Response of Soil Microbial Population and Activity to Sunn Hemp Cover Crop, Combined Nano Zinc and Copper and Nitrogen Fertiliser Application After Canola Cultivation
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site Description
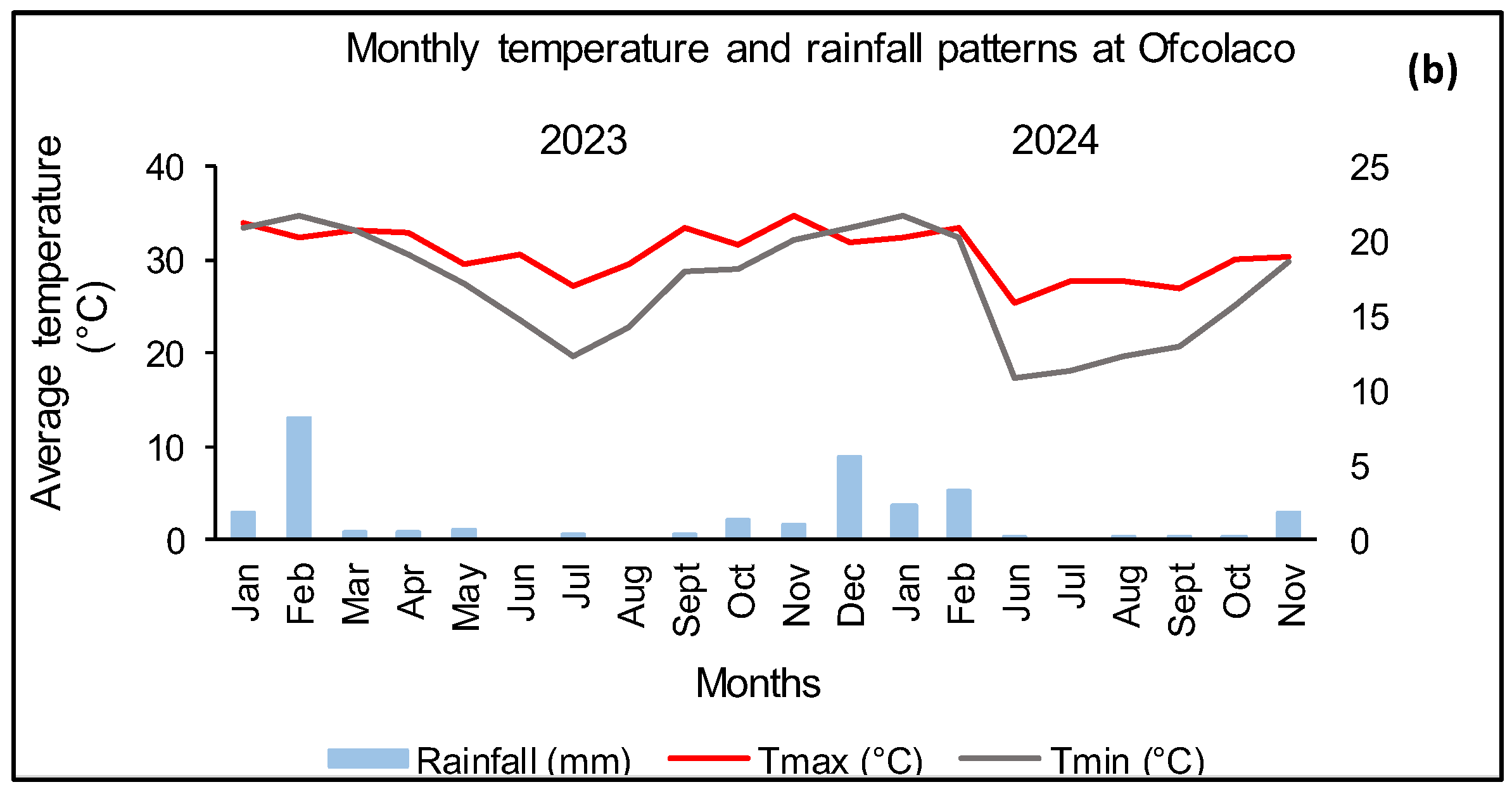
2.2. Weather Conditions for the 2023 Growing Season
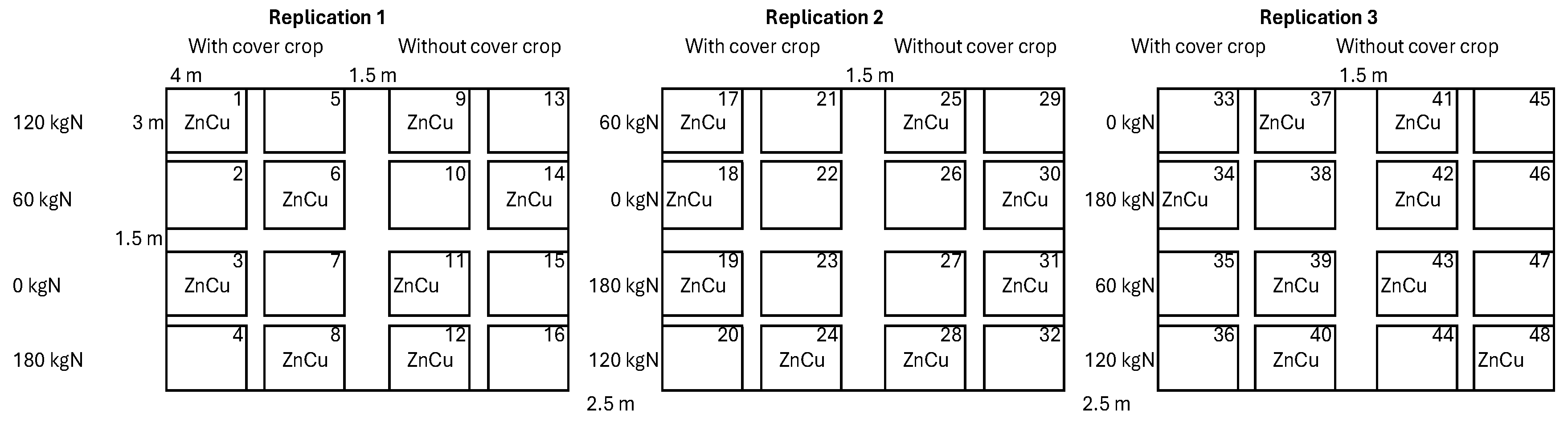
2.3. Experimental Layout and Treatments
2.4. Soil Sampling and Analysis
2.5. Statistical Analysis
3. Results
3.1. Pre-Sown Soil Properties of the Experimental Field
3.2. Treatment Effects on Soil Bacterial Populations
3.2.1. Syferkuil
3.2.2. Ofcolaco
3.3. Treatment Effects on Soil Fungal Populations
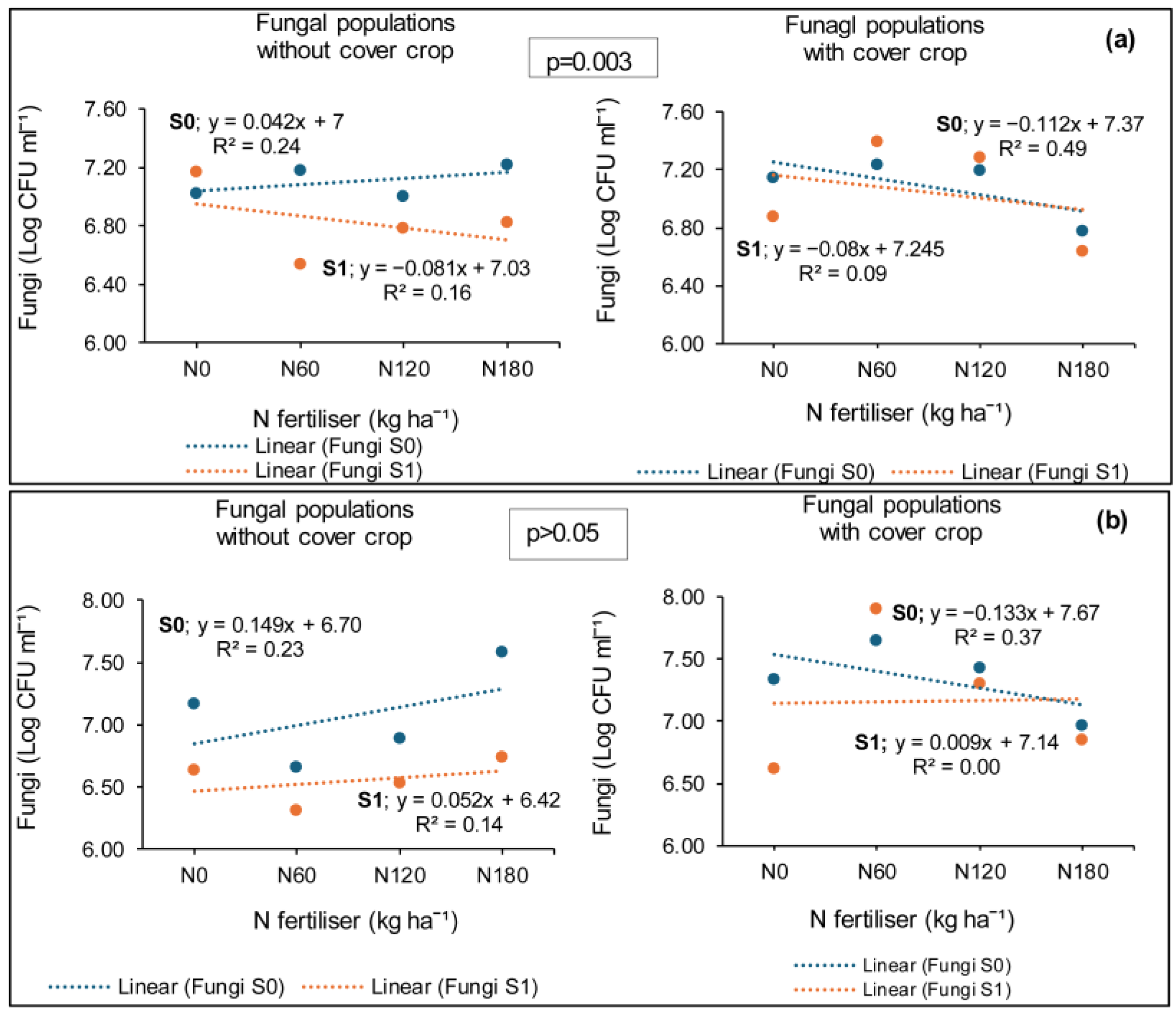
3.3.1. Syferkuil
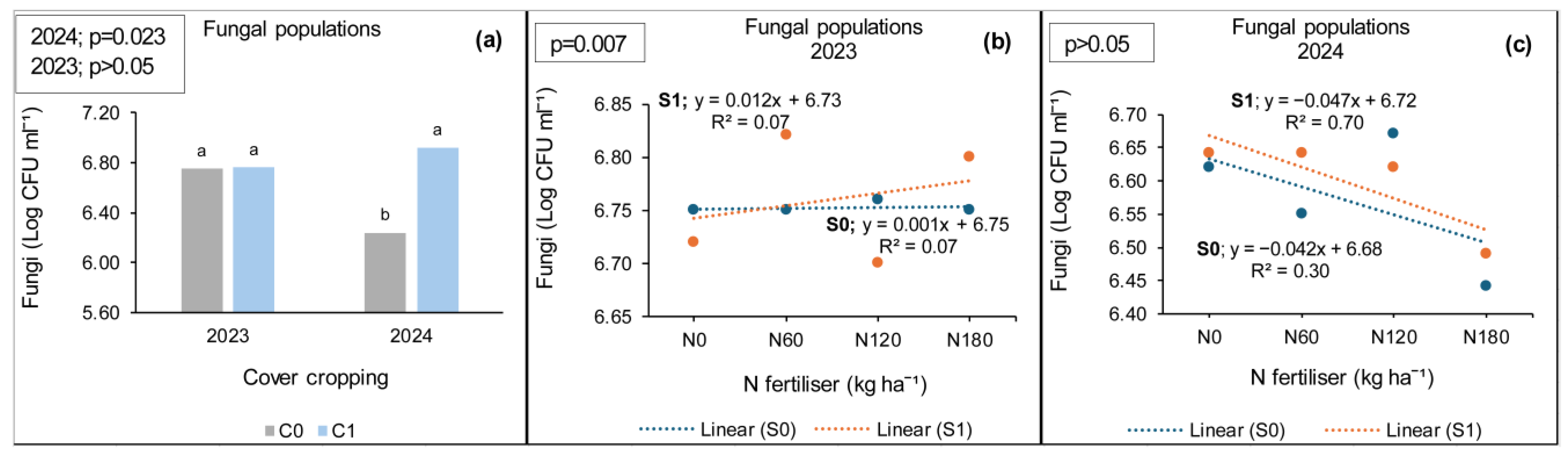
3.3.2. Ofcolaco
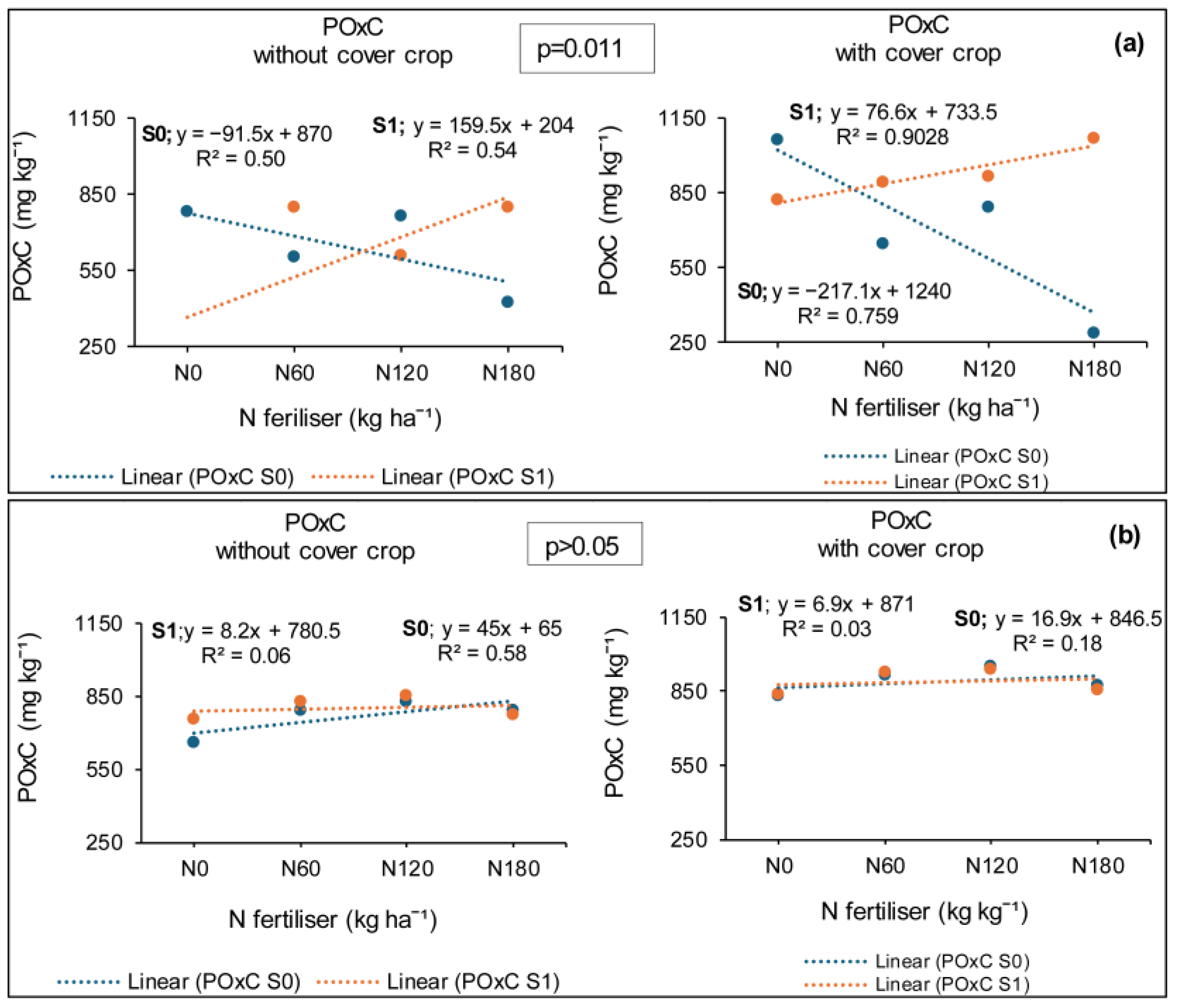
3.4. Treatment Effects on Soil Active Carbon (POxC)
3.4.1. Syferkuil
3.4.2. Ofcolaco
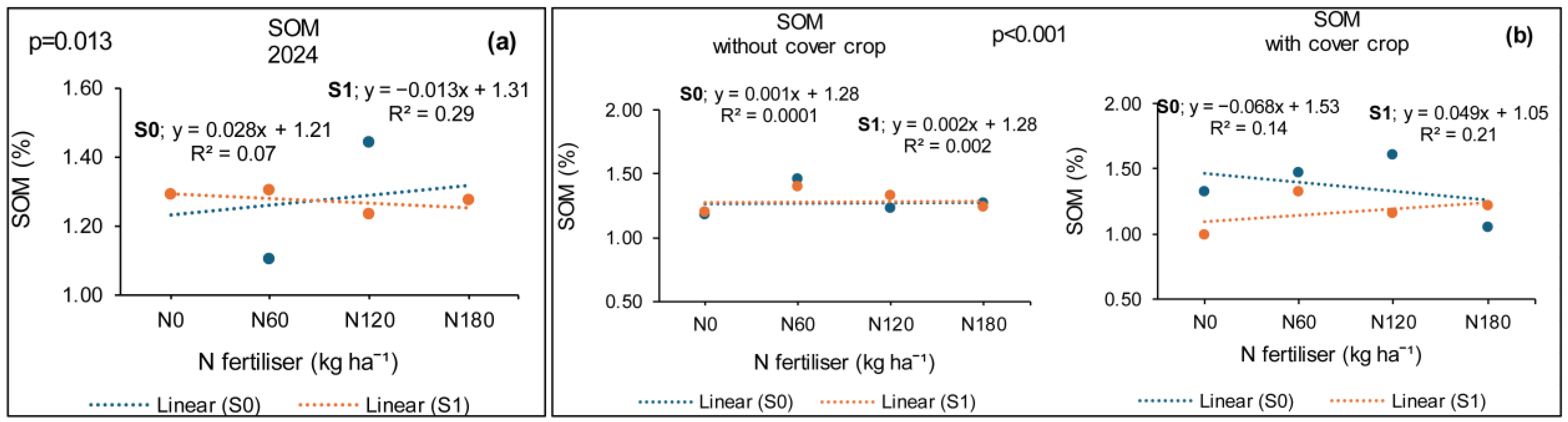
3.5. Treatment Effects on Soil Organic Matter (SOM)
3.5.1. Syferkuil
3.5.2. Ofcolaco
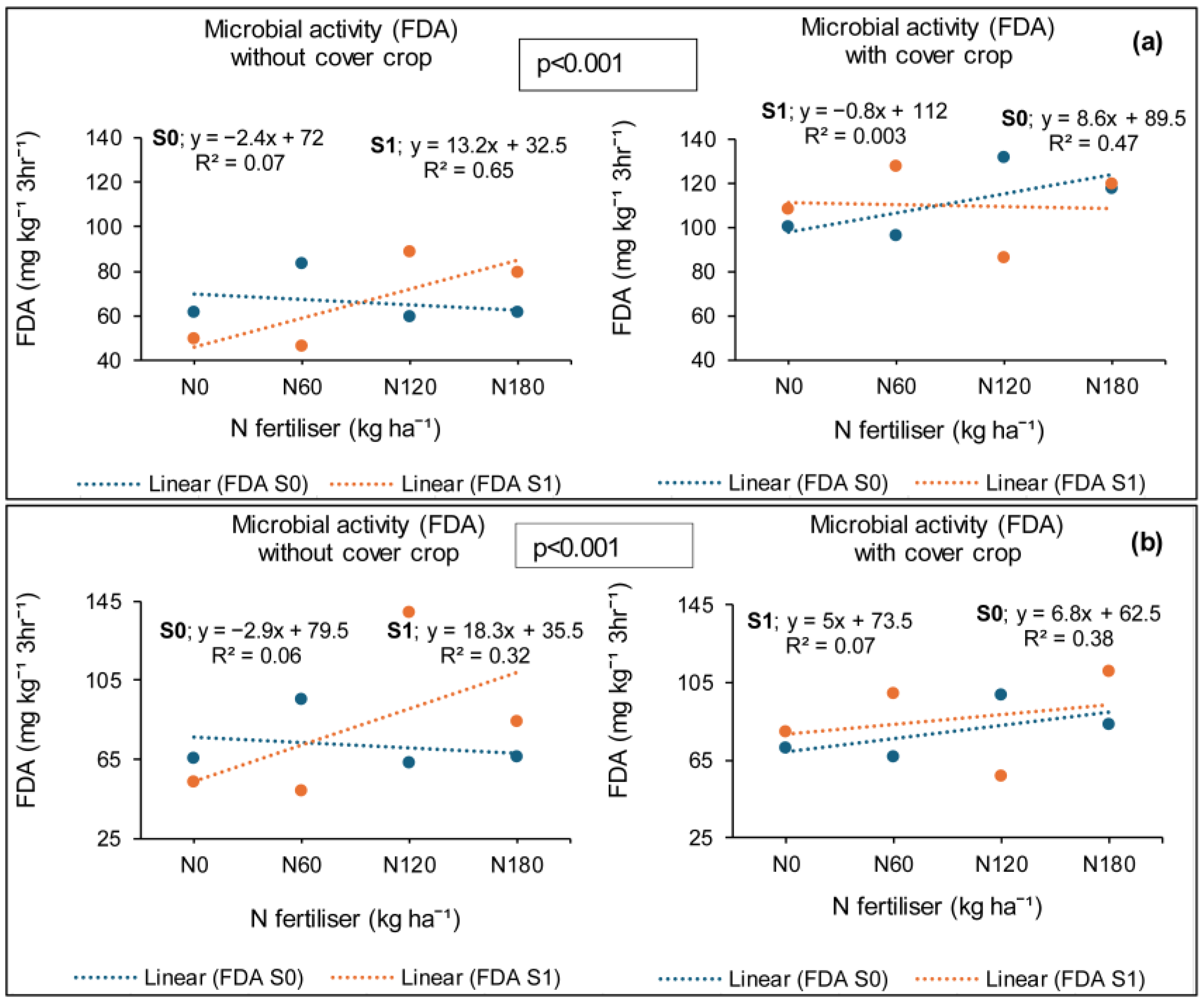
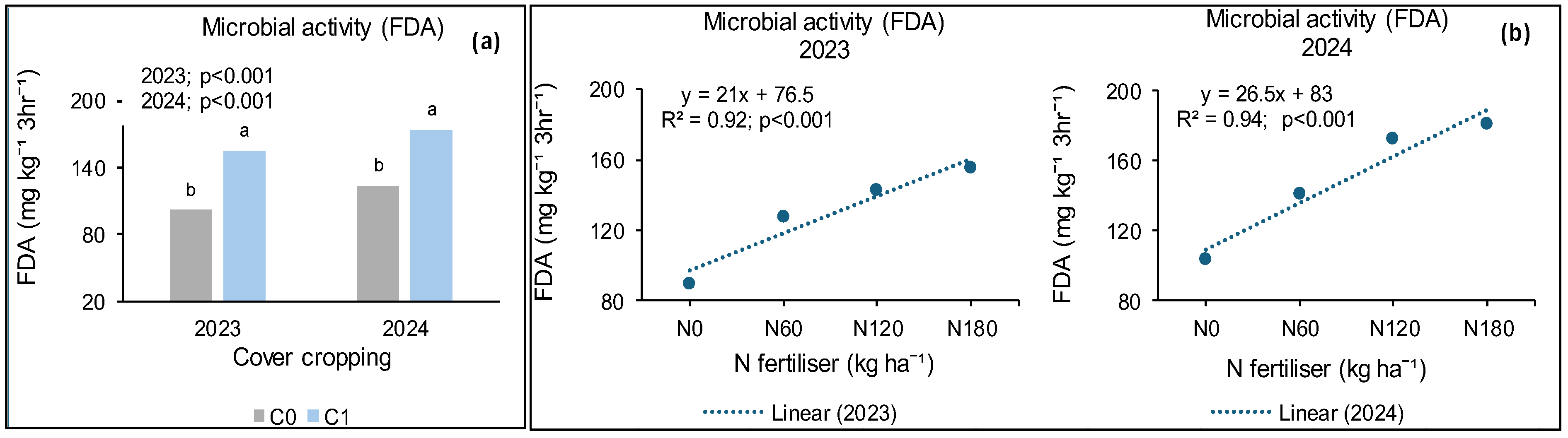
3.6. Treatment Effects on Soil Microbial Activity (FDA)
3.6.1. Syferkuil
3.6.2. Ofcolaco
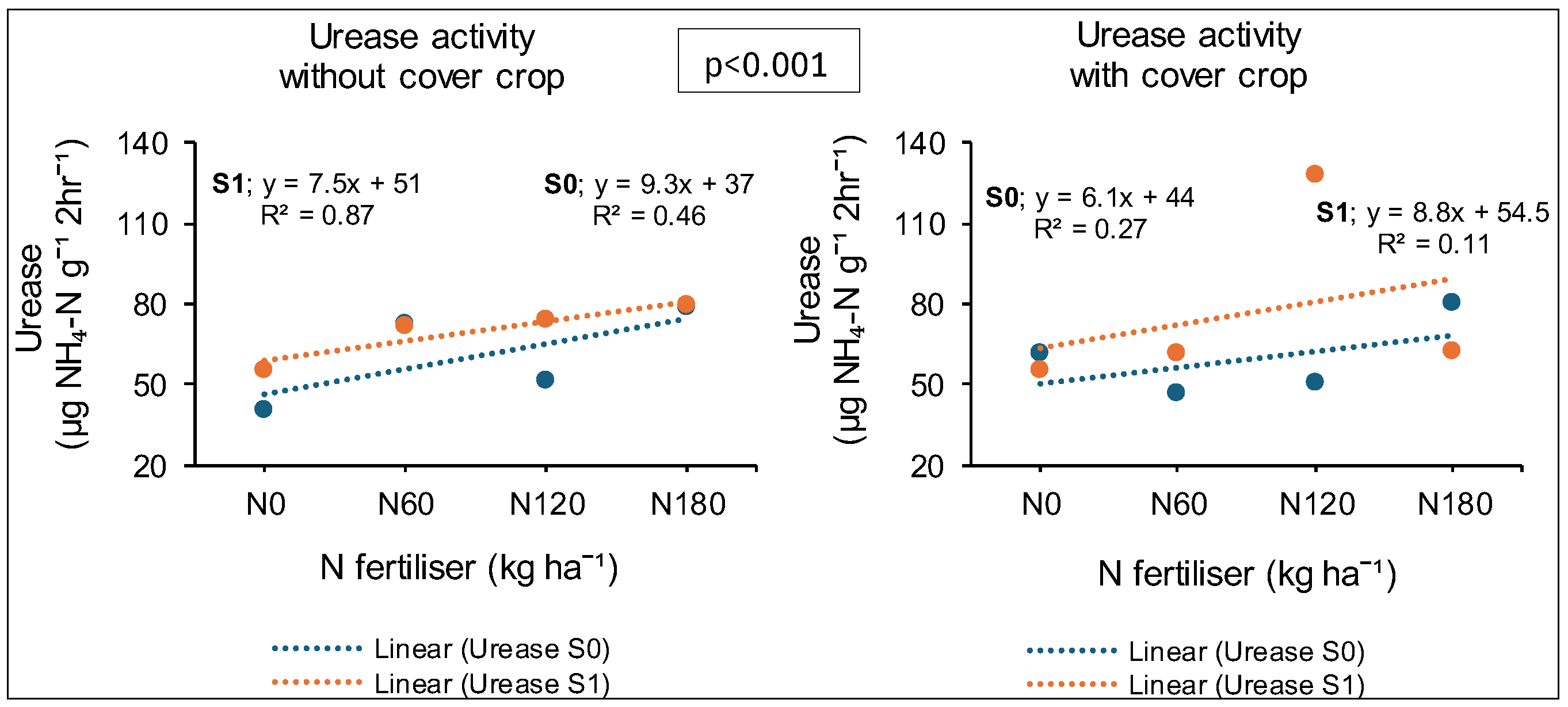
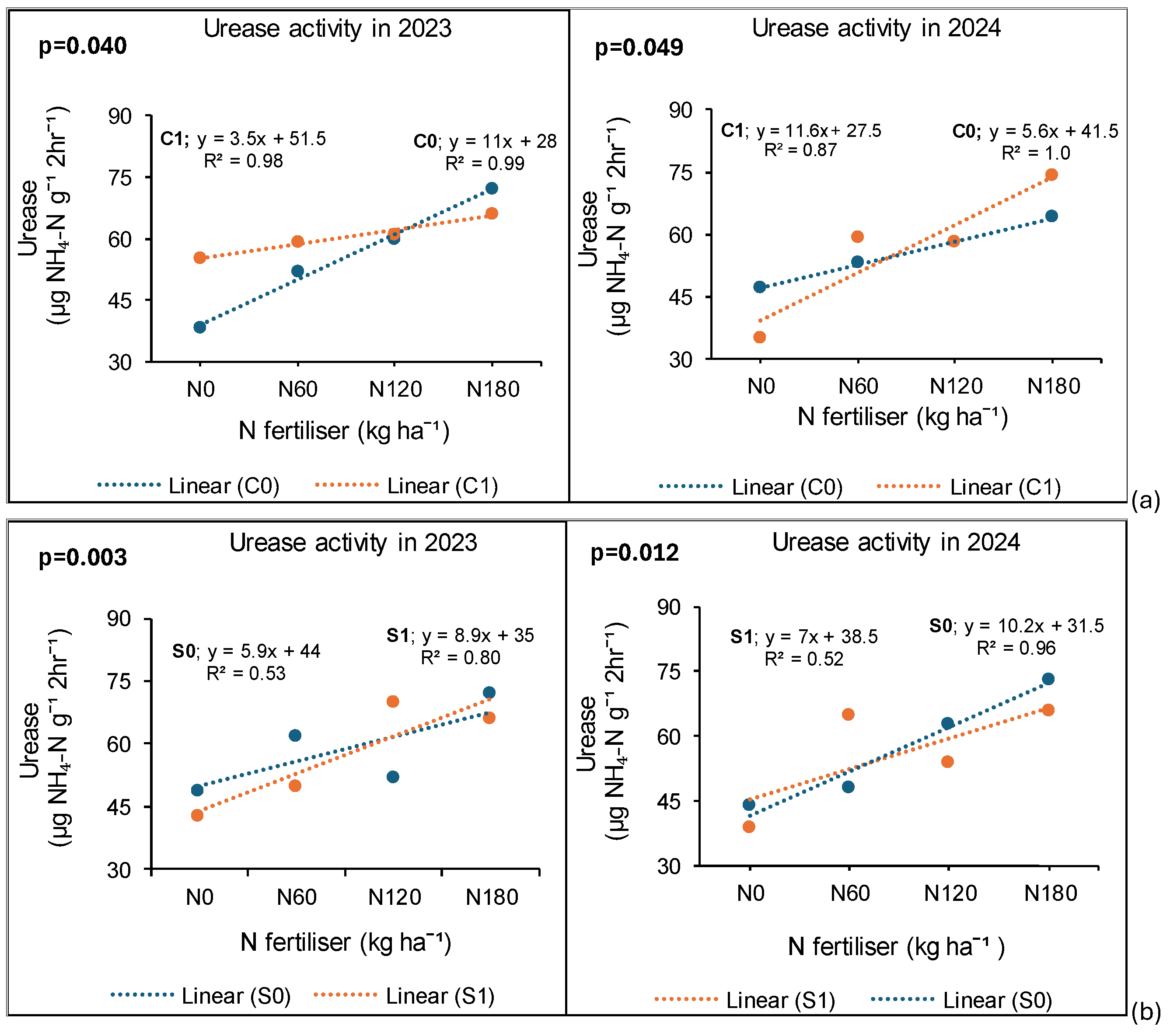
3.7. Treatment Effects on Urease Enzyme Activity
3.7.1. Syferkuil
3.7.2. Ofcolaco
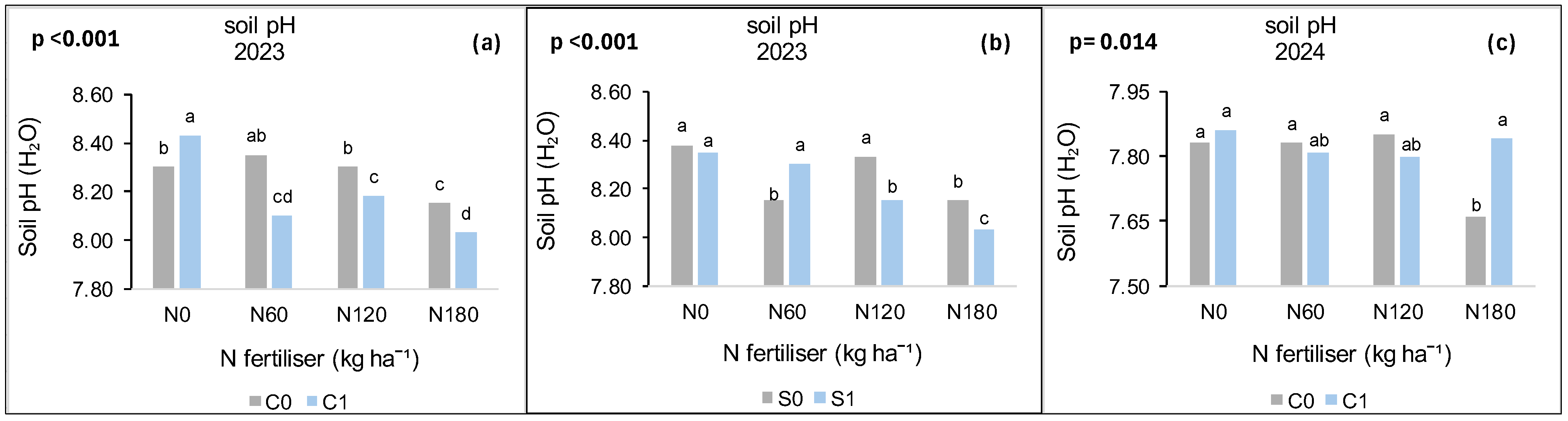
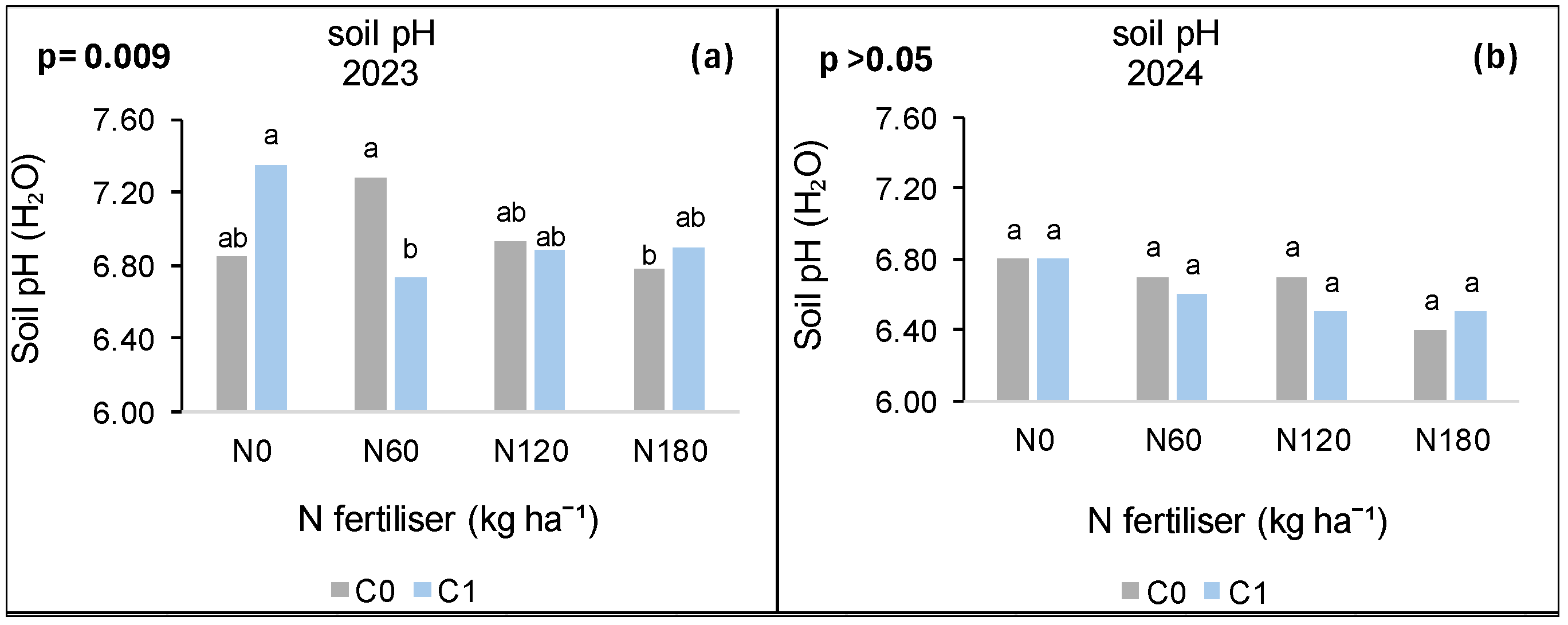
3.8. Treatment Effects on Soil pH
3.8.1. Syferkuil
3.8.2. Ofcolaco
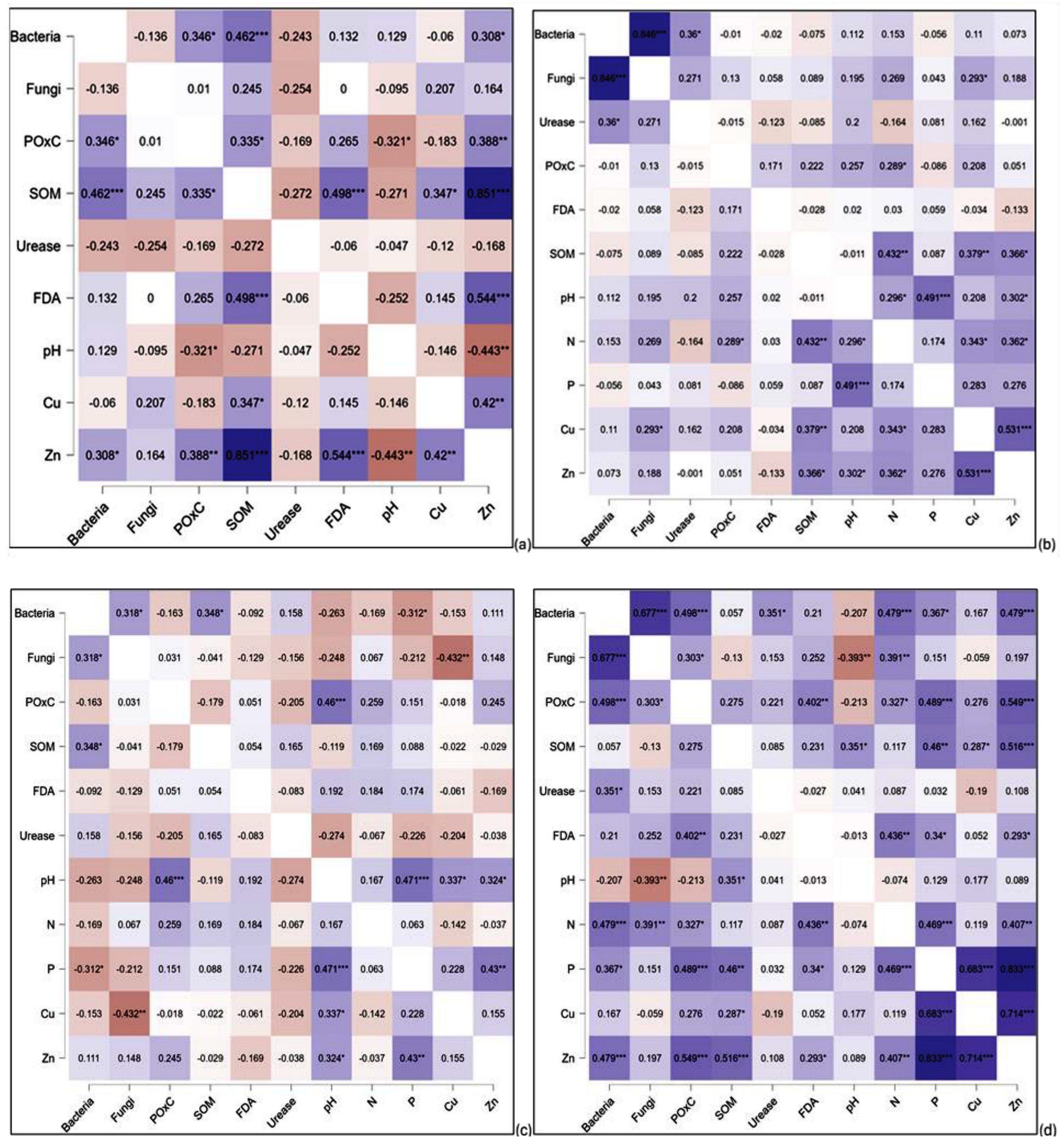
3.9. Correlation Relationships Between the Selected Soil Biological Properties
3.9.1. Syferkuil
3.9.2. Ofcolaco
4. Discussion
4.1. Bacterial Populations
4.2. Fungal Populations
4.3. Active Carbon (POxC)
4.4. Microbial Activity (FDA)
4.5. Soil Organic Matter
4.6. Urease Activity
4.7. Soil pH
5. Conclusions and Recommendations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Calderon, F.J.; Nielsen, D.; Acosta-Martinez, V.; Vigil, M.F.; Drew, L. Cover crop and irrigation effects on soil microbial communities and enzymes in semiarid agroecosystems of the central Great Plains of North America. Pedosphere 2016, 26, 192–205. [Google Scholar] [CrossRef]
- Haney, R.L.; Haney, E.B.; Smith, D.R.; Harmel, R.D.; White, M.J. The soil health tool-Theory and initial broad-scale application. Appl. Soil. Ecol. 2018, 125, 162–168. [Google Scholar] [CrossRef]
- Chen, Y.X.; Chen, X.H.; Tang, Y.Q.; Zhang, F.S.; Chen, X.P.; Zhang, C.C.; Liu, J.; Xu, K.W. Effect of nitrogen fertiliser on dry matter accumulation and yield in wheat/maize/soybean intercropping systems. Acta Pratacult. Sin. 2014, 23, 73. [Google Scholar]
- Delgado-Baquerizo, M.; Maestre, F.T.; Gallardo, A.; Bowker, M.A.; Wallenstein, M.D.; Quero, J.L.; Ochoa, V.; Gozalo, B.; García-Gómez, M.; Soliveres, S.; et al. Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature 2013, 502, 672–676. [Google Scholar] [CrossRef]
- Shukla, A.K.; Behera, S.K.; Prakash, C.; Patra, A.K.; Rao, C.S.; Chaudhari, S.K.; Das, S.; Singh, A.K.; Green, A. Assessing multi-micronutrients deficiency in agricultural soils of India. Sustainability 2021, 13, 9136. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Srivastava, A.K.; Rajput, V.D.; Chauhan, P.K.; Bhojiya, A.A.; Jain, D.; Chaubey, G.; Dwivedi, P.; Sharma, B.; Minkina, T. Root Exudates: Mechanistic Insight of Plant Growth Promoting Rhizobacteria for Sustainable Crop Production. Front. Microbiol. 2022, 13, 916488. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Chen, H.Y.H.; Ruan, H. Global negative effects of nitrogen deposition on soil microbes. ISME J. 2018, 12, 1817–1825. [Google Scholar] [CrossRef]
- Galindo, F.S.; Strock, J.S.; Pagliari, P.H. Impacts of corn stover management and fertiliser application on soil nutrient availability and enzymatic activity. Sci. Rep. 2022, 12, 1985. [Google Scholar] [CrossRef]
- Bebber, D.P.; Richards, V.R. A meta-analysis of the effect of organic and mineral fertilisers on soil microbial diversity. Appl. Soil Ecol. 2022, 175, 104450. [Google Scholar] [CrossRef]
- Lori, M.; Symnaczik, S.; Mäder, P.; De Deyn, G.; Gattinger, A. Organic farming enhances soil microbial abundance and activity-A meta-analysis and meta-regression. PLoS ONE 2017, 12, e0180442. [Google Scholar] [CrossRef]
- Nagula, S.; Ramanjaneyulu, A.V. Nano fertilisers: The next generation fertiliser. Biot. Res. Today 2020, 2, 905–907. [Google Scholar]
- Babu, S.; Singh, R.; Yadav, D.; Rathore, S.S.; Raj, R.; Avasthe, R.; Yadav, S.K.; Das, A.; Yadav, V.; Yadav, B.; et al. Nano fertilisers for agricultural and environmental sustainability. Chemosphere 2022, 292, 133451. [Google Scholar] [CrossRef]
- Wang, J.; Liu, L.; Gao, X.; Hao, J.; Wang, M. Elucidating the effect of biofertilisers on bacterial diversity in maize rhizosphere soil. PLoS ONE 2021, 16, e0249834. [Google Scholar] [CrossRef]
- Koudahe, K.; Samuel, C.; Djaman, A.K. Critical review of the impact of cover crops on soil properties. Int. Soil Water Conserv. Res. 2022, 10, 343–354. [Google Scholar] [CrossRef]
- Xie, Y.; Black, Z.E.; Xu, N.; Brecht, J.K.; Huff, D.M.; Zhao, X. Influence of Sunn Hemp Biomass Incorporation on Organic Strawberry Production. Horticulturae 2023, 9, 1247. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Holman, J.D.; Schlegel, A.J.; Tatarko, J.; Shaver, T.M. Replacing fallow with cover crops in a semiarid soil: Effects on soil properties. Soil Sci. Soc. Am. J. 2013, 77, 1026–1034. [Google Scholar] [CrossRef]
- Blanco-Canqui, H.; Shapiro, C.A.; Wortmann, C.S.; Drijber, R.A.; Mamo, M.; Shaver, T.M.; Ferguson, R.B. Soil organic carbon: The value to soil properties. J. Soil Water Conserv. 2013, 68, 129A–134A. [Google Scholar] [CrossRef]
- DeLaune, P.D.; Mubvumba, P.; Lewis, K.L.; Keeling, J.W. Rye cover crop impacts soil properties in a long-term cotton system. Soil Sci. Soc. Am. J. 2019, 83, 1451–1458. [Google Scholar] [CrossRef]
- Saleem, M.; Pervaiz, Z.H.; Contreras, J.; Lindenberger, J.H.; Hupp, B.M.; Chen, D.; Zhang, Q.; Wang, C.; Iqbal, J.; Twigg, P. Cover crop diversity improves multiple soil properties via altering root architectural traits. Rhizosphere 2020, 16, 100248. [Google Scholar] [CrossRef]
- Hu, J.; Xianyu, Y. When nano meets plants: A review on the interplay between nanoparticles and plants. Nano Today 2021, 38, 101143. [Google Scholar] [CrossRef]
- Singh, P.; Singh, A.P. Nano materials in Soil Health Management and Crop Production: Potentials and Limitations. In Handbook of Nano Materials and Nanocomposites for Energy and Environmental Applications; Springer: Cham, Switzerland, 2021; pp. 1–25. [Google Scholar]
- Kah, M.; Kookana, R.S.; Gogos, A.; Bucheli, T.D. A critical evaluation of nanopesticides and nano fertilisers against their conventional analogues. Nat. Nanotechnol. 2018, 13, 677–684. [Google Scholar] [CrossRef]
- Elizabath, A.; Babychan, M.; Mathew, A.M.; Syriac, G.M. Application of Nanotechnology in Agriculture. Int. J. Pure App. Biosci. 2019, 7, 131–139. [Google Scholar] [CrossRef]
- Irewale, A.T.; Dimpka, C.O.; Agunbiade, F.O.; Oyeboade, O.A.; Elemike, E.E.; Oguzie, E.E. Unlocking sustainable agricultural development in Africa via bio-nanofertiliser application-challenges, opportunities and prospects. Sci. Afr. 2024, 25, e02276. [Google Scholar] [CrossRef]
- Mahapatra, D.M.; Satapathy, K.H.; Panda, B. Biofertilisers and nanofertilisers for sustainable agriculture: Phycoprospects and challenges. Sci. Total Environ. 2022, 803, 149990. [Google Scholar] [CrossRef] [PubMed]
- Hirzel, J.; Undurraga, P.; León, L.; Panichini, M.; Carrasco, J.; González, J.; Matus, I. Canola production and effect on soil chemical properties in response to different residue levels from three biannual crop rotations. Plant Prod. Sci. 2021, 24, 287–296. [Google Scholar] [CrossRef]
- Elsawy, H.; El-shahawy, A.; Ibrahim, M.; El-Halim, A.E.-H.A.; Talha, N.; Sedky, A.; Alfwuaires, M.; Alabbad, H.; Almeri, N.; Mahmoud, E. Properties of Recycled Nanomaterials and Their Effect on Biological Activity and Yield of Canola in Degraded Soils. Agriculture 2022, 12, 2096. [Google Scholar] [CrossRef]
- Kah, M.; Tufenkji, N.; White, J.C. Nano-enabled strategies to enhance crop nutrition and protection. Nat. Nanotechnol. 2019, 14, 532–540. [Google Scholar] [CrossRef] [PubMed]
- Duhan, J.S.; Kumar, R.; Kumar, N.; Kaur, P.; Nehra, K.; Duhan, K. Nanotechnology: The new perspective in precision agriculture. Biotechnol. Rep. 2017, 15, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Soil Classification Working Group. Soil Classification. A Taxonomic System for South Africa; The Department of Agricultural Development: Pretoria, South Africa, 1991. [Google Scholar]
- Masete, F.M.; Munjonji, L.; Ayisi, K.K.; Bopape-Mabapa, M.P. Cowpea Growth and Nitrogen Fixation Performance under Different Mulch Treatments. Agriculture 2022, 12, 1144. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-total. In Methods of Soil Analysis, Part 3, 5th ed.; Soil Science Society of America Book Series; SSSA: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Blanck, E. Handbook of Soil Science; Springer: Berlin, Germany, 1931. [Google Scholar]
- Okalebo, J.; Gathua, K.; Woomer, P. Laboratory Methods of Soil and Plant Analysis: A Working Manual; TSBF-CIAT and SACRED Africa: Nairobi, Kenya, 2002. [Google Scholar]
- Garcia, D.J.; Cegarra, M.P.; Bernal, M.P.; Navarro, A.F. Comparative evaluation of methods employing alkali and sodium pyrophosphate to extract humic substances from peat. Commun. Soil Sci. Plant Anal. 1993, 24, 1481–1494. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis, Part 3, 5th ed.; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Thomas, G.W., Eds.; SSSA: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Walkley, A.; Black, I.A. An examination of the Degtjaref method for determining soil organic matter and a proposed modification of the chromic acid titration method. Soil Sci. 1934, 37, 29–38. [Google Scholar] [CrossRef]
- Cooper, H.V.; Girkin, N.T.; Rosolem, C.; Sjögersten, S.; Lark, R.M. Longterm zero-tillage enhances the protection of soil carbon in tropical agriculture. Eur. J. Soil Sci. 2021, 72, 2477–2492. [Google Scholar] [CrossRef]
- Sanders, E.R. Aseptic laboratory techniques: Plating methods. J. Vis. Exp. 2012, 63, e3064. [Google Scholar] [CrossRef] [PubMed]
- Weil, R.R.; Islam, K.R.; Stine, M.A.; Gruver, J.B.; Samson-Liebig, S.E. Estimating active carbon for soil quality assessment: A simplified method for laboratory and field use. Am. J. Altern. Agric. 2003, 18, 3–17. [Google Scholar] [CrossRef]
- Prosser, J.A.; Speir, T.W.; Stott, D.E. Soil oxido-reductases and FDA hydrolysis. In Methods of Soil Enzymology; Dick, R.P., Ed.; SSSA Book Series 9; SSSA: Madison, WI, USA, 2011. [Google Scholar]
- Tabatabai, M.A.; Bremner, J.M. Assay of urease activity in soils. Soil Biol. Biochem. 1972, 4, 479–487. [Google Scholar] [CrossRef]
- Fertiliser Society of South Africa (FSSA-MVSA). Fertiliser Handbook, 6th ed.; The Fertiliser Society of South Africa: Pretoria, South Africa, 2007. [Google Scholar]
- Landon, J.R. Booker Tropical Soil Manual: A Handbook for Soil Survey and Agricultural Land Evaluation in the Tropics and Subtropics; Longman Scientific and Technical, Booker Tate Limited: London, UK, 1991. [Google Scholar]
- Havlin, J.L.; Tisdale, S.L.; Nelson, W.L.; Beaton, J.D. Soil Fertility and Fertilisers, 8th ed.; Prentice Hall: Upper Saddle River, NJ, USA, 2013; 528p. [Google Scholar]
- Horneck, D.A.; Sullivan, D.M.; Owen, J.S.; Hart, J.M. Soil Test Interpretation Guide, Oregon State University Extension Service. 2011. Available online: https://extension.oregonstate.edu/catalog/ec-1478-soil-test-interpretation-guide (accessed on 14 June 2023).
- Blanco-Canqui, H.; Shaver, T.M.; Lindquist, J.L.; Shapiro, C.A.; Elmore, R.W.; Francis, C.A.; Hergert, G.W. Cover crops and ecosystem services: Insights from studies in temperate soils. Agron. J. 2015, 107, 2449–2474. [Google Scholar] [CrossRef]
- Carver, R.E.; Nelson, N.O.; Abel, D.S.; Roozeboom, K.; Kluitenberg, G.J.; Tomlinson, P.J.; Williams, J.R. Impact of cover crops and phosphorus fertiliser management on nutrient cycling in no-tillage corn-soybean rotation. Kans. Agric. Exp. Stn. Res. Rep. 2017, 3, 5. [Google Scholar] [CrossRef]
- Ropper, W.R. Evaluating Soil Health in North Carolina. Ph.D. Thesis, North Carolina State University, Raleigh, NC, USA, 2018. [Google Scholar]
- Gupta, C.; Prakash, D. Effect of Nano-Fertilizers on Soil Microflora. Ann. Plant Sci. 2020, 9, 3846–3859. [Google Scholar]
- Khardia, N.; Meena, R.H.; Jat, G.; Sharma, S.; Kumawat, H.; Dhayal, S.; Meena, K.A.; Sharma, K. Soil Properties Influenced by the Foliar Application of Nano Fertilisers in Maize (Zea mays L.) Crop. Int. J. Plant Soil Sci. 2022, 34, 99–111. [Google Scholar] [CrossRef]
- Meena, R.H.; Jat, G.; Jain, D. Impact of foliar application of different nano-fertilisers on soil microbial properties and yield of Wheat. J. Environ. Biol. 2021, 42, 302–308. [Google Scholar] [CrossRef]
- Nibin, P.M.; Ushakumari, K.; Ishrath, P.K. Organic nano NPK formulations on soil microbial and enzymatic activities on post-harvest soil of Bhindi. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1814–1819. [Google Scholar] [CrossRef]
- Rajput, V.D.; Minkina, T.; Sushkova, S.; Tsitsuashvili, V.; Mandzhieva, S.; Gorovtsov, A.; Nevidomskyaya, D.; Gromakova, N. Effect of nanoparticles on crops and soil microbial communities. J. Soils Sediments 2018, 18, 2179–2187. [Google Scholar] [CrossRef]
- Kumar, Y. Nano fertilisers for sustainable crop production, higher nutrient use efficiency and enhanced profitability. Indian J. Fertil. 2021, 17, 1206–1214. [Google Scholar]
- Basak, N.; Biswas, S.; Tamang, A.; Basak, P. Impact of organic and mineral inputs onto soil biological and metabolic activities under long term rice-wheat cropping system in subtropical Indian inceptisols. J. Environ. Biol. 2016, 37, 83–89. [Google Scholar]
- Dinca, L.; Grenni, P.; Onet, C.; Onet, A. Fertilisation and soil microbial community: A review. Appl. Sci. 2022, 12, 1198. [Google Scholar] [CrossRef]
- Luo, P.; Han, X.; Wang, Y.; Han, M.; Shi, H.; Liu, N.; Bai, H. Influence of long-term fertilisation on soil microbial biomass, dehydrogenase activity, and bacterial and fungal community structure in a brown soil of northeast China. Ann. Microbiol. 2015, 65, 533–542. [Google Scholar] [CrossRef] [PubMed]
- Jian, S.; Li, J.; Chen, J.; Wang, G.; Mayes, M.A.; Dzantor, K.E.; Hui, D.; Luo, Y. Soil extracellular enzyme activities, soil carbon and nitrogen storage under nitrogen fertilisation: A meta-analysis. Soil Biol. Biochem. 2016, 101, 32–43. [Google Scholar] [CrossRef]
- Yadav, A.; Yadav, K.; Abd-Elsalam, K.A. Nanofertilisers: Types, Delivery and Advantages in Agricultural Sustainability. Agrochemicals 2023, 2, 296–336. [Google Scholar] [CrossRef]
- Woodings, F.S.; Margenot, A.J. Revisiting the permanganate oxidizable carbon (POXC) assay assumptions: POXC is lignin sensitive. Agric. Environ. Lett. 2023, 8, e20108. [Google Scholar] [CrossRef]
- Howe, J.A.; McDonald, M.D.; Burke, J.; Robertson, I.; Coker, H.; Gentry, T.J.; Lewis, K.L. Influence of fertiliser and manure inputs on soil health: A review. Soil Secur. 2024, 16, 100155. [Google Scholar] [CrossRef]
- Rayne, N.; Aula, L. Livestock Manure and the Impacts on Soil Health: A Review. Soil Syst. 2020, 4, 64. [Google Scholar] [CrossRef]
- Abdalla, Z.F.; El-Sawy, S.; El-Bassiony, A.E.M.; Jun, H.; Shedeed, S.; Okasha, A.M.; Bayoumi, Y.; El-Ramady, H.; Prokisch, J. Smart Fertilisers vs. Nano-fertilisers: A Pictorial Overview. Environ. Biodivers. Soil Secur. 2022, 6, 191–204. [Google Scholar] [CrossRef]
- Vilakazi, B.S.; Zengeni, R.; Mafongoya, P. The effects of different tillage techniques and N fertiliser rates on nitrogen and phosphorus in dry land agriculture. Agronomy 2022, 12, 2389. [Google Scholar] [CrossRef]
- Lange, M.; Eisenhauer, N.; Sierra, C.A.; Bessler, H.; Engels, C.; Griffiths, R.I.; Mellado-Vázquez, P.G.; Malik, A.A.; Roy, J.; Scheu, S.; et al. Plant diversity increases soil microbial activity and soil carbon storage. Nat. Commun. 2015, 6, 6707. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, Y.; Zong, Y.; Hu, Z.; Wu, S.; Zhou, J.I.; Jin, Y.; Zou, J. Response of soil carbon dioxide fluxes, soil organic carbon and microbial biomass carbon to biochar amendment: A meta-analysis. Glob. Change Biol. Bioenergy 2016, 8, 392–406. [Google Scholar] [CrossRef]
- Li, J.; Zhao, X.; Maltais-Landry, G.; Paudel, B.R. Dynamics of Soil Nitrogen Availability Following Sunn Hemp Residue Incorporation in Organic Strawberry Production Systems. Hortscience 2021, 56, 138–146. [Google Scholar] [CrossRef]
- Khemtong, J.; Phakamas, N.; Somchit, P. Effects of urea and sunn hemp on nitrogen use efficiency and physiological traits related to Japonica rice yield. Int. J. Agric. Technol. 2023, 19, 997–1010. [Google Scholar]
- Mbuthia, L.W.; Acosta-Martínez, V.; DeBruyn, J.; Schaeffer, S.; Tyler, D.; Odoi, E.; Mpheshea, M.; Walker, F.; Eash, N. Long term tillage, cover crop, and fertilisation effects on microbial community structure, activity: Implications for soil quality. Soil Biol. Biochem. 2015, 89, 24–34. [Google Scholar] [CrossRef]
- Rahman, M.H.; Haque, K.S.; Khan, M.Z.H. A review on application of controlled released fertilisers influencing the sustainable agricultural production: A Cleaner production process. Environ. Technol. Innov. 2021, 23, 101697. [Google Scholar] [CrossRef]
- Pokhrel, S. Effects of Cover Crop Management on Biologically Related Soil Properties in a Mississippi Dryland Soybean System. Ph.D. Thesis, Mississippi State University, Mississippi State, MS, USA, 2013. [Google Scholar]
- Nascente, A.S.; Li, Y.C.; Crusciol, C.A.C. Cover crops and no-till effects on physical fractions of soil organic matter. Soil Tillage Res. 2013, 130, 52–57. [Google Scholar] [CrossRef]
- Sharma, B.; Tiwari, S.; Kumawat, K.C.; Cardinale, M. Nano-biofertilisers as bio-emerging strategies for sustainable agriculture development: Potentiality and their limitations. Sci. Total Environ. 2023, 860, 160476. [Google Scholar] [CrossRef]
- Pruthviraj, N.; Geetha, K.; Prakash, S.; Jayadeva, H.; Pushpa, K.; Shankar, A. Impact of different methods of nano fertilisers application on soil chemical properties and fertility status in sunflower growing soils. Mysore J. Agric. Sci. 2022, 56, 275–284. [Google Scholar]
- Meena, A.; Rao, K.S. Assessment of soil microbial and enzyme activity in the rhizosphere zone under different land use/cover of a semiarid region, India. Ecol. Process. 2021, 10, 16. [Google Scholar] [CrossRef]
- Moghimian, N.; Hosseini, S.M.; Kooch, Y.; Darki, B.Z. Impacts of changes in land use/cover on soil microbial and enzyme activity. Catena 2017, 157, 407–414. [Google Scholar] [CrossRef]
- Van der Voort, A.R.; Arai, Y. The role of nanotechnology in the fortification of plant nutrients and improvement of crop production. Appl. Sci. 2019, 9, 499. [Google Scholar] [CrossRef]
- Dotaniya, M.L.; Aparna, K.; Dotaniya, C.K.; Singh, M.; Regar, K.L. Role of soil enzymes in sustainable crop production. In Enzymes in Food Biotechnology; Kuddus, M., Ed.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 569–589. [Google Scholar]
- Adetunji, A.T.; Ncube, B.; Mulidzi, R.; Lewu, F.B. Management impact and benefit of cover crops on soil quality: A review. Soil Tillage Res. 2020, 204, 104717. [Google Scholar] [CrossRef]
- Tang, L. Soil Fertility, Plant Nutrition and Nutrient Management. Plants 2025, 14, 34. [Google Scholar] [CrossRef] [PubMed]
- Uzoma, A.O. Bacteria and Fungi Population of Surface Soils under Various Land Use Types in Minna, Southern Guinea Savanna. Agric. Ext. J. 2019. Available online: https://ssrn.com/abstract=3669543 (accessed on 13 May 2025).
- Guo, M.; Song, W.; Kazda, R. Fertiliser value of lime-stabilized biosolids as a soil amendment. Agron. J. 2012, 104, 1679–1686. [Google Scholar] [CrossRef]
- Liang, Q.; Chen, H.; Gong, Y.; Fan, M.; Yang, H.; Lal, R.; Kuzyakov, Y. Effects of 15 years of manure and inorganic fertilisers on soil organic carbon fractions in a wheat- maize system in the North China Plain. Nutr. Cycl. Agroecosystems 2012, 92, 3. [Google Scholar] [CrossRef]
- Liebig, M.A.; Hendrickson, J.R.; Archer, D.W.; Schmer, M.A.; Nichols, K.A.; Tanaka, D.L. Short-term soil responses to late-seeded cover crops in a semi-arid environment. Agron. J. 2015, 107, 2011–2019. [Google Scholar] [CrossRef]
- Kutamahufa, M.; Matare, L.; Soropa, G.; Mashavakure, N.; Svotwa, E.; Mashingaidze, A.B. Forage legumes exhibit a differential potential to compete against maize and weeds and to restore soil fertility in a maize-forage legume intercrop. Acta Agric. Scand. Sect. B Soil Plant Sci. 2022, 72, 127–141. [Google Scholar] [CrossRef]
- Bhayal, D.; Khaddar, V.K.; Bhayal, L.; Yadav, T.C.; Bangar, K.S.; Singh, B. Effects of Sunnhemp green manuring and intercropping on soil properties. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 371–384. [Google Scholar] [CrossRef]





| Syferkuil | Ofcolaco | * Soil Limits | |||||
|---|---|---|---|---|---|---|---|
| Before Sunn Hemp | After Sunn Hemp | Difference | Before Sunn Hemp | After Sunn Hemp | Difference | ||
| pH (H2O) | 8.88 | 8.70 | - | 7.91 | 6.78 | - | - |
| ¥ Ca | 846 | 799 | - | 1208 | 728 | - | >2000 |
| ¥ Mg | 619 | 629 | + | 394 | 163 | - | 84–600 |
| ¥ K | 316 | 261 | - | 220 | 143 | - | 80–200 |
| ¥ P | 64 | 69 | + | 117 | 104 | - | >15 |
| ¥ Cu | 4.61 | 3.73 | - | 7.41 | 5.19 | - | 0.75–100 |
| ¥ Zn | 5.98 | 6.76 | + | 7.43 | 8.88 | + | 3–150 |
| N (%) | 0.08 | 0.09 | + | 0.06 | 0.07 | + | 0.15 |
| OC (%) | 0.77 | 0.85 | + | 0.75 | 1.15 | + | - |
| MWD | 1.11 | 1.21 | + | 1.32 | 1.70 | + | - |
| Texture | Sandy clay loam | Clay loam | |||||
| Bacterial Populations (Log CFU mL−1) | ||
|---|---|---|
| 2023 | 2024 | |
| Cover cropping (C) | ||
| C0 | 7.77 b (0.07) | 7.25 b (0.38) |
| C1 | 7.82 a (0.08) | 8.11 a (0.45) |
| HSD (p < 0.05) | *** | * |
| Nano Zn and Cu (S) | ||
| S0 | 7.82 a (0.09) | 7.70 a (0.45) |
| S1 | 7.77 b (0.07) | 7.65 a (0.47) |
| HSD (p < 0.05) | ** | ns |
| Nitrogen (N) | ||
| N0 | 7.82 ab (0.07) | 7.75 a (0.47) |
| N60 | 7.83 a (0.09) | 7.76 a (0.46) |
| N120 | 7.78 bc (0.07) | 7.71 a (0.46) |
| N180 | 7.74 c (0.07) | 7.50 a (0.49) |
| HSD (p < 0.05) | *** | ns |
| Bacterial Populations (Log CFU mL−1) | ||
|---|---|---|
| 2023 | 2024 | |
| Cover cropping (C) | ||
| C0 | 7.99 a (0.19) | 7.25 b (0.49) |
| C1 | 8.04 a (0.25) | 8.40 a (0.87) |
| HSD (p < 0.05) | ns | *** |
| Nano Zn and Cu (S) | ||
| S0 | 8.09 a (0.19) | 7.74 a (0.78) |
| S1 | 7.95 b (0.24) | 7.54 a (0.83) |
| HSD (p < 0.05) | * | ns |
| Nitrogen (N) | ||
| N0 | 7.95 a (0.19) | 7.20 b (0.49) |
| N60 | 7.97 a (0.17) | 7.80 ab (0.79) |
| N120 | 8.12 a (0.27) | 7.95 a (1.02) |
| N180 | 8.02 a (0.23) | 7.62 ab (0.71) |
| HSD (p < 0.05) | ns | * |
| POxC (mg kg−1) | ||
|---|---|---|
| 2023 | 2024 | |
| Cover crop (C) | ||
| C0 | 622 b (208) | 783 b (98) |
| C1 | 811 a (250) | 888 a (104) |
| Pr > F (C) | *** | ** |
| Nano Zn and Cu (S) | ||
| S0 | 669 b (232) | 827 a (118) |
| S1 | 764 a (258) | 845 a (110) |
| Pr > F (S) | *** | ns |
| N fertiliser (N) | ||
| N0 | 717 a (120) | 768 b (100) |
| N60 | 735 a (339) | 862 ab (100) |
| N120 | 769 a (128) | 891 a (105) |
| N180 | 645 b (325) | 823 ab (120) |
| Pr > F (N) | *** | * |
| POxC (mg kg−1) | ||
|---|---|---|
| 2023 | 2024 | |
| Cover crop (C) | ||
| C0 | 932 a (165) | 587 b (131) |
| C1 | 982 a (121) | 762 a (103) |
| Pr > F (C) | ns | *** |
| Nano Zn and Cu (S) | ||
| S0 | 967 a (151) | 680 a (129) |
| S1 | 945 a (141) | 668 a (164) |
| Pr > F (S) | ns | ns |
| N fertiliser (N) | ||
| N0 | 1000 a (92) | 609 b (109) |
| N60 | 939 a (149) | 634 ab (142) |
| N120 | 950 a (161) | 716 ab (148) |
| N180 | 937 a (174) | 741 a (156) |
| Pr > F (N) | ns | * |
| Urease (µg NH4-N g−1 2 hr−1) | ||
|---|---|---|
| 2023 | 2024 | |
| Cover cropping (C) | ||
| C0 | 65 a (27) | 35 a (13) |
| C1 | 68 a (16) | 39 a (13) |
| Pr > F (C) | ns | ns |
| Nano Zn and Cu (S) | ||
| S0 | 60 b (18) | 34 b (12) |
| S1 | 73 a (24) | 41 a (13) |
| Pr > F (S) | *** | * |
| N fertiliser (N) | ||
| N0 | 53 b (34) | 37 ab (14) |
| N60 | 62 b (12) | 46 a (12) |
| N120 | 76 a (12) | 33 b (6) |
| N180 | 75 a (13) | 32 b (13) |
| Pr > F (N) | *** | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mokgophi, M.M.; Ayisi, K.K.; Kgopa, P.M.; Kena, M.A. Response of Soil Microbial Population and Activity to Sunn Hemp Cover Crop, Combined Nano Zinc and Copper and Nitrogen Fertiliser Application After Canola Cultivation. Sustainability 2025, 17, 9407. https://doi.org/10.3390/su17219407
Mokgophi MM, Ayisi KK, Kgopa PM, Kena MA. Response of Soil Microbial Population and Activity to Sunn Hemp Cover Crop, Combined Nano Zinc and Copper and Nitrogen Fertiliser Application After Canola Cultivation. Sustainability. 2025; 17(21):9407. https://doi.org/10.3390/su17219407
Chicago/Turabian StyleMokgophi, Mahlare Mapula, Kingsley Kwabena Ayisi, Pholosho Mmateko Kgopa, and Mapotso Anna Kena. 2025. "Response of Soil Microbial Population and Activity to Sunn Hemp Cover Crop, Combined Nano Zinc and Copper and Nitrogen Fertiliser Application After Canola Cultivation" Sustainability 17, no. 21: 9407. https://doi.org/10.3390/su17219407
APA StyleMokgophi, M. M., Ayisi, K. K., Kgopa, P. M., & Kena, M. A. (2025). Response of Soil Microbial Population and Activity to Sunn Hemp Cover Crop, Combined Nano Zinc and Copper and Nitrogen Fertiliser Application After Canola Cultivation. Sustainability, 17(21), 9407. https://doi.org/10.3390/su17219407







