Valorization of Agro-Industry-Rejected Common Bean Grains for Starch Film Development: Advancing Sustainable and Comprehensive Resource Utilization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Starch Extraction and Composition
2.3. Determination of Amylose/Amylopectin Contents
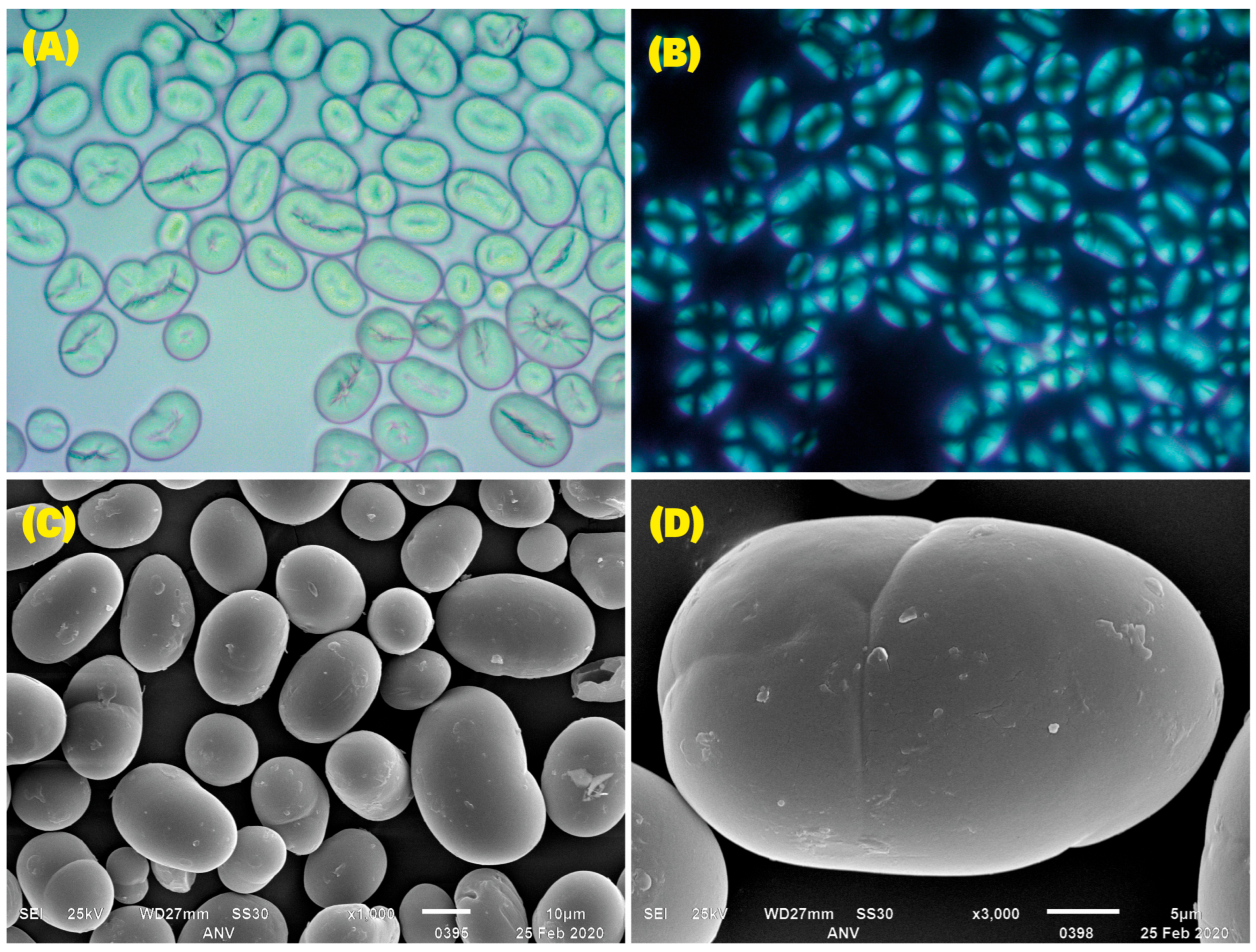
2.4. Granule Morphology
2.5. Starch Pasting
2.6. Starch Film Formulation
2.7. Starch Film Morphology
2.8. Starch Film Color Parameters
2.9. Water Activity
2.10. Tensile Strength
2.11. Statistical Analysis and Design of Experimental Approach
3. Results and Discussion
3.1. Starch Extraction and Chemical Composition
3.2. Morphological Characteristics of Starch Granules According to OM and SEM
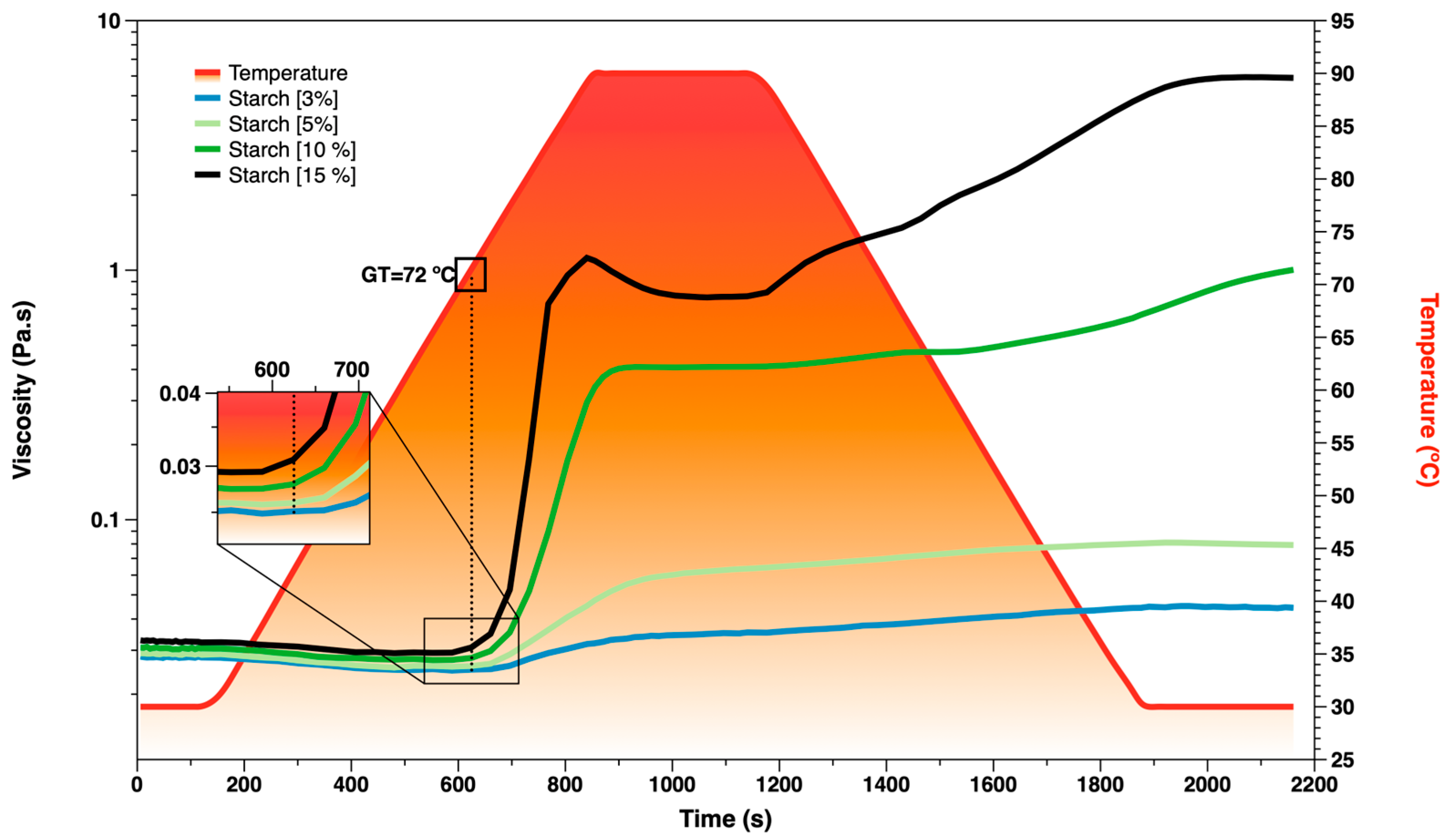
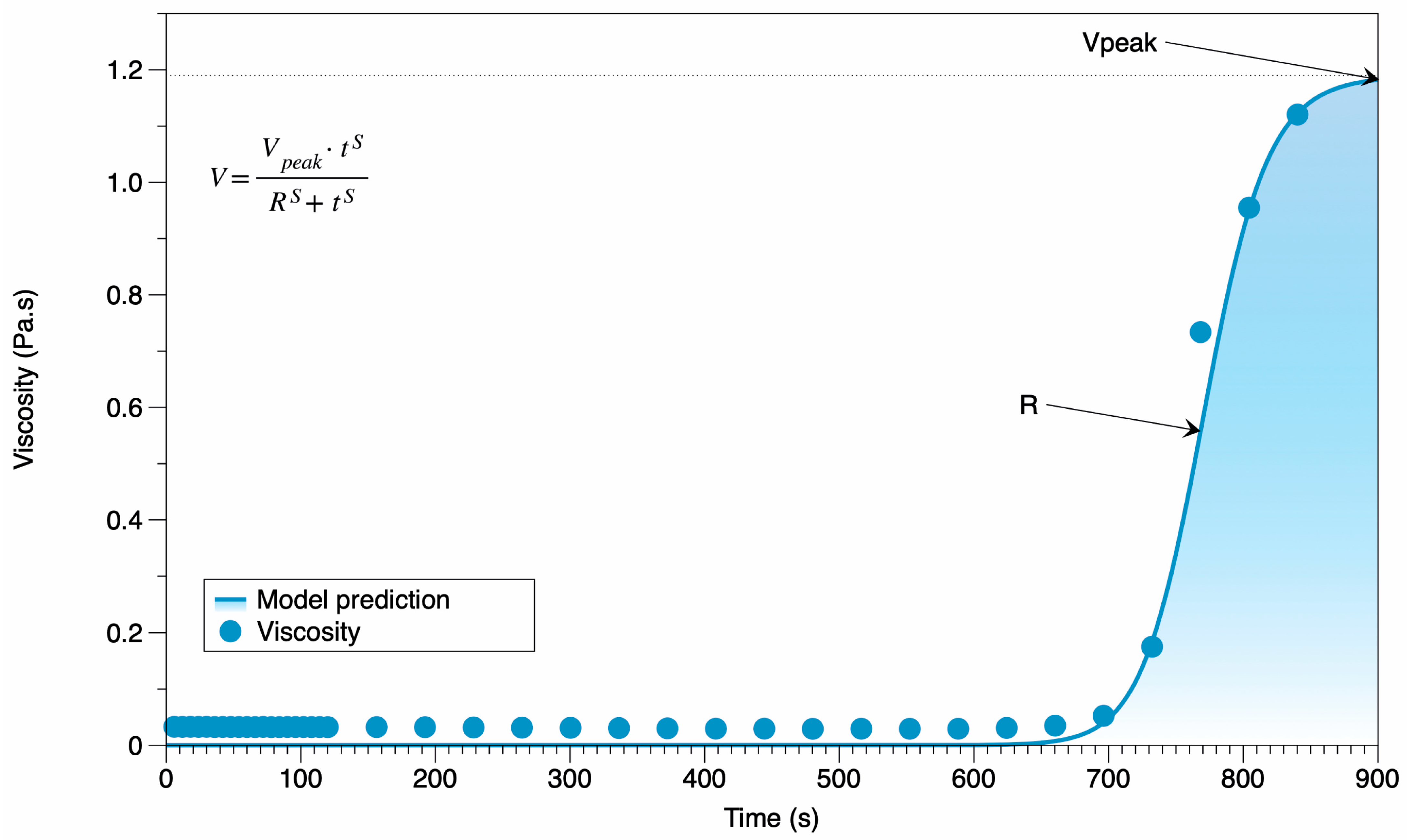
3.3. Starch Pasting Analysis
3.4. Visual Aspect, Color, and Water Activity Parameters of the Obtained Films
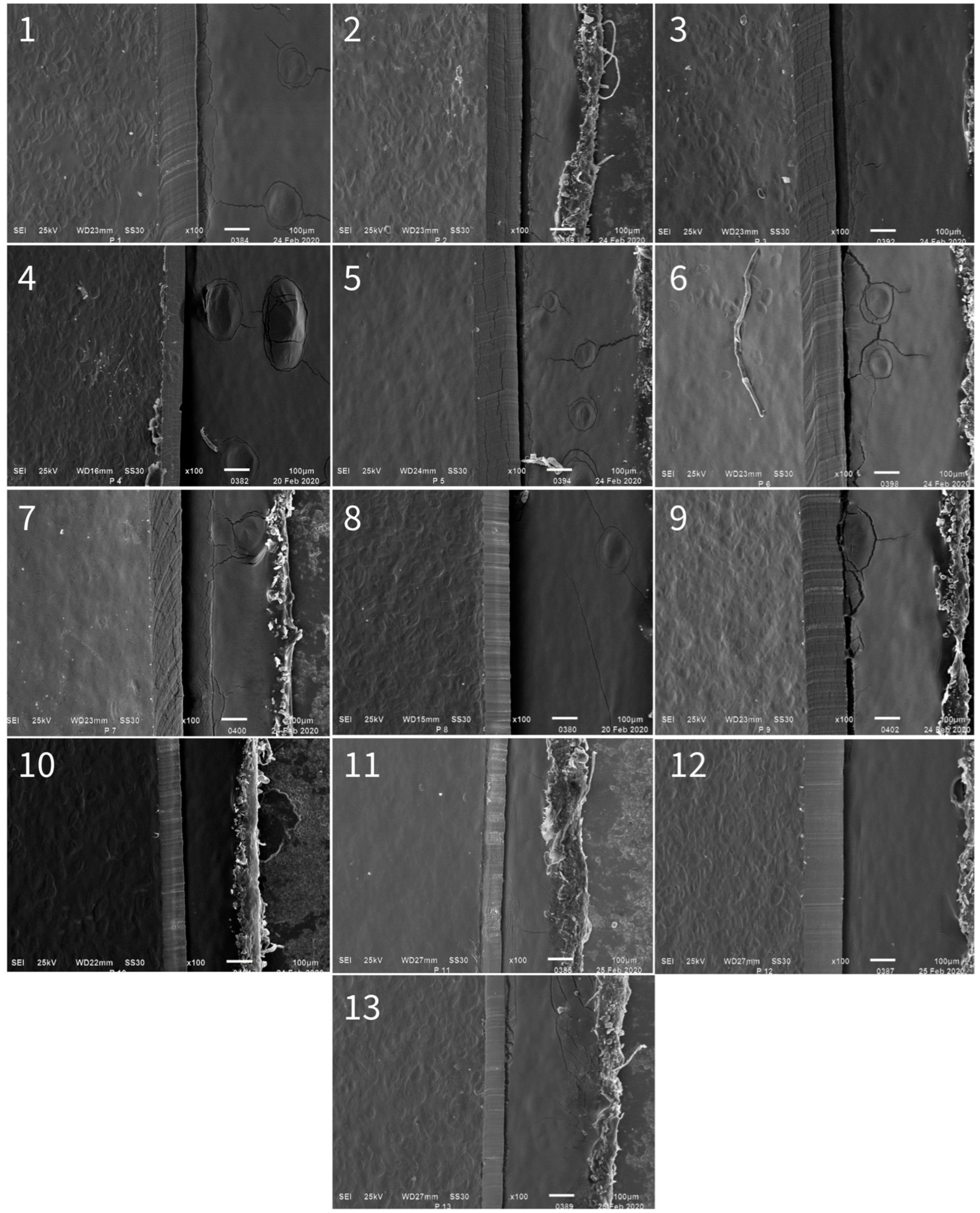
3.5. Film Surface Study
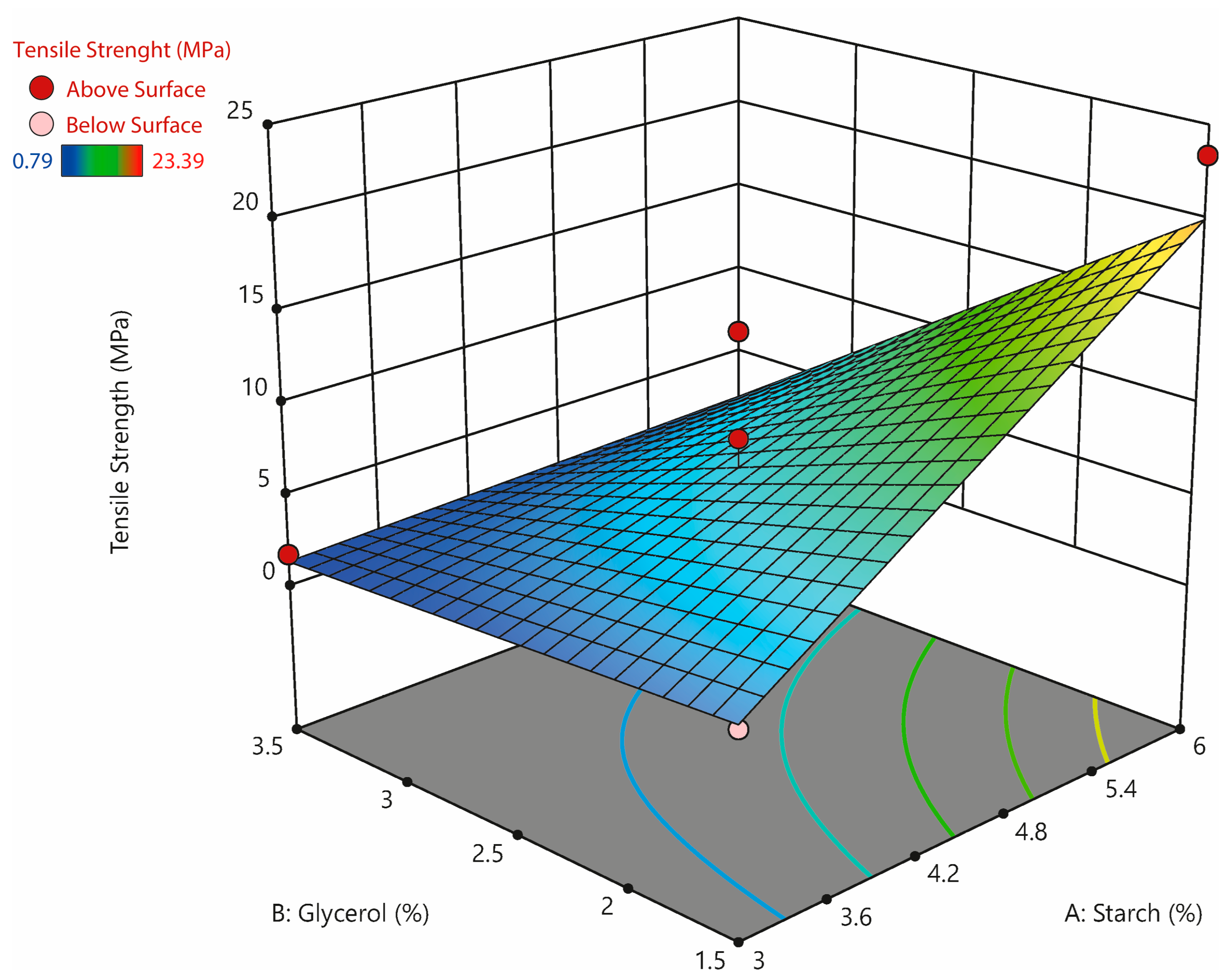
3.6. Mechanical Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| aw | Water activity |
| Fv | Final viscosity |
| GT | Pasting temperature |
| MT | Metric tons |
| OM | Optical microscopy |
| PCs | Pasting curves |
| Pv | Peak viscosity |
| RCCD | Rotatable central composite design |
| RGs | Rejected grains |
| SBv | Setback viscosity |
| SEM | Scanning electron microscopy |
| TS | Tensile strength |
| Tv | Trough viscosity |
References
- Sirbu, E.-E.; Dinita, A.; Tănase, M.; Portoacă, A.-I.; Bondarev, A.; Enascuta, C.-E.; Calin, C. Influence of Plasticizers Concentration on Thermal, Mechanical, and Physicochemical Properties on Starch Films. Processes 2024, 12, 2021. [Google Scholar] [CrossRef]
- Romero-Bastida, C.A.; Bello-Pérez, L.A.; García, M.A.; Martino, M.N.; Solorza-Feria, J.; Zaritzky, N.E. Physicochemical and Microstructural Characterization of Films Prepared by Thermal and Cold Gelatinization from Non-Conventional Sources of Starches. Carbohydr. Polym. 2005, 60, 235–244. [Google Scholar] [CrossRef]
- Fama, L.; Bittante, A.M.B.Q.; Sobral, P.J.A.; Goyanes, S.; Gerschenson, L.N. Garlic Powder and Wheat Bran as Fillers: Their Effect on the Physicochemical Properties of Edible Biocomposites. Mater. Sci. Eng. C 2010, 30, 853–859. [Google Scholar] [CrossRef]
- Mahmood, K.; Kamilah, H.; Shang, P.L.; Sulaiman, S.; Ariffin, F.; Alias, A.K. A Review: Interaction of Starch/Non-Starch Hydrocolloid Blending and the Recent Food Applications. Food Biosci. 2017, 19, 110–120. [Google Scholar] [CrossRef]
- He, W.; Wei, C. Progress in C-Type Starches from Different Plant Sources. Food Hydrocoll. 2017, 73, 162–175. [Google Scholar] [CrossRef]
- Obadi, M.; Qi, Y.; Xu, B. High-Amylose Maize Starch: Structure, Properties, Modifications and Industrial Applications. Carbohydr. Polym. 2023, 299, 120185. [Google Scholar] [CrossRef]
- Martins, P.C.; Latorres, J.M.; Martins, V.G. Impact of Starch Nanocrystals on the Physicochemical, Thermal and Structural Characteristics of Starch-Based Films. LWT 2022, 156, 113041. [Google Scholar] [CrossRef]
- Moo-Huchin, V.M.; Ac-Chim, D.M.; Chim-Chi, Y.A.; Ríos-Soberanis, C.R.; Ramos, G.; Yee-Madeira, H.T.; Ortiz-Fernández, A.; Estrada-León, R.J.; Pérez-Pacheco, E. Huaya (Melicoccus bijugatus) Seed Flour as a New Source of Starch: Physicochemical, Morphological, Thermal and Functional Characterization. Food Meas. 2020, 14, 3299–3309. [Google Scholar] [CrossRef]
- Tagliapietra, B.L.; Felisberto, M.H.F.; Sanches, E.A.; Campelo, P.H.; Clerici, M.T.P.S. Non-Conventional Starch Sources. Curr. Opin. Food Sci. 2021, 39, 93–102. [Google Scholar] [CrossRef]
- Carvalho, H.J.M.; Barcia, M.T.; Schmiele, M. Non-Conventional Starches: Properties and Potential Applications in Food and Non-Food Products. Macromol 2024, 4, 886–909. [Google Scholar] [CrossRef]
- Fonseca, L.M.; Gonçalves, J.R.; El Halal, S.L.M.; Pinto, V.Z.; Dias, A.R.G.; Jacques, A.C.; Zavareze, E.D.R. Oxidation of Potato Starch with Different Sodium Hypochlorite Concentrations and Its Effect on Biodegradable Films. LWT-Food Sci. Technol. 2015, 60, 714–720. [Google Scholar] [CrossRef]
- Bangar, S.P.; Whiteside, W.S.; Ashogbon, A.O.; Kumar, M. Recent Advances in Thermoplastic Starches for Food Packaging: A Review. Food Packag. Shelf Life 2021, 30, 100743. [Google Scholar] [CrossRef]
- Abera, G.; Woldeyes, B.; Demash, H.D.; Miyake, G. The Effect of Plasticizers on Thermoplastic Starch Films Developed from the Indigenous Ethiopian Tuber Crop Anchote (Coccinia abyssinica) Starch. Int. J. Biol. Macromol. 2020, 155, 581–587. [Google Scholar] [CrossRef]
- Basiak, E.; Lenart, A.; Debeaufort, F. How Glycerol and Water Contents Affect the Structural and Functional Properties of Starch-Based Edible Films. Polymers 2018, 10, 412. [Google Scholar] [CrossRef]
- Adhikari, B.; Chaudhary, D.S.; Clerfeuille, E. Effect of Plasticizers on the Moisture Migration Behavior of Low-Amylose Starch Films during Drying. Dry. Technol. 2010, 28, 468–480. [Google Scholar] [CrossRef]
- Lourdin, D.; Bizot, H.; Colonna, P. Antiplasticization in Starch-Glycerol Films? J. Appl. Polym. Sci. 1997, 63, 1047–1053. [Google Scholar] [CrossRef]
- Lee, H.; Htoon, A.; Paterson, J. Alkaline Extraction of Starch from Australian Lentil Cultivars Matilda and Digger Optimised for Starch Yield and Starch and Protein Quality. Food Chem. 2007, 102, 551–559. [Google Scholar] [CrossRef]
- AOAC 930.04:2007; Loss on Drying (Moisture) in Plants. AOAC International: Rockville, MD, USA, 2007.
- AOAC 930.05:2007; Ash of Plants. AOAC International: Rockville, MD, USA, 2007.
- AOAC 930.09:2007; Ether Extract of Plants. AOAC International: Rockville, MD, USA, 2007.
- AOAC 978.04:2007; Nitrogen (Total) (Crude Protein) in Plants. AOAC International: Rockville, MD, USA, 2007.
- Nogueira, G.F.; Soares, C.T.; Cavasini, R.; Fakhouri, F.M.; De Oliveira, R.A. Bioactive Films of Arrowroot Starch and Blackberry Pulp: Physical, Mechanical and Barrier Properties and Stability to pH and Sterilization. Food Chem. 2019, 275, 417–425. [Google Scholar] [CrossRef]
- Miranda, C.S.; Ferreira, M.S.; Magalhães, M.T.; Bispo, A.P.G.; Oliveira, J.C.; Silva, J.B.A.; José, N.M. Starch-Based Films Plasticized with Glycerol and Lignin from Piassava Fiber Reinforced with Nanocrystals from Eucalyptus. Mater. Today Proc. 2015, 2, 134–140. [Google Scholar] [CrossRef]
- ASTM D882-02; Standard Test Method for Tensile Properties of Thin Plastic Sheeting. ASTM International: West Conshohocken, PA, USA, 2010.
- Vithu, P.; Dash, S.K.; Rayaguru, K.; Panda, M.K.; Nedunchezhiyan, M. Optimization of Starch Isolation Process for Sweet Potato and Characterization of the Prepared Starch. Food Meas. 2020, 14, 1520–1532. [Google Scholar] [CrossRef]
- Hoover, R.; Ratnayake, W.S. Starch Characteristics of Black Bean, Chick Pea, Lentil, Navy Bean and Pinto Bean Cultivars Grown in Canada. Food Chem. 2002, 78, 489–498. [Google Scholar] [CrossRef]
- Ovando-Martínez, M.; Bello-Pérez, L.A.; Whitney, K.; Osorio-Díaz, P.; Simsek, S. Starch Characteristics of Bean (Phaseolus vulgaris L.) Grown in Different Localities. Carbohydr. Polym. 2011, 85, 54–64. [Google Scholar] [CrossRef]
- Santana, Á.L.; Zabot, G.L.; Osorio-Tobón, J.F.; Johner, J.C.F.; Coelho, A.S.; Schmiele, M.; Steel, C.J.; Meireles, M.A.A. Starch Recovery from Turmeric Wastes Using Supercritical Technology. J. Food Eng. 2017, 214, 266–276. [Google Scholar] [CrossRef]
- Policegoudra, R.S.; Aradhya, S.M. Structure and Biochemical Properties of Starch from an Unconventional Source—Mango Ginger (Curcuma amada Roxb.) Rhizome. Food Hydrocoll. 2008, 22, 513–519. [Google Scholar] [CrossRef]
- Hughes, T.; Hoover, R.; Liu, Q.; Donner, E.; Chibbar, R.; Jaiswal, S. Composition, Morphology, Molecular Structure, and Physicochemical Properties of Starches from Newly Released Chickpea (Cicer arietinum L.) Cultivars Grown in Canada. Food Res. Int. 2009, 42, 627–635. [Google Scholar] [CrossRef]
- Zhou, Y.; Hoover, R.; Liu, Q. Relationship between α-Amylase Degradation and the Structure and Physicochemical Properties of Legume Starches. Carbohydr. Polym. 2004, 57, 299–317. [Google Scholar] [CrossRef]
- Hoover, R. Composition, Molecular Structure, and Physicochemical Properties of Tuber and Root Starches: A Review. Carbohydr. Polym. 2001, 45, 253–267. [Google Scholar] [CrossRef]
- Hayat, I.; Ahmad, A.; Masud, T.; Ahmed, A.; Bashir, S. Nutritional and Health Perspectives of Beans (Phaseolus vulgaris L.): An Overview. Crit. Rev. Food Sci. Nutr. 2014, 54, 580–592. [Google Scholar] [CrossRef]
- Hoover, R.; Swamidas, G.; Vasanthan, T. Studies on the Physicochemical Properties of Native, Defatted, and Heat-Moisture Treated Pigeon Pea (Cajanus cajan L.) Starch. Carbohydr. Res. 1993, 246, 185–203. [Google Scholar] [CrossRef]
- Wani, I.A.; Sogi, D.S.; Hamdani, A.M.; Gani, A.; Bhat, N.A.; Shah, A. Isolation, Composition, and Physicochemical Properties of Starch from Legumes: A Review. Starch-Stärke 2016, 68, 834–845. [Google Scholar] [CrossRef]
- Braşoveanu, M.; Nemţanu, M.R. Pasting Properties Modeling and Comparative Analysis of Starch Exposed to Ionizing Radiation. Radiat. Phys. Chem. 2020, 168, 108492. [Google Scholar] [CrossRef]
- Alcázar-Alay, S.C.; Meireles, M.A.A. Physicochemical Properties, Modifications and Applications of Starches from Different Botanical Sources. Food Sci. Technol. 2015, 35, 215–236. [Google Scholar] [CrossRef]
- Mauro, R.R.; Vela, A.J.; Ronda, F. Impact of Starch Concentration on the Pasting and Rheological Properties of Gluten-Free Gels. Effects of Amylose Content and Thermal and Hydration Properties. Foods 2023, 12, 2281. [Google Scholar] [CrossRef]
- Nguetcho, V.; Abdou, A.; Nkintang, N. Pasting Properties and Some Functional Properties of Starches from 8 Tropical Legumes Grown in Central Africa. Nutr. Food Toxicol. 2018, 2, 477–487. [Google Scholar]
- Palabiyik, İ.; Toker, O.S.; Karaman, S.; Yildiz, Ö. A Modeling Approach in the Interpretation of Starch Pasting Properties. J. Cereal Sci. 2017, 74, 272–278. [Google Scholar] [CrossRef]
- Fei, W.; Rong, L.; Qi, X.; Chen, X.; Luo, Y.; Wen, H.; Xie, J. Effects of Premna Microphylla Turcz Polysaccharide on Rheological, Gelling, and Structural Properties of Mung Bean Starch and Their Interactions. Food Res. Int. 2024, 189, 114561. [Google Scholar] [CrossRef]
- Nogueira, G.F.; Fakhouri, F.M.; de Oliveira, R.A. Extraction and Characterization of Arrowroot (Maranta arundinaceae L.) Starch and Its Application in Edible Films. Carbohydr. Polym. 2018, 186, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Kaya, S.; Maskan, A. Water Vapor Permeability of Pestil (a Fruit Leather) Made from Boiled Grape Juice with Starch. J. Food Eng. 2003, 57, 295–299. [Google Scholar] [CrossRef]
- Luchese, C.L.; Frick, J.M.; Patzer, V.L.; Spada, J.C.; Tessaro, I.C. Synthesis and Characterization of Biofilms Using Native and Modified Pinhão Starch. Food Hydrocoll. 2015, 45, 203–210. [Google Scholar] [CrossRef]
- Toro-Márquez, L.A.; Merino, D.; Gutiérrez, T.J. Bionanocomposite Films Prepared from Corn Starch With and Without Nanopackaged Jamaica (Hibiscus sabdariffa) Flower Extract. Food Bioprocess Technol. 2018, 11, 1955–1973. [Google Scholar] [CrossRef]
- Esmaeili, M.; Pircheraghi, G.; Bagheri, R. Optimizing the Mechanical and Physical Properties of Thermoplastic Starch via Tuning the Molecular Microstructure through Co-plasticization by Sorbitol and Glycerol. Polym. Int. 2017, 66, 809–819. [Google Scholar] [CrossRef]
- Domene-López, D.; García-Quesada, J.C.; Martin-Gullon, I.; Montalbán, M.G. Influence of Starch Composition and Molecular Weight on Physicochemical Properties of Biodegradable Films. Polymers 2019, 11, 1084. [Google Scholar] [CrossRef]
- Wang, Y.; Meng, Y.; Ji, Z.; Meng, X.; Song, X.; Lu, P.; Chen, F. Bioinspired Colored Degradable Starch-Based Films with Excellent Tensile Strength. Ind. Crops Prod. 2021, 167, 113525. [Google Scholar] [CrossRef]
- Tarique, J.; Sapuan, S.M.; Khalina, A. Effect of Glycerol Plasticizer Loading on the Physical, Mechanical, Thermal, and Barrier Properties of Arrowroot (Maranta arundinacea) Starch Biopolymers. Sci. Rep. 2021, 11, 13900. [Google Scholar] [CrossRef]
- Frangopoulos, T.; Marinopoulou, A.; Goulas, A.; Likotrafiti, E.; Rhoades, J.; Petridis, D.; Kannidou, E.; Stamelos, A.; Theodoridou, M.; Arampatzidou, A.; et al. Optimizing the Functional Properties of Starch-Based Biodegradable Films. Foods 2023, 12, 2812. [Google Scholar] [CrossRef] [PubMed]
- Santana, R.F.; Bonomo, R.C.F.; Gandolfi, O.R.R.; Rodrigues, L.B.; Santos, L.S.; Dos Santos Pires, A.C.; De Oliveira, C.P.; Da Costa Ilhéu Fontan, R.; Veloso, C.M. Characterization of Starch-Based Bioplastics from Jackfruit Seed Plasticized with Glycerol. J. Food Sci. Technol. 2018, 55, 278–286. [Google Scholar] [CrossRef] [PubMed]

| Coded Variables | Uncoded Variables | ||||
|---|---|---|---|---|---|
| Std Order | Run | Factor 1: Starch | Factor 2: Glycerol | Factor 1: Starch (%) | Factor 2: Glycerol (%) |
| 13 | 1 | 0 | 0 | 4.5 | 2.5 |
| 3 | 2 | −1 | 1 | 3 | 3.5 |
| 5 | 3 | −1.41421 | 0 | 2.37868 | 2.5 |
| 8 | 4 | 0 | 1.41421 | 4.5 | 3.91421 |
| 6 | 5 | 1.41421 | 0 | 6.62132 | 2.5 |
| 7 | 6 | 0 | −1.41421 | 4.5 | 1.08579 |
| 1 | 7 | −1 | −1 | 3 | 1.5 |
| 12 | 8 | 0 | 0 | 4.5 | 2.5 |
| 4 | 9 | 1 | 1 | 6 | 3.5 |
| 10 | 10 | 0 | 0 | 4.5 | 2.5 |
| 2 | 11 | 1 | −1 | 6 | 1.5 |
| 11 | 12 | 0 | 0 | 4.5 | 2.5 |
| 9 | 13 | 0 | 0 | 4.5 | 2.5 |
| [Starch] % | GT (°C) | Pv (Pa·s) | SBv (Pa·s) |
|---|---|---|---|
| 3 | 74.44 ± 0.04 | 0.032 ± 0.00 | 0.01 ± 0.00 |
| 5 | 74.51 ± 0.11 | 0.047 ± 0.00 | 0.01 ± 0.00 |
| 10 | 72.38 ± 0.05 | 0.340 ± 0.00 | 0.59 ± 0.01 |
| 15 | 72.55 ± 0.01 | 1.09 ± 0.02 | 5.11 ± 0.09 |
| Coefficients | Value | Confidence Intervals | |||
|---|---|---|---|---|---|
| Low | High | ||||
| Vpeak | 1.19 | 1.0749 | 1.3066 | 0.9814 | 0.9804 |
| S | 32.7991 | 24.7918 | 40.8098 | ||
| R | 774.2395 | 763.4950 | 778.9840 | ||
| Run | Factor | |||||
|---|---|---|---|---|---|---|
| Starch | Glycerol | L | a | b | aw | |
| 1 | 4.5 | 2.5 | 19.16 cd | −0.2 abcd | −2.21 abc | 0.28 cde |
| 2 | 4.5 | 2.5 | 19.11 cd | −0.07 abc | −1.72 a | 0.35 ab |
| 3 | 4.5 | 3.91 | 18.53 fg | −0.3 abcd | −1.70 a | 0.31 bcd |
| 4 | 2.37 | 2.5 | 16.72 i | −0.4 abcd | −3.00 de | 0.4 a |
| 5 | 4.5 | 2.5 | 19.6 b | −0.16 abcd | −2.19 abc | 0.27 de |
| 6 | 4.5 | 2.5 | 19.5 bc | −0.31 abcd | −2.70 cd | 0.22 e |
| 7 | 3 | 3.5 | 18.13 gh | 0.12 a | −2.48 bcd | 0.25 e |
| 8 | 6 | 1.5 | 18.68 ef | −0.32 abcd | −2.70 cd | 0.35 ab |
| 9 | 6.62 | 2.5 | 20.17 a | −0.003 ab | −1.97 ab | 0.39 a |
| 10 | 4.5 | 1.08 | 18.9 de | −0.54 abcd | −2.97 de | 0.32 bcd |
| 11 | 6 | 3.5 | 19.84 ab | −0.64 bcd | −2.66 cd | 0.33 bc |
| 12 | 3 | 1.5 | 15.93 j | −0.82 cd | −3.59 de | 0.27 de |
| 13 | 4.5 | 2.5 | 17.91 h | −0.75 d | −3.07 e | 0.33 b |
| Uncoded Variables | Response | |||
|---|---|---|---|---|
| Std Order | Run | Factor 1: Starch (%) | Factor 2: Glycerol (%) | Tensile Strength (Mpa) |
| 13 | 1 | 4.5 | 2.5 | 3.65 ± 0.12 f,g |
| 3 | 2 | 3 | 3.5 | 1.73 ± 0.12 h,i |
| 5 | 3 | 2.3 | 2.5 | 2.10 ± 0.01 h |
| 8 | 4 | 4.5 | 3.9 | 0.79 ± 0.01 i |
| 6 | 5 | 6.6 | 2.5 | 9.82 ± 0.13 c |
| 7 | 6 | 4.5 | 1 | 15.13 ± 0.41 b |
| 1 | 7 | 3 | 1.5 | 2.57 ± 0.01 g,h |
| 12 | 8 | 4.5 | 2.5 | 3.54 ± 0.02 f,g |
| 4 | 9 | 6 | 3.5 | 6.28 ± 0.31 e |
| 10 | 10 | 4.5 | 2.5 | 3.94 ± 0.75 f |
| 2 | 11 | 6 | 1.5 | 23.39 ± 0.91 a |
| 11 | 12 | 4.5 | 2.5 | 4.55 ± 0.27 f |
| 9 | 13 | 4.5 | 2.5 | 8.20 ± 0.26 d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Graciano-de la Cruz, V.G.; Morales-Contreras, B.E.; Núñez-Bretón, L.C.; Fonseca-Florido, H.A.; Morales-Castro, J.; Gallegos-Infante, J.A.; Rosas-Flores, W. Valorization of Agro-Industry-Rejected Common Bean Grains for Starch Film Development: Advancing Sustainable and Comprehensive Resource Utilization. Sustainability 2025, 17, 9466. https://doi.org/10.3390/su17219466
Graciano-de la Cruz VG, Morales-Contreras BE, Núñez-Bretón LC, Fonseca-Florido HA, Morales-Castro J, Gallegos-Infante JA, Rosas-Flores W. Valorization of Agro-Industry-Rejected Common Bean Grains for Starch Film Development: Advancing Sustainable and Comprehensive Resource Utilization. Sustainability. 2025; 17(21):9466. https://doi.org/10.3390/su17219466
Chicago/Turabian StyleGraciano-de la Cruz, Victoria Guadalupe, Blanca Elizabeth Morales-Contreras, Lucila Concepción Núñez-Bretón, Heidi Andrea Fonseca-Florido, Juliana Morales-Castro, José Alberto Gallegos-Infante, and Walfred Rosas-Flores. 2025. "Valorization of Agro-Industry-Rejected Common Bean Grains for Starch Film Development: Advancing Sustainable and Comprehensive Resource Utilization" Sustainability 17, no. 21: 9466. https://doi.org/10.3390/su17219466
APA StyleGraciano-de la Cruz, V. G., Morales-Contreras, B. E., Núñez-Bretón, L. C., Fonseca-Florido, H. A., Morales-Castro, J., Gallegos-Infante, J. A., & Rosas-Flores, W. (2025). Valorization of Agro-Industry-Rejected Common Bean Grains for Starch Film Development: Advancing Sustainable and Comprehensive Resource Utilization. Sustainability, 17(21), 9466. https://doi.org/10.3390/su17219466







