Spatiotemporal Variation of Soil Enzyme Activities and Their Dominant Drivers in Salinized Wheat Fields of the Yellow River Delta
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Region
2.2. Materials
2.3. Experiment Design and Sample Treatment
2.4. Measurement of SWC and Salinity
2.5. Measurement of Nutrient Components Content
2.6. Measurement of Enzyme Activity
2.7. Data Processing and Analysis
3. Results
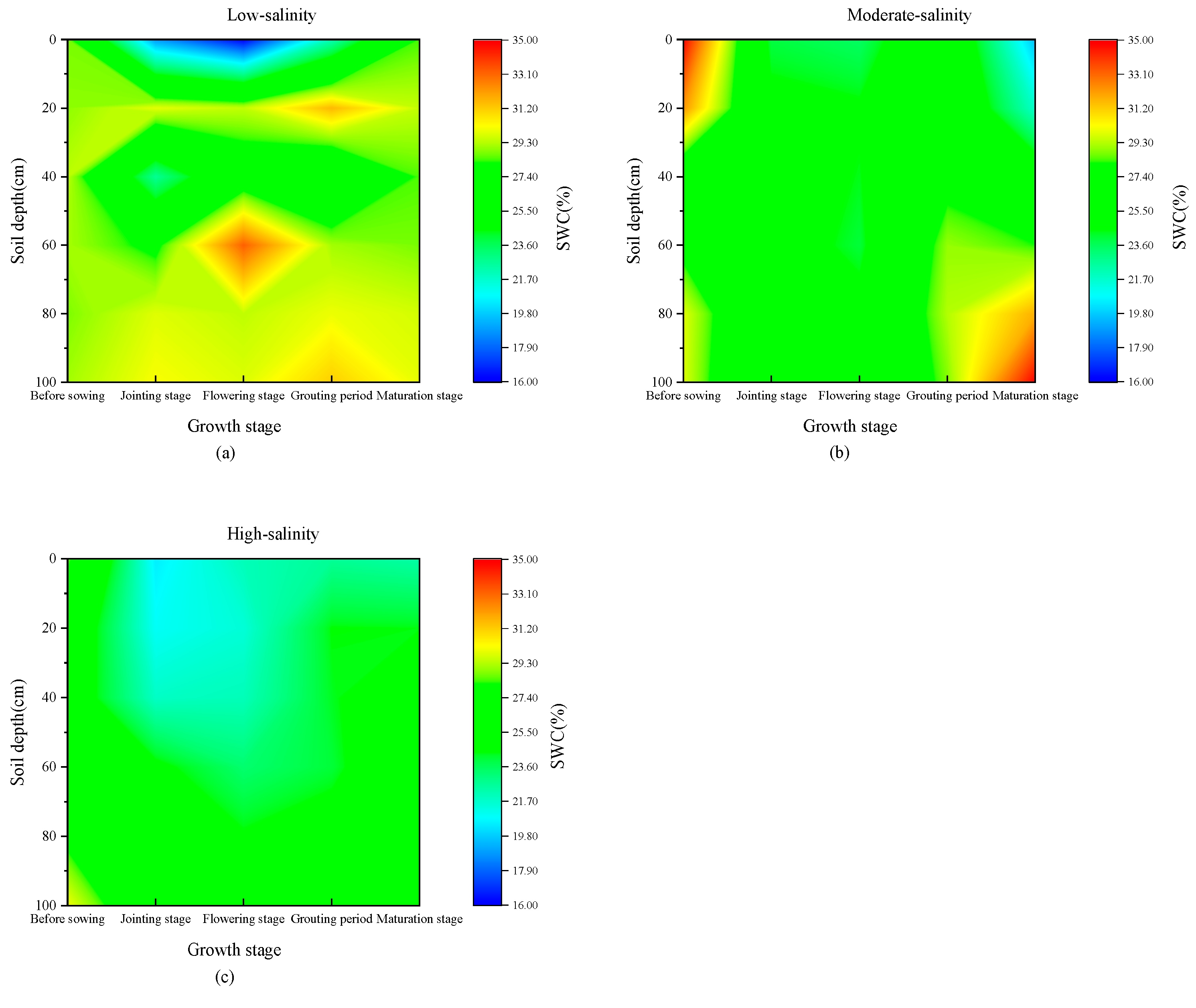
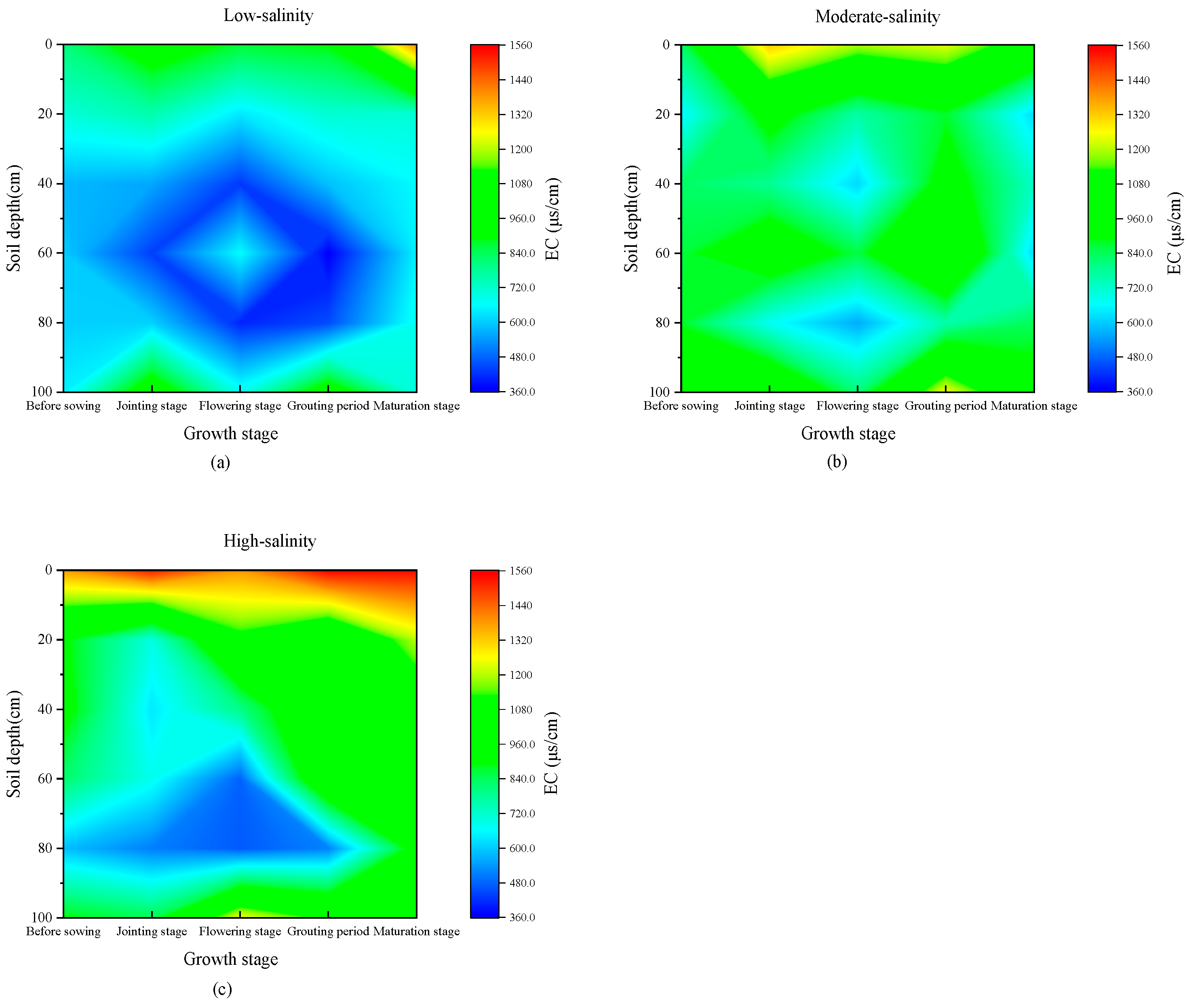
3.1. Spatial–Temporal Distribution Characteristics of Soil Water and Salt Under Wheat Fields with Different Salinity
3.2. Spatial–Temporal Distribution Characteristics of Nutrient Component Under Wheat Fields with Different Salinity
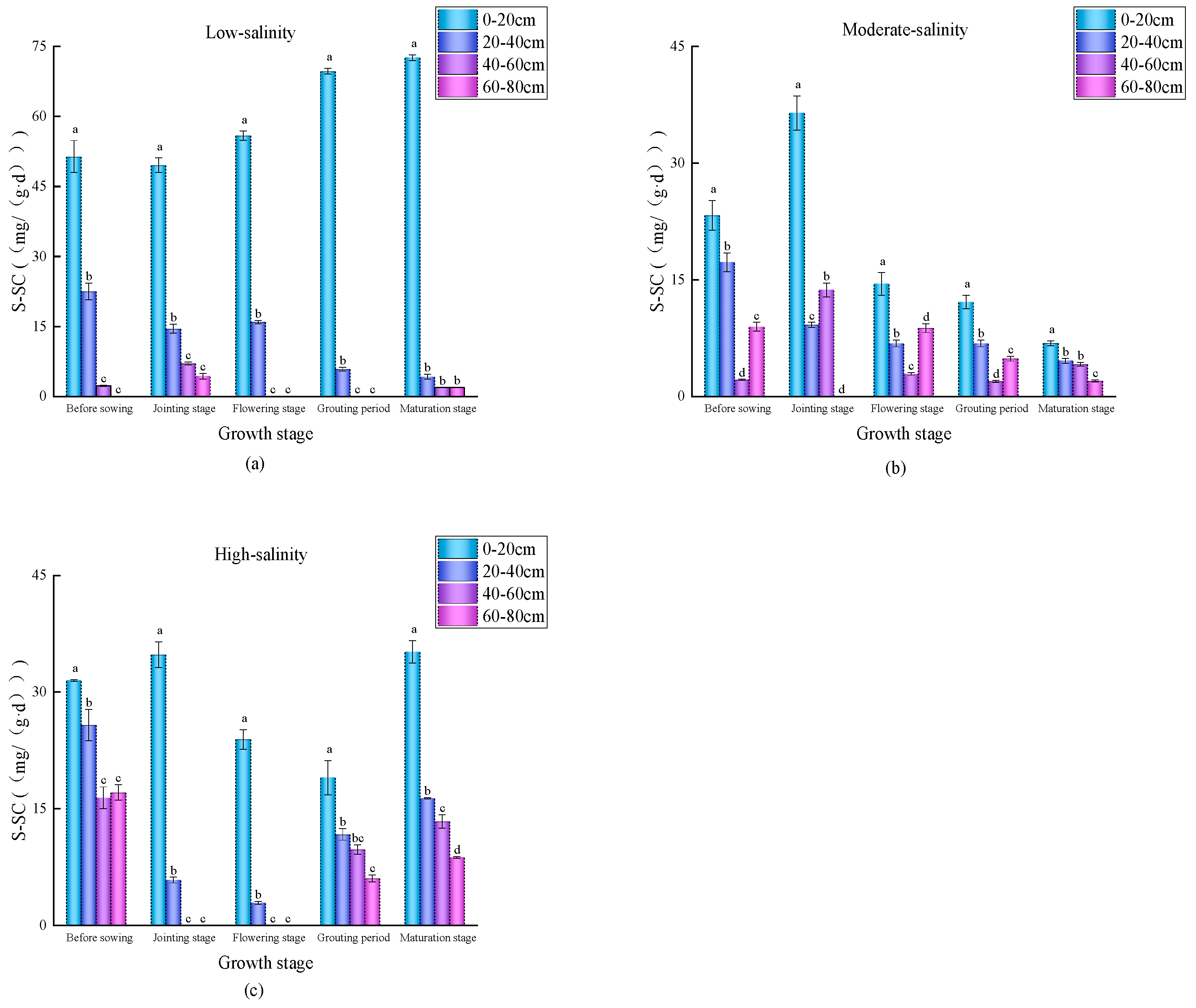
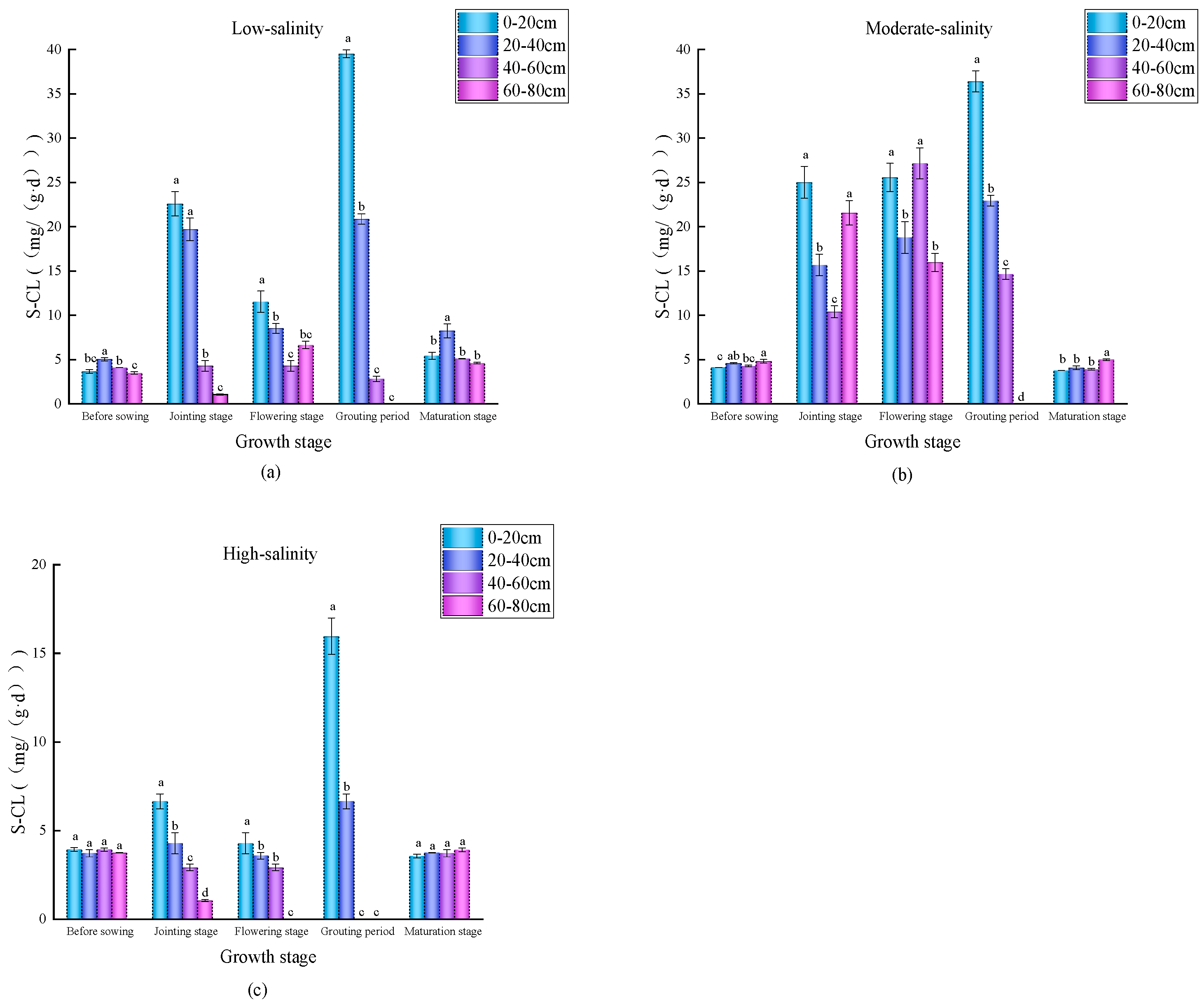
3.3. Spatial–Temporal Distribution Characteristics of Soil Enzyme Activities in Wheat Fields with Different Salinity Levels
3.4. Correlation Between Soil Physicochemical Properties and Enzyme Activities
4. Discussion
4.1. Characteristics of Water and Salt Transport in Different Salinized Wheat Fields
4.2. Temporal and Spatial Variation Characteristics of Soil Nutrients and Enzyme Activities in Differently Salinized Wheat Fields
4.3. Correlations Between Soil Physicochemical Factors and the Enzyme Activity of Carbon Metabolism in Different Salinized Wheat Fields
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| SWC | Soil water content |
| EC | Electrical conductivity |
| A-P | Available phosphorus |
| AK | Available potassium |
| SOM | Soil organic matter |
| TN | Total nitrogen |
| S-SC | Sucrase |
| S-AL | Amylase |
| S-CL | Cellulase |
| RDA | Redundancy analysis |
References
- Pistocchi, C.; Ragaglini, G.; Colla, V.; Branca, T.A.; Tozzini, C.; Romaniello, L. Exchangeable Sodium Percentage Decrease in Saline Sodic Soil after Basic Oxygen Furnace Slag Application in a Lysimeter Trial. J. Environ. Manag. 2017, 203, 896–906. [Google Scholar] [CrossRef]
- Daliakopoulos, I.N.; Tsanis, I.K.; Koutroulis, A.; Kourgialas, N.N.; Varouchakis, A.E.; Karatzas, G.P.; Ritsema, C.J. The Threat of Soil Salinity: A European Scale Review. Sci. Total Environ. 2016, 573, 727–739. [Google Scholar] [CrossRef] [PubMed]
- Xu, C.; Dong, Y.; Lu, J.; Zhang, Y.; Cheng, M.; Cheng, M.; Ma, J. Research Progress of Soil Improvement and Soil Resources Utilization of Coastal Saline-alkaline Land in China. World For. Res. 2020, 33, 68–73. (In Chinese) [Google Scholar] [CrossRef]
- Li, X.; Xia, J.; Zhao, X.; Chen, Y. Effects of Planting Tamarix Chinensis on Shallow Soil Water and Salt Content under Different Groundwater Depths in the Yellow River Delta. Geoderma 2019, 335, 104–111. [Google Scholar] [CrossRef]
- Sun, H.; Qu, J.; Wang, X.; Zheng, W.; Li, C.; Liu, Y. The response of soil organic nitrogen fractions and nitrogen availability to salinity in saline soils of the Yellow River Delta. Chin. J. Eco-Agric. 2021, 29, 1397–1404. (In Chinese) [Google Scholar] [CrossRef]
- De Souza Cruz, D.L.; Do Vale Júnior, J.F.; Timoni Camargo Neves, L.; De Brito Carvalho, L.; Koblam Ahouangbonou, O.R. Soil Physical Quality: A Comprehensive Analysis of Its Importance for Agricultural Production. AGRO@MBIENTE ON-LINE 2024, 18, 1–23. [Google Scholar] [CrossRef]
- Wang, Y.; Guo, H.; Li, J.; Shi, C. Influence of Different Soil Organic Reconstruction Methods on Microstructure of Saline-Alkali Soil. J. Irrig. Drain. 2019, 38, 75–78. (In Chinese) [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, L.; Yi, H.; Lan, S.; Chen, L.; Han, G. Nitrogen Addition Alters Plant Growth in China’s Yellow River Delta Coastal Wetland through Direct and Indirect Effects. Front. Plant Sci. 2022, 13, 1016949. [Google Scholar] [CrossRef]
- Alharbi, K.; Alnusairi, G.S.H.; Alnusaire, T.S.; Alghanem, S.M.S.; Alsudays, I.M.; Alaklabi, A.; Soliman, M.H. Potassium Silica Nanostructure Improved Growth and Nutrient Uptake of Sorghum Plants Subjected to Drought Stress. Front. Plant Sci. 2024, 15, 1425834. [Google Scholar] [CrossRef] [PubMed]
- Song, H.J.; Galgo, S.J.C.; Turner, B.L.; Mishra, U.; Kim, P.J. Greater Root Biomass Offsets Soil Organic Carbon Loss under Climate Impact in Rice Paddies. Soil Biol. Biochem. 2025, 209, 109888. [Google Scholar] [CrossRef]
- Duan, F.; Tan, F.; Zhao, X.; Feng, H.; Wang, J.; Peng, H.; Zhang, N.; Huang, Y. Microbial Life Strategies-Mediated Differences in Carbon Metabolism Explain the Variation in SOC Sequestration between Kandelia Obovata and Sonneratia Apetala. For. Ecosyst. 2025, 14, 100341. [Google Scholar] [CrossRef]
- Xu, X.; Schimel, J.P.; Janssens, I.A.; Song, X.; Song, C.; Yu, G.; Sinsabaugh, R.L.; Tang, D.; Zhang, X.; Thornton, P.E. Global Pattern and Controls of Soil Microbial Metabolic Quotient. Ecol. Monogr. 2017, 87, 429–441. [Google Scholar] [CrossRef]
- Wang, H.; Wu, J.; Li, G.; Yan, L. Changes in Soil Carbon Fractions and Enzyme Activities under Different Vegetation Types of the Northern Loess Plateau. Ecol. Evol. 2020, 10, 12211–12223. [Google Scholar] [CrossRef] [PubMed]
- Lebedev, V.; Lebedeva, T.; Tikhonova, E.; Shestibratov, K. Assessing Impacts of Transgenic Plants on Soil Using Functional Indicators: Twenty Years of Research and Perspectives. Plants 2022, 11, 2439. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Chu, S.; Ouyang, X.; Zou, Z.; Fu, H.; Liu, Y.; Shi, X.; Zhang, Y.; Ouyang, K.; Zhang, L.; et al. Effects of Cow-Dung Vermicomposting on Soil Carbon Mineralization and Temperature Sensitivity in Camellia Oleifera Forest. Agriculture 2024, 14, 1973. [Google Scholar] [CrossRef]
- Han, D.; Zhang, D.; Han, D.; Ren, H.; Wang, Z.; Zhu, Z.; Sun, H.; Wang, L.; Qu, Z.; Lu, W.; et al. Effects of Salt Stress on Soil Enzyme Activities and Rhizosphere Microbial Structure in Salt-Tolerant and-Sensitive Soybean. Sci. Rep. 2023, 13, 17057. [Google Scholar] [CrossRef]
- Sritongon, N.; Sarin, P.; Theerakulpisut, P.; Riddech, N. The Effect of Salinity on Soil Chemical Characteristics, Enzyme Activity and Bacterial Community Composition in Rice Rhizospheres in Northeastern Thailand. Sci. Rep. 2022, 12, 20360. [Google Scholar] [CrossRef]
- Qu, Y.; Tang, J.; Liu, B.; Lyu, H.; Duan, Y.; Yang, Y.; Wang, S.; Li, Z. Rhizosphere Enzyme Activities and Microorganisms Drive the Transformation of Organic and Inorganic Carbon in Saline–Alkali Soil Region. Sci. Rep. 2022, 12, 1314. [Google Scholar] [CrossRef]
- Cheng, Y.; Bai, T.; Hu, J.; Yang, J.; Liu, Q.; Ge, Y.; Xiao, X.; Zhou, Z.; He, H. Drainage for decreasing salt affects the soil properties and microbial community diversity in coastal saline-alkali land. Acta Microbiol. Sin. 2025, 65, 2479–2498. (In Chinese) [Google Scholar] [CrossRef]
- Xie, X.; Pu, L.; Wang, Q.; Zhu, M.; Xu, Y.; Zhang, M. Response of Soil Physicochemical Properties and Enzyme Activities to Long-Term Reclamation of Coastal Saline Soil, Eastern China. Sci. Total Environ. 2017, 607–608, 1419–1427. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.-G.; Bao, X.-G.; Li, X.-F.; Jin, X.; Zhao, J.-H.; Sun, J.-H.; Christie, P.; Li, L. Intercropping Maintains Soil Fertility in Terms of Chemical Properties and Enzyme Activities on a Timescale of One Decade. Plant Soil 2015, 391, 265–282. [Google Scholar] [CrossRef]
- Meena, S.N.; Sharma, S.K.; Singh, P.; Meena, B.P.; Ram, A.; Meena, R.L.; Singh, D.; Meena, R.B.; Nogiya, M.; Jain, D.; et al. Comparative Analysis of Soil Quality and Enzymatic Activities under Different Tillage Based Nutrient Management Practices in Soybean–Wheat Cropping Sequence in Vertisols. Sci. Rep. 2024, 14, 6840. [Google Scholar] [CrossRef] [PubMed]
- Shiferaw, B.; Smale, M.; Braun, H.-J.; Duveiller, E.; Reynolds, M.; Muricho, G. Crops That Feed the World 10. Past Successes and Future Challenges to the Role Played by Wheat in Global Food Security. Food Secur. 2013, 5, 291–317. [Google Scholar] [CrossRef]
- O’Kelly, B.C. Accurate Determination of Moisture Content of Organic Soils Using the Oven Drying Method. Dry. Technol. 2004, 22, 1767–1776. [Google Scholar] [CrossRef]
- Holford, I.C.R. Soil Phosphorus: Its Measurement, and Its Uptake by Plants. Soil Res. 1997, 35, 227. [Google Scholar] [CrossRef]
- Jadoon, S.; Aljaff, H.K.; Hamawandy, J.K.; Pashdary, S.S.; Zakhoy, A.S. Assessment of the Available Potassium in the Soil of Baharka District, Kurdistan-Iraq. Der Pharma Chem. 2015, 7, 1–8. [Google Scholar]
- Liu, B.; Chen, H. The Method of Measuring Organic Matter in Soil. Guangdong Chem. Ind. 2017, 44, 238–240. (In Chinese) [Google Scholar]
- Sáez-Plaza, P.; Michałowski, T.; Navas, M.J.; Asuero, A.G.; Wybraniec, S. An Overview of the Kjeldahl Method of Nitrogen Determination. Part I. Early History, Chemistry of the Procedure, and Titrimetric Finish. Crit. Rev. Anal. Chem. 2013, 43, 178–223. [Google Scholar] [CrossRef]
- Feng, C.; Yi, Z.; Qian, W.; Liu, H.; Jiang, X. Rotations Improve the Diversity of Rhizosphere Soil Bacterial Communities, Enzyme Activities and Tomato Yield. PLoS ONE 2023, 18, e0270944. [Google Scholar] [CrossRef]
- Zuo, Q.; Wu, X.; Shi, J.; Wang, Q.; Liu, Z.; Zhu, A.; Yin, D.; Feng, Q.; Ji, W.; Kang, S. Constraints and Coordinated Allocation Strategies of Water and Land Resources for Sustainable Use of Coastal Saline-Alkali Land in the Yellow River Delta. Strateg. Study CAE 2023, 25, 169–179. (In Chinese) [Google Scholar] [CrossRef]
- Fan, X.; Liu, G.; Tang, Z.; Shu, L. Analysis on Main Contributors Influencing Soil Salinization of Yellow River Delta. J. Soil Water Conserv. 2010, 24, 139–144. (In Chinese) [Google Scholar] [CrossRef]
- Zhao, D.; Ma, H.; Li, S.; Qi, W. Seed Germination Demonstrates Inter-Annual Variations in Alkaline Tolerance: A Case Study in Perennial Leymus Chinensis. BMC Plant Biol. 2024, 24, 397. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Shen, S.; Wang, X.; Lv, Q.; Zhang, M.; Zhou, Y.; Yu, X. Distribution characteristics of soil carbon metabolism related enzyme activities under different vegetation types in Xianghai Wetland. J. Northeast. Norm. Univ. Nat. Sci. Ed. 2023, 55, 119–126. (In Chinese) [Google Scholar] [CrossRef]
- Gao, P. Spatial Multi-Scale Variation, Driving Mechanism, Prediction and Early Warning of Soil Salinization in the Coastal Area of the Yellow River Delta. Ph.D. Thesis, Shandong Agricultural University, Taian, China, 2022. (In Chinese). [Google Scholar]
- Li, Y.; Zhao, S.; Liu, G.; Li, J.; Siddique, K.H.M.; Zhao, D. Agronomic Traits, Nutrient Accumulation, and Their Correlations in Wheat, as Affected by Nitrogen Supply in Rainfed Coastal Saline Soils. Plants 2025, 14, 1022. [Google Scholar] [CrossRef]
- Xu, Y.; Kong, F.; Zhang, M.; Du, H.; Dai, S.; Zhang, Z. Effects of Initial Water and Salt Content on Permeability and Microstructure of Sodic-Saline Loessal Soils. Bull. Eng. Geol. Environ. 2024, 83, 496. [Google Scholar] [CrossRef]
- Kordlaghari, P.K.; Panahikordlaghari, S.; Rahimi, A. Variation in soil available phosphorus and potassium and their relationships with soil properties in some Isfahan soil series of Iran. Res. Crops 2013, 14, 604–607. [Google Scholar]
- Liu, S.; Xie, X.; Wang, X.; Feng, X.; Hou, X.; Wang, S.; Lin, K.; Huang, M.; Jia, S.; Hou, Y.; et al. Distribution Characteristics and Prediction Model of Farmland Soil Organic Carbon in Eastern China. Environ. Res. Commun. 2022, 4, 055012. [Google Scholar] [CrossRef]
- Ma, Q.; Wang, Z.; Hui, X.; Zhang, X.; Zhang, Y.; Hou, S.; Huang, N.; Luo, L.; Zhang, S.; Dang, H. Optimization of Phosphorus Rate and Soil Available Phosphorus Based on Grain Yield and Nutrient Contents in Dryland Wheat Production Scientia. Agric. Sin. 2019, 52, 73–85. (In Chinese) [Google Scholar]
- Li, M.; Yan, Y.; Li, M.; Shi, L.; Kem, S.P.D.; Wang, X. Effects of rice straw returning combined with nitrogen fertilizer on carbon and nitrogen of soil, and growth characteristics and yield of wheat. Hubei Agric. Sci. 2025, 64, 33–41, 55. (In Chinese) [Google Scholar] [CrossRef]
- Sun, Y.; Ye, S.; Fan, G.; Zhang, Y.; Liu, X.; Wu, D.; Wang, F. Isolation and Phosphate Solubilizing Capability of Phosphate-Solubilizing Bacteria in a Wheat Field. Acta Agric. Boreali-Occident. Sin. 2022, 31, 379–387. (In Chinese) [Google Scholar]
- Gao, W.; Yang, W.; He, J.; Jia, Y.; Xu, W.; Ma, H. Effects of biochar on soil microbial community metabolism in wheat field. Chin. J. Ecol. 2020, 39, 3998–4004. (In Chinese) [Google Scholar] [CrossRef]
- Chen, X.; Mi, Q.; Xu, X. Soil Microbial Community Construction Processes and Its Driving Factors in Wheat Field at Local-Scale. Soils 2023, 55, 812–820. (In Chinese) [Google Scholar] [CrossRef]
- Hou, J.; Zhang, S.; Yuan, J.; Cheng, S.; Liu, J.; Liu, S.; Liang, Y.; Ren, J.; Cai, H. Effects of maize straw-derived organic materials on improving soil nutrient availability and enzyme activities in a Mollisol. J. Plant Nutr. Fertil. 2021, 27, 610–618. (In Chinese) [Google Scholar]
- Wang, N.; Ai, Z.; Zhang, Q.; Leng, P.; Qiao, Y.; Li, Z.; Tian, C.; Shi, X.; Cheng, H.; Chen, G.; et al. The Variations of Wheat–Maize Production, Soil Organic Carbon, and Carbon Footprints: Insights from a 20–Year on–Farm Observational Experiment in the North China Plain. Front. Plant Sci. 2025, 16, 1547431. [Google Scholar] [CrossRef]
- Feng, J.; Yu, D.; Sinsabaugh, R.L.; Moorhead, D.L.; Andersen, M.N.; Smith, P.; Song, Y.; Li, X.; Huang, Q.; Liu, Y.; et al. Trade-Offs in Carbon-Degrading Enzyme Activities Limit Long-Term Soil Carbon Sequestration with Biochar Addition. Biol. Rev. 2023, 98, 1184–1199. [Google Scholar] [CrossRef]
- Yin, Y.; Zhang, Z.; Yang, K.; Gu, P.; Liu, S.; Jia, Y.; Zhang, Z.; Wang, T.; Yin, J.; Miao, H. Deeper Insight into the Effect of Salinity on the Relationship of Enzymatic Activity, Microbial Community and Key Metabolic Pathway during the Anaerobic Digestion of High Strength Organic Wastewater. Bioresour. Technol. 2022, 363, 127978. [Google Scholar] [CrossRef]
- Yang, Z.; Nan, H.; Lu, X.; Dai, M.; Zhao, K.; Fan, Y.; Zhu, X.; Zhang, M.; Wang, L.; Sun, Y.; et al. GhDMT7-mediated DNA Methylation Dynamics Enhance Starch and Sucrose Metabolism Pathways to Confer Salt Tolerance in Cotton. Plant J. 2025, 123, e70364. [Google Scholar] [CrossRef]
- Yang, Q.; Sun, Y.; Wang, H.; Pan, H.; Lou, Y.; Zhu, G. Research Progress and Prospect of Saline-alkali Land Improvement and Comprehensive Utilization Technology. J. Shandong Agric. Univ. Nat. Sci. Ed. 2025, 56, 384–395. (In Chinese) [Google Scholar]
- Chen, M.; Kuzyakov, Y.; Zhou, J.; Zamanian, K.; Wang, S.; Abdalla, K.; Wang, J.; Li, X.; Li, H.; Zhang, H.; et al. High Soil Salinity Reduces Straw Decomposition but Primes Soil Organic Carbon Loss. Soil Biol. Biochem. 2025, 207, 109835. [Google Scholar] [CrossRef]
- Li, W.; Wu, M.; Liu, M.; Jiang, C.; Chen, X.; Kuzyakov, Y.; Rinklebe, J.; Li, Z. Responses of Soil Enzyme Activities and Microbial Community Composition to Moisture Regimes in Paddy Soils Under Long-Term Fertilization Practices. Pedosphere 2018, 28, 323–331. [Google Scholar] [CrossRef]
- Tang, J.; Zhang, L.; Zhang, J.; Ren, L.; Zhou, Y.; Zheng, Y.; Luo, L.; Yang, Y.; Huang, H.; Chen, A. Physicochemical Features, Metal Availability and Enzyme Activity in Heavy Metal-Polluted Soil Remediated by Biochar and Compost. Sci. Total Environ. 2020, 701, 134751. [Google Scholar] [CrossRef] [PubMed]
- Ma, Z.; Xu, W.; Chen, Y.; Liu, M.; Wen, J. A Study of the Influence of the Type of Land Use on the Enzymatic Activity of Soils in Southwestern China. Forests 2024, 15, 581. [Google Scholar] [CrossRef]
- Deng, D.; Li, D.; Zhai, Z.; Deng, T.; Wang, J.; Huang, J. Effects of Applying Microbial Fertilizer on Physical and Chemical Properties and Enzyme Activities of Citrus Soil under Salt Stress. Nat. Sci. Hainan Univ. 2020, 38, 226–232. (In Chinese) [Google Scholar] [CrossRef]
- Zou, X.; Zheng, X.; Lie, Z.; Xue, L. Responses of soil element and enzyme activity of landscape plants of three species to salt stress. J. Cent. South Univ. For. Technol. 2018, 38, 41–49. (In Chinese) [Google Scholar] [CrossRef]
- Yadav, D.; Singh, A.; Mathur, N.; Agarwal, A.; Sharma, J. Isolation of Halophilic Bacteria and Their Screening for Extracellular Enzyme Production. J. Sci. Ind. Res. 2021, 80, 617–622. [Google Scholar] [CrossRef]
- Purohit, M.S.; Tipre, D.R.; Shah, M.B.; Dave, S.R. Isolation of halotolerant and halophilic bacteria and screening of their poly enzyme potential. Indian J. Mar. Sci. 2016, 45, 129–136. [Google Scholar]
- Sun, J.; Wang, X. Effects of Salt Stress on Gene Expression of Peroxidase Isozyme in Wheat. J. Triticeae Crops 2006, 42–44, 61. (In Chinese) [Google Scholar]







Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, M.; Guo, S.; Xu, J.; Guan, S.; Zhao, D.; Wang, Y.; Song, X.; Li, J.; Wang, J.; Zhao, S. Spatiotemporal Variation of Soil Enzyme Activities and Their Dominant Drivers in Salinized Wheat Fields of the Yellow River Delta. Sustainability 2025, 17, 8566. https://doi.org/10.3390/su17198566
Li M, Guo S, Xu J, Guan S, Zhao D, Wang Y, Song X, Li J, Wang J, Zhao S. Spatiotemporal Variation of Soil Enzyme Activities and Their Dominant Drivers in Salinized Wheat Fields of the Yellow River Delta. Sustainability. 2025; 17(19):8566. https://doi.org/10.3390/su17198566
Chicago/Turabian StyleLi, Minghui, Sijia Guo, Jikun Xu, Sai Guan, Deyong Zhao, Yuxia Wang, Xianrui Song, Jian Li, Jianlin Wang, and Shuaipeng Zhao. 2025. "Spatiotemporal Variation of Soil Enzyme Activities and Their Dominant Drivers in Salinized Wheat Fields of the Yellow River Delta" Sustainability 17, no. 19: 8566. https://doi.org/10.3390/su17198566
APA StyleLi, M., Guo, S., Xu, J., Guan, S., Zhao, D., Wang, Y., Song, X., Li, J., Wang, J., & Zhao, S. (2025). Spatiotemporal Variation of Soil Enzyme Activities and Their Dominant Drivers in Salinized Wheat Fields of the Yellow River Delta. Sustainability, 17(19), 8566. https://doi.org/10.3390/su17198566






