Comprehensive Review of Recent Trends in the Use of Deep Eutectic Solvents for the Valorization of Secondary Lignocellulosic Biomass
Abstract
1. Introduction
2. Current DES Types, Composition, and Characteristics
3. Conventional Solvents vs. DES in the Biomass Valorization and Biorefinery
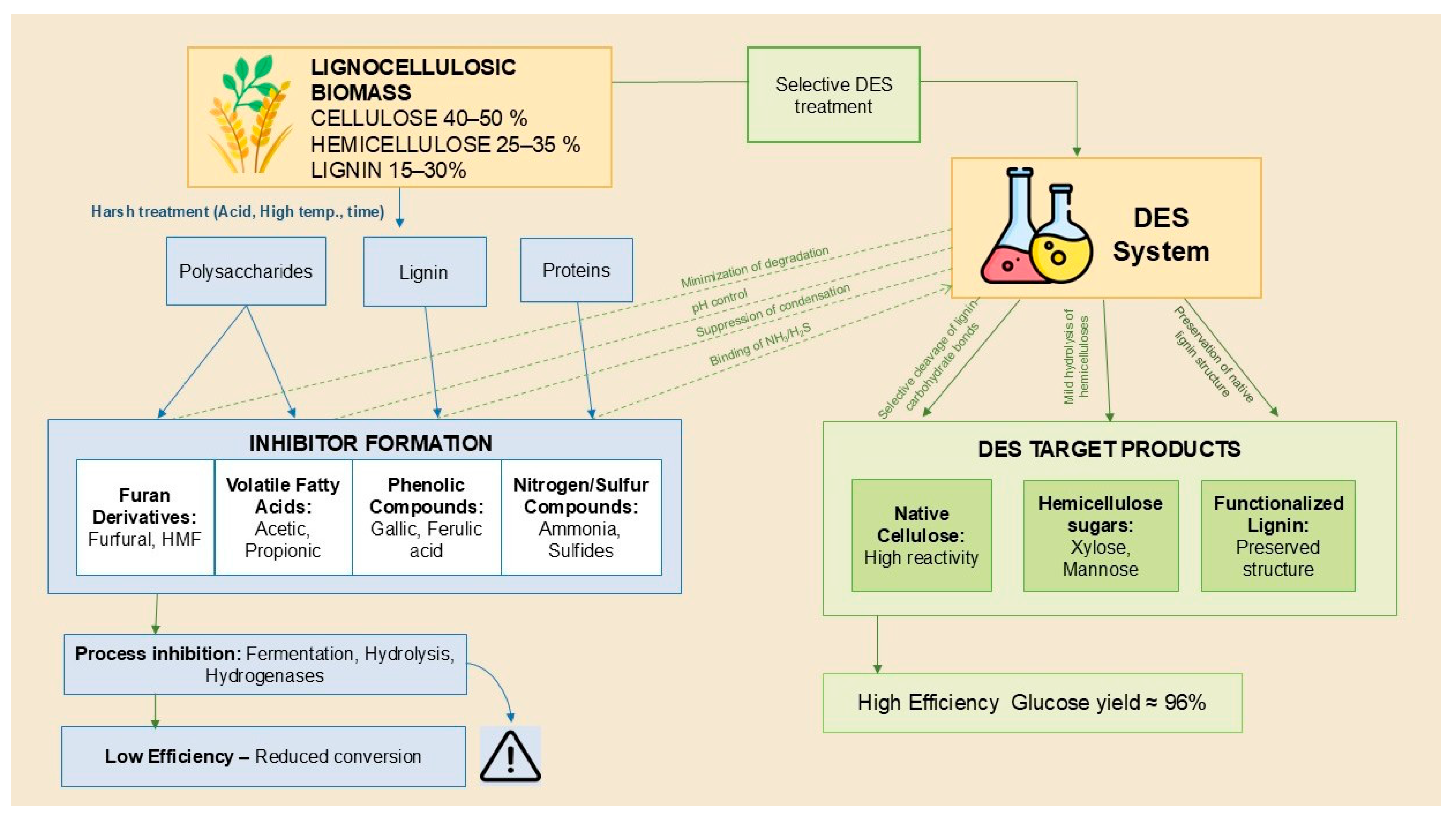
4. Application of DES in Secondary Lignocellulosic Biomass Pretreatment
5. Applications of DES in the Extraction Process
6. Application of DES for Catalytic Conversion
7. Integration of DES with Other Techniques in Valorization of Secondary Lignocellulosic Biomass
8. Challenges and Future Directions
9. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jing, Y.; Li, F.; Li, Y.; Jiang, D.; Lu, C.; Zhang, Z.; Zhang, Q. Biohydrogen Production by Deep Eutectic Solvent Delignification-Driven Enzymatic Hydrolysis and Photo-Fermentation: Effect of Liquid–Solid Ratio. Bioresour. Technol. 2022, 349, 126867. [Google Scholar] [CrossRef] [PubMed]
- Chandel, H.; Kumar, P.; Chandel, A.K.; Verma, M.L. Biotechnological Advances in Biomass Pretreatment for Bio-Renewable Production through Nanotechnological Intervention. Biomass Convers. Biorefin. 2024, 14, 2959–2981. [Google Scholar] [CrossRef]
- Arhin, S.G.; Cesaro, A.; Di Capua, F.; Esposito, G. Recent Progress and Challenges in Biotechnological Valorization of Lignocellulosic Materials: Towards Sustainable Biofuels and Platform Chemicals Synthesis. Sci. Total Environ. 2023, 857, 159333. [Google Scholar] [CrossRef] [PubMed]
- Singh, D.; Dhiman, S.K.; Kumar, V.; Babu, R.; Shree, K.; Priyadarshani, A.; Singh, A.; Shakya, L.; Nautiyal, A.; Saluja, S. Crop Residue Burning and Its Relationship between Health, Agriculture Value Addition, and Regional Finance. Atmosphere 2022, 13, 1405. [Google Scholar] [CrossRef]
- Mathur, R.; Srivastava, V.K. Crop Residue Burning: Effects on Environment. In Greenhouse Gas Emissions: Challenges, Technologies and Solutions; Springer: Singapore, 2018; pp. 127–140. [Google Scholar]
- Lackner, M.; Besharati, M. Agricultural Waste: Challenges and Solutions, a Review. Waste 2025, 3, 18. [Google Scholar] [CrossRef]
- Sharma, S.; Tsai, M.L.; Sharma, V.; Sun, P.P.; Nargotra, P.; Bajaj, B.K.; Chen, C.W.; Dong, C.D. Environment Friendly Pretreatment Approaches for the Bioconversion of Lignocellulosic Biomass into Biofuels and Value-Added Products. Environments 2023, 10, 6. [Google Scholar] [CrossRef]
- Beig, B.; Riaz, M.; Raza Naqvi, S.; Hassan, M.; Zheng, Z.; Karimi, K.; Pugazhendhi, A.; Atabani, A.E.; Thuy Lan Chi, N. Current Challenges and Innovative Developments in Pretreatment of Lignocellulosic Residues for Biofuel Production: A Review. Fuel 2021, 287, 119670. [Google Scholar] [CrossRef]
- Bao, Y.; Wang, Y.; Yan, C.; Xue, Z. Deep Eutectic Solvents for Fractionation and Valorization of Lignocellulose. Green Chem. Eng. 2025, 6, 21–35. [Google Scholar] [CrossRef]
- Adewuyi, A. Underutilized Lignocellulosic Waste as Sources of Feedstock for Biofuel Production in Developing Countries. Front. Energy Res. 2022, 10, 741570. [Google Scholar] [CrossRef]
- Ünlü, A.E.; Arlkaya, A.; Takaç, S. Use of Deep Eutectic Solvents as Catalyst: A Mini-Review. Green Process. Synth. 2019, 8, 355–372. [Google Scholar] [CrossRef]
- Zhao, Z.; Chen, X.; Ali, M.F.; Abdeltawab, A.A.; Yakout, S.M.; Yu, G. Pretreatment of Wheat Straw Using Basic Ethanolamine-Based Deep Eutectic Solvents for Improving Enzymatic Hydrolysis. Bioresour. Technol. 2018, 263, 325–333. [Google Scholar] [CrossRef] [PubMed]
- Dwamena, A.K. Recent Advances in Hydrophobic Deep Eutectic Solvents for Extraction. Separations 2019, 6, 9. [Google Scholar] [CrossRef]
- Procentese, A.; Raganati, F.; Olivieri, G.; Russo, M.E.; Rehmann, L.; Marzocchella, A. Deep Eutectic Solvents Pretreatment of Agro-Industrial Food Waste. Biotechnol. Biofuels 2018, 11, 37. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Ji, X.; Lu, X. Choline-Based Deep Eutectic Solvents for CO2 Separation: Review and Thermodynamic Analysis. Renew. Sustain. Energy Rev. 2018, 97, 436–455. [Google Scholar] [CrossRef]
- Wang, Y.; Kim, K.H.; Jeong, K.; Kim, N.K.; Yoo, C.G. Sustainable Biorefinery Processes Using Renewable Deep Eutectic Solvents. Curr. Opin. Green Sustain. Chem. 2021, 27, 100396. [Google Scholar] [CrossRef]
- Amesho, K.T.T.; Chen, S.C.; Wu, T.Y.; Ponnusamy, V.K.; Lin, Y.C. Green Synthesis of 5-Hydroxymethylfurfural from Biomass-Derived Carbohydrates Using Deep Eutectic Solvents as Environmentally Benign Catalyst. Environ. Technol. Innov. 2023, 29, 102982. [Google Scholar] [CrossRef]
- del Mar Contreras-Gámez, M.; Galán-Martín, Á.; Seixas, N.; da Costa Lopes, A.M.; Silvestre, A.; Castro, E. Deep Eutectic Solvents for Improved Biomass Pretreatment: Current Status and Future Prospective towards Sustainable Processes. Bioresour. Technol. 2023, 369, 128396. [Google Scholar] [CrossRef]
- Ibrahim, A.; Tshibangu, M.M.; Coquelet, C.; Espitalier, F. Ternary Choline Chloride-Based Deep Eutectic Solvents: A Review. ChemEngineering 2025, 9, 84. [Google Scholar] [CrossRef]
- Smith, E.L.; Abbott, A.P.; Ryder, K.S. Deep Eutectic Solvents (DESs) and Their Applications. Chem. Rev. 2014, 114, 11060–11082. [Google Scholar] [CrossRef]
- Gao, Y.; Fan, M.; Cheng, X.; Liu, X.; Yang, H.; Ma, W.; Guo, M.; Li, L. Deep Eutectic Solvent: Synthesis, Classification, Properties and Application in Macromolecular Substances. Int. J. Biol. Macromol. 2024, 278, 134593. [Google Scholar] [CrossRef]
- Abranches, D.O.; Martins, M.A.R.; Silva, L.P.; Schaeffer, N.; Pinho, S.P.; Coutinho, J.A.P. Phenolic Hydrogen Bond Donors in the Formation of Non-Ionic Deep Eutectic Solvents: The Quest for Type V Des. Chem. Commun. 2019, 55, 10253–10256. [Google Scholar] [CrossRef] [PubMed]
- Wu, K.; Ren, J.; Wang, Q.; Nuerjiang, M.; Xia, X.; Bian, C. Research Progress on the Preparation and Action Mechanism of Natural Deep Eutectic Solvents and Their Application in Food. Foods 2022, 11, 3528. [Google Scholar] [CrossRef]
- Li, D. Natural Deep Eutectic Solvents in Phytonutrient Extraction and Other Applications. Front. Plant Sci. 2022, 13, 1004332. [Google Scholar] [CrossRef]
- Dai, Y.; van Spronsen, J.; Witkamp, G.J.; Verpoorte, R.; Choi, Y.H. Natural Deep Eutectic Solvents as New Potential Media for Green Technology. Anal. Chim. Acta 2013, 766, 61–68. [Google Scholar] [CrossRef]
- Rinaldi, R. Solvents and Solvent Effects in Biomass Conversion. In Catalytic Hydrogenation for Biomass Valorization; Rinaldi, R., Ed.; The Royal Society of Chemistry: Cambridge, UK, 2014; pp. 74–98. ISBN 978-1-84973-801-9. [Google Scholar]
- Monroy, A.F.; Caicedo, G.A.; Martínez, J.J.; Romanelli, G.P. Utilization of Deep Eutectic Solvents in the Production of High-value Compounds from Biomass. Biofuels Bioprod. Biorefin. 2024, 18, 1821–1865. [Google Scholar] [CrossRef]
- Quraishi, R.; Haldar, D. Technological Advancement in Product Valorization of Agricultural Wastes Treated with Deep Eutectic Solvents: A Review. ChemBioEng Rev. 2025, 12, e202400054. [Google Scholar] [CrossRef]
- Scelsi, E.; Angelini, A.; Pastore, C. Deep Eutectic Solvents for the Valorisation of Lignocellulosic Biomasses towards Fine Chemicals. Biomass 2021, 1, 29–59. [Google Scholar] [CrossRef]
- Hussain, M.; Ashraf, H.T.; Vasudev, V.; Yasin, S.; Jamil, M.I.; Saleem, M.; Aziz, T.; Al Afroz, E.; Feichao, Z.; Huapeng, Z.; et al. Deep Eutectic Solvents in Biomass Pretreatment for Green Approach: A Comprehensive Review. Polym. Bull. 2025, 82, 10513–10552. [Google Scholar] [CrossRef]
- Hanafi, M.A.; Anwar, F.; Saari, N. Valorization of Biomass for Food Protein via Deep Eutectic Solvent Extraction: Understanding the Extraction Mechanism and Impact on Protein Structure and Properties. Food Front. 2024, 5, 1265–1301. [Google Scholar] [CrossRef]
- Cantero, D.; Jara, R.; Navarrete, A.; Pelaz, L.; Queiroz, J.; Rodríguez-Rojo, S.; Cocero, M.J. Pretreatment Processes of Biomass for Biorefineries: Current Status and Prospects. Annu. Rev. Chem. Biomol. Eng. 2019, 10, 289–310. [Google Scholar] [CrossRef]
- Alam, M.A.; Muhammad, G.; Khan, M.N.; Mofijur, M.; Lv, Y.; Xiong, W.; Xu, J. Choline Chloride-Based Deep Eutectic Solvents as Green Extractants for the Isolation of Phenolic Compounds from Biomass. J. Clean. Prod. 2021, 309, 127445. [Google Scholar] [CrossRef]
- Usman, M.; Nakagawa, M.; Cheng, S. Emerging Trends in Green Extraction Techniques for Bioactive Natural Products. Processes 2023, 11, 3444. [Google Scholar] [CrossRef]
- Singh, S.; Cheng, G.; Sathitsuksanoh, N.; Wu, D.; Varanasi, P.; George, A.; Balan, V.; Gao, X.; Kumar, R.; Dale, B.E.; et al. Comparison of Different Biomass Pretreatment Techniques and Their Impact on Chemistry and Structure. Front. Energy Res. 2015, 2, 62. [Google Scholar] [CrossRef]
- Kassaye, S.; Pant, K.K.; Jain, S. Hydrolysis of Cellulosic Bamboo Biomass into Reducing Sugars via a Combined Alkaline Solution and Ionic Liquid Pretreament Steps. Renew. Energy 2017, 104, 177–184. [Google Scholar] [CrossRef]
- Matsakas, L.; Raghavendran, V.; Yakimenko, O.; Persson, G.; Olsson, E.; Rova, U.; Olsson, L.; Christakopoulos, P. Lignin-First Biomass Fractionation Using a Hybrid Organosolv–Steam Explosion Pretreatment Technology Improves the Saccharification and Fermentability of Spruce Biomass. Bioresour. Technol. 2019, 273, 521–528. [Google Scholar] [CrossRef]
- Isci, A.; Erdem, G.M.; Bagder Elmaci, S.; Sakiyan, O.; Lamp, A.; Kaltschmitt, M. Effect of Microwave-Assisted Deep Eutectic Solvent Pretreatment on Lignocellulosic Structure and Bioconversion of Wheat Straw. Cellulose 2020, 27, 8949–8962. [Google Scholar] [CrossRef]
- Liu, S. A Synergetic Pretreatment Technology for Woody Biomass Conversion. Appl. Energy 2015, 144, 114–128. [Google Scholar] [CrossRef]
- Zhao, X.; Luo, K.; Zhang, Y.; Zheng, Z.; Cai, Y.; Wen, B.; Cui, Z.; Wang, X. Improving the Methane Yield of Maize Straw: Focus on the Effects of Pretreatment with Fungi and Their Secreted Enzymes Combined with Sodium Hydroxide. Bioresour. Technol. 2018, 250, 204–213. [Google Scholar] [CrossRef] [PubMed]
- Alexandropoulou, M.; Antonopoulou, G.; Fragkou, E.; Ntaikou, I.; Lyberatos, G. Fungal Pretreatment of Willow Sawdust and Its Combination with Alkaline Treatment for Enhancing Biogas Production. J. Environ. Manag. 2017, 203, 704–713. [Google Scholar] [CrossRef]
- Zheng, J.; Choo, K.; Bradt, C.; Lehoux, R.; Rehmann, L. Enzymatic Hydrolysis of Steam Exploded Corncob Residues after Pretreatment in a Twin-Screw Extruder. Biotechnol. Rep. 2014, 3, 99–107. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Zhou, T.; Li, X.; Xu, W.; Shi, J. Efficient Green Pre-Treatment of Coniferous Biomass Using Alkali-Assisted Deep Eutectic Solvents for Tall Oil Production and Lignin Component Separation. Sep. Purif. Technol. 2025, 361, 131474. [Google Scholar] [CrossRef]
- Hideno, A.; Inoue, H.; Tsukahara, K.; Fujimoto, S.; Minowa, T.; Inoue, S.; Endo, T.; Sawayama, S. Wet Disk Milling Pretreatment without Sulfuric Acid for Enzymatic Hydrolysis of Rice Straw. Bioresour. Technol. 2009, 100, 2706–2711. [Google Scholar] [CrossRef]
- Lin, H.; Zhi, T.; Zhang, L.; Liu, C.; Dong, Y. Effects of Acid/Alkali-Pretreated Peanut Shells as a Cheap Carbon Source for the Bio-Reduction of Sulfate. J. Clean. Prod. 2023, 385, 135753. [Google Scholar] [CrossRef]
- Zheng, Q.; Zhou, T.; Wang, Y.; Cao, X.; Wu, S.; Zhao, M.; Wang, H.; Xu, M.; Zheng, B.; Zheng, J.; et al. Pretreatment of Wheat Straw Leads to Structural Changes and Improved Enzymatic Hydrolysis. Sci. Rep. 2018, 8, 1321. [Google Scholar] [CrossRef] [PubMed]
- Madyira, D.M.; Olatunji, K.O. Alkali Pretreatment of Lignocellulose Feedstock Improves Morphological Structure and Biomethane Yield. Sustainability 2025, 17, 534. [Google Scholar] [CrossRef]
- Kalhor, P.; Ghandi, K. Deep Eutectic Solvents for Pretreatment, Extraction, and Catalysis of Biomass and Food Waste. Molecules 2019, 24, 4012. [Google Scholar] [CrossRef] [PubMed]
- Jönsson, L.J.; Martín, C. Pretreatment of Lignocellulose: Formation of Inhibitory By-Products and Strategies for Minimizing Their Effects. Bioresour. Technol. 2016, 199, 103–112. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Zhai, R.; Shi, K.; Yuan, Y.; Dale, B.E.; Gao, Z.; Jin, M. Mixing Alkali Pretreated and Acid Pretreated Biomass for Cellulosic Ethanol Production Featuring Reduced Chemical Use and Decreased Inhibitory Effect. Ind. Crops Prod. 2018, 124, 719–725. [Google Scholar] [CrossRef]
- Sodré, V.; Vilela, N.; Tramontina, R.; Squina, F.M. Microorganisms as Bioabatement Agents in Biomass to Bioproducts Applications. Biomass Bioenergy 2021, 151, 106161. [Google Scholar] [CrossRef]
- Palmqvist, E.; Hahn-Hägerdal, B. Fermentation of Lignocellulosic Hydrolysates. II: Inhibitors and Mechanisms of Inhibition. Bioresour. Technol. 2000, 74, 25–33. [Google Scholar] [CrossRef]
- Humbird, D.; Davis, R.; Tao, L.; Kinchin, C.; Hsu, D.; Aden, A.; Schoen, P.; Lukas, J.; Olthof, B.; Worley, M.; et al. Process Design and Economics for Biochemical Conversion of Lignocellulosic Biomass to Ethanol: Dilute-Acid Pretreatment and Enzymatic Hydrolysis of Corn Stover; National Renewable Energy Laboratory: Golden, CO, USA, 2011.
- Kazi, F.K.; Fortman, J.A.; Anex, R.P.; Hsu, D.D.; Aden, A.; Dutta, A.; Kothandaraman, G. Techno-Economic Comparison of Process Technologies for Biochemical Ethanol Production from Corn Stover. Fuel 2010, 89, S20–S28. [Google Scholar] [CrossRef]
- Mesa, L.; González, E.; Cara, C.; González, M.; Castro, E.; Mussatto, S.I. The Effect of Organosolv Pretreatment Variables on Enzymatic Hydrolysis of Sugarcane Bagasse. Chem. Eng. J. 2011, 168, 1157–1162. [Google Scholar] [CrossRef]
- Travaini, R.; Otero, M.D.M.; Coca, M.; Da-Silva, R.; Bolado, S. Sugarcane Bagasse Ozonolysis Pretreatment: Effect on Enzymatic Digestibility and Inhibitory Compound Formation. Bioresour. Technol. 2013, 133, 332–339. [Google Scholar] [CrossRef]
- Qiu, Z.; Aita, G.M.; Walker, M.S. Effect of Ionic Liquid Pretreatment on the Chemical Composition, Structure and Enzymatic Hydrolysis of Energy Cane Bagasse. Bioresour. Technol. 2012, 117, 251–256. [Google Scholar] [CrossRef]
- Thoresen, P.P.; Matsakas, L.; Rova, U.; Christakopoulos, P. Recent Advances in Organosolv Fractionation: Towards Biomass Fractionation Technology of the Future. Bioresour. Technol. 2020, 306, 123189. [Google Scholar] [CrossRef]
- Den, W.; Sharma, V.K.; Lee, M.; Nadadur, G.; Varma, R.S. Lignocellulosic Biomass Transformations via Greener Oxidative Pretreatment Processes: Access to Energy and Value-Added Chemicals. Front. Chem. 2018, 6, 141. [Google Scholar] [CrossRef]
- Mani, K.A.; Kumar, L.; Barrios, N.; Agate, S.; Mittal, A.; Yarbrough, J.; Jameel, H.; Lucia, L.; Pal, L. Emergence of Deep Eutectic Solvents (DES): Chemistry, Preparation, Properties, and Applications in Biorefineries and Critical Materials. Prog. Mater. Sci. 2026, 157, 101586. [Google Scholar] [CrossRef]
- Kunz, W.; Häckl, K. The Hype with Ionic Liquids as Solvents. Chem. Phys. Lett. 2016, 661, 6–12. [Google Scholar] [CrossRef]
- Wang, W.; Lee, D.J. Lignocellulosic Biomass Pretreatment by Deep Eutectic Solvents on Lignin Extraction and Saccharification Enhancement: A Review. Bioresour. Technol. 2021, 339, 125587. [Google Scholar] [CrossRef] [PubMed]
- MacFarlane, D.R.; Golding, J.; Forsyth, S.; Forsyth, M.; Deacon, G.B. Low Viscosity Ionic Liquids Based on Organic Salts of the Dicyanamide Anion. Chem. Commun. 2001, 16, 1430–1431. [Google Scholar] [CrossRef]
- Jiménez-Amezcua, I.; López-Martínez, M.I.; Ruiz-Matute, A.I.; Sanz, M.L. Advances and Challenges in the Selective Extraction of Low Molecular Weight Carbohydrates Using Ionic Liquids and Deep Eutectic Solvents. TrAC Trends Anal. Chem. 2024, 171, 117507. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel Solvent Properties of Choline Chloride/Urea Mixtures. Chem. Commun. 2003, 39, 70–71. [Google Scholar] [CrossRef] [PubMed]
- Anastas, P.; Eghbali, N. Green Chemistry: Principles and Practice. Chem. Soc. Rev. 2010, 39, 301–312. [Google Scholar] [CrossRef]
- Tan, Y.T.; Ngoh, G.C.; Chua, A.S.M. Evaluation of Fractionation and Delignification Efficiencies of Deep Eutectic Solvents on Oil Palm Empty Fruit Bunch. Ind. Crops Prod. 2018, 123, 271–277. [Google Scholar] [CrossRef]
- Procentese, A.; Johnson, E.; Orr, V.; Garruto Campanile, A.; Wood, J.A.; Marzocchella, A.; Rehmann, L. Deep Eutectic Solvent Pretreatment and Subsequent Saccharification of Corncob. Bioresour. Technol. 2015, 192, 31–36. [Google Scholar] [CrossRef]
- Fischer, V.; Kunz, W. Properties of Sugar-Based Low-Melting Mixtures. Mol. Phys. 2014, 112, 1241–1245. [Google Scholar] [CrossRef]
- Shekaari, H.; Ahadzadeh, I.; Karimi, S. Understanding Solvation Behavior of Glucose in Aqueous Solutions of Some Deep Eutectic Solvents by Thermodynamic Approach. J. Mol. Liq. 2019, 289, 111000. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T. Ionic Liquids and Deep-Eutectic Solvents in Extractive Metallurgy: Mismatch Between Academic Research and Industrial Applicability. J. Sustain. Metall. 2023, 9, 423–438. [Google Scholar] [CrossRef]
- Brodeur, G.; Yau, E.; Badal, K.; Collier, J.; Ramachandran, K.B.; Ramakrishnan, S. Chemical and Physicochemical Pretreatment of Lignocellulosic Biomass: A Review. Enzym. Res. 2011, 2011, 787532. [Google Scholar] [CrossRef]
- Galbe, M.; Wallberg, O. Pretreatment for Biorefineries: A Review of Common Methods for Efficient Utilisation of Lignocellulosic Materials. Biotechnol. Biofuels 2019, 12, 294. [Google Scholar] [CrossRef] [PubMed]
- Loow, Y.L.; New, E.K.; Yang, G.H.; Ang, L.Y.; Foo, L.Y.W.; Wu, T.Y. Potential Use of Deep Eutectic Solvents to Facilitate Lignocellulosic Biomass Utilization and Conversion. Cellulose 2017, 24, 3591–3618. [Google Scholar] [CrossRef]
- Francisco, M.; Van Den Bruinhorst, A.; Kroon, M.C. New Natural and Renewable Low Transition Temperature Mixtures (LTTMs): Screening as Solvents for Lignocellulosic Biomass Processing. Green Chem. 2012, 14, 2153–2157. [Google Scholar] [CrossRef]
- Singhvi, M.S.; Chaudhari, S.; Gokhale, D.V. Lignocellulose Processing: A Current Challenge. RSC Adv. 2014, 4, 8271–8277. [Google Scholar] [CrossRef]
- Maibam, P.D.; Goyal, A. Approach to an Efficient Pretreatment Method for Rice Straw by Deep Eutectic Solvent for High Saccharification Efficiency. Bioresour. Technol. 2022, 351, 127057. [Google Scholar] [CrossRef]
- Kumar, N.; Gautam, R.; Stallings, J.D.; Coty, G.G.; Lynam, J.G. Secondary Agriculture Residues Pretreatment Using Deep Eutectic Solvents. Waste Biomass Valorization 2021, 12, 2259–2269. [Google Scholar] [CrossRef]
- Xu, G.C.; Ding, J.C.; Han, R.Z.; Dong, J.J.; Ni, Y. Enhancing Cellulose Accessibility of Corn Stover by Deep Eutectic Solvent Pretreatment for Butanol Fermentation. Bioresour. Technol. 2016, 203, 364–369. [Google Scholar] [CrossRef]
- Zhang, C.W.; Xia, S.Q.; Ma, P.S. Facile Pretreatment of Lignocellulosic Biomass Using Deep Eutectic Solvents. Bioresour. Technol. 2016, 219, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Filippi, K.; Stylianou, E.; Pateraki, C.; Koutinas, A.; Ladakis, D. Pretreatment of Grape Pomaces and Stalks Using Deep Eutectic Solvents for Succinic Acid Production Integrated in a Biorefinery Concept. Waste Biomass Valorization 2023, 14, 2857–2872. [Google Scholar] [CrossRef]
- Jose, D.; Tawai, A.; Divakaran, D.; Bhattacharyya, D.; Venkatachalam, P.; Tantayotai, P.; Sriariyanun, M. Integration of Deep Eutectic Solvent in Biorefining Process of Lignocellulosic Biomass Valorization. Bioresour. Technol. Rep. 2023, 21, 101365. [Google Scholar] [CrossRef]
- Roslan, M.F.; Luthfi, A.A.I.; Salleh, M.Z.M.; Manaf, S.F.A.; Nasoha, N.Z.; Hariz, H.B.; Tan, J.P.; Abdul, P.M. Deep Eutectic Solvent Pretreatment for Enhanced Enzymatic Hydrolysis of Pineapple Biomass via Response Surface Methodology. Biomass Convers. Biorefin. 2025, 15, 12825–12842. [Google Scholar] [CrossRef]
- Li, X.; Tang, W.; He, Y.C. Integrated Understanding of Acidic Deep Eutectic Solvent Choline Chloride: Oxalic Acid Pretreatment to Enhance the Enzymatic Hydrolysis of Rape Straw. Ind. Crops Prod. 2023, 206, 117691. [Google Scholar] [CrossRef]
- Ma, H.; Fu, P.; Zhao, J.; Lin, X.; Wu, W.; Yu, Z.; Xia, C.; Wang, Q.; Gao, M.; Zhou, J. Pretreatment of Wheat Straw Lignocelluloses by Deep Eutectic Solvent for Lignin Extraction. Molecules 2022, 27, 7955. [Google Scholar] [CrossRef] [PubMed]
- Jančíková, V.; Jablonský, M. Exploiting Deep Eutectic Solvent-like Mixtures for Fractionation Biomass, and the Mechanism Removal of Lignin: A Review. Sustainability 2024, 16, 504. [Google Scholar] [CrossRef]
- Zhai, R.; Hu, J.; Jin, M. Towards Efficient Enzymatic Saccharification of Pretreated Lignocellulose: Enzyme Inhibition by Lignin-Derived Phenolics and Recent Trends in Mitigation Strategies. Biotechnol. Adv. 2022, 61, 108044. [Google Scholar] [CrossRef]
- Lyu, Q.; Dar, R.A.; Baganz, F.; Smoliński, A.; Rasmey, A.-H.M.; Liu, R.; Zhang, L. Effects of Lignocellulosic Biomass-Derived Hydrolysate Inhibitors on Cell Growth and Lipid Production During Microbial Fermentation of Oleaginous Microorganisms—A Review. Fermentation 2025, 11, 121. [Google Scholar] [CrossRef]
- Xie, F.; Zhang, W.; Gong, S.; Wang, Z. Inhibitory Effect of Lignin from Canna Edulis Ker Residues on Trypsin: Kinetics and Molecular Docking Studies. J. Sci. Food Agric. 2021, 101, 2090–2099. [Google Scholar] [CrossRef] [PubMed]
- Tan, Y.T.; Chua, A.S.M.; Ngoh, G.C. Evaluation on the Properties of Deep Eutectic Solvent-Extracted Lignin for Potential Aromatic Bioproducts conversion. Ind. Crops Prod. 2020, 154, 112729. [Google Scholar] [CrossRef]
- Zhu, Y.; Yang, T.X.; Qi, B.K.; Li, H.; Zhao, Q.S.; Zhao, B. Acidic and Alkaline Deep Eutectic Solvents (DESs) Pretreatment of Grapevine: Component Analysis, Characterization, Lignin Structural Analysis, and Antioxidant Properties. Int. J. Biol. Macromol. 2023, 236, 123977. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Wang, A.; Yan, C.; Liu, S.; Li, L.; Wu, Q.; Liu, Y.; Liu, Y.; Nie, G.; Nie, S.; et al. Study on the Solubility of Industrial Lignin in Choline Chloride-Based Deep Eutectic Solvents. Sustainability 2023, 15, 7118. [Google Scholar] [CrossRef]
- Julshahril, N.H.; Phuah, E.T.; Rambli, M.M. Deep Eutectic Solvents in the Extraction of Bioactive Compounds in Agri-Food Industry. Food Humanit. 2025, 4, 100468. [Google Scholar] [CrossRef]
- Cvjetko Bubalo, M.; Ćurko, N.; Tomašević, M.; Kovačević Ganić, K.; Radojcic Redovnikovic, I. Green Extraction of Grape Skin Phenolics by Using Deep Eutectic Solvents. Food Chem. 2016, 200, 159–166. [Google Scholar] [CrossRef]
- Ozturk, B.; Parkinson, C.; Gonzalez-Miquel, M. Extraction of Polyphenolic Antioxidants from Orange Peel Waste Using Deep Eutectic Solvents. Sep. Purif. Technol. 2018, 206, 1–13. [Google Scholar] [CrossRef]
- Deniz, S.; Ünlü, A.E.; Takaç, S. Ultrasound-Assisted Natural Deep Eutectic Solvent Extraction of Phenolic Compounds from Apple Pomace. Sep. Sci. Technol. 2023, 58, 302–313. [Google Scholar] [CrossRef]
- Hamieau, M.; Loulergue, P.; Szydłowska-Czerniak, A. Green Solvent Extraction of Antioxidants from Herbs and Agro-Food Wastes: Optimization and Capacity Determination. Appl. Sci. 2024, 14, 2936. [Google Scholar] [CrossRef]
- Li, G.; Zhu, T.; Row, K.H. Isolation of Ferulic Acid from Wheat Bran with a Deep Eutectic Solvent and Modified Silica Gel. Anal. Lett. 2017, 50, 1926–1938. [Google Scholar] [CrossRef]
- Yoo, D.E.; Jeong, K.M.; Han, S.Y.; Kim, E.M.; Jin, Y.; Lee, J. Deep Eutectic Solvent-Based Valorization of Spent Coffee Grounds. Food Chem. 2018, 255, 357–364. [Google Scholar] [CrossRef]
- Wahlström, R.; Rommi, K.; Willberg-Keyriläinen, P.; Ercili-Cura, D.; Holopainen-Mantila, U.; Hiltunen, J.; Mäkinen, O.; Nygren, H.; Mikkelson, A.; Kuutti, L. High Yield Protein Extraction from Brewer’s Spent Grain with Novel Carboxylate Salt–Urea Aqueous Deep Eutectic Solvents. ChemistrySelect 2017, 2, 9355–9363. [Google Scholar] [CrossRef]
- Bleus, D.; Blockx, H.; Gesquiere, E.; Adriaensens, P.; Samyn, P.; Marchal, W.; Vandamme, D. High-Temperature Hydrothermal Extraction of Phenolic Compounds from Brewer’s Spent Grain and Malt Dust Biomass Using Natural Deep Eutectic Solvents. Molecules 2024, 29, 1983. [Google Scholar] [CrossRef]
- Makrygiannis, I.; Athanasiadis, V.; Bozinou, E.; Chatzimitakos, T.; Makris, D.P.; Lalas, S.I. Combined Effects of Deep Eutectic Solvents and Pulsed Electric Field Improve Polyphenol-Rich Extracts from Apricot Kernel Biomass. Biomass 2023, 3, 66–77. [Google Scholar] [CrossRef]
- Lazzarini, C.; Casadei, E.; Valli, E.; Tura, M.; Ragni, L.; Bendini, A.; Toschi, T.G. Sustainable Drying and Green Deep Eutectic Extraction of Carotenoids from Tomato Pomace. Foods 2022, 11, 405. [Google Scholar] [CrossRef]
- Popovic, B.M.; Micic, N.; Potkonjak, A.; Blagojevic, B.; Pavlovic, K.; Milanov, D.; Juric, T. Novel Extraction of Polyphenols from Sour Cherry Pomace Using Natural Deep Eutectic Solvents—Ultrafast Microwave-Assisted NADES Preparation and Extraction. Food Chem. 2022, 366, 130562. [Google Scholar] [CrossRef]
- Sportiello, L.; Marchesi, E.; Tolve, R.; Favati, F. Green Extraction of Carotenoids from Pumpkin By-Products Using Natural Hydrophobic Deep Eutectic Solvents: Preliminary Insights. Molecules 2025, 30, 548. [Google Scholar] [CrossRef]
- Vázquez-González, M.; Fernández-Prior, Á.; Bermúdez Oria, A.; Rodríguez-Juan, E.M.; Pérez-Rubio, A.G.; Fernández-Bolaños, J.; Rodríguez-Gutiérrez, G. Utilization of Strawberry and Raspberry Waste for the Extraction of Bioactive Compounds by Deep Eutectic Solvents. LWT 2020, 130, 109645. [Google Scholar] [CrossRef]
- Kalhor, P.; Ghandi, K. Deep Eutectic Solvents as Catalysts for Upgrading Biomass. Catalysts 2021, 11, 178. [Google Scholar] [CrossRef]
- Bu, C.Y.; Yan, Y.X.; Zou, L.H.; Ouyang, S.P.; Zheng, Z.J.; Ouyang, J. Comprehensive Utilization of Corncob for Furfuryl Alcohol Production by Chemo-Enzymatic Sequential Catalysis in a Biphasic System. Bioresour. Technol. 2021, 319, 124156. [Google Scholar] [CrossRef] [PubMed]
- Arslanoğlu, A.; Sert, M. Direct Conversion of Biomass to Platform Chemicals, Catalyzed Using a Deep Eutectic Solvent of N,N Diethyl Ethanol Ammonium Chloride-Oxalic Acid in a Microwave Reactor. Fuel 2019, 258, 116142. [Google Scholar] [CrossRef]
- Kumar, A.K.; Parikh, B.S.; Pravakar, M. Natural Deep Eutectic Solvent Mediated Pretreatment of Rice Straw: Bioanalytical Characterization of Lignin Extract and Enzymatic Hydrolysis of Pretreated Biomass Residue. Environ. Sci. Pollut. Res. 2016, 23, 9265–9275. [Google Scholar] [CrossRef] [PubMed]
- Mamilla, J.L.K.; Novak, U.; Grilc, M.; Likozar, B. Natural Deep Eutectic Solvents (DES) for Fractionation of Waste Lignocellulosic Biomass and Its Cascade Conversion to Value-Added Bio-Based Chemicals. Biomass Bioenergy 2019, 120, 417–425. [Google Scholar] [CrossRef]
- Gawade, A.B.; Yadav, G.D. Microwave Assisted Synthesis of 5-Ethoxymethylfurfural in One Pot from D-Fructose by Using Deep Eutectic Solvent as Catalyst under Mild Condition. Biomass Bioenergy 2018, 117, 38–43. [Google Scholar] [CrossRef]
- Lee, K.M.; Quek, J.D.; Tey, W.Y.; Lim, S.; Kang, H.S.; Quen, L.K.; Mahmood, W.A.W.; Jamaludin, S.I.S.; Teng, K.H.; Khoo, K.S. Biomass Valorization by Integrating Ultrasonication and Deep Eutectic Solvents: Delignification, Cellulose Digestibility and Solvent Reuse. Biochem. Eng. J. 2022, 187, 108587. [Google Scholar] [CrossRef]
- Okonkwo, C.E.; Hussain, S.Z.; Manzoor, S.; Naseer, B.; Taiwo, A.E.; Ayyash, M.; Al-Marzouqi, A.H.; Kamal-Eldin, A. A Comprehensive Review on the Use of Deep Eutectic Solvents for Biomass Processing, and the Synergistic Coupling with Physical Technology and Biological Method. Bioresour. Technol. Rep. 2023, 23, 101577. [Google Scholar] [CrossRef]
- Husanu, E.; Mero, A.; Rivera, J.G.; Mezzetta, A.; Ruiz, J.C.; D’andrea, F.; Pomelli, C.S.; Guazzelli, L. Exploiting Deep Eutectic Solvents and Ionic Liquids for the Valorization of Chestnut Shell Waste. ACS Sustain. Chem. Eng. 2020, 8, 18386–18399. [Google Scholar] [CrossRef]
- Manivannan, H.; Anguraj, B.L. Valorization of Fruit Waste Using DES Pretreatment and Hydrolysis over a Heterogeneous Catalyst for Bioethanol Production. Biomass Convers. Biorefin. 2023, 13, 5731–5741. [Google Scholar] [CrossRef]
- Guo, Z.; Ling, Z.; Wang, C.; Zhang, X.; Xu, F. Integration of Facile Deep Eutectic Solvents Pretreatment for Enhanced Enzymatic Hydrolysis and Lignin Valorization from Industrial Xylose Residue. Bioresour. Technol. 2018, 265, 334–339. [Google Scholar] [CrossRef]
- Vladić, J.; Jakovljević Kovač, M.; Pavić, V.; Jokić, S.; Simić, S.; Paiva, A.; Jerković, I.; Duarte, A.R. Towards a Greener Approach for Biomass Valorization: Integration of Supercritical Fluid and Deep Eutectic Solvents. Antibiotics 2023, 12, 1031. [Google Scholar] [CrossRef]
- Plaza, A.; Tapia, X.; Yañez, C.; Vilches, F.; Candia, O.; Cabezas, R.; Romero, J. Obtaining Hydroxytyrosol from Olive Mill Waste Using Deep Eutectic Solvents and Then Supercritical CO2. Waste Biomass Valorization 2020, 11, 6273–6284. [Google Scholar] [CrossRef]
- Kim, K.H.; Yoo, C.G. Challenges and Perspective of Recent Biomass Pretreatment Solvents. Front. Chem. Eng. 2021, 3, 785709. [Google Scholar] [CrossRef]
- Satlewal, A.; Agrawal, R.; Bhagia, S.; Sangoro, J.; Ragauskas, A.J. Natural Deep Eutectic Solvents for Lignocellulosic Biomass Pretreatment: Recent Developments, Challenges and Novel Opportunities. Biotechnol. Adv. 2018, 36, 2032–2050. [Google Scholar] [CrossRef]
- Liang, X.; Fu, Y.; Chang, J. Effective Separation, Recovery and Recycling of Deep Eutectic Solvent after Biomass Fractionation with Membrane-Based Methodology. Sep. Purif. Technol. 2019, 210, 409–416. [Google Scholar] [CrossRef]
- Isci, A.; Kaltschmitt, M. Recovery and Recycling of Deep Eutectic Solvents in Biomass Conversions: A Review. Biomass Convers. Biorefin. 2022, 12, 197–226. [Google Scholar] [CrossRef]
- Sun, Y.Q.; Yuan, Y.; Dai, K.X.; Xiu, Z.L. The Pretreatment of the Sustainable Biomass Feedstock of Pennisetum Giganteum for Biorefinery Using Deep Eutectic Solvents. Bioresour. Technol. 2023, 384, 129289. [Google Scholar] [CrossRef] [PubMed]
- Panakkal, E.J.; Cheenkachorn, K.; Chuetor, S.; Dasari, S.; Katam, K.; Phusantisampan, T.; Cheng, Y.-S.; Sriariyanun, M. A Comparative Study on Effectiveness and Recyclability of Three Different Deep Eutectic Solvents for Biomass Fractionation. Biomass Convers. Biorefin. 2024, 15, 13393–13407. [Google Scholar] [CrossRef]
- Zhou, M.; Fakayode, O.A.; Ahmed Yagoub, A.E.G.; Ji, Q.; Zhou, C. Lignin Fractionation from Lignocellulosic Biomass Using Deep Eutectic Solvents and Its Valorization. Renew. Sustain. Energy Rev. 2022, 156, 111986. [Google Scholar] [CrossRef]
- Zaib, Q.; Eckelman, M.J.; Yang, Y.; Kyung, D. Are Deep Eutectic Solvents Really Green?: A Life-Cycle Perspective. Green Chem. 2022, 24, 7924–7930. [Google Scholar] [CrossRef]
- Nejrotti, S.; Antenucci, A.; Pontremoli, C.; Gontrani, L.; Barbero, N.; Carbone, M.; Bonomo, M. Critical Assessment of the Sustainability of Deep Eutectic Solvents: A Case Study on Six Choline Chloride-Based Mixtures. ACS Omega 2022, 7, 47449–47461. [Google Scholar] [CrossRef]
- Yiin, C.L.; Lai, Z.Y.; Chin, B.L.F.; Lock, S.S.M.; Cheah, K.W.; Taylor, M.J.; Al-Gailani, A.; Kolosz, B.W.; Chan, Y.H. Green Pathways for Biomass Transformation: A Holistic Evaluation of Deep Eutectic Solvents (DESs) through Life Cycle and Techno-Economic Assessment. J. Clean. Prod. 2024, 470, 143248. [Google Scholar] [CrossRef]
- Zang, G.; Shah, A.; Wan, C. Techno-Economic Analysis of Co-Production of 2,3-Butanediol, Furfural, and Technical Lignin via Biomass Processing Based on Deep Eutectic Solvent Pretreatment. Biofuels Bioprod. Biorefin. 2020, 14, 326–343. [Google Scholar] [CrossRef]
- Song, W.; He, Y.; Huang, R.; Li, J.; Yu, Y.; Xia, P. Life Cycle Assessment of Deep-Eutectic-Solvent-Assisted Hydrothermal Disintegration of Microalgae for Biodiesel and Biogas Co-Production. Appl. Energy 2023, 335, 120758. [Google Scholar] [CrossRef]

| Hydrogen Bond Acceptor | Hydrogen Bond Donor |
|---|---|
| Choline Chloride | Urea |
| Tetrabutylammonium Chloride | Glycerol |
| Methyltriphenylphosphonium Bromide | Malonic Acid |
| Imidazolium Chloride | Citric Acid |
| Ethylammonium Nitrate | Lactic Acid |
| Dimethylsulfoxide | Tartaric Acid |
| Ethylene Glycol | Acetic Acid |
| Triethylammonium Chloride | Levulinic Acid |
| Tetraethylammonium Bromide | Propionic Acid |
| Dimethylacetamide | Glucose |
| Dimethylformamide | Sorbitol |
| Diethyl Ether | Mannitol |
| Dimethyl Carbonate | Polyethylene Glycol (PEG) |
| Acetonitrile | Ethylene Glycol |
| N, N-Dimethylformamide | Xylitol |
| Biomass Type | DES Composition | Pretreatment Time (h) | Pretreatment Temperature (°C) | Biomass to Solvent Ration | Lignin Removal (%) | Cellulose/Sugar Yield (%) | Key Observations/Trends | References | |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Rice straw | ChCl–acetic acid | 2.5 | 126 | 01:03.6 | 83.1 | - | ChCl–acetic acid pretreatment produced cellulose-rich rice straw, yielding high reducing sugar and proving its efficacy | [77] |
| 2 | Wheat Straw | Choline chloride–Monoethanolamine | 9 | 70 | 1:20 | 71.4 | 93.7 | Choline chloride: monoethanolamine at pretreatment time 9 h and 70 °C was the best solvent among these DES’s. | [12] |
| Choline chloride–Monoethanolamine | 12 | 90 | 1:20 | 81 | 90.8 | ||||
| Choline chloride–Glycerol | 12 | 90 | 1:20 | 24.7 | 97.8 | ||||
| Choline chloride–Urea | 12 | 90 | 1:20 | 27.7 | 95.9 | ||||
| Choline chloride–Diethanolamine | 12 | 90 | 1:02 | 73.5 | 98 | ||||
| 3 | Brewers’ spent grain | Choline chloride–glycerol (1:2) | 3 | 60 | 1:08 | - | 81 | In both DES treatments low temperature and small solid to solvent ratio—increased the amount of recovered biomass. | [14] |
| 3 | 60 | 1:16 | - | 80 | |||||
| 3 | 60 | 1:32 | - | 80 | |||||
| 3 | 115 | 1:08 | - | 75 | |||||
| 3 | 115 | 1:16 | - | 74 | |||||
| 3 | 115 | 1:32 | - | 73 | |||||
| Choline chloride–ethylene glycol (1:2) | 3 | 60 | 1:08 | - | 80 | ||||
| 3 | 60 | 1:16 | - | 80 | |||||
| 3 | 60 | 1:32 | - | 79 | |||||
| 3 | 115 | 1:08 | - | 76 | |||||
| 3 | 115 | 1:16 | - | 76 | |||||
| 3 | 115 | 1:32 | - | 74 | |||||
| 4 | Rice hulls | Choline chloride-Formic acid (1:2) | 2 | 155 | 1:10 | 48 | 60 | Overall, the biomass that underwent pretreatment was more cellulosic, and less concentrated in lignin compared to raw biomass. DES composed of Choline Chloride-Formic acid was more efficient as pretreatment solution for rice hulls. | [78] |
| Choline chloride-Lactic acid (1:10) | 2 | 155 | 1:10 | 24 | 55 | ||||
| Choline chloride-Acetic acid (1:2) | 2 | 155 | 1:10 | 28 | 58 | ||||
| Betaine–Lactic acid (1:2) | 2 | 155 | 1:10 | 0 | 44 | ||||
| Proline -Lactic acid (1:3.3) | 2 | 155 | 1:10 | 0 | 43 | ||||
| 5 | Sugarcane Bagasse | Choline chloride-Formic acid (1:2) | 2 | 155 | 1:10 | 79 | 60 | DESs with choline chloride as hydrogen bond acceptor were effective compared to proline as hydrogen bond acceptor. | [78] |
| Choline chloride-Lactic acid (1:10) | 2 | 155 | 1:10 | 79 | 60 | ||||
| Choline chloride-Acetic acid (1:2) | 2 | 155 | 1:10 | 76 | 62 | ||||
| Betaine–Lactic acid (1:2) | 2 | 155 | 1:10 | - | - | ||||
| Proline -Lactic acid (1:3.3) | 2 | 155 | 1:10 | 42 | 47 | ||||
| 6 | Corn stover | Choline chloride:formic acid | 2 | 130 | 1:20 | 23.8 | 48 | Choline chloride–formic acid was more efficient as DES. | [79] |
| 1-Butyl-3-methylimidazolium chloride | 2 | 130 | 1:20 | 8.5 | 36 | ||||
| 7 | Corncob | Choline chloride–Lactic acid (1:2) | - | 90 | 1:20 | 64.7 | 81.6 | Choline chloride–Lactic acid (1:15) was the most efficient solvent in removing lignin, while Choline chloride–Glycerol (1:2) was more successful for yield of glucose. | [80] |
| Choline chloride–Lactic acid (1:5) | - | 90 | 1:20 | 77.9 | 83.5 | ||||
| Choline chloride–Lactic acid (1:10) | - | 90 | 1:20 | 86.1 | 83.2 | ||||
| Choline chloride–Lactic acid (1:15) | - | 90 | 1:20 | 93.1 | 79.1 | ||||
| Choline chloride–Oxalic acid (1:1) | - | 90 | 1:20 | 98.5 | 45.2 | ||||
| Choline chloride–Ethylene glycol (1:2) | - | 90 | 1:20 | 87.6 | 85.3 | ||||
| Choline chloride–Glycerol (1:2) | - | 90 | 1:20 | 71.3 | 96.4 | ||||
| 8 | Grape pomace and stalks | Choline chloride–Formic acid (1:2) | 2 | 120 | 1:10 | 47.9 | - | Choline chloride–Formic acid (1:2) and Choline chloride–Lactic acid (1:10) were more effective in lignin removal. | [81] |
| Choline chloride–Acetic acid (1:2) | 2 | 120 | 1:10 | 22.5 | - | ||||
| Choline chloride–Oxalic acid (1:1) | 2 | 120 | 1:10 | 7.8 | - | ||||
| Choline chloride–Lactic acid (1:10) | 2 | 120 | 1:10 | 47.1 | - | ||||
| Choline chloride–Lactic acid (1:2) | 2 | 120 | 1:10 | 19.6 | - | ||||
| Choline chloride–Lactic acid (1:5) | 2 | 120 | 1:10 | 22.7 | - | ||||
| Choline chloride–Lactic acid (1:15) | 2 | 120 | 1:10 | 43 | - | ||||
| 9 | Potato peels | Choline chloride–glycerol | 3 | 60 | 1:08 | 33 | 85 | Lower solid-to-solvent ratio (1:8) and lower temperature (60 °C) yielded higher compound recovery. | [14] |
| 1:32 | 83 | ||||||||
| 115 | 1:08 | 80 | |||||||
| 1:32 | 75 | ||||||||
| 1:08 | 53 | ||||||||
| 150 | 1:32 | 52 | |||||||
| 10 | Pineapple peel | Choline chloride–Oxalic acid (1:1) | 1 | 99.65 | 1:01 | 72.4 | 88.35 | Acidic DES (CC–OA) showed superior cellulose recovery and hemicellulose/lignin removal compared with other DESs. | [83] |
| Choline chloride–Lactic acid (1:2) | |||||||||
| Choline chloride–Ethylene glycol (1:2) | |||||||||
| Choline chloride–Ethylene glycol (1:2) | |||||||||
| Choline chloride–Glycerol (1:2) | |||||||||
| 11 | Rape straw | Choline chloride–Oxalic acid (1:1) | 1 | 130 | 1:10 | 83.6 | Choline chloride–Oxalic acid (3:1) gave highest delignification (83.6%) and enzymatic efficiency (96%) with improved cellulose accessibility. | [84] | |
| Choline chloride–Oxalic acid (3:1) |
| # | Biomass Type | DES Composition | Target Compounds | Extraction Conditions | Extraction Efficiency | Additional Notes | References |
|---|---|---|---|---|---|---|---|
| 1 | Grape skin | ChCl/glucose | Phenolics | 80 °C for 2–6 h | Among the DES tested, ChCl/oxalic acid with 25% water was the most effective solvent for extracting grape skin phenolic compounds, outperforming conventional organic solvents. | [94] | |
| ChCl/sorbose | |||||||
| ChCl/glycerol | |||||||
| ChCl/proline | |||||||
| ChCl/malic acid | |||||||
| ChCl/oxalic acid | |||||||
| 2 | Orange peel waste | ChCl/EG (1:4) | Phenolic compounds | 60 °C, 100 min | DES outperformed the conventional solvents by providing higher total phenolic content (TPC) and antioxidant potential. | [95] | |
| 3 | Apple pomace | Choline chloride-lactic acid (1:1) | Phenolic compounds | 60 °C | The highest total flavonoid content, 17.30 mg EPE/g apple pomace, was obtained with choline chloride:urea, while the NADES composed of choline chloride:lactic acid exhibited significant antioxidant activity. | In addition to deep eutectic solvent, ultrasound-assisted extraction was employed | [96] |
| Choline chloride-lactic acid (1:6) | 60 °C | ||||||
| Choline chloride-lactic acid (1:9) | 60 °C | ||||||
| Choline chloride-malic acid (1:1) | 70 °C | ||||||
| Choline chloride-citric acid (1:1) | 80 °C | ||||||
| Choline chloride-citric acid (3:1) | 80 °C | ||||||
| Choline chloride–sucrose-water (1:1:11) | 50 °C | ||||||
| Glucose-fructose-water (1:1:11) | 50 °C | ||||||
| Fructose-sucrose-water (1:1:11) | 50 °C | ||||||
| Glucose-sucrose-water (1:1:11) | 50 °C | ||||||
| Choline chloride-ethylene glycol (1:2) | 80 °C | ||||||
| Choline chloride-glycerol (1:2) | 80 °C | ||||||
| Choline chloride-urea (1:2) | 80 °C | ||||||
| 4 | Buckwheat husk | Choline chloride/Citric acid (1:1) Glucose/Citric acid (1:1) | Antioxidants | 80 °C for 2–6 h | Glucose/Urea (1:1) solvent with various amounts of water achieved the best extraction efficiency for antioxidants from Buckwheat husk | [97] | |
| Glucose/Urea (1:1) | |||||||
| Glucose/Urea (1:1) | |||||||
| Betaine/Citric acid (1:1) | |||||||
| Betaine/Urea (1:1) | |||||||
| 5 | Wheat bran | Choline chloride-glycerol (1:1) | Ferulic acid | 80 °C for 2 h | The DES with choline chloride-glycerol at a 1:2 molar ratio provided the highest extraction yield of ferulic acid from wheat bran under optimal conditions of 50% DES, 15 mL/g liquid-to-material ratio, and 35 min of extraction | [98] | |
| Choline chloride-glycerol (1:2) | |||||||
| Choline chloride-glycerol (1:3) | |||||||
| Choline chloride-glycerol (1:4) | |||||||
| Choline chloride-glycerol (1:5) | |||||||
| 6 | Spent coffee grounds | Choline chloride-Urea (1:2) | Phenolic compounds | The mixtures was vigorously agitated at 80 °C until a homogeneous liquid formed | The testing and adjusting of solvents showed that a DES made of 1,6-hexanediol and ChCl in a 7:1 ratio (called HC-6) was the most effective. | Additionally, UAE (Ultrasound-Assisted Extraction) was used for extraction. | [99] |
| Choline chloride-Acetamide(1:2) | |||||||
| Choline chloride-Glycerol (1:2) | |||||||
| Choline chloride-Sorbitol (1:2) | |||||||
| Choline chloride-Ethylene glycol (1:2) | |||||||
| Choline chloride-1,4-Butanediol (1:2) | |||||||
| Choline chloride-1,6-Hexanediol (1:2) | |||||||
| Choline chloride-Malonic acid (1:2) | |||||||
| Choline chloride-Citric acid (1:2) | |||||||
| Choline chloride-Fructose-Water (5:2:5) | |||||||
| Choline chloride-Xylose-Water (2:1:2) | |||||||
| Choline chloride-Sucrose-Water (4:1:4) | |||||||
| Choline chloride-Glucose-Water (5:2:5) | |||||||
| 7 | Brewer’s Spent Grain | NaAcO:Urea (1:2) | Protein | 80 °C for 20 h | The extraction using 90 wt% sodium acetate and urea (1:2) was more effective than choline chloride and urea (1:2), achieving up to 79% protein yield from brewer’s spent grain. | [100] | |
| NaAcO:Urea (1:3) | |||||||
| KAcO:Urea (1:2) | |||||||
| KAcO:Urea (1:3) | |||||||
| NaForm:Urea (1:2) | |||||||
| NaForm:Urea (1:3) | |||||||
| 8 | Brewer’s Spent Grain and malt dust | malic acid-choline chloride (1:1) | Phenolic compounds | 120 °C | Using high-temperature hydrothermal extraction with acidic NADESs significantly boosts polyphenolic compound yields from brewer’s spent grain and malt dust compared to other methods | [101] | |
| glycerol-choline chloride (1:1) | |||||||
| 9 | Apricot Kernel Biomass | Glycerol–choline chloride (2:1) | Polyphenol rich extract | The mixture was heated at 80–90 °C for 90 min under stirring until a transparent liquid was formed. | Using DES increased polyphenol content by approximately 70%, and combining DES with PEF resulted in a 173% increase | Pulsed electric field (PEF) extraction methods used as complementary. | [102] |
| 10 | Tomato pomace | Ethyl acetate:ethyl lactate | carotenoids | Ultrasound-assisted extraction; solvent preheated; 60 °C, 20 min | Ethyl acetate:ethyl lactate with non-thermal air-drying yielded the highest lycopene (75.86 μg/g) and β-carotene (3950.08 μg/g) contents. | Non-thermal air-drying | [103] |
| 11 | Sour cherry pomace | ChCl:malic acid (1:1); ChCl:urea (1:2); ChCl:fructose (1:1) | Polyphenols | Microwave-assisted; 80–100 °C, 5–10 min | Yields up to 35 mg GAE/g dry weight with ChCl:malic acid, 1.5–2x higher than conventional solvents | Microwave-assisted NADES preparation and extraction; extracts exhibited >80% DPPH inhibition and antimicrobial effects against E. coli. | [104] |
| 12 | Pumpkin peels | DL-menthol:lactic acid (1:2); menthol:acetic acid (1:1) | β-carotene | Conventional shaking; 50 °C, 30 min, 10 mL/g | 0.823 mg/mL β-carotene yield (93.95% of acetone reference), with menthol:lactic acid (1:2) most effective. | Natural Hydrophobic Deep Eutectic Solvents as sustainable alternative; high efficiency without toxic solvents; potential for direct food/cosmetic use. | [105] |
| 13 | Blueberry leaves | Lactic acid:sodium acetate:water (3:1:2) | Phenolic compounds | Samples were sonicated 45 min at 65 °C using 15:1 and 75:1 (v/w) solvent ratios. | 1.6–2.2x higher phenolics and 1.6–2.8x antioxidants than conventional solvents. | [105] | |
| ChCl:oxalic acid (1:1) | |||||||
| 14 | Strawberry and raspberry waste | Choline chloride-Glycerol (1:2) | Phenolic compounds | DES preparation: Heated to 60 °C with agitation until viscous liquid formed | Choline chloride:Glycolic acid:Oxalic acid (1:1.7:0.3, 0% H2O) yielded highest phenolics from raspberry and strawberry | [106] | |
| Choline chloride:Sucrose (1:2, 25% H2O) | |||||||
| Choline chloride:1,4-Butanediol (1:5, 0% H2O) | |||||||
| Choline chloride:1,2-Propanediol (1:1, 7.5% H2O) | |||||||
| Betaine:Sucrose (2:1, 13% H2O) | |||||||
| Betaine:Levulinic acid (1:2, 0% H2O) | |||||||
| Choline chloride:Glycolic acid:Oxalic acid (1:1.7:0.3, 0% H2O) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toleugazykyzy, A.; Bekbayev, K.; Bolkenov, B.; Alwazeer, D.; Rskeldiyev, B.; Kuterbekov, K.; Bekmyrza, K.; Kabyshev, A.; Kubenova, M.; Opakhai, S. Comprehensive Review of Recent Trends in the Use of Deep Eutectic Solvents for the Valorization of Secondary Lignocellulosic Biomass. Sustainability 2025, 17, 9492. https://doi.org/10.3390/su17219492
Toleugazykyzy A, Bekbayev K, Bolkenov B, Alwazeer D, Rskeldiyev B, Kuterbekov K, Bekmyrza K, Kabyshev A, Kubenova M, Opakhai S. Comprehensive Review of Recent Trends in the Use of Deep Eutectic Solvents for the Valorization of Secondary Lignocellulosic Biomass. Sustainability. 2025; 17(21):9492. https://doi.org/10.3390/su17219492
Chicago/Turabian StyleToleugazykyzy, Akerke, Kairat Bekbayev, Bakytzhan Bolkenov, Duried Alwazeer, Berdikul Rskeldiyev, Kairat Kuterbekov, Kenzhebatyr Bekmyrza, Asset Kabyshev, Marzhan Kubenova, and Serikzhan Opakhai. 2025. "Comprehensive Review of Recent Trends in the Use of Deep Eutectic Solvents for the Valorization of Secondary Lignocellulosic Biomass" Sustainability 17, no. 21: 9492. https://doi.org/10.3390/su17219492
APA StyleToleugazykyzy, A., Bekbayev, K., Bolkenov, B., Alwazeer, D., Rskeldiyev, B., Kuterbekov, K., Bekmyrza, K., Kabyshev, A., Kubenova, M., & Opakhai, S. (2025). Comprehensive Review of Recent Trends in the Use of Deep Eutectic Solvents for the Valorization of Secondary Lignocellulosic Biomass. Sustainability, 17(21), 9492. https://doi.org/10.3390/su17219492







