Abstract
Hydrogen is emerging as a critical component in the global energy transition, providing a low-carbon alternative for sectors such as industry and transportation. This paper aims to comprehensively address water usage in hydrogen production by exploring the water demands of different production methods and their implications for water management, particularly in Texas. Key variables influencing water consumption are identified, and potential water demands under different hydrogen market scenarios are estimated. Using spatial analysis, regions where hydrogen production may stress local water resources are identified, alongside policy recommendations for sustainable water use.
1. Introduction
Hydrogen is emerging as a critical component in the global energy transition, providing a flexible and low-carbon alternative for hard-to-abate applications, including those in the industrial, transportation, and power sectors. As countries strive to meet their decarbonization targets, many include hydrogen as part of their energy policy portfolios, recognizing its potential to serve as a clean energy carrier and chemical feedstock. The hydrogen value chain encompasses a wide range of activities, from production and storage to distribution and utilization, highlighting its versatility and potential as a transformative energy resource, as described in Figure 1.
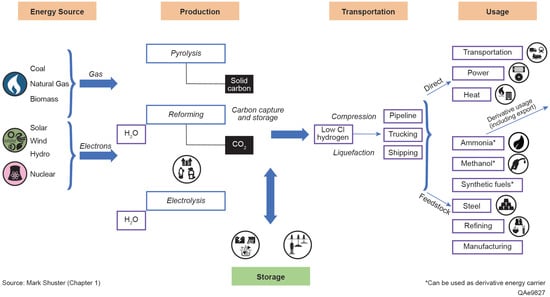
Figure 1.
Hydrogen value chain, based on data and concepts from DOE (2022), with modifications by authors to enhance clarity and provide updated context [].
In the United States, there are 11 million metric tons per annum (MMTPA) of hydrogen production, which translates to Joules of energy, given the energy content of hydrogen is approximately 120 MJ per kilogram. The total U.S. energy system is estimated at 100 quads, where 1 quad equals Joules []. Therefore, hydrogen currently constitutes approximately 1.25% of the total U.S. energy system, mainly serving refining and petrochemicals, including ammonia, apart from a range of smaller industrial uses, including metal production []. Figure 2 shows the current distribution of hydrogen production facilities across the U.S., with capacity indicated by the size of each dot. The Texas-Louisiana Gulf Coast region stands out for its concentration of hydrogen production facilities. This area is intricately connected to the refining and chemical industries and boasts extensive natural gas and hydrogen pipeline networks. The future demand for hydrogen in the U.S. is set to grow in sectors where electrification is challenging, like in hard-to-decarbonize sectors such as heavy-duty transportation, steelmaking, and the production of synthetic fuels for marine and aviation applications. Hydrogen is also expected to play a crucial role in long-duration energy storage and stationary heat and power generation, complementing other decarbonization strategies where electrification is less feasible []. The National Petroleum Council (NPC) study concluded that without hydrogen, the cost of achieving net-zero in the U.S. by year 2050 could increase by an additional 0.5–1% of gross domestic production (GDP) annually, amounting to USD 160–USD 260 billion each year by 2050. Under the net-zero by 2050 scenario, hydrogen demand is projected to increase nearly sevenfold, reaching 75 MMTPA by 2050 from 11 MMTPA in 2021.
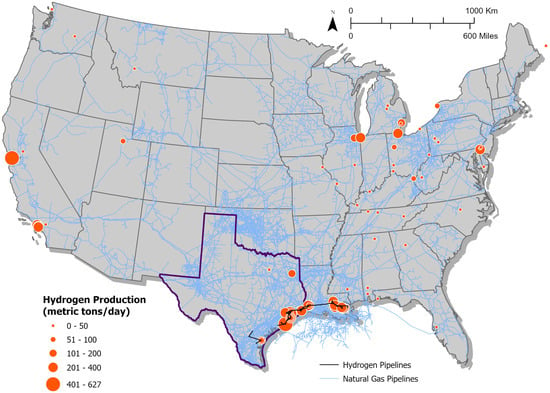
Figure 2.
Hydrogen production and pipelines in the U.S. This map highlights the state of Texas with a distinct bold outline. Note: Sources: Hydrogen production [], natural gas pipelines [], and hydrogen pipelines [,].
To accelerate hydrogen adoption, the U.S. Department of Energy (DOE) launched the Hydrogen Earthshot initiative in June 2021 []. The initiative aims to reduce the cost of clean hydrogen to 1 USD per kilogram by 2032, an 80% reduction from the current average production cost of around USD 5 per kilogram. Complementing this initiative, the Inflation Reduction Act (IRA) introduces the 45V Production Tax Credit (PTC) for low-carbon hydrogen, driving investments in production technologies []. Furthermore, the DOE demonstrated its commitment by announcing USD 8 billion in funding for seven regional clean hydrogen hubs in October 2023 []. These hubs are strategically located to leverage regional strength, including the HyVelocity Hub in Texas. The HyVelocity Hub aims to harness the region’s existing infrastructure and resources to support applications ranging from refining and chemicals to power generation and transportation and has successfully executed a cooperative agreement with the U.S. DOE to receive up to USD 1.2 billion in federal funding to build and expand low-carbon hydrogen and hydrogen infrastructure across Texas and the Gulf Coast in November 2024 []. Other hubs focus on different strengths, such as the Appalachian Hydrogen Hub’s emphasis on natural gas with carbon capture and storage (CCS) and the California Hub’s focus on green hydrogen from renewables. This network of hubs is expected to facilitate hydrogen production, storage, and distribution nationwide, reducing costs and promoting widespread adoption.
Hydrogen energy systems present a dual opportunity for Texas: driving economic growth while addressing decarbonization goals. As a capital-intensive sector, hydrogen development contributes significantly to GDP, tax revenue, and job creation across its value chain, from production and storage to distribution and utilization. For example, the NPC study emphasizes that hydrogen development has the potential to enhance economic resilience by supporting traditional energy sectors while fostering innovation in cleaner technologies. Moreover, the expansion of hydrogen infrastructure has the potential to increase regional competitiveness by creating high-skilled jobs and attracting downstream industries like manufacturing and advanced transportation technologies. However, this economic promise comes with significant resource considerations, particularly for energy and water. Hydrogen production is inherently water-intensive, and this raises critical questions about the sustainability of large-scale deployment in a state already grappling with water management challenges. Texas has long struggled with water scarcity, particularly in its semi-arid western regions, where demands from agriculture, industry, and a growing population have increasingly strained available water supplies. The Texas Water Development Board (TWDB) projects that water demand will continue to rise while available supplies may decline due to factors such as drought and climate change []. In this context, the introduction of new hydrogen projects, particularly in regions like West Texas, could exacerbate resource competition and spark community concerns about equitable water allocation [,].
The NPC study’s societal considerations further emphasize the importance of balancing economic benefits with resource management. While hydrogen development can stimulate local economies and increase public revenues, it must also address potential environmental and social impacts. Communities near hydrogen production sites may express concerns about water use, particularly in regions already experiencing high water stress. Engaging local stakeholders early in the planning process and incorporating their feedback into project design will be crucial for ensuring equitable and sustainable hydrogen development. By considering these societal factors, hydrogen projects can build public trust and foster long-term support. With hydrogen’s significance growing in Texas’ energy transition and economic development, addressing its resource requirements is critical. Understanding and managing these water demands is essential not only for the success of hydrogen projects but also for maintaining the long-term sustainability of Texas’s water resources.
This paper investigates water requirements for hydrogen production and their potential impacts on Texas water resources. The analysis begins with a comprehensive review of water use across different hydrogen production pathways, distinguishing between water withdrawal (the total volume of water drawn from a source) and water consumption (the portion of water permanently used or lost in the process). This review establishes a baseline understanding of water requirements and highlights key variables, such as production methods, water treatment needs, and regional availability, which influence hydrogen’s water footprint. Building on this foundational understanding, the paper estimates future water demand for hydrogen production in Texas under various market scenarios. These projections consider anticipated growth in hydrogen demand and the adoption of diverse production technologies, assessing whether Texas’s water resources can sustainably support the anticipated expansion of the hydrogen industry. In addition, the study will evaluate the local impacts of hydrogen development on water resources at the county level. By utilizing spatial analysis, the research maps the water balance across different regions of Texas, comparing the availability of water resources to the potential locations of hydrogen production facilities. This analysis helps to identify regions where hydrogen development may place additional stress on already water-stressed communities. Finally, the paper will consider the social and environmental implications of increased water use, including potential conflicts with other water users and the long-term sustainability of water resources. This balanced approach aims to provide actionable insights for policymakers and industry leaders seeking to develop Texas’s hydrogen sector sustainably.
2. Literature Review
The relationship between water and energy, often referred to as the “water-energy nexus”, is critical when evaluating the sustainability of hydrogen production []. As hydrogen becomes one of the solutions for the global energy transition, water requirements will become a key factor in the sustainability of hydrogen production [,]. Many local regions currently face water stress, which could be worsened by the added demands of hydrogen production. Hence, hydrogen production facilities must be designed with regional water availability in mind []. Figure 3 categorizes major hydrogen production pathways into fossil fuel-based and renewable sources. Each method has distinct energy and water requirements, influencing its suitability for various applications.
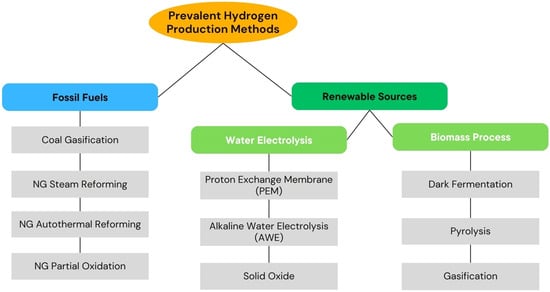
Figure 3.
Hydrogen production methods []. Note: NG refers to natural gas as feedstock. The figure highlights major pathways, illustrating that hydrogen can be produced from multiple primary energy sources and production processes.
While water is required for nearly all hydrogen production methods, the volume consumed varies across different technologies. Figure 4 illustrates the average water withdrawal and consumption for various hydrogen production pathways. Water withdrawal refers to the total amount of water source for the process, while consumption represents the water permanently used (e.g., lost to evaporation or incorporated into products). The whisker plots indicate the variability range in reported values due to differences in operational conditions, technologies, and region locations. This section primarily focuses on articulating the use of water in hydrogen production, the volume of water consumed and withdrawal, the quality standards needed, and source considerations in the hydrogen production pathways.
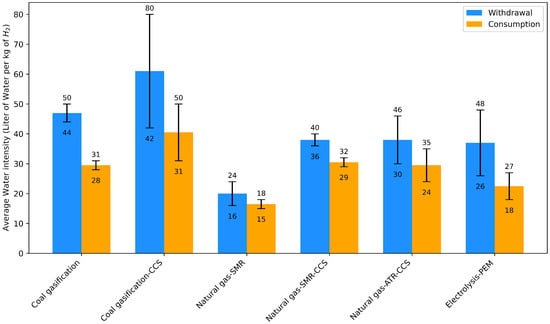
Figure 4.
Average water use (withdrawal and consumption) for hydrogen production pathways (L/kg H2) [,,,]. Note: Withdrawal refers to all the water taken from the source that has a quality similar to tap water. Consumption refers to water that is permanently used up in the process of Hydrogen production (including cooling, lost to evaporation, or incorporated into products).
2.1. Water Use in Hydrogen Production
Coal gasification is one of the most water-intensive hydrogen production technologies. According to the International Renewable Energy Agency (IRENA) report, coal gasification without carbon capture and storage (CCS) consumes approximately 31 L/kg H2 and withdraws about 50 L/kg H2. When equipped with CCS, these figures increase substantially to 49.4 L/kg H2 for consumption and 80.2 L/kg H2 for withdrawal, making it the most water-intensive hydrogen production technology []. The high water requirements make coal gasification with CCS particularly challenging in regions facing water scarcity. For example, a coal gasification hydrogen plant producing 237 kilotons of hydrogen per year with CCS would withdraw about 19 million cubic meters of water annually [].
Figure 5 provides a detailed schematic of natural gas-based hydrogen production pathways, comparing steam methane reforming (SMR) and autothermal reforming (ATR) processes, both with integrated carbon capture and storage (CCS). Key process steps include feedstock pretreatment, primary reforming, the water–gas shift reaction, and hydrogen purification via Pressure Swing Adsorption (PSA). PSA is a gas separation process that isolates hydrogen by adsorbing impurities like CO2, N2, and residual methane under varying pressures. Additional outputs, such as CO2 for sequestration and tail gases, are integrated into heat recovery systems to optimize efficiency. This pathway highlights the critical role of CO2 capture and removal in achieving decarbonization goals for hydrogen production. These processes are water-intensive, as both SMR and ATR require significant volumes of water for various functions, including the water–gas shift reaction, cooling, and solvent regeneration. The water demands are particularly heightened with the inclusion of CCS technologies, which increase cooling and solvent recycling requirements.
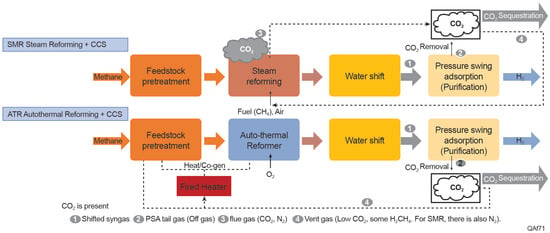
Figure 5.
Natural gas-based hydrogen production pathway. Processes include SMR and ATR with CCS. PSA refers to the “Pressure Swing Adsorption” process used for purification, separating hydrogen from gas streams.
SMR is the dominant method of hydrogen production globally, accounting for approximately 95% of hydrogen generated from fossil fuels. In SMR, methane reacts with steam in an endothermic process, requiring substantial energy inputs and water. The water–gas shift reaction converts CO to CO2 using additional steam, increasing the overall water consumption. Elgowainy et al. (2015) evaluated the connections between hydrogen production and water consumption for both central and distributed SMR facilities. Their analysis, informed by industry data, utilized steam-to-carbon ratios of 2.8 (with a range of 2.5 to 3) for central production and 4.2 (ranging from 4 to 5) for distributed production. Water consumption is 6.43 L/kg H2 for central SMR and 9.46 L/kg H2 for distributed SMR. Additionally, cooling water requirements further increase total water use []. When CCS is added to SMR, water consumption increases primarily due to the additional cooling and solvent regeneration needs in the CCS process. SMR with CCS consumes 32.2 L/kg H2 and withdraws 36.7 L/kg H2 []. Up to 92% of the total water use is attributed to cooling needs, particularly due to the CCS system []. The addition of CCS to SMR processes increases water consumption by approximately 1.8 L/ kg H2 produced []. It is worth noting that water consumption can vary across studies, indicating that there is no standardized design for these processes nor these estimates []. While CCS significantly reduces greenhouse gas emissions, it introduces a trade-off by increasing water and electricity consumption, underscoring the necessity for multi-criteria assessments in life cycle analyses [].
Different from SMR, ATR integrates partial oxidation of methane with steam reforming, utilizing both exothermic and endothermic reactions in a single reactor. ATR has an advantage in terms of energy efficiency due to the autonomous nature of the reactions, which reduces the need for external energy inputs. When combined with CCS, ATR demonstrates lower water consumption compared to SMR with CCS. ATR-CCS has a water consumption intensity of 24.2 L/kg H2 and a withdrawal intensity of 30.8 L/kg H2 []. Despite the lower water consumption compared to SMR-CCS, ATR still requires more water than electrolysis-based methods. A significant portion of the water is used for cooling, and the addition of CCS further increases water requirements, particularly for solvent regeneration and cooling systems []. The implementation of CCS technologies, including ATR+CCS, could exacerbate water stress in regions already experiencing scarcity [], underscoring the importance of considering water requirements and regional water availability when evaluating the feasibility and sustainability of ATR+CCS for hydrogen production. The available data points for ATR are limited due to its relative novelty compared to other methods.
Electrolysis, an alternative to fossil fuel-based hydrogen production, splits water into hydrogen and oxygen shown in Figure 6. This reaction takes place in a unit called an electrolyzer, consisting of an anode and a cathode separated by an electrolyte. Alkaline water (AWE) electrolyzer, polymer electrolyte membrane (PEM) electrolyzer and solid oxide electrolyzer are the common technologies currently, distinguished by the different types of electrolyte material involved and the ionic species it conducts. AWE operate via transport of hydroxide ions (OH-) through the electrolyte from the cathode to the anode with hydrogen being generated on the cathode side, using a liquid alkaline solution of sodium or potassium hydroxide as the electrolyte. AWE for hydrogen production has distinct water requirements and operational characteristics compared to other electrolysis technologies. AWE typically operates at temperatures between 30–90 °C, utilizing a 20–30% concentrated potassium hydroxide (KOH) or sodium hydroxide (NaOH) aqueous solution as the electrolyte []. AWE has a water consumption intensity of 22.3 L/kg H2 and a withdrawal intensity of 32.2 L/kg H2 []. AWE systems require ultrapure make-up water, which is mixed with KOH to achieve the desired electrolyte concentration []. The highly alkaline environment presents unique challenges for water purity maintenance, primarily focusing on particle removal rather than ionic contamination due to the salting-out effect in the concentrated electrolyte []. Typically, AWE systems use lye filters—specialized filtration systems integrated into the deionized water/lye loop—to remove particles and impurities from the highly alkaline solution. These filters prevent clogging and scaling within the electrolyzer, ensuring efficient and stable operation over time.
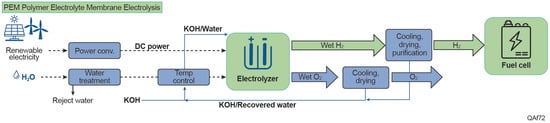
Figure 6.
Electrolysis hydrogen production pathway.
In a PEM electrolyzer, where the electrolyte is a solid specialty plastic material, water reacts at the anode to form oxygen and positively charged hydrogen ions (protons). The electrons flow through an external circuit, and the hydrogen ions selectively move across the membrane to the cathode. At the cathode, hydrogen ions combine with electrons from the external circuit to form hydrogen gas. PEM electrolysis is considered one of the most water-efficient hydrogen production methods. It consumes 17.5 L/kg H2, significantly less than AWE and SMR-CCS systems []. While the theoretical minimum water requirement for electrolysis is 9 L/kg H2, practical applications typically require 20–30 L/kg H2 when accounting for purification and cooling needs []. Similar to AWE, PEM requires ultrapure water but with stricter purity standards to avoid contamination of the proton exchange membrane. PEM electrolysis typically consume 45–55 kWh per kg of hydrogen, equating to 0.16–0.2 L of ultrapure water per kWh [,]. While AWE generally allows for higher conductivity levels (1–5 μS/cm) compared to PEM systems, maintaining water quality remains crucial to both pathways for long-term operational stability and efficiency []. Cooling water demands in electrolysis vary significantly. In smaller or more efficient setups, cooling water use may be minimal. However, in larger-scale industrial operations, particularly those using cooling towers, water consumption can range from 26.5 L per kilogram of hydrogen produced when powered by solar or wind energy to as much as 89 L per kilogram of hydrogen when powered by nuclear energy []. The difference in cooling water usage stems from factors such as the local climate, the efficiency of the electrolyzer, and the specific cooling technology employed. While electrolysis is generally more water-efficient compared to SMR or ATR with CCS, the variability in water use, particularly for cooling, underscores the need for careful water management.
2.2. Water Quality
Water serves essential functions such as cooling, solvent regeneration, and chemical reactions in various hydrogen production processes. The quality and treatment of water significantly affect operational efficiency, costs, and the sustainability of hydrogen production plants. PEM electrolysis, for example, requires ultrapure water to avoid damaging the electrolyzer membrane, necessitating advanced treatment systems such as reverse osmosis (RO) and electrodeionization (EDI). Similarly, SMR and ATR processes require high-purity water to prevent scaling and corrosion in reactors, although their water quality requirements are less stringent than PEM electrolysis. These differences in water quality needs across production methods emphasize the importance of tailored water treatment technologies to meet the specific requirements of each pathway and also impact the total water withdrawal volume versus the water consumption in the process after treatments.
Table 1 summarizes the water quality requirements and treatment methods across different hydrogen production pathways.

Table 1.
Water Quality and Treatment by Hydrogen Production Pathway [,,].
The yield rate of reverse osmosis (RO) systems varies significantly depending on the water quality requirements of different hydrogen production pathways and the nature of the feedwater. For most industrial applications, RO systems typically achieve recovery rates of 75–85%, sufficient for pathways of SMR and ATR where water is used primarily as a reactant or coolant. In these systems, the focus is on balancing water treatment costs with operational efficiency and avoiding operational issues such as scaling or fouling. However, in pathways requiring ultrapure, water recovery rates are often intentionally reduced, sometimes as low as 25–50% []. This is because ultrapure water, with conductivity requirements as low as <0.1 μS/cm [], imposes stringent demands on water treatment systems. While PEM electrolysis demands ultrapure water with conductivity levels as low as <0.1 μS/cm, AWE electrolysis is slightly less stringent, allowing conductivity up to 1–5 μS/cm. Despite these differences, both pathways rely on similar treatment technologies, such as RO and particle filtration, to maintain the required standards. To mitigate these risks, conservative recovery rates are maintained, ensuring that impurities such as chloride ions, heavy metals, and other dissolved solids are effectively removed before the water enters subsequent polishing stages, such as electrodeionization (EDI) [] or mixed-bed ion exchange [,]. Overall, the choice of RO recovery rate reflects a balance between process water quality needs, feedwater characteristics, and operational priorities. These distinctions highlight the trade-offs between water withdrawal, consumption, and treatment costs in hydrogen production. High recovery rates can concentrate impurities in the brine stream, increasing the likelihood of fouling, scaling, or damage to RO membranes, which would compromise the consistency of ultrapure water production.
2.3. Water Source Considerations
The choice of water source for hydrogen production, whether freshwater, groundwater, treated wastewater, brackish water, seawater, or recycled water, has significant implications for operational costs, water treatment requirements, and environmental sustainability. Treatment intensity, recovery rates, and ecological impacts vary based on the source.
Freshwater from rivers, lakes, and reservoirs is the most accessible source but requires treatment to meet the high-purity needs of hydrogen production. While minimal pre-treatment may be sufficient for cooling, more advanced and the most commonly used methods like RO and deionization are necessary for processing water in SMR, ATR, and PEM systems. In water-scarce regions like Texas, reliance on freshwater could exacerbate existing shortages, necessitating careful management to avoid conflicts with agriculture and municipal needs.
Groundwater generally has higher quality than surface water and may require less treatment. However, excessive extraction risks aquifer depletion, particularly in areas already facing water scarcity.
Treated wastewater (reclaimed water) offers a sustainable alternative by reducing pressure on natural water sources. Urban areas where municipal and industrial wastewater is abundant hold potential for hydrogen production. However, treated wastewater often requires additional steps—ultrafiltration, UV treatment, or advanced oxidation—to meet hydrogen production standards. Recovery rates for these systems range from 90% to 95%, depending on the incoming water quality and treatment technologies.
Brackish water (TDS ranging from 1 to 10 g/L) is less saline than seawater but still necessitates desalination. Desalinating brackish water is less energy-intensive than seawater desalination, with recovery rates between 60% and 85%, depending on the technology used. In Texas, brackish water presents a viable alternative, especially when coupled with advanced desalination methods [].
Seawater can be an attractive option for hydrogen production in coastal areas, particularly when desalination plants are already operational or strategically designed to meet the demands of multiple sectors, such as municipalities and industries.
Produced water from Texas’s oil and gas operations also represents a potential water source for hydrogen production. The salinity of produced water often exceeds 100 g/L, far surpassing the levels typically encountered in brackish or even seawater. Additionally, it contains a complex mixture of contaminants such as benzene, toluene, ethylbenzene, and xylene (BTEX), with cumulative concentrations reaching several grams per liter (g/L), as well as ammonia and other impurities [,]. Desalination and purification processes are necessary to meet the required quality standards. Pilot projects in the Midland and Delaware Basins show promise for industrial reuse, but scaling these technologies for hydrogen production in West Texas requires improvements in treatment efficiency and cost []. While treatment is more feasible for lower-salinity water (below 20 g/L), meeting the stringent quality standards needed for hydrogen production, particularly electrolysis, remains a challenge. RO and thermal distillation technologies show potential but are constrained by their high energy demands.
The regional climate also plays a crucial role in shaping the water and cooling requirements for hydrogen production. In colder climates, improved cooling efficiency due to naturally lower ambient temperatures can reduce the withdrawal and consumption of water for cooling systems, especially in water-intensive pathways such as SMR with CCS and PEM electrolysis. However, these advantages must be carefully balanced against challenges unique to colder environments. Hydrogen production facilities in such regions often incur additional costs for winterization to prevent the freezing of equipment, pipelines, and storage tanks []. Furthermore, production facilities in remote or colder regions must consider the increased logistics costs associated with transporting hydrogen to high-demand areas.
The transition from water treatment discussions to source considerations emphasizes the trade-offs inherent in hydrogen production. While treated wastewater and brackish water represent sustainable alternatives, their lower recovery rates and higher treatment intensity introduce additional operational costs. For PEM electrolysis, using brackish or treated wastewater lowers the recovery rate even further to avoid overwhelming the polishing stages with impurities, which could compromise the electrolyzer’s performance and lifespan [,]. In contrast, freshwater sources for SMR and ATR pathways allow for higher recovery rates with less stringent purity demands, though their use in arid regions exacerbates water resource challenges. In balancing water source selection with treatment requirements, a comprehensive strategy must account for regional water availability, feedwater quality, and technology constraints. Regions like Texas, where water scarcity and salinity variability are critical concerns, require innovative approaches, such as integrating brackish water desalination with wastewater reuse or coupling desalination with renewable energy sources to offset energy demands. Ultimately, the choice of water source must balance operational reliability, environmental sustainability, and economic feasibility, aligning with the broader goals of hydrogen production in diverse contexts.
2.4. Fate of Water After Hydrogen Production
The fate of water used in hydrogen production depends on the specific production pathway and the source of water. For coal gasification and SMR/ATR with CCS, water is primarily consumed during the gasification or reforming reactions and in cooling processes. Cooling towers, commonly used in these pathways, account for substantial evaporation losses, representing a major portion of water consumption. Additional wastewater is generated during CCS processes, particularly from solvent regeneration systems, which produce high-salinity or chemical-laden streams. These wastewater streams require advanced treatment, such as RO or thermal distillation, to meet environmental discharge standards or to be recycled within the facility. In some cases, treated wastewater can be reused for non-critical operations like auxiliary cooling or dust suppression, reducing overall withdrawal requirements [,].
For electrolysis-based pathways, particularly PEM and AWE, nearly all consumed water is used in the hydrogen-splitting process, leaving little opportunity for direct recycling. However, the production of ultrapure water required for electrolysis generates rejected streams from RO and deionization systems. These streams can be repurposed for industrial applications like cooling or treated for further reuse, reducing overall waste. Cooling water, when used in electrolysis, is often recycled through closed-loop systems, which minimize withdrawal rates and environmental impacts [,].
The choice of water source also significantly impacts its fate after hydrogen production. Freshwater withdrawal from rivers or lakes may lead to partial returns after treatment but poses risks of depletion and competition with agricultural or municipal uses. Treated wastewater offers a sustainable alternative, with the potential for full recycling within hydrogen production facilities []. However, treated wastewater typically requires additional purification to meet the stringent quality requirements for processes like PEM electrolysis []. Brackish water and seawater, after desalination, produce brine as a byproduct, which must be managed carefully to avoid ecological harm []. Advancements in recycling technologies, integrated water management systems, and careful selection of water sources hold the potential to mitigate water-related impacts of hydrogen production [,].
3. Water Consumption Estimation for Hydrogen Production in Texas
In the current section, we estimate the future water consumption for hydrogen production in Texas under various scenarios based on projected hydrogen demand through 2050. Our analysis considers the water requirements for the production of hydrogen from natural gas with CCS (SMR or ATR) and from PEM electrolysis powered by renewable energy sources, such as solar and wind. These pathways were chosen due to their prominence in large-scale, low-carbon hydrogen production and their distinct water consumption characteristics. The water requirement for hydrogen production for the state is aggregated by combining the projected hydrogen demand and estimated water requirement by pathway.
3.1. Water Consumption and Withdrawal Assumption
To provide transparency in the analysis, Table 2 outlines the assumptions used for different hydrogen production technologies. These values are calculated using simplified average yield rates for process and cooling water. While these values represent a baseline for this analysis, they do not capture the full variability observed across different projects, regions, and operational conditions. Actual water withdrawal and consumption can differ significantly depending on factors such as operational efficiency, local climate, technology-specific configurations, and water recycling measures. The values presented in Table 2 are not definitive or universally applicable. They reflect single assumptions used for this analysis to estimate state-level impacts and are sufficient for providing actionable insights into Texas’s water resource management.

Table 2.
Water amount required by the production path unit: L/kg H2 [], cooling assumptions [].
As shown in Table 2, the average water requirements provide a baseline for comparing the relative efficiency across hydrogen production pathways, particularly when examining both water consumption and withdrawal. While SMR without CCS demonstrates the lowest total water consumption at 16.86 L/kg H2, this pathway conflicts with the broader decarbonization objectives driving the transition to low-carbon hydrogen production. Consequently, such units are likely to be retrofitted with CCS or replaced by alternative, more sustainable technologies. Among the decarbonized options, SMR with CCS emerges as relatively efficient, consuming 26.90 L/kg H2 with a withdrawal of 33.42 L/kg H2. Its slightly lower cooling water demands (1.45 L/kg H2) compared to ATR with CCS (3.33 L/kg H2, total withdrawal of 36.13 L/kg H2) make it a favorable option from a water efficiency perspective. In contrast, PEM electrolysis presents unique challenges, particularly due to its high cooling water demands. While its total consumption of 24.00 L/kg H2 places it in a moderately efficient category, the associated water withdrawal of 52.67 L/kg H2 significantly exceeds that of other pathways. This disparity highlights the importance of cooling water withdrawal in determining the overall water demand and underscores the potential for improvement through advanced cooling and recycling technologies. For PEM systems, the high withdrawal rate is primarily driven by the need for ultrapure water and the corresponding reject streams from reverse osmosis and deionization processes, which contribute to water losses unless effectively recycled. As discussed earlier in Section 2.4, the potential for water recycling plays a critical role in reducing the environmental impact of hydrogen production. Technologies like PEM electrolysis, with high water purity requirements, produce rejected streams that can be repurposed or treated for reuse, offsetting a portion of their water demand. Similarly, advanced closed-loop cooling systems can significantly reduce water withdrawal and evaporation losses across pathways reliant on cooling water.
Despite these opportunities for recycling and efficiency improvements, it is essential to focus on water withdrawal when assessing the water impact of hydrogen production on local communities because it captures the total demand placed on regional water resources, regardless of whether it is returned. In local communities with limited water resources, water withdrawal provides a more accurate representation of the stress placed on local water systems. High withdrawal rates can exacerbate resource scarcity, especially during drought periods or in areas where aquifers and surface water systems are already overexploited. While some water may be returned to the source after use, the initial withdrawal can create temporary shortages and disrupt water availability for competing needs, including agriculture, municipal supply, and ecosystem health. Moreover, water withdrawal reflects the strain placed on local infrastructure, such as treatment facilities and distribution systems. High volumes of extraction, even when partially recycled, can overwhelm these systems, leading to operational challenges and increased costs for local communities. Environmental impacts, such as reduced river flow, habitat alteration, or changes in water temperature, are also tied more closely to withdrawal rates than to net consumption.
3.2. Water Cost and Its Impact on the Levelized Cost of Hydrogen
Water treatment costs depend on the source of the water and the required quality. Using brackish water or treated wastewater can reduce costs but requires more sophisticated treatment facilities. Conversely, using high-quality freshwater minimizes treatment costs but raises concerns about sustainability, particularly in water-stressed regions like Texas.
For example, the use of brackish water, though more sustainable, can add USD 0.05 to USD 0.10 per gallon to the water cost due to desalination or other treatment processes. Cooling water is necessary for both ATR with CCS and large-scale electrolysis plants. The cost of cooling water can vary depending on the system’s efficiency and the cooling technology used (e.g., cooling towers versus dry cooling). Figure 7 shows the breakdown of the levelized cost of hydrogen production for both ATR with CCS and wind-based PEM in 2021 dollars []. First of all, the water cost can impact the levelized cost of hydrogen production for SMR and ATR with CCS. As the water unit cost increases, the levelized cost of hydrogen also rises. More specifically, a difference of half a cent per unit of water can result in about a 5-cent difference in the levelized cost of hydrogen per kg, according to Kowal et al. (2024) for wind-based electrolyzer production in Texas. The total cost of water, excluding equipment, accounts for approximately 2% of the total levelized cost of hydrogen. This relatively small impact of water on overall costs creates an opportunity for hydrogen producers to consider alternative water sources, such as treated-produced water or brackish water, to align with sustainability or emissions reduction goals. By prioritizing alternative water sources, hydrogen producers can balance cost considerations with environmental criteria, reducing their impact on freshwater resources in water-stressed regions. Investments in advanced treatment technologies and recycling systems could further enable the use of less conventional water sources without significantly affecting production economics. This approach highlights the potential for hydrogen projects to incorporate sustainable water practices while maintaining economic viability.
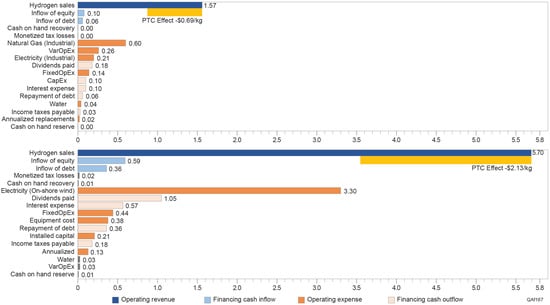
Figure 7.
Real levelized cost breakdown of hydrogen production for ATR with CCS (left) and wind-based PEM electrolysis (right) in 2021 dollars. Note: The year reference reflects constant dollar values adjusted for inflation to maintain comparability across time. The ’PTC Effect’ (yellow bar) represents the impact of a Production Tax Credit, reducing the levelized cost of hydrogen production. Costs are categorized into operating revenues (blue), operating expenses (orange), financing cash inflows (light blue), and financing cash outflows (light orange). The data and assumptions for the calculation references from Lin et al. (2024), Kowal et al. (2024), and the National Renewable Energy Laboratory H2A-lite hydrogen production model [,,,].
3.3. Aggregated Water Demand for Hydrogen in Texas
To estimate the total water withdrawal for hydrogen production in Texas, we first need to determine how much hydrogen is projected to be produced in the state. Given the uncertainties surrounding this emerging sector, we adopt a scenario-based approach, referencing the NPC study [], which provides two scenarios: the more aggressive net-zero scenario and the more conservative stated policy scenario. These scenarios explore different trajectories for hydrogen demand in the United States, with the net-zero scenario assuming the U.S. achieves carbon neutrality by 2050 and the stated policy scenario reflecting currently implemented policies without additional decarbonization commitments. Under these scenarios, hydrogen demand in the Gulf Coast region (encompassing Texas and Louisiana) is projected to grow significantly. The net-zero scenario forecasts an increase from 14.2 MMTPA in 2030 to 44.3 MMTPA by 2050, shown in Figure 8, while the stated policy scenario anticipates growth from 9.5 MMTPA in 2030 to 30.1 MMTPA by 2050. Since intra-regional trade for hydrogen is expected to remain minimal, the Gulf Coast’s demand is assumed to be met entirely by local production. This assumption is particularly reasonable for the Gulf Coast region, which benefits from abundant natural gas, renewable energy potential, and extensive existing infrastructure, making it highly competitive for hydrogen production.
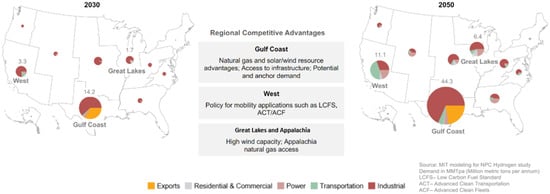
Figure 8.
NPC study projected hydrogen demand by region in the net-zero scenario [].
Considering water withdrawal varies greatly across different pathways, discussed in Section 2 and Table 2, the following three production mix scenarios are considered for both the net-zero and stated policy scenarios:
- Scenario 1: 80% blue hydrogen (SMR/ATR + CCS) and 20% green hydrogen (electrolysis).
- Scenario 2: 20% blue hydrogen and 80% green hydrogen.
- Scenario 3: 50% blue hydrogen and 50% green hydrogen.
To further refine the analysis, we applied a static split between Texas and Louisiana to divide regional demand into state-level projections. This division is based on the current hydrogen production capacities of the two states, as indicated in recent studies []. While this method simplifies the dynamics between the states, it provides a reasonable estimate of future hydrogen demand for Texas. Such an approach is sufficient for this study, which focuses on the water impacts of hydrogen production rather than a detailed exploration of state-level hydrogen trade or production dynamics.
Once total hydrogen production for Texas is established, it becomes essential to differentiate production pathways to estimate water requirements accurately. For simplicity and clarity, we use the common color-based terminology []: blue hydrogen, derived from natural gas through SMR or ATR with CCS, and green hydrogen, produced via electrolysis powered by renewable electricity. More specifically, we use ATR with CCS to represent the water withdrawal for blue hydrogen and wind-based PEM to represent that for green hydrogen. For this analysis, the color-based terminology serves to streamline the presentation of results. At the same time, it is important to recognize that such categorization can oversimplify significant differences in emissions, water requirements, and other factors depending on the specific technology designs and configurations.
To assess the water impact of hydrogen production for the state, we calculate the total water withdrawal for each scenario by combining the projected hydrogen production for each pathway with the corresponding withdrawal assumptions from Table 2. For a given scenario, the total water withdrawal is determined using the following equation:
Note: i refers to the hydrogen production pathway (blue or green), and n is the number of pathways considered in the scenario.
The total water withdrawal is calculated in liters and converted to cubic meters for consistency with international standards.
Figure 9 summarizes the resulting estimated water withdrawal for each net-zero and stated policy hydrogen production scenario. As hydrogen demand increases over time, water withdrawal follows suit, with stated policy scenarios ranging from 0.2 to 0.23 billion m3 in 2030 and rising to 0.4 to 0.44 billion m3 of water by 2050. In comparison, net-zero scenarios require 0.35 to 0.39 billion m3 in 2030, escalating significantly to 1.2 to 1.5 billion m3 of water by 2050.
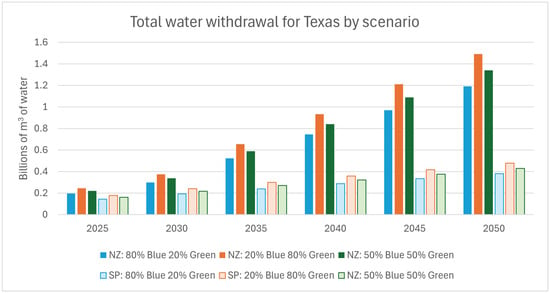
Figure 9.
Estimated water withdrawal by scenario for hydrogen production in Texas. NZ: net-zero scenario; SP: stated policy scenario.
3.4. Texas Water Demand Projections
To thoroughly assess the water impacts of hydrogen production on Texas, it is essential to account for the state’s overall water demand trends and future projections and to assess whether total water withdrawal from hydrogen production could become a significant portion of Texas’s water resources, particularly in scenarios pursuing aggressive hydrogen production for decarbonization.
Every five years, the Texas Water Development Board (TWDB) releases a comprehensive state water plan that forecasts water supply and demand for each decade over the next 50 years. For these projections, the TWDB collaborates with regional water planning groups, incorporating data on population growth, economic trends, and historical water use across sectors such as agriculture, industry, and municipal services. The 2026 Texas State Water Plan estimates annual water demand across these sectors will range from 21.4 billion m3 in 2030 to 21.9 billion m3 in 2050 [], as detailed in Table 3. While irrigation demand is expected to decline, municipal water demand is projected to rise due to population growth.

Table 3.
2026 Texas State Water Plan’s Regional Water Demand Projections for 2030–2050 by demand sector as percentages of total water demand in Texas [].
When compared to the TWDB’s water demand projections, the hydrogen industry’s water withdrawal is estimated to account for approximately 1% to 1.8% of Texas’s total water demand by 2030, potentially increasing to 2.0% to 6.8% by 2050 across both net-zero and stated policy scenarios, for the 50% blue and 50% green supply mix. These percentages are comparable to the water demand of the livestock sector by 2030 and the combined demands of the livestock and steam electric power sectors by 2050, as shown in Table 3. Table 3 presents the projected water demand for Texas by sector, as provided by the TWDB, alongside the estimated water withdrawal for the hydrogen sector. The original percentages for demand sectors reflect raw data directly from the TWDB’s projections without integrating the water withdrawal from future hydrogen production. The hydrogen sector’s percentages, calculated independently, are shown separately to allow a direct comparison of its water withdrawal relative to other sectors. Adding hydrogen’s water withdrawal to the total would slightly adjust the percentages of the other sectors. However, this adjustment is not made here to maintain the integrity of the original TWDB data. Furthermore, it is important to note that existing hydrogen production, which is currently accounted for within sectors like manufacturing, is not included in the hydrogen sector estimate. The fate of existing plants—whether retrofitted with CCS, shut down, or operated without modification—introduces uncertainties. Retrofitted plants may result in minor double-counting if included in new hydrogen production estimates. However, these uncertainties do not significantly impact the broader narrative of hydrogen’s water withdrawal compared to the state’s overall water demand. Table 3 allows for a clear and consistent comparison while highlighting the emerging role of hydrogen in Texas’s water resource planning. This analysis suggests that the hydrogen industry’s water withdrawal will be moderately considerable in the near future, requiring careful planning to ensure that hydrogen production scales sustainably while preserving water availability for other essential sectors.
4. Locational Consideration and Water Management Strategy for Texas
Although the aggregated usage of water from hydrogen is not as considerable as other sectors like agriculture and municipal services, it could still impact local water balances as water availability varies across Texas. Locational consideration of water by hydrogen and related projects is important, particularly for counties with a high concentration of new projects or already-strained water resources.
In their state-wide water plan, TWDB identifies regions that might face shortages and provides water management strategy plans, including water conservation, drought management, and aquifer restoration strategies to mitigate water shortages. It is important to note that the term “shortage” here does not imply inevitable future water deficits; rather, it highlights regions where additional infrastructure or management interventions may be needed to meet projected water demand (refer to Section 3.4 and Table 3). Their findings project an average statewide water shortage of approximately 6.17 billion m3 by 2040 [,]. However, there are notable regional variations at the county level, as shown in Figure 10. While some counties are projected to face water shortages if no new source is identified, other counties may face a water surplus. Large metropolitan cities such as Austin, Houston, and the Dallas-Ft. Worth metropolitan areas are expected to encounter significant water shortages due to projected population growth. In contrast, northern Texas near the city of Amarillo shows a regional water surplus, driven by a projected decrease in irrigation demand, minimal population growth, and the availability of larger water reservoirs.
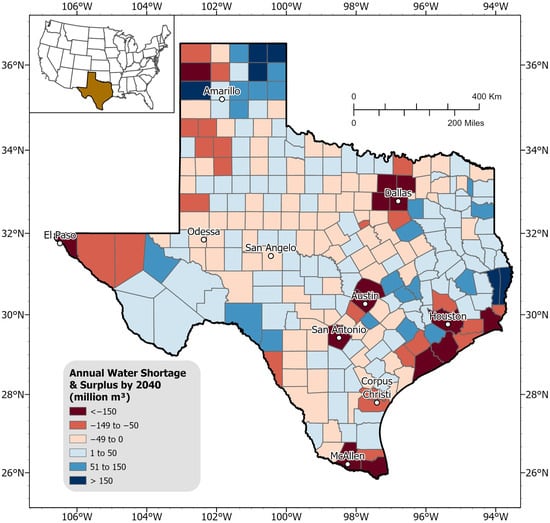
Figure 10.
Projected annual water needs (in terms of shortage and surplus) per county by 2040 (units: million m3). Shades of red indicate areas of projected water shortage, while shades of blue represent water surplus. Deeper shades of red or blue correspond to more severe shortages or surpluses, respectively. Note: Water supply projection data was obtained from the TWDB’s 2022 State Water Plan [], while the water demand projection data were sourced from their newest 2026 State Water Plan []. Fresh groundwater supply projections are considered only.
The Gulf Coast region of Texas currently hosts a well-established hydrogen market, with facilities and pipeline infrastructure primarily centered around the city of Houston, as shown in Figure 11. This area features one of the longest hydrogen pipeline networks in the country, spanning approximately 1900 km in length [], and supports around 5 million kg per day of hydrogen production capacity []. The HyVelocity Hub, as one of seven Regional Clean Hydrogen Hubs in the USA, is centered around the Great Houston area and will leverage existing hydrogen infrastructure, including pipelines and production facilities, to expand the hydrogen market []. The HyVelocity hub already has at least four new proposed clean hydrogen facilities in the area. In addition, southwest of Houston, there are more proposed facilities near Corpus Christi, in southern Texas, plans to produce, store, and build new pipelines to transport up to 280,000 metric tons of green hydrogen annually [].
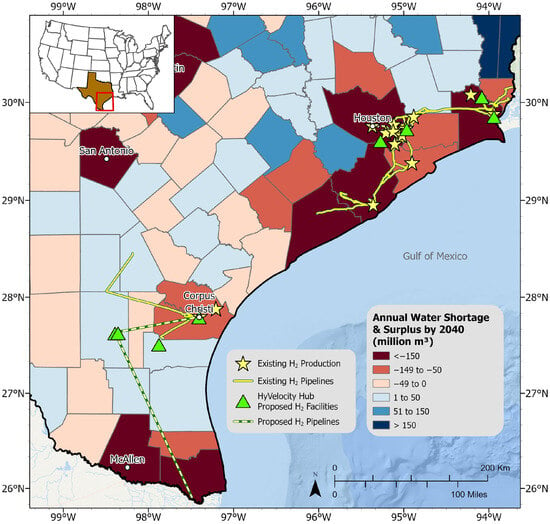
Figure 11.
Existing and proposed hydrogen infrastructure as of 2024 in the Gulf Coast region of Texas, USA overlaying projected water needs by 2040 per county. Note: Existing hydrogen sources from 2016 H2Tools database []. Proposed clean hydrogen facilities include both green (production tech: electrolysis) and blue (production tech: ATR or SMR with CCS) production facilities. Existing hydrogen pipelines sourced from the Texas Department of Transportation []. Proposed hydrogen pipelines sourced from Hydrogen City’s official project website [].
Figure 11 highlights the importance of regional water resource management for sustainable hydrogen infrastructure growth in the Gulf Coast region of Texas, USA. Most existing and proposed hydrogen production infrastructures are within projected water-strained cities and counties, such as Houston in Harris County and Corpus Christi in Nueces County. As the market expands, facilities may need to rely increasingly on desalination or treatment of lower-quality water, such as brackish water, or arrange for transportation from alternative sources from nearby counties to meet their water needs.
Historically, the distribution of hydrogen infrastructure has closely followed industrial demand, particularly in the petrochemical and refining sectors. For example, the Gulf Coast region’s extensive hydrogen infrastructure reflects its established role as a hub for these industries rather than a direct correlation to population or general economic activity. Looking ahead, this pattern may evolve as hydrogen applications expand into areas such as transportation and residential or commercial energy use. These new applications could lead to hydrogen infrastructure aligning more closely with population centers and regions of economic activity, where demand for hydrogen-based solutions like fuel cells or gas blending is higher. However, the primary driver for hydrogen distribution will likely remain tied to resource availability, cost considerations, and industrial policy. For instance, hydrogen hubs in other parts of the U.S., such as Appalachia or California, highlight diverse strategies driven by regional strengths like natural gas reserves or renewable energy potential. This suggests that future hydrogen distribution will prioritize resources and infrastructure, with population and economic activity serving as secondary influences.
5. Conclusions
This study provides a comprehensive analysis of the water requirements for hydrogen production in Texas, emphasizing the critical role of water management in the state’s energy transition. While overall water withdrawal from hydrogen production is projected to account for a considerable 2.0% to 6.8% of Texas’s total water demand by 2050, its localized impact could be more pronounced in regions already facing water scarcity.
5.1. Key Findings
Water requirement varies significantly across production pathways. Blue hydrogen, referring to natural gas-based hydrogen with CCS, withdraws 33–36 L/kg and is expected to dominate early deployment due to its reliance on existing infrastructure and lower costs. Green hydrogen, which requires 52 L/kg for the wind-based PEM electrolysis, will likely play a larger role as costs decline and decarbonization goals intensify. However, its higher water intensity makes it less suitable for water-stressed regions, necessitating careful planning.
Water quality is another critical factor. PEM electrolysis requires ultrapure water, demanding advanced treatment systems such as reverse osmosis and electrodeionization. SMR and ATR processes, while requiring lower water purity, still necessitate significant treatment to prevent scaling and corrosion. These differences in water quality needs highlight the importance of technology-specific water management strategies.
Although hydrogen’s total water demand appears moderately considerable compared to other water demand sectors like agriculture and manufacturing, its local impact can be prominent. For instance, planned HyVelocity Hub facilities are located in water-stressed Gulf Coast counties, emphasizing the importance of careful site selection and regional water management to ensure sustainability.
5.2. Policy Recommendations and Measures
To balance hydrogen development with sustainable water use, the following measures are recommended:
- Integrate hydrogen into regional water planning: Collaborate with the TWDB and local water boards to prioritize regions with surplus water availability for hydrogen projects.
- Promote water recycling and reuse: Incentivize the use of treated wastewater or brackish water for hydrogen production, particularly in water-scarce regions.
- Conduct water use audits: Require comprehensive assessments of water footprints as part of hydrogen project proposals to align production with local water availability.
- Encourage efficient technologies: Develop policies that support the adoption of water-efficient hydrogen production methods, especially for green hydrogen.
- Engage local community: Early and transparent engagement with affected communities can build trust and support for hydrogen development.
Hydrogen development presents a significant opportunity for economic growth and decarbonization, but it requires careful management of natural resources, particularly water. A regionally tailored, forward-looking approach will help Texas balance hydrogen development with sustainable water use.
5.3. Limitations and Future Research
This study is based on projected hydrogen demand and assumptions about the future development of the sector. Technological advancements, policy changes, and market dynamics could significantly alter water usage estimates. Future research should refine these projections using life cycle analyses and explore water-saving technologies to reduce the sector’s water footprint. Additionally, local social, economic, and environmental impacts should be assessed to ensure equitable and sustainable hydrogen deployment.
Author Contributions
Conceptualization, N.L. and J.-P.N.; methodology, N.L.; software, N.L.; validation, N.L., M.A. and E.R.C.; formal analysis, N.L.; investigation, N.L.; resources, J.-P.N.; data curation, N.L.; writing—original draft preparation, N.L.; writing—review and editing, M.A. and E.R.C.; visualization, N.L.; supervision, J.-P.N.; project administration, N.L.; funding acquisition, J.-P.N. All authors have read and agreed to the published version of the manuscript.
Funding
This work was partially supported by the State of Texas Advanced Resource Recovery (STARR) program and the GeoH2 industrial affiliates program at the Bureau of Economic Geology (BEG).
Institutional Review Board Statement
Not applicable. This study did not involve humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data supporting the reported results were obtained from publicly available sources.
Acknowledgments
This article is an extended version of a presentation entitled Lin, M.; Arzumanyan, M.; Rodriguez Calzado, E.; Childers, G.L. “Hydrogen Energy Value Chain and Impacts on Water” at 2024 Texas Groundwater Summit, San Antonio, TX, USA, 20 August 2024 [].
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| Abbreviation | Definition |
| ATR | autothermal reforming |
| AWE | alkaline water electrolysis |
| CCS | carbon capture and storage |
| CO2 | carbon dioxide |
| DOE | United States Department of Energy |
| EDI | electrodeionization |
| GDP | gross domestic product |
| GHG | greenhouse gas |
| GREET | greenhouse gases, regulated emissions, and energy use in technologies (model) |
| H2 | molecular hydrogen |
| IRENA | International Renewable Energy Agency |
| kWh | kilowatt-hour |
| KOH | potassium hydroxide |
| MMTPA | million metric tons per annum |
| NaOH | sodium hydroxide |
| NPC | National Petroleum Council |
| PEM | proton exchange membrane |
| ppm | parts per million |
| PTC | production tax credit |
| RO | reverse osmosis |
| SMR | steam methane reforming |
| TDS | total dissolved solids |
| TWDB | Texas Water Development Board |
| µS/cm | microsiemens per centimeter (a unit of conductivity) |
References
- U.S. Department of Energy. DOE National Clean Hydrogen Strategy and Roadmap; U.S. Department of Energy: Washington, DC, USA, 2022.
- Lawrence Livermore National Laboratory. Estimated U.S. Energy Consumption in 2022; Lawrence Livermore National Laboratory: Livermore, CA, USA, 2023.
- Department of Energy. Hydrogen Shot; Department of Energy: Washington, DC, USA, 2021.
- National Petroleum Council. HARNESSING HYDROGEN—A Key Element of the U.S. Energy Future; National Petroleum Council: Washington, DC, USA, 2024. [Google Scholar]
- Hydrogen Analysis Resource Center. H2 Tools. In North America Merchant Hydrogen Plants by City; Hydrogen Analysis Resource Center: Washington, DC, USA, 2016. [Google Scholar]
- Energy Information Administration (EIA). Natural Gas Interstate and Intrastate Pipelines. In U.S. Energy Atlas; Energy Information Administration (EIA): Washington, DC, USA, 2023. [Google Scholar]
- Texas Department of Transportation (TxDOT). Statewide Pipelines GIS Data; Data originally from Railroad Commission; Texas Department of Transportation (TxDOT): Austin, TX, USA, 2022.
- RBN Energy. U.S. Gulf Coast Hydrogen Infrastructure Map; RBN Energy: Houston, TX, USA, 2024. [Google Scholar]
- Miller, D.E.L. The Hydrogen Energy Earthshot and H2@Scale: Importance to Industrial Decarbonizatio; U.S. Department of Energy: Washington, DC, USA, 2022.
- US Treasury. Section 45V Credit for Production of Clean Hydrogen; Section 48(a)(15) Election To Treat Clean Hydrogen Production Facilities as Energy Property. In Federal Register; Government Publishing Office: Washington, DC, USA, 2023. [Google Scholar]
- The White House. Biden-Harris Administration Announces Regional Clean Hydrogen Hubs to Drive Clean Manufacturing and Jobs; The White House: Washington, DC, USA, 2023.
- U.S. Department of Energy. Biden-Harris Administration Announces Awards for Up to $2.2 Billion for Two Regional Clean Hydrogen Hubs to Bolster America’s Global Clean Energy Competitiveness and Strengthen Our National Energy Security; U.S. Department of Energy: Washington, DC, USA, 2024.
- Texas Water Development Board. Sixth Cycle of Regional Water Planning Cycle Information; Texas Water Development Board: Austin, TX, USA, 2023.
- Robles, C. Texas Water Fight Shows Pushback on ‘Clean’ Hydrogen; FCHEA: Washington, DC, USA, 2024. [Google Scholar]
- Baddour, D. Water Scarcity and Clean Energy Collide in South Texas; The Texas Tribune: Austin, TX, USA, 2024. [Google Scholar]
- Hamiche, A.M.; Stambouli, A.B.; Flazi, S. A review of the water-energy nexus. Renew. Sustain. Energy Rev. 2016, 65, 319–331. [Google Scholar] [CrossRef]
- Lampert, D.J.; Cai, H.; Wang, Z.; Keisman, J.; Wu, M.; Han, J.; Dunn, J.; Sullivan, J.L.; Elgowainy, A.; Wang, M.; et al. Development of a Life Cycle Inventory of Water Consumption Associated with the Production of Transportation Fuels; U.S. Department of Energy: Washington, DC, USA, 2015. [CrossRef]
- Elaouzy, Y.; Fadar, A.E. Water-energy-carbon-cost nexus in hydrogen production, storage, transportation and utilization. Int. J. Hydrogen Energy 2024, 53, 1190–1209. [Google Scholar] [CrossRef]
- Lee, U.; Xu, H.; Daystar, J.; Elgowainy, A.; Wang, M.Q. AWARE-US: Quantifying water stress impacts of energy systems in the United States. Sci. Total Environ. 2019, 648, 1313–1322. [Google Scholar] [CrossRef]
- Kumar, S.S.; Himabindu, V. Hydrogen production by PEM water electrolysis—A review. Mater. Sci. Energy Technol. 2019, 2, 442–454. [Google Scholar] [CrossRef]
- Sun, P.; Elowainy, A. Water Consumption in Hydrogen Production Processes. 2019. Available online: https://greet.anl.gov/files/smr_h2_2019 (accessed on 23 December 2023).
- Katakam, V.S.S.; Bahadur, V. Reverse osmosis-based water treatment for green hydrogen production. Desalination 2024, 581, 117588. [Google Scholar] [CrossRef]
- National Energy Technology Laboratory. Comparison of Commercial State-of-Art Fossil-Based Hydrogen Production Technologies; Technical report; U.S. Department of Energy: Washington, DC, USA, 2022.
- IRENA; Bluerisk. Water for Hydrogen Production; International Renewable Energy Agency, Bluerisk: Abu Dhabi, United Arab Emirates, 2023. [Google Scholar]
- Midilli, A.; Kucuk, H.; Topal, M.E.; Akbulut, U.; Dincer, I. A comprehensive review on hydrogen production from coal gasification: Challenges and Opportunities. Int. J. Hydrogen Energy 2021, 46, 25385–25412. [Google Scholar] [CrossRef]
- Elgowainy, A.; Wu, M.; Lampert, D.; Cai, H.; Han, J.; Wang, M. Life-Cycle Analysis of Water Consumption for Hydrogen Production Pathways. 2014 DOE Hydrogen and Fuel Cells Program Annual Merit Review; U.S. Department of Energy: Washington, DC, USA, 2014.
- Mehmeti, A.; Angelis-Dimakis, A.; Arampatzis, G.; McPhail, S.J.; Ulgiati, S. Life Cycle Assessment and Water Footprint of Hydrogen Production Methods: From Conventional to Emerging Technologies. Environments 2018, 5, 24. [Google Scholar] [CrossRef]
- Rosa, L.; Mazzotti, M. Potential for hydrogen production from sustainable biomass with carbon capture and storage. Renew. Sustain. Energy Rev. 2022, 157, 112123. [Google Scholar] [CrossRef]
- Webber, M.E. The water intensity of the transitional hydrogen economy. Environ. Res. Lett. 2007, 2, 034007. [Google Scholar] [CrossRef]
- Ansar, A.S.; Gago, A.S.; Razmjooei, F.; Reißner, R.; Xu, Z.; Friedrich, K.A. Chapter 5—Alkaline electrolysis—Status and prospects. In Electrochemical Power Sources: Fundamentals, Systems, and Applications; Smolinka, T., Garche, J., Eds.; Elsevier: Amsterdam, The Netherlands, 2022; pp. 165–198. [Google Scholar] [CrossRef]
- Yu, Z.Y.; Duan, Y.; Feng, X.Y.; Yu, X.; Gao, M.R.; Yu, S.H. Clean and Affordable Hydrogen Fuel from Alkaline Water Splitting: Past, Recent Progress, and Future Prospects. Adv. Mater. 2021, 33, e2007100. [Google Scholar] [CrossRef] [PubMed]
- Wang, T.; Cao, X.; Jiao, L. PEM water electrolysis for hydrogen production: Fundamentals, advances, and prospects. Carbon Neutrality 2022, 1, 21. [Google Scholar] [CrossRef]
- Simon, A.; Daily, W.; White, R. Hydrogen and Water: An Engineering, Economic and Environmental Analysis; U.S. Department of Energy: Washington, DC, USA, 2010. [CrossRef][Green Version]
- Madsen, H.T. Water Treatment for Green Hydrogen: What You Need to Know. 2022. Available online: https://hydrogentechworld.com/water-treatment-for-green-hydrogen-what-you-need-to-know (accessed on 10 October 2024).
- Haug, P.; Kreitz, B.; Koj, M.; Turek, T. Process modelling of an alkaline water electrolyzer. Int. J. Hydrogen Energy 2017, 42, 15689–15707. [Google Scholar] [CrossRef]
- Niekerk, R.; Manita, R. Thermal Management in Green Hydrogen Production: Design Considerations. 2022. Available online: https://hydrogentechworld.com/thermal-management-in-green-hydrogen-production-design-considerations (accessed on 10 October 2024).
- Hamer, P. Present-Day Feed-Water Treatment for High-Pressure Boilers. Proc. Inst. Mech. Eng. 1960, 174, 69–94. [Google Scholar] [CrossRef]
- Layman, C.M. Boiler Water Chemistry: Getting From the Source to the Boiler. Available online: https://www.cibomembers.org/wp-content/uploads/2017/05/EE_June2017_Layman-Presentation.pdf (accessed on 10 October 2024).
- Shokri, A.; Sanavi Fard, M. Principles, operational challenges, and perspectives in boiler feedwater treatment process. Environ. Adv. 2023, 13, 100389. [Google Scholar] [CrossRef]
- Mässgård, H.; Jonsson, A. An Industrial Perspective on Ultrapure Water Production for Electrolysis: A Techno-Economic Assessment of Membrane Distillation for Electrolysis—Synergies, Performance, Costs, and Value Propositions; KTH: Stockholm, Sweden, 2021. [Google Scholar]
- Kumar, P.; Date, A.; Mahmood, N.; Kumar Das, R.; Shabani, B. Freshwater supply for hydrogen production: An underestimated challenge. Int. J. Hydrogen Energy 2024, 78, 202–217. [Google Scholar] [CrossRef]
- Kjellsson, J.B.; Webber, M.E. The Energy-Water Nexus: Spatially-Resolved Analysis of the Potential for Desalinating Brackish Groundwater by Use of Solar Energy. Resources 2015, 4, 476–489. [Google Scholar] [CrossRef]
- Kar, A.; Bahadur, V. Using excess natural gas for reverse osmosis-based flowback water treatment in US shale fields. Energy 2020, 196, 117145. [Google Scholar] [CrossRef]
- Kharaka, Y.K.; Hanor, J.S. 7.14—Deep Fluids in Sedimentary Basins. In Treatise on Geochemistry, 2nd ed.; Holland, H.D., Turekian, K.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 471–515. [Google Scholar] [CrossRef]
- Texas Produced Water Consorium. Beneficial Use of Produced Water in Texas—Texas Produced Water Consortium Report to the Texas Legislature 2024; Texas Produced Water Consorium: Lubbock, TX, USA, 2024. [Google Scholar]
- Oyewunmi, T. Resilience, reliability and gas to power systems in the USA: An energy policy outlook in the era of decarbonization. J. World Energy Law Bus. 2021, 14, 257–276. [Google Scholar] [CrossRef]
- Wang, J.; Cai, S.; Chen, R.; Tu, Z.; Li, S. Operation strategy optimization of an integrated proton exchange membrane water electrolyzer and batch reverse osmosis desalination system powered by offgrid wind energy. Energy Convers. Manag. 2024, 22, 100607. [Google Scholar] [CrossRef]
- Sztekler, K.; Kalawa, W.; Bujok, T.; Boruta, P.; Radomska, E.; Mika, L.; Mlonka-Medrala, A.; Nowak, W.; Sloma, J.; Wojcikowski, A.; et al. Hybrid desalination system for Baltic Sea water: A preliminary study. Desalination 2024, 574, 117269. [Google Scholar] [CrossRef]
- Nemitallah, M.A.; Alnazha, A.A.; Ahmed, U.; El-Adawy, M.; Habib, M.A. Review on techno-economics of hydrogen production using current and emerging processes: Status and perspectives. Results Eng. 2024, 21, 101890. [Google Scholar] [CrossRef]
- Gutierrez, W.; Enrique, F. A Comparative Study of Low-Emissions Hydrogen Production Processes: Technical Limitations and Future Trends; Aalto University: Espoo, Finland, 2023. [Google Scholar]
- Yun, S.; Lee, J.; Cho, H.; Kim, J. Oxy-fuel combustion-based blue hydrogen production with the integration of water electrolysis. Energy Convers. Manag. 2023, 291, 117275. [Google Scholar] [CrossRef]
- Qin, B.; Wang, H.; Li, F.; Liu, D.; Liao, Y.; Li, H. Towards zero carbon hydrogen: Co-production of photovoltaic electrolysis and natural gas reforming with CCS. Int. J. Hydrogen Energy 2024, 78, 604–609. [Google Scholar] [CrossRef]
- Tang, Y.; Li, Y. Comparative analysis of the levelized cost of hydrogen production from fossil energy and renewable energy in China. Energy Sustain. Dev. 2024, 83, 101588. [Google Scholar] [CrossRef]
- Rahbari, A.; Shirazi, A.; Venkataraman, M.B.; Pye, J. Solar fuels from supercritical water gasification of algae: Impacts of low-cost hydrogen on reformer configurations. Appl. Energy 2021, 288, 116620. [Google Scholar] [CrossRef]
- Ghernaout, D. Brine Recycling: Towards Membrane Processes as the Best Available Technology. Appl. Eng. 2019, 3, 71–84. [Google Scholar] [CrossRef]
- Yan, Y.; Manovic, V.; Anthony, E.J.; Clough, P.T. Techno-economic analysis of low-carbon hydrogen production by sorption enhanced steam methane reforming (SE-SMR) processes. Energy Convers. Manag. 2020, 226, 113530. [Google Scholar] [CrossRef]
- Oni, A.O.; Anaya, K.; Giwa, T.; Di Lullo, G.; Kumar, A. Comparative assessment of blue hydrogen from steam methane reforming, autothermal reforming, and natural gas decomposition technologies for natural gas-producing regions. Energy Convers. Manag. 2022, 254, 115245. [Google Scholar] [CrossRef]
- Slanger, D. Hydrogen Reality Check: Distilling Green Hydrogen’s Water Consumption. 2023. Available online: https://rmi.org/hydrogen-reality-check-distilling-green-hydrogens-water-consumption/ (accessed on 16 August 2024).
- Kowal, A.; Childers, G.; Beagle, E.; Lewis, M. Leveraging Permian Basin Assets for the Emerging Clean Hydrogen Economy. In Proceedings of the 2024 IEEE Power and Energy Conference at Illinois (PECI), Urbana, IL, USA, 19 April 2024. [Google Scholar] [CrossRef]
- Lin, N.; Chen, Y.; Madariaga, M.P. Route-to-market strategy for low-carbon hydrogen from natural gas in the Permian Basin. Fuel 2024, 355, 129420. [Google Scholar] [CrossRef]
- Lin, N.; Xu, L. Navigating the Implementation of Tax Credits for Natural-Gas-Based Low-Carbon-Intensity Hydrogen Projects. Energies 2024, 17, 1604. [Google Scholar] [CrossRef]
- National Renewable Energy Laboratory. H2A-Lite: Hydrogen Analysis Lite Production Model; National Renewable Energy Laboratory: Golden, CO, USA, 2022.
- Upton, G.; Mandalika, A. The Potential for Hydrogen in Louisiana; Presentation to Clean Hydrogen Task Force; Louisiana State University: Baton Rouge, LA, USA, 2024. [Google Scholar]
- Texas Water Development Board. 2026 Water Demand Projections. 2023. Available online: https://www.twdb.texas.gov/waterplanning/data/projections/2027/projections.asp (accessed on 15 September 2024).
- Texas Water Development Board. 2022 State Water Plan. 2021. Available online: https://www.twdb.texas.gov/waterplanning/swp/2022/index.asp (accessed on 15 September 2024).
- Department of Energy. Regional Clean Hydrogen Hubs Selections for Award Negotiations; Department of Energy: Washington, DC, USA, 2023.
- GHI Corp. Hydrogen City Project; GHI Corp.: Mequon, WI, USA, 2024. [Google Scholar]
- Lin, M.; Arzumanyan, M.; Rodriguez Calzado, E.; Childers, G.L. Hydrogen Energy Value Chain and Impacts on Water. In Proceedings of the 2024 Texas Groundwater Summit, San Antonio, TX, USA, 20 August 2024. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).