The Biosorption of Cadmium, Lead, and Arsenic Using Garlic Byproducts and Their Potential for Metal Immobilization in Soil
Abstract
1. Introduction
2. Materials and Methods
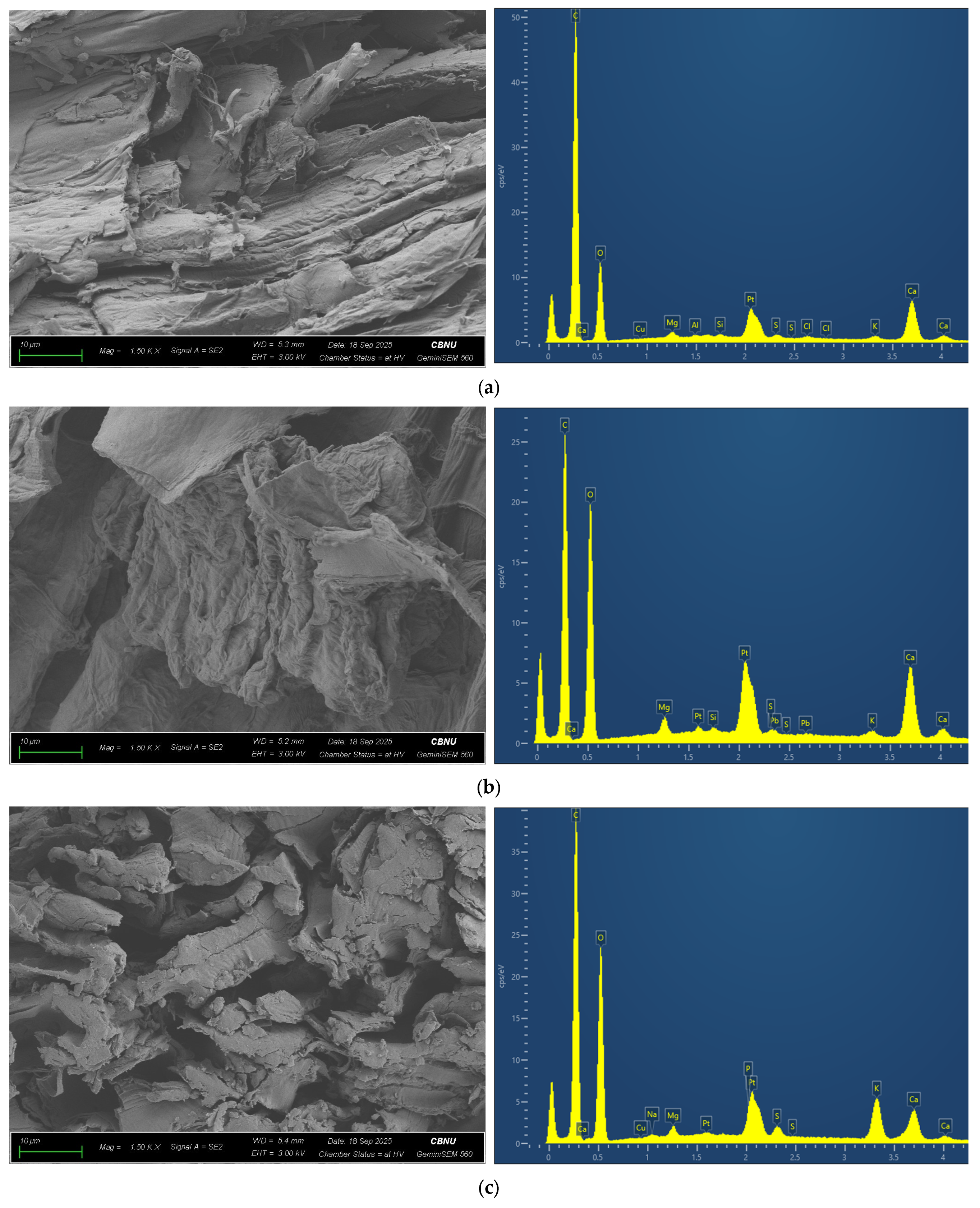
2.1. Preparation of Soil and Garlic Byproducts
2.2. Batch Adsorption of Cd, Pb, and As by Garlic Stem and Peel
2.3. Incubation of Metal Contaminated Soil with Garlic Stem and Peel
2.4. Column Incubation of Pb-Contaminated Soil with Garlic Stem
2.5. Statistical Analysis
3. Results and Discussion
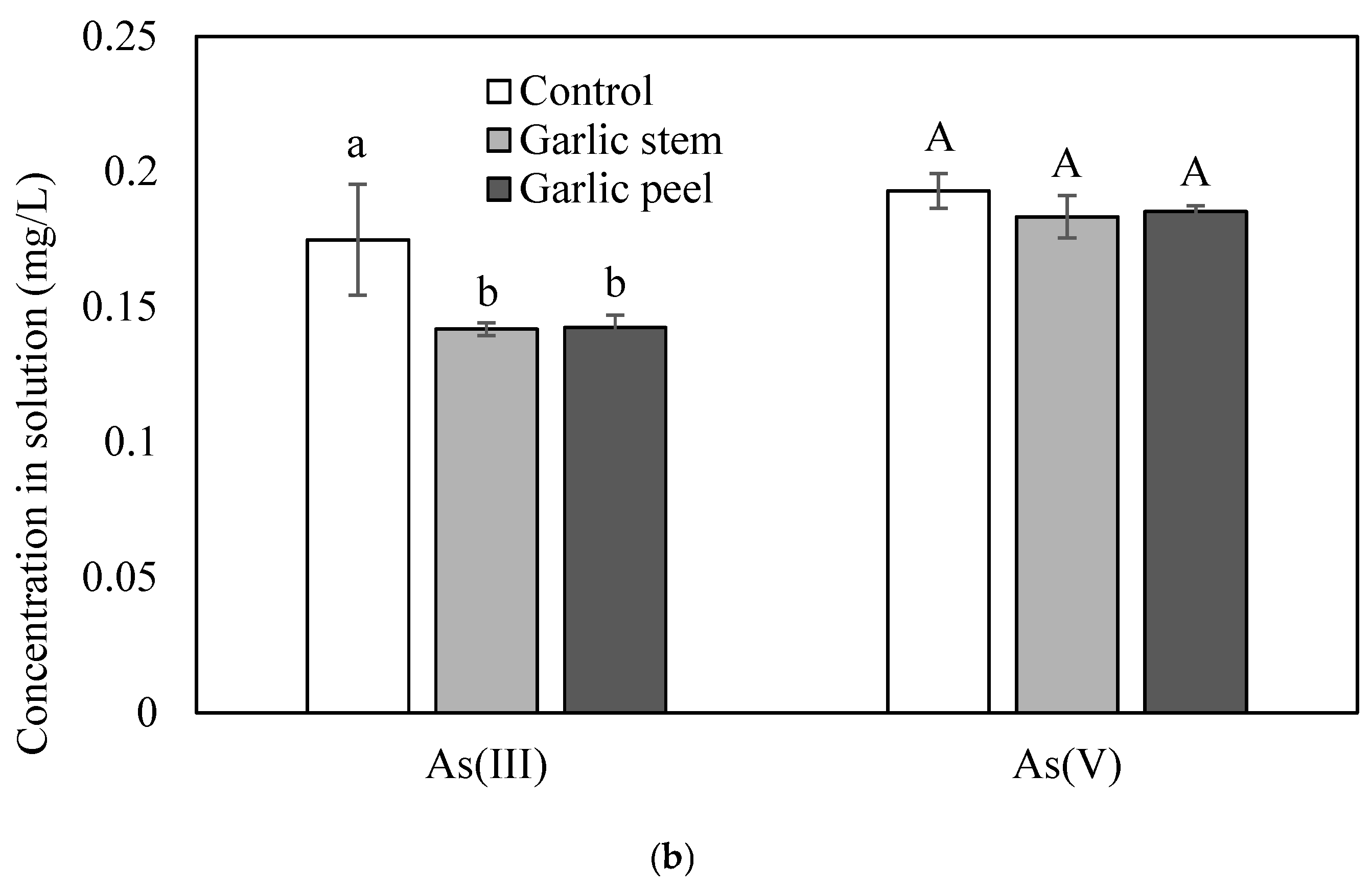
3.1. Removal of Cd, Pb and As Using Garlic Stem and Peel
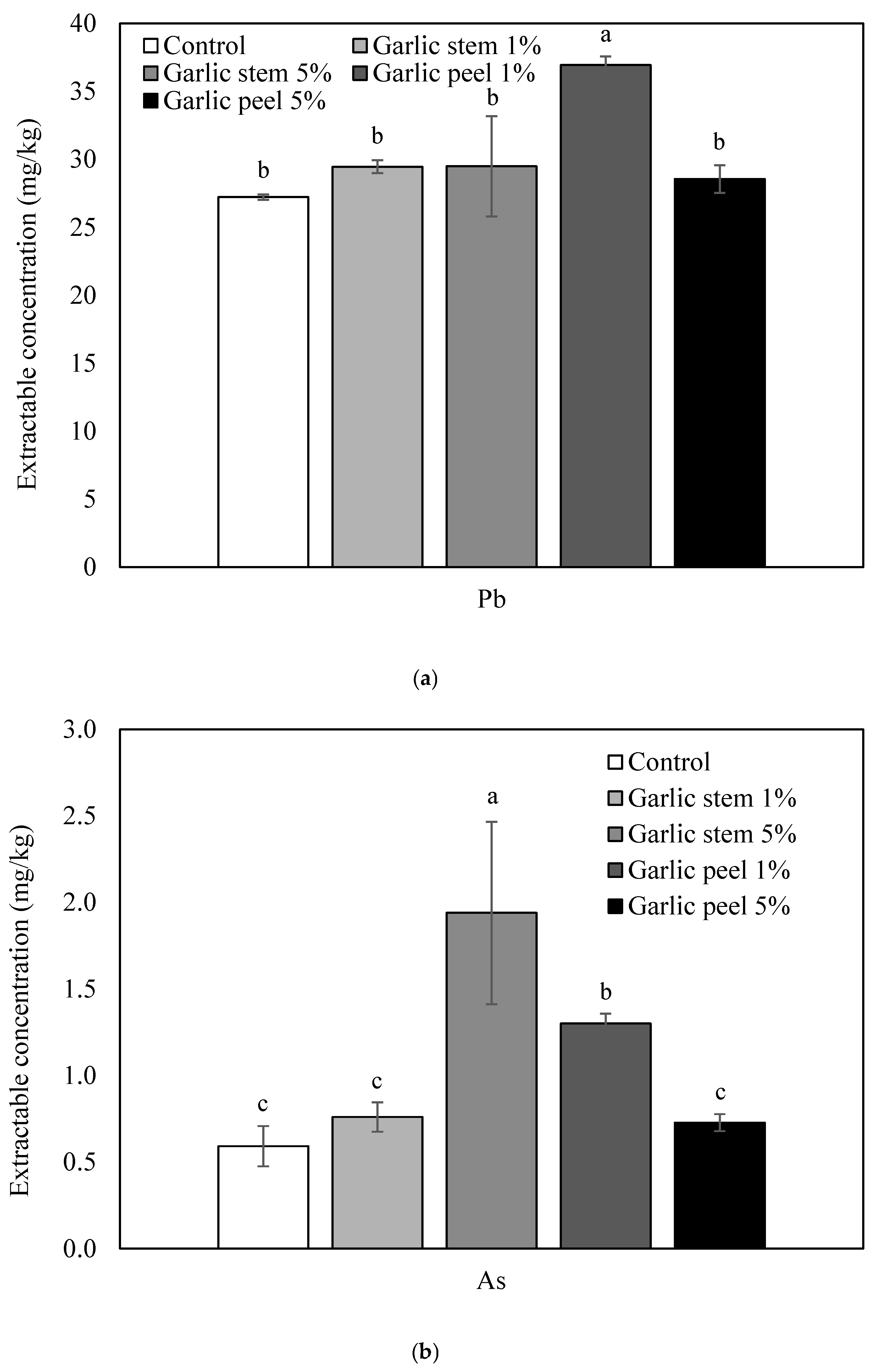
3.2. Immobilization of Pb and As Using Garlic Stem and Peel
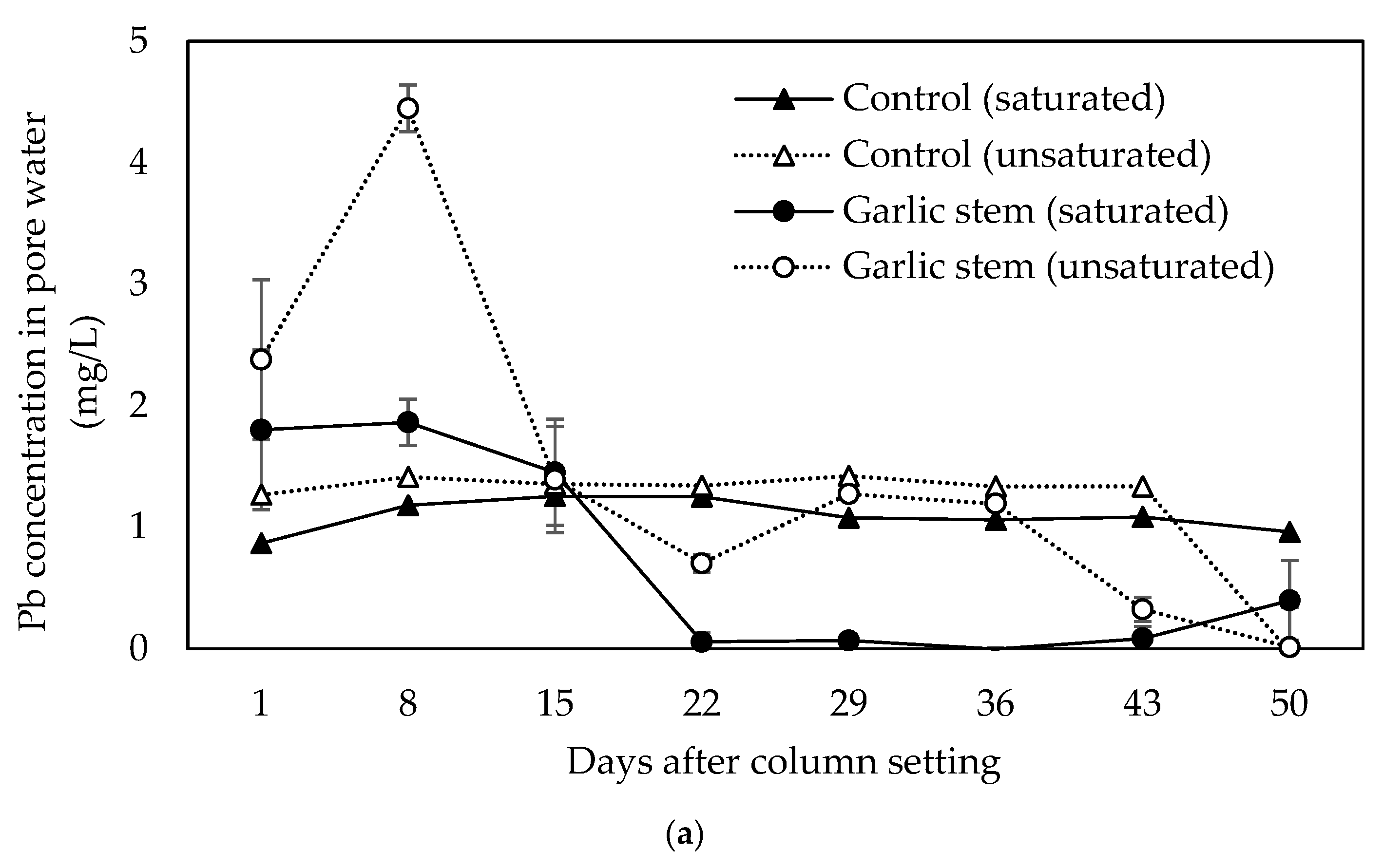
3.3. Lead Release in Saturated and Unsaturated Soil Column
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ICP-OES | Inductively coupled plasma–optical emission spectroscopy |
| EC | Electrical conductivity |
References
- Niyogi, A.; Sarkar, P.; Bhattacharyya, S.; Pal, S.; Mukherjee, S. Harnessing the potential of agriculture biomass: Reuse, transformation and applications in energy and environment. Environ. Sci. Pollut. Res. 2024, 32, 19180–19203. [Google Scholar] [CrossRef]
- Alalwan, H.A.; Kadhom, M.A.; Alminshid, A.H. Removal of heavy metals from wastewater using agricultural byproducts. J. Water Supply Res. Technol. AQUA 2020, 69, 99–112. [Google Scholar] [CrossRef]
- Liu, G.; Dai, Z.; Liu, X.; Dahlgren, R.A.; Xu, J. Modification of agricultural wastes to improve sorption capacities for pollutant removal from water—A review. Carbon Res. 2022, 1, 24. [Google Scholar] [CrossRef]
- Simón, D.; Palet, C.; Costas, A.; Cristóbal, A. Agro-industrial waste as potential heavy metal adsorbents and subsequent safe disposal of spent adsorbents. Water 2022, 14, 3298. [Google Scholar] [CrossRef]
- Ungureanu, E.L.; Mocanu, A.L.; Stroe, C.A.; Panciu, C.M.; Berca, L.; Sionel, R.M.; Mustatea, G. Agricultural byproducts used as low-cost adsorbents for removal of potentially toxic elements from wastewater: A comprehensive review. Sustainability 2023, 15, 5999. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- Nguyen, T.A.; Ngo, H.H.; Guo, W.S.; Zhang, J.; Liang, S.; Yue, Q.Y.; Li, Q.; Nguyen, T.V. Applicability of agricultural waste and by-products for adsorptive removal of heavy metals from wastewater. Bioresour. Technol. 2013, 148, 574–585. [Google Scholar] [CrossRef]
- Elhafez, S.A.; Hamad, H.A.; Zaatout, A.A.; Malash, G.F. Management of agricultural waste for removal of heavy metals from aqueous solution: Adsorption behaviors, adsorption mechanisms, environmental protection, and techno-economic analysis. Environ. Sci. Pollut. Res. 2017, 24, 1397–1415. [Google Scholar] [CrossRef]
- Herrera, A.; Tejada-Tovar, C.; González-Delgado, Á.D. Enhancement of cadmium adsorption capacities of agricultural residues and industrial fruit byproducts by the incorporation of Al2O3 nanoparticles. ACS Omega 2020, 5, 23645–23653. [Google Scholar] [CrossRef]
- Oliveira, E.A.; Montanher, S.F.; Andrade, A.D.; Nobrega, J.A.; Rollemberg, M.C. Equilibrium studies for the sorption of chromium and nickel from aqueous solutions using raw rice bran. Process Biochem. 2005, 40, 3485–3490. [Google Scholar] [CrossRef]
- Zhu, C.S.; Wang, L.P.; Chen, W.B. Removal of Cu (II) from aqueous solution by agricultural by-product: Peanut hull. J. Hazard. Mater. 2009, 168, 739–746. [Google Scholar] [CrossRef]
- Chang, Q.; Li, P.; Han, Y.; Guan, X.; Xiong, J.; Li, Q.; Zhang, H.; Huang, K.; Zhang, X.; Xie, H.; et al. Ultra-low concentration terbium (Tb) adsorption on garlic peels biosorbent and its application for Nd-Fe-B scraps recovery. J. Environ. Chem. Eng. 2023, 11, 109997. [Google Scholar] [CrossRef]
- Sun, J.; Li, X.; Ai, X.; Liu, J.; Yin, Y.; Huang, Y.; Zhou, H.; Huang, K. Efficient removal of cadmium from soil-washing effluents by garlic peel biosorbent. Environ. Sci. Pollut. Res. 2018, 25, 19001–19011. [Google Scholar] [CrossRef] [PubMed]
- Benkeblia, N.; Lanzotti, V. Allium thiosulfinates: Chemistry, biological properties and their potential utilization in food preservation. Food 2007, 1, 193–201. [Google Scholar]
- Pohl, A. Removal of heavy metal ions from water and wastewaters by sulfur-containing precipitation agents. Water Air Soil Pollut. 2020, 231, 503. [Google Scholar] [CrossRef]
- Raj, D.; Maiti, S.K. Sources, bioaccumulation, health risks and remediation of potentially toxic metal(loid)s (As, Cd, Cr, Pb and Hg): An epitomised review. Environ. Monit. Assess. 2020, 192, 108. [Google Scholar] [CrossRef]
- Bouida, L.; Rafatullah, M.; Kerrouche, A.; Qutob, M.; Alosaimi, A.M.; Alorfi, H.S.; Hussein, M.A. A review on cadmium and lead contamination: Sources, fate, mechanism, health effects and remediation methods. Water 2022, 14, 3432. [Google Scholar] [CrossRef]
- Sani, A.H.; Amanabo, M. Lead: A concise review of its toxicity, mechanism and health effect. GSC Biol. Pharm. Sci. 2021, 15, 55–62. [Google Scholar]
- Ng, J.C. Environmental contamination of arsenic and its toxicological impact on humans. Environ. Chem. 2005, 2, 146–160. [Google Scholar] [CrossRef]
- Kallel, F.; Ellouz Chaabouni, S. Perspective of garlic processing wastes as low-cost substrates for production of high-added value products: A review. Environ. Prog. Sustain. Energy 2017, 36, 1765–1777. [Google Scholar] [CrossRef]
- Sebeia, N.; Jabli, M.; Sonsudin, F. Garlic Cellulosic Powders with Immobilized AgO and CuO Nanoparticles: Preparation, Characterization of the Nanocomposites, and Application to the Catalytic Degradation of Azo Dyes. Polymers 2024, 16, 1661. [Google Scholar] [CrossRef]
- Oyaluna, Z.E.; Abolaji, A.O.; Bodede, O.; Olanlokun, J.O.; Prinsloo, G.; Steenkamp, P.; Babalola, C.P. Chemical analysis of Alliin-Rich Allium sativum (Garlic) extract and its safety evaluation in Drosophila melanogaster. Toxicol. Rep. 2024, 13, 101760. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, Y.; Tao, Y.; Yu, Y.; Jiang, H.; Lian, H. Comparative study of adsorption of Pb (II) on native garlic peel and mercerized garlic peel. Environ. Sci. Pollut. Res. 2014, 21, 2054–2063. [Google Scholar] [CrossRef] [PubMed]
- Hoa, T.T.; Liamleam, W.; Annachhatre, A.P. Lead removal through biological sulfate reduction process. Bioresour. Technol. 2007, 98, 2538–2548. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Xu, G.; Yu, H. Removal of lead from aqueous solutions by ferric activated sludge-based adsorbent derived from biological sludge. Arab. J. Chem. 2019, 12, 4142–4149. [Google Scholar] [CrossRef]
- Soleymani-Bonoti, F.; Kazemi, D.; Hajimiri, I.; Imani, A. Biosorption of Cd2+, Ni2+ and Zn2+ from Contaminated Well Water by Garlic Peel. Fine Chem. Eng. 2024, 5, 304–318. [Google Scholar] [CrossRef]
- Liang, S.; Guo, X.; Tian, Q. Adsorption of Pb2+, Cu2+ and Ni2+ from aqueous solutions by novel garlic peel adsorbent. Desalin. Water Treat. 2013, 51, 7166–7171. [Google Scholar] [CrossRef]
- Sharma, G.; Verma, Y.; Lai, C.W.; Naushad, M.; Iqbal, J.; Kumar, A.; Dhiman, P. Biochar and biosorbents derived from biomass for arsenic remediation. Heliyon 2024, 10, e36288. [Google Scholar] [CrossRef]
- Kamala, C.T.; Chu, K.H.; Chary, N.S.; Pandey, P.K.; Ramesh, S.L.; Sastry, A.R.; Sekhar, K.C. Removal of arsenic (III) from aqueous solutions using fresh and immobilized plant biomass. Water Res. 2005, 39, 2815–2826. [Google Scholar] [CrossRef]
- Prajapati, A.K.; Mondal, M.K. Hazardous As (III) removal using nanoporous activated carbon of waste garlic stem as adsorbent: Kinetic and mass transfer mechanisms. Korean J. Chem. Eng. 2019, 36, 1900–1914. [Google Scholar] [CrossRef]
- Wang, W.; Zhu, Y.; Qu, J. Effect of DOM derived from composting on the changes of Pb bioactivity in black soil. J. Environ. Chem. Eng. 2024, 12, 112232. [Google Scholar] [CrossRef]
- Martínez, C.E.; Jacobson, A.R.; McBride, M.B. Aging and temperature effects on DOC and elemental release from a metal contaminated soil. Environ. Pollut. 2003, 122, 135–143. [Google Scholar] [CrossRef]
- Kalbitz, K.; Wennrich, R. Mobilization of heavy metals and arsenic in polluted wetland soils and its dependence on dissolved organic matter. Sci. Total Environ. 1998, 209, 27–39. [Google Scholar] [CrossRef]
- Qiao, W.; Guo, H.; He, C.; Shi, Q.; Xiu, W.; Zhao, B. Molecular evidence of arsenic mobility linked to biodegradable organic matter. Environ. Sci. Technol. 2020, 54, 7280–7290. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.B.; Kim, S.H.; Jeon, E.K.; Kim, D.H.; Tsang, D.C.; Alessi, D.S.; Kwon, E.E.; Baek, K. Effect of dissolved organic carbon from sludge, rice straw and spent coffee ground biochar on the mobility of arsenic in soil. Sci. Total Environ. 2018, 636, 1241–1248. [Google Scholar] [CrossRef] [PubMed]
- Brink, H.G.; Hörstmann, C.; Peens, J. Microbial Pb(II)-precipitation: The influence of oxygen on Pb(II)-removal from aqueous environment and the resulting precipitate identity. Int. J. Environ. Sci. Technol. 2020, 17, 409–420. [Google Scholar] [CrossRef]
- Bloem, E.; Haneklaus, S.; Schnug, E. Influence of nitrogen and sulfur fertilization on the alliin content of onions and garlic. J. Plant Nutr. 2005, 27, 1827–1839. [Google Scholar] [CrossRef]
- Schwab, A.P.; He, Y.; Banks, M.K. The influence of organic ligands on the retention of lead in soil. Chemosphere 2005, 61, 856–866. [Google Scholar] [CrossRef]
- Yang, L.; Chen, L.; Zhuang, W.E.; Zhu, Z. Unveiling changes in the complexation of dissolved organic matter with Pb (II) by photochemical and microbial degradation using fluorescence EEMs-PARAFAC. Environ. Pollut. 2024, 341, 122982. [Google Scholar] [CrossRef]




| Soil Texture | CEC (cmolc/kg) | As (mg/kg) | Cd (mg/kg) | Cu (mg/kg) | Pb (mg/kg) | Zn (mg/kg) | |
|---|---|---|---|---|---|---|---|
| Soil for incubation | Sandy loam | 3.01 | 254 ± 21 | 22 ± 1 | ND * | 2032 ± 194 | 222 ± 8 |
| Soil for column | Sandy loam | 2.82 | 20 ± 3 | 7.7 ± 2.5 | 116 ± 66 | 1613 ± 166 | 88 ± 14 |
| Ca (mg/kg) | K (mg/kg) | Mg (mg/kg) | Fe (mg/kg) | P (mg/kg) | S (mg/kg) | Cd (mg/kg) | Pb (mg/kg) | As (mg/kg) | |
|---|---|---|---|---|---|---|---|---|---|
| Garlic stem | 873.9 ± 28.5 | 2445.1 ± 44.2 | 137.9 ± 0.7 | 7.2 ± 0.1 | 604.8 ± 17.7 | 1138 ± 34.4 | ND * | ND | 0.044 ± 0.01 |
| Garlic peel | 983.1 ± 33 | 634.7 ± 3 | 100.4 ± 2.1 | 6.3 ± 1.4 | 549.7 ± 25.9 | 1323.1 ± 56.1 | ND | ND | 0.079 ± 0.034 |
| pH | EC (μS/cm) | DOC (mg/kg) | |
|---|---|---|---|
| Control | 5.10 ± 0.14 b | 82.7 ± 4.1 e | 68.4 ± 0.6 e |
| Garlic stem 1% | 5.76 ± 0.36 a | 322.1 ± 6.0 b | 860.1 ± 5.7 c |
| Garlic stem 5% | 5.93 ± 0.04 a | 143.0 ± 0.9 d | 4140.2 ± 16.7 a |
| Garlic peel 1% | 5.99 ± 0.51 a | 177.7 ± 5.0 c | 480.4 ± 1.2 d |
| Garlic peel 5% | 5.66 ± 0.05 a | 632.1 ± 29.2 a | 3751.0 ± 9.7 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.H. The Biosorption of Cadmium, Lead, and Arsenic Using Garlic Byproducts and Their Potential for Metal Immobilization in Soil. Sustainability 2025, 17, 8857. https://doi.org/10.3390/su17198857
Park JH. The Biosorption of Cadmium, Lead, and Arsenic Using Garlic Byproducts and Their Potential for Metal Immobilization in Soil. Sustainability. 2025; 17(19):8857. https://doi.org/10.3390/su17198857
Chicago/Turabian StylePark, Jin Hee. 2025. "The Biosorption of Cadmium, Lead, and Arsenic Using Garlic Byproducts and Their Potential for Metal Immobilization in Soil" Sustainability 17, no. 19: 8857. https://doi.org/10.3390/su17198857
APA StylePark, J. H. (2025). The Biosorption of Cadmium, Lead, and Arsenic Using Garlic Byproducts and Their Potential for Metal Immobilization in Soil. Sustainability, 17(19), 8857. https://doi.org/10.3390/su17198857








