Biodegradation of Low-Density Polyethylene by Native Aspergillus Strains Isolated from Plastic-Contaminated Soil
Abstract
1. Introduction
2. Materials and Methods
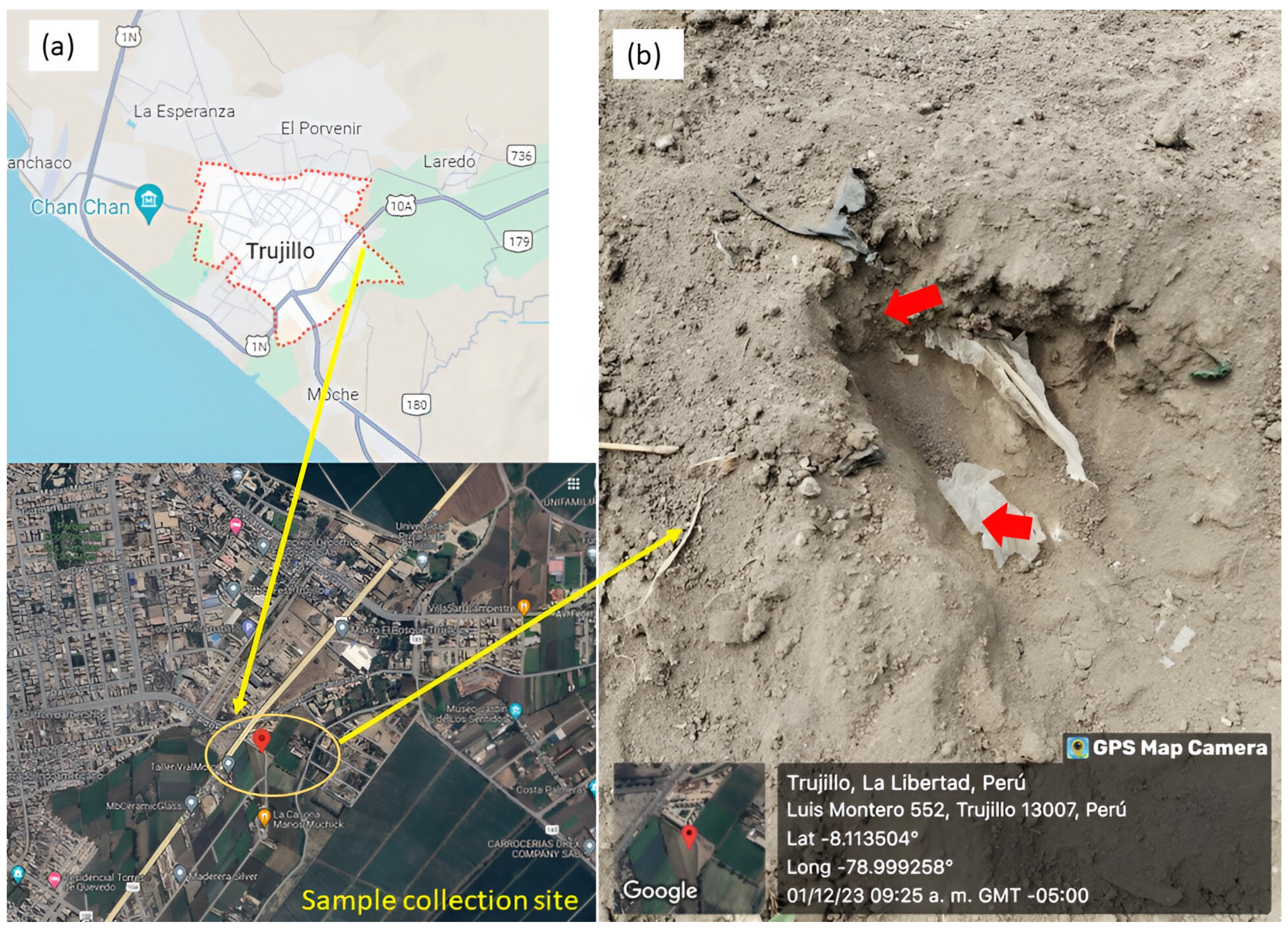
2.1. Sampling Site
2.2. Sampling of LDPE Residues from Contaminated Soil
2.3. LDPE-Degrading Aspergillus Isolation and Preliminary Enzymatic Assessment
2.3.1. Isolation on Solid Medium
2.3.2. Preliminary Enzymatic Assessment Using Model Substrates
2.4. Molecular Identification of Aspergillus spp.
2.5. In Vitro Fungal Systems for the Assessment of LDPE Biodegradation
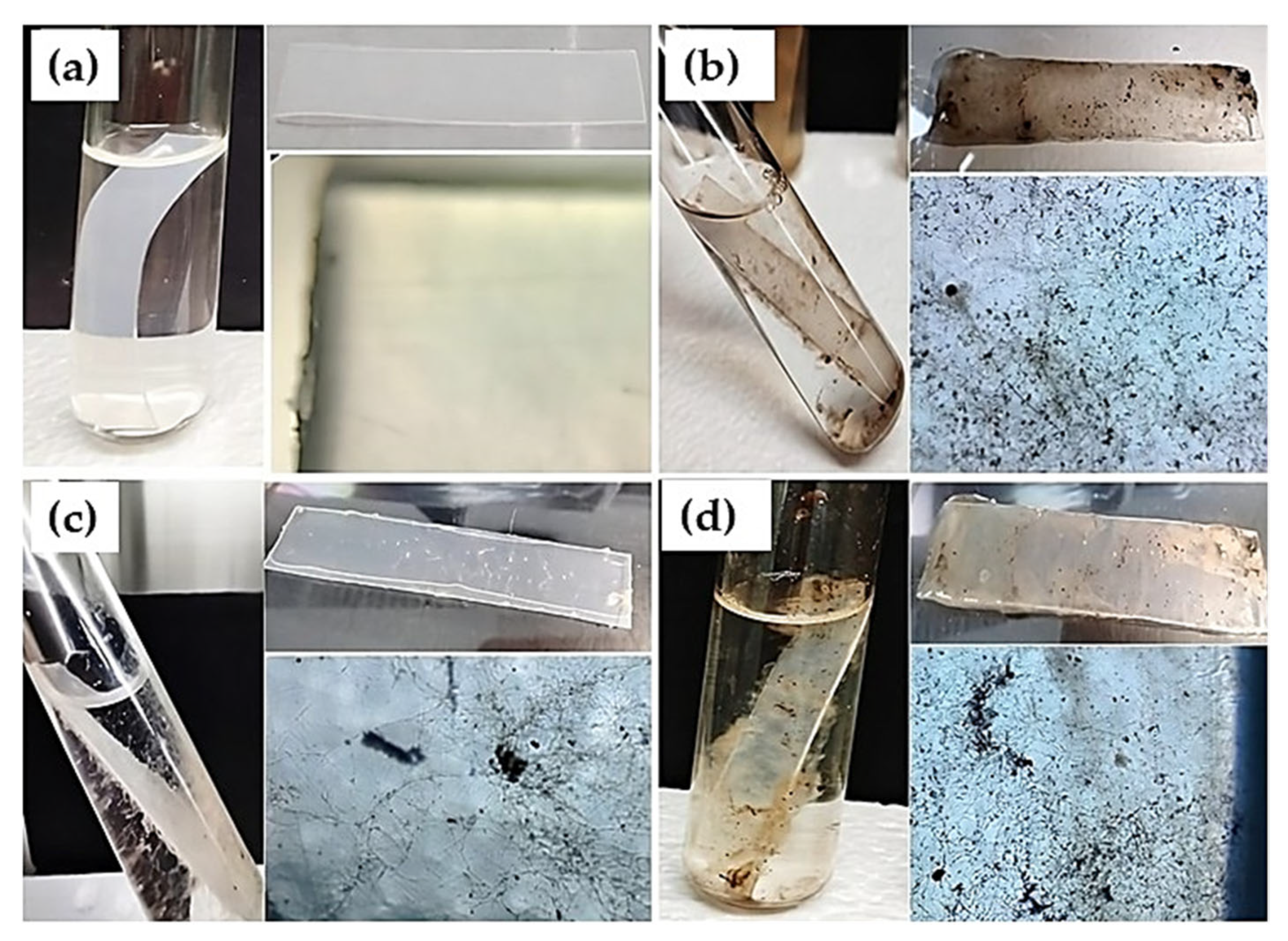
LDPE Strips and Fungal Inoculum
2.6. LDPE Biodegradation Assessment
2.6.1. Preparing Tube Systems to Evaluate LDPE Biodegradation
2.6.2. Aspergillus Strains Growth on LDPE Strips
2.6.3. pH Measurement
2.6.4. Determine Weight Loss and Half-Life (t1/2) of LDPE Strips
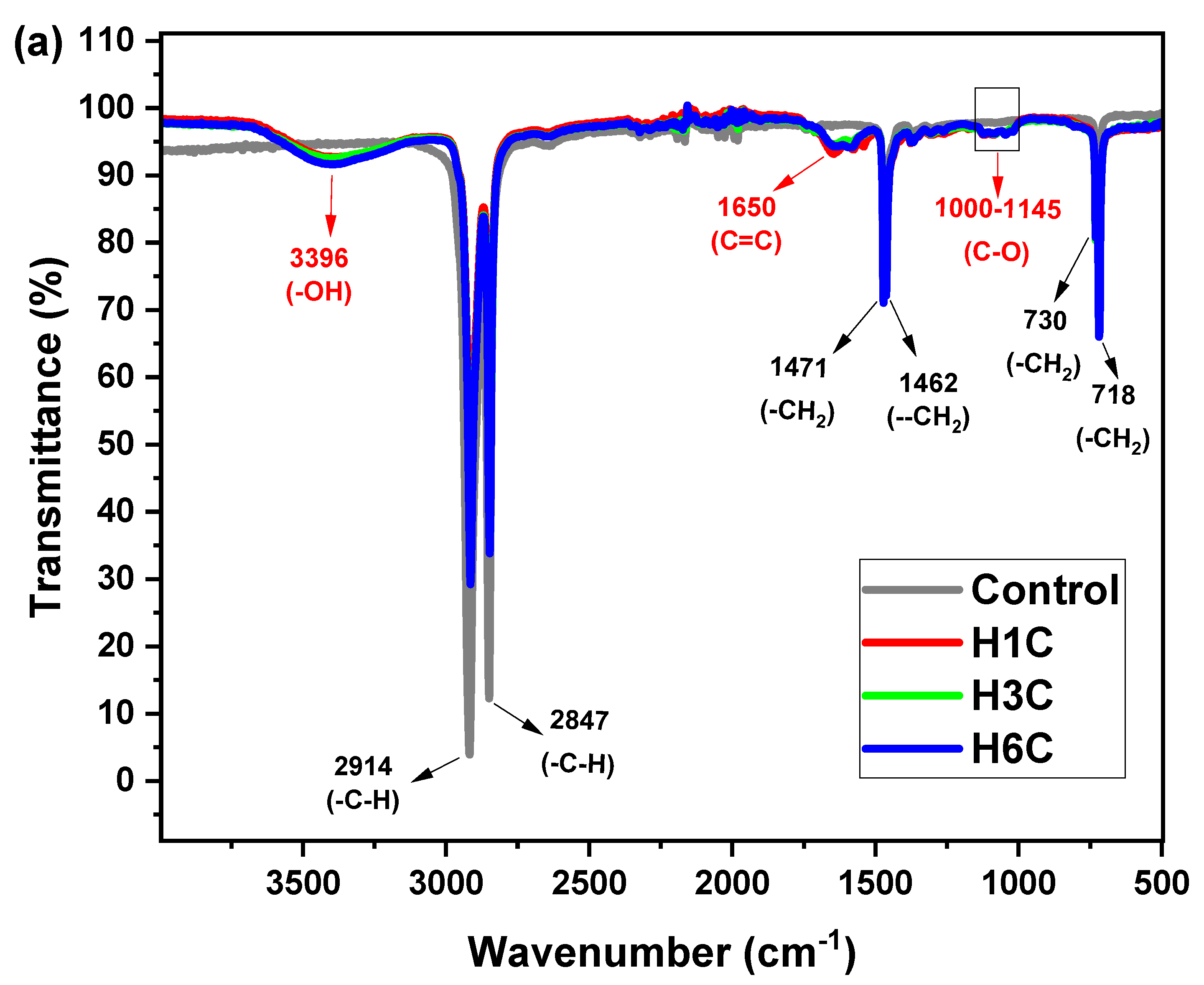
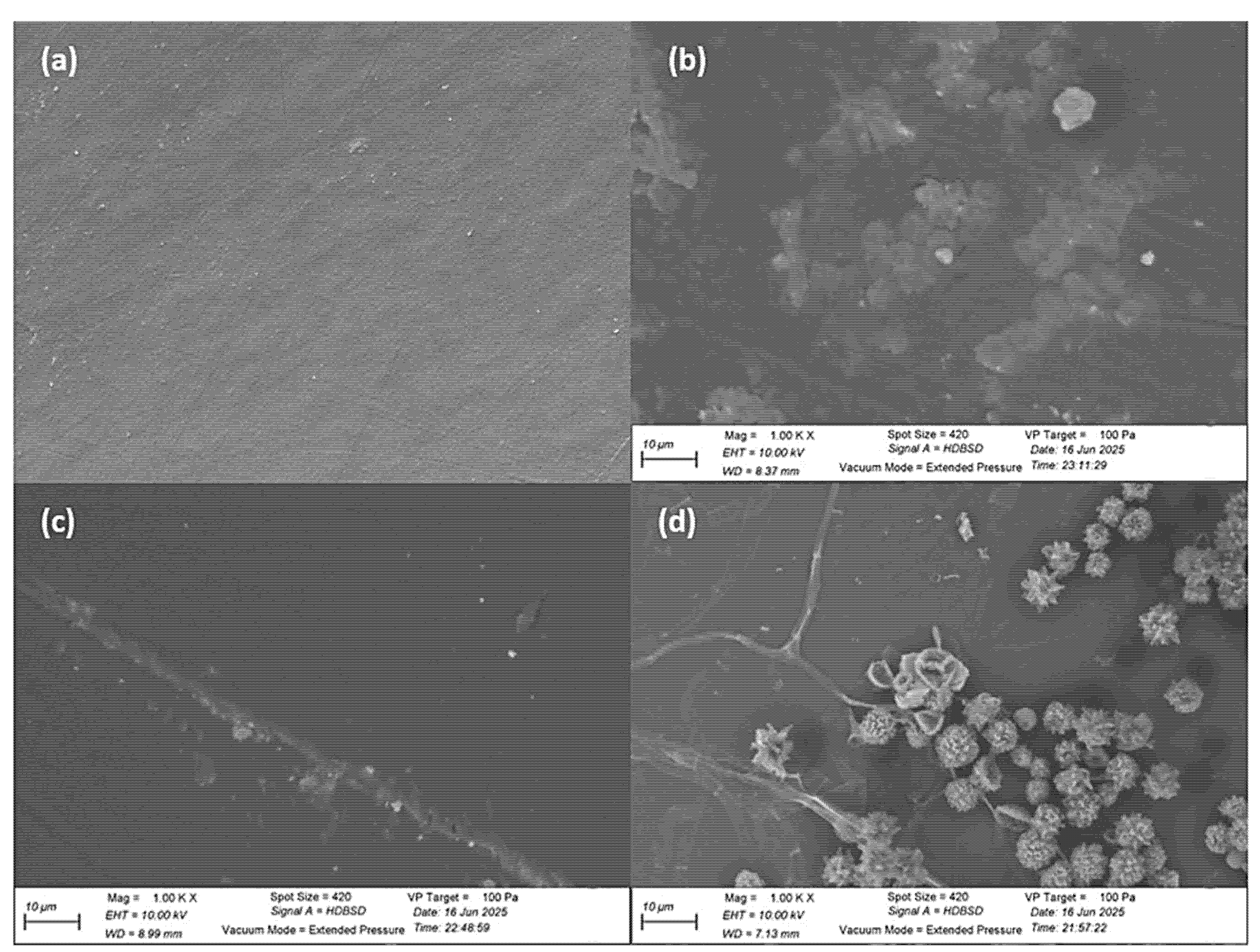
2.6.5. Fourier Transform Infrared (FTIR) and Scanning Electron Microscope (SEM) Analysis
2.7. Statistics Analysis
3. Results
4. Discussion
5. Research Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bahl, S.; Dolma, J.; Jyot Singh, J.; Sehgal, S. Biodegradation of Plastics: A State of the Art Review. Mater. Today Proc. 2021, 39, 31–34. [Google Scholar] [CrossRef]
- Zeenat; Elahi, A.; Bukhari, D.A.; Shamim, S.; Rehman, A. Plastics Degradation by Microbes: A Sustainable Approach. J. King Saud Univ. Sci. 2021, 33, 101538. [Google Scholar] [CrossRef]
- Adeniran, A.A.; Shakantu, W. The Health and Environmental Impact of Plastic Waste Disposal in South African Townships: A Review. Int. J. Environ. Res. Public Health 2022, 19, 779. [Google Scholar] [CrossRef]
- North, E.J.; Halden, R.U. Plastics and Environmental Health: The Road Ahead. Rev. Environ. Health 2013, 28, 1–8. [Google Scholar] [CrossRef]
- Sa’adu, I.; Farsang, A. Plastic Contamination in Agricultural Soils: A Review. Environ. Sci. Eur. 2023, 35, 13. [Google Scholar] [CrossRef]
- Geyer, R.; Jambeck, J.R.; Law, K.L. Production, Use, and Fate of All Plastics Ever Made. Sci. Adv. 2017, 3, e1700782. [Google Scholar] [CrossRef]
- Kadac-Czapska, K.; Knez, E.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Grembecka, M. Microplastics Derived from Food Packaging Waste-Their Origin and Health Risks. Materials 2023, 16, 674. [Google Scholar] [CrossRef] [PubMed]
- Us Epa, O. Plastics: Material-Specific Data. Available online: https://www.epa.gov/facts-and-figures-about-materials-waste-and-recycling/plastics-material-specific-data (accessed on 24 November 2024).
- Fayshal, M.A. Current practices of plastic waste management, environmental impacts, and potential alternatives for reducing pollution and improving management. Heliyon 2024, 10, e40838. [Google Scholar] [CrossRef]
- Kotova, I.B.; Taktarova, Y.V.; Tsavkelova, E.A.; Egorova, M.A.; Bubnov, I.A.; Malakhova, D.V.; Shirinkina, L.I.; Sokolova, T.G.; Bonch-Osmolovskaya, E.A. Microbial Degradation of Plastics and Approaches to Make It More Efficient. Microbiology 2021, 90, 671–701. [Google Scholar] [CrossRef]
- Shi, C.; Zhang, Y.; Shao, Y.; Ray, S.S.; Wang, B.; Zhao, Z.; Yu, B.; Zhang, X.; Li, W.; Ding, J.; et al. A review on the occurrence, detection methods, and ecotoxicity of biodegradable microplastics in the aquatic environment: New cause for concern. TrAC Trends Anal. Chem. 2024, 178, 117832. [Google Scholar] [CrossRef]
- Kumar, R.; Verma, A.; Shome, A.; Sinha, R.; Sinha, S.; Jha, P.K.; Kumar, R.; Kumar, P.; Shubham Das, S.; Sharma, P. Impacts of Plastic Pollution on Ecosystem Services, Sustainable Development Goals, and Need to Focus on Circular Economy and Policy Interventions. Sustainability 2021, 13, 9963. [Google Scholar] [CrossRef]
- Iroegbu, A.O.C.; Ray, S.S.; Mbarane, V.; Bordado, J.C.; Sardinha, J.P. Plastic Pollution: A Perspective on Matters Arising: Challenges and Opportunities. ACS Omega 2021, 6, 19343–19355. [Google Scholar] [CrossRef]
- Thushari, G.G.N.; Senevirathna, J.D.M. Plastic Pollution in the Marine Environment. Heliyon 2020, 6, e04709. [Google Scholar] [CrossRef]
- Wayman, C.; Niemann, H. The Fate of Plastic in the Ocean Environment—A Minireview. Environ. Sci. Process. Impacts 2021, 23, 198–212. [Google Scholar] [CrossRef] [PubMed]
- Watt, E.; Picard, M.; Maldonado, B.; Abdelwahab, M.A.; Mielewski, D.F.; Drzal, L.T.; Misra, M.; Mohanty, A.K. Ocean Plastics: Environmental Implications and Potential Routes for Mitigation—A Perspective. RSC Adv. 2021, 11, 21447–21462. [Google Scholar] [CrossRef]
- Evode, N.; Qamar, S.A.; Bilal, M.; Barceló, D.; Iqbal, H.M.N. Plastic Waste and Its Management Strategies for Environmental Sustainability. Case Stud. Chem. Environ. Eng. 2021, 4, 100142. [Google Scholar] [CrossRef]
- Zhang, F.; Zhao, Y.; Wang, D.; Yan, M.; Zhang, J.; Zhang, P.; Ding, T.; Chen, L.; Chen, C. Current Technologies for Plastic Waste Treatment: A Review. J. Clean. Prod. 2021, 282, 124523. [Google Scholar] [CrossRef]
- Damayanti, D.; Saputri, D.R.; Marpaung, D.S.S.; Yusupandi, F.; Sanjaya, A.; Simbolon, Y.M.; Asmarani, W.; Ulfa, M.; Wu, H.-S. Current Prospects for Plastic Waste Treatment. Polymers 2022, 14, 3133. [Google Scholar] [CrossRef]
- Mohanan, N.; Montazer, Z.; Sharma, P.K.; Levin, D.B. Microbial and Enzymatic Degradation of Synthetic Plastics. Front. Microbiol. 2020, 11, 580709. [Google Scholar] [CrossRef]
- Srikanth, M.; Sandeep, T.S.R.S.; Sucharitha, K.; Godi, S. Biodegradation of Plastic Polymers by Fungi: A Brief Review. Bioresour. Bioprocess. 2022, 9, 42. [Google Scholar] [CrossRef]
- Shilpa; Basak, N.; Meena, S.S. Microbial Biodegradation of Plastics: Challenges, Opportunities, and a Critical Perspective. Front. Environ. Sci. Eng. 2022, 16, 161. [Google Scholar] [CrossRef]
- Vaksmaa, A.; Guerrero-Cruz, S.; Ghosh, P.; Zeghal, E.; Hernando-Morales, V.; Niemann, H. Role of Fungi in Bioremediation of Emerging Pollutants. Front. Mar. Sci. 2023, 10, 1070905. [Google Scholar] [CrossRef]
- Ekanayaka, A.H.; Tibpromma, S.; Dai, D.; Xu, R.; Suwannarach, N.; Stephenson, S.L.; Dao, C.; Karunarathna, S.C. A Review of the Fungi That Degrade Plastic. J. Fungi 2022, 8, 772. [Google Scholar] [CrossRef]
- Malcolm, D.; Richardson, K.B.; Hope, W. Aspergillus. In Clinical Mycology; Elsevier: Amsterdam, The Netherlands, 2009; pp. 271–296. ISBN 9781416056805. [Google Scholar]
- Mohamed, A.H.; Balbool, B.A.; Abdel-Azeem, A.M. Aspergillus from Different Habitats and Their Industrial Applications. In Fungal Biology; Springer International Publishing: Cham, Switzerland, 2021; pp. 85–106. ISBN 9783030675608. [Google Scholar]
- Mousavi, B.; Hedayati, M.T.; Hedayati, N.; Ilkit, M.; Syedmousavi, S. Aspergillus Species in Indoor Environments and Their Possible Occupational and Public Health Hazards. Curr. Med. Mycol. 2016, 2, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Mukherjee, A. Role of Aspergillus in Bioremediation Process. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2016; pp. 209–214. ISBN 9780444635051. [Google Scholar]
- Munir, E.; Harefa, R.S.M.; Priyani, N.; Suryanto, D. Plastic Degrading Fungi Trichoderma Viride and Aspergillus Nomius Isolated from Local Landfill Soil in Medan. IOP Conf. Ser. Earth Environ. Sci. 2018, 126, 012145. [Google Scholar] [CrossRef]
- Pu, S.; Ma, H.; Deng, D.; Xue, S.; Zhu, R.; Zhou, Y.; Xiong, X. Isolation, Identification, and Characterization of an Aspergillus Niger Bioflocculant-Producing Strain Using Potato Starch Wastewater as Nutrilite and Its Application. PLoS ONE 2018, 13, e0190236. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.I.; Ahmed, S.; Robson, G.; Javed, I.; Ali, N.; Atiq, N.; Hameed, A. Isolation and Molecular Characterisation of Polyvinyl Chloride (PVC) Plastic Degrading Fungal Isolates: Fungal Degradation of PVC Plastics. J. Basic Microbiol. 2014, 54, 18–27. [Google Scholar] [CrossRef] [PubMed]
- Guerrero Cabrera, J.P.; Cabello Torres, R.J.; Valdiviezo Gonzales, L.G.; Garzon Flores, A.; Reynoso Quispe, P. Application of Fungal Microorganisms for the Degradation “in Vitro” of Synthetic Polymers. In Proceedings of the 20th LACCEI International Multi-Conference for Engineering, Education, and Technology: “Education, Research and Leadership in Post-pandemic Engineering: Resilient, Inclusive and Sustainable Actions”, Hybrid Event, Boca Raton, FL, USA, 18–22 July 2022; Latin American and Caribbean Consortium of Engineering Institutions: Miami Lakes, FL, USA, 2022. [Google Scholar] [CrossRef]
- Russell, J.R.; Huang, J.; Anand, P.; Kucera, K.; Sandoval, A.G.; Dantzler, K.W.; Hickman, D.; Jee, J.; Kimovec, F.M.; Koppstein, D.; et al. Biodegradation of Polyester Polyurethane by Endophytic Fungi. Appl. Environ. Microbiol. 2011, 77, 6076–6084. [Google Scholar] [CrossRef]
- Méndez, C.R.; Vergaray, G.; Béjar, V.R.; Cárdenas, K.J. Aislamiento y Caracterización de Micromicetos Biodegradadores de Polietileno. Rev. Peru. Biol. 2006, 13, 203–205. [Google Scholar] [CrossRef]
- Uribe, D.; Giraldo, D.; Gutiérrez, S.; Merino, F. Biodegradación de Polietileno de Baja Densidad Por Acción de Un Consorcio Microbiano Aislado de Un Relleno Sanitario, Lima, Perú. Rev. Peru. Biol. 2011, 17, 133–136. [Google Scholar] [CrossRef]
- Ibrahim, S.S.; Ionescu, D.; Grossart, H.-P. Tapping into Fungal Potential: Biodegradation of Plastic and Rubber by Potent Fungi. Sci. Total Environ. 2024, 934, 173188. [Google Scholar] [CrossRef] [PubMed]
- Saira Abdullah Maroof, L.; Iqbal, M.; Farman, S.; Lubna Faisal, S. Biodegradation of Low-Density Polyethylene (LDPE) Bags by Fungi Isolated from Waste Disposal Soil. Appl. Environ. Soil Sci. 2022, 2022, 8286344. [Google Scholar] [CrossRef]
- Nademo, Z.M.; Shibeshi, N.T.; Gemeda, M.T. Isolation and Screening of Low-Density Polyethylene (LDPE) Bags Degrading Bacteria from Addis Ababa Municipal Solid Waste Disposal Site “Koshe”. Ann. Microbiol. 2023, 73, 6. [Google Scholar] [CrossRef]
- Gupta, K.K.; Devi, D.; Rana, D. Isolation And Screening of Low Density Polyethylene (Ldpe) Degrading Bacterial Strains from Waste Disposal Sites. World J. Pharm. Res. 2016, 5, 1633–1643. Available online: https://www.semanticscholar.org/paper/ISOLATION-AND-SCREENING-OF-LOW-DENSITY-POLYETHYLENE-Gupta-Devi/98c2a520cf88266f61f2fee8fe65c00df1f342e5 (accessed on 27 September 2024).
- Khan, S.; Ali, S.A.; Ali, A.S. Biodegradation of Low Density Polyethylene (LDPE) by Mesophilic Fungus “Penicillium citrinum” Isolated from Soils of Plastic Waste Dump Yard, Bhopal, India. Environ. Technol. 2023, 44, 2300–2314. [Google Scholar] [CrossRef]
- Kim, D.-W.; Lim, E.S.; Lee, G.H.; Son, H.F.; Sung, C.; Jung, J.-H.; Park, H.J.; Gong, G.; Ko, J.K.; Um, Y.; et al. Biodegradation of Oxidized Low Density Polyethylene by Pelosinus Fermentans Lipase. Bioresour. Technol. 2024, 403, 130871. [Google Scholar] [CrossRef]
- Machuca-Guevara, J.I.; Suárez-Peña, E.A.; Motte Darricau, E.; Mialhe-Matonnier, E.L. Caracterización Molecular de Los Microorganismos Presentes Durante El Proceso Fermentativo de Los Granos de Cacao (Theobroma cacao). Rev. Peru. Biol. 2019, 26, 535–542. [Google Scholar] [CrossRef]
- Kuchkarova, N.; Han, C.; Toshmatov, Z.; Chen, H.; Shao, H. Diversity of Fungal Endophytes Isolated from the Invasive Plant Solanum Rostratum. Acta Bot. Croat. 2024, 83, 76–79. [Google Scholar] [CrossRef]
- Gong, Z.; Jin, L.; Yu, X.; Wang, B.; Hu, S.; Ruan, H.; Sung, Y.-J.; Lee, H.-G.; Jin, F. Biodegradation of Low Density Polyethylene by the Fungus Cladosporium sp. Recovered from a Landfill Site. J. Fungi 2023, 9, 605. [Google Scholar] [CrossRef] [PubMed]
- Bustillo, A. Método para Cuantificar Suspensiones de Esporas de Hongos y Otros Organismos; Universidad Nacional de Colombia: Palmira, Colombia, 2010; Volume 10. [Google Scholar]
- Sathiyabama, M.; Boomija, R.V.; Sathiyamoorthy, T.; Mathivanan, N.; Balaji, R. Mycodegradation of Low-Density Polyethylene by Cladosporium Sphaerospermum, Isolated from Platisphere. Sci. Rep. 2024, 14, 8351. [Google Scholar] [CrossRef]
- E13 Committee Practice for General Techniques for Obtaining Infrared Spectra for Qualitative Analysis. Available online: https://store.astm.org/e1252-98r21.html (accessed on 20 December 2024).
- Esmaeili, A.; Pourbabaee, A.A.; Alikhani, H.A.; Shabani, F.; Esmaeili, E. Biodegradation of Low-Density Polyethylene (LDPE) by Mixed Culture of Lysinibacillus Xylanilyticus and Aspergillus Niger in Soil. PLoS ONE 2013, 8, e71720. [Google Scholar] [CrossRef]
- Mori-Mestanza, D.; Valqui-Rojas, I.; Caetano, A.C.; Culqui-Arce, C.; Cruz-Lacerna, R.; Cayo-Colca, I.S.; Castro-Alayo, E.M.; Balcázar-Zumaeta, C.R. Physicochemical Properties of Nanoencapsulated Essential Oils: Optimizing D-Limonene Preservation. Polymers 2025, 17, 348. [Google Scholar] [CrossRef]
- Nasrabadi, A.E.; Ramavandi, B.; Bonyadi, Z. Recent Progress in Biodegradation of Microplastics by Aspergillus Sp. in Aquatic Environments. Colloids Interface Sci. Commun. 2023, 57, 100754. [Google Scholar] [CrossRef]
- d’Halewyn, M.-A.; Chevalier, P. Aspergillus Niger. Available online: https://www.inspq.qc.ca/en/moulds/fact-sheets/aspergillus-niger (accessed on 21 January 2025).
- Nyongesa, B.W.; Okoth, S.; Ayugi, V. Identification Key for Aspergillus Species Isolated from Maize and Soil of Nandi County, Kenya. Adv. Microbiol. 2015, 05, 205–229. [Google Scholar] [CrossRef]
- Visagie, C.M.; Varga, J.; Houbraken, J.; Meijer, M.; Kocsubé, S.; Yilmaz, N.; Fotedar, R.; Seifert, K.A.; Frisvad, J.C.; Samson, R.A. Ochratoxin Production and Taxonomy of the Yellow Aspergilli (Aspergillus Section Circumdati). Stud. Mycol. 2014, 78, 1–61. [Google Scholar] [CrossRef]
- Akter, H.; Ara, I.; Alam, N.B.; Alam, N. The First Report of Aspergillus Ochraceopetaliformis Bat. & Maia from Small Indigenous Dry Fish Setipinna Phasa (Hamilton 1822) in Bangladesh. Jahangirnagar Univ. J. Biol. Sci. 2024, 12, 87–95. [Google Scholar] [CrossRef]
- Sharma, R.; Talukdar, D.; Bhardwaj, S.; Jaglan, S.; Kumar, R.; Kumar, R.; Akhtar, M.S.; Beniwal, V.; Umar, A. Bioremediation Potential of Novel Fungal Species Isolated from Wastewater for the Removal of Lead from Liquid Medium. Environ. Technol. Innov. 2020, 18, 100757. [Google Scholar] [CrossRef]
- Gowthami, A.; Syed Marjuk, M.; Raju, P.; Nanthini Devi, K.; Santhanam, P.; Dinesh Kumar, S.; Perumal, P. Biodegradation Efficacy of Selected Marine Microalgae against Low-Density Polyethylene (LDPE): An Environment Friendly Green Approach. Mar. Pollut. Bull. 2023, 190, 114889. [Google Scholar] [CrossRef]
- Zhang, J.; Gao, D.; Li, Q.; Zhao, Y.; Li, L.; Lin, H.; Bi, Q.; Zhao, Y. Biodegradation of Polyethylene Microplastic Particles by the Fungus Aspergillus Flavus from the Guts of Wax Moth Galleria Mellonella. Sci. Total Environ. 2020, 704, 135931. [Google Scholar] [CrossRef]
- Sánchez, C. Fungal Potential for the Degradation of Petroleum-Based Polymers: An Overview of Macro- and Microplastics Biodegradation. Biotechnol. Adv. 2020, 40, 107501. [Google Scholar] [CrossRef]
- Dharshni, S.; Kanchana, M. Kanchana Microbial Degradation of Low-Density Polyethylene (Ldpe) by Fungus Isolated from Landfill Soil. Plant Arch. 2021, 21, 809–815. [Google Scholar] [CrossRef]
- Das, M.P.; Kumar, S.; Das, J. Fungal-Mediated Deterioration and Biodegradation Study of Low-Density Polyethylene (LDPE) Isolated from Municipal Dump Yard in Chennai, India. Energy Ecol. Environ. 2018, 3, 229–236. [Google Scholar] [CrossRef]
- Temporiti, M.E.E.; Nicola, L.; Nielsen, E.; Tosi, S. Fungal Enzymes Involved in Plastics Biodegradation. Microorganisms 2022, 10, 1180. [Google Scholar] [CrossRef]
- Muhonja, C.N.; Makonde, H.; Magoma, G.; Imbuga, M. Biodegradability of Polyethylene by Bacteria and Fungi from Dandora Dumpsite Nairobi-Kenya. PLoS ONE 2018, 13, e0198446. [Google Scholar] [CrossRef]
- Jayaprakash, V.; Maheswari Devi Palempalli, U. Effect of Palmitic Acid in the Acceleration of Polyethylene Biodegradation by Aspergillus Oryzae. J. Pure Appl. Microbiol. 2018, 12, 2259–2268. [Google Scholar] [CrossRef]
- Raaman, N.; Rajitha, N.; Jayshree, A.; Jegadeesh, R. Biodegradation of Plastic by Aspergillus spp. Isolated from Polythene Polluted Sites around Chennai. J. Acad. Indus. Res. 2014, 16, 313–316. Available online: https://api.semanticscholar.org/CorpusID:3464885 (accessed on 25 December 2024).
- Makut, M.D.; Okey-Ndeche, N.F. Optimisation of Some Conditions for the Biodegradation of Low-Density Polyethylene Strips by Fungi Isolated from Parts of North Central Nigeria. Int. J. Latest Technol. Eng. Manag. Appl. Sci. 2024, 13, 35–42. [Google Scholar] [CrossRef]
- DSouza, G.C.; Sheriff, R.S.; Ullanat, V.; Shrikrishna, A.; Joshi, A.V.; Hiremath, L.; Entoori, K. Fungal Biodegradation of Low-Density Polyethylene Using Consortium of Aspergillus Species under Controlled Conditions. Heliyon 2021, 7, e07008. [Google Scholar] [CrossRef]
- Cabeza Vásquez, H.T.; Reaño Segundo, Y.J. Potencial En La Degradación de Polietileno de Baja Densidad En Consorcios de Bacterias Del Botadero de Residuos Sólidos “Pampas de Reque”, 2022; Universidad Nacional Pedro Ruíz Gallo: Lambayeque, Perú, 2024; Available online: https://hdl.handle.net/20.500.12893/13172 (accessed on 25 December 2024).
- Maan Shake, M.; Khlaif, A.T. Bioremediation of Low-Density Polyethylene (PE) by Fungi. J. Neonatal Surg. 2025, 14, 57–63. [Google Scholar] [CrossRef]
- Yang, S.; Yang, X.; Xie, J.; Hou, Q.; Liu, Y. Degradation Effect and Degradation Mechanism of Aspergillus niger on Polyethylene Components of Waste Cables. Trans. China Electrotech. Soc. 2024, 39, 76–87. [Google Scholar] [CrossRef]
- Sutkar, P.R.; Hadkar, S.S.; Kamble, S.A.; Dhulap, V.P. Biodegradation Potential of Low-Density Polyethylene (LDPE) Using Aspergillus Niger and Phanerochaete Chrysosporium. Discov. Environ. 2025, 3, 7. [Google Scholar] [CrossRef]
- Ahmed, M.; Iram, S.; Tabassum, N.; Sajid, M.; Paseutsakoun, K.; Aleksza, L.; Székács, A. Biodegradation Efficacy of Aspergillus niger and Trichoderma harzianum on Low-Density Polyethylene. Polymers 2025, 17, 1303. [Google Scholar] [CrossRef]
- Safdar, A.; Ismail, F.; Imran, M. Biodegradation of Synthetic Plastics by the Extracellular Lipase of Aspergillus Niger. Environ. Adv. 2024, 17, 100563. [Google Scholar] [CrossRef]
- Abraham, J.; Ghosh, E.; Mukherjee, P.; Gajendiran, A. Microbial Degradation of Low Density Polyethylene. Environ. Prog. Sustain. Energy 2017, 36, 147–154. [Google Scholar] [CrossRef]
- El-Sayed, M.T.; Rabie, G.H.; Hamed, E.A. Biodegradation of Low-Density Polyethylene (LDPE) Using the Mixed Culture of Aspergillus Carbonarius and A. Fumigates. Environ. Dev. Sustain. 2021, 23, 14556–14584. [Google Scholar] [CrossRef]




| Fungi Strains | W0 (g) | W1 (g) | % Loss of Weight ± SD | Reduction Rate Constant (k) of LDPE (g·day−1) | Half-Life (t1/2) of the Treated LDPE (Days) |
|---|---|---|---|---|---|
| A. niger H1C | 0.20 | 0.19 | 4.25 ± 1.67b * | 0.0009 ± 0.0035 | 897.89 |
| A. ochraceopetaliformis H3C | 0.19 | 0.18 | 1.98 ± 0.37ab | 0.0004 ± 0.0002 | 1757.33 |
| A. tamarii H6C | 0.25 | 0.24 | 3.79 ± 1.52b | 0.0008 ± 0.0004 | 901.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rojas-Villacorta, W.; Cruz-Noriega, M.D.L.; Otiniano, N.M.; Terrones-Rodríguez, N.; Quiñones-Cerna, C. Biodegradation of Low-Density Polyethylene by Native Aspergillus Strains Isolated from Plastic-Contaminated Soil. Sustainability 2025, 17, 8983. https://doi.org/10.3390/su17208983
Rojas-Villacorta W, Cruz-Noriega MDL, Otiniano NM, Terrones-Rodríguez N, Quiñones-Cerna C. Biodegradation of Low-Density Polyethylene by Native Aspergillus Strains Isolated from Plastic-Contaminated Soil. Sustainability. 2025; 17(20):8983. https://doi.org/10.3390/su17208983
Chicago/Turabian StyleRojas-Villacorta, Walter, Magaly De La Cruz-Noriega, Nélida Milly Otiniano, Nicole Terrones-Rodríguez, and Claudio Quiñones-Cerna. 2025. "Biodegradation of Low-Density Polyethylene by Native Aspergillus Strains Isolated from Plastic-Contaminated Soil" Sustainability 17, no. 20: 8983. https://doi.org/10.3390/su17208983
APA StyleRojas-Villacorta, W., Cruz-Noriega, M. D. L., Otiniano, N. M., Terrones-Rodríguez, N., & Quiñones-Cerna, C. (2025). Biodegradation of Low-Density Polyethylene by Native Aspergillus Strains Isolated from Plastic-Contaminated Soil. Sustainability, 17(20), 8983. https://doi.org/10.3390/su17208983








