Three-Dimensional Dual-Network Gel-Immobilized Mycelial Pellets: A Robust Bio-Carrier with Enhanced Shear Resistance and Biomass Retention for Sustainable Removal of SMX
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Experimental Materials and Mycelium Pellets
2.1.1. Fungal Strains and Culture Conditions
2.1.2. PVA-SA Gel Embedding Protocol
2.2. Fabrication of Functionalized Mycelial Pellets (MPs-AFP)
2.3. Material Characterization Methods
2.3.1. Mechanical Property Testing
2.3.2. Morphological and Structural Analysis
2.4. Physicochemical Property Assessments
2.4.1. Specific Oxygen Uptake Rate (SOUR)
2.4.2. Mass Transfer Efficiency Experiments
2.4.3. Settling Velocity Determination
2.4.4. Shear Resistance Test
2.4.5. Diffusion Inhibition Assay
2.5. Sulfamethoxazole (SMX) Concentration Analysis
2.6. Statistical Analysis
3. Results and Discussion
3.1. Effect of PVA-SA Concentration on Tensile Force and Ductility of Materials
3.2. Immobilization Effects on Aerobic Metabolism
3.3. PVA-SA-Reinforced Fungal Mycelial Pellets
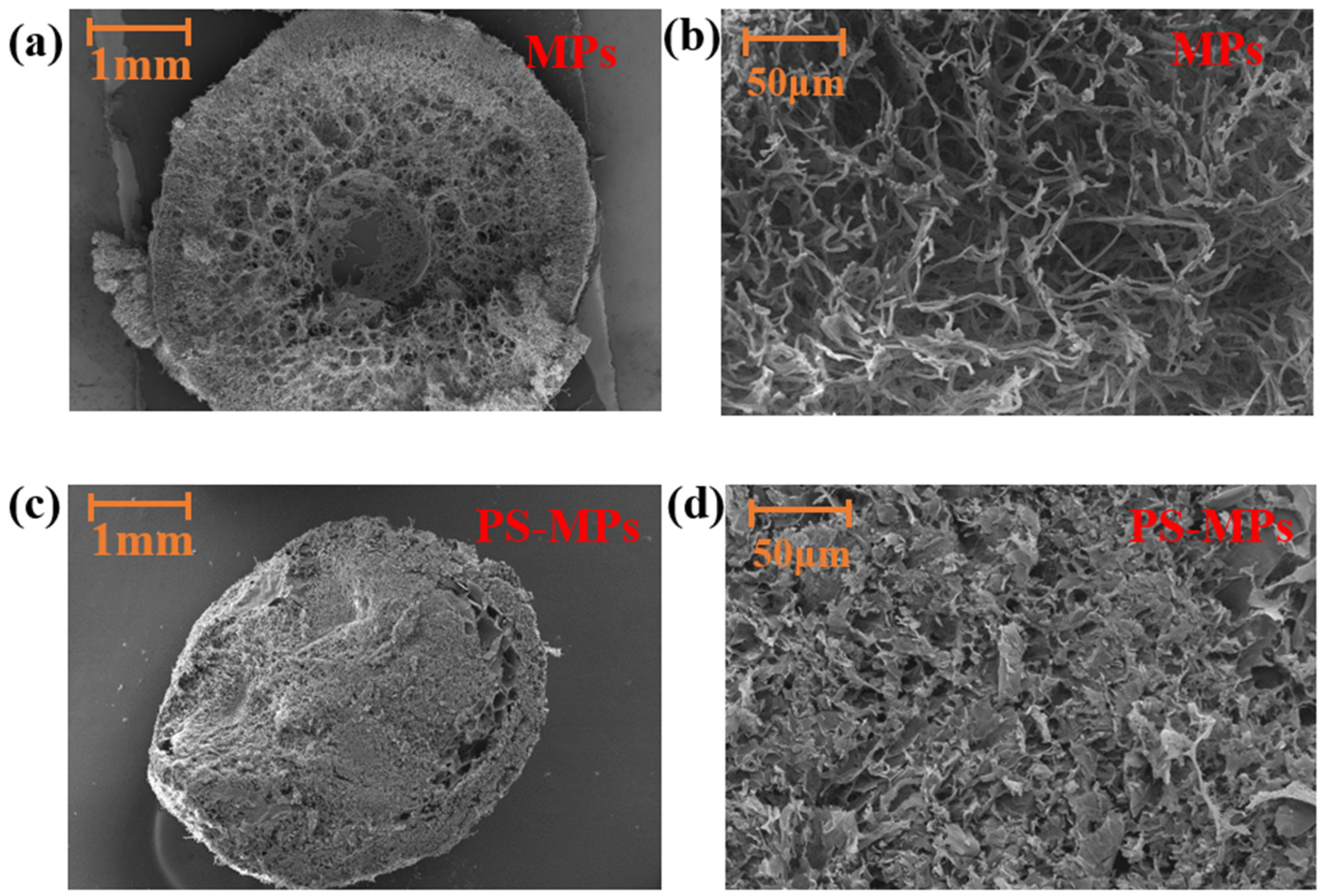
3.3.1. Morphological Characterization of PVA-SA-MPs
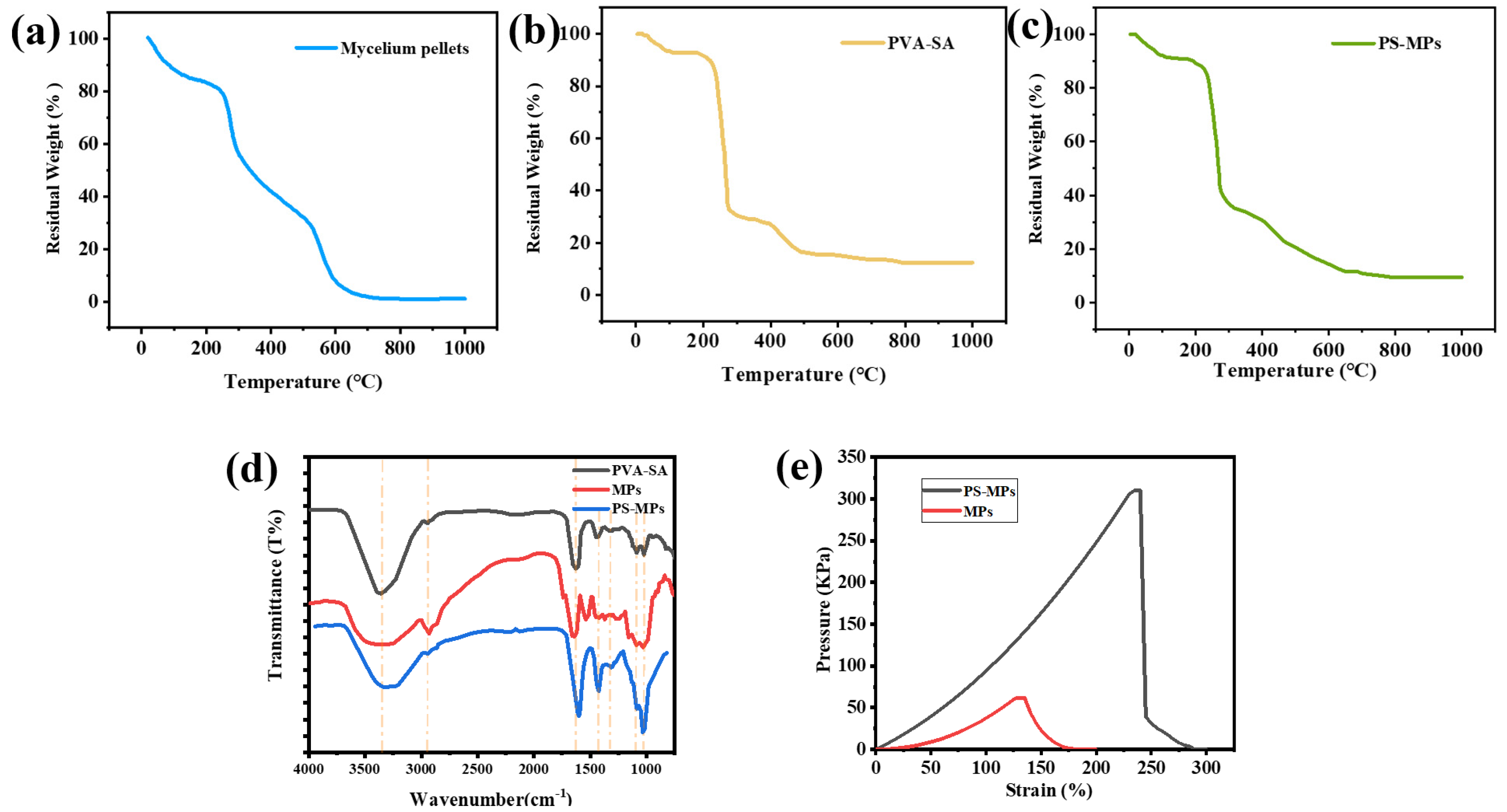
3.3.2. FTIR Analysis
3.3.3. TGA Analysis
3.3.4. Tensile Strength and Strain Tolerance
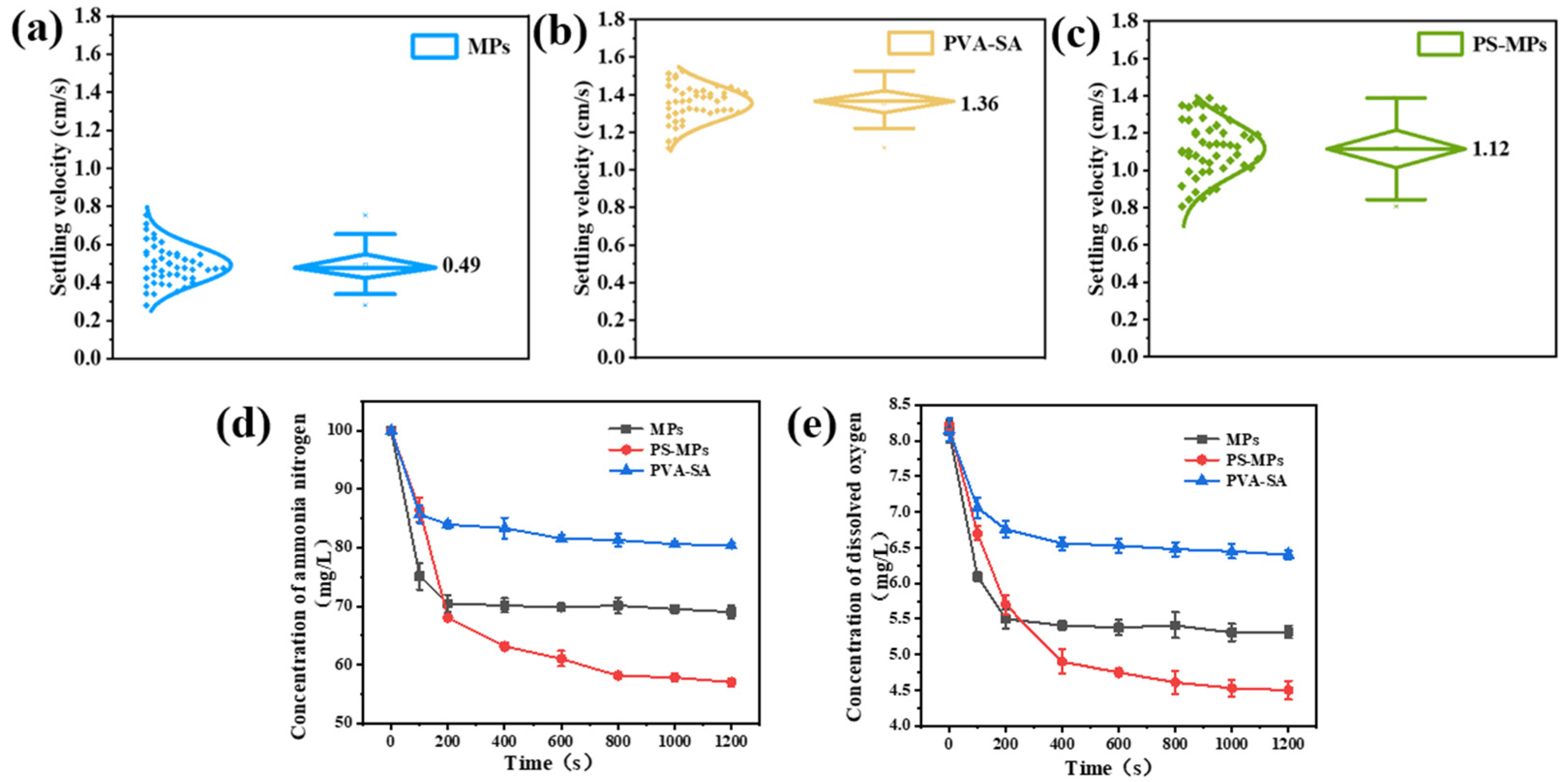
3.3.5. Porosity–Diffusion Trade-Off in Gel-Embedded Mycelial Pellets
3.3.6. Influence of Gel Embedding on Settling Velocity
3.3.7. Controlled Experiment on the Suppression of Hyphal Dispersion
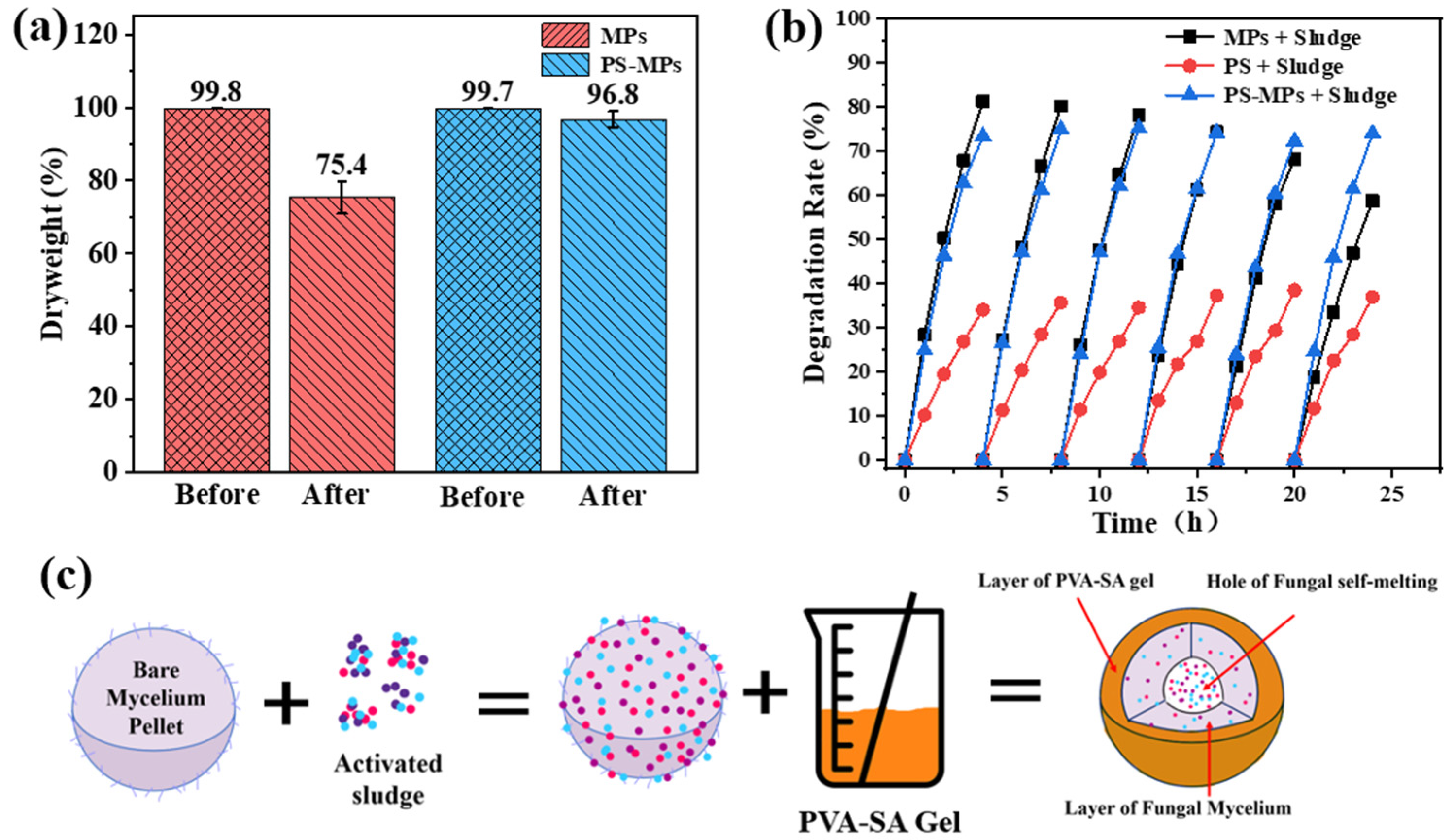
3.3.8. Agitation Tolerance and Structural Integrity
3.4. Engineered Bio-Carrier with Acclimated Sludge for SMX Degradation
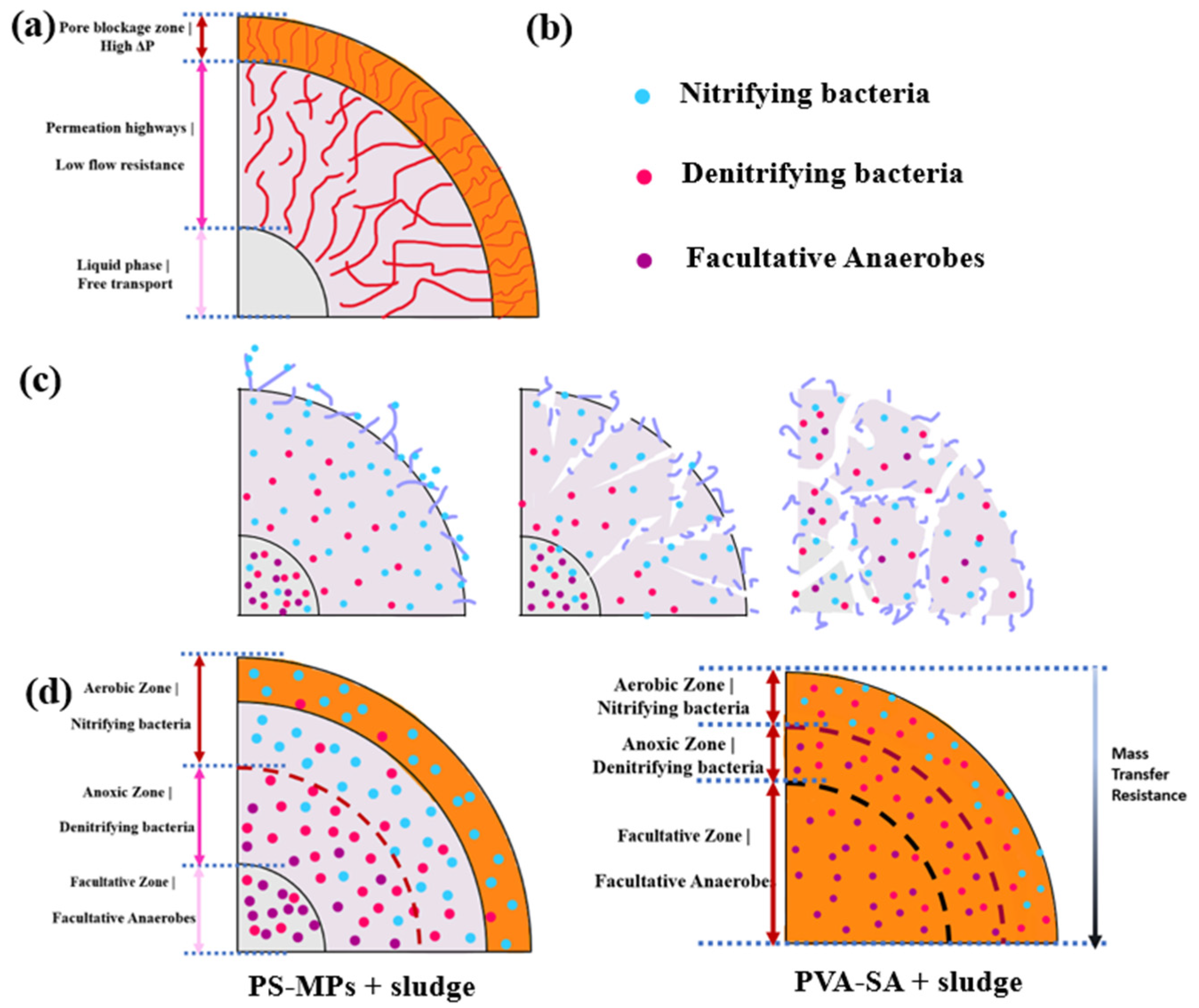
3.5. Mechanism of Enhanced SMX Degradation via PS-MPs-AFP
3.5.1. Robust Biomass Retention Through Structural Reinforcement
3.5.2. Optimized Substrate Accessibility via Hierarchical Mass Transfer
3.5.3. Microenvironment-Driven Microbial Syntrophy
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Li, Y.; Huang, P.; Guo, S.; Nie, M. A promising and green strategy for recycling waste oyster shell powder as bio-filler in polypropylene via mycelium-enlightened interfacial interlocking. J. Clean. Prod. 2020, 272, 122694. [Google Scholar] [CrossRef]
- Ahn, H.; Rehman, J.U.; Kim, T.; Oh, M.S.; Yoon, H.Y.; Kim, C.; Lee, Y.; Shin, S.G.; Jeon, J.-R. Fungal mycelia functionalization with halloysite nanotubes for hyphal spreading and sorption behavior regulation: A new bio-ceramic hybrid for enhanced water treatment. Water Res. 2020, 186, 116380. [Google Scholar] [CrossRef]
- Camilleri, E.; Narayan, S.; Lingam, D.; Blundell, R. Mycelium-based composites: An updated comprehensive overview. Biotechnol. Adv. 2025, 79, 108517. [Google Scholar] [CrossRef]
- Espinosa-Ortiz, E.J.; Rene, E.R.; Pakshirajan, K.; Van Hullebusch, E.D.; Lens, P.N.L. Fungal pelleted reactors in wastewater treatment: Applications and perspectives. Chem. Eng. J. 2016, 283, 553–571. [Google Scholar] [CrossRef]
- Volk, R.; Schröter, M.; Saeidi, N.; Steffl, S.; Javadian, A.; Hebel, D.E.; Schultmann, F. Life cycle assessment of mycelium-based composite materials. Resour. Conserv. Recycl. 2024, 205, 107579. [Google Scholar] [CrossRef]
- Li, L.; Liang, T.; Zhao, M.; Lv, Y.; Song, Z.; Sheng, T.; Ma, F. A review on mycelial pellets as biological carriers: Wastewater treatment and recovery for resource and energy. Bioresour. Technol. 2022, 355, 127200. [Google Scholar] [CrossRef]
- Sánchez-Vargas, J.; Valdés-Parada, F.J.; Peraza-Reyes, L.; Lasseux, D.; Trujillo-Roldán, M.A. Flow modeling and structural characterization in fungal pellets. J. Theor. Biol. 2024, 590, 111853. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Wang, L.; Wang, Y.; Wang, X.; Wei, J.; Yu, T.; Ma, F. Functional fungal pellets self-immobilized by mycelium fragments of Irpex lacteus WRF-IL for efficient degradation of sulfamethazine as the sole carbon source. Bioresour. Technol. 2023, 385, 129376. [Google Scholar] [CrossRef]
- Li, S.; Li, Z.; Liu, D.; Yin, Z.; Hu, D.; Yu, Y.; Li, Z.; Zhu, L. Response of fungi-microalgae pellets to copper regulation in the removal of sulfonamides and release of dissolved organic matters. J. Hazard. Mater. 2022, 434, 128932. [Google Scholar] [CrossRef]
- Min, Y.; Xu, L.; Su, J.; Ma, J.; Ali, A.; Li, X. Enhanced ammonia nitrogen and phenol removal by immobilized bacteria through composite mycelium pellet-driven quinone redox cycle. J. Environ. Manag. 2023, 345, 118893. [Google Scholar] [CrossRef]
- Han, X.; Niu, X.; Jin, Y.; Yu, J. Rapid cultivation of aerobic granular sludge for shale gas flowback water treatment by bioaugmentation with inoculating multifunctional fungal pellets. J. Clean. Prod. 2024, 457, 142483. [Google Scholar] [CrossRef]
- Womer, S.; Huynh, T.; John, S. Hybridizations and reinforcements in mycelium composites: A review. Bioresour. Technol. Rep. 2023, 22, 101456. [Google Scholar] [CrossRef]
- Zhang, S.; Wang, Y.; Wang, B.; Wang, S. A review on superiority of mycelial pellets as bio-carriers: Structure, surface properties, and bioavailability. J. Water Process Eng. 2024, 58, 104745. [Google Scholar] [CrossRef]
- Li, K.; Wei, Z.; Jia, J.; Xu, Q.; Liu, H.; Zhong, C.; Huang, H. Engineered living materials grown from programmable Aspergillus niger mycelial pellets. Mater. Today Bio 2023, 19, 100545. [Google Scholar] [CrossRef] [PubMed]
- Dai, L.; Xie, Y.; Zhang, Y.; Wang, Y. Fe2P/N-doped biocarbon composite derived from mycelial pellet for bisphenol AF removal through peroxymonosulfate activation. J. Environ. Chem. Eng. 2023, 11, 109130. [Google Scholar] [CrossRef]
- Jin, Y.; Liu, D.; Xiong, W.; Wu, Z.; Xiao, G.; Wang, S.; Su, H. Enhancing nitrogen removal performance using immobilized aerobic denitrifying bacteria by modified polyvinyl alcohol/sodium alginate (PVA/SA). Chemosphere 2024, 357, 141954. [Google Scholar] [CrossRef]
- Zhou, X.; Feng, M.-G. Improved sporulation of alginate pellets entrapping Pandora nouryi and millet powder and their potential to induce an aphid epizootic in field cages after release. Biol. Control 2010, 54, 153–158. [Google Scholar] [CrossRef]
- Wang, H.; Neal, B.; White, B.; Nelson, B.; Lai, J.; Long, B.; Arreola-Vargas, J.; Yu, J.; Banik, M.T.; Dai, S.Y. Microplastics removal in the aquatic environment via fungal pelletization. Bioresour. Technol. Rep. 2023, 23, 101545. [Google Scholar] [CrossRef]
- Xie, S.; Wei, H.; Xu, L.; Pu, K.; Li, X.; Su, J. Sludge reduction using polyvinyl alcohol-sodium alginate (PVA/SA)-immobilized refined iron ore and microorganisms: Optimization and mechanism. Chem. Eng. J. 2025, 510, 161864. [Google Scholar] [CrossRef]
- Sun, Y.; Su, J.; Ali, A.; Wang, Z.; Zhang, S.; Zheng, Z.; Min, Y. Fungal-sponge composite carriers coupled with denitrification and biomineralization bacteria to remove nitrate, calcium, and cadmium in a bioreactor. Bioresour. Technol. 2022, 355, 127259. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhang, Q.; Bai, D.; Cai, L.; Lu, T.; Yao, S. Removal process and mechanism of hexavalent chromium by adsorption-coupled reduction with marine-derived Aspergillus niger mycelial pellets. Chin. J. Chem. Eng. 2022, 49, 198–204. [Google Scholar] [CrossRef]
- Liu, Y.; Han, M.; Li, F.; Zhang, N.; Lu, S.; Liu, X.; Wu, F. Performance and mechanism of SMX removal by an electrolysis-integrated ecological floating bed at low temperatures: A new perspective of plant activity, iron plaque, and microbial functions. J. Hazard. Mater. 2024, 463, 132802. [Google Scholar] [CrossRef]
- Geng, M.; You, S.; Guo, H.; Ma, F.; Xiao, X.; Zhang, J. Impact of fungal pellets dosage on long-term stability of aerobic granular sludge. Bioresour. Technol. 2021, 332, 125106. [Google Scholar] [CrossRef]
- Zhang, B.; He, Y.; Shi, W.; Liu, L.; Li, L.; Liu, C.; Lens, P.N.L. Biotransformation of sulfamethoxazole (SMX) by aerobic granular sludge: Removal performance, degradation mechanism and microbial response. Sci. Total Environ. 2023, 858, 159771. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Wu, Y.; Chen, H.; Gao, Y.; Zhou, L.; Wan, J.; Li, Y.; Tang, M.; Peng, Y.; Wang, B.; et al. Efficient phosphate removal from water by multi-engineered PVA/SA matrix double network hydrogels: Influencing factors and removal mechanism. Sep. Purif. Technol. 2024, 336, 126261. [Google Scholar] [CrossRef]
- Yu, T.; Wang, L.; Ma, F.; Wang, Y.; Bai, S. A bio-functions integration microcosm: Self-immobilized biochar-pellets combined with two strains of bacteria to remove atrazine in water and mechanisms. J. Hazard. Mater. 2020, 384, 121326. [Google Scholar] [CrossRef]
- Zheng, Z.; Ali, A.; Su, J.; Huang, T.; Wang, Y.; Zhang, S. Fungal pellets immobilized bacterial bioreactor for efficient nitrate removal at low C/N wastewater. Bioresour. Technol. 2021, 332, 125113. [Google Scholar] [CrossRef]
- Chen, M.; Jin, L.; Liu, X.; Li, R.; Xian, H.; Guo, C. Immobilization of ammonia-oxidizing bacteria using mycelial pellets: Preparation, characteristics, and application for nitritation. Bioresour. Technol. 2025, 419, 132083. [Google Scholar] [CrossRef]
- Zheng, Z.; Ali, A.; Su, J.; Zhang, S.; Fan, Y.; Sun, Y. Self-immobilized biochar fungal pellet combined with bacterial strain H29 enhanced the removal performance of cadmium and nitrate. Bioresour. Technol. 2021, 341, 125803. [Google Scholar] [CrossRef]
- Huang, Y.; Dong, T.; Zhang, X.; Xu, S.; Song, X.; Wang, Z.; Qin, M.; Deng, L.; Li, Y. Preparation of PVA/SA Interpenetrating Double Network Municipal Sludge Hydrogel and the Study of pH Response. J. Polym. Mater. 2025, 42, 151–172. [Google Scholar] [CrossRef]
- Xiong, Y.; Hu, D.; Huang, L.; Fang, Z.; Jiang, H.; Mao, Q.; Wang, H.; Tang, P.; Li, J.; Wang, G.; et al. Ultra-high strength sodium alginate/PVA/PHMB double-network hydrogels for marine antifouling. Prog. Org. Coat. 2024, 187, 108175. [Google Scholar] [CrossRef]
- Xiao, X.; Guo, H.; Ma, F.; You, S.; Geng, M.; Kong, X. Biological mechanism of alleviating membrane biofouling by porous spherical carriers in a submerged membrane bioreactor. Sci. Total Environ. 2021, 792, 148448. [Google Scholar] [CrossRef] [PubMed]
- Xiang, X.; Yi, X.; Zheng, W.; Li, Y.; Zhang, C.; Wang, X.; Chen, Z.; Huang, M.; Ying, G.-G. Enhanced biodegradation of thiamethoxam with a novel polyvinyl alcohol (PVA)/sodium alginate (SA)/biochar immobilized Chryseobacterium sp H5. J. Hazard. Mater. 2023, 443, 130247. [Google Scholar] [CrossRef] [PubMed]
- Hou, L.; Hu, K.; Huang, F.; Pan, Z.; Jia, X.; Liu, W.; Yao, X.; Yang, Z.; Tang, P.; Li, J. Advances in immobilized microbial technology and its application to wastewater treatment: A review. Bioresour. Technol. 2024, 413, 131518. [Google Scholar] [CrossRef] [PubMed]
- Zhou, H.; Li, X.; Hu, B.; Wu, M.; Zhang, Y.; Yi, X.; Liu, Y. Assembly of fungal mycelium-carbon nanotube composites and their application in pyrene removal. J. Hazard. Mater. 2021, 415, 125743. [Google Scholar] [CrossRef]
- Phu, N.A.M.M.; Wi, E.; Jeong, G.; Kim, H.; Singha, N.R.; Chang, M. Highly efficient dye adsorption by hierarchical porous SA/PVA/ZIF-8 composite microgels prepared via microfluidics. Carbohydr. Polym. 2025, 350, 123016. [Google Scholar] [CrossRef]
- Maneechote, W.; Cheirsilp, B.; Angelidaki, I.; Suyotha, W.; Boonsawang, P. Chitosan-coated oleaginous microalgae-fungal pellets for improved bioremediation of non-sterile secondary effluent and application in carbon dioxide sequestration in bubble column photobioreactors. Bioresour. Technol. 2023, 372, 128675. [Google Scholar] [CrossRef]
- Li, B.; Jiang, Y.; Wang, Y.; Li, X.; Xia, K.; Tian, M.; He, X. Activity enhancement and the anammox mechanism under low temperature via PVA-SA and nano Fe2O3-PVA-SA entrapped beads. Sci. Total Environ. 2022, 845, 157306. [Google Scholar] [CrossRef]
- Sun, Y.; Ali, A.; Zheng, Z.; Su, J.; Zhang, S.; Min, Y.; Liu, Y. Denitrifying bacteria immobilized magnetic mycelium pellets bioreactor: A new technology for efficient removal of nitrate at a low carbon-to-nitrogen ratio. Bioresour. Technol. 2022, 347, 126369. [Google Scholar] [CrossRef]
- Zou, J.J.; Dai, C.; Hu, J.; Tong, W.K.; Gao, M.; Zhang, Y.; Leong, K.H.; Fu, R.; Zhou, L. A novel mycelial pellet applied to remove polycyclic aromatic hydrocarbons: High adsorption performance & its mechanisms. Sci. Total Environ. 2024, 922, 171201. [Google Scholar] [CrossRef]
- Ma, J.; Min, Y.; Su, J.; Huang, T.; Ali, A.; Wang, Y.; Li, X. Simultaneous removal of ammonia nitrogen, phosphate, zinc, and phenol by degradation of cellulose in composite mycelial pellet bioreactor: Enhanced performance and community co-assembly mechanism. Environ. Res. 2024, 252, 118780. [Google Scholar] [CrossRef]
- Villena, G.K.; Fujikawa, T.; Tsuyumu, S.; Gutiérrez-Correa, M. Structural analysis of biofilms and pellets of Aspergillus niger by confocal laser scanning microscopy and cryo scanning electron microscopy. Bioresour. Technol. 2010, 101, 1920–1926. [Google Scholar] [CrossRef] [PubMed]
- Muller, E.; Schussler, W.; Horn, H.; Lemmer, H. Aerobic biodegradation of the sulfonamide antibiotic sulfamethoxazole by activated sludge applied as cosubstrate and sole carbon and nitrogen source. Chemosphere 2013, 92, 969–978. [Google Scholar] [CrossRef]
- Deng, Y.; Mao, Y.; Li, B.; Yang, C.; Zhang, T. Aerobic degradation of sulfadiazine by arthrobacter spp: kinetics, pathways, and genomic characterization. Environ. Sci. Technol. 2016, 50, 9566–9575. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.F.; Francis, A.J.; Bollag, J.M. Microbial metabolism of homocyclic and heterocyclic aromatic compounds under anaerobic conditions. Microbiol. Rev. 1987, 51, 43. [Google Scholar] [CrossRef]
- Mohatt, J.L.; Hu, L.; Finneran, K.T.; Strathmann, T.J. Microbially mediated abiotic transformation of the antimicrobial agent sulfamethoxazole under ironreducing soil conditions. Environ. Sci. Technol. 2011, 45, 4793–4801. [Google Scholar] [CrossRef]
- Zhang, D.; Raghavan, N.; Chen, S.Y.; Zhang, H.; Quan, M.; Lecureux, L.; Patrone, L.M.; Lam, P.Y.; Bonacorsi, S.J.; Knabb, R.M.; et al. Reductive isoxazole ring opening of the anticoagulant razaxaban is the major metabolic clearance pathway in rats and dogs. Drug Metab. Dispos. 2008, 36, 303–315. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Q.; Guo, H.; Zhang, J.; Ma, F. Three-Dimensional Dual-Network Gel-Immobilized Mycelial Pellets: A Robust Bio-Carrier with Enhanced Shear Resistance and Biomass Retention for Sustainable Removal of SMX. Sustainability 2025, 17, 8765. https://doi.org/10.3390/su17198765
Zhang Q, Guo H, Zhang J, Ma F. Three-Dimensional Dual-Network Gel-Immobilized Mycelial Pellets: A Robust Bio-Carrier with Enhanced Shear Resistance and Biomass Retention for Sustainable Removal of SMX. Sustainability. 2025; 17(19):8765. https://doi.org/10.3390/su17198765
Chicago/Turabian StyleZhang, Qingyu, Haijuan Guo, Jingyan Zhang, and Fang Ma. 2025. "Three-Dimensional Dual-Network Gel-Immobilized Mycelial Pellets: A Robust Bio-Carrier with Enhanced Shear Resistance and Biomass Retention for Sustainable Removal of SMX" Sustainability 17, no. 19: 8765. https://doi.org/10.3390/su17198765
APA StyleZhang, Q., Guo, H., Zhang, J., & Ma, F. (2025). Three-Dimensional Dual-Network Gel-Immobilized Mycelial Pellets: A Robust Bio-Carrier with Enhanced Shear Resistance and Biomass Retention for Sustainable Removal of SMX. Sustainability, 17(19), 8765. https://doi.org/10.3390/su17198765




