by emergence) but rather due to germination inhibition, since at the end of the test period, the seeds were recovered intact. In short, the “active seed bank” of this species, although soil texture-dependent, is unquestionably superficial and therefore strictly linked to soil tillage management. Conservation tillage practices, particularly sod-seeding, appear to favour the emergence of the C. rotundus seed bank. This “gamic” infestation is probably more manageable (both chemically and mechanically) compared to vegetative infestations (tubers). Unfortunately, it is less visible to the eye and, therefore, more neglected from an agronomical standpoint in this first phase of agroecosystem colonization.
As expected, the higher energy-dense tubers permitted the formation of an “active propagule bank” that extended to greater soil depths (Figure 5). It is worth noting that in the context of the tubers, the soil depth scale is expressed not in mm but in cm. In this case, the emergence dynamics were modulated not only by the depth and the soil texture but also by the tuber size. Indeed, the larger tubers were shown to emerge from greater depths. This confirmed that the inhibition of tuber sprouting was inversely proportional to the level of energy reserves. The lower “active” propagule bank, specific to the smaller tubers, confirmed the efficacy of the agronomic strategy of including frequently mown forage crops [44] and/or summer cover crops [45] in the crop rotation that hindered the transfer of photosynthetic agents towards the tubers [46]. However, the inhibition of both the smaller tubers and those occurring at greater depths prevented “suicide” sprouting due to the exhaustion of energy reserves prior to reaching the soil surface and transitioning to the autotrophic phase. In terms of “emergence rate” potential, the sandy soil showed consistent or non-negligible sprouting even from depths of 40 and 35 cm, showing a good capacity of the species to colonize sandy soils. The tubers of C. rotundus showed an even greater capacity for emergence than C. aromaticus, which were completely inhibited at a depth of 15 cm [47].
Unlike with the seed bank, these results highlighted that the architectural positioning pattern of tubers in the ground was not as important, provided that it occurred within a range of 0–30 cm. Even periodic ploughing (annual or every two or more years) would not be effective in reducing the aggressiveness of C. rotundus towards the crop since tuber arrangement, even with conventional ploughing (30–40 cm), does not sufficiently hinder sprouting potential. In addition to this evident tolerance, not even a soil covering with plastic mulch could be envisaged to effectively hinder the emergence dynamics of the tubers of this species [47].
The type of ecological conditions that would render C. rotundus persistence dynamics vulnerable remains to be investigated. Figure 6 shows how sun exposure for 1–2 days caused a rapid dehydration capable of eliminating tuber vitality. After 3 days, the tubers were shown to lose their entire initial water content (47.7%) and were no longer viable. The water content limit resulting in the complete loss of viability was below 15%. Separate from the visual evidence, the complete loss of vitality was confirmed by the rehydration and incubation of the tubers under optimal conditions, which then did not show any sprouting. In practice, the “Achilles’ heel” of this species is drying intolerance. This contrasts with the related species, C. esculentus which store large amounts of lipids, similar to those of the typically dry-tolerant seeds. Instead, the tubers of C. rotundus do not accumulate oil and are not desiccation-tolerant [48], especially when the water content declines below 15% [49]. This is confirmed from the worldwide distribution pattern of this weed species, which is found mostly in tropical and Mediterranean climates where soil moisture is not limiting [50]. On the other hand, this species is particularly aggressive in agronomic areas characterized by very shallow water tables, such as in the agro-ecosystems located near the mouth of the Po River, on both sides towards Veneto and Emilia-Romagna regions. This suggests that such moisture in the various soil layers is crucial for the survival and vitality of the tubers. This hypothesis was confirmed by exposing the tubers to drying out due to their exposure to sunlight during summer periods.
The desiccation vulnerability of the tubers provides a potential agronomic strategy involving the use of a subsoiler during the summer periods. Exposing tubers, raised from the ground by soil tillage and/or other frequent tillage techniques [51], to the sun for a few days shows promise as a crucial strategy to “reclaim” highly infested areas. In the context of a crop rotation, the most suitable period for the application of this soil tillage practice is envisaged to be the one following the wheat harvest. In addition, the possible introduction of perennial forage crops, such as alfalfa (Medicago sativa), and/or summer cover crops, can also be an effective agronomic practice to complement summer tillage by using subsoiler. This additional agronomic management could not only hinder the growth of C. rotundus following frequent mowing but also allow for sustainable “conservative” management, by including the perspective of protecting soil organic matter.
5. Conclusions
C. rotundus possesses a dual survival strategy. The first is based on persistence over time, through a dormant soil seed bank. The second is based on a persistence in space, as tubers capable of both: (i) giving rise to vertical seedling emergence from considerable soil depths and (ii) colonizing horizontally through their characteristic chains. The extent of pre-emergence in autotrophic tuber growth was found to be directly proportional to the size of the soil particles (maximum in sandy soil) and the tuber size. Consequently, the growth of this perennial weed under conditions of little or no crop competition (inter-crop periods) appears to amplify aggressiveness by ensuring seedlings emergence from extreme depths, especially in sandy soils. These inter-crop periods can be managed by summer cover crops in order to increase the shading of C. rotundus, thus reducing the level of energy-rich substances necessary to increase the vigour of the tubers. However, the present study highlighted an important “Achilles’ heel”: the desiccation of the exposed tubers within a few days. This confirmed the typical diffusion of this species in alluvial areas, often reclaimed with very high phreatic water. Soil tillage by means of a subsoiler during the summer periods (once or more in a summer season) as an agronomic strategy shows promise in reclaiming unsustainable weedy situations. In summary, these experiments highlight that integrating summer soil exposure with cover cropping can substantially weaken C. rotundus infestations, offering a path toward more sustainable weed management. Further studies on tuber vulnerability as a function of soil tillage modalities, geographic agro-environments and genetic ecotype could play a crucial role in rendering integrated cropping systems sustainable.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Restrictions apply to the availability of these data. Data were obtained from SSRN and are available at https://doi.org/10.2139/ssrn.5396449 [52] (accessed on 15 September 2025) with the permission of Elsevier.
Conflicts of Interest
The author declares no conflict of interest.
References
- Peerzada, A.M. Biology, agricultural impact, and management of Cyperus rotundus L.: The world’s most tenacious weed. Acta Phys. Plant. 2017, 39, 270. [Google Scholar] [CrossRef]
- Bendixen, L.E.; Nandihalli, U.B. Worldwide distribution of purple and yellow nutsedge (Cyperus rotundus and C. esculentus). Weed Technol. 1987, 1, 61–65. [Google Scholar] [CrossRef]
- Zhang, H.Y.; Hanna, M.A.; Ali, Y.; Nan, L. Yellow nut-sedge (Cyperus esculentus L.) tuber oil as a fuel. Ind. Crops Prod. 1996, 5, 177–181. [Google Scholar] [CrossRef]
- Wills, G.D. Description of purple and yellow nutsedge (Cyperus rotundus and C. esculentus). Weed Technol. 1987, 1, 2–9. [Google Scholar] [CrossRef]
- Horowitz, M. Growth, tuber formation and spread of Cyperus rotundus L. from single tubers. Weed Res. 1972, 12, 348–363. [Google Scholar] [CrossRef]
- Williams, R.D. Growth and reproduction of Cyperus esculentus L. and Cyperus rotundus L. Weed Res. 1982, 22, 149–154. [Google Scholar] [CrossRef]
- Alsaadawi, I.S.; Salih, N.M.M. Allelopathic potential of Cyperus rotundus LI Interference with crops. Allelopath. J. 2009, 23, 297–304. [Google Scholar]
- Wang, C.; Hu, Y.; Wu, H.; Wang, Z.; Cai, J.; Liu, H.; Ren, W.; Yang, N.; Wang, Z.; Jiang, Y.; et al. No-tillage practice enhances soil total carbon content in a sandy Cyperus esculentus L. field. Ecol. Process. 2025, 14, 9. [Google Scholar] [CrossRef]
- Srivastava, R.; Singh, K.P. Diversity in weed seed production and the soil seed bank: Contrasting responses between two agroecosystems. Weed Biol. Manag. 2014, 14, 21–30. [Google Scholar] [CrossRef]
- Benvenuti, S.; Mazzoncini, M. “Active” weed seed bank: Soil texture and seed weight as key factors of burial-depth inhibition. Agronomy 2021, 11, 210. [Google Scholar] [CrossRef]
- Bryson, C.T.; Reddy, K.N.; Molin, W.T. Purple nutsedge (Cyperus rotundus) population dynamics in narrow row transgenic cotton (Gossypium hirsutum) and soybean (Glycine max) rotation. Weed Technol. 2003, 17, 805–810. [Google Scholar] [CrossRef]
- Benvenuti, S.; Pardossi, A. Weed seedbank dynamics in Mediterranean organic horticulture. Sci. Hortic. 2017, 221, 53–61. [Google Scholar] [CrossRef]
- Pena-Fronteras, J.T.; Villalobos, M.C.; Baltazar, A.M.; Merca, F.E.; Ismail, A.M.; Johnson, D.E. Adaptation to flooding in upland and lowland ecotypes of Cyperus rotundus, a troublesome sedge weed of rice: Tuber morphology and carbohydrate metabolism. Ann. Bot. 2009, 103, 295–302. [Google Scholar] [CrossRef]
- Yi, S.C.; Wei, C.Y.; Tong, Y.; Xu, L.; Fan, D.L.; Yu, S.X.; Liu, S.Y.; Wu, R.H.; Liu, X.L.; Tang, W.W. Mature tubers of purple nutsedge (Cyperus rotundus) confer flooding tolerance by adopting a low-oxygen quiescence strategy that may contribute to its emergence in rice fields. Weed Sci. 2024, 72, 761–773. [Google Scholar] [CrossRef]
- Kumar, M.; Das, T.K.; Yaduraju, N.T. An integrated approach for management of Cyperus rotundus (purple nutsedge) in soybean–wheat cropping system. Crop Prot. 2012, 33, 74–81. [Google Scholar] [CrossRef]
- Webster, T.M.; Grey, T.L. Halosulfuron reduced purple nutsedge (Cyperus rotundus) tuber production and viability. Weed Sci. 2014, 62, 637–646. [Google Scholar] [CrossRef]
- De Ryck, S.; Reheul, D.; De Cauwer, B. Impact of regular mowing, mowing height, and grass competition on tuber number and tuber size of yellow nutsedge clonal populations (Cyperus esculentus L.). Weed Res. 2023, 63, 371–381. [Google Scholar] [CrossRef]
- Bangarwa, S.K.; Norsworthy, J.K.; Jha, P.; Malik, M. Purple nutsedge (Cyperus rotundus) management in an organic production system. Weed Sci. 2008, 56, 606–613. [Google Scholar] [CrossRef]
- Warren, L.S., Jr.; Coble, H.D. Managing purple nutsedge (Cyperus rotundus) populations utilizing herbicide strategies and crop rotation sequences. Weed Technol. 1999, 13, 494–503. [Google Scholar] [CrossRef]
- Phatak, S.C.; Callaway, M.B.; Vavrina, C.S. Biological control and its integration in weed management systems for purple and yellow nutsedge (Cyperus rotundus and C. esculentus). Weed Technol. 1997, 1, 84–91. [Google Scholar] [CrossRef]
- Kadir, J.B.; Charudattan, R.; Stall, W.M.; Brecke, B.J. Field efficacy of Dactylaria higginsii as a bioherbicide for the control of purple nutsedge (Cyperus rotundus). Weed Technol. 2000, 14, 1–6. [Google Scholar] [CrossRef]
- Webster, T.M. Mulch type affects growth and tuber production of yellow nutsedge (Cyperus esculentus) and purple nutsedge (Cyperus rotundus). Weed Sci. 2005, 53, 834–838. [Google Scholar] [CrossRef]
- Nath, C.P.; Kumar, N.; Dutta, A.; Hazra, K.K.; Praharaj, C.S.; Kumar, D.; Dixit, G.P. Cover crop and herbicides can control purple nutsedge (Cyperus rotundus L.) and increase crop yields in conservation agriculture-based crop rotations. Crop Prot. 2025, 187, 106974. [Google Scholar] [CrossRef]
- Lamour, A.; Lotz, L.A. The importance of tillage depth in relation to seedling emergence in stale seedbeds. Ecol. Model. 2007, 201, 536–546. [Google Scholar] [CrossRef]
- Riemens, M.M.; Van der Weide, R.Y.; Runia, W.T. Biology and Control of Cyperus rotundus and Cyperus esculentus, Review of a Literature Survey; Plant Research International: Wageningen, The Netherlands, 2008. [Google Scholar]
- Steel, R.G.; Torrie, D. Principles and Procedures of Statistics: A Biometrical Approach, 2nd ed.; McGraw-Hill Inc.: Columbus, OH, USA, 1980. [Google Scholar]
- Singh, S.; Singh, M. Effect of temperature, light and pH on germination of twelve weed species. Indian J. Weed Sci. 2009, 41, 113–126. [Google Scholar]
- Penfield, S. Seed dormancy and germination. Curr. Biol. 2017, 27, R874–R878. [Google Scholar] [CrossRef]
- Zivanai, M.; Ronald, M.; Nester, M. The role of tillage, allelopathy, dormancy-breaking mechanisms and wind in the spread of purple nutsedge (Cyperus rotundus) in Zimbabwe. Agric. Res. 2019, 8, 461–466. [Google Scholar] [CrossRef]
- Leck, M.A.; Schütz, W. Regeneration of Cyperaceae, with particular reference to seed ecology and seed banks. Perspect. Plant Ecol. Evol. Syst. 2005, 7, 95–133. [Google Scholar] [CrossRef]
- Rosbakh, S.; Hülsmann, L.; Weinberger, I.; Bleicher, M.; Poschlod, P. Bleaching and cold stratification can break dormancy and improve seed germination in Cyperaceae. Aquat. Bot. 2019, 158, 103128. [Google Scholar] [CrossRef]
- Casal, J.J.; Sánchez, R.A. Phytochromes and seed germination. Seed Sci. Res. 1998, 8, 317–329. [Google Scholar] [CrossRef]
- Benvenuti, S. Soil light penetration and dormancy of jimsonweed (Datura stramonium) seeds. Weed Sci. 1995, 43, 389–393. [Google Scholar] [CrossRef]
- Milberg, P.; Andersson, L.; Thompson, K. Large-seeded spices are less dependent on light for germination than small-seeded ones. Seed Sci. Res. 2000, 10, 99–104. [Google Scholar] [CrossRef]
- Neeser, C.; Aguero, R.; Swanton, C.J. Survival and dormancy of purple nutsedge (Cyperus rotundus) tubers. Weed Sci. 1997, 45, 784–790. [Google Scholar] [CrossRef]
- Du, L.; Gao, X.; Qu, C.; Bai, S.; Shi, C.; Jiang, X.; Li, X.; Ju, Q.; Qu, M. Identification of purple nutsedge (Cyperus rotundus L.) ecotypes and the effect of environmental factors on tuber sprouting in China. Weed Res. 2022, 62, 360–371. [Google Scholar] [CrossRef]
- Kawabata, O.; Nishimoto, R.K. Temperature and rhizome chain effect on sprouting of purple nutsedge (Cyperus rotundus) ecotypes. Weed Sci. 2003, 51, 348–355. [Google Scholar] [CrossRef]
- Taylorson, R.B. Seasonal variation in sprouting and available carbohydrate in yellow nutsedge tubers. Weeds 1967, 15, 22–24. [Google Scholar] [CrossRef]
- Dor, E.; Hershenhorn, J. Effect of low temperature on purple nutsedge (Cyperus rotundus) reproductive biology. Weed Sci. 2013, 61, 239–243. [Google Scholar] [CrossRef]
- Mijani, S.; Rastgoo, M.; Ghanbari, A.; Nassiri Mahallati, M.; González-Andújar, J.L. Development of a new thermal time model for describing tuber sprouting of Purple nutsedge (Cyperus rotundus L.). Weed Res. 2021, 61, 431–442. [Google Scholar] [CrossRef]
- Benvenuti, S. Soil texture involvement in germination and emergence of buried weed seeds. Agron. J. 2003, 95, 191–198. [Google Scholar] [CrossRef]
- Benvenuti, S.; Macchia, M.; Miele, S. Quantitative analysis of emergence of seedlings from buried weed seeds with increasing soil depth. Weed Sci. 2001, 49, 528–535. [Google Scholar] [CrossRef]
- Benvenuti, S.; Mazzoncini, M. Soil physics involvement in the germination ecology of buried weed seeds. Plants 2018, 8, 7. [Google Scholar] [CrossRef]
- Bezuidenhout, S.R.; Reinhardt, C.F.; Whitwell, M.I. Cover crops of oats, stooling rye and three annual ryegrass cultivars influence maize and Cyperus esculentus growth. Weed Res. 2012, 52, 153–160. [Google Scholar] [CrossRef]
- Wauters, V.M.; Grossman, J.M.; Pfeiffer, A.; Cala, R. Ecosystem services and cash crop tradeoffs of summer cover crops in northern region organic vegetable rotations. Front. Sustain. Food Syst. 2021, 5, 635955. [Google Scholar] [CrossRef]
- Bangarwa, S.K.; Norsworthy, J.K.; Gbur, E.E. Effects of shoot clipping–soil disturbance frequency and tuber size on aboveground and belowground growth of purple and yellow nutsedge (Cyperus rotundus and Cyperus esculentus). Weed Technol. 2012, 26, 813–817. [Google Scholar] [CrossRef]
- Chadha, A.; Florentine, S.K.; Dhileepan, K.; Turville, C. Effect of rhizome fragment length and burial depth on the emergence of a tropical invasive weed Cyperus aromaticus (Navua Sedge). Plants 2022, 11, 3331. [Google Scholar] [CrossRef]
- Niemeyer, P.W.; Irisarri, I.; Scholz, P.; Schmitt, K.; Valerius, O.; Braus, G.H.; Herrfurth, C.; Feussner, I.; Sharma, S.; Carlsson, A.S.; et al. A seed-like proteome in oil-rich tubers. Plant J. 2022, 112, 518–534. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, R.S. Ecology of Cyperus rotundus L.-3. Population of tubers at different depths of the soil and their sprouting response to air drying. Proc. Nat. Acad. Sci. India 1969, 39, 140–142. [Google Scholar]
- Henry, G.M.; Elmore, M.T.; Gannon, T.W. Cyperus esculentus and Cyperus rotundus. In Biology and Management of Problematic Crop Weed Species; Academic Press: Cambridge, MA, USA, 2021; pp. 151–172. [Google Scholar]
- Follak, S.; Belz, R.; Bohren, C.; De Castro, O.; Del Guacchio, E.; Pascual-Seva, N.; Schwarz, M.; Verloove, F.; Essl, F. Biological flora of central Europe: Cyperus esculentus L. Perspect. Plant Ecol. Evol. Syst. 2016, 23, 33–51. [Google Scholar] [CrossRef]
- Benvenuti, S. Ecology of Cyperus Rotundus Seedling Emergence and Agronomic Strategies for Sustainable Management. Available online: https://ssrn.com/abstract=5396449 (accessed on 15 September 2025).
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).