Abstract
Water is an essential resource whose quality is threatened by emerging pollutants, including microplastics (MP), whose persistence, bioaccumulation capacity and ecotoxic potential pose a growing risk to ecosystems and human health. Wastewater treatment plants (WWTPs) have been identified as one of the main sources of these pollutants, as conventional treatments are insufficient to remove them completely. In response to this problem and with the aim of finding more efficient and sustainable solutions, a study has been carried out at WWTP with a pilot MP capture plant capable of detecting, quantifying and removing these particles from different wastewater sources with high precision and sustainability. This proposal represents a significant advance in the mitigation of invisible pollution, contributing to the protection of the environment and public health, achieving an efficiency of over 80% in the removal of plastic particles. This system not only addresses the challenge of environmental protection but also represents an unavoidable commitment to a healthier, more equitable, and sustainable development model for current and future generations, directly contributing to strategic action to advance the fulfillment of several Sustainable Development Goals (SDGs) promoted by the UN (SDG 3, SDG 6, SDG 12, SDG 14 and SDG 15).
1. Introduction
Water is a fundamental resource for any type of life on the planet; however, the constant pressure exerted on this resource, changes in water cycles and the environmental impact produced by human activities have led to a situation of depletion and poor condition of natural waters in many parts of the world, which is especially worrying in the case of freshwater available for human consumption. Pollution is one of the main problems facing this precious resource, and a wide range of physical, chemical and biological pollutants have been studied. However, there is a type of pollutant, known as emerging pollutants, which has attracted attention in recent years and whose magnitude and effects on the environment have yet to be extensively studied [1]. Within these emerging pollutants, microplastics (MPs) are of particular concern due to their presence in all natural compartments, from rivers [2,3], seas and oceans [4,5], lakes [6,7], coasts [8,9], soils [10,11] and air [12,13,14]. MPs have been found in some of the most remote areas of the planet such as the poles [15,16] or the Himalayas [17], even in biota [18,19] and in humans [20,21,22], in addition to their high resistance and persistence in the environment and the negative effects they may have on living beings, which are still being evaluated. The introduction of this contaminant from the lowest link in the food chain could be a problem for human health, as contaminated food is consumed. Micro- and nanoplastics have been detected in products such as table salt, honey, sugar and bottled water [23]. They are introduced into organisms through ingestion and respiration, causing damage to these systems and may cause growth retardation, neurological damage, hormonal changes and cancer due to the presence of additives and substances adsorbed on their surface. MPs of different sizes and composition have even been found in the human placenta of several pregnant women studied, without knowing the effects they may cause in the fetus [24].
Plastic has become an essential material in our society, used in a wide variety of industries and sectors. In the 1950s, 1.5 million tons of plastic were produced, an amount that increased until 2016, when 335 million tons were produced. The increase in the generation of this plastic waste and, in many cases, its mismanagement, cause the contamination of ecosystems [25]. When plastic waste reaches the environment, it undergoes physical, chemical and biological degradation processes, forming smaller plastics, the MP, which measure less than 5 mm. These MP can be divided into two classifications: first, we have primary MP, which are manufactured in this size and added to everyday products, such as toothpaste, exfoliants and cosmetics, among others [26]. Second, we have secondary MP, which are those that degrade from larger plastics due to environmental conditions such as sunlight, wind and water currents [26]. In turn, these MPs can degrade into smaller particles, nanoplastics, which are between 0.001 and 0.1 μm in size.
These pollutants are of great concern because of their potential ecotoxicity, their ability to bioaccumulate in living organisms together with substances adsorbed on them (pharmaceuticals, heavy metals and pathogenic microorganisms, among others) and because of the magnification of their effects in the food chain [27]. They are considered contaminants of emerging concern [28], that is, unknown or unrecognized compounds whose presence in the environment, food or water, in any natural or man-made product, or in any living being is not necessarily new, but the knowledge of the possible harmful consequences of their existence is.
The negative effects of the presence of these micro- and nanoplastics have been extensively studied in aquatic ecosystems, but they have also been found in freshwater ecosystems, sediments, soils and in the atmosphere [29,30]. There are a wide variety of studies in the literature that address this issue, especially from a toxicological point of view. Regarding the interaction between MPs and terrestrial and aquatic plants [31], there are negative effects on seed germination, plant size, number of leaves, etc. in plants exposed to polyethylene (PE) MPs [32]. Algae can also suffer negative effects from exposure to polystyrene (PS) microparticles, such as inhibition of Chlorella vulgaris colony formation and decrease in photosynthetic pigment content [33].
The vast majority of these MPs end up in the environment and are difficult to degrade, as would be the case for polyethylene terephthalate (PET) as it has an aliphatic ring; polyethylene degradation times are long due to its high molecular weight, and not many species have been studied that degrade polypropylene (PP); and regarding polystyrene (PS), it is also a recalcitrant compound, but many bacteria use styrene monomer as a carbon source, such as Pseudomonas, Rhodococcus and Nocardia, among others [30].
The presence of these compounds in the environment has created a global problem that requires analysis and understanding from multiple perspectives to assess its scope and develop alternatives and solutions that can reduce pollution caused by substances derived directly from human production and consumption activities.
Knowing the ways in which these pollutants reach the environment is the first step in planning strategies to prevent this type of pollution, and at this point we find that, in the case of natural waters, discharges of treated wastewater have been identified as one of the most important access routes for these pollutants from land, wastewater treatment plants (WWTPs) are considered to be the main source of MP emissions to the aquatic ecosystem, acting as a meeting point for waste from atmospheric deposition and from domestic and industrial waters [29,34]. WWTPs manage to retain a large part of the MPs, but they do not have specific treatments for them as they are particles that, due to their size and density, can easily escape conventional treatments, with significant quantities remaining in the treated water that is returned to the environment [35,36,37,38,39,40], to the aquatic environment [29] and to the soil through the application of sewage sludge as an agricultural soil amendment [41,42,43,44].
Although WWTPs were not specifically designed to remove solid particles (PM), the physical, chemical and biological processes involved in their various stages contribute significantly to their retention. Various studies have shown that conventional plants can remove up to 70%, and in plants with membrane technology, up to 98%, of the PM present in the wastewater, depending on the design, the degree of treatment applied and the characteristics of the particles [45,46,47].
Considering conventional treatment plants, in the primary stage, the screening and sedimentation processes preferentially remove the largest and densest particles, reaching efficiencies of 65% [44]. In the secondary stage, biological processes, such as activated sludge or biofilters, provide an additional 5% removal, mainly through adsorption on flocs [46]. Finally, tertiary stages, especially those incorporating membrane filtration or membrane bioreactors (MBR), can increase overall efficiency to values above 90–99% [47,48]. Despite these high efficiencies, WWTPs do not completely remove MPs, as the smallest fractions (<50 µm) or elongated fibers often escape into the final effluent [45,49]. Therefore, the application of advanced treatments emerges as a possible solution to reduce the millions of MPs that are discharged daily into the environment from these facilities.
In this regard, various studies have been conducted in wastewater treatment plants (WWTPs) to remove MPs through different mechanisms, including both physical processes (rapid sand filtration or coagulation and advanced oxidation processes) and chemical or biological degradation processes (advanced oxidation processes).
Physical processes, based on rapid sand filters, are filters that use quartz and anthracite sand as filter materials. Although this is an effective mechanism, it depends on hydrophilic interactions between the MPs and the sand, as they can be irreversibly retained [50].
Adsorption could be a valid method for the removal of MPs. Different adsorbent media such as graphene oxide and chitin are presented for adsorption of MPs via hydrogen bonds and electrostatic attractions [51]. Alginate secreted by algae and microalgae would also be a good adsorbent. Granular activated carbon is also a good adsorbent capable of removing MPs from wastewater. In nanoplastics, the use of biodegradable cellulose fibers has been found to function as an adsorbent material [52]. Coagulation allows coagulants by the addition of coagulants to trap the MPs in the granules. Combined with a sedimentation process, the process is improved. The coagulant that works best is aluminum sulphate [50]. Regarding the removal of MPs by coagulation, high efficiencies are shown using calcium–aluminum ions [52].
Membrane bioreactors, membrane systems, usually micro- and ultrafiltration membranes (pore sizes are 0.1 to 50 μm and 0.001 to 0.1 μm, respectively) coated with biofilms, which allow trapping of contaminants, are effective in removing MPs from wastewater [34,53], but are very expensive systems.
Advanced oxidation processes (AOPs) are widely used in wastewater treatment plants due to their ability to oxidize and degrade organic pollutants, including microplastics (MPs). Their main objective is the complete mineralization of these compounds—that is, their conversion into CO2, H2O and inorganic salts—however, incomplete degradation can generate smaller or partially oxidized fragments, which may retain or even increase their toxicity.
In some treatments, such as chlorination, chlorinated organic compounds (with C-Cl bonds), such as trihalomethanes or haloacetic acids, can form, which are potentially toxic or persistent. Nevertheless, chlorination is mainly used as a disinfection process, and its application requires a balance between microbicidal efficacy and minimization of unwanted by-products [54]. During these treatments, some of the microplastics and their degradation products may be retained in the treatment systems, while another fraction may be dehydrated and accumulated in sewage sludge [51,54].
Given this scenario, it is necessary to find complementary methods for the selective capture of fine fractions and irregular fibers of MP that escape conventional wastewater treatment, without the need for structural modifications to existing plants, that are sustainable and have a reduced environmental impact.
Despite being a very important issue, legislation on the presence of MPs in wastewater is an evolving issue, both at European and Spanish level. Currently, there is no specific regulation that establishes maximum limits for MPs in wastewater, but there are important advances and regulations that address the problem indirectly. The regulations associated with microplastics in urban wastewater are mainly Royal Decree 1085/2024 [55] on water reuse, Directive (EU) 2024/3019 (TARU) [56] on urban wastewater treatment, Directive 86/278/EEC on the use of sewage sludge in agriculture and Regulation 2019/2023 [57] on eco-design requirements for domestic washing machines and tumble dryers, and for industrial wastewater there would be Directive EU2013/904 [58] on the control of intentional and unintentional discharge of plastics into the environment. Existing regulations only indirectly address the presence of microplastics, and the establishment of specific quantitative limits remains an area pending regulatory development.
The quantification of MP represents a major challenge due to the lack of standardized methods, which leads to poorly reproducible results and large discrepancies between studies. For WWTPs, this challenge is even greater, as the MPs present can interact with other suspended solids, which complicates their extraction and analysis. In general, most of the methods developed have focused on the numerical assessment of particles, but measurement in terms of mass concentration remains a pending task.
In this context, this study pursues the following objectives, listed in order:
- To develop a standardized and reproducible method for quantifying PM at strategic points along the water line of the experimental WWTP. The experiments conducted in this study were carried out in collaboration with Captoplastic, using a pilot plant for PM recovery that incorporates an online analysis method, allowing continuous sample collection at different points along the water line of the WWTP itself. This technology allows for both the quantification of PM at the water line and their identification and removal.
- To estimate the PM content in the different industrial water streams and the removal efficiency with the conventional processes existing at WWTP under study. Furthermore, these concentrations and removal efficiency are simulated, considering the application of external PM recovery technology, by installing a demonstration pilot plant at the different points of the plant evaluated. The results are extrapolated to the actual flow rates treated at the experimental WWTP and compared.
- To verify the study’s contribution toward achieving the Sustainable Development Goals established by the UN.
2. Materials and Methods
2.1. Description of the Facility Under Study
The facility under study is an urban WWTP with total reuse of treated wastewater, a design flow of 2500 m3/day and direct application of the sludge generated on agricultural land. In this WWTP, the presence of MPs has been detected in the raw water received, so the approach of a system for the elimination of MPs is interesting and a real challenge.
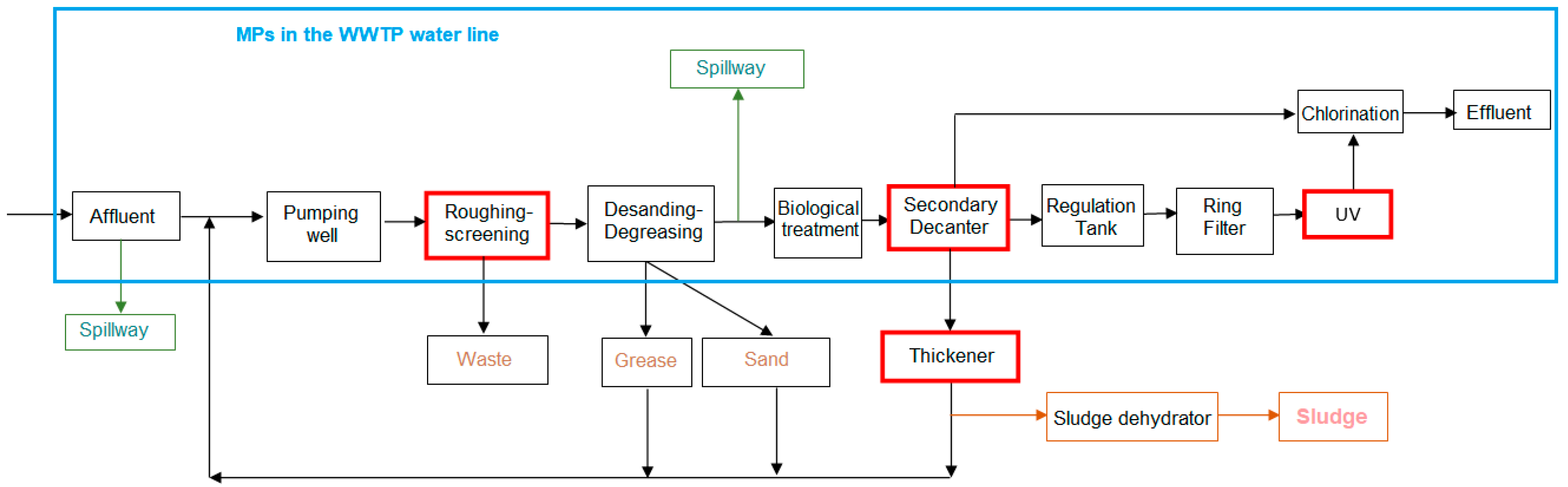
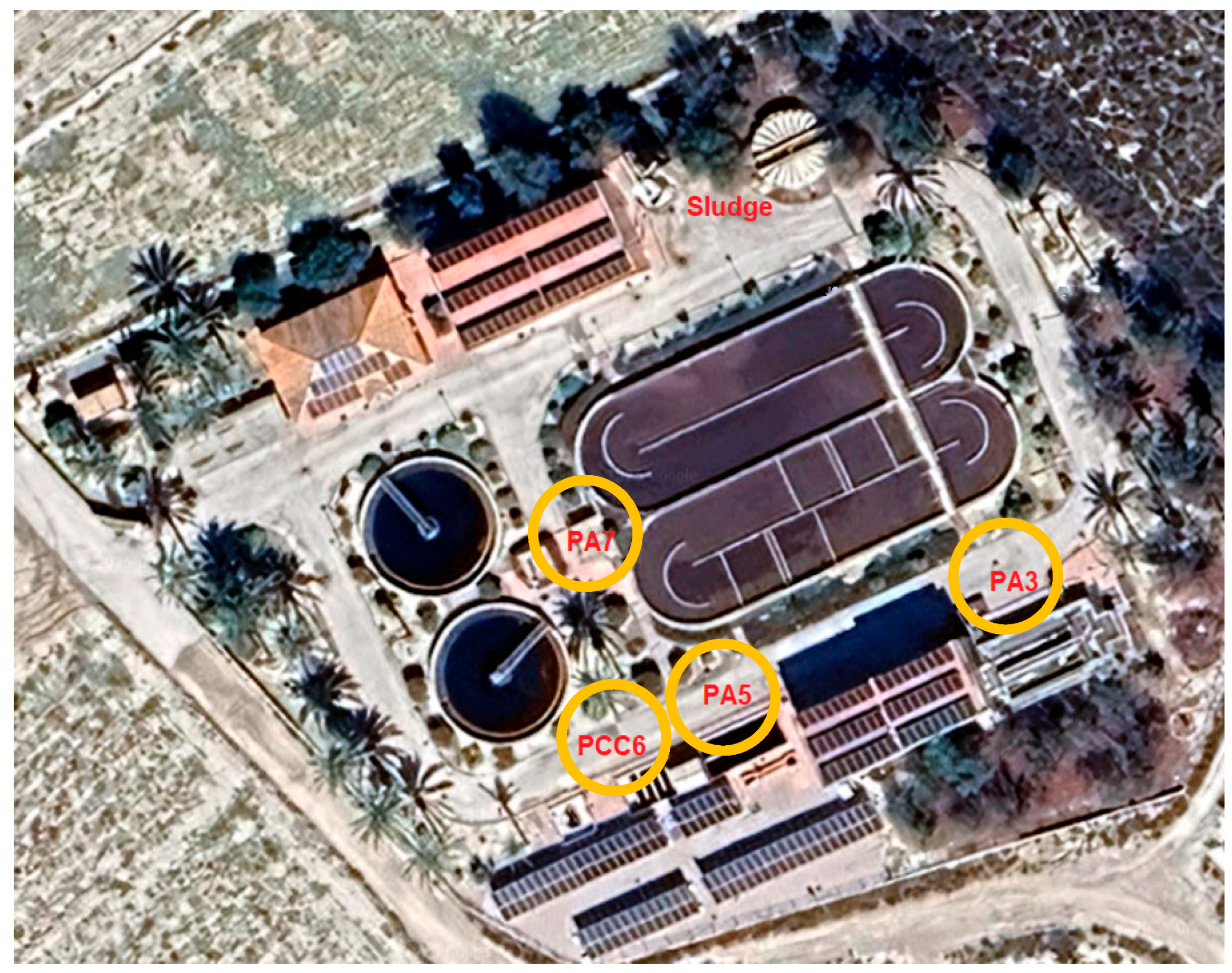
The first step was to identify the points in the WWTP where it is most interesting to verify and quantify the presence of MPs in the water flow, which are then marked on a process flow diagram and on a satellite image of the WWTP (Figure 1 and Figure 2). The most representative points of the water line selected for the study were:
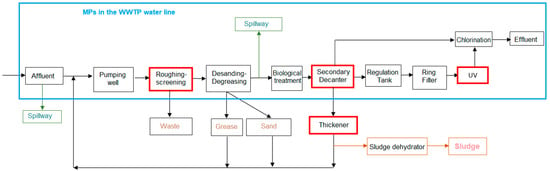
Figure 1.
Flow diagram of experimental WWTP process.
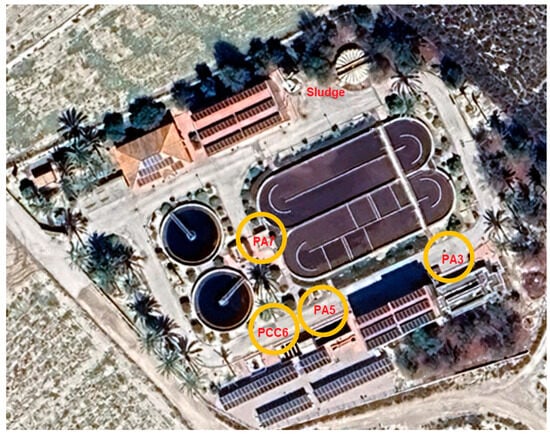
Figure 2.
Identification of sampling points in experimental WWTP.
- -
- Pretreatment outlet (PA3)
- -
- Secondary treatment outlet (PA5)
- -
- Tertiary treatment outlet (PCC6)
2.2. Application of the Demonstration Plant for the Quantification and Removal of MPs in the Aqueous Streams of the WWTP
A mobile pilot plant owned by Captoplastic, company located in Madrid (Spain) (Figure 3), with a treatment capacity of up to 5000 L/h, was used to quantify and remove microplastics from the different streams. It is the only microplastic recovery technology that has an analysis method for quantifying microplastics, allowing continuous sampling at the WWTP. The flow rate treated in the pilot plant (5 m3 d−1) is considered representative of medium-sized full-scale treatment plants, such as the reference WWTP (2500 m3 d−1). In pilot-scale studies, flow rates are typically between 0.1% and 1% of the full-scale facility’s design flow rate, a range within which the system used falls (0.2%). This ratio allows for comparable hydraulic and mass loading ratios, ensuring the validity of the extrapolation of results to real-life operating conditions [59].

Figure 3.
Image of pilot plant for quantification and removal of MP.
The technology used integrates a combination of several processes:
- -
- Filtration, comfortably covering particle removal ranges between ~1 µm and <5 mm. The use of polypropylene filters with pores of 100 µm, 50 µm and 20 µm is combined for efficient MP removal in the field. The parameters to be controlled were pressure and flow rate. Pressure values did not exceed 1.5–2 bar for treatment flow rates of 5 m3/h [60].
- -
- Magnetic extraction methods to separate MPs from other components of the sample Bare Fe3O4 nanoaggregates are added with direct adsorption onto MPs. The approximate dose is 1% by mass of MP (~1g/L), controlling the time and neutral pH. The aggregates formed with the MPs present in the water are separated by a magnetic field with an intensity of between 0.3–0.5 T, sufficient to recover the magnetic fraction without dragging MP particles. Once the MPs are retained, the iron nanoaggregates are released and can be reused in the process, minimizing the generation of new waste [61].
- -
- Finally, selective oxidation is carried out to remove non-plastic organic matter, leaving only the MPs for quantification. The equipment can handle various reagents, and as it is a pilot plant, it can be adapted to the installation and the nature of the MPs received. The reagent used was Fenton (0.05–0.1 M Fe2+; 0.1–0.3 M H2O2), maintaining the reaction at room temperature, although it is possible to work at higher temperatures when required. This treatment oxidizes more than 90% of the organic matter, preserving the polymers present in the water (PE, PP, PS and PET). Since Fe3O4 magnetic nanoparticles can also act as Fenton-type heterogeneous catalysts, their presence promotes the localized generation of hydroxyl radicals (OH), accelerating selective oxidation and reducing the need to add soluble iron. At the end of the oxidation process, the samples are rinsed with ultrapure water and filtered before proceeding to the spectroscopic identification of the polymers (µ-FTIR or µ-Raman) [62,63].
In addition, this plant offers a measurement system to identify and quantify the presence of MPs in the water, which is crucial for the standardization of the analysis and the evaluation of the effectiveness of the technology at the facility itself.
The methodology used for microplastic (MP) analysis included the use of Captoplastic’s Captolab equipment, a semiautomatic system designed for the quantification of microplastics in complex aquatic matrices. This equipment allows direct measurement in mg/L using a patented method that does not require highly specialized personnel.
The following reagents were used: Hydrogen peroxide (H2O2, 30%) for the digestion of organic matter, sodium sulfate (Na2SO4, 0.9 M) as a density separation medium and ethanol (C2H5OH, 96%) for sample cleaning and contaminant reduction.
The accuracy range of the Captolab system is ±5% for concentrations between 0.1 and 10 mg/L of microplastics, with a detection limit of 0.05 mg/L and repeatability of >95% in replicate tests.
This plant was transferred to the WWTP to analyze its effectiveness in removing MPs at each stage of the process. Water flowed through the demonstration plant, and the concentration of MP was analyzed before and after each treatment at different time intervals, to evaluate the overall performance of the system. The tests carried out with the pilot plant were divided into three stages, and applied to each sample analyzed:
- -
- Stage 1: Incoming water characterization tests, whereby the concentration of total suspended solids (TSS), volatile suspended solids (VSS) and fixed suspended solids (FSS) are determined.
- -
- Stage 2: Quantitative analysis of the water sample to determine the amount of microplastics (mg/L) present.
- -
- Stage 3: In this stage, the microplastics contained in the receiving water are identified. The identification is carried out using micro-FTIR (Fourier transform infrared spectrometry), analyzing the signals obtained in the spectra and comparing these spectra with the database available in the equipment.
On the first day of testing, the pilot plant was located at the previously identified PCC6 point, as tertiary treatment and disinfection effluent (ring filtration and UV, with hypochlorite dosing as a safety measure) before discharge into the environment. The pilot plant then evaluated point PA5, the effluent from the secondary sedimentation tanks before entering the ring filtration stage. Finally, the test was carried out at point PA3 with water from the sand traps and grease traps.
All the tests lasted approximately four hours, where the water was collected from each of the sampling points continuously and was treated by the pilot plant. To check the efficiency in the elimination of the equipment, a sample was taken at the inlet and outlet of the equipment (two bottles of 20 L for each of the inlets), collecting an approximate volume of four liters each hour of the test.
To ensure the correct homogenization of the samples throughout the process, the incoming bottles were shaken with the help of a bottle turner. And since the results obtained in this article focus on the analysis of MPs, all characterization, quantification and identification tests are performed by passing the samples through a 5 mm sieve, since the US National Oceanic and Atmospheric Administration (NOAA) defines MPs as small pieces of plastic, less than 5 mm in diameter, that contaminate the environment.
2.3. Technology for the Characterization, Quantification and Identification of MPs
In parallel with the experimental development and the quantification and elimination tests for continuous monitoring carried out on site at WWTP itself using the mobile treatment plant, periodic sampling was carried out for detailed analysis in the laboratory for verification, characterization and polymer identification. This combination of on-site analysis and laboratory verification provides us with the certainty that the results obtained are reliable, representative and reproducible, validating both the effectiveness of the system in detecting and removing microplastics and the consistency of the data recorded continuously.
For spot quantification in the laboratory, Captoplastic MP quantification equipment was used, which combines infrared spectroscopy with traditional optical microscopy and requires a starter kit for sample treatment prior to analysis, as well as a license to access the MP quantification and identification software (Figure 4).

Figure 4.
Captoplastic brand MP quantification equipment.
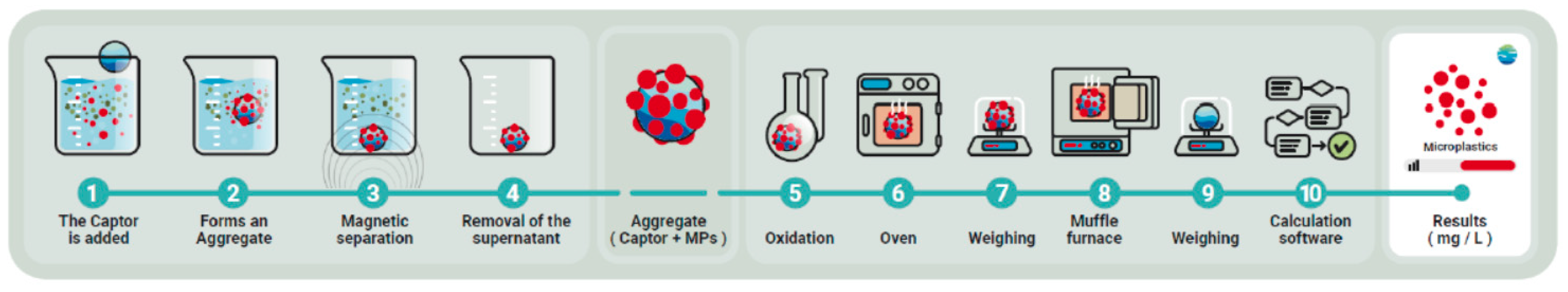
This is a semi-automated system for quantifying MP in complex aquatic matrices, such as urban and industrial wastewater. The determination method combines several techniques, including filtration, magnetic extraction and selective oxidation, to effectively isolate and measure MP. For more details, see Figure 5.
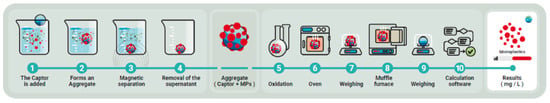
Figure 5.
Flowchart of MP quantification process.
Before applying the method, the sample was prepared: one liter of water was kept under agitation to ensure homogenization. It was also necessary to determine the concentration of total suspended solids, as well as the proportion of fixed and volatile solids, as shown below. To determine the concentration of suspended solids, a known volume of the aqueous matrix to be analyzed (one liter of sample) was filtered using a vacuum or low-pressure apparatus through a glass fiber filter of known weight. The filter containing the retained particles was then dried at 85 °C and the mass of the particles was determined by the difference in weight from the original filter. The filter with the solids was then dried in an oven for at least two hours at 85 °C. Once dry and cooled, the filter containing the solids was weighed on a precision balance and the total mass of solids was determined by the difference in weight from the same filter. To determine the fixed (inorganic) and volatile (organic) solid particles in suspension, the filter containing the solid particles was retained and dried at 85 °C and calcined at 550 °C for at least two hours until a constant mass value was reached. Once cooled, the filter with the solids was weighed on a precision balance and the mass of fixed solid particles in suspension was determined by the difference in weight with the same filter. The mass of volatile suspended solid particles was determined by the difference between the total mass of solids and the mass of fixed solids.
Once the concentration of total suspended solids, including fixed and volatile solids in the sample, was known, the MP was quantified based on the analytical determination of the MP using a Captoplastic laboratory kit (CAPTOPLASTIC, S.L., Madrid, Spain) on another aliquot of the sample.
The procedure for quantifying MP was carried out in two main phases: separation and oxidation, with the aim of isolating and quantifying MP, distinguishing it from the organic matter (OM) and inorganic matter (IM) present in the samples.
In the separation phase, a magnetic collector based on iron oxide nanoparticles was used, capable of selectively capturing MP from the sample, although it can also separate part of the OM and, to a lesser extent, the IM. The magnetic aggregates formed were separated from the sample by applying a magnetic field (magnet). The supernatant containing the particles not retained by the magnet was filtered and dried for subsequent gravimetric analysis. All the magnetically retained material was suspended in deionized water (volume of 50 mL) to proceed to the oxidation stage.
In the oxidation phase, all the material previously recovered by magnetic means was subjected to ten consecutive cycles of microwave-assisted oxidation to completely remove the retained organic matter without damaging the magnetic particles. The reagents used in the oxidation process were supplied in the determination kit described above and are based on the Fenton process, which requires prior adjustment of the sample pH to pH0 = 3. Once the oxidation cycles were completed, the resulting solution was filtered again, collecting a second filter that, in principle, should only contain the MP and the magnetic collector. Figure 6 shows an image of the filter, covered by the dark-colored magnetic collector, in which the PET particles can be seen.

Figure 6.
Image of filter obtained after oxidation process.
Both filters, the first (containing the inorganic components derived from the separation stage) and the second (containing the MP and magnetic material after oxidation), underwent heat treatment in a muffle furnace at 550 °C for two hours. This incineration process allowed for the complete removal of the MP, leaving only the inorganic fraction, corresponding to the magnetic material (and part of the MI that could have been removed in the separation phase), as residue in the filters. The difference in weight recorded before and after heat treatment was used to calculate the number of MPs that were removed in the muffle furnace and were therefore present in the sample.
Finally, the weight data obtained in each phase, together with the differences recorded after treatment in the muffle furnace, were processed using App Captoplastic software. This analysis made it possible to accurately determine the final concentration of MP in the samples expressed in mg/L, ensuring accurate quantification.
The images were then analyzed using ImageJ software, which made it possible to calculate the projected area of the retained particles. This analysis made it possible to estimate the concentration of MP in the original sample using the following Equation (1):
where [MPs] is the concentration of MP in the original sample (in mg/g); AMPs is the total projected area of the retained MP, measured with ImageJ software (mm2); fvol is the volume factor, which is used to convert the projected area into an estimate of the mass of the MP, taking into account its density and geometric characteristics (mg/mm2); and msample is the mass of the sample used (g).
[MPs] = (AMPs × fvol)/msample
Next, the microplastics contained in the water samples are identified using the PerkinElmer Spotlight 200i, owned by the Autonomous University of Madrid (Spain),system for the identification and quantification of microplastics by Fourier transform infrared spectroscopy (FTIR) with an imaging system/FTIR Imaging system, which analyzes the signals obtained in the spectra of the detected polymers and compares them with the database available to the equipment. It has a spatial resolution of 10 µm, which allows it analyzing individual microplastic particles with high precision, widely used in environmental studies for microplastics, with FPA detector and identification software.
The microplastic removal rate in water streams, expressed as a percentage, is calculated using Equation (2):
where CMPe and CMPs are the MP concentrations in the inlet and outlet streams, respectively, to the treatment unit at the WWTP or pilot plant, as appropriate, expressed in mg/L. All tests in this project were conducted in triplicate, and the results presented in tables are presented as mean values with their corresponding confidence intervals.
Removal rate (%) = (CMPe − CMPs)/CMPe × 100
3. Results and Discussion
3.1. Analysis and Discussion of the Results of the Quantification of MPs at Different Points of the Water Line
This section shows the results of the characterization, quantification and identification of the samples by means of a demonstration of pilot plant placed at different stages in the experimental WWTP treatment process. The same steps were followed for all streams (Table 1).

Table 1.
Pilot plant input and output samples at different process stages.
The first step for the quantification of MPs was the characterization of samples, i.e., the calculation of total suspended solids and volatiles. For the inlet and outlet samples to pretreatment (PA3), the characterization results were as follows (Table 2):

Table 2.
Average characterization results, sample PA3.
In the three characterization tests, for each of the samples, a low deviation and CV are observed, which indicates good homogenization and characterization of the samples.
The microplastic quantification results provided by the detection equipment, for the input and output samples to pilot plant PA3, are presented in Table 3.

Table 3.
MPs concentration in pretreatment input and output samples (PA3).
As can be seen, in the effluent of the pretreatment (PA3), the concentration of MPs was 26.58 mg/L. This value is consistent with typical levels observed in urban wastewater, as the pretreatment will have very little impact on the concentration of MPs present in the water. After passing through the pilot plant for MPs recovery, it can be observed that the concentration of MPs decreases considerably as it passes through the pilot plant (MPs in the plant outlet stream of 6.38 mg/L), achieving a removal efficiency of 76%.
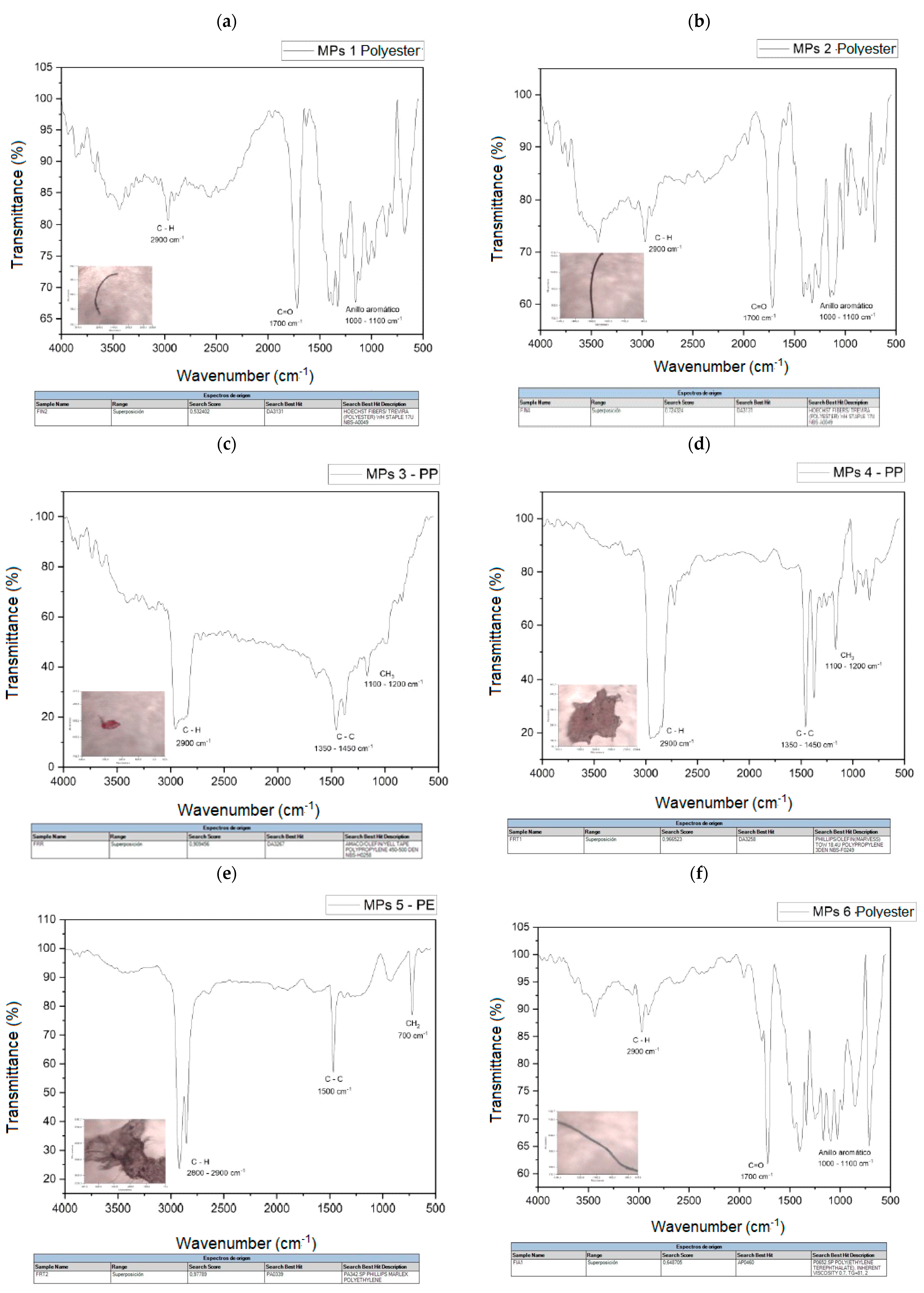
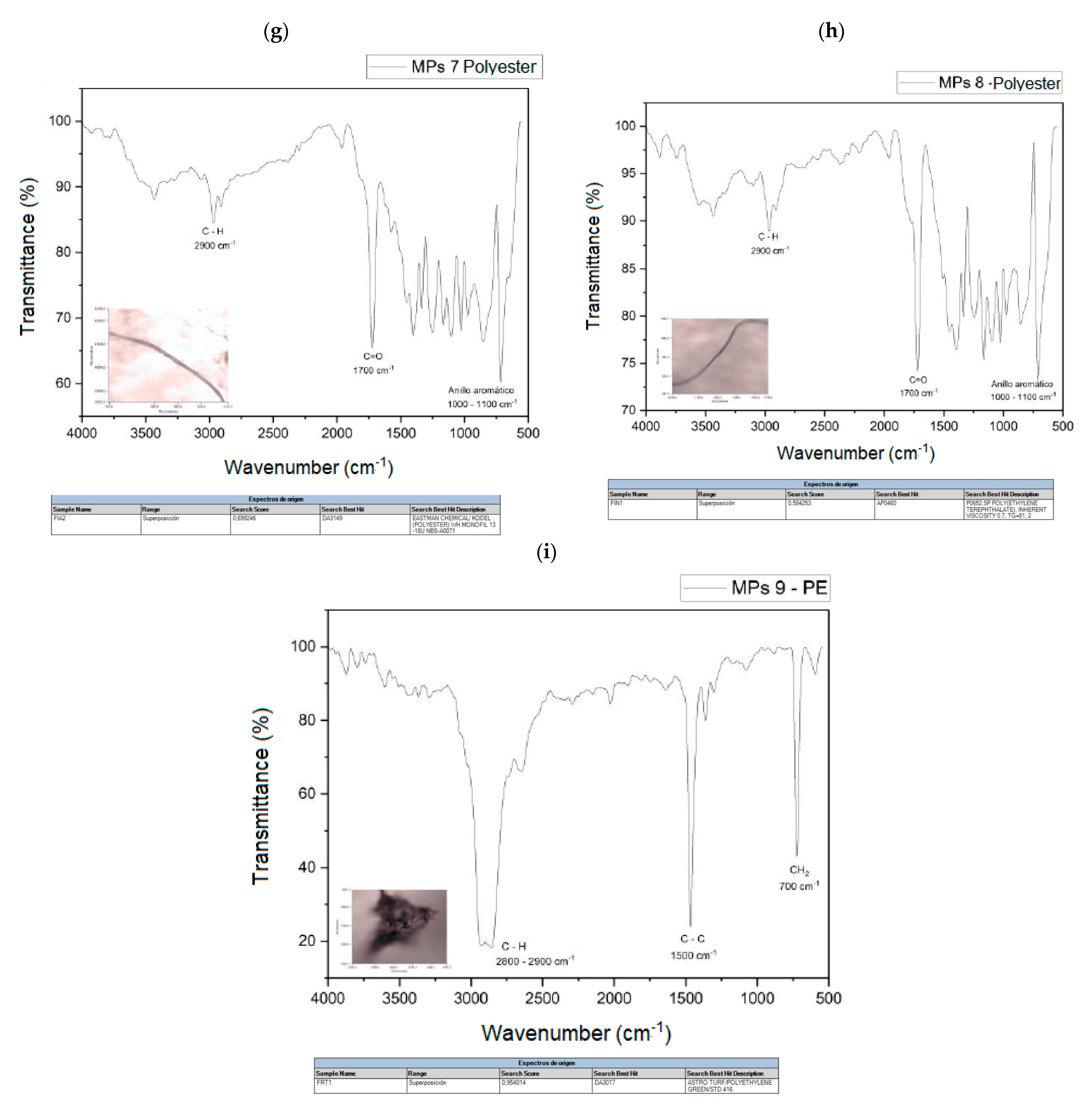
The spectra of the analyzed polymers, identified using micro-FTIR, are shown in Figure 7a–i. The signals obtained in the spectra were analyzed and the spectra were compared with the database available in the equipment.
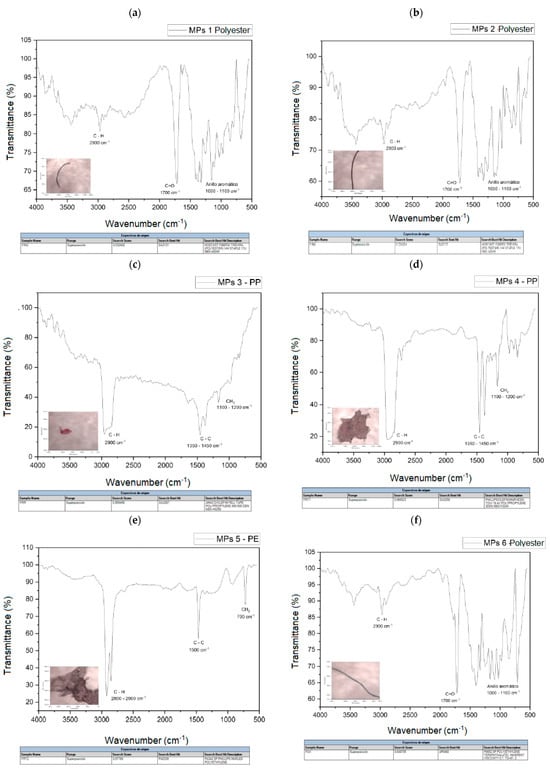
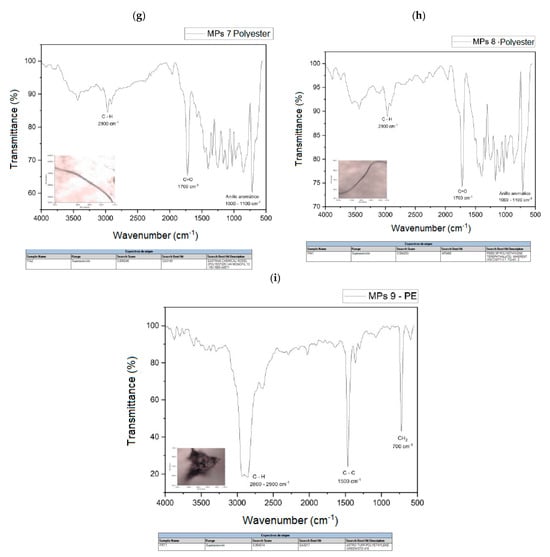
Figure 7.
(a) MPs 1 and MPs 1 library search, (b) MPs 2 and MPs 2 library search, (c) MPs 3 and MPs 3 library search, (d) MPs 4 and MPs 4 library search, (e) MPs 5 and MPs 5 library search, (f). MPs 6 and MPs 6 library search, (g) MPs 7 and library search MPs 7, (h) MPs 8 and MPs 8 library search, (i) MPs 9 and MPs 9 library search.
Table 4 shows a summary of the MPs identified in these samples, the most frequently identified types are polyester fibers (PET), where a total of five microfibers have been found, followed by PP and PE. The main polymers identified are also consistent with those usually reported in urban wastewater.

Table 4.
Summary table of results obtained in identification of MPs in sample PA3.
The same analysis was carried out for the rest of the samples, PA5 and PCC6, and the results of the quantification of microplastics provided by the detection equipment for the inlet and outlet samples to the corresponding pilot plant PA5 and PCC6 are summarized below (Table 5) and a summary of the MPs identified is included in Table 6 and Table 7, respectively.

Table 5.
Concentration of MPs in secondary treatment input and output samples (PA5) and tertiary treatment input and output samples (PCC6).

Table 6.
Summary table of results obtained in identification of MPs in sample PA5.

Table 7.
Summary table of results obtained in identification of MPs in sample PCC6.
It was obtained for the output of the secondary treatment that the concentration of MPs was notably lower, with a value of approximately 1 mg/L. It is important to bear in mind that this value is precisely the detection limit of the method used, as it is specially designed for wastewater in which higher values of this type of particle are expected. Nevertheless, it can be assured that the concentration of MPs has decreased by almost 96%. Again, the composition of the polymers present in the recovered MPs was mainly PET, PE and PP, which is consistent with the composition of the treated influent. Finally, at the outlet of the tertiary treatment, the concentration of MPs was below 1 mg/L, with an average value of 0.61 mg/L, and the identified MPs were mainly PET and PP. This result is also within the detection limit of the method. However, it confirms that the tertiary treatment did not completely remove the MPs from the effluent.
If we evaluate the performance of the pilot plant in removing the MPs present in the process PA5, corresponding to the outlet of the secondary settling stage, the concentration of MPs at the inlet was 1.08 mg/L and the average concentration of MPs at the outlet <1 mg/L (mean value 0.16 mg/L). We found that the MPs concentration at the plant outlet was considerably lower than the detection limit of the method (<1 mg/L); by using a larger sample volume, it was possible to quantify the MPs concentration of the plant outlet effluent. Although the concentrations of MPs are very low both at the inlet and outlet of the pilot plant, it was also observed by MPs identification that the concentration of MPs decreases as it passes through the pilot plant.
The same occurred for the sampling at point PCC6 (tertiary effluent treatment), although it was observed that there is a decrease in concentration at the plant outlet due to lower identification of MPs in the stream, which indicates a removal of MPs, the measurement obtained of MPs in the pilot plant outlet stream was very small <<1 mg/L, it was necessary to use a larger sample volume to obtain a concrete value of removal performance (0.10 mg/L).
The low concentration of MPs present in the inlet stream to the pilot plant at some selected points means that it is not possible to determine the actual yields obtained.
3.2. Estimation of the MPs Content in the Different Streams of the Wastewater Treatment Plant
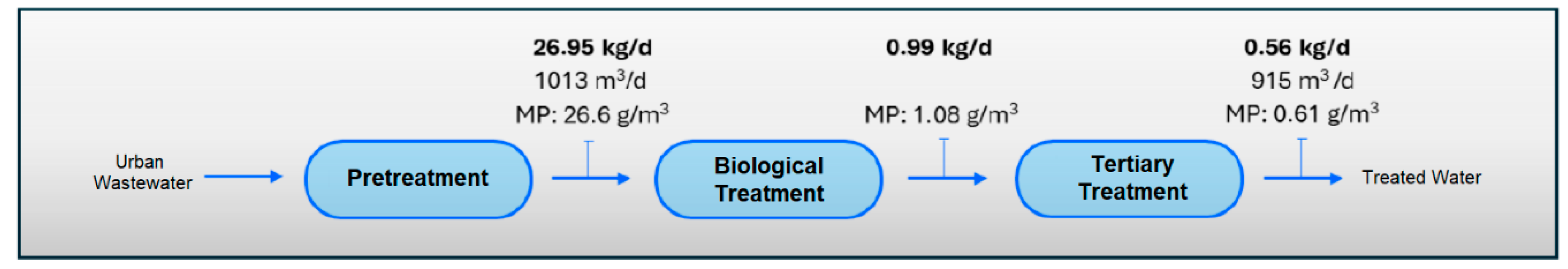
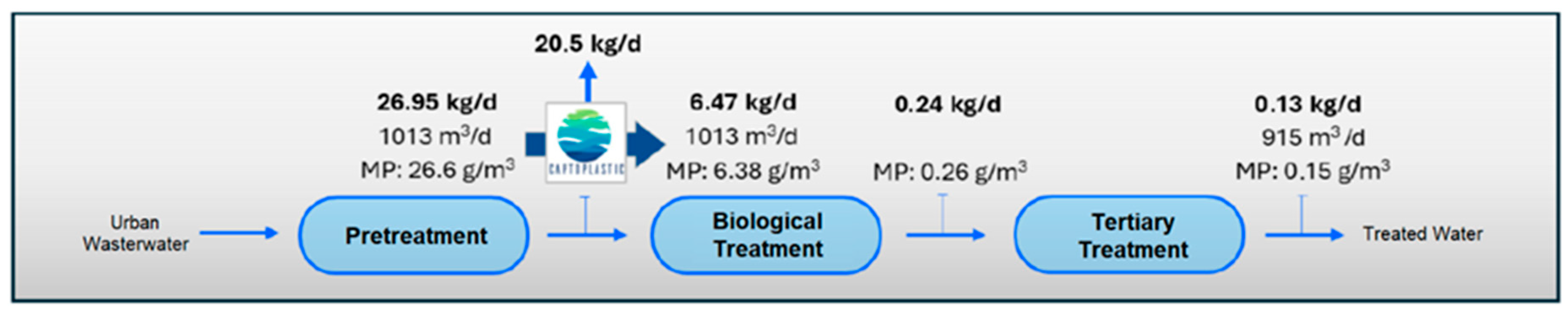
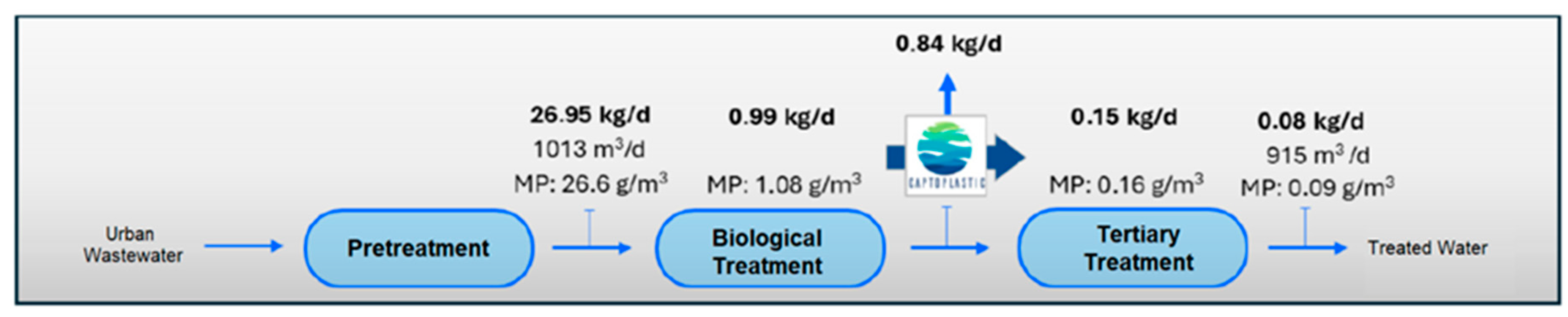
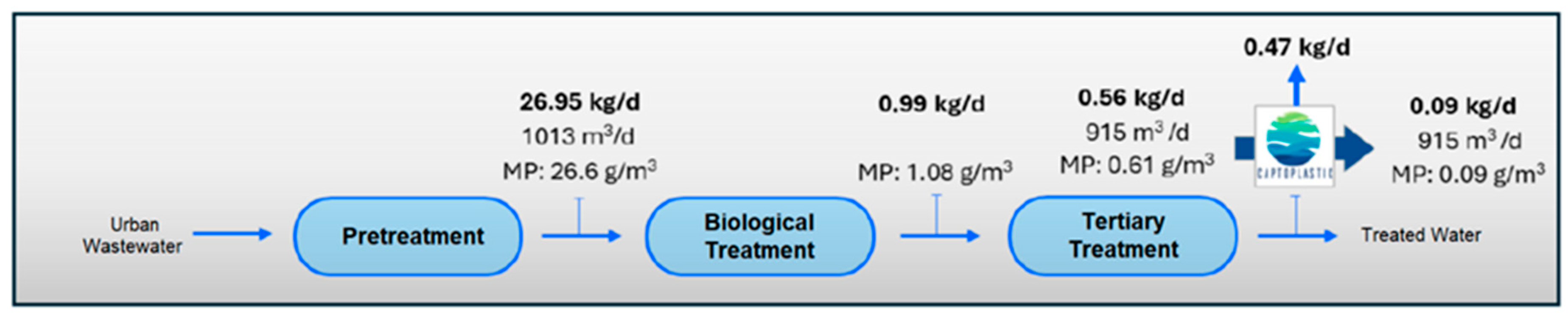
If we consider the results obtained in the quantification of MPs in the different sampling points in the water line at the WWTP (Table 8), and the results of the quantification of MPs in the outflows of the pilot plant (Table 9), we estimate the load of MPs present in the water line at the WWTP and their removal from the system through the installation of the pilot plant (see Figure 8, Figure 9, Figure 10 and Figure 11).

Table 8.
Summary table of results obtained in quantification of MPs in water line at WWTP.

Table 9.
Summary table of results obtained in quantification of MPs in pilot plant outlet water line.
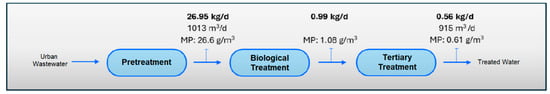
Figure 8.
MPs load in water line in Scenario 1 (without application of MP retention technology).
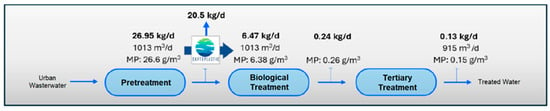
Figure 9.
Water line MPs load in Scenario 2 (with application of MP retention technology at outlet of pretreatment stage).

Figure 10.
MPs load in water line in Scenario 3 (with application of MP retention technology at outlet of secondary settler).
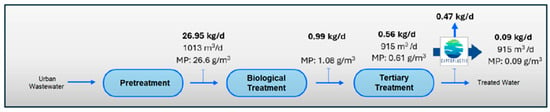
Figure 11.
MPs load in water line in Scenario 4 (with application of MP retention technology at outlet of tertiary treatment (UV).
The results show a clear accumulation of MPs mainly in the secondary treatment line (biological reactor) and in the tertiary treatment line (ring filter), with return to the treatment system by recirculating sludge back to the biological reactor and through the drain line to raw water well. Of the 26.95 kg/d of MPs, 0.56 kg/d would be discharged into the environment. This reinforces the results of the first phase of this study, dedicated entirely to the evaluation of the presence of MPs in the sludge generated at the WWTP, in which the return flow of the secondary sedimented had a remarkably high concentration of MPs, reaching almost 450 mg/L, with a predominant composition of PET, PE and PP. However, the concentration of total suspended solids was also considerable (6.5 g/L), which means that MPs represented 6.9% of the total solids present. These MPs that are removed from the water line in the WWTP end up in the sludge, which acts as a “sink” for these pollutants and where they tend to accumulate [64], which represents a serious problem if we consider that this return flow from the settler is the starting point of the sludge treatment stage with final destination agricultural application.
The incorporation of the elimination plant achieves considerable minimization in the emission of MPs in the water line, with a total load eliminated per day estimated at 21.81 kg/d of microplastics, which means almost 8 Tonsof microplastics per year. If we consider the input of MPs to the WWTP, in the pre-treated stream, of 26.95 k/day and a removal of 21.81 kg/day, we would be guaranteeing a removal performance of more than 80%, with reduced MPs accumulation in the system, which also guarantees that the MPs load in the sludge is also substantially reduced and therefore its impact on the soil. The values obtained are within the ranges reported by other authors in studies of this type [65,66,67]. However, despite the high efficiency in the removal of MPs obtained, it is essential to continue researching new technologies and complementary strategies to optimize the current processes and improve the percentages obtained in the removal.
4. Conclusions
The main conclusions obtained in the experimental study are summarized below:
- The Captoplastic analytical method combines filtration processes and magnetic extraction methods to separate MP, with selective oxidation processes to remove non-plastic organic matter and leave only MP for quantification. Its application at different points in the experimental WWTP allowed the MP present in the water line to be quantified and identified. The results obtained at key points in the plant showed variable concentrations of MP: in the pretreatment effluent, the concentration was 26.6 ± 1.80 mg/L; in secondary treatment, it was reduced to 1.08 ± 0.88 mg/L; and in tertiary treatment, the concentration was 0.61 ± 0.49 mg/L. These results demonstrate the method’s ability to detect and analyze MP in the different treatment streams of the WWTP. The MP identified were mainly PET, PE and PP, with fibers being the most common morphology.
- The experiment carried out at the WWTP with the MP capture plant allowed us to evaluate the degree of MP removal at different points in the water line. An MP removal rate of 76% was observed in the pretreatment effluent, which is lower than the usual removal performance for this type of water, which is usually over 80%. In secondary and tertiary treatment, the degree of removal could not be demonstrated, as the effluents obtained had concentrations well below the detection limit, requiring the use of a larger sample volume for determination. The PM concentrations in these effluents were 0.16 and 0.10 mg/L, respectively, with PM removal results of 85% for these stages.
- The application of MP capture technology in the WWTP could significantly reduce MP emissions, with reductions of up to 80% in the water line, highlighting its potential to reduce MP pollution. The incorporation of the removal plant achieves a considerable minimization of MP emissions in the water line, with an estimated total load removed per day of 21.81 kg/d of microplastics, which amounts to almost 8 tons of microplastics per year. If we extrapolate these results to all WWTPs nationwide, the reduction potential is very significant. Considering that there are approximately 2000 wastewater treatment plants in operation in Spain, the application of this technology could prevent the release of more than 15,000 tons of microplastics into the aquatic environment each year. This data reflects the high positive environmental impact that the widespread implementation of the system would have, contributing directly to the fulfillment of the objectives of the European Plastics Strategy and the Water Framework Directive in terms of reducing emerging pollutants.
- The technology piloted at WWTP offers an integrated solution for the continuous detection and removal of MP in WWTP water lines, combining real-time in situ monitoring with high capture capacity. Its plug-and-play design allows for easy installation without altering the hydraulic regime, significantly reducing dependence on laboratory analysis and associated operating costs. Furthermore, its autonomous and scalable operation, demonstrated in the pilot project, positions it as a viable, cost-effective and sustainable technology for mitigating microplastic pollution in wastewater treatment.
- The microplastic removal technology studied contributes directly to the strategic action to advance the fulfillment of several SDGs promoted by the UN. Reducing the presence of these particles in water, air and soil not only protects human health (SDG 3),but also improves water quality (SDG 6) by reducing pollution levels that affect people and ecosystems. At the same time, it promotes more responsible production and consumption patterns (SDG 12) by preventing the generation of plastic waste at source and reinforces the conservation of underwater life (SDG 14) and terrestrial life (SDG 15) by mitigating adverse impacts on biodiversity. Overall, addressing the challenge of microplastics goes beyond protecting the environment: it represents an unavoidable commitment to a healthier, more equitable and sustainable development model for current and future generations.
Author Contributions
Conceptualization, A.B.L.A.; methodology, A.B.L.A.; formal analysis, A.B.L.A., G.M.M. and F.d.C.V.; investigation, A.B.L.A., G.M.M. and F.d.C.V.; data curation, G.M.M. and F.d.C.V.; writing—original draft, A.B.L.A.; writing—review and editing, G.M.M. and F.d.C.V.; project administration, A.B.L.A.; supervision, G.M.M. and F.d.C.V.; validation, G.M.M. and F.d.C.V.; visualization, A.B.L.A., G.M.M. and F.d.C.V. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The authors do not have permission to share data.
Acknowledgments
The authors would like to thank the staff of the Murcia region sanitation authority and the WWTP staff for making the on-site work for this study possible, as well as the staff of Captoplastic and the University of Madrid for their technical support throughout the project.
Conflicts of Interest
The authors declare that they have no competing interests.
References
- Lozano Avilés, A.B.; Del Cerro Velázquez, F.; Lozano Rivas, F. Ultrafiltration membranes system: A proposal to remove emerging pollutants in urban wastewater. Membranes 2022, 12, 1234. [Google Scholar] [CrossRef] [PubMed]
- Kunz, A.; Schneider, F.; Anthony, N.; Lin, H.-T. Microplastics in rivers along an urban-rural gradient in an urban agglomeration: Correlation with land use, potential sources and pathways. Environ. Pollut. 2023, 321, 121096. [Google Scholar] [CrossRef]
- Chauhan, J.S.; Semwal, D.; Nainwal, M.; Badola, N.; Thapliyal, P. Investigation of microplastic pollution in river Alaknanda stretch of Uttarakhand. Environ. Dev. Sustain. 2021, 23, 16819–16833. [Google Scholar] [CrossRef]
- Russell, M.; Webster, L. Microplastics in sea surface waters around Scotland. Mar. Pollut. Bull. 2021, 166, 112210. [Google Scholar] [CrossRef] [PubMed]
- Biswas, T.; Pal, S.C.; Saha, A.; Ruidas, D.; Shit, M.; Islam, A.R.M.T.; Malafaia, G. Microplastics in the coral ecosystems: A threat which needs more global attention. Ocean Coast. Manag. 2024, 249, 107012. [Google Scholar] [CrossRef]
- Chen, L.; Zhou, S.; Zhang, Q.; Su, B.; Yin, Q.; Zou, M. Global occurrence characteristics, drivers and environmental risk assessment of microplastics in lakes: A meta-analysis. Environ. Pollut. 2024, 344, 123321. [Google Scholar] [CrossRef]
- Mercy, F.T.; Rashidul Alam, A.K.M.; Ahedul Akbor, M. Abundance and characteristics of microplastics in major urban lakes of Dhaka, Bangladesh. Heliyon 2023, 6, E14587. [Google Scholar] [CrossRef]
- Bissen, R.; Chawchai, S. Microplastics on beaches along the eastern Gulf of Thailand—A preliminary study. Mar. Pollut. Bull. 2020, 157, 111345. [Google Scholar] [CrossRef]
- Abelouah, M.R.; Ben-Haddad, M.; Rangel-Buitrago, N.; Haiji, S.; El Alem, N.; Ait Alla, A. Microplastics pollution along the central Atlantic coastline of Morocco. Mar. Pollut. Bull. 2022, 174, 113190. [Google Scholar] [CrossRef]
- Guo, J.-J.; Huang, X.-P.; Xiang, L.; Wang, Y.-Z.; Li, Y.-W.; Li, H.; Cai, Q.-Y.; Mo, C.-H.; Wong, M.-H. Source, migration and toxicology of microplastics in soil. Environ. Int. 2020, 137, 105263. [Google Scholar] [CrossRef] [PubMed]
- Jacques, O.; Prosser, R.S. A probabilistic risk assessment of microplastics in soil ecosystems. Sci. Total Environ. 2021, 757, 143987. [Google Scholar] [CrossRef]
- O’Brien, S.; Rauert, C.; Ribeiro, F.; Okoffo, E.D.; Burrows, S.D.; O’Brien, J.W.; Wang, X.; Wright, S.L.; Thomas, K.V. There’s something in the air: A review of sources, prevalence and behaviour of microplastics in the atmosphere. Sci. Total Environ. 2023, 874, 162193. [Google Scholar] [CrossRef]
- Revell, L.E.; Kuma, P.; Le Ru, E.C.; Somerville, W.R.C.; Gaw, S. Direct radiative effects of airborne microplastics. Nature 2021, 598, 462–467. [Google Scholar] [CrossRef]
- Xiao, S.; Cui, Y.; Brahney, J.; Mahowald, N.M.; Li, Q. Long-distance atmospheric transport of microplastic fibres influenced by their shapes. Nat. Geosci. 2023, 16, 863–870. [Google Scholar] [CrossRef]
- Bergmann, M.; Collard, F.; Fabres, J.; Gabrielsen, G.W.; Provencher, J.F.; Rochman, C.M.; van Sebille, E.; Tekman, M.B. Plastic pollution in the Arctic. Nat. Rev. Earth Environ. 2022, 3, 323–337. [Google Scholar] [CrossRef]
- Waller, C.L.; Griffiths, H.J.; Waluda, C.M.; Thorpe, S.E.; Loaiza, I.; Moreno, B.; Pacherres, C.O.; Hughes, K.A. Microplastics in the Antarctic marine system: An emerging area of research. Sci. Total Environ. 2017, 598, 202–227. [Google Scholar] [CrossRef] [PubMed]
- Talukdar, A.; Bhattacharya, S.; Bandyopadhyay, A.; Dey, A. Microplastic pollution in the Himalayas: Occurrence, distribution, accumulation and environmental impacts. Sci. Total Environ. 2023, 874, 162495. [Google Scholar] [CrossRef]
- Fraissinet, S.; de Benedetto, G.E.; Malitesta, C.; Holzinger, R.; Materić, D. Microplastics and nanoplastics size distribution in farmed mussel tissues. Commun. Earth Environ. 2024, 5, 128. [Google Scholar] [CrossRef]
- Meaza, I.; Toyoda, J.H.; Wise, J.P., Sr. Microplastics in sea turtles, marine mammals and humans: A one environmental health perspective. Front. Environ. Sci. 2021, 16, 575614. [Google Scholar] [CrossRef]
- Prata, J.C.; Patrício Silva, A.L.; da Costa, J.P.; Dias-Pereira, P.; Carvalho, A.; Silva Fernandes, A.J.; Mendes da Costa, F.; Duarte, A.C.; Rocha-Santos, T. Microplastics in internal tissues of companion animals from urban environments. Animals 2022, 12, 1979. [Google Scholar] [CrossRef]
- Hu, C.J.; Garcia, M.A.; Nihart, A.; Liu, R.; Yin, L.; Adolphi, N.; Gallego, D.F.; Kang, H.; Campen, M.J.; Yu, X. Microplastic presence in dog and human testis and its potential association with sperm count and weights of testis and epididymis. Toxicol. Sci. 2024, 200, 235–240. [Google Scholar] [CrossRef]
- Saha, S.C.; Saha, G. Effect of microplastics deposition on human lung airways: A review with computational benefits and challenges. Heliyon 2024, 10, e24355. [Google Scholar] [CrossRef]
- Jiang, B.; Kauffman, A.E.; Li, L.; McFee, W.; Cai, B.; Weinstein, J.; Lead, J.R.; Chatterjee, S.; Scott, G.I.; Xiao, S. Health impacts of environmental contamination of micro- and nanoplastics: A review. Environ. Health Prev. Med. 2020, 25, 29. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Godoy, V.; Blázquez, G.; Calero, M.; Quesada, L.; Martín-Lara, M.A. The potential of microplastics as carriers of metals. Environ. Pollut. 2022, 255, 113363. [Google Scholar] [CrossRef]
- Ahmed, R.; Hamid, A.K.; Krebsbach, S.A.; He, J.; Wang, D. Critical review of microplastics removal from the environment. Chemosphere 2022, 293, 133557. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Zhang, Y.; Wangjin, X.; Wang, Y.; Meng, G.; Chen, Y. The adsorption behavior of metals in aqueous solution by microplastics effected by UV radiation. J. Environ. Sci. 2020, 87, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Azizi, N.; Nasseri, S.; Nodehi, R.N.; Jaafarzadeh, N.; Pirsaheb, M. Evaluation of conventional wastewater treatment plants efficiency to remove microplastics in terms of abundance, size, shape, and type: A systematic review and Meta-analysis. Mar. Pollut. Bull. 2022, 177, 113462. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, J.; Liu, H.; Guo, X.; Zhang, X.; Yao, X.; Cao, Z.; Zhang, T. A review of the removal of microplastics in global wastewater treatment plants: Characteristics and mechanisms. Environ. Int. 2021, 146, 106277. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Kumar, D.; Yoo, C.G.; Gitsov, I.; Majumder, E.L.W. Conversion and removal strategies for microplastics in wastewater treatment plants and landfills. Chem. Eng. J. 2021, 406, 126715. [Google Scholar] [CrossRef]
- Wu, X.; Liu, Y.; Yin, S.; Xiao, K.; Xiong, Q.; Bian, S.; Liang, S.; Hou, H.; Hu, J.; Yang, J. Metabolomics revealing the response of rice (Oryza sativa L.) exposed to polystyrene microplastics. Environ. Pollut. 2020, 266, 115159. [Google Scholar] [CrossRef]
- Pignatelli, S.; Broccoli, A.; Renzi, M. Physiological responses of garden cress (L. sativum) to different types of microplastics. Sci. Total Environ. 2020, 727, 138609. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Y.; Zhao, L.; Wan, Q.; Ma, L.; Liang, J.; Li, H.; Dong, J.; Zhang, M. Effects of microplastics on the growth, photosynthetic efficiency and nutrient composition in freshwater algae Chlorella vulgaris Beij. Aquat. Toxicol. 2023, 261, 106615. [Google Scholar] [CrossRef] [PubMed]
- Turan, N.B.; Erkan, H.S.; Engin, G.O. Microplastics in wastewater treatment plants: Occurrence, fate and identification. Process Saf. Environ. Prot. 2021, 146, 77–84. [Google Scholar] [CrossRef]
- An, L.; Liu, Q.; Deng, Y.; Wu, W.; Gao, Y.; Ling, W. Sources of microplastics in the environment. In Microplastics in Terrestrial Environments; He, D., Luo, Y., Eds.; The Handbook of Environmental Chemistry Series; Springer: Cham, Switzerland, 2020; Volume 95. [Google Scholar] [CrossRef]
- Ziajahromi, S.; Neale, P.A.; Rintoul, L.; Leusch, F.D. Wastewater treatment plants as a pathway for microplastics: Development of a new approach to sample wastewater-based microplastics. Water Res. 2017, 112, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Vercauteren, M.; Semmouri, I.; Van Acker, E.; Pequeur, E.; Janssen, C.R.; Asselman, J. Toward a better understanding of the contribution of wastewater treatment plants to microplastic pollution in receiving waterways. Environ. Toxicol. Chem. 2022, 42, 642–654. [Google Scholar] [CrossRef]
- Jiang, L.; Chen, M.; Huang, Y.; Peng, J.; Zhao, J.; Chan, F.; Yu, X. Effects of different treatment processes in four municipal wastewater treatment plants on the transport and fate of microplastics. Sci. Total Environ. 2022, 831, 154946. [Google Scholar] [CrossRef]
- Zeri, C.; Adamopoulou, A.; Koi, A.; Koutsikos, N.; Lytras, E.; Dimitriou, E. Rivers and wastewater-treatment plants as microplastics pathways to eastern Mediterranean waters: First records for the Aegean Sea, Greece. Sustainability 2021, 13, 5328. [Google Scholar] [CrossRef]
- Canal de Isabel II. Available online: https://www.canaldeisabelsegunda.es (accessed on 25 August 2025).
- Collivignarelli, M.C.; Miino, M.C.; Caccamo, F.M.; Milanese, C. Microplastics in sewage sludge: A K nown but underrated pathway in wastewater treatment plants. Sustainability 2021, 13, 12591. [Google Scholar] [CrossRef]
- Hatinoğlu, M.D.; Sanin, F.D. Sewage sludge as a source of microplastics in the environment: A review of occurrence and fate during sludge treatment. J. Environ. Manag. 2021, 295, 113028. [Google Scholar] [CrossRef]
- Franco, A.A.; Martín-García, A.P.; Egea-Corbacho, A.; Arellano, J.M.; Albendín, G.; Rodríguez-Barroso, R.; Quiroga, J.M.; Coello, M.D. Assessment and accumulation of microplastics in sewage sludge at wastewater treatment plants located in Cádiz, Spain. Environ. Pollut. 2023, 317, 120689. [Google Scholar] [CrossRef]
- De Souza Machado, A.A.; Lau, C.W.; Kloas, W.; Bergmann, J.; Bachelier, J.B.; Faltin, E.; Becker, R.; Görlich, A.S.; Rillig, M.C. Microplastics can change soil properties and affect plant performance. Environ. Sci. Technol. 2019, 53, 6044–6052. [Google Scholar] [CrossRef] [PubMed]
- Iyare, P.U.; Ouki, S.K.; Bond, T. Microplastic removal in wastewater treatment plants: A critical review. Environ. Sci Water Res Technol. 2020, 6, 2664–2675. [Google Scholar] [CrossRef]
- Edo, C.; González-Pleiter, M.; Leganés, F.; Fernández-Piñas, F.; Rosal, R. Fate of microplastics through wastewater treatment plants and their environmental dispersion with effluent and sludge: A review. Sci Total Environ. 2020, 746, 141097. [Google Scholar] [CrossRef]
- Sun, J.; Dai, X.; Wang, Q.; van Loosdrecht, M.C.M.; Ni, B.J. Microplastics in wastewater treatment plants: Detection, occurrence and removal. Water Res. 2019, 152, 21–37. [Google Scholar] [CrossRef]
- Mintenig, S.M.; Löder, M.G.J.; Primpke, S.; Gerdts, G. Low numbers of microplastics detected in drinking water from ground water sources. Sci Total Environ. 2019, 648, 631–635. [Google Scholar] [CrossRef]
- Edo, C.; Corrales, J.; Gómez-Cerezo, M.N.; González-Pleiter, M.; Fernández-Piñas, F.; Rosal, R. Removal of microplastics from primary and secondary effluents in a conventional wastewater treatment plant (WWTP). Water 2021, 13, 1339. [Google Scholar] [CrossRef]
- Xu, J.; Wang, X.; Zhang, Z.; Yan, Z.; Zhang, Y. Effects of chronic exposure to different sizes and polymers of microplastics on the characteristics of activated sludge. Sci. Total Environ. 2021, 783, 146954. [Google Scholar] [CrossRef] [PubMed]
- Joseph, A.; Naseem, A.; Vijayanandan, A. Microplastics in Wastewater Treatment Plants: Occurrence, Fate and Mitigation Strategies. In Energy, Environment, and Sustainability; Springer Nature: London, UK, 2022; pp. 81–100. [Google Scholar] [CrossRef]
- Ali, I.; Ding, T.; Peng, C.; Naz, I.; Sun, H.; Li, J.; Liu, J. Micro- and nanoplastics in wastewater treatment plants: Occurrence, removal, fate, impacts and remediation technologies—A critical review. Chem. Eng. J. 2021, 423, 130205. [Google Scholar] [CrossRef]
- Richardson, S.D.; Plewa, M.J.; Wagner, E.D.; Schoeny, R.; DeMarini, D.M. Occurrence, genotoxicity, and carcinogenicity of disinfection by-products in drinking water: A review and roadmap for research. Water Res. 2011, 45, 461–492. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, J.; Li, J. The removal of microplastics in the wastewater treatment process and their potential impact on anaerobic digestion due to pollutants association. Chemosphere 2020, 251, 126360. [Google Scholar] [CrossRef]
- Royal Decree 1085/2024 of 22 October, Approving the Regulation on Water Reuse and Amending Various Royal Decrees Regulating Water Management. Spain. Official State Gazette 256, 23 October 2024. Available online: https://www.boe.es/eli/es/rd/2024/10/22/1085 (accessed on 6 August 2025).
- Directive (EU) 2024/3019 of the European Parliament and of the Council of 27 November 2024 on Urban Wastewater Treatment (Recast). European Union. Official Journal of the European Union, L 2024/3019. 12 December 2024. Available online: https://eur-lex.europa.eu/eli/dir/2024/3019/oj (accessed on 25 August 2025).
- Council Directive 86/278/EEC of 12 June 1986 on the Protection of the Environment, and in Particular of the Soil, When Sewage Sludge is Used in Agriculture. European Union. Official Journal of the European Communities L 181. 4 July 1986, pp. 6–12. Available online: https://eur-lex.europa.eu/legal-content/ES/TXT/?uri=CELEX%3A31986L0278 (accessed on 6 August 2025).
- Council Decision (EU) 2023/904 of 7 March 2023 Approving the Voluntary Partnership Agreement Between the European Union and the Co-Operative Republic of Guyana on Forest Law Enforcement, Governance and Trade in Timber Products to the European Union. European Union. Official Journal of the European Union, L 121. 5 May 2023, pp. 1–2. Available online: https://eur-lex.europa.eu/eli/dec/2023/904/oj (accessed on 21 July 2025).
- Metcalf Eddy Inc. Wastewater Engineering: Treatment and Resource Recovery, 5th ed.; McGraw-Hill Education: New York, NY, USA, 2014. [Google Scholar]
- Bodzek, M.; Pohl, A.; Rosik-Dulewska, C. Microplastics in wastewater treatment plants: Characteristics, occurrence and removal technologies. Water 2024, 16, 3574. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, L.; Wang, P. Magnetic Fenton-assisted removal of microplastics from water: Pilot-scale studies. Water 2025, 17, 2686. [Google Scholar] [CrossRef]
- Ortiz, D.; Munoz, M.; Nieto-Sandoval, J.; Romera-Castillo, C. Insights into the degradation of microplastics by Fenton oxidation: From surface modification to mineralization. Chemosphere 2022, 309 Pt 2, 136809. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, B.; Pileggi, V.; Chang, S. Methods to recover and characterize microplastics in wastewater treatment plants. Case Stud. Chem. Environ. Eng. 2022, 5, 100183. [Google Scholar] [CrossRef]
- Franco, A.A.; Arellano, J.M.; Albendín, G.; Rodríguez-Barroso, R.; Zahedi, S.; Quiroga, J.M.; Coello, M.D. Mapping microplastics in Cádiz (Spain): Occurrence of microplastics in municipal and industrial wastewaters. J. Water Process Eng. 2020, 38, 101596. [Google Scholar] [CrossRef]
- Pittura, L.; Magni, S.; Binelli, A.; Valsecchi, S. Microplastics in real wastewater treatment schemes: Occurrence, fate and removal in a full-scale plant in Central Italy. Sci. Total Environ. 2021, 758, 143561. [Google Scholar] [CrossRef]
- Gies, E.A.; Ross, P.S. Metro Vancouver Retention of microplastics in a major secondary wastewater treatment plant in Vancouver, Canada. Mar. Pollut. Bull. 2018, 136, 460–468. [Google Scholar] [CrossRef]
- Bayo, J.; Pérez, L.; Martínez, A.; García, R. Characterization and removal efficiencies of microplastics in wastewater treatment plants in Southeast Spain. Sci. Total Environ. 2023, 888, 164637. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).