Electrostatic Interactions Override Surface Area Effects in Size-Dependent Adsorptive Removal of Microplastics by Fe3O4 Nanoparticles
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Adsorption Experiment of MPs by Fe3O4 Nanoparticles
2.3. Adsorption Model Analysis
3. Results and Discussion
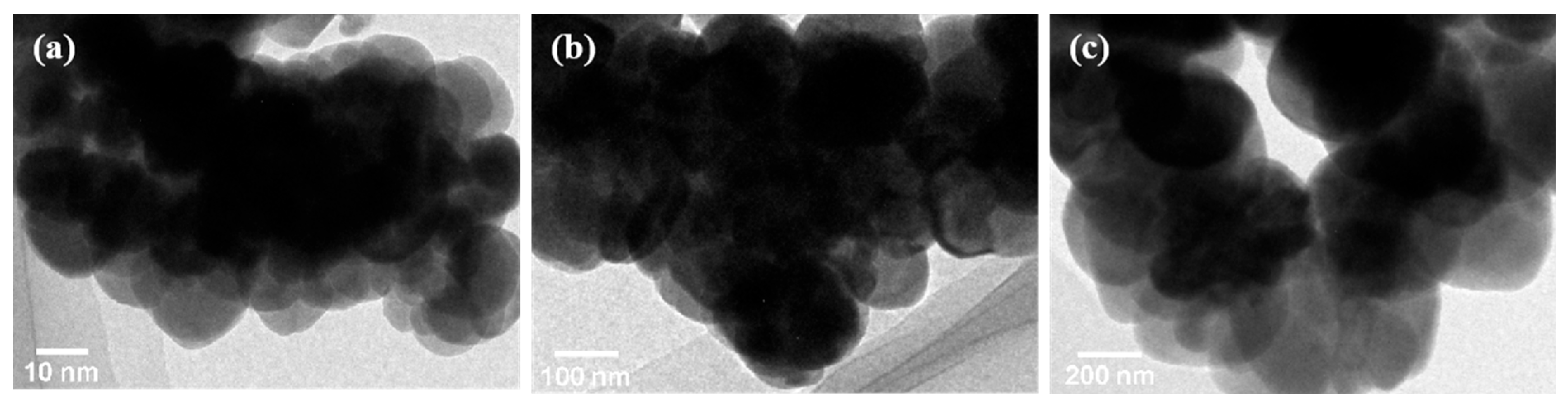
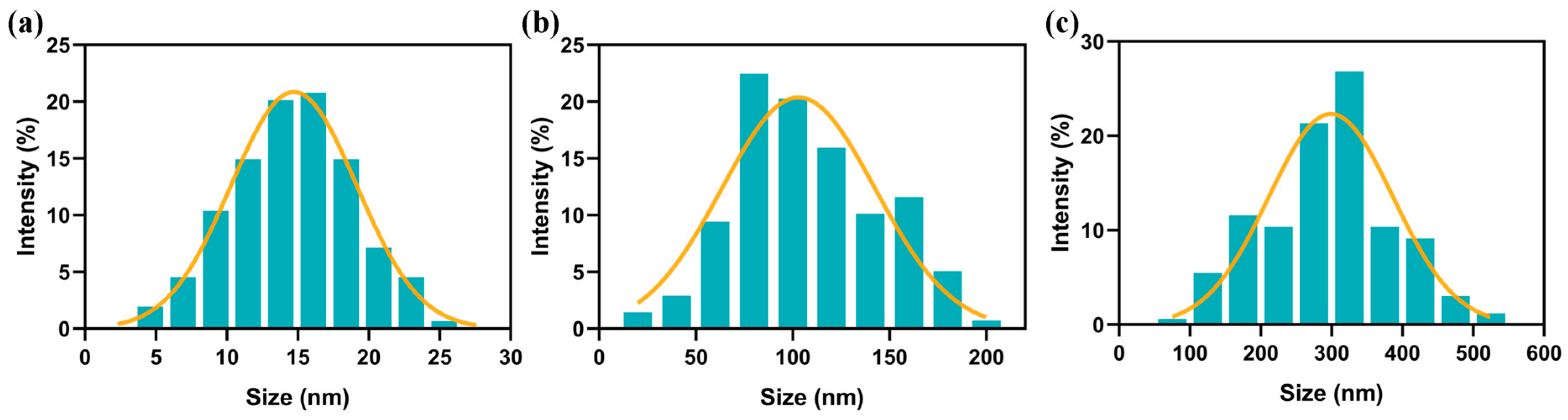
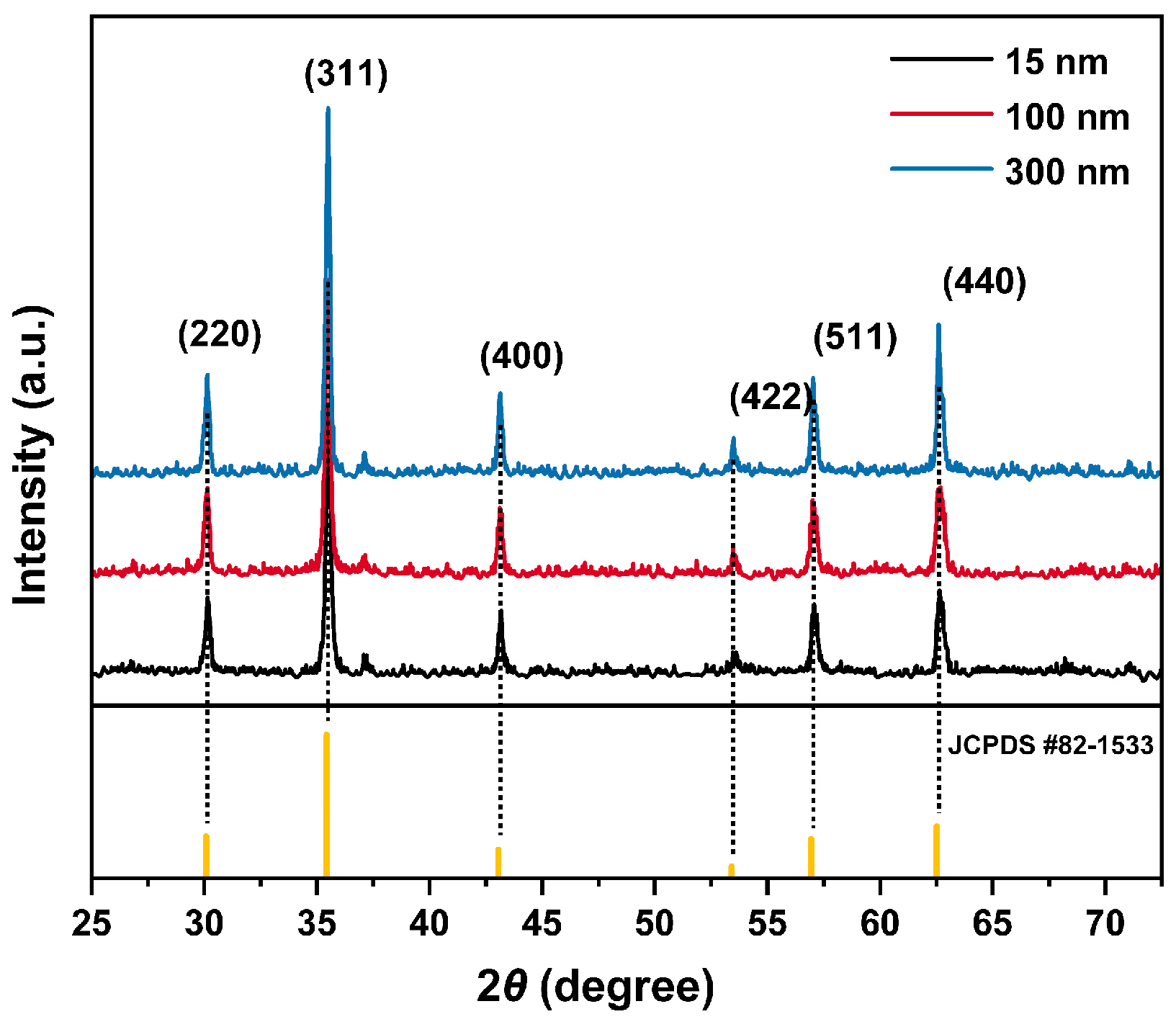
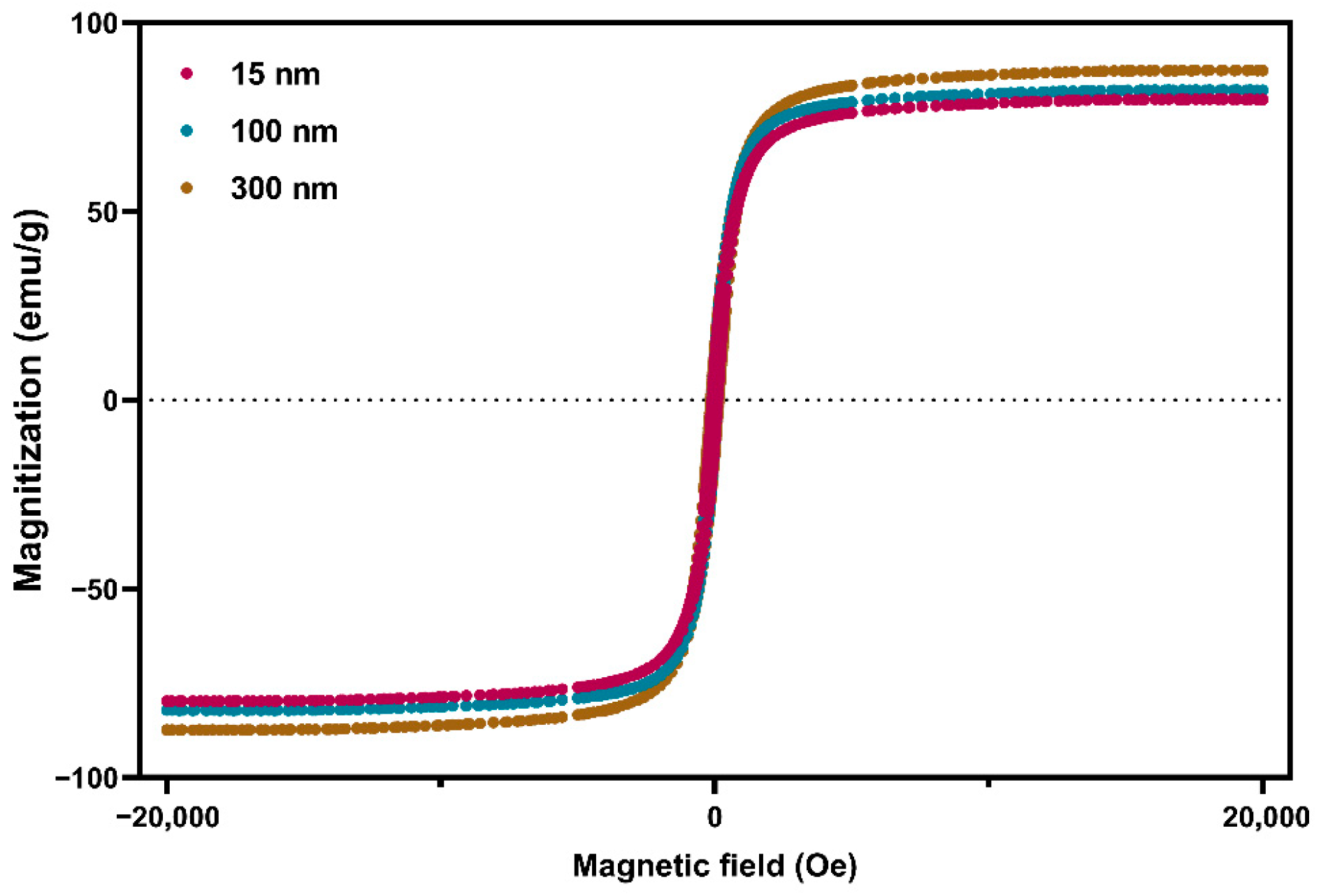
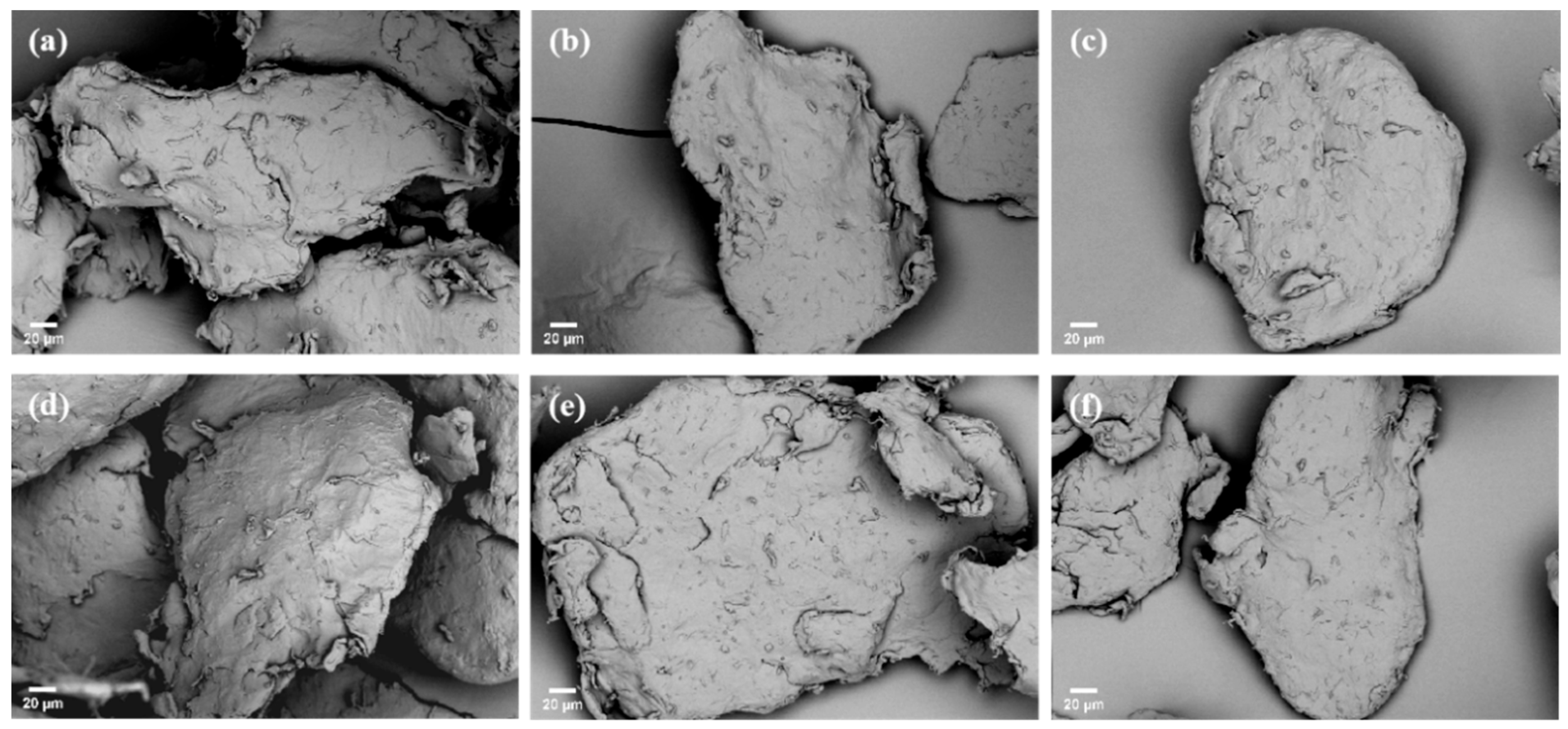
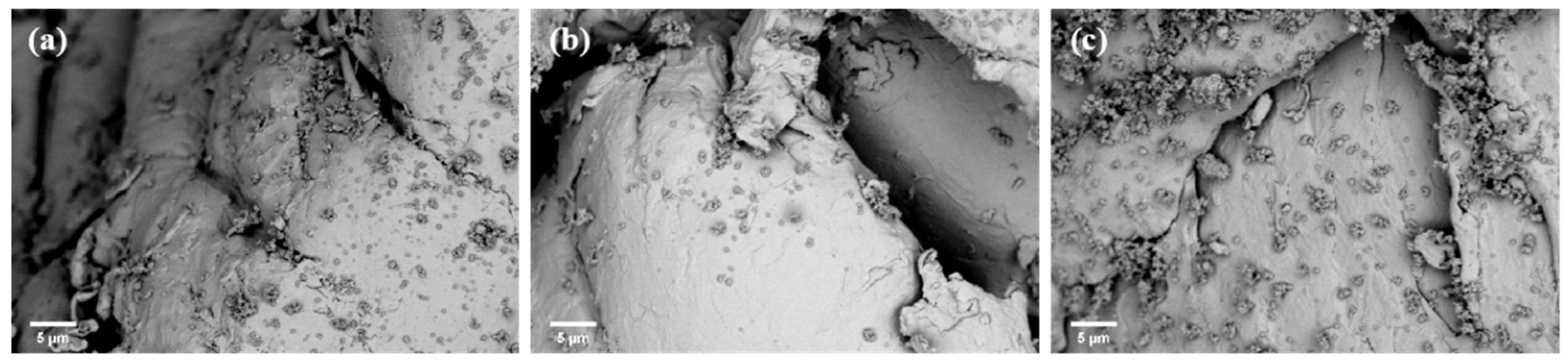
3.1. Characterization of Fe3O4 Nanoparticles and MPs
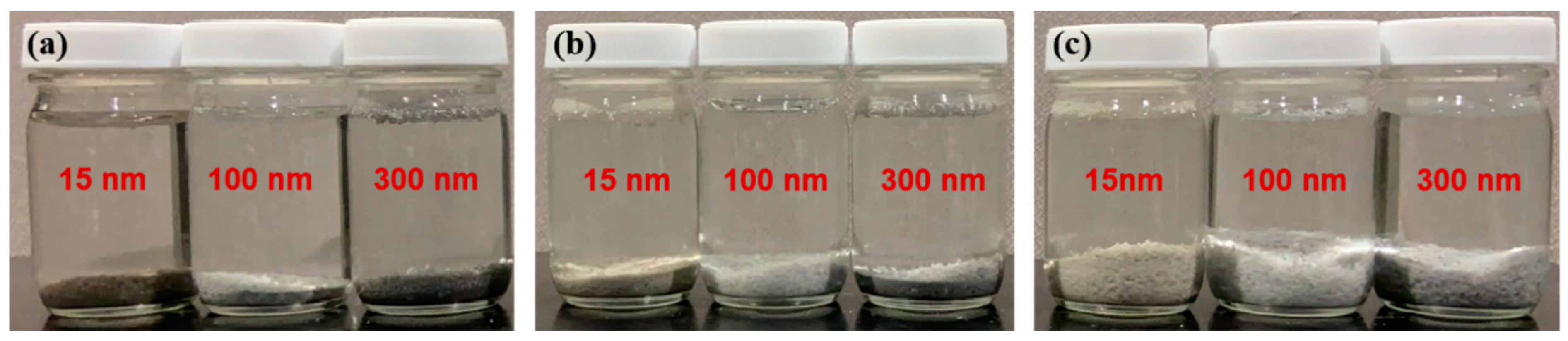
3.2. MPs Adsorption Experiments
3.3. Adsorption Isotherms
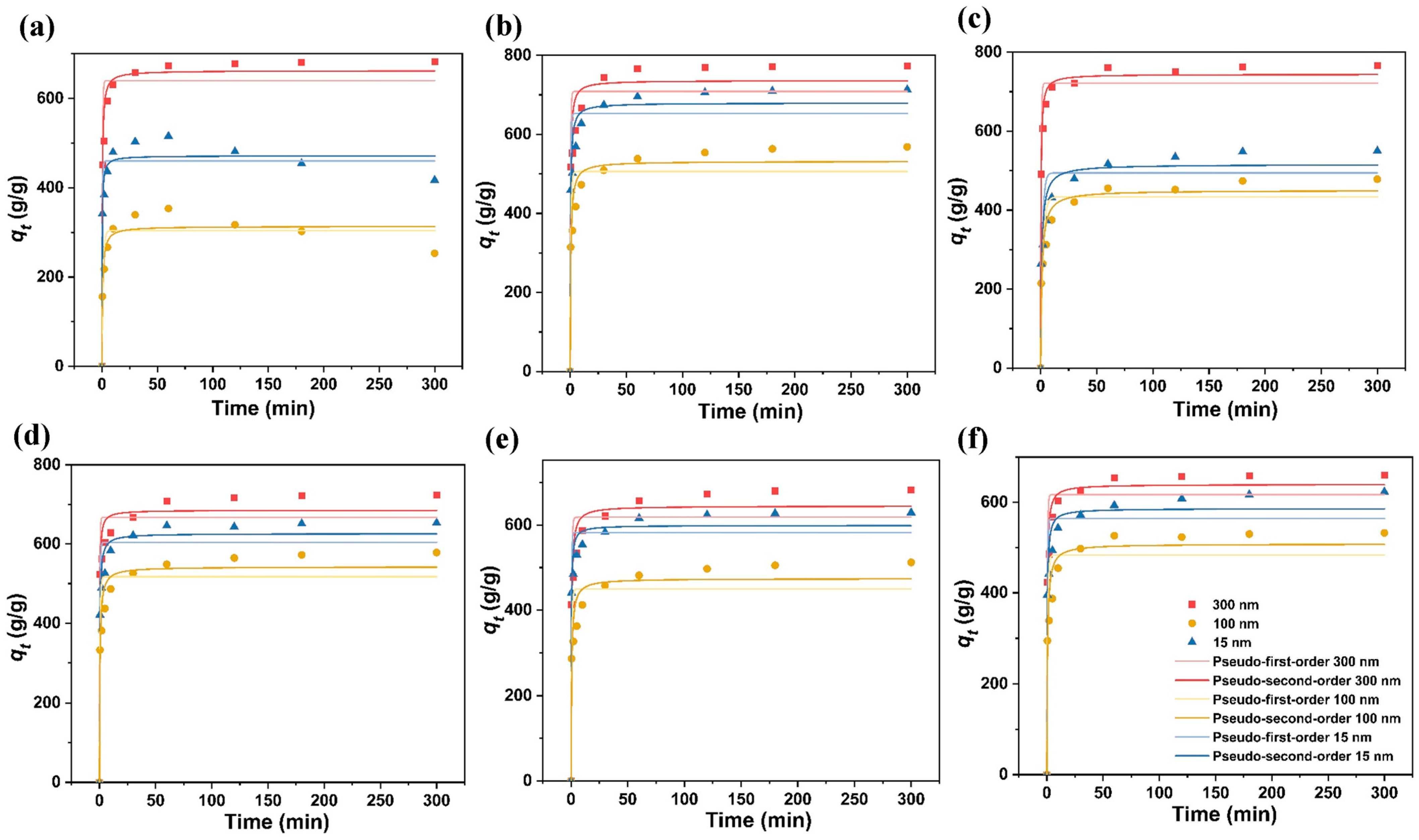
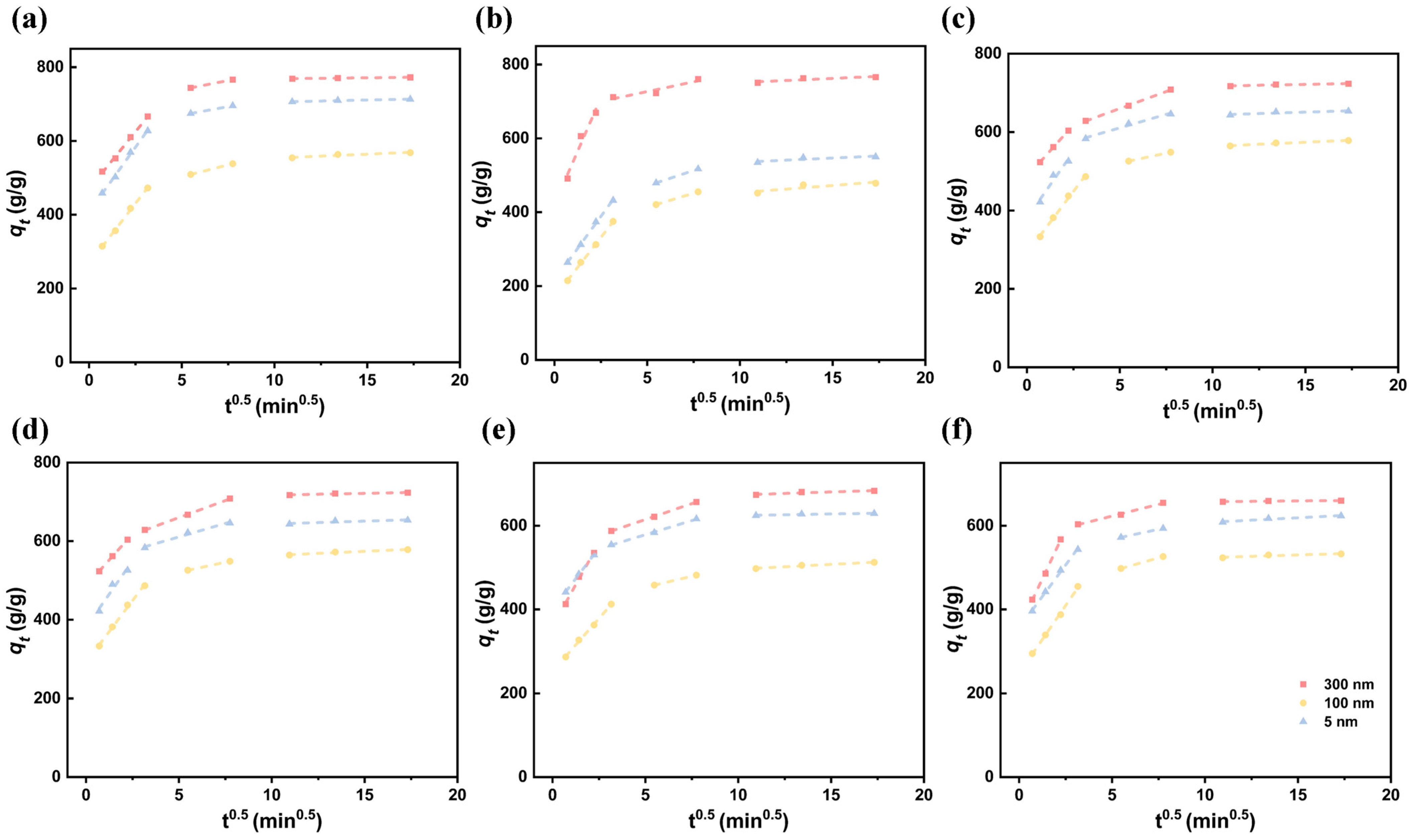
3.4. Adsorption Kinetics
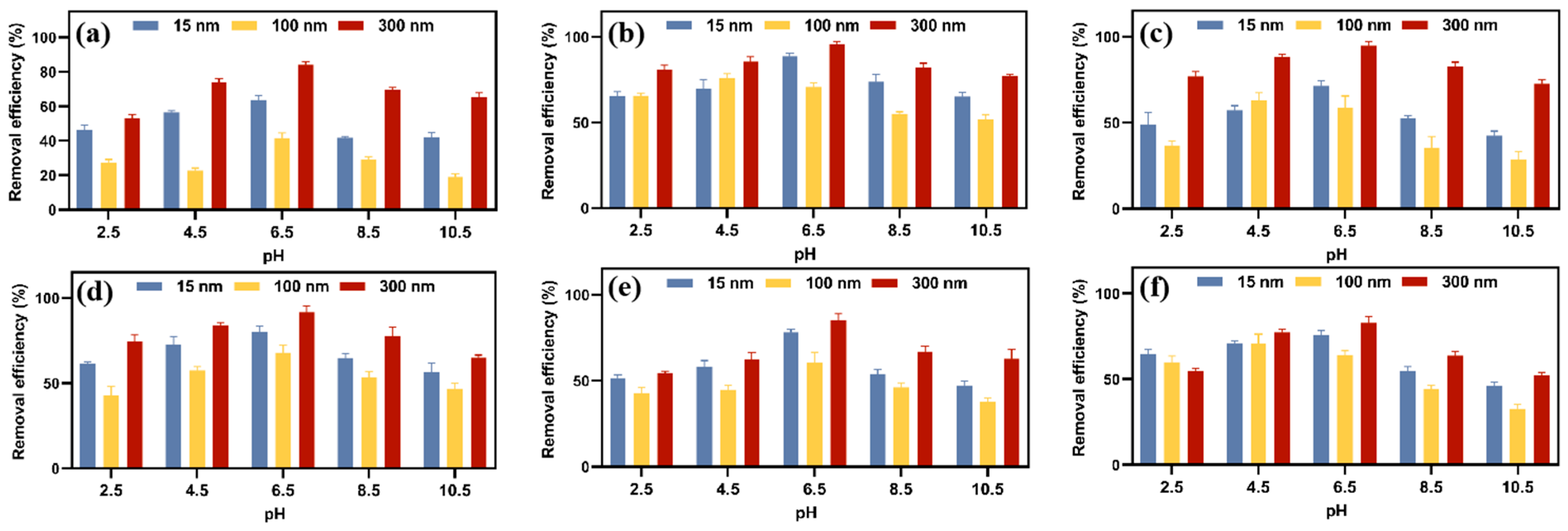
3.5. Effect of pH on MPs Adsorption
3.6. Mechanism of Size-Dependent Adsorption of MPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HDPE | High-density polyethylene |
| IEP | Isoelectric point |
| LDPE | Low-density polyethylene |
| MP | Microplastic |
| PET | Polyethylene terephthalate |
| PP | Polypropylene |
| PS | Polystyrene |
| PVC | Polyvinyl chloride |
| POP | Persistent organic pollutant |
| XRD | X-ray diffraction |
| Ms | Saturation magnetization |
| SEM | Scanning electron microscopy |
| TEM | Transmission electron microscopy |
| VSM | Vibrating sample magnetometer |
References
- Ding, H.; Liao, S.; Tu, D.; Hua, P.; Zhang, J. Trade drives leakage of life-cycle carbon dioxide emissions from plastics in China over 2010–2021. J. Clean. Prod. 2023, 417, 137994. [Google Scholar] [CrossRef]
- Zhuang, S.; Wang, J. Interaction between antibiotics and microplastics: Recent advances and perspective. Sci. Total Environ. 2023, 897, 165414. [Google Scholar] [CrossRef]
- Ompala, C.; Renault, J.P.; Taché, O.; Cournède, É.; Devineau, S.; Chivas-Joly, C. Stability and dispersibility of microplastics in experimental exposure medium and their dimensional characterization by SMLS, SAXS, Raman microscopy, and SEM. J. Hazard. Mater. 2024, 469, 134083. [Google Scholar] [CrossRef] [PubMed]
- Thompson, R.C.; Courtene-Jones, W.; Boucher, J.; Pahl, S.; Raubenheimer, K.; Koelmans, A.A. Twenty years of microplastic pollution research—What have we learned? Science 2024, 386, eadl2746. [Google Scholar] [CrossRef]
- Marcelo, L.R.; de Gois, J.S.; da Silva, A.A.; Cesar, D.V. Synthesis of iron-based magnetic nanocomposites and applications in adsorption processes for water treatment: A review. Environ. Chem. Lett. 2021, 19, 1229–1274. [Google Scholar] [CrossRef]
- Bostan, N.; Ilyas, N.; Akhtar, N.; Mehmood, S.; Saman, R.U.; Sayyed, R.Z.; Shatid, A.A.; Alfaifi, M.Y.; Elbehairi, S.E.I.; Pandiaraj, S. Toxicity assessment of microplastic (MPs); a threat to the ecosystem. Environ. Res. 2023, 234, 116523. [Google Scholar] [CrossRef]
- Kinigopoulou, V.; Pashalidis, I.; Kalderis, D.; Anastopoulos, I. Microplastics as carriers of inorganic and organic contaminants in the environment: A review of recent progress. J. Mol. Liq. 2022, 350, 118580. [Google Scholar] [CrossRef]
- Huang, W.; Song, B.; Liang, J.; Niu, Q.; Zeng, G.; Shen, M.; Deng, J.; Luo, Y.; Wen, X.; Zhang, Y. Microplastics and associated contaminants in the aquatic environment: A review on their ecotoxicological effects, trophic transfer, and potential impacts to human health. J. Hazard. Mater. 2021, 405, 124187. [Google Scholar] [CrossRef] [PubMed]
- Amato-Lourenço, L.F.; Carvalho-Oliveira, R.; Júnior, G.R.; dos Santos Galvão, L.; Ando, R.A.; Mauad, T. Presence of airborne microplastics in human lung tissue. J. Hazard. Mater. 2021, 416, 126124. [Google Scholar] [CrossRef]
- Ragusa, A.; Svelato, A.; Santacroce, C.; Catalano, P.; Notarstefano, V.; Carnevali, O.; Papa, F.; Rongioletti, M.C.A.; Baiocco, F.; Draghi, S.; et al. Plasticenta: First evidence of microplastics in human placenta. Environ. Int. 2021, 146, 106274. [Google Scholar] [CrossRef]
- Leslie, H.A.; van Velzen, M.J.; Brandsma, S.H.; Vethaak, A.D.; Garcia-Vallejo, J.J.; Lamoree, M.H. Discovery and quantification of plastic particle pollution in human blood. Environ. Int. 2022, 163, 107199. [Google Scholar] [CrossRef]
- Nihart, A.J.; Garcia, M.A.; El Hayek, E.; Liu, R.; Olewine, M.; Kingston, J.D.; Castillo, E.F.; Gullapalli, R.R.; Howard, T.; Bleske, B.; et al. Bioaccumulation of microplastics in decedent human brains. Nat. Med. 2025, 31, 1114–1119. [Google Scholar] [CrossRef]
- Marfella, R.; Prattichizzo, F.; Sardu, C.; Fulgenzi, G.; Graciotti, L.; Spadoni, T.; D’Onofrio, N.; Scisciola, L.; La Grotta, R.; Frigé, C.; et al. Microplastics and nanoplastics in atheromas and cardiovascular events. N. Engl. J. Med. 2024, 390, 900–910. [Google Scholar] [CrossRef]
- Winiarska, E.; Jutel, M.; Zemelka-Wiacek, M. The potential impact of nano- and microplastics on human health: Understanding human health risks. Environ. Res. 2024, 251, 118535. [Google Scholar] [CrossRef]
- Luo, Q.; Tan, H.; Ye, M.; Jho, E.H.; Wang, P.; Iqbal, B.; Zhao, X.; Shi, H.; Lu, H.; Li, G. Microplastics as an emerging threat to human health: An overview of potential health impacts. J. Environ. Manag. 2025, 387, 125915. [Google Scholar] [CrossRef]
- Padervand, M.; Lichtfouse, E.; Robert, D.; Wang, C. Removal of microplastics from the environment. A review. Environ. Chem. Lett. 2020, 18, 807–828. [Google Scholar] [CrossRef]
- Ahmed, R.; Hamid, A.K.; Krebsbach, S.A.; He, J.; Wang, D. Critical review of microplastics removal from the environment. Chemosphere 2022, 293, 133557. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Zhang, Y.; Mo, A.; Jiang, J.; Liang, Y.; Cao, X.; He, D. Removal of microplastics in water: Technology progress and green strategies. Green Anal. Chem. 2022, 3, 100042. [Google Scholar] [CrossRef]
- Ramos, R.L.; dos Santos, C.R.; Drumond, G.P.; de Souza Santos, L.V.; Amaral, M.C.S. Critical review of microplastic in membrane treatment plant: Removal efficiency, environmental risk assessment, membrane fouling, and MP release. Chem. Eng. J. 2024, 480, 148052. [Google Scholar] [CrossRef]
- Pan, X.; Ji, J.; Zhang, N.; Xing, M. Research Progress of Graphene-Based Nanomaterials for the Environmental Remediation. Chin. Chem. Lett. 2020, 31, 1462–1473. [Google Scholar] [CrossRef]
- Pan, X.; Kong, F.; Xing, M. Spatial Separation of Photo-Generated Carriers in g-C3N4/MnO2/Pt with Enhanced H2 Evolution and Organic Pollutant Control. Res. Chem. Intermed. 2022, 48, 2837–2855. [Google Scholar] [CrossRef]
- Liu, Q.; Chen, Y.; Chen, Z.; Yang, F.; Xie, Y.; Yao, W. Current status of microplastics and nanoplastics removal methods: Summary, comparison and prospect. Sci. Total Environ. 2022, 851, 157991. [Google Scholar] [CrossRef]
- Wang, J.; Sun, C.; Huang, Q.X.; Chi, Y.; Yan, J.H. Adsorption and thermal degradation of microplastics from aqueous solutions by Mg/Zn modified magnetic biochars. J. Hazard. Mater. 2021, 419, 126486. [Google Scholar] [CrossRef]
- Sun, C.; Wang, Z.; Chen, L.; Li, F. Fabrication of robust and compressive chitin and graphene oxide sponges for removal of microplastics with different functional groups. Chem. Eng. J. 2020, 393, 124796. [Google Scholar] [CrossRef]
- Garfansa, M.P.; Zalizar, L.; Husen, S.; Triwanto, J.; Iswahyudi, I.; Bakhtiar, A.; Lasaksi, P.; Ekalaturrahmah, Y.A.C. Addition of biochar-based activated carbon into sand filtration columns to improve removal efficiency of microplastic from water. Bioresour. Technol. Rep. 2025, 30, 102099. [Google Scholar]
- Kegl, T.; Košak, A.; Lobnik, A.; Novak, Z.; Kralj, A.K.; Ban, I. Adsorption of rare earth metals from wastewater by nanomaterials: A review. J. Hazard. Mater. 2020, 386, 121632. [Google Scholar] [CrossRef] [PubMed]
- Rasheed, T. Magnetic nanomaterials: Greener and sustainable alternatives for the adsorption of hazardous environmental contaminants. J. Clean. Prod. 2022, 362, 132338. [Google Scholar] [CrossRef]
- Vijayakumar, G.; Wijayaratne, K.B.; Perera, C.S. Enhanced mercury removal from water using Fe3O4/MgO composite adsorbent. Water Air Soil Pollut. 2025, 236, 444. [Google Scholar] [CrossRef]
- Zou, Z.; Chen, Y.; Sun, R.; Shi, B.; Jiang, L.; Huang, F. Ti3C2/Fe3O4-based surface plasmon resonance imaging biosensor for efficient separation and sensitive detection of CA125 in serum. Microchim. Acta 2025, 192, 168. [Google Scholar] [CrossRef]
- Heo, Y.; Lee, E.H.; Lee, S.W. Adsorptive removal of micron-sized polystyrene particles using magnetic iron oxide nanoparticles. Chemosphere 2022, 307, 135672. [Google Scholar] [CrossRef]
- Zandieh, M.; Liu, J. Removal and degradation of microplastics using the magnetic and nanozyme activities of bare iron oxide nanoaggregates. Angew. Chem. 2022, 134, e202212013. [Google Scholar] [CrossRef]
- Wang, J.; Yue, D.; Wang, H. In situ Fe3O4 nanoparticle coating of polymers for separating hazardous PVC from microplastic mixtures. Chem. Eng. J. 2021, 407, 127170. [Google Scholar] [CrossRef]
- Wang, H.P.; Huang, X.H.; Chen, J.N.; Dong, M.; Nie, C.Z.; Qin, L. Modified superhydrophobic magnetic Fe3O4 nanoparticles for removal of microplastics in liquid foods. Chem. Eng. J. 2023, 476, 146562. [Google Scholar] [CrossRef]
- Li, J.; Zhang, Y.; Zeng, C.; Xiong, Q.; Zhang, H.; Guo, Q.; Wang, L.; Ma, J.; Chen, S. Enhanced removal of nanoplastics from freshwater using in-situ synthesized and anchored nano-Fe3O4 via two-step ferrous oxidation. Chem. Eng. J. 2024, 499, 156205. [Google Scholar] [CrossRef]
- Yan, R.; Lin, S.; Jiang, W.; Yu, X.; Zhang, L.; Zhao, W.; Sui, Q. Effect of aggregation behavior on microplastic removal by magnetic Fe3O4 nanoparticles. Sci. Total Environ. 2023, 898, 165431. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, X.; Wang, H.; Wang, Z.; Wang, X.; Dai, Y.; Yue, T.; Zhang, X.; Xing, B. Size-selective attachment of polyvinyl chloride microplastics on iron oxides in aqueous environments. Chem. Eng. J. 2024, 488, 150721. [Google Scholar] [CrossRef]
- Fayyadh, S.N.; Tahrim, N.A.; Mokhtar, W.N.A.W. Predicting particle size of iron magnetic nanoparticles synthesized from celery extract using artificial neural networks and regression learner models. Adsorption 2025, 31, 57. [Google Scholar] [CrossRef]
- Hou, L.; Liang, Q.; Wang, F. Mechanisms that control the adsorption–desorption behavior of phosphate on magnetite nanoparticles: The role of particle size and surface chemistry. RSC Adv. 2020, 10, 2378–2388. [Google Scholar] [CrossRef] [PubMed]
- Phearom, S.; Shahid, M.K.; Choi, Y. Nature of surface interactions among Fe3O4 particles and arsenic species during static and continuous adsorption processes. Groundw. Sustain. Dev. 2022, 18, 100789. [Google Scholar] [CrossRef]
- Shen, Y.F.; Tang, J.; Nie, Z.H.; Wang, Y.D.; Ren, Y.; Zuo, L. Tailoring size and structural distortion of Fe3O4 nanoparticles for the purification of contaminated water. Bioresour. Technol. 2009, 100, 4139–4146. [Google Scholar] [CrossRef]
- Lin, C.C.; Lin, Y.S.; Ho, J.M. Adsorption of Reactive Red 2 from aqueous solutions using Fe3O4 nanoparticles prepared by co-precipitation in a rotating packed bed. J. Alloys Compd. 2016, 666, 153–158. [Google Scholar] [CrossRef]
- Luo, S.; Qin, J.; Wu, Y.; Feng, F. Tetracycline adsorption on magnetic sludge biochar: Size effect of the Fe3O4nanoparticles. R. Soc. Open Sci. 2022, 9, 210805. [Google Scholar] [CrossRef]
- Ulusal, F.; Bilici, Z.; Ozay, Y.; Özdemir, N.; Dizge, N. Synthesis of mesoporous magnetic Fe3O4nanoparticles with different pore sizes and investigation of dye adsorption capacities. Part. Sci. Technol. 2024, 42, 804–814. [Google Scholar] [CrossRef]
- Cao, Y.; Sathish, C.I.; Guan, X.; Wang, S.; Palanisami, T.; Vinu, A.; Yi, J. Advances in magnetic materials for microplastic separation and degradation. J. Hazard. Mater. 2024, 461, 132537. [Google Scholar] [CrossRef]
- Kumar, C.; Singh, H.; Kaur, M.; Arya, S.K.; Khatri, M. Remediation strategies for micro/nanoplastic pollution using magnetic nanomaterials. Environ. Sci. Pollut. Res. 2025, 32, 15574–15603. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Feng, J.C.; Li, C.; Liang, J.; Zhang, S.; Yang, Z. Global occurrence, drivers, and environmental risks of microplastics in marine environments. J. Environ. Manag. 2023, 329, 116961. [Google Scholar] [CrossRef] [PubMed]
- Kakade, A.; Mi, J.; Long, R. Microplastics in the world oceans and strategies for their control. Rev. Environ. Contam. Toxicol. 2024, 262, 20. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, S.; Wu, J.; Liu, F.; Chen, C.; Ding, B.; Zhou, X.; Deng, H. Revivable self-assembled supramolecular biomass fibrous framework for efficient microplastic removal. Sci. Adv. 2024, 10, eadn8662. [Google Scholar] [CrossRef]
- Lyu, W.; Li, J.; Trchová, M.; Wang, G.; Liao, Y.; Bober, P.; Stejskal, J. Fabrication of polyaniline/poly(vinyl alcohol)/montmorillonite hybrid aerogels toward efficient adsorption of organic dye pollutants. J. Hazard. Mater. 2022, 435, 129004. [Google Scholar] [CrossRef]
- Al-Ghouti, M.A.; Da’ana, D.A. Guidelines for the use and interpretation of adsorption isotherm models: A review. J. Hazard. Mater. 2020, 393, 122383. [Google Scholar] [CrossRef]
- Zhu, Z.; Wu, X.; Wang, C.; Meng, Z.; Sun, C.; Wang, Z. Effect of carbon black particle size in chitin sponges on microplastics adsorption. Sep. Purif. Technol. 2023, 322, 124321. [Google Scholar] [CrossRef]
- Akinola, A.O.; Prabakaran, E.; Govender, K.; Pillay, K. Cetyltrimethylammonium bromide modified magnetic apricot shells for removing Congo red dye and an artificial neural network model. New. J. Chem. 2025, 49, 5529–5544. [Google Scholar] [CrossRef]
- Sahoo, A.K.; Sangita Nanda, S.; Nayak, P.; Dash, S. Structural, magnetic and optical properties of Fe3O4@NaGdF4 core-shell composite. Mater. Today Proc. 2023, 91, 167–171. [Google Scholar] [CrossRef]
- Debnath, B.; Salunke, H.G.; Bhattacharyya, S. Spin disorder and particle size effects in cobalt ferrite nanoparticles with unidirectional anisotropy and permanent magnet-like characteristics. J. Phys. Chem. C 2020, 124, 25992–26000. [Google Scholar] [CrossRef]
- Unnimaya, S.; Mithun, N.; Lukose, J.; Nair, M.P.; Gopinath, A.; Chidangil, S. Identification of microplastics using a custom-built micro-Raman spectrometer. J. Phys.: Conf. Ser. 2023, 2426, 012007. [Google Scholar] [CrossRef]
- Zhuang, J.; Rong, N.; Wang, X.; Chen, C.; Xu, Z. Adsorption of small size microplastics based on cellulose nanofiber aerogel modified by quaternary ammonium salt in water. Sep. Purif. Technol. 2022, 293, 121133. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, X.; Dong, W.; Zhang, L.; Kong, Q.; Wang, W. Efficient adsorption of sulfamethazine onto modified activated carbon: A plausible adsorption mechanism. Sci. Rep. 2017, 7, 12437. [Google Scholar] [CrossRef]
- Shi, X.; Zhang, X.; Gao, W.; Zhang, Y.; He, D. Removal of microplastics from water by magnetic nano-Fe3O4. Sci. Total Environ. 2022, 802, 149838. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, Y.; Qian, Z.; Wang, Q.; Yalikun, N.; Jiang, H.; Wang, C.; Wang, H. A hydrophilic/hydrophobic switch on polymer surface triggered by calcite towards separation of hazardous PVC from plastic mixtures. J. Hazard. Mater. 2025, 483, 136667. [Google Scholar] [CrossRef] [PubMed]
- Shi, C.; Geng, J.; Qu, X.; Niu, F.; Yang, J.; Wang, J. Experimental study on enhanced magnetic removal of microplastics in water using modified Maifanite. J. Environ. Chem. Eng. 2025, 13, 116014. [Google Scholar] [CrossRef]
- Grbic, J.; Nguyen, B.; Guo, E.; You, J.B.; Sinton, D.; Rochman, C.M. Magnetic extraction of microplastics from environmental samples. Environ. Sci. Technol. Lett. 2019, 6, 68–72. [Google Scholar] [CrossRef]
- Sarcletti, M.; Park, H.; Wirth, J.; Englisch, S.; Eigen, A.; Drobek, D.; Vivod, D.; Friedrich, B.; Tietze, R.; Alexiou, C.; et al. The remediation of nano-/microplastics from water. Mater. Today 2021, 48, 38–46. [Google Scholar] [CrossRef]
- Matar, G.H.; Dikbas, C.; Andac, M. Efficient magnetic adsorption of polystyrene nanoplastic from aqueous solutions by eco-friendly Fe3O4nanoparticles: Removal, kinetic and isotherm modeling studies. J. Environ. Health Sci. Eng. 2024, 23, 4. [Google Scholar] [CrossRef]
- Zhang, Y.; Duan, J.; Liu, R.; Petropoulos, E.; Feng, Y.; Xue, L.; Yang, L.; He, S. Efficient magnetic capture of PE microplastic from water by PEG modified Fe3O4nanoparticles: Performance, kinetics, isotherms and influence factors. J. Environ. Sci. 2025, 147, 677–687. [Google Scholar] [CrossRef]
- Ranjbar, D.; Raeiszadeh, M.; Lewis, L.; MacLachlan, M.J.; Hatzikiriakos, S.G. Adsorptive removal of Congo Red by surfactant modified cellulose nanocrystals: A kinetic, equilibrium, and mechanistic investigation. Cellulose 2020, 27, 3211–3232. [Google Scholar] [CrossRef]
- Dos Reis, L.G.T.; Robaina, N.F.; Pacheco, W.F.; Cassella, R.J. Separation of Malachite Green and Methyl Green cationic dyes from aqueous medium by adsorption on Amberlite XAD-2 and XAD-4 resins using sodium dodecylsulfate as carrier. Chem. Eng. J. 2011, 171, 532–540. [Google Scholar] [CrossRef]
- Nguyen, T.B.; Ho, T.B.C.; Huang, C.P.; Chen, C.W.; Chen, W.H.; Hsieh, S.; Hsieh, S.L.; Dong, C.D. Adsorption of lead(II) onto PE microplastics as a function of particle size: Influencing factors and adsorption mechanism. Chemosphere 2022, 304, 135276. [Google Scholar] [CrossRef]
- Zhao, H.; Huang, X.; Wang, L.; Zhao, X.; Yan, F.; Yang, Y.; Li, G.; Gao, P.; Ji, P. Removal of polystyrene nanoplastics from aqueous solutions using a novel magnetic material: Adsorbability, mechanism, and reusability. Chem. Eng. J. 2022, 430, 133122. [Google Scholar] [CrossRef]
- Zhao, H.; Wu, J.; Su, F.; He, X. Removal of polystyrene nanoplastics from aqueous solutions by a novel magnetic zeolite adsorbent. Hum. Ecol. Risk Assess. 2023, 29, 327–346. [Google Scholar] [CrossRef]
- Kim, J.; Lee, Y.G.; Kim, H.; Chon, K.; Phae, C. One-step synthesis of magnetic biochar via co-pyrolysis of walnut shells and Fe-rich mine tails for adsorption capacity improvement of polystyrene sulfonate microplastics: Role of microplastic size. Environ. Technol. Innov. 2024, 34, 103624. [Google Scholar] [CrossRef]
- Liu, Z.; Qin, Q.; Hu, Z.; Yan, L.; Ieong, U.I.; Xu, Y. Adsorption of chlorophenols on polyethylene terephthalate microplastics from aqueous environments: Kinetics, mechanisms and influencing factors. Environ. Pollut. 2020, 265, 114926. [Google Scholar] [CrossRef]
- Suttiponparnit, K.; Jiang, J.; Sahu, M.; Suvachittanont, S.; Charinpanitkul, T.; Biswas, P. Role of surface area, primary particle size, and crystal phase on titanium dioxide nanoparticle dispersion properties. Nanoscale Res. Lett. 2010, 6, 27. [Google Scholar] [CrossRef] [PubMed]
- He, Y.T.; Wan, J.; Tokunaga, T. Kinetic stability of hematite nanoparticles: The effect of particle sizes. J. Nanopart. Res. 2008, 10, 321–332. [Google Scholar] [CrossRef]
- Robinson, M.R.; Coustel, R.; Abdelmoula, M.; Mallet, M. As(V) and As(III) sequestration by starch-functionalized magnetite nanoparticles: Influence of the synthesis route on the trapping efficiency. Sci. Technol. Adv. Mater. 2020, 21, 524–539. [Google Scholar] [CrossRef]
- Iida, H.; Takayanagi, K.; Nakanishi, T.; Osaka, T. Synthesis of Fe3O4nanoparticles with various sizes and magnetic properties by controlled hydrolysis. J. Colloid Interface Sci. 2007, 314, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Yun, T.Y.; Chandler, B.D. Surface hydroxyl chemistry of titania- and alumina-based supports: Quantitative titration and temperature dependence of surface Brønsted acid–base parameters. ACS Appl. Mater. Interfaces 2023, 15, 6868–6876. [Google Scholar] [CrossRef]
- Sun, C.; Berg, J.C. A review of the different techniques for solid surface acid–base characterization. Adv. Colloid Interface Sci. 2003, 105, 151–175. [Google Scholar] [CrossRef]
- Yates, P.D.; Franks, G.V.; Biggs, S.; Jameson, G.J. Heteroaggregation with nanoparticles: Effect of particle size ratio on optimum particle dose. Colloids Surf. A 2005, 255, 85–90. [Google Scholar] [CrossRef]




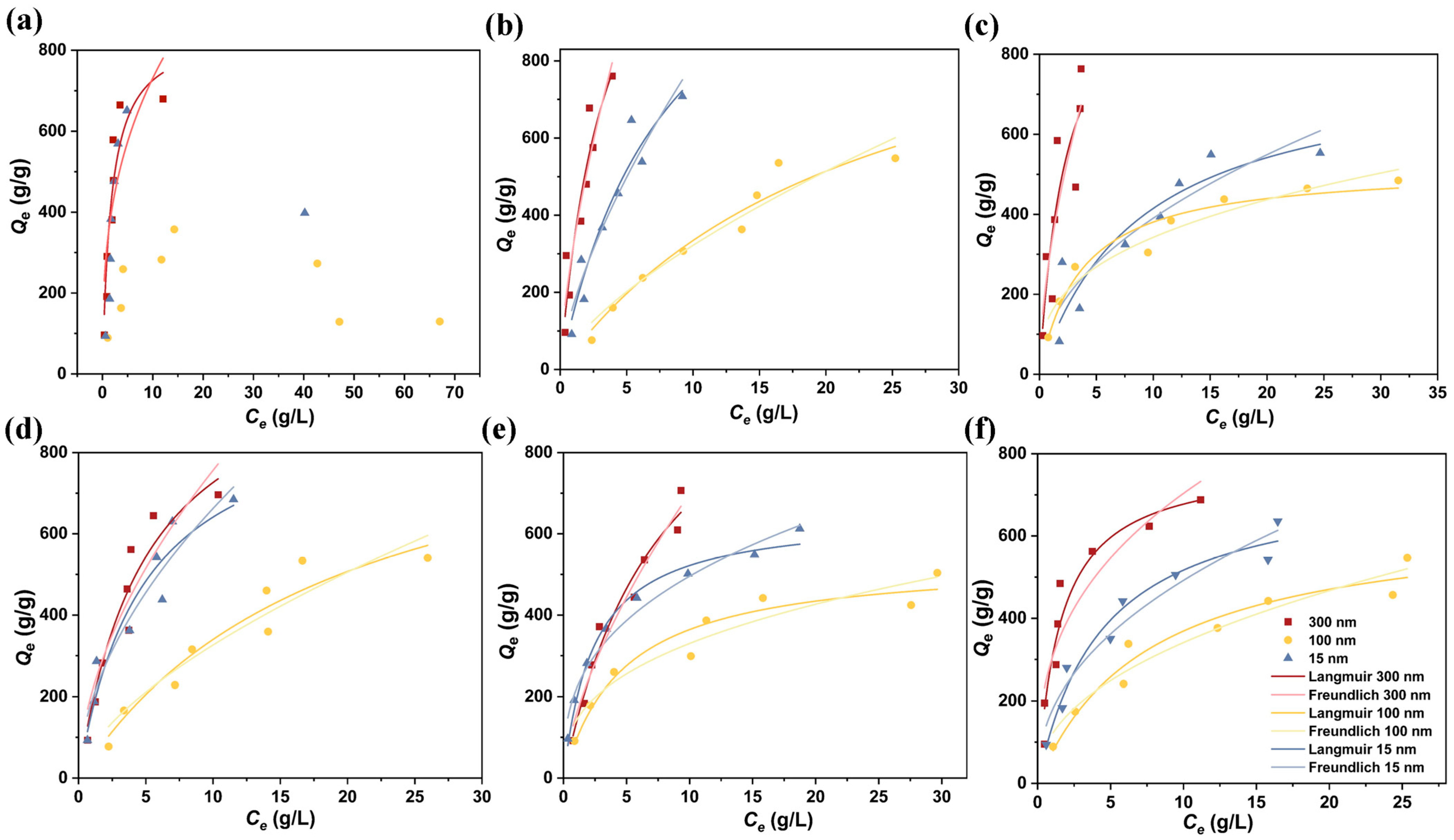

| MPs | Fe3O4 (nm) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
(L/g) | (g/g) | (gg−1(g/L)−1/n) | |||||
| PET | 300 | 0.569 | 853.460 | 0.877 | 326.756 | 2.857 | 0.714 |
| 100 | – | – | – | – | – | – | |
| 15 | – | – | – | – | – | – | |
| PS | 300 | 0.284 | 1460.071 | 0.894 | 323.077 | 1.515 | 0.891 |
| 100 | 0.043 | 1109.075 | 0.951 | 68.111 | 1.481 | 0.930 | |
| 15 | 0.120 | 1378.877 | 0.929 | 168.539 | 1.486 | 0.911 | |
| PP | 300 | 0.422 | 1224.195 | 0.760 | 316.067 | 1.715 | 0.759 |
| 100 | 0.274 | 519.388 | 0.950 | 152.876 | 2.857 | 0.948 | |
| 15 | 0.113 | 780.546 | 0.855 | 125.722 | 2.033 | 0.844 | |
| LDPE | 300 | 0.196 | 1096.714 | 0.920 | 210.944 | 1.802 | 0.869 |
| 100 | 0.052 | 886.367 | 0.929 | 76.290 | 1.585 | 0.904 | |
| 15 | 0.207 | 951.324 | 0.919 | 190.520 | 1.848 | 0.918 | |
| HDPE | 300 | 0.123 | 1089.197 | 0.966 | 154.176 | 1.522 | 0.963 |
| 100 | 0.210 | 537.237 | 0.939 | 140.871 | 2.703 | 0.921 | |
| 15 | 0.410 | 648.890 | 0.986 | 217.015 | 2.793 | 0.975 | |
| PVC | 300 | 0.633 | 784.372 | 0.929 | 303.680 | 2.740 | 0.847 |
| 100 | 0.132 | 647.696 | 0.950 | 119.869 | 2.203 | 0.940 | |
| 15 | 0.226 | 745.052 | 0.962 | 177.223 | 2.257 | 0.946 | |
| MPs | Fe3O4 (nm) | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
(min−1) | (gg−1) | (g(g·min)−1) | (gg−1) | ||||
| PET | 300 | 2.271 | 640.144 | 0.936 | 0.005 | 662.312 | 0.978 |
| 100 | 0.922 | 304.001 | 0.892 | 0.005 | 313.481 | 0.925 | |
| 15 | 2.631 | 459.826 | 0.933 | 0.010 | 471.456 | 0.956 | |
| PS | 300 | 2.467 | 708.695 | 0.900 | 0.004 | 736.272 | 0.950 |
| 100 | 1.406 | 506.015 | 0.865 | 0.003 | 531.725 | 0.943 | |
| 15 | 2.229 | 652.827 | 0.906 | 0.004 | 679.310 | 0.958 | |
| PP | 300 | 2.141 | 721.654 | 0.960 | 0.004 | 744.072 | 0.990 |
| 100 | 0.503 | 433.282 | 0.859 | 0.002 | 450.147 | 0.941 | |
| 15 | 0.645 | 494.642 | 0.849 | 0.002 | 515.854 | 0.936 | |
| LDPE | 300 | 3.001 | 667.169 | 0.938 | 0.007 | 685.172 | 0.966 |
| 100 | 1.688 | 517.281 | 0.884 | 0.004 | 542.101 | 0.952 | |
| 15 | 2.231 | 604.105 | 0.926 | 0.005 | 626.179 | 0.970 | |
| HDPE | 300 | 1.948 | 618.445 | 0.904 | 0.004 | 644.903 | 0.960 |
| 100 | 1.597 | 450.065 | 0.856 | 0.004 | 474.185 | 0.933 | |
| 15 | 2.750 | 582.209 | 0.939 | 0.007 | 599.239 | 0.971 | |
| PVC | 300 | 2.115 | 617.204 | 0.934 | 0.005 | 639.578 | 0.978 |
| 100 | 1.280 | 483.548 | 0.866 | 0.003 | 507.887 | 0.945 | |
| 15 | 2.234 | 564.049 | 0.912 | 0.005 | 586.105 | 0.961 | |
| MPs | Fe3O4 (nm) | (g/(g·min1/2)) | (g/(g·min1/2)) | (g/(g·min1/2)) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PET | 300 | 93.501 | 380.760 | 0.989 | 9.258 | 603.370 | 0.982 | 0.813 | 669.165 | 0.815 |
| 100 | 61.200 | 122.125 | 0.979 | 6.347 | 304.435 | 0.999 | −10.312 | 434.269 | 0.972 | |
| 15 | 56.530 | 304.129 | 0.996 | 5.510 | 473.122 | 0.999 | −10.006 | 590.081 | 0.999 | |
| PS | 300 | 61.842 | 470.493 | 0.997 | 9.785 | 690.604 | 0.999 | 0.556 | 763.073 | 0.983 |
| 100 | 65.190 | 267.748 | 0.998 | 12.827 | 439.046 | 0.999 | 2.125 | 532.467 | 0.906 | |
| 15 | 70.093 | 407.856 | 0.997 | 9.300 | 623.660 | 0.999 | 1.088 | 694.841 | 0.965 | |
| PP | 300 | 115.345 | 421.399 | 0.956 | 10.582 | 673.869 | 0.898 | 2.236 | 728.932 | 0.787 |
| 100 | 64.618 | 170.124 | 0.999 | 15.251 | 337.168 | 0.999 | 3.898 | 414.026 | 0.768 | |
| 15 | 69.022 | 216.045 | 0.999 | 16.661 | 388.143 | 0.999 | 2.262 | 512.897 | 0.746 | |
| LDPE | 300 | 52.656 | 486.419 | 0.999 | 17.386 | 573.043 | 0.999 | 0.921 | 707.922 | 0.894 |
| 100 | 62.856 | 291.336 | 0.996 | 10.138 | 470.173 | 0.999 | 2.096 | 542.605 | 0.960 | |
| 15 | 67.698 | 380.538 | 0.953 | 13.732 | 542.098 | 0.986 | 1.470 | 628.841 | 0.783 | |
| HDPE | 300 | 79.570 | 359.495 | 0.993 | 15.139 | 539.147 | 0.999 | 1.434 | 659.076 | 0.864 |
| 100 | 50.539 | 252.042 | 0.998 | 10.534 | 400.100 | 0.999 | 2.274 | 473.364 | 0.972 | |
| 15 | 58.273 | 400.727 | 0.999 | 13.479 | 511.045 | 0.998 | 0.749 | 616.726 | 0.878 | |
| PVC | 300 | 94.258 | 355.193 | 0.999 | 11.187 | 576.029 | 0.995 | 0.400 | 653.148 | 0.818 |
| 100 | 64.802 | 247.078 | 0.998 | 12.694 | 427.971 | 0.999 | 1.367 | 509.566 | 0.840 | |
| 15 | 60.150 | 355.573 | 0.998 | 9.477 | 520.194 | 0.999 | 2.284 | 584.626 | 0.945 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, L.; Zhou, J.; Kitazawa, D. Electrostatic Interactions Override Surface Area Effects in Size-Dependent Adsorptive Removal of Microplastics by Fe3O4 Nanoparticles. Sustainability 2025, 17, 8878. https://doi.org/10.3390/su17198878
Hu L, Zhou J, Kitazawa D. Electrostatic Interactions Override Surface Area Effects in Size-Dependent Adsorptive Removal of Microplastics by Fe3O4 Nanoparticles. Sustainability. 2025; 17(19):8878. https://doi.org/10.3390/su17198878
Chicago/Turabian StyleHu, Lei, Jinxin Zhou, and Daisuke Kitazawa. 2025. "Electrostatic Interactions Override Surface Area Effects in Size-Dependent Adsorptive Removal of Microplastics by Fe3O4 Nanoparticles" Sustainability 17, no. 19: 8878. https://doi.org/10.3390/su17198878
APA StyleHu, L., Zhou, J., & Kitazawa, D. (2025). Electrostatic Interactions Override Surface Area Effects in Size-Dependent Adsorptive Removal of Microplastics by Fe3O4 Nanoparticles. Sustainability, 17(19), 8878. https://doi.org/10.3390/su17198878







