Phosphogypsum as the Secondary Source of Rare Earth Elements
Abstract
1. Introduction
2. Industrial Production of Phosphoric Acid
3. Rare Earth Elements Usage, World Reserves, and Demand
3.1. REEs from Primary Raw Material
3.2. REEs from Secondary Raw Material
3.3. REEs and Trace Metals in Phosphate Rock
3.4. Incorporation and Occurrence of Metal Ions in PG
- ▪
- Interstitial incorporation
- ▪
- Coprecipitation
- ▪
- Isomorphous substitution
- ▪
- Diffusion from the bulk solution towards the crystal surface
- ▪
- Adsorption at the surface
- ▪
- Surface diffusion towards the steps upon the surface
- ▪
- Incorporation in the kink sites
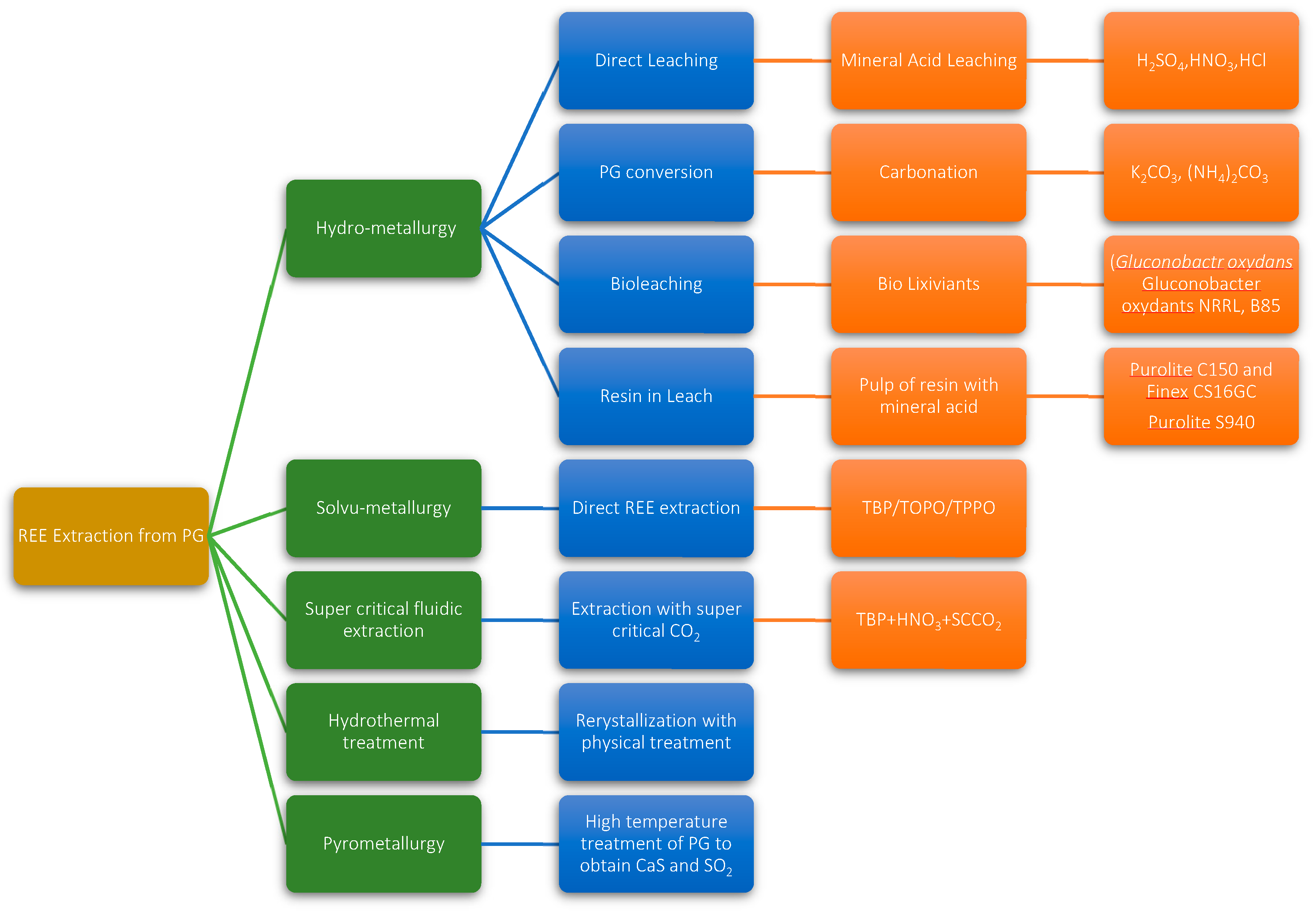
4. Review of Existing Research on REE Recovery from Phosphogypsum
- Hydrometallurgical methods
- Solvo-metallurigical methods
- Supercritical CO2 extraction
- Hydrothermal methods
- Pyrometallurgical methods
4.1. Hydrometallurgical Methods
- ▪
- Leaching (dissolution)
- ▪
- Purification and concentration
- ▪
- Metal recovery (precipitation or electrowinning)
4.1.1. Mineral Acid Leaching
- Effect of Leaching Parameters on REE Leaching Efficiency
- Acid Type and Concentration
- Leaching Temperature
- Particle Size
- Solid-to-Liquid Ratio
- Leaching Kinetics
- Multi-Parameter Effects on REE Recovery from PG
4.1.2. Phosphogypsum Conversion
4.1.3. Bioleaching
4.1.4. Resin in Leach
4.2. Solvo-Metallurgical Leaching
4.3. Super Critical Fluidic Extraction
4.4. Hydrothermal Treatment of Phosphogypsum
4.5. Thermal Decomposition of Phosphogypsum
4.6. Comparative Overview of REE Recovery Methods
5. Summary of Current Challenges
- Origin of phosphate rock: Sedimentary versus igneous rocks have different accessory minerals and trace elements.
- Production process: Dihydrate, hemihydrate, or hemi-dihydrate processes yield PG with different crystal structures and impurity levels.
- Impurities mixed in the crystal lattice or surface: PG often holds silica, fluorides, phosphates, clays, and traces of metals (Fe, Al, Zn, Cd, Cu, etc.) and radionuclides (U, Th, Ra).
- Particle size distribution: Some PG is fine and porous, while others are coarse and crystalline, which may affect leaching and adsorption.
- Industrial bottlenecks: The very low concentration of REEs in PG (generally 0.2–1 wt% of total PG, often < 0.1% recoverable), the need to process large volumes of waste, high chemical consumption, scaling issues when moving from batch leaching to continuous systems, and the lack of standardized pretreatment methods to handle impurities and radionuclides.
- Policy support suggestions: Lack of regulatory frameworks to encourage PG valorization instead of stockpiling, international collaboration to establish safety and reuse standards for decontaminated PG residues, and government incentives (e.g., subsidies or tax credits) for pilot plants that integrate REE recovery with PG decontamination.
Future Directions
- Open-Access Global PG Characterization Database: Collaboration of international institutes and industries is necessary to create a shared database with PG characterization data (REE, impurities, particle size, etc.). A research and industry platform to standard processes and help knowledge sharing for adapting REE recovery to various PG sources.
- Pretreatment Protocol Based on PG Typology: Categorization of PG based on origin (igneous, sedimentary, weathered) and production process (dihydrate, hemihydrate, etc.), then create modular pretreatment protocols (e.g., washing, pH adjustment, thermal treatment, flotation). AI-assisted database matching PG type to develop more specific pretreatment recipes to maximize REE mobilization and impurity removal.
- Adaptive design for Resin-In-Leach Modules: Develop switchable resins/solvents (pH or ion-specific triggers) that adapt their affinity based on the PG composition, increasing selectivity across PG types.
- Co-Product Valorization for Circularity: To minimize treatment cost it will be beneficial to convert decontaminated PG residues into construction materials, agricultural additives, or fillers. Develop standards for safe reuse based on metal leachability and REE residue levels to turn waste into certified products, offsetting treatment costs.
- Utilize Machine Learning for Process Predictability: Apply machine learning models trained on datasets from various PG sources to predict best leaching, sorption, and precipitation conditions. A smart PG profiler tool that shows the ideal treatment path using real-time PG characterization data (XRF, ICP, pH, etc.).
- Modular Pilot Plant Concept for Flexible Scaling: Design a containerized pilot system with interchangeable modules for different steps (pretreatment, leaching, separation). Enables on-site testing and adaptation to local PG composition without building entirely new infrastructure.
- Techno-Economic and LCA: Techno-economic assessment (TEA) and life cycle analysis (LCA) must be included from the first design stage. A dynamic tool can be used to update environmental and cost indicators in real time as the process adopts different PG types or scales.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
Abbreviations
| PR | Phosphate rock |
| PA | Phosphoric acid |
| PG | Phosphogypsum |
| WPA | Wet phosphoric acid |
| REE | Rare earth elements |
| S | Sedimentary |
| M | Magmatic |
| NR | Not reported |
Appendix A
| Rock Type | Sedimentary | Magmatic | Mixed | |||
|---|---|---|---|---|---|---|
| Method | HDH Hitashi | HDH Hydro | DH Jacob | DH Prayon (S) | DH Prayon (M) | HRC Kemira |
| CaO% | 30.53% | 33.65% | 33.73% | 35.20% | 35.85% | 36.42% |
| SO3% | 44.83% | 45.82% | 42.50% | 44.47% | 43.49% | NR |
| SiO2% | 4.70% | 6.82% | 4.38% | 5.13% | 2.39% | 3.25% |
| Al2O3% | 0.08% | 0.14% | 0.36% | 0.19% | 0.36% | ND |
| Fe2O3% | 0.18% | 0.06% | 0.12% | 0.18% | 0.25% | 0.03% |
| P2O5% | 1.31% | 0.28% | 0.78% | 0.52% | 0.70% | 0.38% |
| Soluble P2O5 | 0.23% | NR 1 | NR | 0.37% | 0.08% | 0.02% |
| Na2O% | 0.06% | 0.06% | 0.08% | 0.04% | 0.07% | 0.02% |
| K2O% | 0.03% | 0.02% | 0.10% | 0.01% | 0.05% | 0.02% |
| TiO2% | 0.03% | 0.02% | 0.03% | 0.03% | 0.29% | ND |
| MgO% | 0.04% | 0.07% | 0.02% | <0.01% | 0.04% | ND |
| MnO% | 0.01% | 0.01% | NR | <0.01% | 0.01% | ND |
| F% | 0.55% | 0.61% | 0.64% | 0.45% | 0.55% | 0.05% |
| Soluble F | 0.21% | 0.35% | 0.11% | 0.31% | 0.24% | 0.11% |
| CO2% | 0.21% | 0.56% | 0.39% | 0.35% | 0.28% | 0.12% |
| Organic C | 0.01% | 0.11% | 0.05% | 0.04% | 0.02% | 0.01% |
| Chloride as Cl | 1.78 mg/L | 1.86 mg/L | 0.88 mg/L | 2.4 mg/L | NR | NR |
| Nitrate | 8.70 mg/L | 6.83 mg/L | 6.86 mg/L | 10.02 mg/L | NR | NR |
| Purity | 93.27% | 94.87% | 91.83% | NR | 95.56% | NR |
| Rock Type | Sedimentary | Magmatic | Mixed | |||
|---|---|---|---|---|---|---|
| Method | HDH Hitashi | HDH Hydro | DH Jacob | DH Prayon (S) | DH Prayon (M) | HRC Kemira |
| As | NR | NR | NR | 2 ppm | NR | NR |
| Co | 1 ppm | 1 ppm | 1 ppm | <1 ppm | 5 ppm | 1 ppm |
| Zn | 49 ppm | 48 ppm | 34 ppm | 44 ppm | 14 ppm | 8 ppm |
| Pb | 6 ppm | 2 ppm | 2 ppm | 4 ppm | <1 ppm | ND |
| Cd | 1 ppm | 2 ppm | 2 ppm | 3 ppm | <1 ppm | ND |
| Ni | 3 ppm | 2 ppm | 1 ppm | 4 ppm | 1 ppm | ND |
| V | 4 ppm | 2 ppm | 4 ppm | 2 ppm | 7 ppm | 1 ppm |
| Cr | 4 ppm | 5 ppm | 7 ppm | 14 ppm | 4 ppm | 3 ppm |
| Cu | 9 ppm | 60 ppm | 1 ppm | 33 ppm | 5 ppm | 10 ppm |
| Ba | 76 ppm | 109 ppm | 150 ppm | 53 ppm | 207 ppm | 39 ppm |
| Sr | 569 ppm | 801 ppm | 402 ppm | NR | 7692 ppm | 2099 ppm |
| Th | 1 ppm | 1 ppm | 1 ppm | 3 ppm | 42 ppm | 4 ppm |
| U | 2 ppm | 5 ppm | 3 ppm | <1 ppm | 52 ppm | 8 ppm |
| Rock Type | Sedimentary | Magmatic | NR 1 | Sedimentary | Magmatic | NR |
|---|---|---|---|---|---|---|
| Method | HDH Hitashi | HDH Hydro | DH Jacob | DH Prayon | DH Prayon | HRC Kemira |
| Y | 18 ppm | 27 ppm | 72 ppm | 140 ppm | 133 ppm | 27 ppm |
| La | 9 ppm | 14 ppm | 39 ppm | 49 ppm | 1215 ppm | 165 ppm |
| Ce | 10 ppm | 15 ppm | 20 ppm | 31 ppm | 1998 ppm | 246 ppm |
| Nd | 7 ppm | 11 ppm | 25 ppm | 37 ppm | 921 ppm | NR |
| Sm | 1 ppm | 2 ppm | 6 ppm | NR | NR 2 | NR |
| Eu | <1 ppm | 1 ppm | 1 ppm | 2 ppm | NR | NR |
| Gd | 1 ppm | 2 ppm | 7 ppm | 10 ppm | NR | NR |
| Tb | <1 ppm | <1 ppm | 1 ppm | 3 ppm | NR | NR |
| Dy | 1 ppm | 2 ppm | 7 ppm | NR | NR | NR |
| Ho | <1 ppm | <1 ppm | 2 ppm | 2 ppm | NR | NR |
| Er | 1 ppm | 1 ppm | 5 ppm | 6 ppm | NR | NR |
| Tm | <1 ppm | <1 ppm | 1 ppm | NR | NR | NR |
| Yb | 1 ppm | 1 ppm | 4 ppm | 8 ppm | NR | NR |
| Lu | <1 ppm | <1 ppm | 1 ppm | 1 ppm | NR | NR |
| ∑REEs + Y | 49 ppm | 78 ppm | 180 ppm | 289 ppm | 3960 ppm | 438 ppm |
| Rock Type | Sedimentary | Magmatic | NR | Sedimentary | Magmatic | NR |
|---|---|---|---|---|---|---|
| Method | HDH Hitashi | HDH Hydro | DH Jacob | DH Prayon | DH Prayon | HRC Kemira |
| 226Ra | 211 Bq/kg | 596 Bq/kg | 414 Bq/kg | 562 Bq/kg | 267 Bq/kg | NR |
| 232Th | 5 Bq/kg | <7 Bq/kg | 5 Bq/kg | 32 Bq/kg | 185 Bq/kg | NR |
| 235U | <7 Bq/kg | <16 Bq/kg | 73 Bq/kg | <9 Bq/kg | 261 Bq/kg | NR |
| 40K | <10 Bq/kg | <26 Bq/kg | 60 Bq/kg | <12 Bq/kg | NR | NR |
| Radio activity | 0.73 | 1.99 | 1.71 | 2.03 | NR | NR |
References
- U.S. Geological Survey. Mineral Commodity Summaries 2025; ver. 1.2, March 2025; U.S. Geological Survey: Reston, VA, USA, 2025; 212p. [Google Scholar] [CrossRef]
- Gilmour, R. Phosphoric Acid: Purification, Uses, Technology, and Economics, 1st ed.; CRC Press: Boca Raton, FL, USA, 2013. [Google Scholar] [CrossRef]
- Nadim, F. Fuleihan Phosphogypsum Disposal-The Pros & Cons of Wet Versus Dry Stacking. Procedia Eng. 2012, 46, 195–205. [Google Scholar] [CrossRef]
- Ben Abdelouahed, H.; Reguigui, N. Radiotracer investigation of phosphoric acid and phosphatic fertilizers production process. J. Radioanal. Nucl. Chem. 2011, 289, 103–111. [Google Scholar] [CrossRef]
- Klugh, B.G. Thermal Production of Phosphoric Acid. Ind. Eng. Chem. 1932, 24, 371–374. [Google Scholar] [CrossRef]
- Leder, F.; Park, W.C.; Chang, P.; Ellis, J.D.; Megy, J.A.; Hard, R.A.; Kyle, H.E.; Mu, J.; Shaw, B.W. New process for technical-grade phosphoric acid. Ind. Eng. Chem. Process Des. Dev. 1985, 24, 688–697. [Google Scholar] [CrossRef]
- Dahlgren, S.-E. Fertilizer Materials, Calcium Sulfate Transitions in Superphosphate. J. Agric. Food Chem. 1960, 8, 411–412. [Google Scholar] [CrossRef]
- Sevim, F.; Saraç, H.; Kocakerim, M.M.; Yartaşı, A. Dissolution Kinetics of Phosphate Ore in H2SO4 Solutions. Ind. Eng. Chem. Res. 2003, 42, 2052–2057. [Google Scholar] [CrossRef]
- Prayon Processes for Phosphoric Acid Production. Available online: https://www.prayon.com/en/processes/dihydrate/ (accessed on 2 May 2025).
- U.S. Geological Survey (USGS). Mineral Commodity Summaries: Rare Earths. 2023. Available online: https://www.usgs.gov (accessed on 2 April 2025).
- Balaram, V. Rare earth elements: A review of applications, occurrence, exploration, analysis, recycling, and environmental impact. Geosci. Front. 2019, 10, 1285–1303. [Google Scholar] [CrossRef]
- Ramos, S.J.; Dinali, G.S.; Oliveira, C.; Martins, G.C.; Moreira, C.G.; Siqueira, J.O.; Guilherme, L.R.G. Rare earth elements in the soil environment. Curr. Pollut. Rep. 2016, 2, 28–50. [Google Scholar] [CrossRef]
- Emsbo, P.; McLaughlin, P.I.; Breit, G.N.; Bray, E.A.D.; Koenig, A.E. Rare earth elements in sedimentary phosphate deposits: Solution to the global REE crisis? Gondwana Res. 2015, 27, 776–785. [Google Scholar] [CrossRef]
- Nugraheni, R.D.; Sunjaya, D.; Burhannudinnur, M. The enrichment mechanism of rare earth elements in weathered granitoids, tin placer and bauxite laterite. Int. J. Sci. Technol. Res. 2020, 9, 1506–1511. [Google Scholar]
- Verplanck, P.L.; Van Gosen, B.S.; Seal, R.R.; McCafferty, A.E. A Deposit Model for Carbonatite and Peralkaline Intrusion-Related Rare Earth Element Deposits; Scientific Investigations Report 2010–5070-J; U.S. Geological Survey: Reston, VA, USA, 2014; p. 58. [Google Scholar] [CrossRef]
- Weng, Z.; Jowitt, S.M.; Mudd, G.M.; Haque, N. A Detailed Assessment of Global Rare Earth Element Resources: Opportunities and Challenges. Econ. Geol. 2015, 110, 1925–1952. [Google Scholar] [CrossRef]
- Möller, P.; Siebert, C. Cycling of calcite and hydrous metal oxides and chemical changes of major element and REE chemistry in monomictic hardwater lake: Impact on sedimentation. Geochemistry 2016, 76, 133–148. [Google Scholar] [CrossRef]
- Zhu, W.; Kennedy, M.; de Leer, E.W.B.; Zhou, H.; Alaerts, G.J.F.R. Distribution and modelling of rare earth elements in Chinese river sediments. Sci. Total Environ. 1997, 204, 233–243. [Google Scholar] [CrossRef]
- Akcil, A.; Akhmadiyeva, N.; Abdulvaliyev, R.; Abhilash; Meshram, P. Overview On Extraction and Separation of Rare Earth Elements from Red Mud: Focus on Scandium. Miner. Process. Extr. Metall. Rev. 2018, 39, 145–151. [Google Scholar] [CrossRef]
- Blissett, R.; Smalley, N.; Rowson, N. An investigation into six coal fly ashes from the United Kingdom and Poland to evaluate rare earth element content. Fuel 2014, 119, 236–239. [Google Scholar] [CrossRef]
- Cao, S.; Zhou, C.; Pan, J.-H.; Liu, C.; Tang, M.; Ji, W.; Hu, T.; Zhang, N. Study on Influence Factors of Leaching of Rare Earth Elements from Coal Fly Ash. Energy Fuels 2018, 32, 8000–8005. [Google Scholar] [CrossRef]
- Mukaba, J.-L.; Eze, C.P.; Pereao, O.; Petrik, L.F. Rare Earths’ Recovery from Phosphogypsum: An Overview on Direct and Indirect Leaching Techniques. Minerals 2021, 11, 1051. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Blanpain, B.; Van Gerven, T.; Pontikes, Y. Towards zero-waste valorisation of rare-earth-containing industrial process residues: A critical review. J. Clean. Prod. 2015, 99, 17–38. [Google Scholar] [CrossRef]
- Ihlen, P.M.; Schiellerup, H.; Gautneb, H.; Skår, Ø. Characterization of apatite resources in Norway and their REE potential—A review. Ore Geol. Rev. 2014, 58, 126–147. [Google Scholar] [CrossRef]
- Keane, E. Neodymium Magnets Provide Key to Understanding Rare Earth Trends: Seeking Alpha, June 23. 2009. Available online: http://seekingalpha.com/instablog/345817-eamon-keane/9675-neodymium-magnets-provide-key-to-understanding-rare-earth-trends (accessed on 10 May 2025).
- Rabe, W.; Kostka, G.; Stegen, K.S. China’s supply of critical raw materials: Risks for Europe’s solar and wind industries? Energy Policy 2017, 101, 692–699. [Google Scholar] [CrossRef]
- Tan, Q.; Li, J. Chapter 15—Rare earth metal recovery from typical e-waste. In Waste Electrical and Electronic Equipment (WEEE) Handbook, 2nd ed.; Woodhead Publishing Series in Electronic and Optical Materials; Goodship, V., Stevels, A., Huisman, J., Eds.; Woodhead Publishing: Cambridge, UK, 2019; pp. 393–421. ISBN 9780081021583. [Google Scholar] [CrossRef]
- Buechler, D.T.; Zyaykina, N.N.; Spencer, C.A.; Lawson, E.; Ploss, N.M.; Hua, I. Comprehensive elemental analysis of consumer electronic devices: Rare earth, precious, and critical elements. Waste Manag. 2020, 103, 67–75. [Google Scholar] [CrossRef]
- Ptáček, P. Phosphate Rocks. In Apatites and Their Synthetic Analogues—Synthesis, Structure, Properties and Applications; InTechOpen: London, UK, 2016. [Google Scholar] [CrossRef]
- Pufahl, P.K.; Groat, L.A. Sedimentary and Igneous Phosphate Deposits: Formation and Exploration: An Invited Paper. Econ. Geol. 2017, 112, 483–516. [Google Scholar] [CrossRef]
- Brown, P.W.; Constantz, B. Hydroxyapatite and Related Materials; CRC Press: Boca Raton, FL, USA, 1994; Volume 368. [Google Scholar] [CrossRef]
- Broom-Fendley, S.; Heaton, T.; Wall, F.; Gunn, G. Tracing the fluid source of heavy REE mineralisation in carbonatites using a novel method of oxygen-isotope analysis in apatite: The example of Songwe Hill, Malawi. Chem. Geol. 2016, 440, 275–287. [Google Scholar] [CrossRef]
- Kechiched, R.; Laouar, R.; Bruguier, O.; Laouar-Salmi, S.; Ameur-Zaimeche, O.; Foufou, A. Preliminary Data of REE in Algerian Phosphorites: A Comparative Study and Paleo-redox Insights. Procedia Eng. 2016, 138, 19–29. [Google Scholar] [CrossRef]
- Valetich, M.; Zivak, D.; Spandler, C.; Degeling, H.; Grigorescu, M. REE enrichment of phosphorites: An example of the Cambrian Georgina Basin of Australia. Chem. Geol. 2022, 588, 120654. [Google Scholar] [CrossRef]
- Li, Z.; Xie, Z.; He, D.; Deng, J.; Zhao, H.; Li, H. Simultaneous leaching of rare earth elements and phosphorus from a Chinese phosphate ore using H3PO4. Green Process. Synth. 2021, 10, 258–267. [Google Scholar] [CrossRef]
- El-Taher, A.; Ashry, A.; Ene, A.; Almeshari, M.; Zakaly, H. Determination of phosphate rock mines signatures using XRF and ICP-MS elemental analysis techniques: Radionuclides, oxides, rare earth, and trace elements. Rom. Rep. Phys. 2023, 75, 701. [Google Scholar]
- Decrée, S.; Savolainen, M.; Mercadier, J.; Debaille, V.; Höhn, S.; Frimmel, H.; Baele, J.-M. Geochemical and spectroscopic investigation of apatite in the Siilinjärvi carbonatite complex: Keys to understanding apatite forming processes and assessing potential for rare earth elements. Appl. Geochem. 2020, 123, 104778. [Google Scholar] [CrossRef]
- Decrée, S.; Cawthorn, G.; Deloule, E.; Mercadier, J.; Frimmel, H.; Baele, J.-M. Unravelling the processes controlling apatite formation in the Phalaborwa Complex (South Africa) based on combined cathodoluminescence, LA-ICPMS and in-situ O and Sr isotope analyses. Contrib. Miner. Pet. 2020, 175, 34. [Google Scholar] [CrossRef]
- Kalashnikov, A.O.; Konopleva, N.G.; Danilin, K.P. Rare earths of the Murmansk Region, NW Russia: Minerals, extraction technologies and value. Appl. Earth Sci. 2023, 132, 52–61. [Google Scholar] [CrossRef]
- Khelifi, F.; Batool, S.; Kechiched, R.; Padoan, E.; Ncibi, K.; Hamed, Y. Abundance, distribution, and ecological/environmental risks of critical rare earth elements (REE) in phosphate ore, soil, tailings, and sediments: Application of spectroscopic fingerprinting. J. Soils Sediments 2024, 24, 2099–2118. [Google Scholar] [CrossRef]
- Khater, A.E.M.; Galmed, M.A.; Nasr, M.M.; El-Taher, A. Uranium and rare earth elements in Hazm El-Jalamid phosphate, Saudi Arabia: Concentrations and geochemical patterns comparison. Environ. Earth Sci. 2016, 75, 1261. [Google Scholar] [CrossRef]
- Chen, M.; Graedel, T.E. The potential for mining trace elements from phosphate rock. J. Clean. Prod. 2015, 91, 337–346. [Google Scholar] [CrossRef]
- Witkamp, G.J. Crystallization of Calcium Sulfate and Uptake of Impurities. Ph.D. Thesis, De Technische Universiteit Delft, Delft, The Netherlands, 1989. [Google Scholar]
- Bilal, E.; Bellefqih, H.; Bourgier, V.; Mazouz, H.; Dumitraş, D.-G.; Bard, F.; Laborde, M.; Caspar, J.P.; Guilhot, B.; Iatan, E.-L.; et al. Phosphogypsum circular economy considerations: A critical review from more than 65 storage sites worldwide. J. Clean. Prod. 2023, 414, 137561. [Google Scholar] [CrossRef]
- Samrane, K.; Bouhaouss, A. Cadmium in Phosphorous Fertilizers: Balance and Trends. Rasayan J. Chem. 2022, 15, 2022. [Google Scholar] [CrossRef]
- Preston, J.; Cole, P.; Craig, W.; Feather, A. The recovery of rare earth oxides from a phosphoric acid by-product. Part 1: Leaching of rare earth values and recovery of a mixed rare earth oxide by solvent extraction. Hydrometallurgy 1996, 41, 1–19. [Google Scholar] [CrossRef]
- De Vreugd, C.; Witkamp, G.; van Rosmalen, G. Growth of gypsum III. Influence and incorporation of lanthanide and chromium ions. J. Cryst. Growth 1994, 144, 70–78. [Google Scholar] [CrossRef]
- Lokshin, E.P.; Vershkova, Y.A.; Vershkov, A.V.; Tareeva, O.A. Efficiency of Sulfuric Acid Leaching of Lanthanides in Relation to Quality of Phosphosemihydrate Obtained from Khibiny Apatite Concentrate. Russ. J. Appl. Chem. 2002, 75, 1572–1576. [Google Scholar] [CrossRef]
- Lokshin, E.P.; Tareeva, O.A. Activation of leaching of rare earth elements from phosphohemihydrate. Russ. J. Appl. Chem. 2013, 86, 1638–1642. [Google Scholar] [CrossRef]
- Lokshin, E.P.; Tareeva, O.A. Specific features of sulfuric acid leaching-out of lanthanides from phosphohemihydrate. Russ. J. Appl. Chem. 2008, 81, 8–13. [Google Scholar] [CrossRef]
- Lokshin, E.; Tareeva, O.A. Solubility of YF3, CeF3, PrF3, NdF3, and DyF3 in solutions containing sulfuric and phosphoric acids. Russ. J. Inorg. Chem. 2007, 52, 1830–1834. [Google Scholar] [CrossRef]
- Koopman, C.; Witkamp, G.J.; VAN Rosmalen, G.M. Removal of Heavy Metals and Lanthanides from Industrial Phosphoric Acid Process Liquors. Sep. Sci. Technol. 1999, 34, 2997–3008. [Google Scholar] [CrossRef]
- Mahrou, A.; Hakkar, M.; Jouraiphy, R.; Arhouni, F.E.; Mazouz, H.; Boukhair, A.; Fahad, M. Rare earth elements distribution during phosphoric acid production. Min. Metall. Explor. 2021, 39, 161–167. [Google Scholar] [CrossRef]
- International Atomic Energy Agency. Recovery of Uranium from Phosphate Ores; IAEA-TECDOC-2086; IAEA: Vienna, Austria, 2025. [Google Scholar] [CrossRef]
- Jarosiński, A.; Kowalczyk, J.; Mazanek, C. Development of the Polish wasteless technology of apatite phosphogypsum utilization with recovery of rare earths. J. Alloys Compd. 1993, 200, 147–150. [Google Scholar] [CrossRef]
- Todorovsky, D.; Terziev, A.; Milanova, M. Influence of mechanoactivation on rare earths leaching from phosphogypsum. Hydrometallurgy 1997, 45, 13–19. [Google Scholar] [CrossRef]
- Lokshin, E.P.; Tareeva, O.A.; Elizarova, I.R. On integrated processing of phosphogypsum. Russ. J. Appl. Chem. 2013, 86, 463–468. [Google Scholar] [CrossRef]
- Kulczycka, J.; Kowalski, Z.; Smol, M.; Wirth, H. Evaluation of the recovery of Rare Earth Elements (REE) from phosphogypsum waste—Case study of the WIZÓW Chemical Plant (Poland). J. Clean. Prod. 2016, 113, 345–354. [Google Scholar] [CrossRef]
- Al-Thyabat, S.; Zhang, P. REE extraction from phosphoric acid, phosphoric acid sludge, and phosphogypsum. Miner. Process. Extr. Metall. 2015, 124, 143–150. [Google Scholar] [CrossRef]
- Hammas-Nasri, I.; Horchani-Naifer, K.; Férid, M.; Barca, D. Rare earths concentration from phosphogypsum waste by two-step leaching method. Int. J. Miner. Process. 2016, 149, 78–83. [Google Scholar] [CrossRef]
- Wildenboer, R.A.; Sandenbergh, R.F. Extraction of Rare Earth Elements from Phalaborwa phosphogypsum. J. South. Afr. Inst. Min. Metall. 2024, 124, 575–582. [Google Scholar] [CrossRef]
- Maina, L.; Kiegiel, K.; Samczyński, Z.; Haneklaus, N.; Zakrzewska-Kołtuniewicz, G. Sulfuric Acid Leaching Recovery of Rare Earth Elements from Wizów’s Phosphogypsum in Poland. Sustainability 2024, 16, 9059. [Google Scholar] [CrossRef]
- Li, S.; Malik, M.; Azimi, G. Extraction of Rare Earth Elements from Phosphogypsum Using Mineral Acids: Process Development and Mechanistic Investigation. Ind. Eng. Chem. Res. 2022, 61, 102–114. [Google Scholar] [CrossRef]
- Rychkov, V.; Kirillov, E.; Kirillov, S.; Semenishchev, V.; Bunkov, G.; Botalov, M.; Smyshlyaev, D.; Malyshev, A. Recovery of rare earth elements from phosphogypsum. J. Clean. Prod. 2018, 196, 674–681. [Google Scholar] [CrossRef]
- Asafar, F.; Amine, M.; Outzourhit, A.; Bilali, L.; Nadifiyine, M. Use of Plackett–Burman Statistical Design to Study Factors Acting on the Nitric Acid Leaching of Rare Earth Elements from Moroccan Phosphogypsum. J. Chem. 2024, 2024, 1311327. [Google Scholar] [CrossRef]
- Cánovas, C.; Chapron, S.; Arrachart, G.; Pellet-Rostaing, S. Leaching of rare earth elements (REEs) and impurities from phosphogypsum: A preliminary insight for further recovery of critical raw materials. J. Clean. Prod. 2019, 219, 225–235. [Google Scholar] [CrossRef]
- Lokshin, E.P.; Tareeva, O.A.; Elizarova, I.P. Processing of phosphodihydrate to separate rare-earth elements and obtain gypsum free from phosphates and fluorides. Russ. J. Appl. Chem. 2011, 84, 1461–1469. [Google Scholar] [CrossRef]
- Liang, H.; Zhang, P.; Jin, Z.; DePaoli, D. Rare earths recovery and gypsum upgrade from Florida phosphogypsum. Min. Metall. Explor. 2017, 34, 201–206. [Google Scholar] [CrossRef]
- Grabas, K.; Pawełczyk, A.; Stręk, W.; Szełęg, E.; Stręk, S. Study on the Properties of Waste Apatite Phosphogypsum as a Raw Material of Prospective Applications. Waste Biomass-Valorization 2019, 10, 3143–3155. [Google Scholar] [CrossRef]
- Masmoudi-Soussi, A.; Hammas-Nasri, I.; Horchani-Naifer, K.; Férid, M. Rare earths recovery by fractional precipitation from a sulfuric leach liquor obtained after phosphogypsum processing. Hydrometallurgy 2020, 191, 105253. [Google Scholar] [CrossRef]
- Zeng, C.-X.; Guan, Q.-J.; Sui, Y.; Yu, W.-J.; Bu, Y.-J.; Liu, C.-F.; Zhang, Z.-Y. Kinetics of nitric acid leaching of low-grade rare earth elements from phosphogypsum. J. Cent. South Univ. 2022, 29, 1869–1880. [Google Scholar] [CrossRef]
- Golahdooz, M.R.; Lashgari, V.A.; Yoozbashizadeh, H. Separation of Rare Earth Elements from Phosphogypsum Obtained from the Processing of Apatite Concentrate from the Chadormalu Mine by Acid Leaching. Trans. Indian Inst. Met. 2023, 76, 1885–1891. [Google Scholar] [CrossRef]
- Antonick, P.J.; Hu, Z.; Fujita, Y.; Reed, D.W.; Das, G.; Wu, L.; Shivaramaiah, R.; Kim, P.; Eslamimanesh, A.; Lencka, M.M.; et al. Bio- and mineral acid leaching of rare earth elements from synthetic phosphogypsum. J. Chem. Thermodyn. 2019, 132, 491–496. [Google Scholar] [CrossRef]
- Ray, A.R.; Tripathy, B.C.; Mishra, S. Leaching of rare earth elements from phosphogypsum via citric acid medium: Optimization through central composite design and kinetics studies. Environ. Sci. Pollut. Res. 2025, 32, 4076–4089. [Google Scholar] [CrossRef]
- Diwa, R.R.; Tabora, E.U.; Haneklaus, N.H.; Ramirez, J.D. Rare earths leaching from Philippine phosphogypsum using Taguchi method, regression, and artificial neural network analysis. J. Mater. Cycles Waste Manag. 2023, 25, 3316–3330. [Google Scholar] [CrossRef]
- Burnett, W.C.; Schultz, M.K.; Hull, C.D. Radionuclide flow during the conversion of phosphogypsum to ammonium sulfate. J. Environ. Radioact. 1996, 32, 33–51. [Google Scholar] [CrossRef]
- Ennaciri, Y.; El Alaoui-Belghiti, H.; Bettach, M. Comparative study of K2SO4 production by wet conversion from phosphogypsum and synthetic gypsum. J. Mater. Res. Technol. 2019, 8, 2586–2596. [Google Scholar] [CrossRef]
- Pyagai, I.; Zubkova, O.; Babykin, R.; Toropchina, M.; Fediuk, R. Influence of Impurities on the Process of Obtaining Calcium Carbonate during the Processing of Phosphogypsum. Materials 2022, 15, 4335. [Google Scholar] [CrossRef]
- Padilla, A.M.; Pana, A.-M.; ad Patel, R.A. Rare Earth Element Recovery from Phosphogypsum using a Biolixiviant. Available online: https://repository.upenn.edu/cbe_sdr/118 (accessed on 28 November 2024).
- Mashkovtsev, M.; Botalov, M.; Smyshlyaev, D.; Pajarre, R.; Kangas, P.; Rychkov, V.; Koukkari, P. Pilot-scale recovery of rare earths and scandium from phosphogypsum and uranium leachates. E3S Web Conf. 2016, 8, 01026. [Google Scholar] [CrossRef]
- Yahorava, O.; Freeman, V. Viability of Phosphogypsum as a Secondary Resource of Rare Earth Elements. In Proceedings of the IMPC 2016: XXVIII International Mineral Processing Congress, Quebec City, QC, Canada, 11–15 September 2016. [Google Scholar]
- Virolainen, S.; Repo, E.; Sainio, T. Recovering rare earth elements from phosphogypsum using a resin-in-leach process: Selection of resin, leaching agent, and eluent. Hydrometallurgy 2019, 189, 105125. [Google Scholar] [CrossRef]
- Ponomareva, M.A.; Cheremisina, O.V.; Mashukova, Y.A.; Lukyantseva, E.S. Increasing the efficiency of rare earth metal recovery from technological solutions during processing of apatite raw materials. J. Min. Inst. 2021, 252, 918–927. [Google Scholar] [CrossRef]
- Kurkinen, S.; Virolainen, S.; Sainio, T. Recovery of rare earth elements from phosphogypsum waste in resin-in-leach process by eluting with biodegradable complexing agents. Hydrometallurgy 2021, 201, 105569. [Google Scholar] [CrossRef]
- El-Didamony, H.; Ali, M.M.; Awwad, N.S.; Fawzy, M.M.; Attallah, M.F. Treatment of phosphogypsum waste using suitable organic extractants. J. Radioanal. Nucl. Chem. 2012, 291, 907–914. [Google Scholar] [CrossRef]
- Tokpayev, R.; Khavaza, T.; Ibraimov, Z.; Kishibayev, K.; Atchabarova, A.; Abdimomyn, S.; Abduakhytova, D.; Nauryzbayev, M. Phosphogypsum conversion under conditions of SC-CO2. J. CO2 Util. 2022, 63, 102120. [Google Scholar] [CrossRef]
- Samsonov, M.D.; Trofimov, T.I.; Kulyako, Y.M.; Malikov, D.A.; Myasoedov, B.F. Supercritical fluid extraction of rare earth elements, thorium and uranium from monazite concentrate and phosphogypsum using carbon dioxide containing tributyl phosphate and di-(2-ethylhexyl) phosphoric acid. Russ. J. Phys. Chem. B 2016, 10, 1078–1084. [Google Scholar] [CrossRef]
- Yahorava, V.; Lakay, E.; Clark, W.; Strauss, J. Hydrothermal Modification of Phosphogypsum to Improve Subsequent Recovery of Rare Earths. In Extraction 2018; The Minerals, Metals & Materials Series; Davis, B., Moats, M.S., Wang, S., Gregurek, D., Kapusta, J., Battle, T.P., Schlesinger, M.E., Flores, G.R.A., Jak, E., Goodall, G., et al., Eds.; Springer: Cham, Switzerland, 2018. [Google Scholar] [CrossRef]
- Lu, S.-B.; Warmadewanthi; Liu, J.-C. Recovery of rare earth elements from phosphogypsum using subcritical water extraction. Chem. Eng. Process.—Process Intensif. 2023, 190, 109433. [Google Scholar] [CrossRef]
- Christophe, N.N.; McCrindle, R.; Maree, J.; Ngole-Jeme, V. The behaviour of selected rare-earth elements during the conversion of phosphogypsum to calcium sulphide and residue. J. Mater. Cycles Waste Manag. 2023, 25, 1658–1671. [Google Scholar] [CrossRef]
- Smerigan, A.; Shi, R. Advancing the Economic and Environmental Sustainability of Rare Earth Element Recovery from Phosphogypsum. Environ. Sci. Technol. 2025, 59, 19755–19767. [Google Scholar] [CrossRef]
- Phalaborwa Stack (Rainbow Rare Earths). Available online: https://www.research-tree.com/newsfeed/article/rainbow-rare-earths-interim-study-confirms-phalaborwa-viability-2662340 (accessed on 15 September 2025).
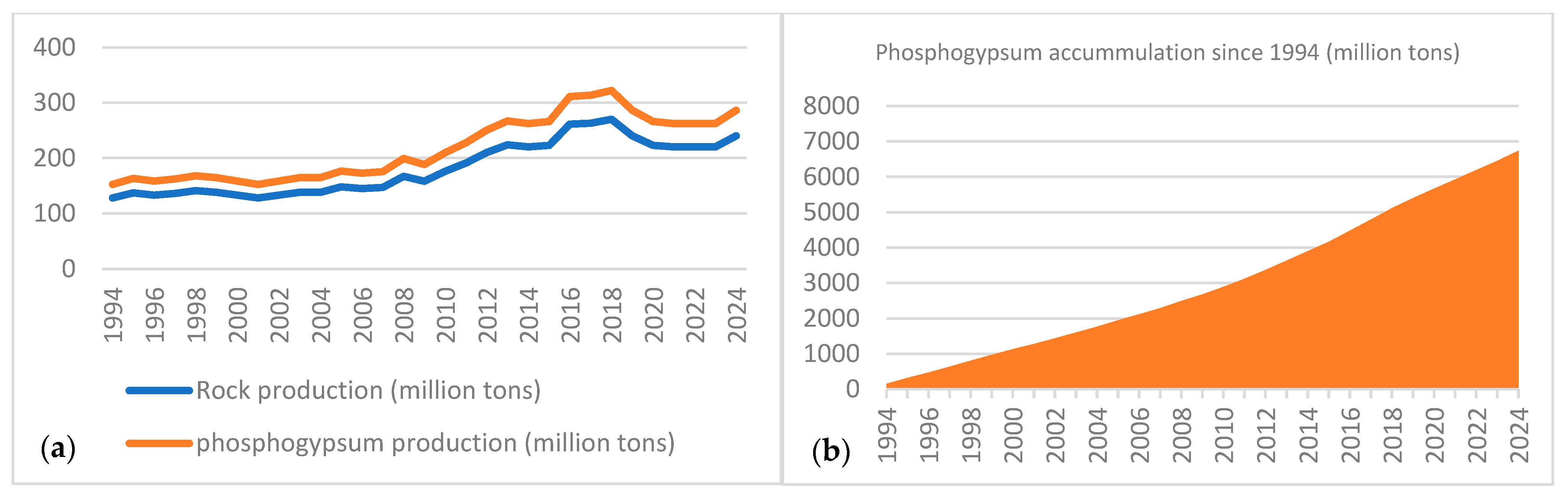
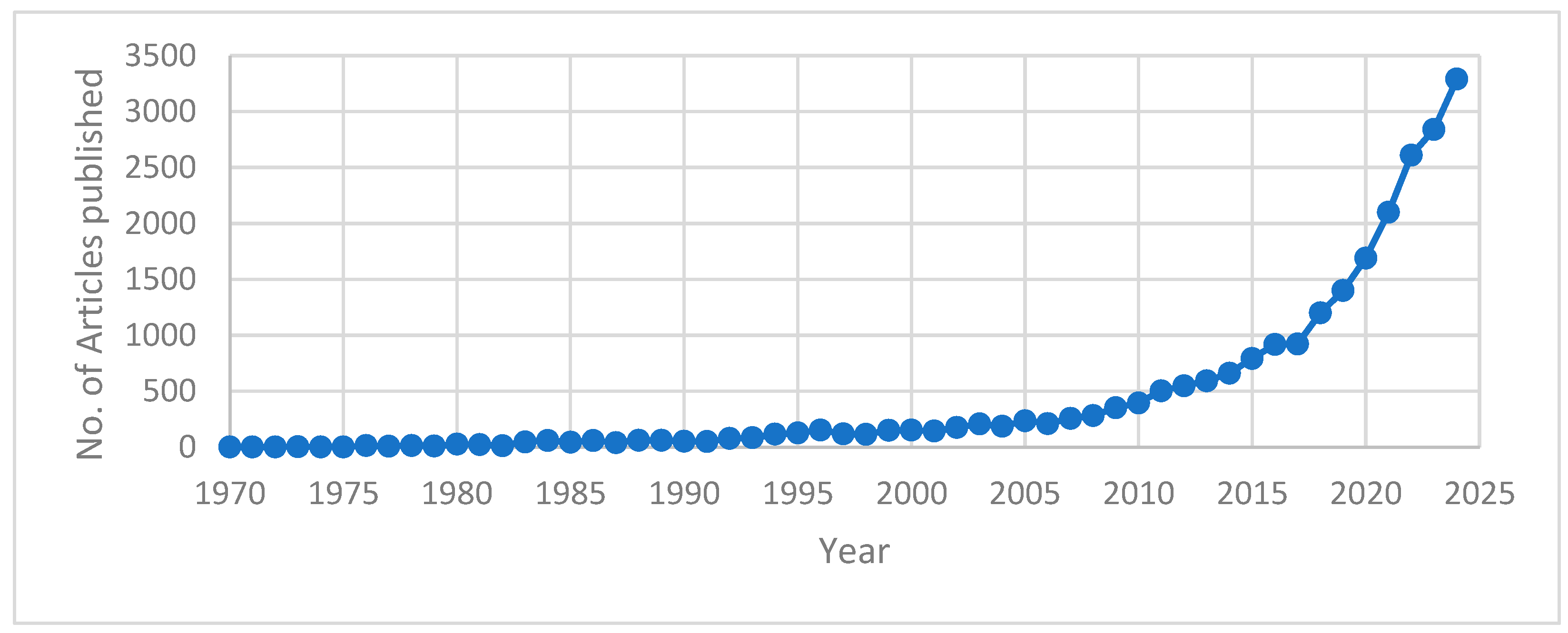
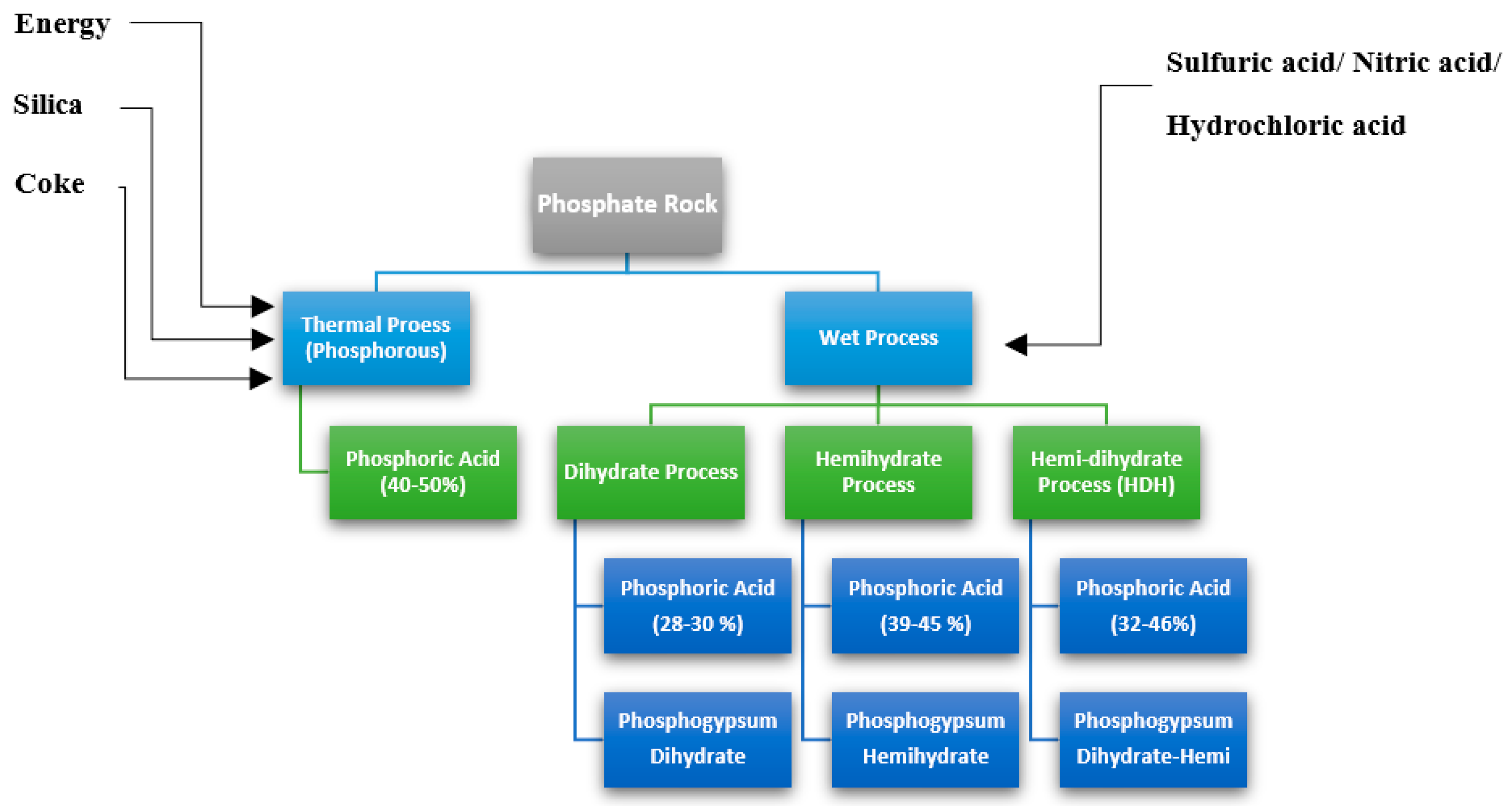
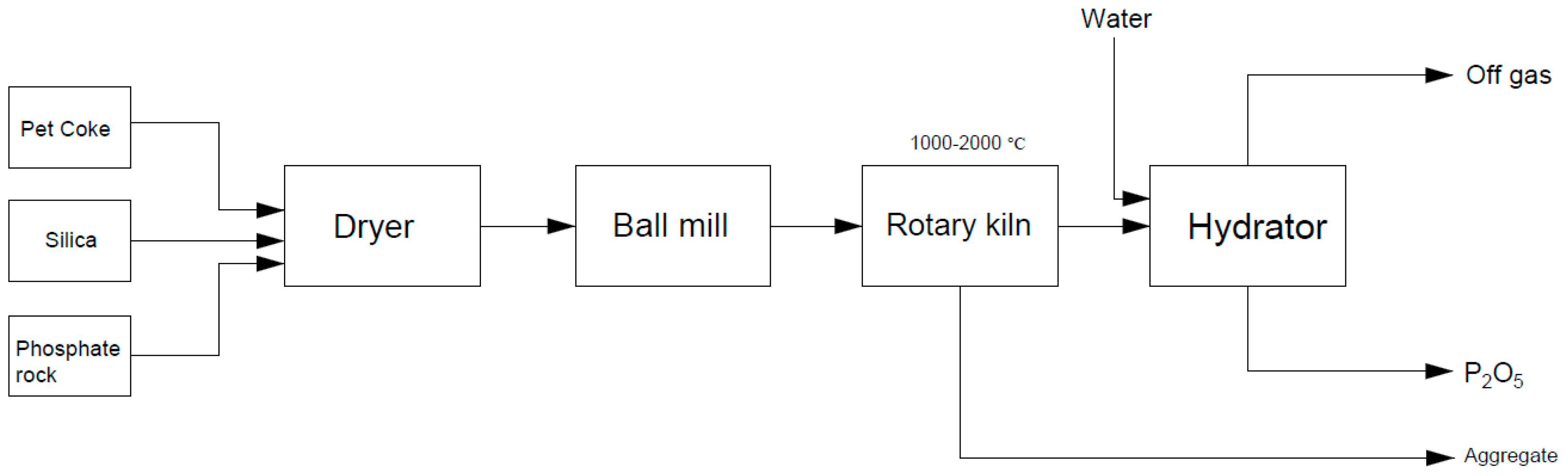
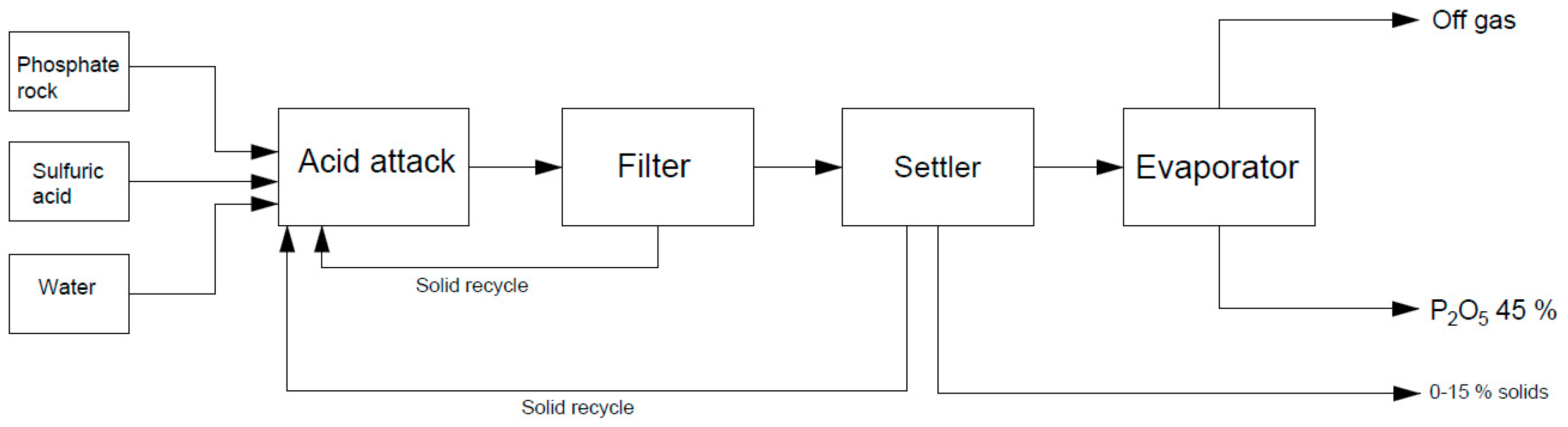
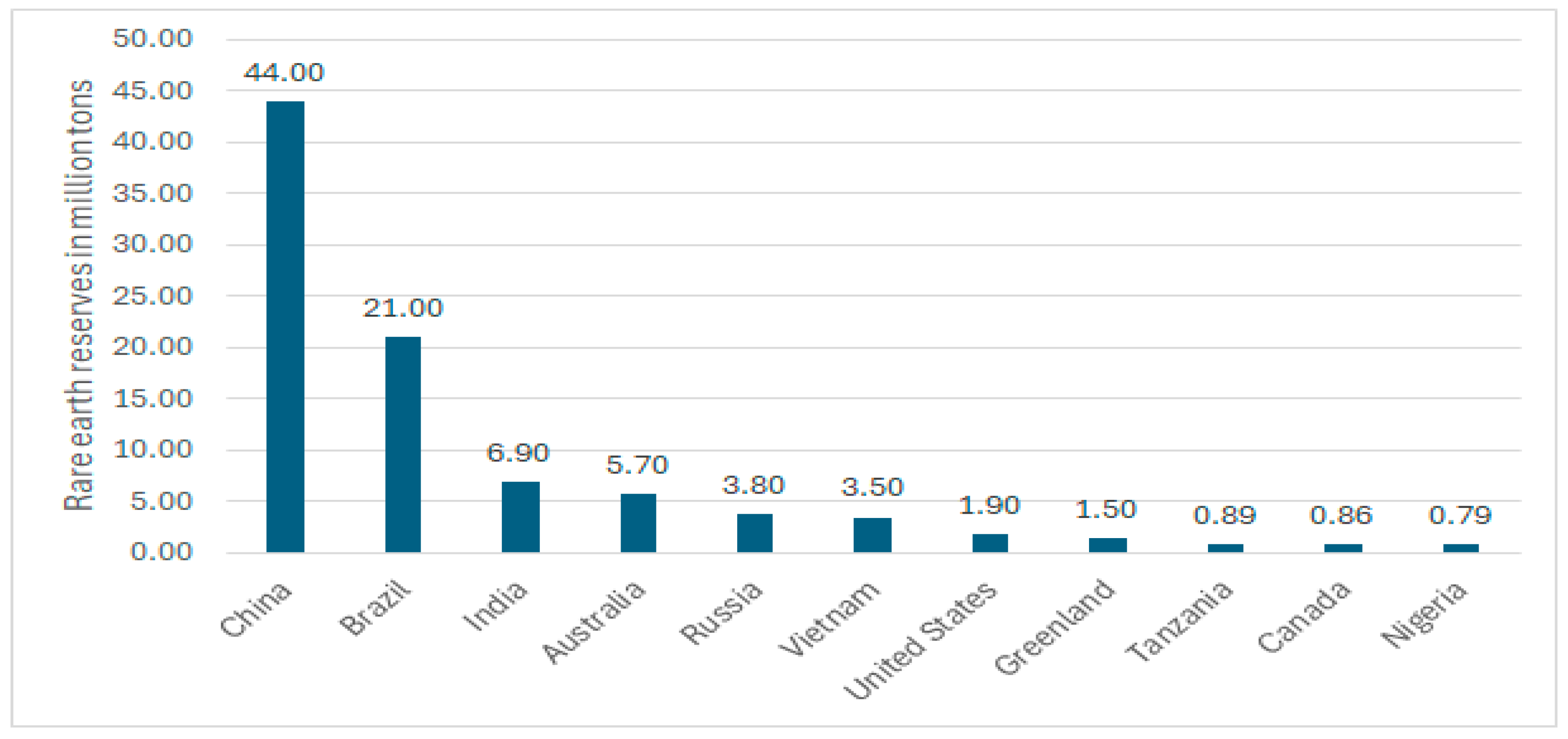
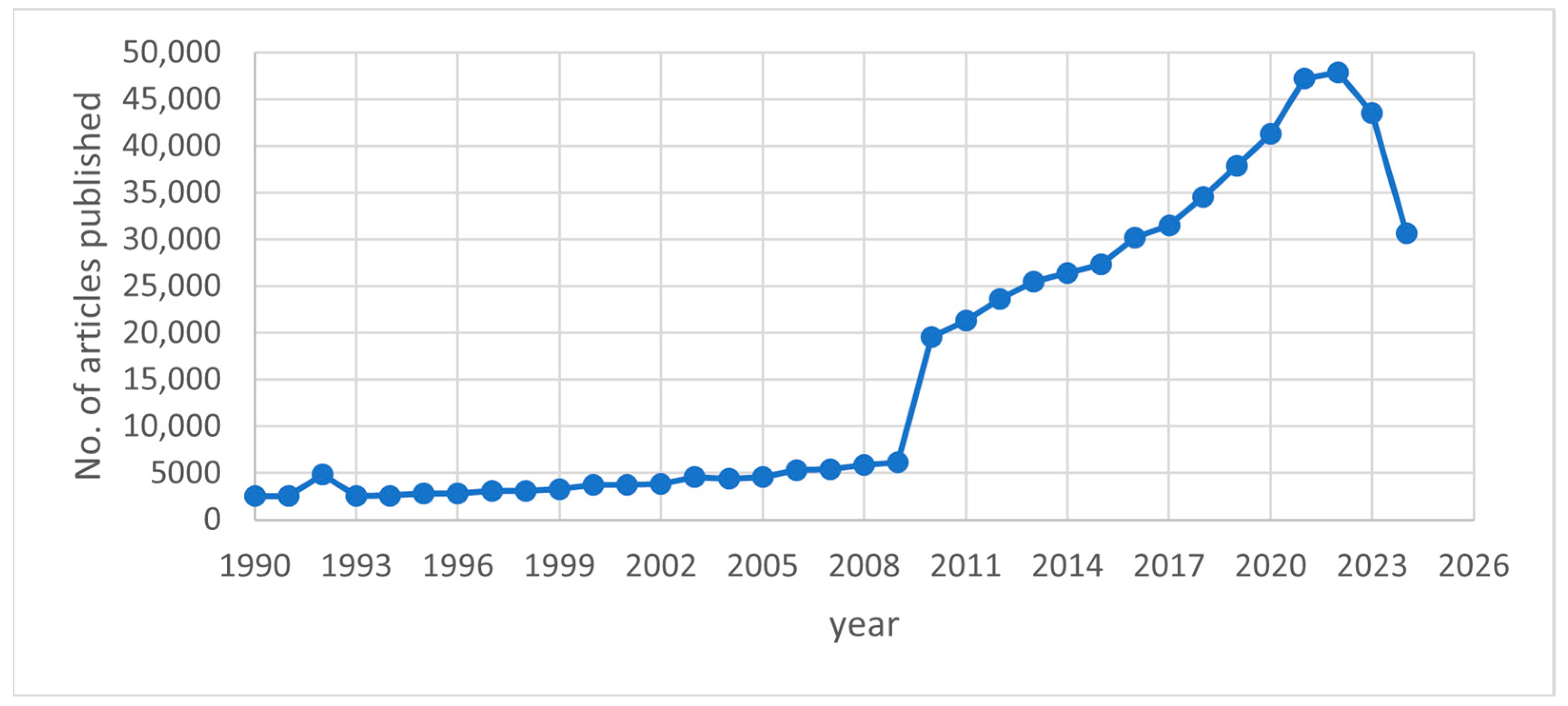
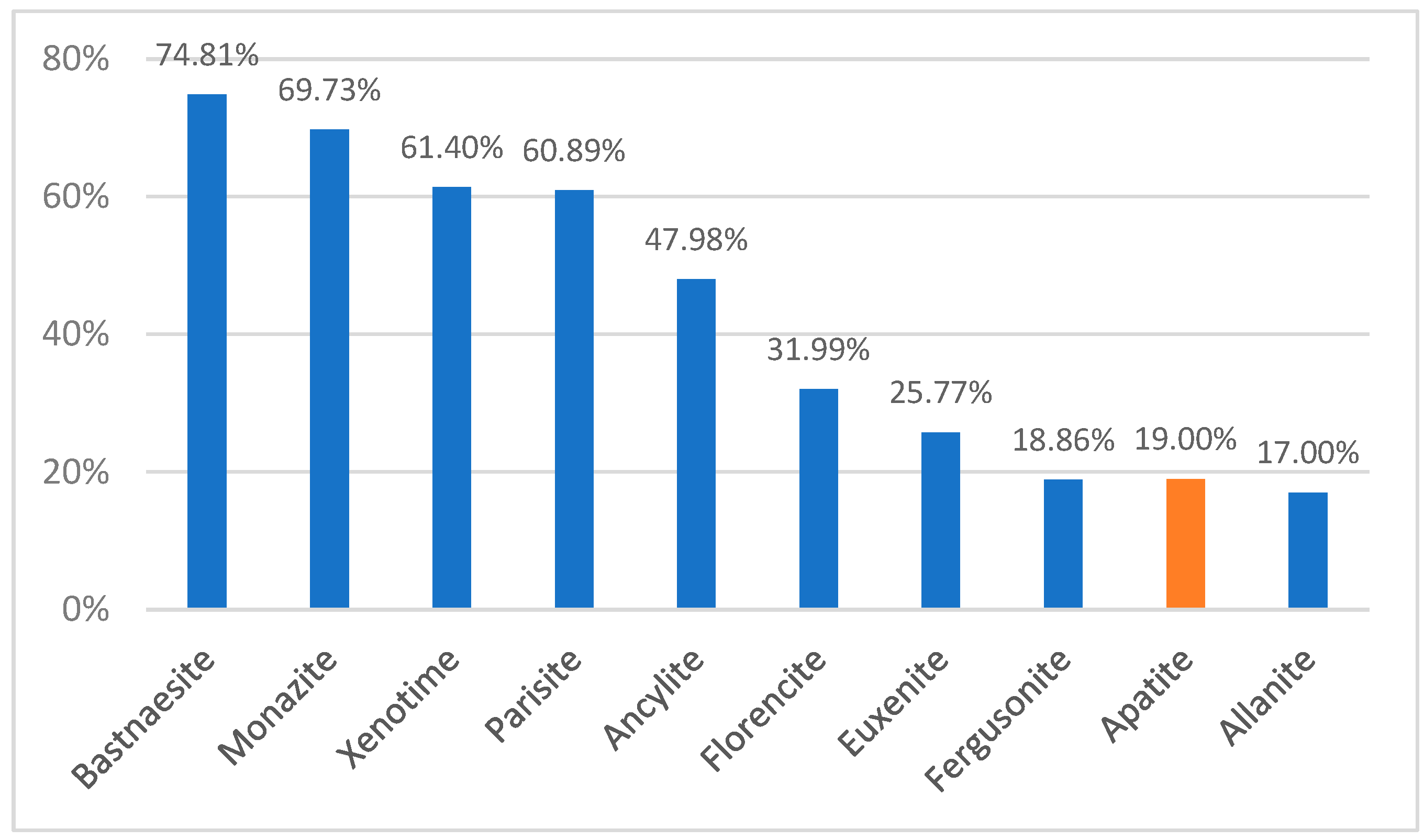
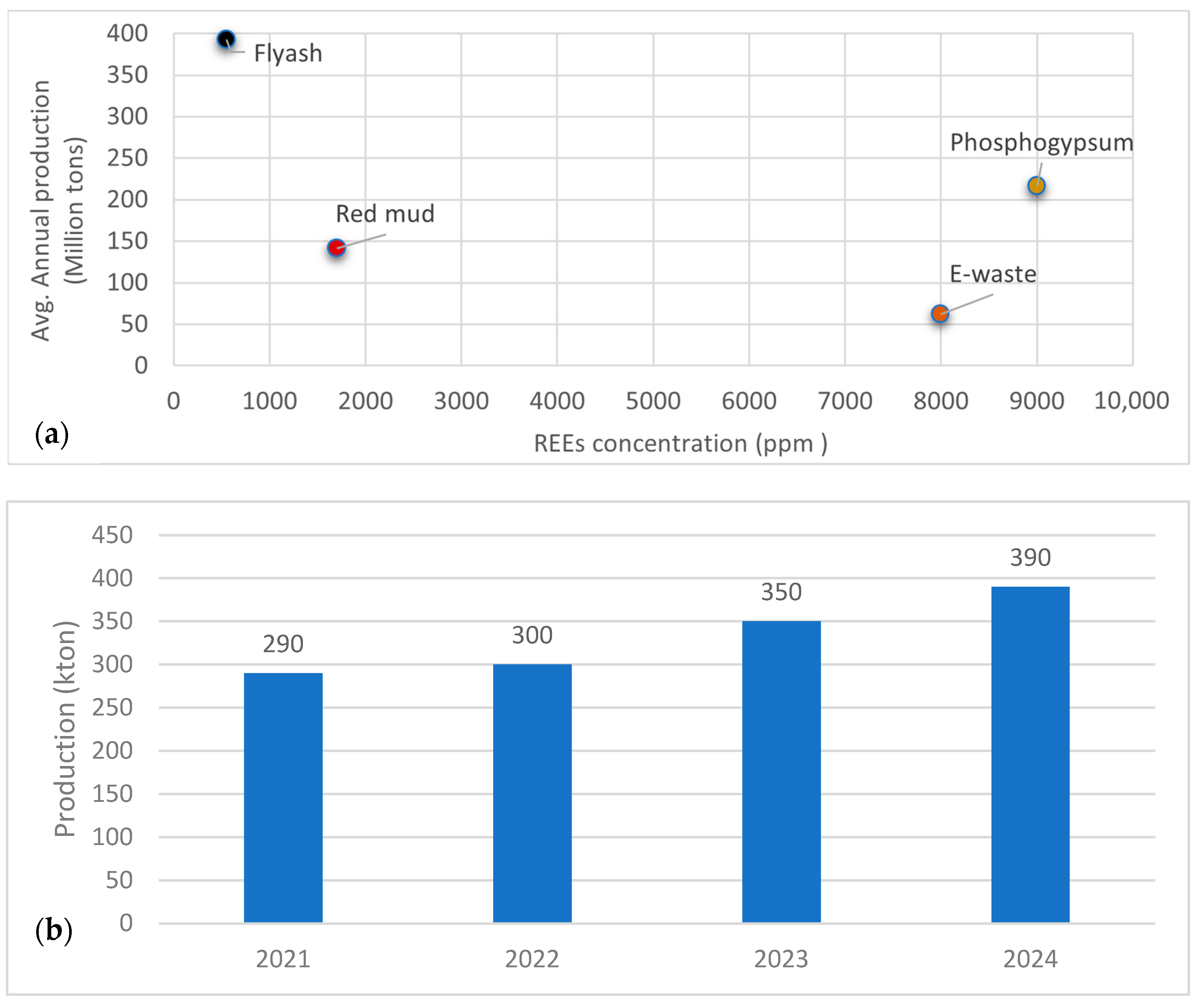
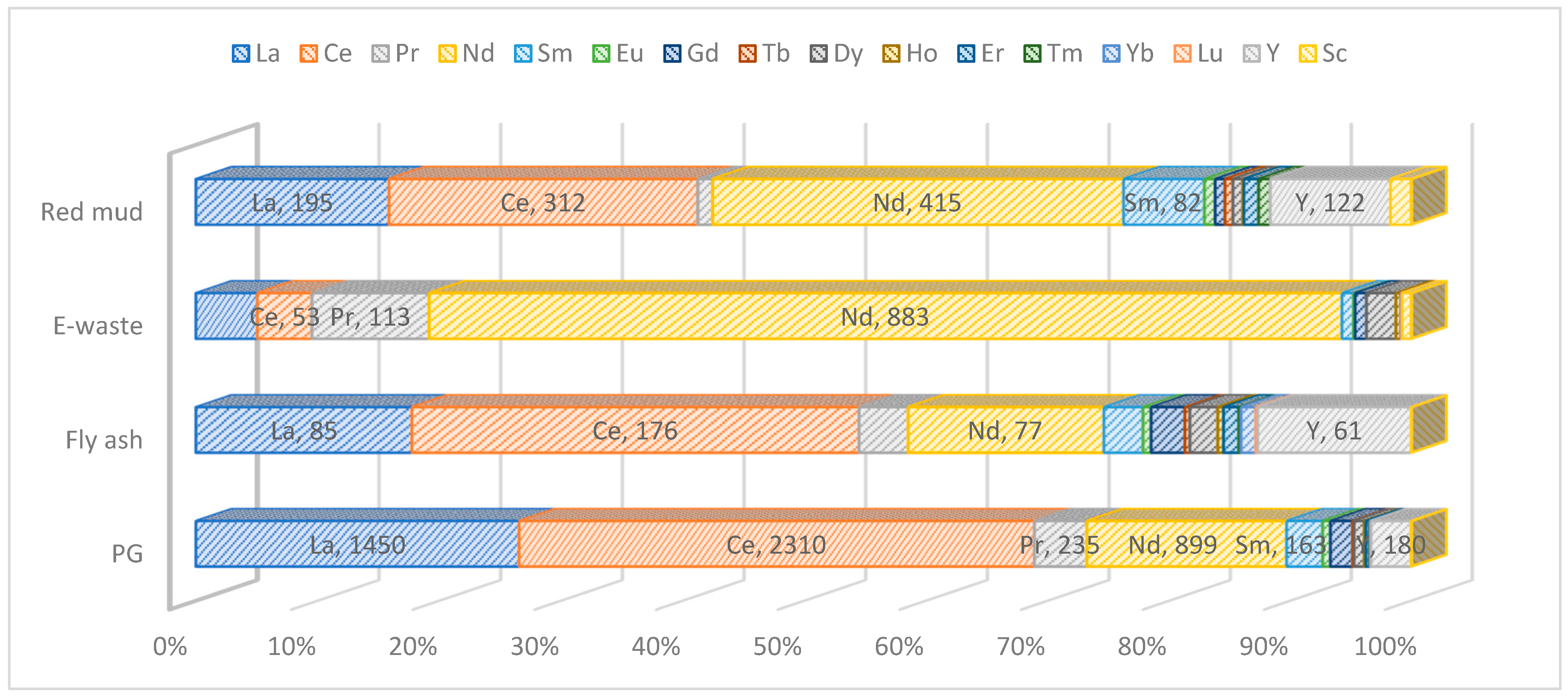
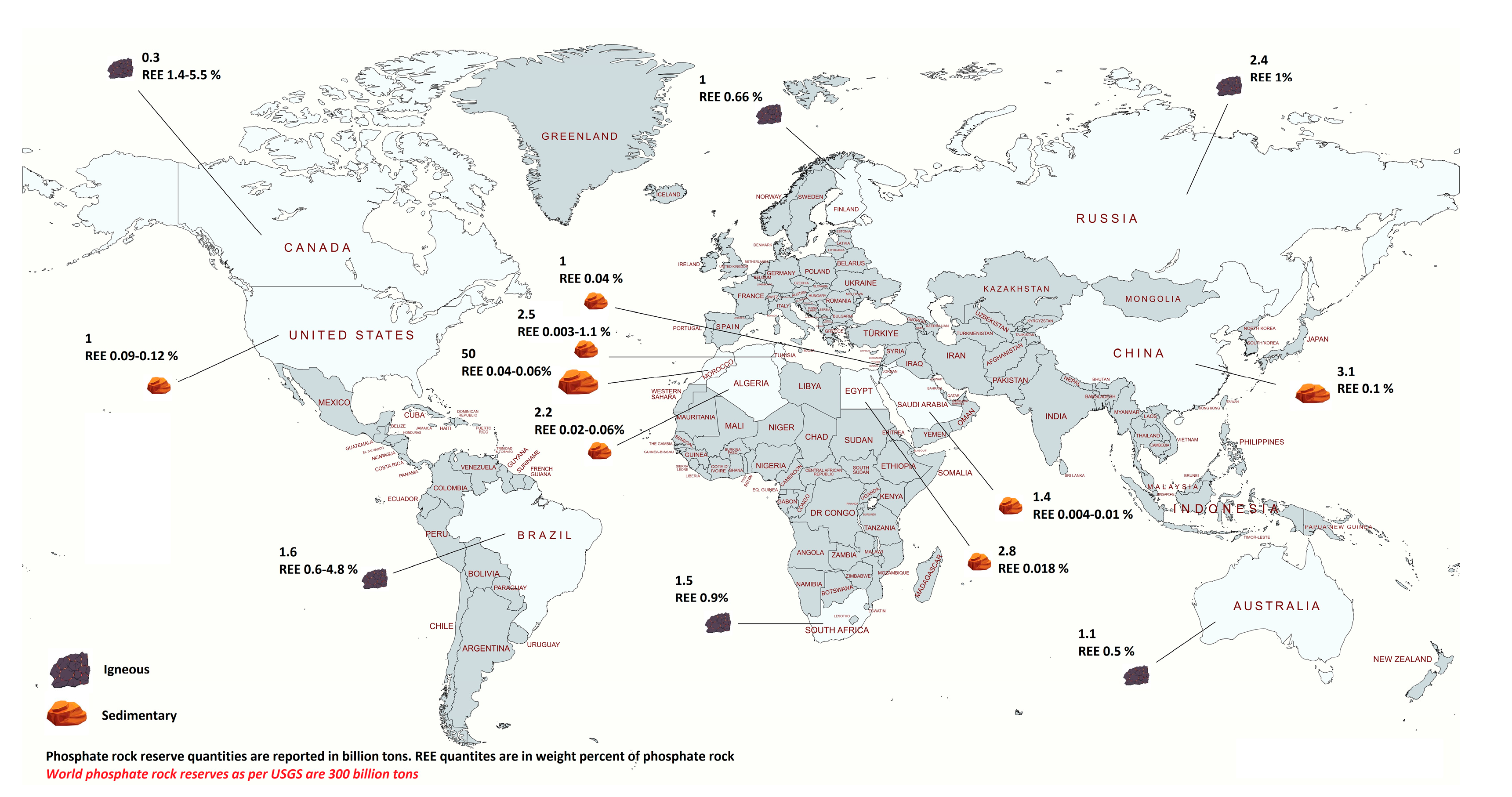

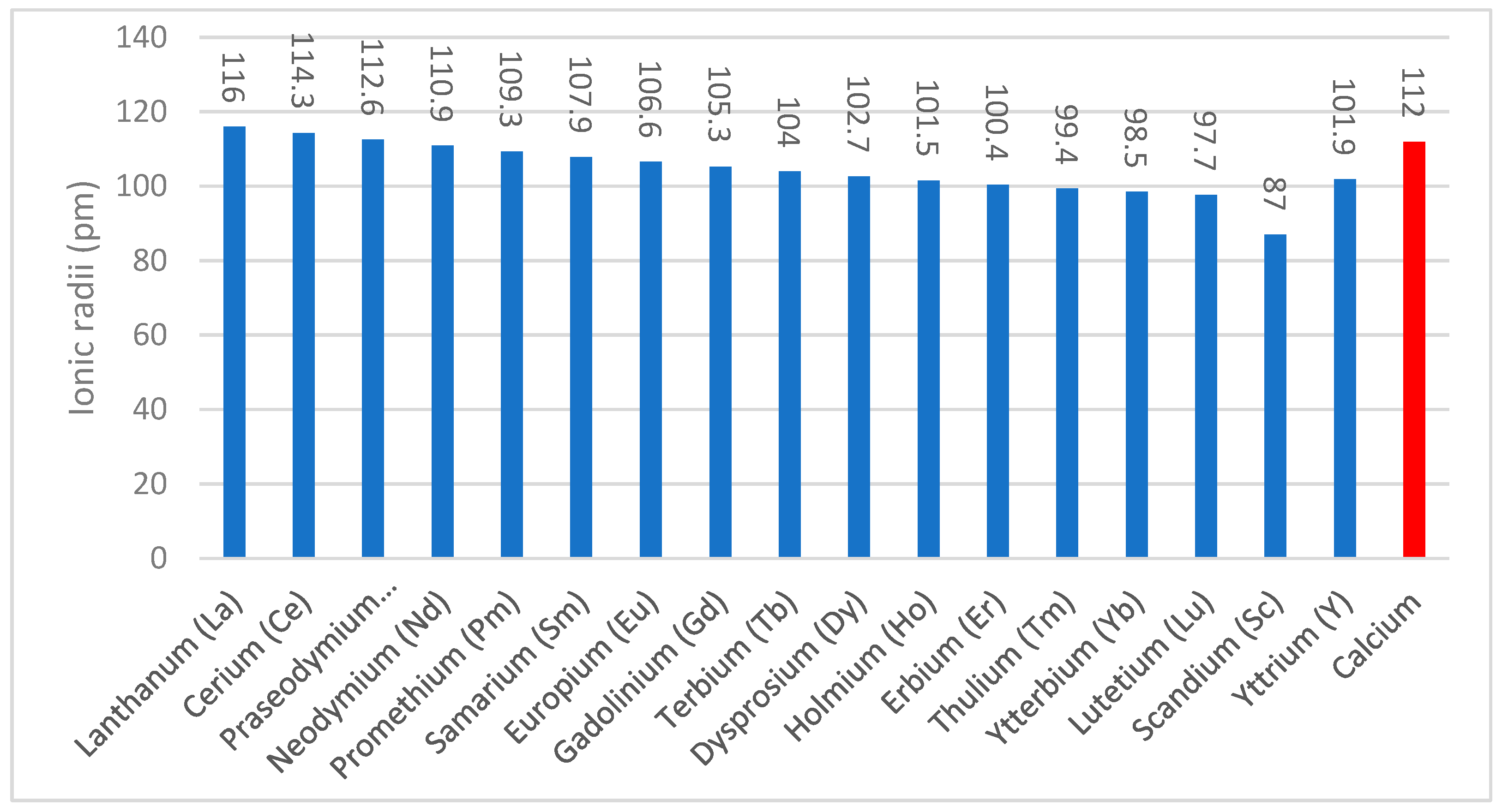
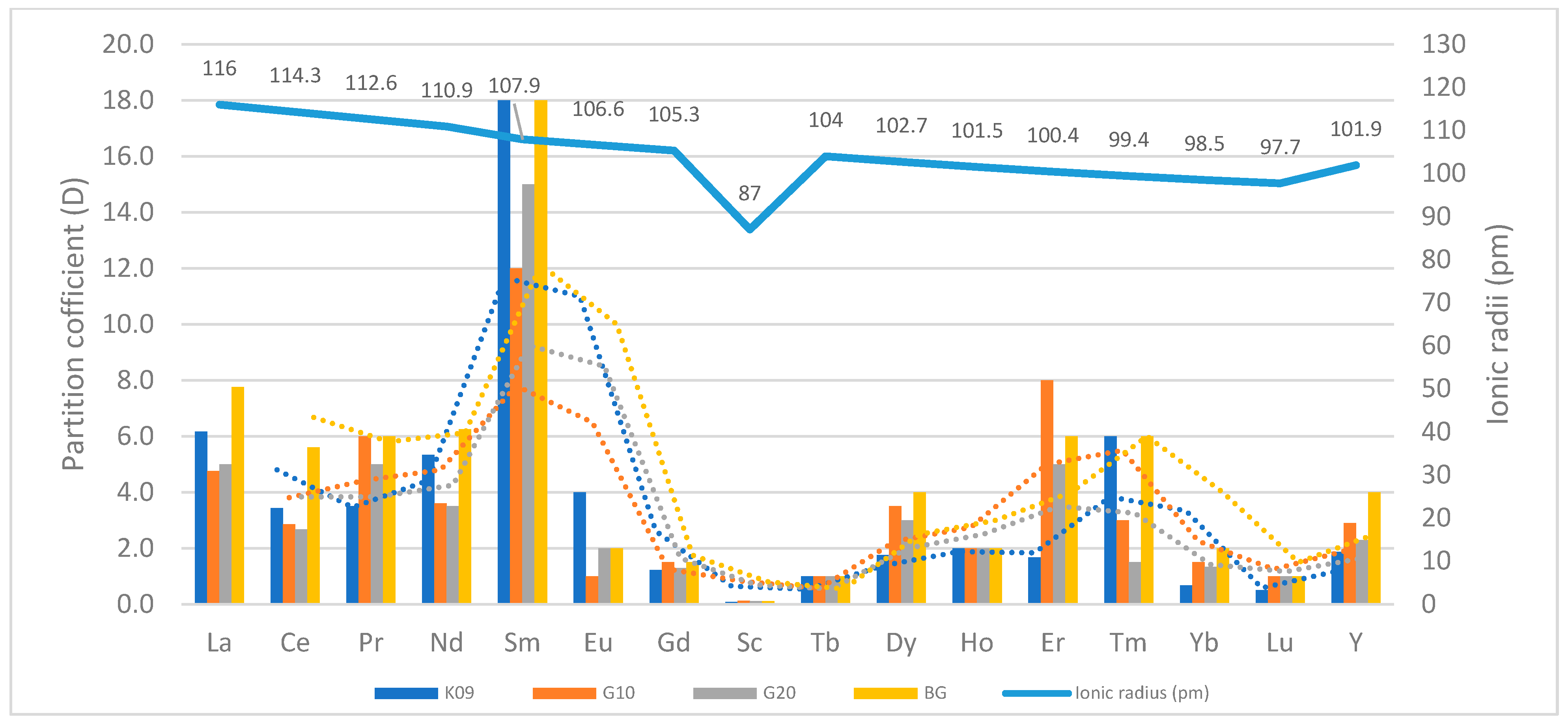
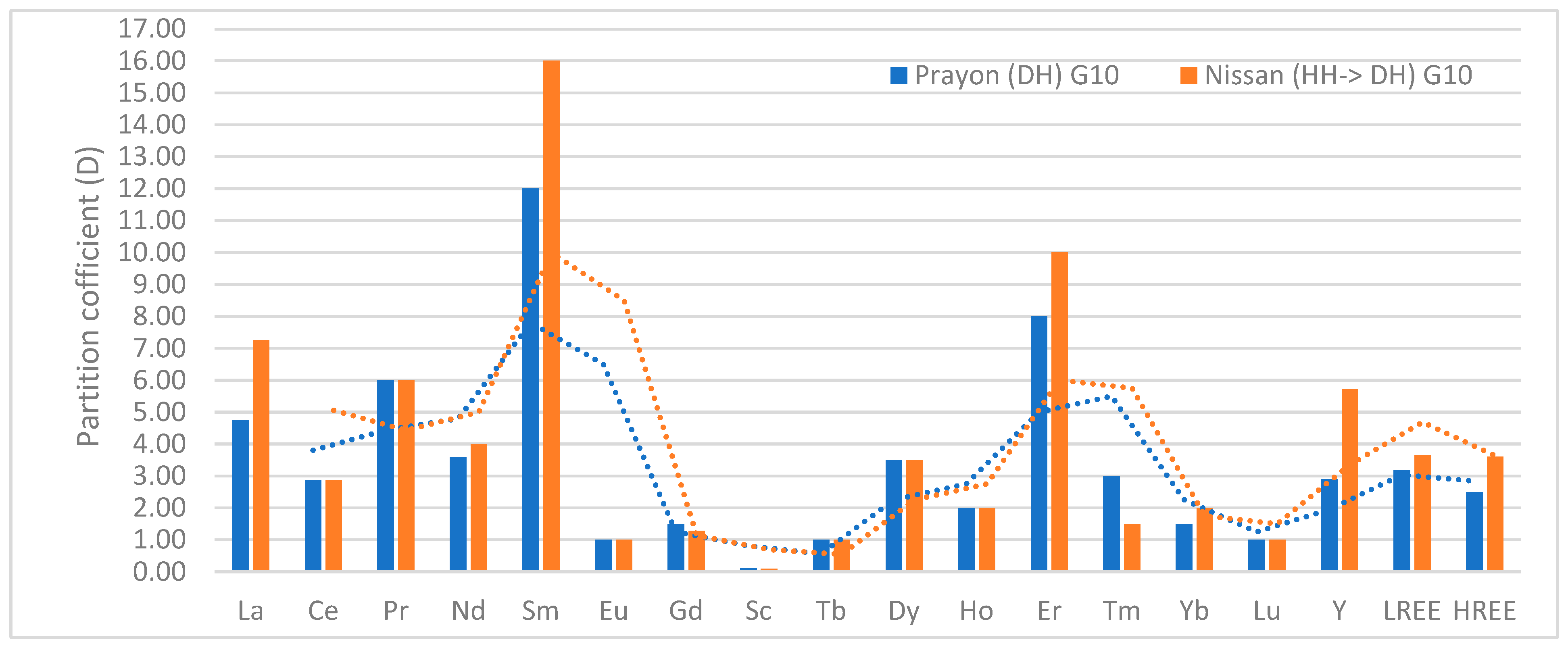
| Process Name | PG Type | P2O5 (%) | Eff. (%) | Key Process Steps/Outcomes |
|---|---|---|---|---|
| Dihydrate process | Dihydrate | 25–30% | 95–96% | In this process, phosphate rock is attacked with sulfuric acid and calcium sulfate as dihydrate crystals are obtained with low concentration of P2O5. |
| Di-hemihydrate | Dihydrate–Hemihydrate | 32–36% | >98% | In this process, sulfuric acid and steam are added to transform the dihydrate solids into hemihydrate. This change in structure breaks open the solid crystals, which helps to release materials that were previously trapped, such as phosphate. |
| Di attack Hemi filtration process | Dihydrate–Hemihydrate | 32–35% | 97–98% | Here, gypsum slurry recrystallizes into hemihydrate calcium sulfate in the conversion section before being filtered to produce the phosphoric acid. |
| Hemi-dihydrate Process | Hemihydrate–Dihydrate | 40–46% | >98.5% | After the first reaction in hemihydrate mode, the product acid is separated as a 46% P2O5. The remaining α-hemihydrate is further processed with sulfuric acid in conditions in which α-hemihydrate is unstable and recrystallizes as gypsum, releasing crystallized and unreacted P2O5. |
| Two stage Hemihydrate process | Hemihydrate | 39–45% | >92–95% | The reaction takes place in two stages. The first stage takes place with low sulfuric acid concentration, while the second stage works at a higher sulfuric concentration. Control of sulfate content and temperature allow the filtration of a slurry holding highly stable hemihydrate crystals that stay in slurry and do not stick easily. |
| Deposit | Mountain Pass | Byan Obo | Mount Weld | Lehat | Longnan | Xunmu | Kola Peninsula |
|---|---|---|---|---|---|---|---|
| Country | USA | China | Australia | Malaysia | China | China | Russia |
| Mineral | Bastnasite | Bastnasite | Monazite | Xenomite | High-Ylateritie | Low-Ylateritie | Loparite |
| La | 33.8 | 23.0 | 25.5 | 1.2 | 1.8 | 43.4 | 25.0 |
| Ce | 49.6 | 50.0 | 46.7 | 3.1 | 0.4 | 2.4 | 50.5 |
| Pr | 4.1 | 6.2 | 5.3 | 0.5 | 0.7 | 9.0 | 5.0 |
| Nd | 11.2 | 18.5 | 18.5 | 1.6 | 3.0 | 31.7 | 15.0 |
| Sm | 0.9 | 0.8 | 2.3 | 1.1 | 2.8 | 3.9 | 0.7 |
| Eu | 0.1 | 0.2 | 0.4 | trace | 0.1 | 0.5 | 0.1 |
| Gd | 0.2 | 0.7 | <0.1 | 3.5 | 6.9 | 3.0 | 0.6 |
| Tb | 0.0 | 0.1 | <0.1 | 0.9 | 1.3 | Trace | trace |
| Dy | 0.0 | 0.1 | 0.1 | 8.3 | 6.7 | Trace | 0.6 |
| Ho | 0.0 | trace | trace | 2.0 | 1.6 | Trace | 0.7 |
| Er | 0.0 | trace | trace | 6.4 | 4.9 | Trace | 0.8 |
| Tm | 0.0 | trace | none | 1.1 | 0.7 | Trace | 0.1 |
| Yb | 0.0 | trace | none | 6.8 | 2.5 | 0.3 | 0.2 |
| Lu | trace | trace | none | 1.0 | 0.4 | 0.1 | 0.2 |
| Y | 0.1 | trace | <0.1 | 61.0 | 65.0 | 8.0 | 1.3 |
| Waste | La | Ce | Pr | Nd | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | Lu | Y | Sc | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Red mud | 195 | 312 | 15 | 415 | 82 | 11 | 10 | 8 | 10 | 1 | 15 | 12 | 0 | 0 | 122 | 21 | [19] |
| Fly ash | 85 | 176 | 19 | 77 | 15 | 3 | 13 | 2 | 11 | 2 | 6 | 1 | 6 | 1 | 61 | 0 | [20,21] |
| PG | 1450 | 2310 | 235 | 899 | 163 | 35 | 99 | 7 | 46 | 7 | 16 | 1 | 6 | 1 | 180 | 1 | [22,23,24] |
| E-waste 1 | 59 | 53 | 113 | 883 | 11 | 2 | 11 | 0 | 29 | 5 | 0 | 0 | 1 | 1 | 0 | 9 | [27,28] |
| PG Type | Operating Conditions | REE Content (%) | Eff. (%) | Ref. | |||
|---|---|---|---|---|---|---|---|
| Lixiviant | Temp (°C) | S/L Ratio | Time (h) | ||||
| Hemihydrate | 5–10% H2SO4 | <20 | 0.5 | 6 | NR 1 | 80 | [55] |
| Dihydrate | 10–15% H2SO4 | 40 | 0.5 | 6 | NR | 52 | |
| Dihydrate | 7% H2SO4 | NR | 0.2 | 2 | 0.37 | 59 | [56] |
| Dihydrate | 10% HCl | NR | 0.2 | 2 | NR | 49 | |
| Dihydrate | 7% H2SO4 | NR | 0.2 | 2 | NR | 62 | |
| Dihydrate | HCl | NR | 0.2 | 2 | NR | 51 | |
| Dihydrate | 7% H2SO4 | NR | 0.2 | 2 | NR | 70 | |
| Hemihydrate | 4% H2SO4 | 25 | 0.22 | NR | 0.57–0.51 | NR | [57] |
| Dihydrate | 4% H2SO4 | 25 | 0.22 | NR | 0.41–0.49 | NR | |
| Dihydrate | 96% H2SO4 + 34% H3PO4 | 72 | 0.14 | 1 | 0.0335 | 49 | [58] |
| Dihydrate | 2 M H2SO4 | 60 | 0.125 | 4 | 0.51 | 92 | [62] |
| Dihydrate | 2.5 M HCl | 45 | 0.033 | 0.3 | NR | 98.5% Y, 94.6% Nd 86.1% Dy | [63] |
| Dihydrate | 2.1 M HNO3 | 85 | 0.036 | NR | NR | 7.6% Nd, 83.5% Y, and 77.8% Dy | |
| Dihydrate | 1.3 H2SO4 | 85 | 0.033 | NR | NR | 47.7% Nd, 57.2% Y, 44.9% Dy | |
| Dihydrate | 3.3 M HNO3 | 75 | 0.111 | 0.3 | 0.036 | 87.55 | [65] |
| Dihydrate | 4% H2SO4 | NR | 0.49 | 1248 | NR | 50.5 | [67] |
| Dihydrate | 5% H2SO4 | 50 | 0.25 | 0.5 | 0.0218 | 43 | [68] |
| Dihydrate | 10–15% H2SO4 | 25 | 0.33 | 4 | 0.34–0.63 | 50.9–51.5 | [69] |
| Dihydrate | 15% H2SO4 | 100 | 0.33 | 2 | 0.041 | NR | [70] |
| Dihydrate | 1.65 M/l HNO3 | 80 | 0.1 | 2 | 0.0208 | 83 | [71] |
| Dihydrate | 3 M HNO3 | 80 | 0.1 | NR | NR | >80% Nd | [72] |
| Synthetic | 0.02 M H3PO4 | 25 | 20 | 24 | Synthetic | 85 (for Y) | [73] |
| Synthetic | 0.02 M H2SO4 | 25 | 20 | 24 | Synthetic | 80 (for Y) | [73] |
| PG Type | Operating Conditions | REE Content (%) | Leaching Eff. (%) | Ref. | |||||
|---|---|---|---|---|---|---|---|---|---|
| Lixiviant | Resin Type | Eluent | Temp (°C) | Resin/PG/Acid Ratio (v/m/v) | Time(h) | ||||
| Dihydrate | 25 g/L H2SO4 | SAC | NR | 40 | Resin/PG/Acid = 1:5:40 | 12 | 0.43–0.45 | 65% | [61] |
| Dihydrate | 10–20 g/L H2SO4 | Purolite C160 | NH4NO3 | NR | Resin/PG/Acid = 4:30:4 | 2 | NR | 15–70% | [64] |
| Dihydrate | 10 g/L H2SO4, | Purolite C150TLH, | NaCl | NR 1 | Resin/PG/Acid = 1:5:20 | 24 | 0.3387 | 15–80% | [81] |
| Dihydrate | 0.1, 1, 5 and 10 g/L H2SO4, HCl, and H3PO4 | Purolite S940, Purolite C150, Finex CS16GC | NaCl, HCl, EDTA, Na-citrate | 20 | Resin/PG/Acid = 1:5:40 | 24 | 0.17 | 45–75% from CHEL resin | [82] |
| Dihydrate | 10 g/L H2SO4 | Purolite S940, Na+ | HCl, GLDA and MGDA | 25 | Resin/PG/Acid = 1:5:40 | 20 | NR | yield 98%, purity 94% | [84] |
| Resin | Type | Key Findings | Advantages | Limitations |
|---|---|---|---|---|
| Chelating resin | Chelating (e.g., iminodiacetic acid or phosphonic acid types) | Achieved 19.2 g REE/kg resin loading and 20% purity with 1 g/L H2SO4 | High selectivity, good performance at low acid concentrations | Requires complex eluents (EDTA or conc. HCl), multi-stage processing |
| Purolite S940 | Chelating resin | Maximum loading: 0.92 eq/kg; 70% Ca-REE purity after 7 RIL cycles; REE elution with biodegradable agents | High selectivity; good for batch RIL with eco-friendly elution | Multiple cycles needed; moderate overall loading capacity |
| Macroporous sulfonated resin (DVB > 12%) | Strong acid cation (SAC) | 32.8% REE extraction; 30–40% scandium recovery | Industrial-scale tested, good for high-throughput | Low selectivity for REEs, moderate efficiency |
| Purolite C150TLH | Strong acid cation exchange | 15–80% efficiency depending on PG source; acid conc. = 10 g/L | Commercially available; effective in RIL at ambient temp | Highly variable efficiency depending on PG origin |
| AN-31 | Anion exchange | 59.7% Pr, 52.8% Sm extraction from sulfate solutions | Good for light REEs; effective at pH 2–4 | Not tested directly on solid PG; more applicable to solution-based systems |
| PG Type | Operating Conditions | REE Content (%) | Leaching Eff. (%) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Lixiviant | Temp (°C) | Pressure (bar) | S/L Ratio | Time (h) | ||||
| Dihydrate | (CO2) = 700–1100 g/min. | 33 | 50.6 | 0.33 | 0.16 | 0.08 | 87% (PG conversion) | [86] |
| Dihydrate | 2 M HNO3, CO2 with TBP, DEHPA | 45 | 200 | 0.7–1 | 1 | 0.42 | 93.50% | [87] |
| PG Type | Operating Conditions | REE Content (%) | Leaching Eff. (%) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Lixiviant | Temp (°C) | Pressure (bar) | S/L Ratio | Time (h) | ||||
| Dihydrate | 100 g/L H2SO4 | 80–200 | 0.78–14.7 | NR 1 | 6 | NR | >80 | [88] |
| Dihydrate | 0.1 mol/L HCl, 0.1 mol/L HNO3, and 0.05 mol/L H2SO4 | 100 | 9.8 | 10 | 0.08 | 0.02 | Max 98.63 (while using HCl) | [89] |
| PG Type | Operating Conditions | REE Content (%) | Leaching Eff. (%) | Ref. | ||||
|---|---|---|---|---|---|---|---|---|
| Lixiviant | Temp (°C) | Pressure (bar) | S/L Ratio | Time (h) | ||||
| Dihydrate | H2S flow 450–1350 mL/min, CO2 450–1340 mL/min | 1100 | Atm | NA | 1 | 0.19 | Enriched REE residue | [90] |
| Extraction Technique | Extraction Eff. | Environmental Impact | Technical Challenges for Scale-Up | Key Economic Cost Drivers | Profitability Outlook and Economic Feasibility |
|---|---|---|---|---|---|
| Acid Leaching | 50–85% | High (Acid waste, radionuclides) | Managing large volumes of corrosive, radioactive waste streams; low selectivity requires extensive downstream purification. | Reagent consumption (H2SO4 or HCl), neutralization costs, waste disposal. OPEX is high. | Uneconomical as a standalone process. High operational and waste management costs outweigh the value of recovered REEs unless they are integrated with PG processing for other purposes (e.g., sulfuric acid production). |
| Resin-in-Leach | 97–99% | Low-Moderate (Exhausted resin is a new waste stream) | Resin degradation/fouling over time; requires efficient solid–liquid separation; elution and regeneration cycles. | Capital cost of columns/reactors, resin cost but possible to reuse, eluent reagents. | Promising and likely feasible. High selectivity reduces downstream costs. Profitability is highly dependent on resin longevity and elution efficiency. OPEX is lower than solvent extraction for certain applications. A key candidate for commercial scale-up. |
| Carbonation | 80–86% | Moderate (Multiple waste streams) | Requires a pure, concentrated CO2 and NH3 source for in situ ammonium carbonate production, production of. Effective leaching of REEs from co-products is challenging. | Reagent cost (ammonium salts or NH3/CO2), energy cost for CO2 compression | Low feasibility. Economics are tied to the sale of main product, ammonium sulfate, and the recovery of REEs from byproducts calcium carbonate. The difficulty to penetrate in the ammonium sulphate market with existing suppliers does not offset the high process costs for REE recovery alone. |
| Bioleaching | 40–60% | Very Low (Environmentally friendly) | Extremely slow leaching kinetics (weeks/months); keeping microbial health at scale; large equipment required to meet the required residence time for scaled plants | Low reagent cost but very high operational cost due to reactor holding time (CAPEX for large tank farms), nutrient costs, aeration/agitation energy. | Uneconomical for large scale. The immense reactor volume and time required to process industrial PG volumes make the capital and operational costs prohibitive. Potential for niche, small-scale applications. |
| Solvent Extraction | 56–69.8% | High (Organic solvent loss, volatile emissions) | Requires very pure leachate (PG liquors are complex and cause crud formation); multi-stage setup is complex; solvent loss is a major operational issue. | High CAPEX for mixer-settlers, reagent cost (extractants like D2EHPA, TBP), solvent makeup cost, scrubbing/stripping reagents. | Economical only after purification. Not feasible for direct leachate. It is the necessary, established downstream step for purifying REE concentrates produced by a more selective primary extraction method (like RIL). Its economics are acceptable only after the bulk of impurities have been removed. |
| Supercritical Fluid Extraction | 93% using TBP, 88% using DHEPA | Low-Moderate (But high energy demand) | Engineering equipment for high-pressure (70–300 bar) continuous operation; material compatibility; efficient recycling of CO2 and modifiers. | Extremely high CAPEX for pressure-rated equipment, high energy cost for compression and heating, cost of CO2 and modifiers (e.g., TBP). | Uneconomical with current technology. The capital intensity and high operating energy costs are far greater than the value of the recovered REEs from PG. Significant technological breakthroughs in pressure vessel design and energy recovery are needed. |
| Hydrothermal Extraction | Almost 100% | Low-Moderate (Uses water, but high energy) | Similarly to SCFE: high-pressure/temperature reactor design; corrosion control; handling of solids in a pressurized system. | High CAPEX for autoclaves, high energy cost for maintaining T and P, reagent cost. | Uneconomical for bulk processing. The energy input required to maintain subcritical conditions for large PG tonnages is cost prohibitive. May have potential for processing specific, high-value waste streams but not for bulk PG. |
| REE Content | Plant Scale | Method | Eff. | Key Economic Metric | Ref. |
|---|---|---|---|---|---|
| REE content of 0.27–0.33 wt.% | 3.3 million tons per annum of phosphogypsum on a dry basis | Resin in leach using sulfuric acid and SAC resin Purolite C150TLH | Tested recovery from PG ranging from 15% to 80% | Economic study suggested that with mixed REE oxide product price > USD 21/kg, even low overall recovery (15%) could be economically favorable. | [81] |
| REE content > 0.5 wt.% needed for profitability | 100 tons of PG per hour | Acid leaching followed by bio-inspired adsorptive separation | 43% REE leaching efficiency | In 87% of baseline simulations and internal rate of return (IRR) > 15% was observed | [91] |
| REE content of 0.44 wt.%, focus on the recovery of Nd, Pr, Dy and Tb | 2.2 Mt/year PG, the plant is expected to produce ca. 1900 t magnet REO annually | Sulfuric acid leaching followed by continuous ion exchange | 65% recovery (magnet REE) | Operating cost USD 40.83/kg magnet REO and USD 12.91/kg total REO with post-tax IRR of 38% and a PBT of 2 years | [92] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Khalil, F.; Pagnanelli, F.; Moscardini, E. Phosphogypsum as the Secondary Source of Rare Earth Elements. Sustainability 2025, 17, 8828. https://doi.org/10.3390/su17198828
Khalil F, Pagnanelli F, Moscardini E. Phosphogypsum as the Secondary Source of Rare Earth Elements. Sustainability. 2025; 17(19):8828. https://doi.org/10.3390/su17198828
Chicago/Turabian StyleKhalil, Faizan, Francesca Pagnanelli, and Emanuela Moscardini. 2025. "Phosphogypsum as the Secondary Source of Rare Earth Elements" Sustainability 17, no. 19: 8828. https://doi.org/10.3390/su17198828
APA StyleKhalil, F., Pagnanelli, F., & Moscardini, E. (2025). Phosphogypsum as the Secondary Source of Rare Earth Elements. Sustainability, 17(19), 8828. https://doi.org/10.3390/su17198828







