Activation of Iron Tailings with Organic Acids: A Sustainable Approach for Soil Amelioration
Abstract
1. Introduction
2. Materials and Methods
2.1. Mineralogical Characterization
2.2. Experimental Design
2.3. Analytical Determination Method
2.4. Data Analysis
3. Results and Analysis
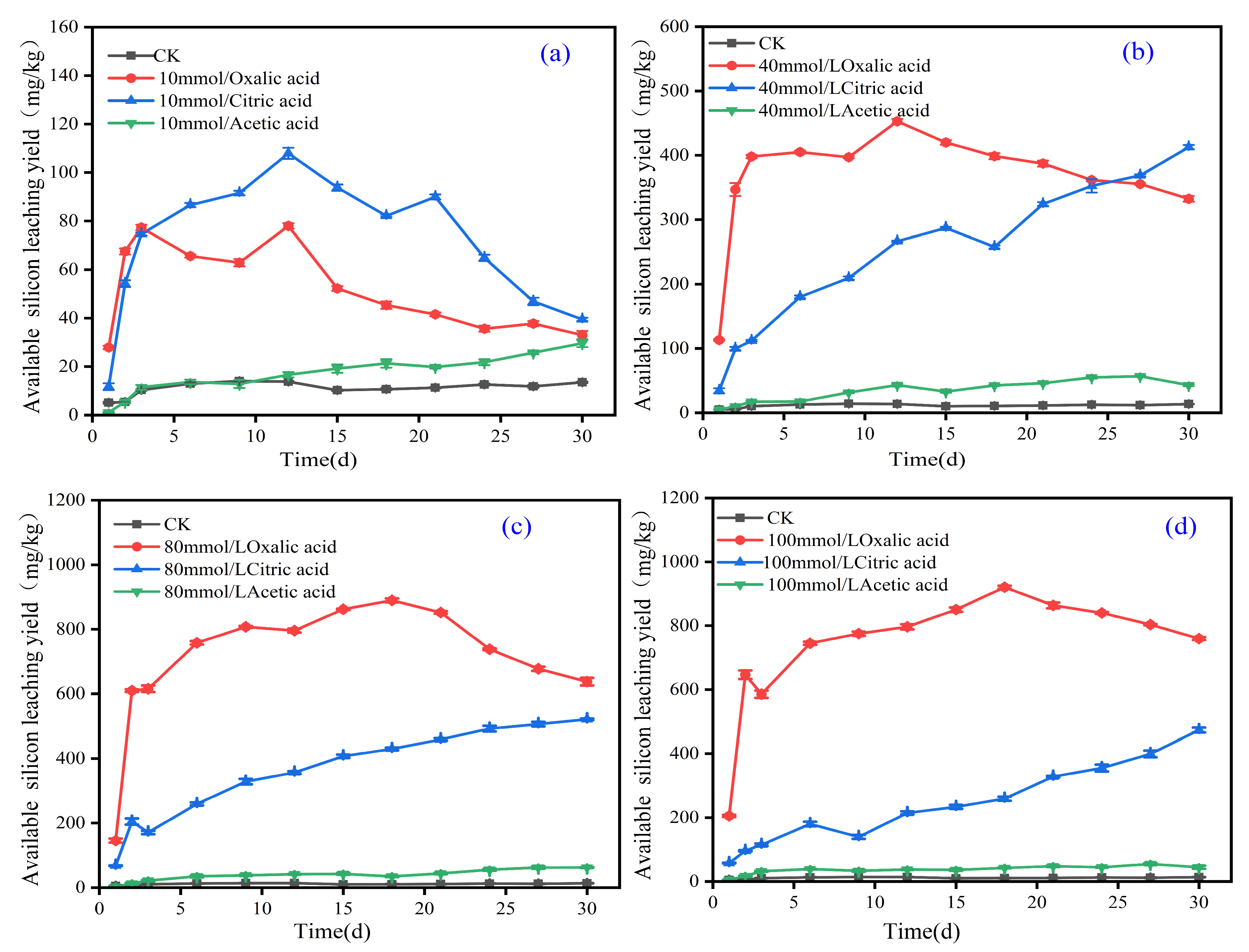
3.1. Effect of Organic Acids on Activation of Effective Silicon in Iron Tailings
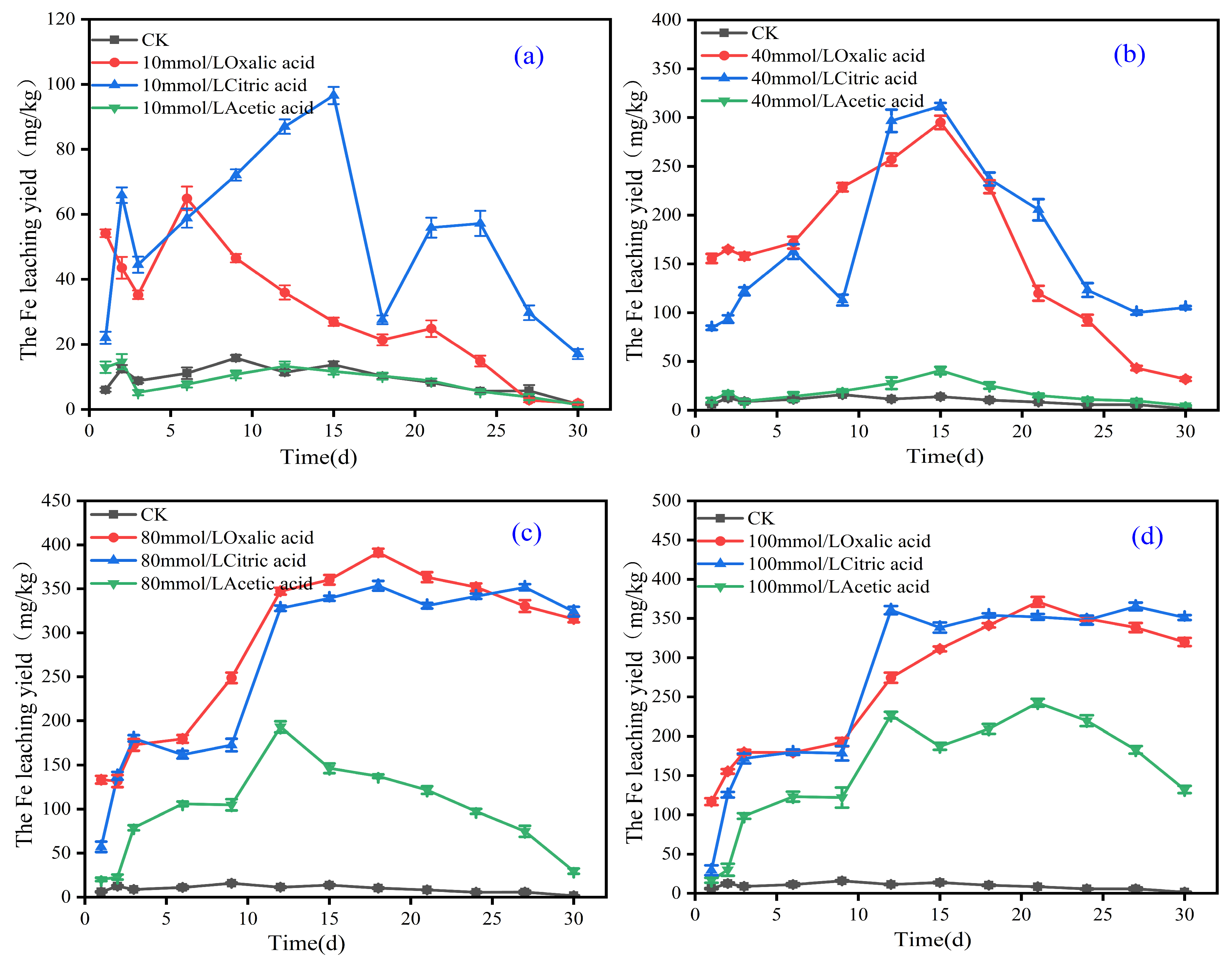
3.2. Effect of Organic Acids on Activation of Total Iron in Iron Tailings
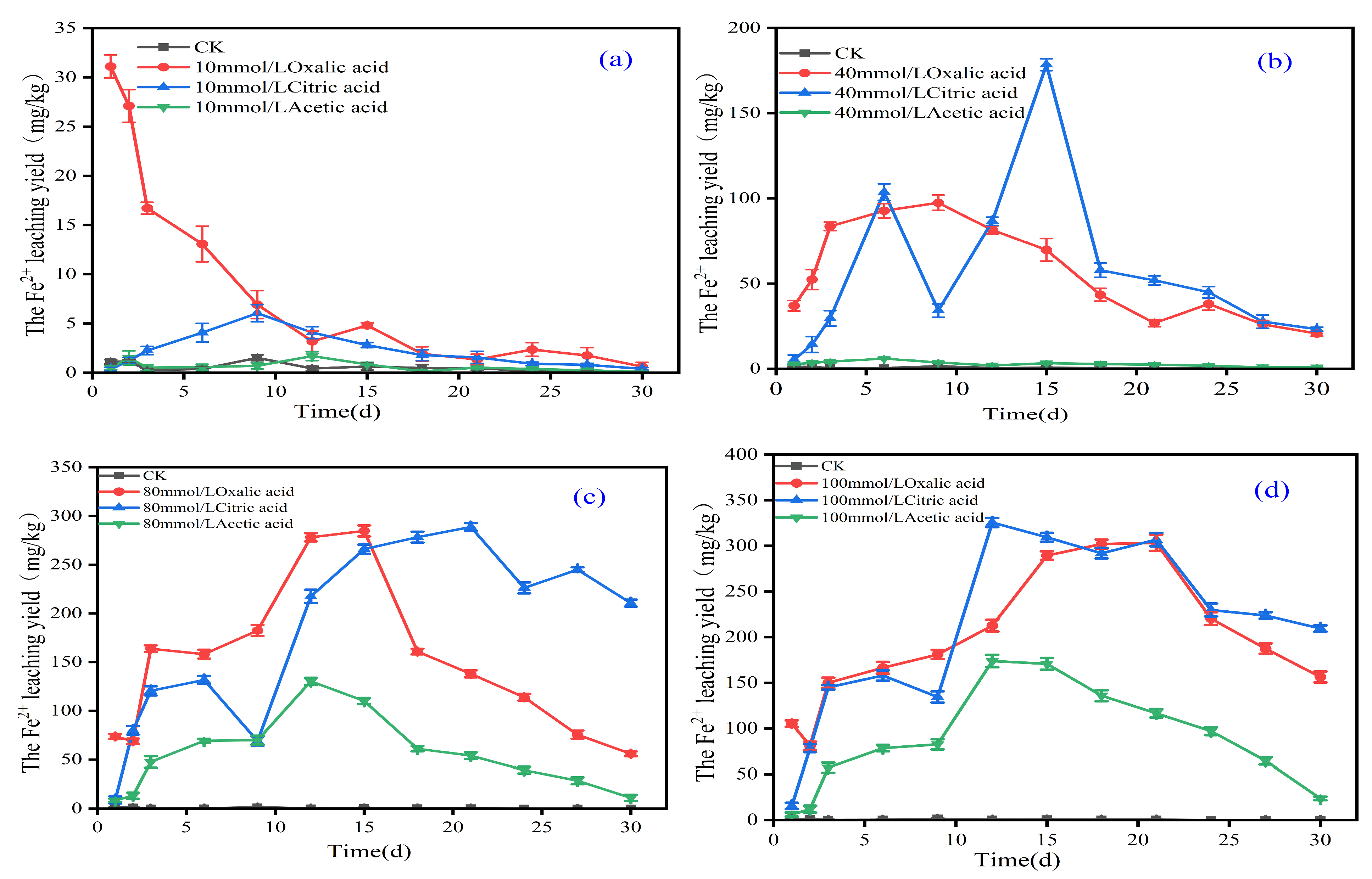
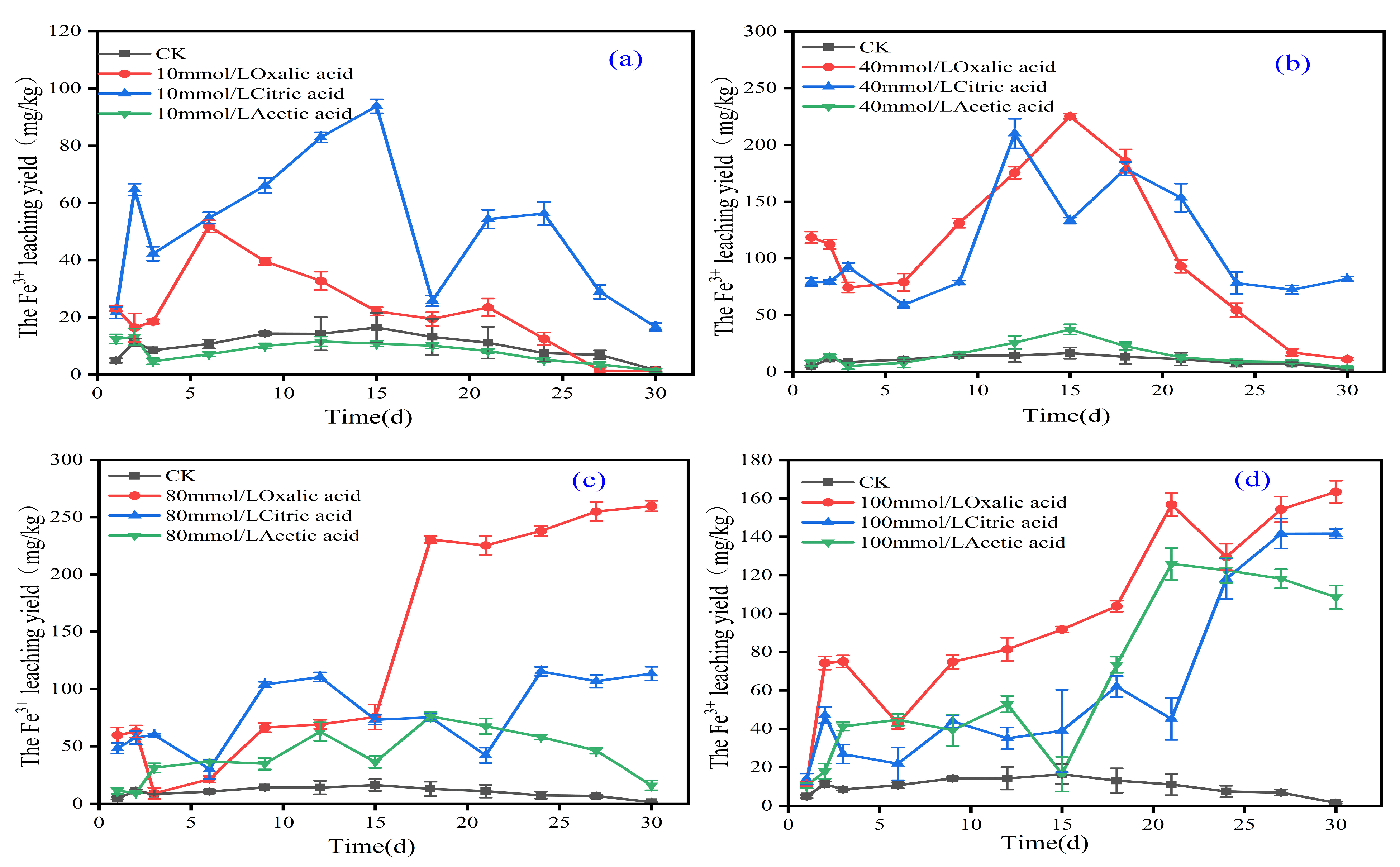
3.3. The Effect of Organic Acids on the Chemical Form of Iron in Iron Tailings
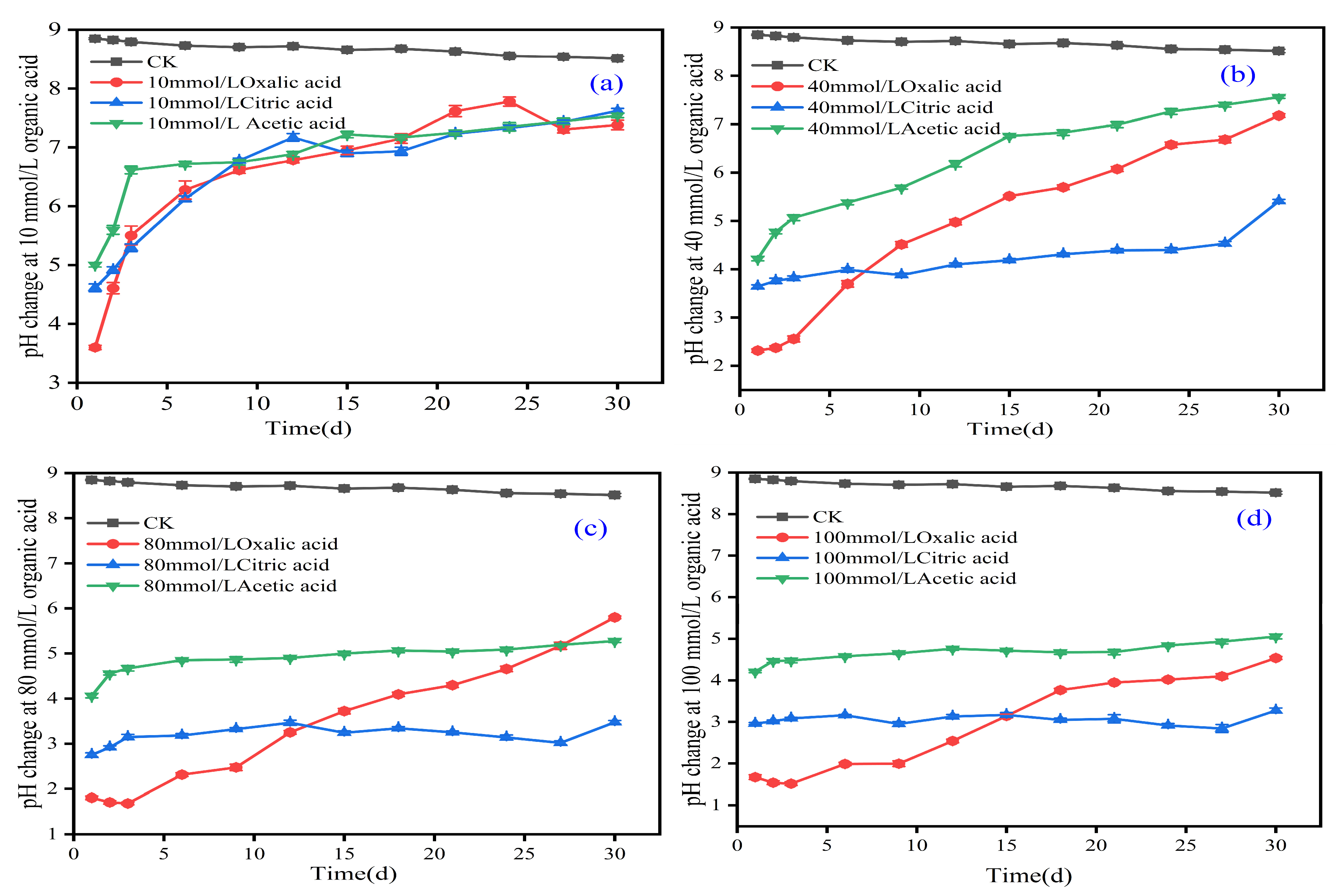
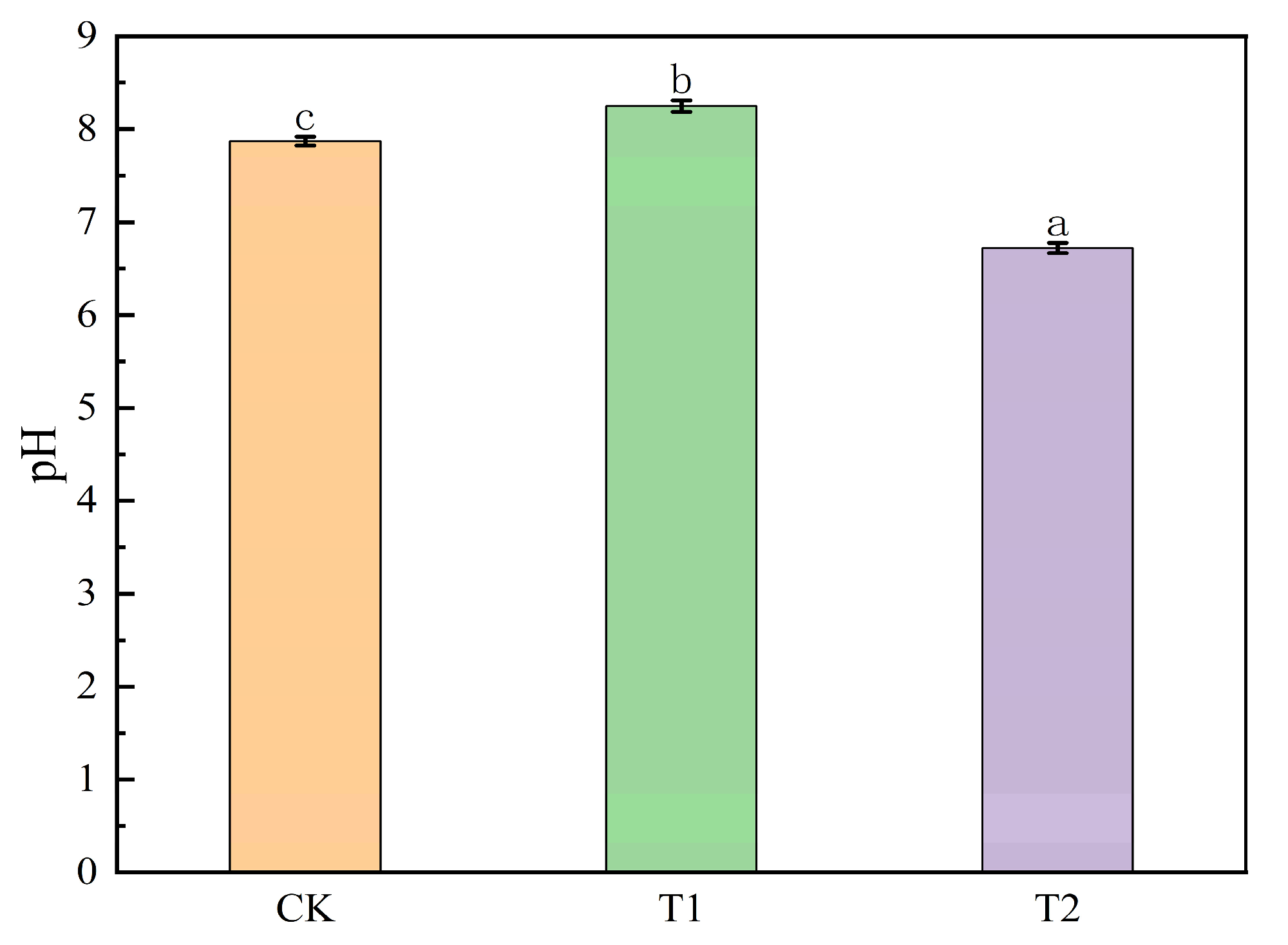
3.4. Dynamic Changes in pH During Activation of Iron Tailings with Different Organic Acids
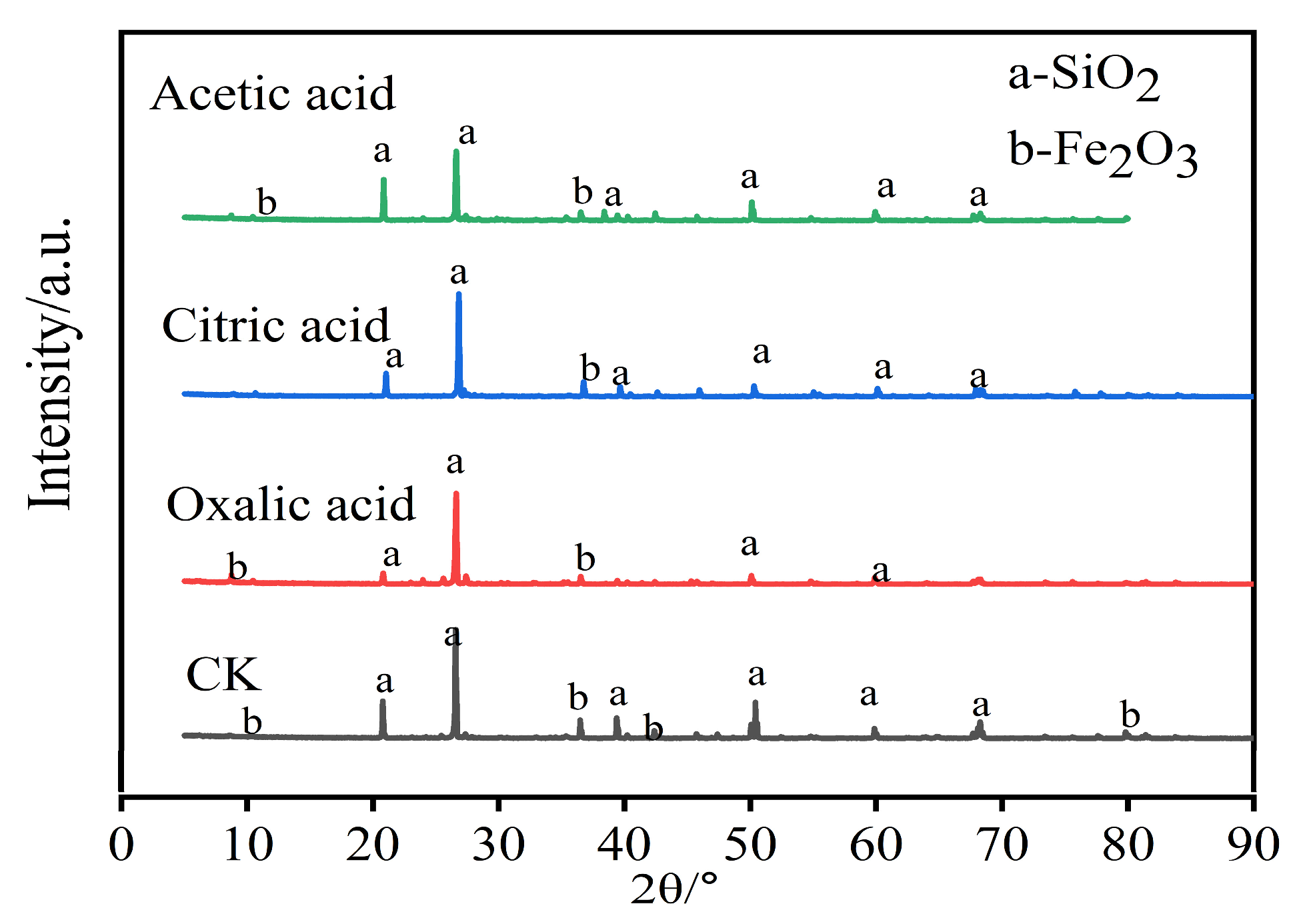
3.5. Changes in Crystal Structure of Iron Tailings
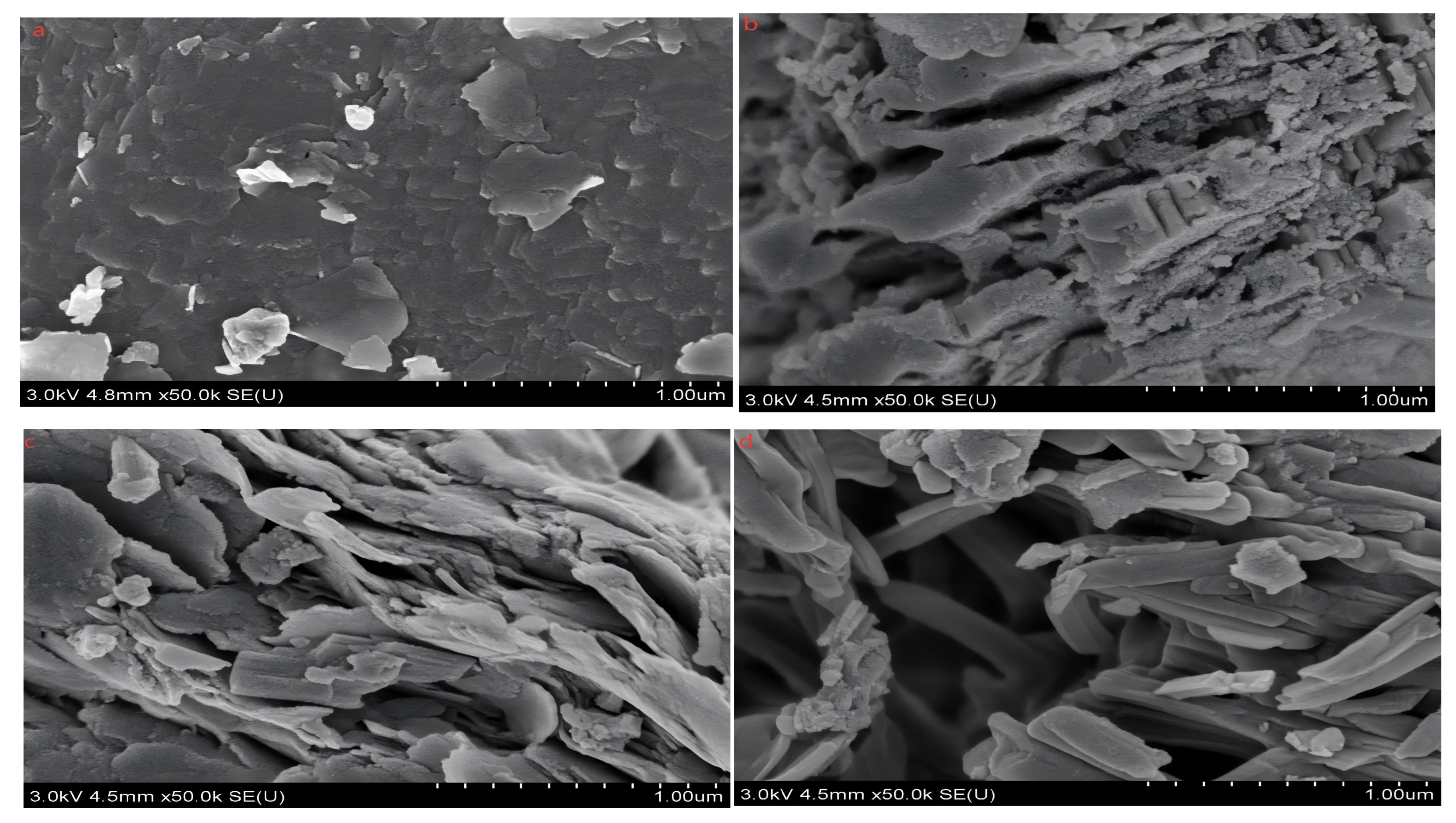
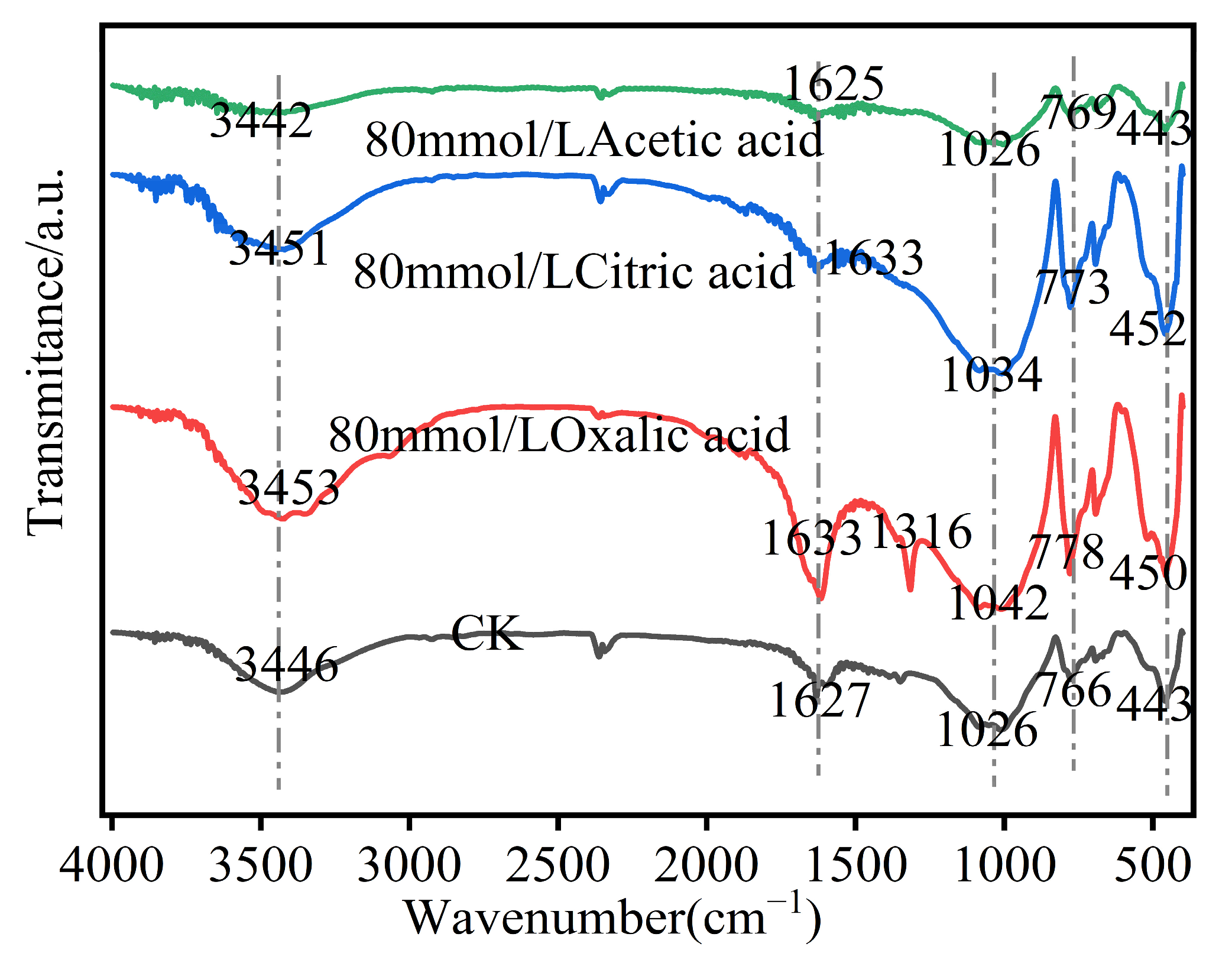
3.6. Characterization of Iron Tailings in Different Organic Acids
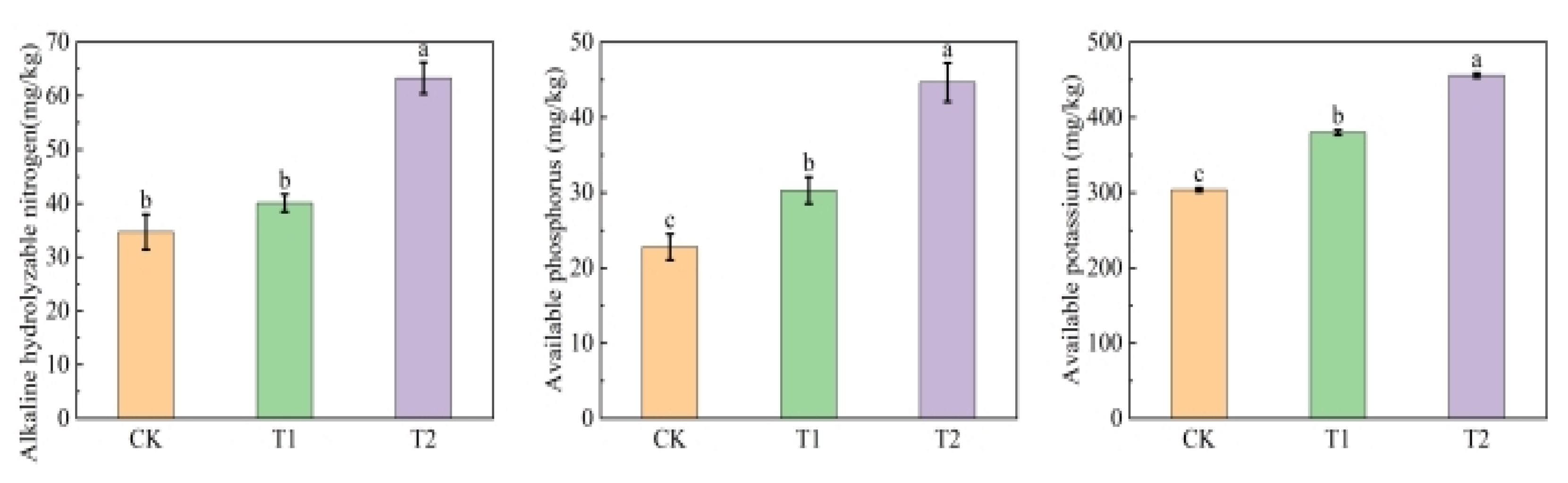
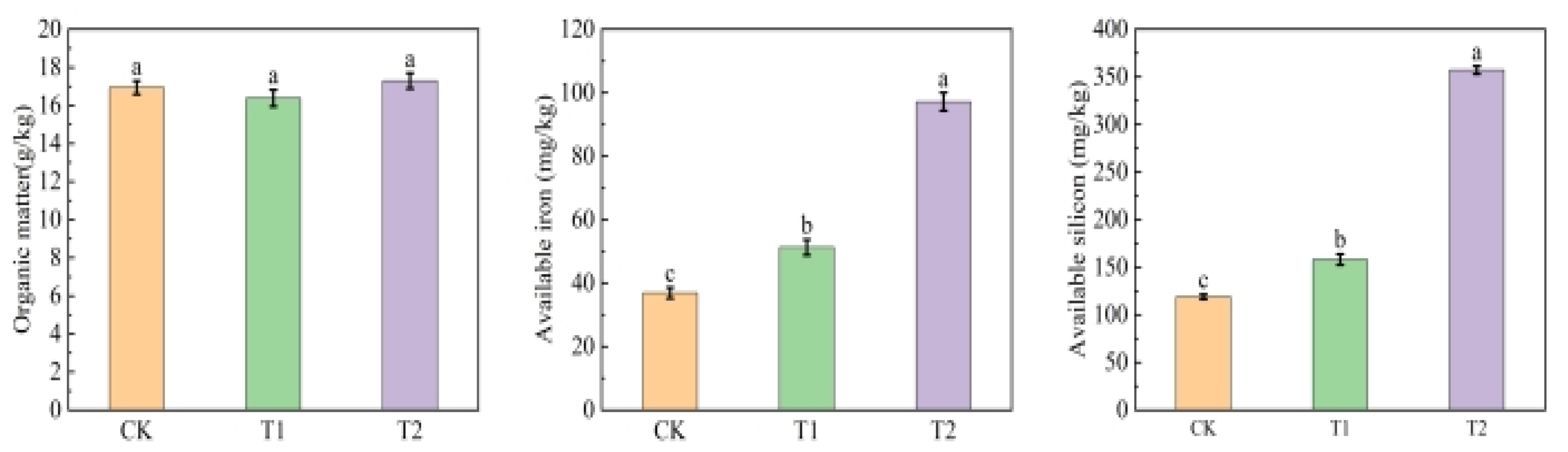
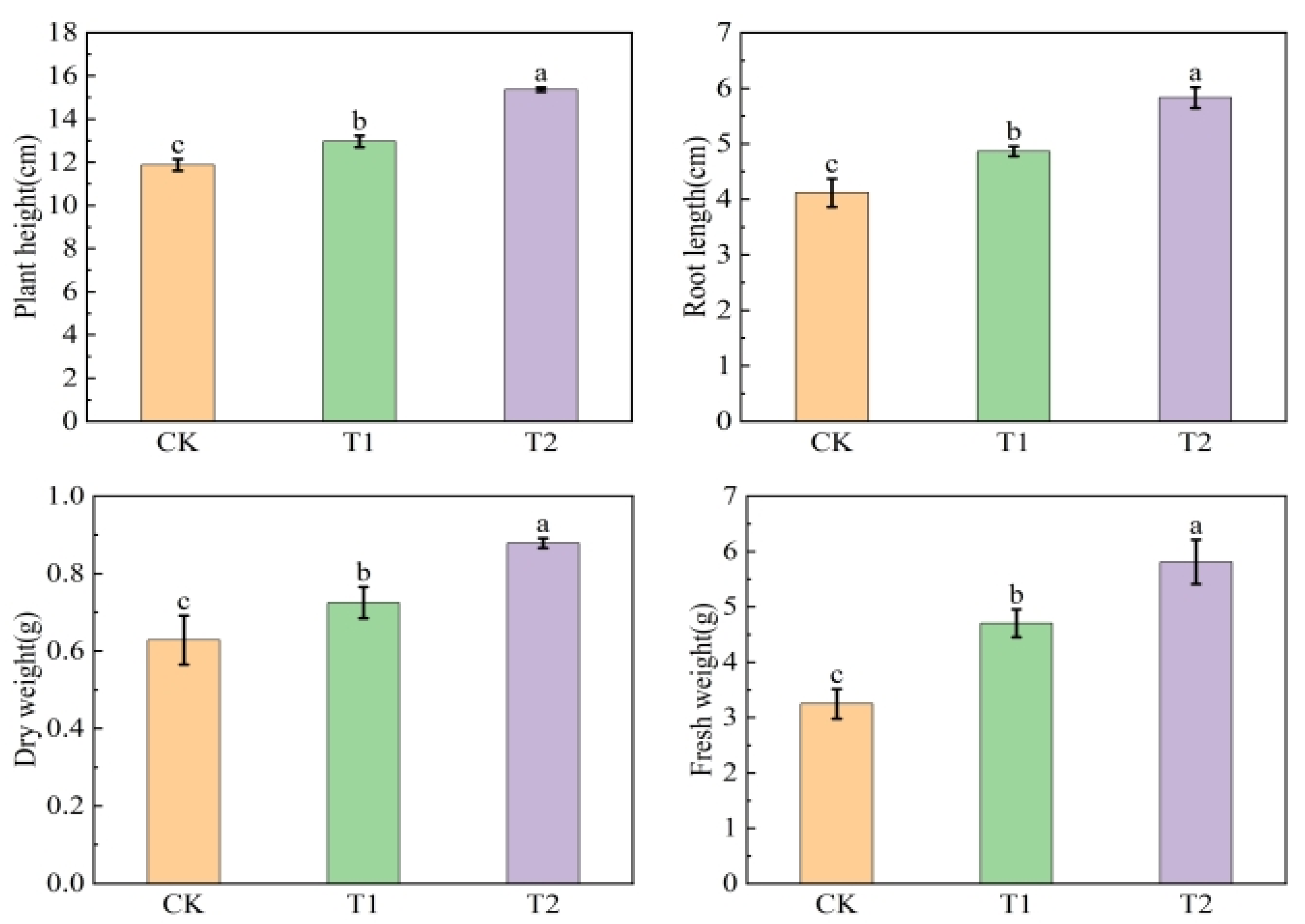
3.7. Soil Improvement Effects of Organic Acid-Iron Tailings Complexes
4. Discussion
4.1. Effect of Organic Acids on Activation of Iron Tailings
4.2. Effects of Organic Acid–Iron Tailings Complexes on Soil Nutrients and Plant Growth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Hua, S.; Pei, D.; Liu, J.; Li, X.; Li, S.; Wang, W. Current status and prospects of iron tailings management and comprehensive utilization. Met. Mines 2025, 5, 243–253. [Google Scholar]
- Yuan, S.; Zhang, Q.; Yin, H.; Li, Y. Efficient iron recovery from iron tailings using advanced suspension reduction technology: A study of reaction kinetics, phase transformation, and structure evolution. J. Hazard. Mater. 2020, 404, 124067. [Google Scholar] [CrossRef]
- Cui, Y.; Si, C.; Li, S.; Jia, Y.; Guo, B. Iron tailings as mineral fillers and their effect on the fatigue performance of asphalt mastic. Materials 2024, 17, 2927. [Google Scholar] [CrossRef]
- Cui, X.; Geng, Y.; Li, T.; Zhao, R.; Li, X.; Cui, Z. Field application and effect evaluation of different iron tailings soil utilization technologies. Resour. Conserv. Recycl. 2021, 173, 105746. [Google Scholar] [CrossRef]
- Zhao, S.; Fan, J.; Sun, W. Utilization of iron ore tailings as fine aggregate in ultra-high performance concrete. Constr. Build. Mater. 2014, 50, 540–548. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, Y.; Chen, J.; Gao, Q. Current status and development strategies for the resource utilization of iron tailings. Sci. Technol. Rev. 2024, 42, 90–103. [Google Scholar]
- Sattar, A.; Cheema, M.; Basra, S.; Wahid, A. Optimization of source and rate of soil applied silicon for improving the growth of wheat. Pak. J. Agric. Sci. 2013, 50, 63–68. [Google Scholar]
- Tozsin, G.; Arol, A. Pyritic tailings as a source of plant micronutrients in calcareous soils. Commun. Soil Sci. Plant Anal. 2015, 46, 1473–1481. [Google Scholar] [CrossRef]
- Jeer, M.; Yele, Y.; Sharma, K.; Prakash, N. Exogenous application of different silicon sources and potassium reduces pink stem borer damage and improves photosynthesis, yield and related parameters in wheat. Silicon 2021, 13, 901–910. [Google Scholar] [CrossRef]
- Rea, R.; Islam, M.; Rahman, M.; Nath, B.; Mix, K. Growth, nutrient accumulation, and drought tolerance in crop plants with silicon application: A review. Sustainability 2022, 14, 4525. [Google Scholar] [CrossRef]
- Kasozi, N.; Tandlich, R.; Fick, M.; Kaiser, H.; Wilhelmi, B. Iron supplementation and management in aquaponic systems: A review. Aquacult. Rep. 2019, 15, 100221. [Google Scholar]
- Zhang, F.; Hou, Y.; Zed, R.; Mauchline, T.H.; Shen, J.; Zhang, F.; Jin, K. Root exudation of organic acid anions and recruitment of beneficial actinobacteria facilitate phosphorus uptake by maize in compacted silt loam soil. Soil Biol. Biochem. 2023, 184, 109074. [Google Scholar] [CrossRef]
- Xiao, L.; Cheng, X.; Zhang, T.; Guo, M.; Zhang, M. Efficient inorganic/organic acid leaching for the remediation of protogenetic lead-contaminated soil. Appl. Sci. 2022, 12, 3395. [Google Scholar]
- Agarwal, C.; Pandey, A.K. Remediation and recycling of inorganic acids and their green alternatives for sustainable industrial chemical processes. Environ. Sci. Adv. 2023, 2, 1306–1339. [Google Scholar] [CrossRef]
- Kirtzel, J.; Ueberschaar, N.; Deckert-Gaudig, T.; Krause, K.; Deckert, V.; Gadd, G.M.; Kothe, E. Organic acids, siderophores, enzymes and mechanical pressure for black slate bioweathering with the basidiomycete Schizophyllum commune. Environ. Microbiol. 2020, 22, 1535–1546. [Google Scholar]
- Sun, X.L.; An, W.D.; Zhang, T.; Zhang, J.; Wang, D.; Hu, R.F. Experimental study on the preparation of soil conditioners using iron tailings and by-products mica powder and dolomite. Met. Mines 2018, 6, 192–196. [Google Scholar]
- Mu, J.; Hu, Z.; Huang, L.; Xie, Z.; Holm, P.E. Preparation of a silicon-iron amendment from acid-extracted copper tailings for remediating multi-metal-contaminated soils. Environ. Pollut. 2020, 257, 113565. [Google Scholar]
- Li, Z.Y.; Li, F.; Hu, L.N. The molybdenum blue spectrophotometric method for the determination of silicon dioxide in copper concentrates. Non-Ferr. Min. Metall. 2006, 3, 67–69. [Google Scholar]
- Zhu, X.D.; Chen, L.; Pumpanen, J.; Keinänen, M.; Laudon, H.; Ojala, A.; Palviainen, M.; Kiirikki, M.; Neitola, K.; Berninger, F. Assessment of a portable UV-Vis spectrophotometer’s performance for stream water DOC and Fe content monitoring in remote areas. Talanta 2021, 224, 121919. [Google Scholar]
- Bao, S.D. Soil and Agricultural Chemistry Analysis; China Agriculture Press: Beijing, China, 2000; pp. 263–270. [Google Scholar]
- Wang, H.; Fan, X.; Wang, Y.; Li, W.; Sun, Y.; Zhan, M.; Wu, G. Comparative leaching of six toxic metals from raw and chemically stabilized MSWI fly ash using citric acid. J. Environ. Manag. 2018, 208, 15–23. [Google Scholar] [CrossRef]
- Li, C.; Zhang, X.; Yin, Y.; Xi, F.; Wang, S.; Hu, Q.; Wang, J.; Bing, L. The mechanism of citric acid and oxalic acid on dissolution of high-silicon iron tailings. J. Ind. Eng. Chem. 2025, 143, 319–326. [Google Scholar] [CrossRef]
- Petcharoen, K.; Sirivat, A. Synthesis and characterization of magnetite nanoparticles via the chemical co-precipitation method. Mater. Sci. Eng. B 2012, 177, 421–427. [Google Scholar] [CrossRef]
- Nikvar-Hassani, A.; Hodges, R.; Zhang, L. Production of green bricks from low-reactive copper mine tailings: Durability and environmental aspects. Constr. Build. Mater. 2022, 337, 127571. [Google Scholar] [CrossRef]
- Ogorodova, L.P.; Vigasina, M.F.; Melchakova, L.V.; Kiseleva, I.A.; Krupskaya, V.V.; Bryzgalov, I.A. Natural Mg-Fe clinochlores: Enthalpies of formation and dehydroxylation derived from calorimetric study. Am. Mineral. 2016, 101, 1431–1437. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, W.; Liu, W.; Tong, K.; Shen, Y.; Zhao, S.; Zhou, S. Efficient separation of magnesite and quartz using eco-friendly Dimethylaminopropyl lauramideexperimental and mechanistic studies. Miner. Eng. 2022, 188, 107814. [Google Scholar] [CrossRef]
- Liu, Q.; Huang, Y.; Zhou, Y.; Chen, Z.; Luo, J.; Yan, X. Impacts of wet-dry alternations on cadmium and zinc immobilisation in soil remediated with iron oxides. J. Environ. Manag. 2023, 326, 116660. [Google Scholar]
- Geng, H.; Wang, F.; Yan, C.; Tian, Z.; Chen, H.; Zhou, B.; Yuan, R.; Yao, J. Leaching behavior of metals from iron tailings under varying pH and low-molecular-weight organic acids. J. Hazard. Mater. 2020, 383, 121136. [Google Scholar] [CrossRef]
- Xia, Z.; Zhang, S.; Cao, Y.; Zhong, Q.; Wang, G.; Li, T.; Xu, X. Remediation of cadmium, lead and zinc in contaminated soil with CETSA and MA/AA. J. Hazard. Mater. 2019, 366, 177–183. [Google Scholar] [CrossRef]
- Fan, Y.; Wang, L.; Putnis, C.V.; Zhang, W. Direct nanoscale imaging reveals the mechanism by which organic acids dissolve vivianite through proton and ligand effects. Inorg. Chem. 2024, 63, 6909–6921. [Google Scholar] [CrossRef]
- Yan, Y.; Wan, B.; Mansor, M.; Wang, X.; Zhang, Q.; Kappler, A.; Feng, X. Co-sorption of metal ions and inorganic anions/organic ligands on environmental minerals: A review. Sci. Total Environ. 2022, 803, 149918. [Google Scholar] [CrossRef]
- El-Nakhel, C.; Geelen, D.; De Paepe, J.; Clauwaert, P.; De Pascale, S.; Rouphael, Y. An appraisal of urine derivatives integrated in the nitrogen and phosphorus inputs of a lettuce soilless cultivation system. Sustainability 2021, 13, 4218. [Google Scholar] [CrossRef]
- Fang, D.; Wei, S.; Xu, Y.; Xiong, J.; Tan, W. Impact of low-molecular weight organic acids on selenite immobilization by goethite: Understanding a competitive-synergistic coupling effect and speciation transformation. Sci. Total Environ. 2019, 684, 694–704. [Google Scholar]
- Xiang, L.; Chen, X.T.; Yu, P.F.; Li, X.H.; Zhao, H.M.; Feng, N.X.; Li, Y.W.; Li, H.; Cai, Q.Y.; Mo, C.H.; et al. Oxalic acid in root exudates enhances accumulation of perfluorooctanoic acid in lettuce. Environ. Sci. Technol. 2020, 54, 13046–13055. [Google Scholar] [CrossRef]
- Ding, Y.; Ye, Q.; Liu, M.; Shi, Z.; Liang, Y. Reductive release of Fe mineral-associated organic matter accelerated by oxalic acid. Sci. Total Environ. 2021, 763, 142937. [Google Scholar] [CrossRef]
- Lin, S.; Wang, W.; Wu, L.; Zhong, M.; Zhang, C.; Yu, Y.; Zhang, Z.; Wu, Y. The effect of oxalic acid and citric acid on the modification of wollastonite surface. Materials 2023, 16, 7704. [Google Scholar] [CrossRef]
- Zeng, L.; Peng, T.; Sun, H.; Zhang, X.; Zhao, D. Comparative study on ionic dissolution and structural changes of montmorillonite, kaolinite and muscovite during interfacial reactions with oxalic acid solution. Mineral. Mag. 2024, 88, 546–556. [Google Scholar] [CrossRef]
- Lodi, L.A.; Klaic, R.; Bortoletto-Santos, R.; Ribeiro, C.; Farinas, C.S. Unveiling the solubilization of potassium mineral rocks in organic acids for application as K-fertilizer. Appl. Biochem. Biotechnol. 2022, 194, 2431–2447. [Google Scholar] [CrossRef]
- Tsakiri, D.; Douni, I.; Taxiarchou, M. Structural and surface modification of oxalic-acid-activated bentonites in various acid concentrations for bleaching earth synthesis—A comparative study. Minerals 2022, 12, 764. [Google Scholar]
- Clarholm, M.; Skyllberg, U.; Rosling, A. Organic acid induced release of nutrients from metal-stabilized soil organic matter—The unbutton model. Soil Biol. Biochem. 2015, 84, 168–176. [Google Scholar]
- Yang, X.; Chen, X.; Guo, E.; Yang, X. Path analysis of phosphorus activation capacity as induced by low-molecular-weight organic acids in a black soil of Northeast China. J. Soils Sediments 2019, 19, 840–947. [Google Scholar]
- Lin, S.M.; Yu, Y.L.; Zhong, M.F.; Yang, H.; Zhang, C.Y.; Zhang, Z.J.; Wu, Y.Y. The dissolution behavior of feldspar minerals in various low-molecular-weight organic acids. Materials 2023, 16, 6704. [Google Scholar] [CrossRef] [PubMed]
- George, S.E.; Wan, Y. Microbial functionalities and immobilization of environmental lead: Biogeochemical and molecular mechanisms and implications for bioremediation. J. Hazard. Mater. 2023, 457, 131738. [Google Scholar] [CrossRef] [PubMed]
- Battini, F.; Cristani, C.; Giovannetti, M.; Agnolucci, M. Multifunctionality and diversity of culturable bacterial communities strictly associated with spores of the plant beneficial symbiont Rhizophagus intraradices. Microbiol. Res. 2016, 183, 68–79. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, X.; Zhang, L.; Mao, W.; Wang, W.; Zhang, M.; Wang, J.; Guan, Y. Co-transport of citrate-modified biochar nanoparticles and released plant-available silicon in saturated porous media: Effect of LMWOAs and solution chemistry. Chemosphere 2024, 365, 143259. [Google Scholar] [CrossRef]

| Chemical Composition | SiO2 | Fe2O3 | Al2O3 | CaO | MgO | K2O | Na2O | P2O5 |
|---|---|---|---|---|---|---|---|---|
| Content (%) | 71.25 | 10.84 | 5.24 | 4.00 | 3.73 | 3.70 | 0.75 | 0.49 |
| Elements | Content (mg/kg) | Standard (mg/kg) |
|---|---|---|
| Cd | 0.085 ± 0.02 | 0.3–0.6 |
| Hg | 0.013 ± 0.01 | 1.3–3.4 |
| As | 3.64 ± 0.27 | 25–40 |
| Pb | 11.59 ± 1.06 | 70–170 |
| Cr | 63.15 ± 0.85 | 150–250 |
| Cu | 23.72 ± 0.19 | 50–100 |
| Ni | 29.48 ± 1.13 | 60–190 |
| Organic Acid | Chemical Structure | pKa1 | pKa2 | pKa3 |
|---|---|---|---|---|
| acetic acid | CH3-COOH | 4.74 | ||
| oxalic acid | HOOCCOOH | 1.27 | 4.27 | |
| citric acid | HOOCOHC-(CH2COOH)2 | 3.13 | 4.76 | 6.40 |
| Sample | Specific Surface Area (m2/g) |
|---|---|
| CK | 0.5640 |
| Oxalic acid treatment | 4.2576 |
| Citric acid treatment | 1.3058 |
| Acetic acid treatment | 0.6817 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.-C.; Zhao, Z.-H.; Han, D.-Y.; Wang, X.-H.; Yuan, X.-T.; Ai, Y.-J. Activation of Iron Tailings with Organic Acids: A Sustainable Approach for Soil Amelioration. Sustainability 2025, 17, 9308. https://doi.org/10.3390/su17209308
Wang H-C, Zhao Z-H, Han D-Y, Wang X-H, Yuan X-T, Ai Y-J. Activation of Iron Tailings with Organic Acids: A Sustainable Approach for Soil Amelioration. Sustainability. 2025; 17(20):9308. https://doi.org/10.3390/su17209308
Chicago/Turabian StyleWang, Hui-Chen, Zi-Hao Zhao, Dong-Yun Han, Xiao-Hong Wang, Xue-Tao Yuan, and Yan-Jun Ai. 2025. "Activation of Iron Tailings with Organic Acids: A Sustainable Approach for Soil Amelioration" Sustainability 17, no. 20: 9308. https://doi.org/10.3390/su17209308
APA StyleWang, H.-C., Zhao, Z.-H., Han, D.-Y., Wang, X.-H., Yuan, X.-T., & Ai, Y.-J. (2025). Activation of Iron Tailings with Organic Acids: A Sustainable Approach for Soil Amelioration. Sustainability, 17(20), 9308. https://doi.org/10.3390/su17209308






