4.1. Influence of Paper Sludge Concentration on Soil Respiration
In accordance with the results published by [
29], which emphasize that dissolved organic matter (DOM) is the most active fraction during composting and its degradability gradually decreases due to the depletion of easily degradable compounds and the accumulation of aromatic components [
29], we observed a similar trend in samples with varying sludge content. The declining trend of oxygen consumption at the end of the test in most of our samples likely reflects the exhaustion of simpler organic compounds that are rapidly metabolized by microorganisms. A more pronounced decrease in oxygen consumption in those samples containing 10% and 20% sludge suggests faster degradation of easily accessible organic compounds, corresponding to the predominance of the labile, hydrophilic fraction of DOM [
29].
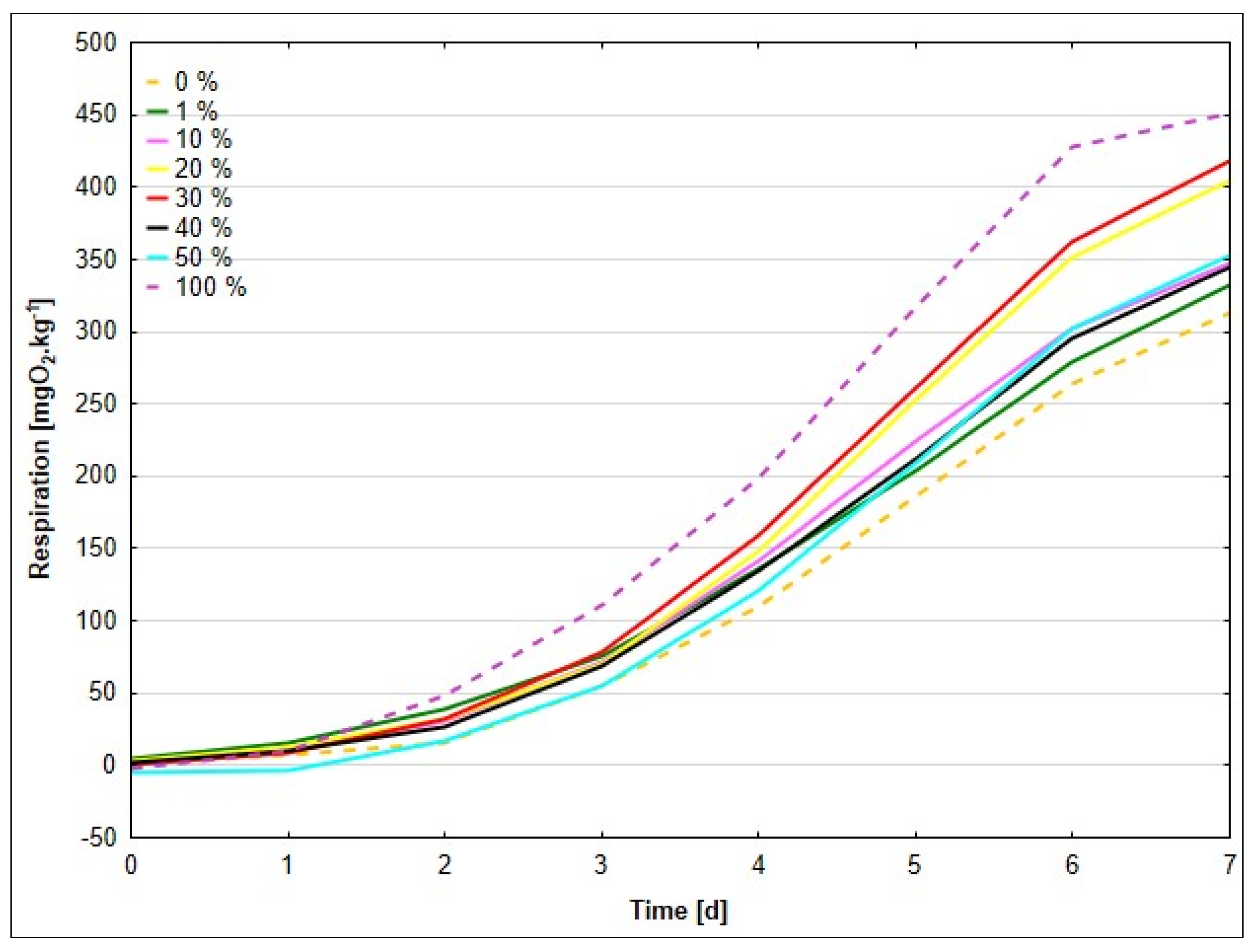
Conversely, samples with a higher sludge content (40% and 50%) exhibited increased and prolonged microbial activity, which may indicate the presence of a larger amount of more complex but still biodegradable organic substances supporting long-term respiration. The delayed increase in oxygen consumption in the 100% sludge sample can be explained by the high sludge density and the absence of original soil microbial communities, which could have slowed the initial microbial adaptation; however, the presence of more complex organic substances allowed a gradual increase in respiration over time.
These findings thus complement and extend the DOM degradation mechanisms described by Pullicino and Gigliotti, emphasizing the importance of sludge concentration on the dynamics of microbial activity and oxygen consumption during composting [
29].
One of the main results was a finding that microbial activity increased with the concentration of paper sludge, as evidenced by soil respiration. This trend confirms that the organic matter content of the sludge supports microbial metabolism, which is a desirable property from the standpoint of waste biodegradability as stated by Liu [
30]. Cited authors also state that in comparison to chemical fertilizer, organic amendments significantly increased total microbial biomass, bacterial biomass (including Gram-positive and Gram-negative bacteria), fungal biomass, and the biomass of Gram-positive and Gram-negative bacteria. The sample with 40% of sludge achieved the highest respiratory activity at the end of the experiment, which indicates its sufficient content of organic material to support long-term microbial activity, as well as the appropriate structure of the paper sludge and soil mixture, which provided sufficient oxygen access to the deeper layers of the sample. Soil structure is a very important factor that influences the diffusion of gases and thus the availability of oxygen in soil layers as reported by Neira et al. [
31] and Ball [
32]. This result is consistent with the work of Kusumarini et al. (2022) [
33], which states that an adequate amount of organic waste can stimulate microbial activity in the soil and improve its fertility.
From the perspective of waste management, this result is relevant because it shows that a substantial portion of paper sludge can be biologically stabilized through soil processes, without the need for energy-intensive incineration or long-term landfilling. The increased respiration rate demonstrates a natural pathway for organic matter mineralization, making land application a viable route for sludge disposal in a circular system [
33].
This approach minimizes landfilling, reduces greenhouse gas emissions from traditional disposal methods, and promotes the restoration of soil organic carbon. At the same time, this process can be a solution for companies trying to achieve goals of zero-waste and lower carbon footprint, making paper sludge an example of implementing circular strategies in the industry [
34].
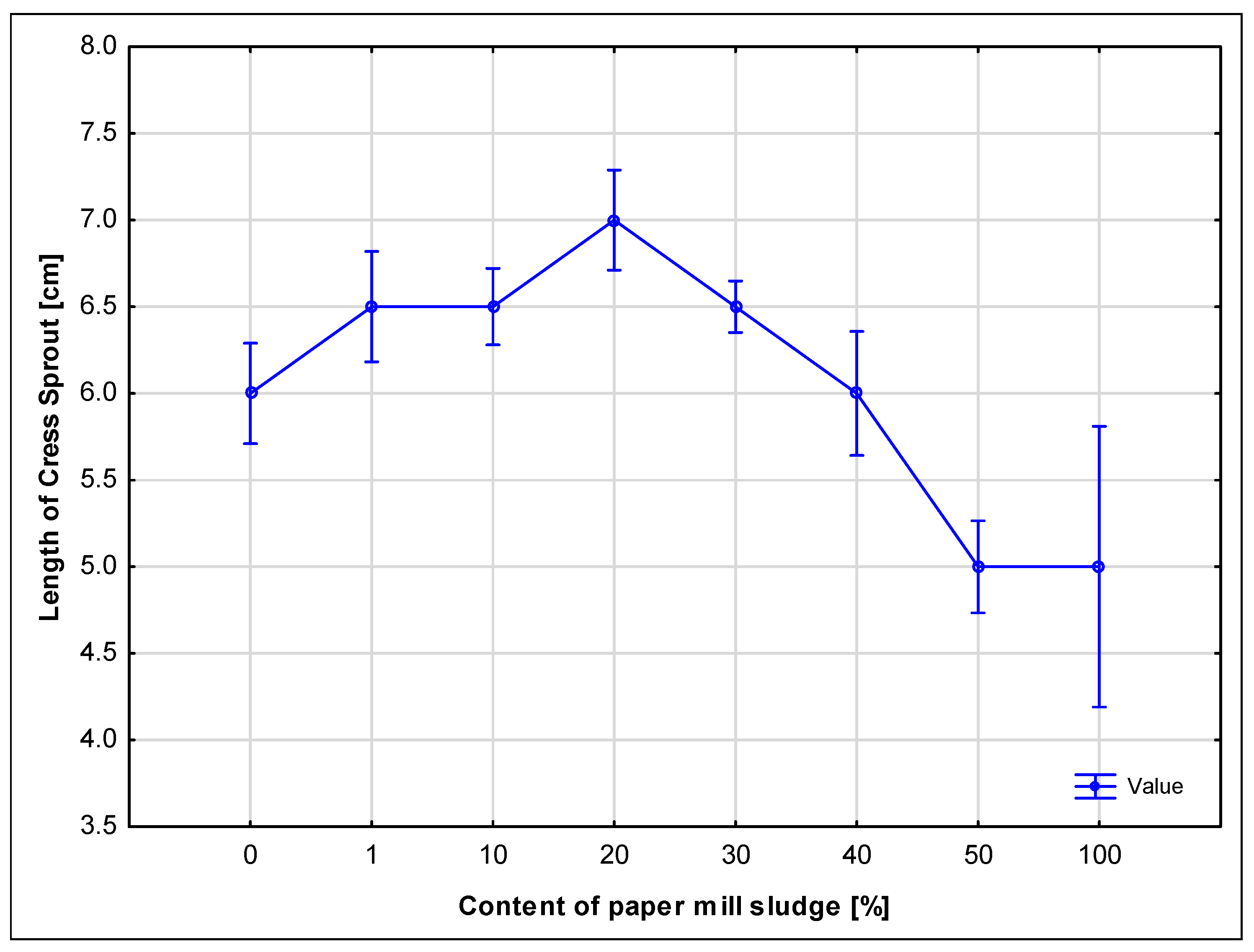
The results indicate that low concentrations of paper sludge (1–20%) can be applied to soil without triggering harmful effects, supporting aerobic microbial activity and allowing for controlled degradation of organic matter, which is consistent with the findings of Kusumarini et al. [
35]. These conditions facilitate the biological stabilization of the sludge, which is essential for its use as an environmentally sustainable disposal method. Concentrations above 20% began to show inhibitory effects, like in the work by A. Singh [
36].
On the other hand, samples with higher concentration of sludge, especially 50% and 100%, showed that excessive microbial activity leads to rapid oxygen depletion as reported by Siedt [
37] and the formation of potentially toxic metabolites. This was also reflected in reduced germination and slower plant growth, which was also evident from the low germination index value (42%). This phenomenon can be attributed mainly to anaerobic conditions caused by compaction of the substrate, by lack of oxygen. Similar results were found also in studies on the impact of high doses of organic waste on soil microflora, where it was shown that excessive microbial activity can create inhibitory conditions for plant growth [
36]. Ballast substances, especially calcium carbonate, may affect the availability of water and nutrients in the soil, which may influence the germination process. Samples with higher concentrations of sludge contained more ballast substances that could create physical barriers for roots and sprouts, thus negatively affecting their development; therefore, the content of inert materials must be considered when evaluating paper sludge for land application [
38].
Samples with higher sludge content showed a peak of respiratory activity in the intermediate phase. Until day 16, the values for samples with 20% and 50% sludge were similar, but the sample with 50% sludge later showed higher values, probably due to the longer adaptation time of microorganisms to high concentrations of organic material [
39].
The highest values of respiratory activity (up to 1487 O
2 kg
−1) were recorded in the samples containing 30% and 40% sludge, which created optimal conditions for microbial decomposition. It has been found that with decreasing concentrations of dissolved organic matter, increased oxygen availability in the soil regulates the composition of dissolved organic matter and enhances its biodegradability, primarily by influencing microbial metabolism and iron oxidation [
31].
These differences show a complex interaction between the availability of organic substances, the ability of microorganisms to adapt and oxygen access, while optimal conditions were achieved in the samples with 30% and 40% sludge.
The addition of paper sludge at concentrations of 10% and higher resulted in increased respiratory activity, with the intensity of respiration generally rising alongside the sludge content. The sample containing 50% sludge exhibited the highest oxygen consumption (338 mg O2 kg−1) during the initial five days, indicating enhanced microbial activity driven by the presence of readily degradable organic compounds. However, in the later stages of the test, samples with 30% and 40% sludge surpassed the 50% variant in terms of respiratory activity.
This shift may be attributed to the inhibitory effects associated with excessive microbial activity at higher sludge concentrations. Intense microbial metabolism in substrates with a high sludge content can lead to the accumulation of phytotoxic intermediates, such as volatile organic acids or ammonia, as well as physical deterioration of the substrate structure. Specifically, sludge-rich substrates may experience compaction and reduced porosity, which restrict oxygen diffusion and limit microbial activity in the later stages of decomposition [
40,
41].
In contrast, the sample composed entirely of sludge (100%) demonstrated markedly lower respiratory activity throughout the test. This was likely due to a limited population of indigenous microorganisms capable of efficiently degrading the organic matter present in the sludge. Previous studies have shown that undiluted sludge may lack the microbial diversity or enzymatic capacity necessary for effective organic matter mineralization, especially under conditions of poor aeration and high pollutant load [
42,
43,
44].
These observations highlight the complexity of microbial responses to varying sludge concentrations and emphasize the need to balance organic input with the biological and physical conditions required to sustain decomposition processes without inducing toxicity or oxygen limitation. The observed outcomes underscore the importance of establishing safe application thresholds, as sludge concentration strongly influences both the rate of decomposition and the potential ecological impact.
4.2. Effect of Paper Sludge Concentration on the Respiration of Germinating Seeds and Growth
Respiration of germinating seeds is an essential indicator of metabolic activity, which affects the ability of seeds to grow and develop the measurement of germination and seed respiration was as a biological sensitivity test to evaluate the potential risks associated with paper sludge application in soil environments [
45]. The results of this study showed that lower sludge concentrations (1% and 10%) did not negatively affect seed respiration or germination. In fact, the sample with 1% sludge achieved the highest germination index (150%), suggesting that at low levels, paper sludge does not pose a toxic effect on germinating seeds, and may even provide a modest stimulus due to microbial stimulation and trace nutrient availability. Mutual interactions among microorganisms and germinating plants are a significant factor in the germination process and subsequent development of plants and can be positive or negative [
35,
46]. This positive effect can be explained by increased nutrients availability and better soil structure, which enables better gas exchange and water transport [
47].
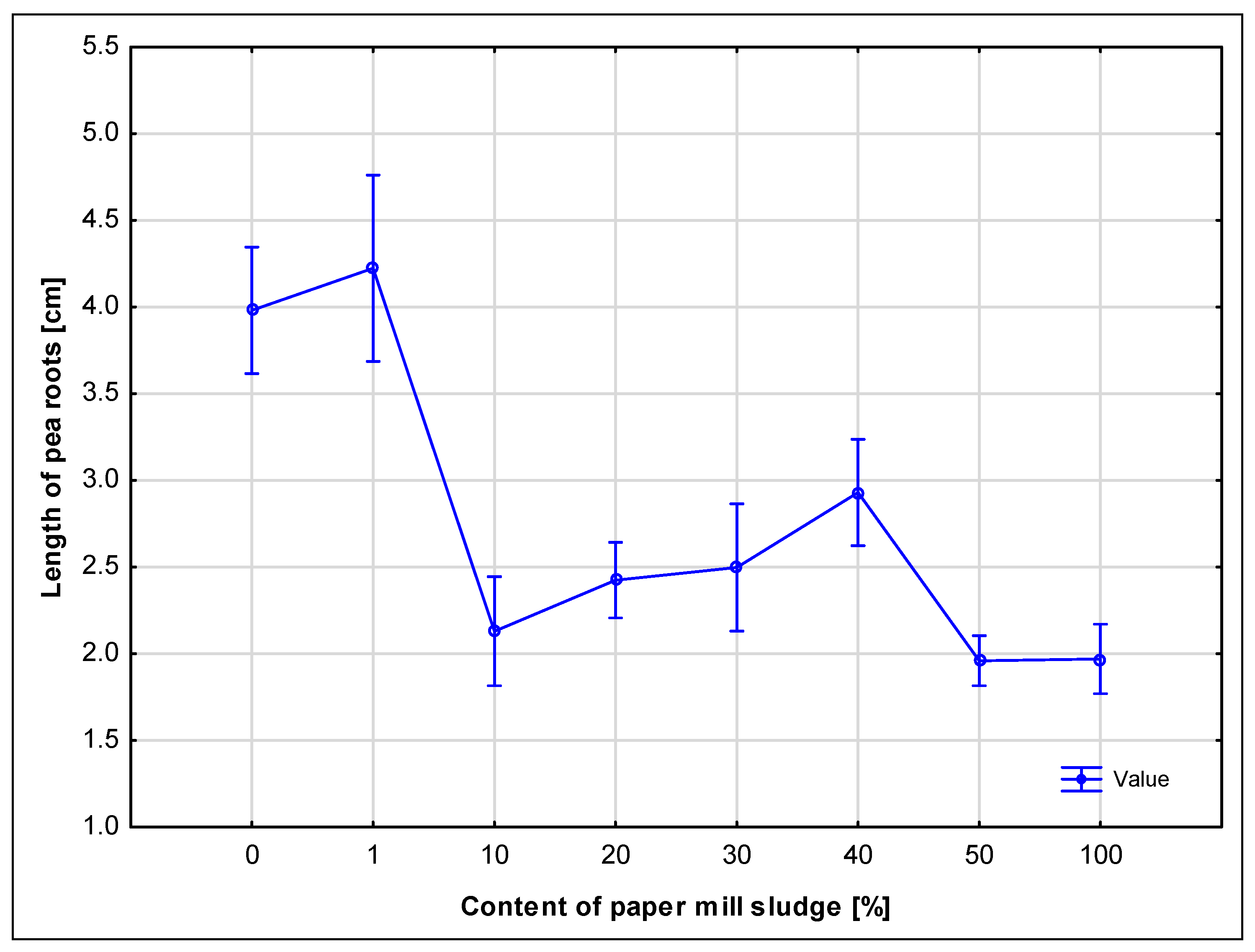
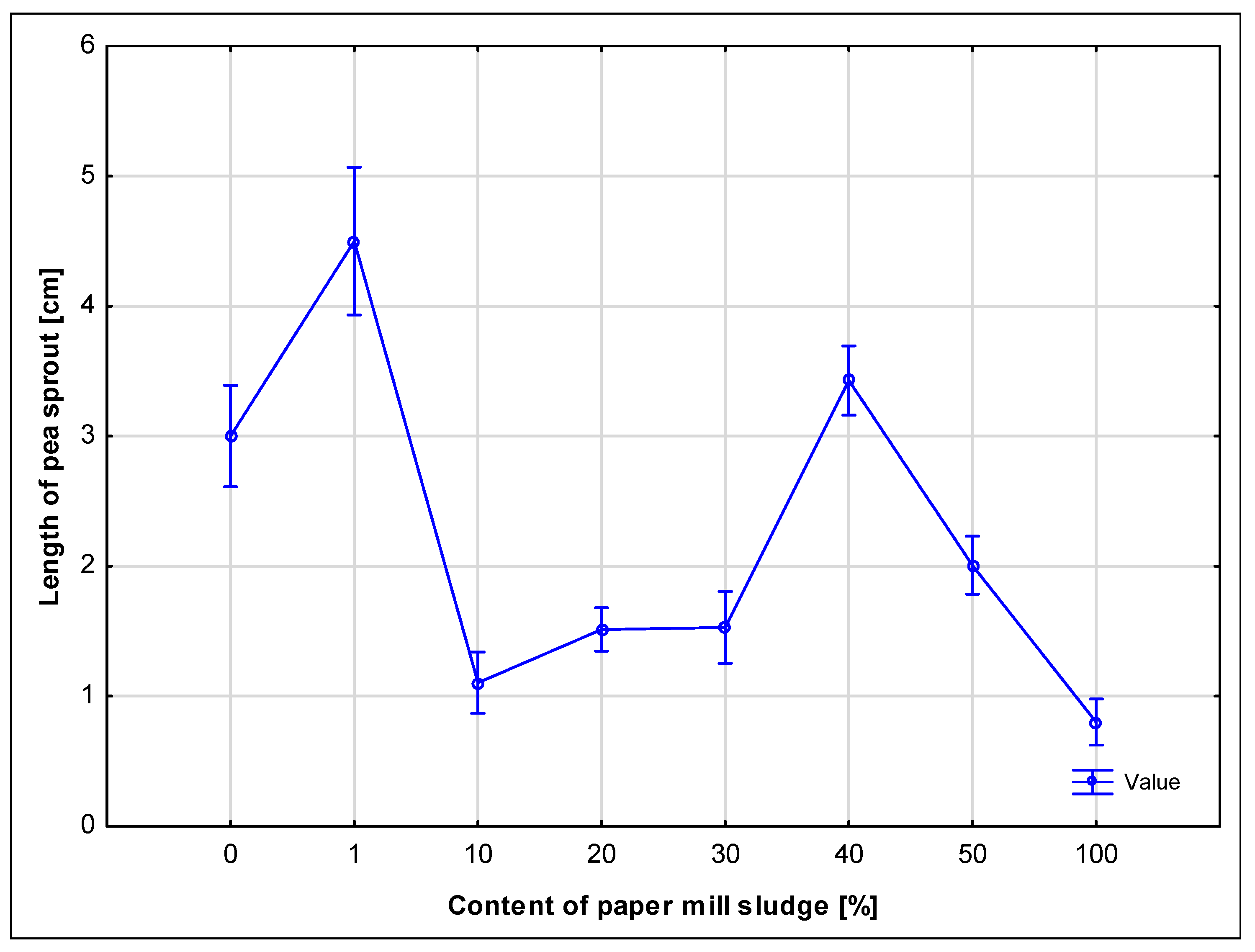
However, higher concentrations of sludge (50% and 100%) had a clearly negative impact on germination and seedling development. While the 100% sludge sample exhibited elevated microbial respiration (as observed in soil tests), the high microbial activity was likely accompanied by oxygen depletion and increased CO
2 levels, leading to respiratory stress in seeds. High levels of CO
2 in the soil negatively affect plant germination as reported in a study by Wenmei He [
48]. Large amounts of paper sludge likely created unsuitable or toxic conditions or bound oxygen, limiting the availability of oxygen to the seeds and reducing their growth. As reported by Norris and Titshall [
49], the application of paper sludge to soil can cause a slight decrease in germination, especially at higher concentrations, which is attributed to the high electrical conductivity (EC) of the sludge, or a study where the germination of seeds watered with paper sludge infusion was assessed [
50]. This is also confirmed by observations where pea seeds showed low germination and limited growth of roots and sprouts. Ballast substances, especially calcium carbonate, may have affected the availability of water and nutrients in the soil, which may have affected the germination process [
51]. Samples with higher concentrations of sludge contained more ballast substances that could create physical barriers for roots and sprouts, thus negatively affecting their development.
These findings are crucial when considering land application of paper sludge as a disposal method, because they highlight the importance of concentration limits to avoid ecological stress and ensure safe application. Germination and seed respiration thus serve as sensitive bioindicators for identifying thresholds beyond which the material could have unintended environmental effects.
During the middle phase of the experiment, an increase in oxygen consumption was observed in the respiration of cress (Lepidium sativum L.), typical of peak metabolic activity during germination. This was accompanied by the active decomposition of storage substances and intensive growth of roots and shoots. The average height of the plants began to vary among the samples, indicating different metabolic responses to the presence of sludge. Higher sludge concentrations may contain substances or properties that prevent effective germination, reduce respiratory efficiency, and limit the growth, possibly due to the presence of toxic substances or excessive amounts of certain ingredients. During final phase (day 6–7) for seeds of pea (Pisum sativum L.), microscopic fungi moulds and fungi were formed on the samples, indicating the presence of microbial contaminants in the substrates containing paper sludge. These microorganisms may have had a negative impact on the germination and growth of seeds, which highlights the importance of assessing microbial activity when using such substrates.
4.3. Microbial Contamination and Its Consequences
The study revealed the presence of the growth of microscopic fungi in the samples with sludge, suggesting that paper sludge naturally contains microorganisms that can affect the soil microbial community and plant physiology. These microorganisms, often identified also in other studies, have the potential to negatively affect plant germination and growth.
While microbial growth may initially raise concerns regarding hygienic safety and potential phytopathogenic effects, it also reflects the biological activity and decomposition potential of the material. Certain fungal species—filamentous fungi (these are known as white-rot fungi) have been shown to possess lignocellulolytic abilities, contributing to the breakdown of complex organic substrates, such as cellulose and lignin, which are abundant in paper sludge [
52]. These microorganisms could be purposefully cultured in composting or bio-remediation practices, reducing the risk of pathogenic effects and increasing the efficiency of decomposition. This approach would support innovative solutions in the field of industrial waste treatment.
The detection of microbial contamination on germinated seeds points to a possible risk for applications of paper sludge as a soil additive. The presence of these microorganisms can pose a challenge to plant health and the overall stability of ecosystems, especially in situations where conditions are suitable for their multiplication. According to the study [
50], the leachate of paper sludges and water from sludge dewatering had a slightly negative effect on the germination of cress (
Lepidium sativum L.) and lettuce (
Lactuca sativa L.) seeds, with germination rates ranging from 83.3% to 100%. The presence of moulds and fungal growth in sludge samples further highlights the need to monitor microbial activity during application. While microbial presence is crucial for decomposition, it must be managed to prevent phytotoxicity or microbial competition.
4.4. Potential Application and Sustainability
The results of this study indicate that land application of paper sludge offers a feasible and potentially sustainable strategy for its disposal, particularly at low concentrations (1–20%). The benefits to crops have been demonstrated emphatically, while negative ecological impacts under typical field application rates have not been observed [
3]. This approach not only reduces the volume of sludge destined for incineration or landfilling but also supports natural biodegradation pathways by utilizing soil microbial communities to decompose organic matter present in the sludge. However, certain carefulness is necessary for higher concentrations, as excessive microbial activity can create unfavourable conditions for plant growth. The proper ratio of paper mill sludge to other components has previously proven to be a necessary condition for the composting of paper mill sludge, as evidenced by increased metabolic activity [
53].
At concentrations of 30–40%, intense microbial respiration was maintained for several weeks, indicating active biodegradation, which is essential for the stabilization and mineralization of sludge components. Soil in this context does not serve as a medium for improving agriculture, but as a bioreactive environment enabling the transformation of organic waste.
However, high sludge concentrations (e.g., 50%) have been associated with excessive microbial activity, leading to oxygen depletion and potentially phytotoxic conditions. According to the study [
54] an increase in sludge concentration led to a decrease in dissolved oxygen. Conversely, a reduction in sludge concentration promoted algal growth but also forced bacteria to rely on the oxygen produced by the algae for their survival. These findings underscore the importance of defining safe application thresholds. Concentrations ranging from 1 to 10% have been shown to prevent such adverse effects while still promoting microbial degradation and are suitable for immediate planting after sludge application to soil.
To mitigate the temporary effects of increased biological oxygen demand, especially at intermediate concentrations, a delayed planting interval (e.g., 21 days) may be recommended to allow the microbial community to stabilize and avoid stress in plant systems. This was supported by the observed decrease in oxygen consumption in the samples after three weeks, indicating that the most intensive phase of decomposition had passed, a conclusion also reached by O’Brien et al. [
55]. When applying paper sludge to soil, the content of ballast substances, especially calcium carbonate and kaolin, should be taken into account, which can alter soil structure and affect nutrient bioavailability [
56].