Enhancing the Performance of Aluminum Anodes in Aqueous Batteries: A Review on Alloying, Microstructure, and Corrosion Inhibition Strategies
Abstract
1. Introduction
2. The Influencing Factors of Al-Negative Electrode
2.1. Microalloying
- (1)
- Damaging the passivation film on the surface of aluminum, reducing the resistance of the oxide film, increasing the dissolution activity of the alloy, and having a high hydrogen evolution overpotential, can effectively inhibit the corrosion of the alloy due to hydrogen evolution. These elements include In, Ga, Sn, and Mg, etc.;
- (2)
- A low eutectic mixture with good fluidity can be formed on the surface of aluminum to improve the microstructure of aluminum alloy, destroy the oxide film on the surface of aluminum alloy, and improve the electrochemical performance of aluminum alloy. These elements include In, Ga, Sn, and Bi, etc.;
- (3)
- Can improve the chemical activity and corrosion resistance of aluminum, such as Pb, Sn, and Hg, etc.;
2.2. Microstructure
3. Negative Electrode Improvement Measures
- (1)
- Material nano materialization involves powder pressing or sintering of materials to obtain particle structures with different nanosizes and morphologies, effectively increasing the alloy surface area and reducing the polarization effect per unit area;
- (2)
- Obtaining high-purity aluminum-based materials through special refining processes reduces the influence of impurities on the open circuit potential, reduces the tendency of self-corrosion, and thus reduces the surface polarization effect caused by self-corrosion products.;
- (3)
- The addition of microalloyed elements can effectively suppress hydrogen evolution, reduce losses caused by self-corrosion of the alloy matrix, improve current efficiency, and improve the microstructure and electrochemical performance of aluminum alloy negative electrode materials.
3.1. Increase Discharge Voltage
3.2. Reduce Hydrogen Evolution Rate
3.3. Inhibiting Self-Corrosion Reactions
3.4. Heat Treatment Improves Material Defects
4. Summary and Outlook
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, S.; Wang, L.; Presser, V. Dual-Use of Seawater Batteries for Energy Storage and Water Desalination. Small 2022, 18, e2107913. [Google Scholar] [CrossRef]
- Das, S.K.; Mahapatra, S.; Lahan, H. Aluminium-ion batteries: Developments and challenges. J. Mater. Chem. A 2017, 5, 6347–6367. [Google Scholar] [CrossRef]
- Elia, G.A.; Marquardt, K.; Hoeppner, K.; Fantini, S.; Lin, R.; Knipping, E.; Peters, W.; Drillet, J.; Passerini, S.; Hahn, R. An Overview and Future Perspectives of Aluminum Batteries. Adv. Mater. 2016, 28, 7564–7579. [Google Scholar] [CrossRef]
- Jiang, M.; Fu, C.; Meng, P.; Ren, J.; Wang, J.; Bu, J.; Dong, A.; Zhang, J.; Xiao, W.; Sun, B. Challenges and Strategies of Low-Cost Aluminum Anodes for High-Performance Al-Based Batteries. Adv. Mater. 2022, 34, 2102026. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, M.; Talib, M.Z.M.; Majlan, E.H.; Tasirin, S.M.; Sahari, J. Recent developments in materials for aluminum-air batteries: A review. J. Ind. Eng. Chem. 2015, 32, 1–20. [Google Scholar] [CrossRef]
- He, X.; Li, Z.; Wang, Y.; Xu, W.; Zhang, Q.; Wang, X.; Liu, H.; Yang, G.; Zhang, H.; Song, J.; et al. A high-purity AgO cathode active material for high-performance aqueous AgO–Al batteries. J. Power Sources 2022, 551, 232151. [Google Scholar] [CrossRef]
- Egan, D.R.; Leoen, C.P.D.; Wood, R.J.K.; Jones, R.L.; Stokes, K.R.; Walsh, F.C. Developments in electrode materials and electrolytes for aluminium-air batteries. J. Power Sources 2013, 236, 293–310. [Google Scholar] [CrossRef]
- Shkolnikov, E.I.; Zhuk, A.Z.; Vlaskin, M.S. Aluminum as energy carrier: Feasibility analysis and current technologies overview. Renew. Sustain. Energ. Rev. 2011, 15, 4611–4623. [Google Scholar] [CrossRef]
- Fan, L.; Lu, H.; Leng, J.; Sun, Z.; Chen, C. The study of industrial aluminum alloy as anodes for aluminum-air batteries in alkaline electrolytes. J. Electrochem. Soc. 2016, 163, A8–A12. [Google Scholar] [CrossRef]
- Peng, G.S.; Huang, J.; Gu, Y.C.; Song, G.S. Self-corrosion, electrochemical and discharge behavior of commercial purity Al anode via Mn modification in Al-air battery. Rare Met. 2021, 40, 3501–3511. [Google Scholar] [CrossRef]
- Rodrigues, S.R.S.; Dalmoro, V.; Santos, J.H.Z. An evaluation of Acacia mearnsii tannin as an aluminum corrosion inhibitor in acid, alkaline, and neutral media. Mater. Corros. 2020, 71, 1160–1174. [Google Scholar] [CrossRef]
- Pan, W.; Wang, Y.; Zhang, Y.; Kwok, H.Y.H.; Wu, M.; Zhao, X.; Leung, D.Y.C. A low-cost and dendrite-free rechargeable aluminium-ion battery with superior performance. J. Mater. Chem. A 2019, 7, 17420–17425. [Google Scholar] [CrossRef]
- Kahveci, O. Preparation of 3-D Porous Pure Al Electrode for Al-Air Battery Anode and Comparison of its Electrochemical Performance with a Smooth Surface Electrode. ChemElectroChem 2023, 10, e202300221. [Google Scholar] [CrossRef]
- Ghosh, M.; Venkatesh, G.; Rao, G.M. Surface modification of vertically aligned graphene nanosheets by microwave assisted etching for application as anode of lithium ion battery. Solid State Ion. 2016, 296, 31–36. [Google Scholar] [CrossRef]
- Liu, X.; Jiao, H.; Wang, M.; Song, W.; Xue, J.; Jiao, S. Current progresses and future prospects on aluminium-air batteries. Int. Mater. Rev. 2022, 67, 734–764. [Google Scholar] [CrossRef]
- Chen, P.; Zheng, Q.; Wang, C.; Dai, P.; Yin, Y.; Chen, J.; Wang, X.; Xu, W.; Ruan, M. Progress in Al/AgO Electrode Materials for Seawater-Activated Batteries. Energies 2025, 18, 4007. [Google Scholar] [CrossRef]
- Zhu, Y.; Hua, Z.; Lu, Y.; Wang, Z.; Tian, Z.; Peng, K. Effect Factors on the Performances of Commercial Al Alloy as Anode for Al-Air Batteries. Energy Fuels 2023, 37, 11367–11375. [Google Scholar] [CrossRef]
- Zhang, Y.; Lv, C.; Zhu, Y.; Kuang, J.; Wang, H.; Li, Y.; Tang, Y. Challenges and Strategies of Aluminum Anodes for High-Performance Aluminum-Air Batteries. Small Methods 2024, 8, 2300911. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Zhu, Y.; Li, Y.; Yang, K.; Lu, W.; Peng, K.; Tian, Z. Investigation of commercial aluminum alloys as anode materials for alkaline aluminum-air batteries. Sustain. Energy Fuels 2022, 7, 300–309. [Google Scholar] [CrossRef]
- Buckingham, R.; Asset, T.; Atanassov, P. Aluminum-air batteries: A review of alloys, electrolytes and design. J. Power Sources 2021, 498, 229762. [Google Scholar] [CrossRef]
- Mori, R. Recent Developments for Aluminum-Air Batteries. Electrochem. Energy Rev. 2020, 3, 344–369. [Google Scholar] [CrossRef]
- Gu, Y.; Jiang, J.; Xie, Q.; Ma, A.; Chen, J.; Wang, G. Effects of hot rolling on the electrochemical behaviors and discharge performance of AZ61-In anodes for seawater activated batteries. J. Mater. Res. Technol. 2024, 28, 591–604. [Google Scholar] [CrossRef]
- Wan, H.; Wang, W.; Bu, L.X. Investigations on Mn shielding the negative effect of Fe in the aluminum alloy anodes used for Al-air batteries. J. Solid State Electrochem. 2024, 28, 2547–2560. [Google Scholar] [CrossRef]
- Moghanni-Bavil-Olyaei, H.; Arjomandi, J. Enhanced electrochemical performance of Al-0.9Mg-1Zn-0.1Mn-0.05Bi-0.02In fabricated from commercially pure aluminum for use as the anode of alkaline batteries. RSC Adv. 2016, 6, 28055–28062. [Google Scholar] [CrossRef]
- Ma, Z.; Li, X. The study on microstructure and electrochemical properties of Al-Mg-Sn-Ga-Pb alloy anode material for Al/AgO battery. J. Solid State Electrochem. 2011, 15, 2601–2610. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, H.; Zou, J.; Qin, K.; Ban, C.; Cui, J.; Nagaumi, H. Effect of microstructure on discharge performance of Al-0.8Sn-0.05Ga-0.9Mg-1.0Zn (wt%) alloy as anode for seawater-activated battery. Mater. Corros. 2020, 71, 1680–1690. [Google Scholar] [CrossRef]
- Gonzalez-Guerrero, M.J.; Gomez, F.A. Miniaturized Al/AgO coin shape and self-powered battery featuring painted paper electrodes for portable applications. Sens. Actuators B Chem. 2018, 273, 101–107. [Google Scholar] [CrossRef]
- Ren, J.; Ma, J.; Zhang, J.; Fu, C.; Sun, B. Electrochemical performance of pure Al, Al-Sn, Al-Mg and Al-Mg-Sn anodes for Al-air batteries. J. Alloys Compd. 2019, 808, 151708. [Google Scholar] [CrossRef]
- Walmsley, J.C.; Sævik, Ø.; Graver, B.; Mathiesen, R.H.; Yu, Y.; Nisancioglu, K. Nature of segregated lead on electrochemically active AlPb model alloy. J. Electrochem. Soc. 2007, 154, C28–C35. [Google Scholar] [CrossRef]
- Xu, X.; Zhang, J.; Jiang, W.; Deng, Y. Influence of adding Mg, Sn and Ga on the discharge property of the Al-Sb anode for Al-air batteries. J. Alloys Compd. 2024, 997, 174986. [Google Scholar] [CrossRef]
- Xia, Q.; Li, Z.; Liu, D.; Song, N.; Zhang, N.; Ma, S.; Wu, Z.; Yuan, W. Highly Effective Electrolytes Toward High-Performance Aluminum/Seawater Batteries. Batter. Supercaps 2024, 7, e202400307. [Google Scholar] [CrossRef]
- Moghanni-Bavil-Olyaei, H.; Arjomandi, J. Performance of Al-1Mg-1Zn-0.1Bi-0.02In as anode for the Al-AgO battery. RSC Adv. 2015, 5, 91273–91279. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Bai, J.; Xue, H.; Tian, N.; Zhao, Z.; Qin, G. Research progress on microstructure tuning of heat-resistant cast aluminum alloys. J. Mater. Sci. 2024, 59, 10035–10057. [Google Scholar] [CrossRef]
- Dai, Y.; Yan, L.; Hao, J. Review on Micro-Alloying and Preparation Method of 7xxx Series Aluminum Alloys: Progresses and Prospects. Materials 2022, 15, 1216. [Google Scholar] [CrossRef] [PubMed]
- Zheng, G.; Wei, S.; Zuo, C.; Sun, Y.; Xia, H.; Han, Z.; Xu, L. Effect of Annealing Process on Anode Discharge Performance of Aluminum-Air Batteries. J. Electrochem. Soc. 2023, 170, 100516. [Google Scholar] [CrossRef]
- Feng, D.; Li, X.D.; Zhang, X.; Liu, S.; Wang, J.; Liu, Y. The novel heat treatments of aluminium alloy characterized by multistage and non-isothermal routes: A review. J. Cent. South Univ. 2023, 30, 2833–2866. [Google Scholar] [CrossRef]
- Sirichaivetkul, R.; Wongpinij, T.; Euaruksakul, C.; Limmaneevichitr, C.; Kajornchaiyakul, J. In-situ study of microstructural evolution during thermal treatment of 6063 aluminum alloy. Mater. Lett. 2019, 250, 42–45. [Google Scholar] [CrossRef]
- Yu, K.; Xiong, H.Q.; Wen, L.; Dai, Y.L.; Yang, S.H.; Fan, S.F.; Teng, F.; Qiao, X.Y. Discharge behavior and electrochemical properties of Mg-Al-Sn alloy anode for seawater activated battery. Trans. Nonferrous Met. Soc. China 2015, 25, 1234–1240. [Google Scholar] [CrossRef]
- Leng, P.S.; Wang, H.B.; Wu, B.F.; Zhao, L.; Deng, Y.J.; Cui, J.T. Ag Decorated Co3O4-Nitrogen Doped Porous Carbon as the Bifunctional Cathodic Catalysts for Rechargeable Zinc-Air Batteries. Sustainability 2022, 14, 13417. [Google Scholar] [CrossRef]
- Liu, Y.; Sun, Q.; Li, W.; Adair, K.R.; Li, J.; Sun, X. A comprehensive review on recent progress in aluminum-air batteries. Green Energy Environ. 2017, 2, 246–277. [Google Scholar] [CrossRef]
- Chen, J.; Xu, W.; Wang, X.; Yang, S.; Xiong, C. Progress and applications of seawater-activated batteries. Sustainability 2023, 15, 1635. [Google Scholar] [CrossRef]
- Gu, Y.; Liu, Y.; Tong, Y.; Qin, Z.; Wu, Z.; Hu, W. Improving Discharge Voltage of Al-Air Batteries by Ga3+ Additives in NaCl-Based Electrolyte. Nanomaterials 2022, 12, 1336. [Google Scholar] [CrossRef]
- Yu, T.; Wang, S.; Liu, X.; Liu, S.; Shi, C.; Du, N. Electrochemical corrosion behavior of 7075 aluminum alloy with different ultrasonic surface rolling microstructures. J. Mater. Res. Technol. 2024, 30, 4702–4713. [Google Scholar] [CrossRef]
- Zhang, P.; Xue, J.; Liu, X.; Wang, Z.; Li, X.; Jiang, K. Improving energy efficiency of commercial aluminum alloy as anodes for Al-air battery through introducing micro-nanoscale AlSb precipitates. Electrochim. Acta 2022, 417, 140331. [Google Scholar] [CrossRef]
- Guo, L.; Huang, Y.; Ritacca, A.G.; Wang, K.; Ritacco, I.; Tan, Y.; Qiang, Y.; Al-Zaqri, N.; Shi, W.; Zheng, X. Effect of Indole-2-carboxylic Acid on the Self-Corrosion and Discharge Activity of Aluminum Alloy Anode in Alkaline Al-Air Battery. Molecules 2023, 28, 4193. [Google Scholar] [CrossRef]
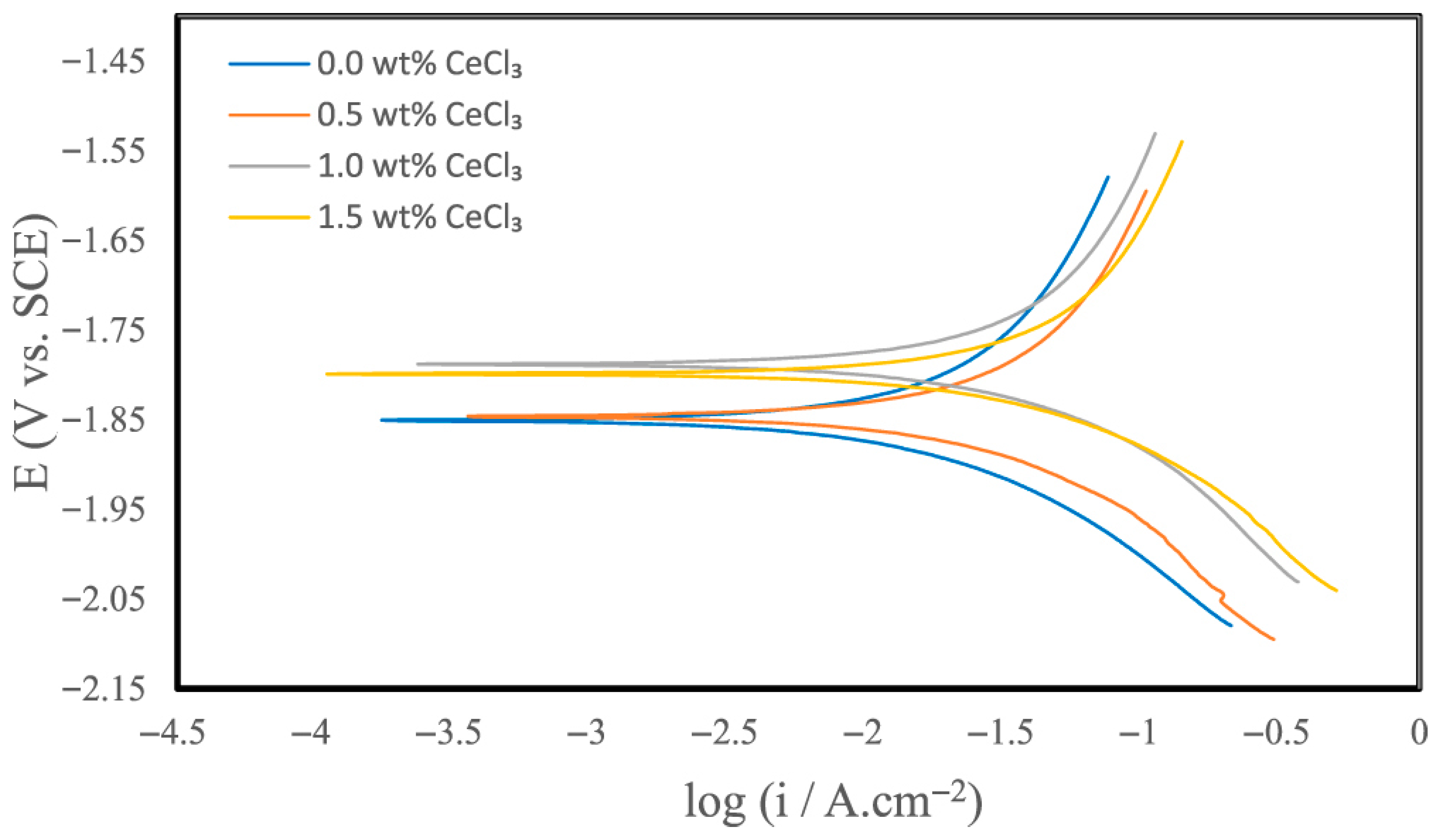
- Harchegani, R.K.; Riahi, A.R. Effect of Cerium Chloride on the Self-Corrosion and Discharge Activity of Aluminum Anode in Alkaline Aluminum-air Batteries. J. Electrochem. Soc. 2022, 170, 030542. [Google Scholar] [CrossRef]
- Asfia, M.P.; Pourfarzad, H.; Kashani, H.; Olia, M.H.; Badrnezhad, R. Study of Uniform and Localized Corrosion Behaviour of Aluminum Alloy 1050 as Al/AgO Battery Anode in Aerated NaCl in the Presence of an Organosulfur Inhibitor. J. Electrochem. Soc. 2020, 167, 140527. [Google Scholar] [CrossRef]
- Farhan, A.M.; Jassim, R.A.; Khammas, S.J. Protection of aluminum from corrosion by nanoparticles. Chem. Pap. 2022, 76, 7847–7854. [Google Scholar] [CrossRef]
- Ren, J.; Liu, T.; Zhang, J.; Jiang, M.; Dong, Q.; Fu, C. Spray-formed commercial aluminum alloy anodes with suppressed self-corrosion for Al-Air batteries. J. Power Sources 2022, 524, 231082. [Google Scholar] [CrossRef]
- Park, I.J.; Choi, S.R.; Kim, J.G. Aluminum anode for aluminum-air battery-Part II: Influence of In addition on the electrochemical characteristics of Al-Zn alloy in alkaline solution. J. Power Sources 2017, 357, 47–55. [Google Scholar] [CrossRef]
- Fan, L.; Lu, H.; Leng, J.; Sun, Z. Performance of Al-0.6 Mg-0.05 Ga-0.1 Sn-0.1 in as anode for Al-air battery in KOH electrolytes. J. Electrochem. Soc. 2015, 162, A2623–A2627. [Google Scholar] [CrossRef]
- Kim, Y.; Harzandi, A.M.; Lee, J.; Choi, Y.; Kim, Y. Design of Large-Scale Rectangular Cells for Rechargeable Seawater Batteries. Adv. Sustain. Syst. 2021, 5, 2000106. [Google Scholar] [CrossRef]
- Wu, Z.; Zhang, H.; Nagaumi, H.; Wang, D.; Luo, S.; Dong, X.; Zou, J.; Yang, D.; Cui, J. Effect of microstructure evolution on the discharge characteristics of Al-Mg-Sn-based anodes for Al-air batteries. J. Power Sources 2022, 521, 230928. [Google Scholar] [CrossRef]



| Number | Elemental Composition | Impact on Discharge Performance |
|---|---|---|
| 1 | Beneficial elements such as Mg, Hg, Ga, In, etc. | Low melting point and high density can cause a significant negative shift in the negative electrode potential of aluminum alloy, resulting in a decrease in negative electrode polarization |
| 2 | Beneficial elements such as Zn, Sn, Pb, Bi, etc. | High resolution hydrogen overpotential elements have an inhibitory effect on hydrogen evolution in aluminum alloy negative electrodes, which can improve their current efficiency and utilization efficiency of aluminum alloy electrodes |
| 3 | Harmful elements such as Cu and Fe | Easy to cause pitting corrosion, severe local corrosion, increased hydrogen evolution corrosion rate, greatly reducing the negative electrode efficiency of aluminum negative electrode alloy |
| Strategy | Key Methods | Mechanism of Action | Advantages | Limitations/Challenges |
|---|---|---|---|---|
| Alloying | Doping with elements like Mg, Sn, Ga, In, etc. | Disrupts the continuous passive oxide film. Increases hydrogen evolution overpotential. Promotes uniform dissolution. | Significantly reduces self-corrosion. Enhances activation and discharge voltage. Mature and scalable fabrication process. | Complex interplay between multiple elements. Risk of promoting galvanic corrosion if elements are improperly selected. May increase material cost. |
| Microstructure Control | Thermo-mechanical processing (e.g., rolling, annealing). Spray forming. | Refines grain size and reduces segregation. Promotes uniform distribution of alloying elements and second phases. | Reduces internal potential differences and intergranular corrosion. Improves overall corrosion resistance and discharge stability. Synergizes effectively with alloying. | Requires precise control of process parameters (temperature, time, deformation). Adds complexity and cost to manufacturing. |
| Nanostructuring | Fabricating nano-sized powders or porous structures. | Dramatically increases specific surface area. Reduces current density per unit area, mitigating polarization. | Enhances electrochemical activity and rate capability. Can lead to very high utilization rates. | High fabrication cost. Severe self-corrosion due to large surface area. Challenges in electrode fabrication and long-term stability. |
| High-Purity Materials | Using high-purity aluminum or removing harmful impurities (Fe, Cu). | Eliminates cathodic sites for hydrogen evolution. Reduces micro-galvanic cell formation. | Effectively lowers the intrinsic self-corrosion rate. Simple conceptual approach. | High cost of purification. Does not address the issue of surface passivation. Limited improvement in activation. |
| Corrosion Inhibitors | Adding organic (e.g., ICA, thiourea) or inorganic (e.g., Ce3+) compounds to the electrolyte. | Adsorbs and forms a protective film on the anode surface. Blocks active corrosion sites. | Directly suppresses self-corrosion without modifying the anode. Can be combined with other strategies. Some inhibitors (e.g., Ce3+) offer self-healing properties. | Consumption over time requires replenishment in a closed system. May increase electrolyte resistance or cause unwanted side reactions. Optimization of concentration is critical. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, P.; Chen, J.; Zheng, Q.; Yin, Y.; Su, X.; Ruan, M.; Huang, L. Enhancing the Performance of Aluminum Anodes in Aqueous Batteries: A Review on Alloying, Microstructure, and Corrosion Inhibition Strategies. Sustainability 2025, 17, 9220. https://doi.org/10.3390/su17209220
Chen P, Chen J, Zheng Q, Yin Y, Su X, Ruan M, Huang L. Enhancing the Performance of Aluminum Anodes in Aqueous Batteries: A Review on Alloying, Microstructure, and Corrosion Inhibition Strategies. Sustainability. 2025; 17(20):9220. https://doi.org/10.3390/su17209220
Chicago/Turabian StyleChen, Peiqiang, Jinmao Chen, Qun Zheng, Yujuan Yin, Xing Su, Man Ruan, and Long Huang. 2025. "Enhancing the Performance of Aluminum Anodes in Aqueous Batteries: A Review on Alloying, Microstructure, and Corrosion Inhibition Strategies" Sustainability 17, no. 20: 9220. https://doi.org/10.3390/su17209220
APA StyleChen, P., Chen, J., Zheng, Q., Yin, Y., Su, X., Ruan, M., & Huang, L. (2025). Enhancing the Performance of Aluminum Anodes in Aqueous Batteries: A Review on Alloying, Microstructure, and Corrosion Inhibition Strategies. Sustainability, 17(20), 9220. https://doi.org/10.3390/su17209220





