Exploration of Influencing Factors and Generation Mechanism of EPFRs in Polycyclic Aromatic Hydrocarbon-Contaminated Soil
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Soil with Various Transition Metal Ion Loading
2.2. Analytical Methods
2.2.1. EPFR Measurements
2.2.2. PAH Measurements
2.3. Data Processing
3. Results and Discussion
3.1. Influence of Environmental Factors on the Generation of EPFRs from Individual Pollutants
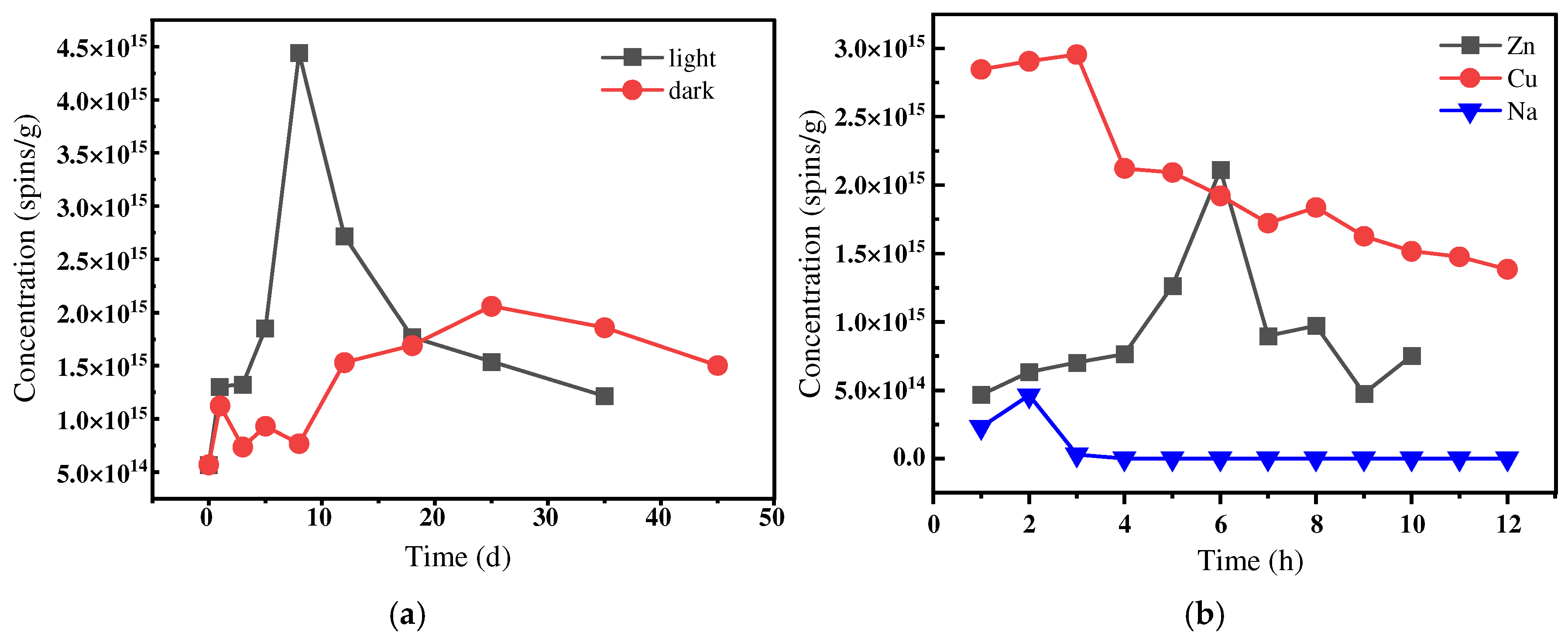
3.1.1. Effects of Light and Darkness on EPFR Spin Concentration
3.1.2. Effects of Different Transition Metal Ions on EPFR Spin Concentration
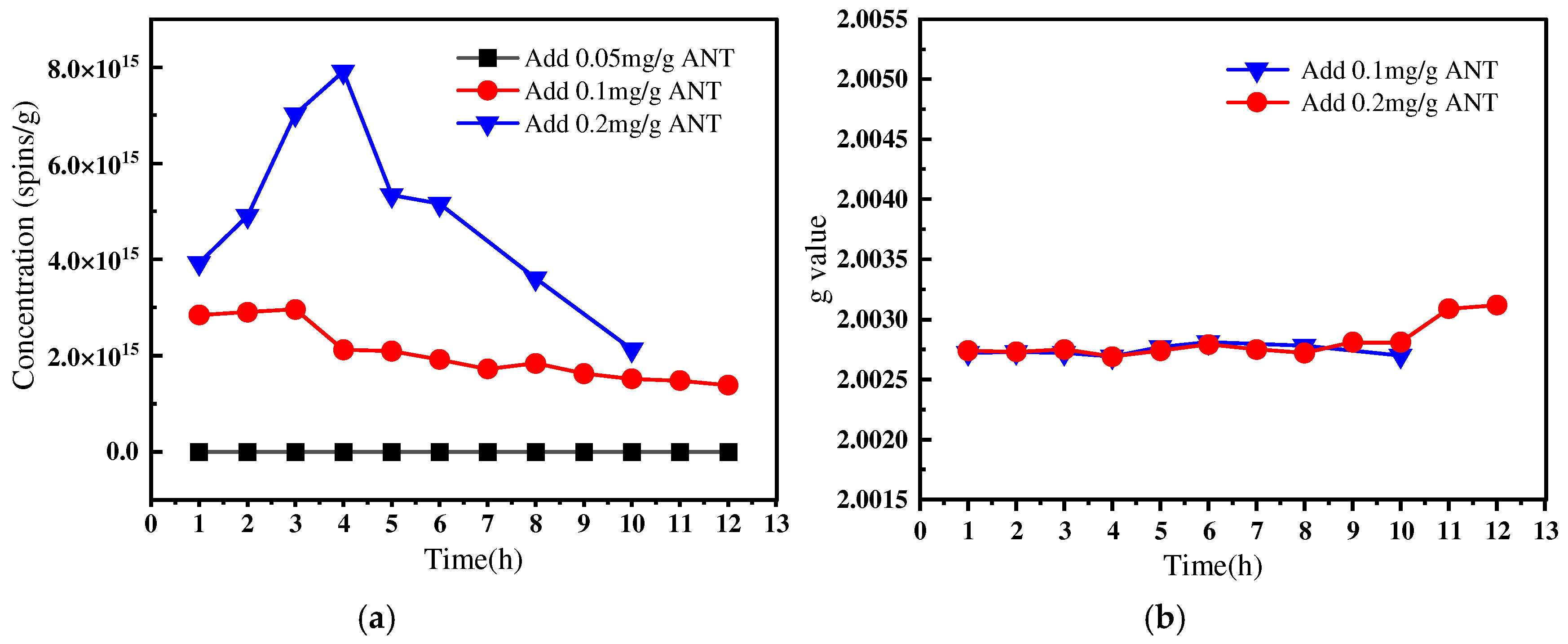
3.1.3. Effects of Precursor Concentration on Spin Concentration and g-Value of EPFRs
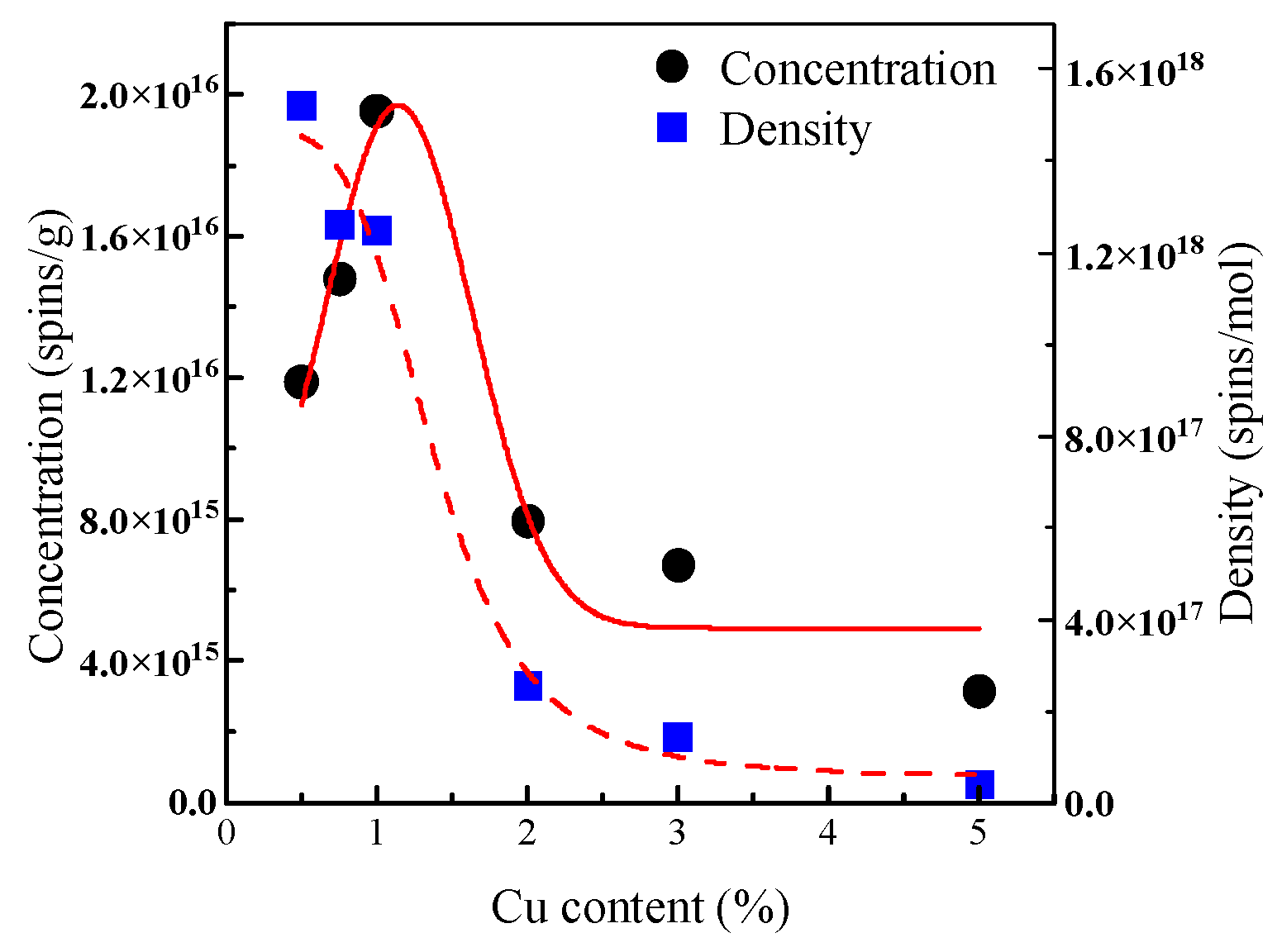
3.1.4. The Effect of Different Concentrations of Cu Loading Systems on the Generation of EPFRs
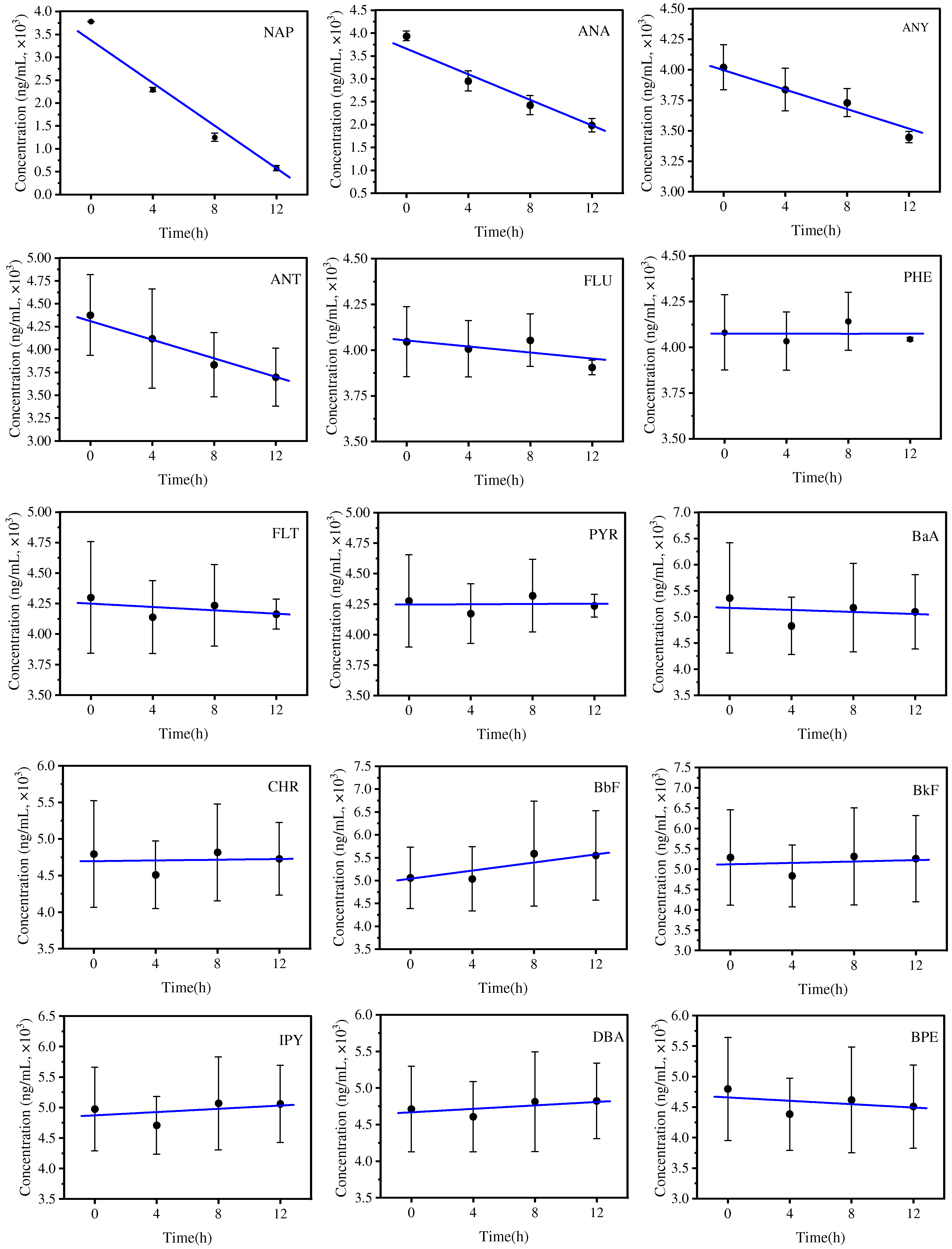
3.2. Generation of EPFRs in the PAH Mixtures and Degradation of Mixed PAHs
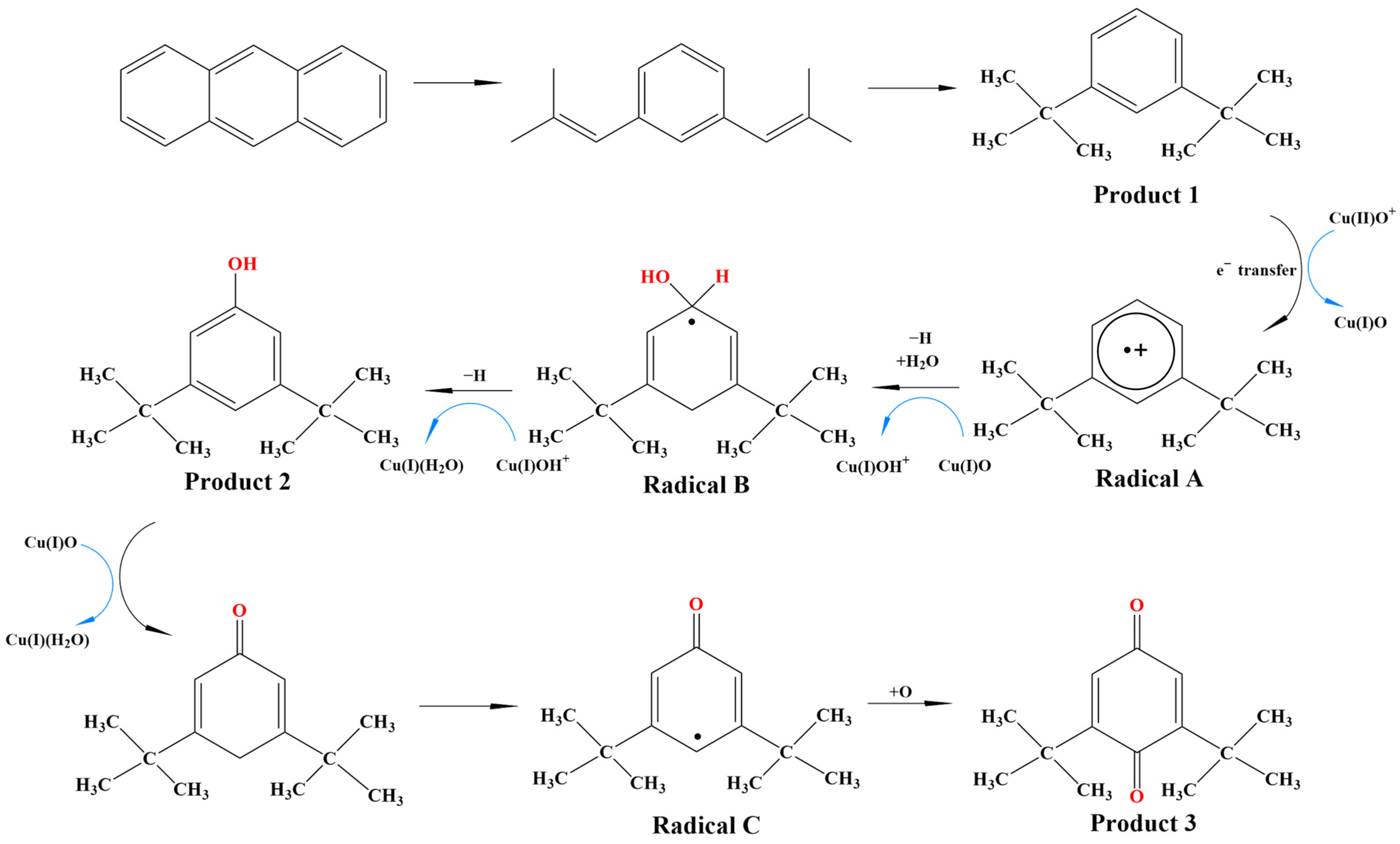
3.3. Free Radical-Meditated Formation Mechanism of Organic Pollutants from Photodegradation Reactions of Anthracene
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, Q.; Sun, H.; Wang, M.; Wang, Y.; Zhang, L.; Han, Y. Environmentally Persistent Free Radical (EPFR) Formation by Visible-Light Illumination of the Organic Matter in Atmospheric Particles. Environ. Sci. Technol. 2019, 53, 10053–10061. [Google Scholar] [CrossRef]
- Squadrito, G.L.; Cueto, R.; Dellinger, B.; Pryor, W.A. Quinoid Redox Cycling as a Mechanism for Sustained Free Radical Generation by Inhaled Airborne Particulate Matter. Free Radic. Biol. Med. 2001, 31, 1132–1138. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Gu, B.; Leboeuf, E.J.; Pan, H.; Dai, S. Spectroscopic Characterization of the Structural and Functional Properties of Natural Organic Matter Fractions. Chemosphere 2002, 48, 59–68. [Google Scholar] [CrossRef]
- Paul, A.; Stösser, R.; Zehl, A.; Zwirnmann, E.; Vogt, R.D.; Steinberg, C.E.W. Nature and Abundance of Organic Radicals in Natural Organic Matter: Effect of pH and Irradiation. Environ. Sci. Technol. 2006, 40, 5897–5903. [Google Scholar] [CrossRef]
- Chen, Q.; Sun, H.; Wang, M.; Mu, Z.; Wang, Y.; Li, Y.; Wang, Y.; Zhang, L.; Zhang, Z. Dominant Fraction of EPFRs from Nonsolvent-Extractable Organic Matter in Fine Particulates over Xi’an, China. Environ. Sci. Technol. 2018, 52, 9646–9655. [Google Scholar] [CrossRef]
- Nam, J.J.; Sweetman, A.J.; Jones, K.C. Polynuclear Aromatic Hydrocarbons (PAHs) in Global Background Soils. J. Environ. Monit. 2009, 11, 45–48. [Google Scholar] [CrossRef]
- Menager, M.; Sarakha, M. Simulated Solar Light Phototransformation of Organophosphorus Azinphos Methyl at the Surface of Clays and Goethite. Environ. Sci. Technol. 2013, 47, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Li, P.; Gong, Z.; Oni, A.A. Photochemical Behavior of Benzo[a]Pyrene on Soil Surfaces under UV Light Irradiation. J. Environ. Sci. 2006, 18, 1226–1232. [Google Scholar] [CrossRef]
- Ahn, M.-Y.; Filley, T.R.; Jafvert, C.T.; Nies, L.; Hua, I.; Bezares-Cruz, J. Photodegradation of Decabromodiphenyl Ether Adsorbed onto Clay Minerals, Metal Oxides, and Sediment. Environ. Sci. Technol. 2006, 40, 215–220. [Google Scholar] [CrossRef] [PubMed]
- dela Cruz, A.L.N.; Cook, R.L.; Lomnicki, S.M.; Dellinger, B. Effect of Low Temperature Thermal Treatment on Soils Contaminated with Pentachlorophenol and Environmentally Persistent Free Radicals. Environ. Sci. Technol. 2012, 46, 5971–5978. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Wang, S.Z.; Xu, D.H.; Gong, Y.M.; Ma, H.H.; Tang, X.Y. Review of Catalytic Supercritical Water Gasification for Hydrogen Production from Biomass. Renew. Sustain. Energy Rev. 2010, 14, 334–343. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, S.; Shi, Y.; Zhu, L.; Wang, C.; Sharma, V.K. Transformation of Polycyclic Aromatic Hydrocarbons and Formation of Environmentally Persistent Free Radicals on Modified Montmorillonite: The Role of Surface Metal Ions and Polycyclic Aromatic Hydrocarbon Molecular Properties. Environ. Sci. Technol. 2018, 52, 5725–5733. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, S.; Shi, Y.; Zhu, K.; Gao, P.; Zhu, L. Mechanisms for Light-Driven Evolution of Environmentally Persistent Free Radicals and Photolytic Degradation of PAHs on Fe(III)-Montmorillonite Surface. J. Hazard. Mater. 2019, 362, 92–98. [Google Scholar] [CrossRef]
- Jia, H.; Li, L.; Chen, H.; Zhao, Y.; Li, X.; Wang, C. Exchangeable Cations-Mediated Photodegradation of Polycyclic Aromatic Hydrocarbons (PAHs) on Smectite Surface under Visible Light. J. Hazard. Mater. 2015, 287, 16–23. [Google Scholar] [CrossRef]
- Kiruri, L.W.; Khachatryan, L.; Dellinger, B.; Lomnicki, S. Effect of Copper Oxide Concentration on the Formation and Persistency of Environmentally Persistent Free Radicals (EPFRs) in Particulates. Environ. Sci. Technol. 2014, 48, 2212–2217. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Tao, S.; Zhang, N.; Zhang, D.Y.; Li, X.Q. The Effect of Soil Organic Matter on Fate of Polycyclic Aromatic Hydrocarbons in Soil: A Microcosm Study. Environ. Pollut. 2010, 158, 1768–1774. [Google Scholar] [CrossRef]
- Koelmans, A.A.; Jonker, M.T.O.; Cornelissen, G.; Bucheli, T.D.; Van Noort, P.C.M.; Gustafsson, Ö. Black Carbon: The Reverse of Its Dark Side. Chemosphere 2006, 63, 365–377. [Google Scholar] [CrossRef]
- Jia, H.; Nulaji, G.; Gao, H.; Wang, F.; Zhu, Y.; Wang, C. Formation and Stabilization of Environmentally Persistent Free Radicals Induced by the Interaction of Anthracene with Fe(III)-Modified Clays. Environ. Sci. Technol. 2016, 50, 6310–6319. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, J.; Fan, X.; Dilimulati, K.; Wang, C. Photodegradation of Phenanthrene on Cation-Modified Clays under Visible Light. Appl. Catal. B Environ. 2012, 123–124, 43–51. [Google Scholar] [CrossRef]
- Jia, H.; Zhao, J.; Li, L.; Li, X.; Wang, C. Transformation of Polycyclic Aromatic Hydrocarbons (PAHs) on Fe(III)-Modified Clay Minerals: Role of Molecular Chemistry and Clay Surface Properties. Appl. Catal. B Environ. 2014, 154–155, 238–245. [Google Scholar] [CrossRef]
- Hammerich, O.; Parker, V.D. Kinetics and Mechanisms of Reactions of Organic Cation Radicals in Solution. In Advances in Physical Organic Chemistry; Gold, V., Bethell, D., Eds.; Academic Press: Cambridge, MA, USA, 1984; Volume 20, pp. 55–189. [Google Scholar] [CrossRef]
- Zhang, W.; Zhuang, L.; Yuan, Y.; Tong, L.; Tsang, D.C.W. Enhancement of Phenanthrene Adsorption on a Clayey Soil and Clay Minerals by Coexisting Lead or Cadmium. Chemosphere 2011, 83, 302–310. [Google Scholar] [CrossRef]
- McBride, M.B.; Mortland, M.M. Copper (II) Interactions with Montmorillonite: Evidence from Physical Methods. Soil Sci. Soc. Am. J. 1974, 38, 408–415. [Google Scholar] [CrossRef]
- Yang, L.; Liu, G.; Zheng, M.; Jin, R.; Zhao, Y.; Wu, X.; Xu, Y. Pivotal Roles of Metal Oxides in the Formation of Environmentally Persistent Free Radicals. Environ. Sci. Technol. 2017, 51, 12329–12336. [Google Scholar] [CrossRef]
- Si, Y.; Wang, S.; Zhou, D.; Chen, H. Adsorption and Photo-Reactivity of Bensulfuron-Methyl on Homoionic Clays. Clays Clay Miner. 2004, 52, 742–748. [Google Scholar] [CrossRef]
- Enid Martínez, C.; Martínez-Villegas, N. Copper−Alumina−Organic Matter Mixed Systems: Alumina Transformation and Copper Speciation as Revealed by EPR Spectroscopy. Environ. Sci. Technol. 2008, 42, 4422–4427. [Google Scholar] [CrossRef]
- de Bethune, A.J.; Loud, N.a.S.; King, C.V. Standard Aqueous Electrode Potentials and Temperature Coefficients at 25 °C. J. Electrochem. Soc. 1965, 112, 107C. [Google Scholar] [CrossRef]
- Stokes, R.H.; Stokes, J.M. A Thermodynamic Study of Bivalent Metal Halides in Aqueous Solution. Part XII. Electromotive Force Measurements on Zinc Bromide Solutions. Trans. Faraday Soc. 1945, 41, 688–695. [Google Scholar] [CrossRef]
- Vagramyan, A.T.; Uvarov, L. Mechanism of Electrodeposition of Nickel from Sulfate Solutions; Defense Technical Information Center: Fort Belvoir, VA, USA, 1962.
- Zhong, W.; Tian, Z. Numerical simulations of chemical reaction kinetics based on Simple Collision Theory. Chem. Eng. (CHINA) 2011, 39, 56–59. [Google Scholar]
- Wang, Z.; Liu, Q.; Yu, J.; Wu, T.; Wang, G. Surface Structure and Catalytic Behavior of Silica-Supported Copper Catalysts Prepared by Impregnation and Sol–Gel Methods. Appl. Catal. Gen. 2003, 239, 87–94. [Google Scholar] [CrossRef]
- Pignatello, J.J.; Oliveros, E.; MacKay, A. Advanced Oxidation Processes for Organic Contaminant Destruction Based on the Fenton Reaction and Related Chemistry. Crit. Rev. Environ. Sci. Technol. 2006, 36, 1–84. [Google Scholar] [CrossRef]
- Fang, G.; Liu, C.; Gao, J.; Dionysiou, D.D.; Zhou, D. Manipulation of Persistent Free Radicals in Biochar to Activate Persulfate for Contaminant Degradation. Environ. Sci. Technol. 2015, 49, 5645–5653. [Google Scholar] [CrossRef] [PubMed]
- Ingram, D.J.E.; Tapley, J.G.; Jackson, R.; Bond, R.L.; Murnaghan, A.R. Paramagnetic Resonance in Carbonaceous Solids. Nature 1954, 174, 797–798. [Google Scholar] [CrossRef]
- Lyons, M.J.; Gibson, J.F.; Ingram, D.J.E. Free-Radicals Produced in Cigarette Smoke. Nature 1958, 181, 1003–1004. [Google Scholar] [CrossRef]
- Maskos, Z.; Dellinger, B. Radicals from the Oxidative Pyrolysis of Tobacco. Energy Fuels 2008, 22, 1675–1679. [Google Scholar] [CrossRef]
- Wagner, C.; Fournier, N.; Ruiz, V.G.; Li, C.; Müllen, K.; Rohlfing, M.; Tkatchenko, A.; Temirov, R.; Tautz, F.S. Non-Additivity of Molecule-Surface van Der Waals Potentials from Force Measurements. Nat. Commun. 2014, 5, 5568. [Google Scholar] [CrossRef]
- Zhu, D.; Herbert, B.E.; Schlautman, M.A.; Carraway, E.R.; Hur, J. Cation-Pi Bonding: A New Perspective on the Sorption of Polycyclic Aromatic Hydrocarbons to Mineral Surfaces. J. Environ. Qual. 2004, 33, 1322–1330. [Google Scholar] [CrossRef]
- Söderström, G.; Sellström, U.; de Wit, C.A.; Tysklind, M. Photolytic Debromination of Decabromodiphenyl Ether (BDE 209). Environ. Sci. Technol. 2004, 38, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Vijay, D.; Sastry, G.N. Exploring the Size Dependence of Cyclic and Acyclic π-Systems on Cation–π Binding. Phys. Chem. Chem. Phys. 2008, 10, 582–590. [Google Scholar] [CrossRef]
- Ohura, T.; Kitazawa, A.; Amagai, T.; Makino, M. Occurrence, Profiles, and Photostabilities of Chlorinated Polycyclic Aromatic Hydrocarbons Associated with Particulates in Urban Air. Environ. Sci. Technol. 2005, 39, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Jin, R.; Zheng, M.; Lammel, G.; Bandowe, B.A.M.; Liu, G. Chlorinated and Brominated Polycyclic Aromatic Hydrocarbons: Sources, Formation Mechanisms, and Occurrence in the Environment. Prog. Energy Combust. Sci. 2020, 76, 100803. [Google Scholar] [CrossRef]
- Gao, D. Photo-Activated Molecular Oxygen Mechanism and Biological Toxicity of Chlorophenol/Anthracene-Derived Environmental Persistent Free Radicals. Master’s Thesis, Huazhong Agriculture University, Wuhan, China, 2024. [Google Scholar]
- Boyd, S.A.; Mortland, M.M. Radical Formation and Polymerization of Chlorophenols and Chloroanisole on Copper(II)-Smectite. Environ. Sci. Technol. 1986, 20, 1056–1058. [Google Scholar] [CrossRef]
- Sorokin, A.; Meunier, B. Oxidation of Polycyclic Aromatic Hydrocarbons Catalyzed by Iron Tetrasulfophthalocyanine FePcS: Inverse Isotope Effects and Oxygen Labeling Studies. Eur. J. Inorg. Chem. 1998, 1998, 1269–1281. [Google Scholar] [CrossRef]

| Time (h) | 0 | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| g value | ANT | 2.00315 | 2.00310 | 2.00310 | 2.00307 | 2.00308 | 2.00309 | 2.00308 | 2.00308 | 2.00306 | 2.00305 | 2.00305 | 2.00307 | 2.00308 |
| PAHs | 2.00301 | 2.00330 | 2.00305 | 2.00328 | 2.00321 | 2.00318 | 2.00358 | 2.00318 | 2.00332 | 2.00313 | 2.00316 | 2.00304 | 2.00348 | |
| ΔHp-p(G) | ANT | 4.2 | 2.2 | 2.1 | 2.0 | 2.3 | 1.8 | 2.1 | 2.2 | 2.3 | 2.3 | 2.6 | 2.0 | 2.1 |
| PAHs | 10.3 | 11.6 | 10.2 | 11.1 | 12.8 | 10.3 | 11.0 | 12.4 | 9.6 | 10.1 | 10.1 | 11.1 | 11.4 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Y.; Su, G.; Xu, Y.; Peng, J.; Meng, J.; Li, Q.; Shi, B. Exploration of Influencing Factors and Generation Mechanism of EPFRs in Polycyclic Aromatic Hydrocarbon-Contaminated Soil. Sustainability 2025, 17, 663. https://doi.org/10.3390/su17020663
Liu Y, Su G, Xu Y, Peng J, Meng J, Li Q, Shi B. Exploration of Influencing Factors and Generation Mechanism of EPFRs in Polycyclic Aromatic Hydrocarbon-Contaminated Soil. Sustainability. 2025; 17(2):663. https://doi.org/10.3390/su17020663
Chicago/Turabian StyleLiu, Yaning, Guijin Su, Yulin Xu, Jiahua Peng, Jing Meng, Qianqian Li, and Bin Shi. 2025. "Exploration of Influencing Factors and Generation Mechanism of EPFRs in Polycyclic Aromatic Hydrocarbon-Contaminated Soil" Sustainability 17, no. 2: 663. https://doi.org/10.3390/su17020663
APA StyleLiu, Y., Su, G., Xu, Y., Peng, J., Meng, J., Li, Q., & Shi, B. (2025). Exploration of Influencing Factors and Generation Mechanism of EPFRs in Polycyclic Aromatic Hydrocarbon-Contaminated Soil. Sustainability, 17(2), 663. https://doi.org/10.3390/su17020663








