Emerging Pollutants as Chemical Additives in the Petroleum Industry: A Review of Functional Uses, Environmental Challenges and Sustainable Control Strategies
Abstract
1. Introduction
2. Functional Uses of EPs
2.1. Oilfield Chemicals
2.1.1. Oil Well Drilling
2.1.2. Oil Well Acidifier
2.1.3. Chemical Oil Displacement Agent
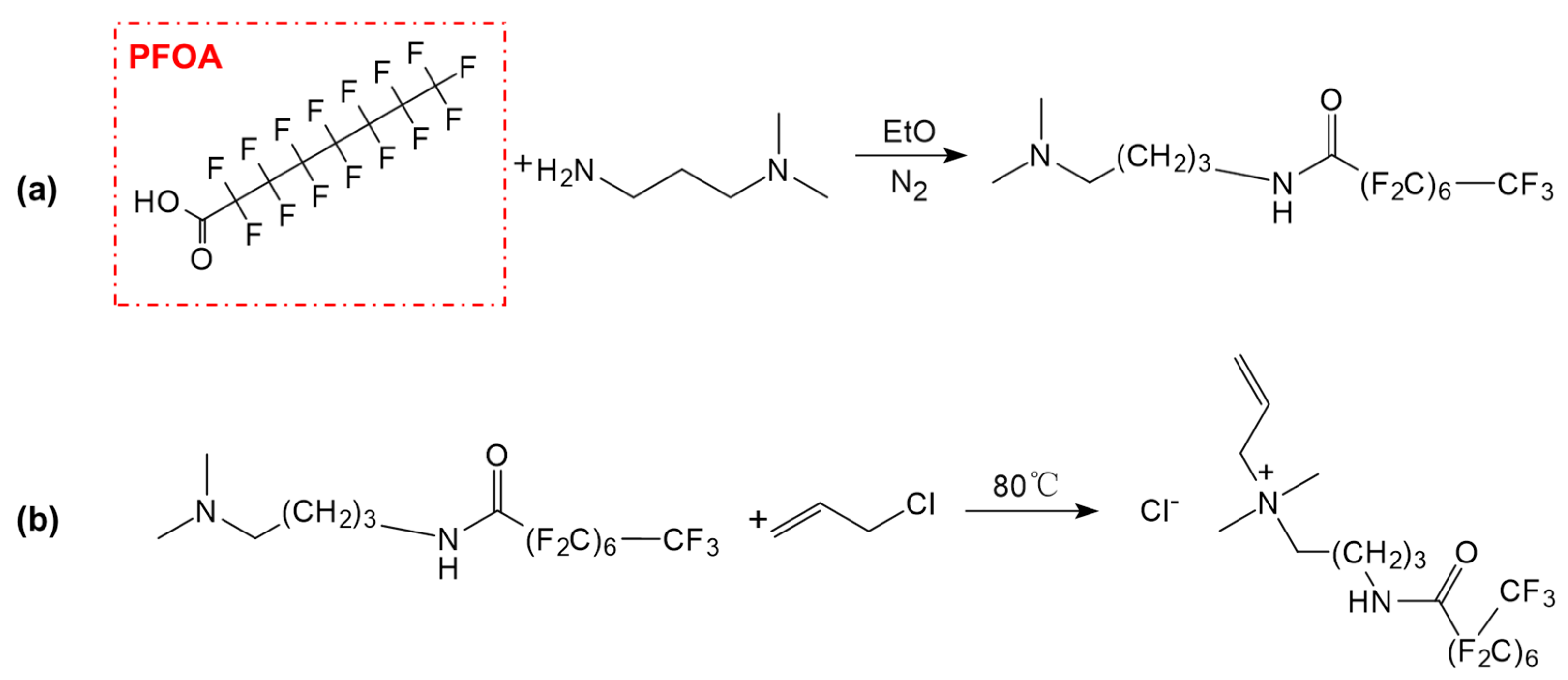
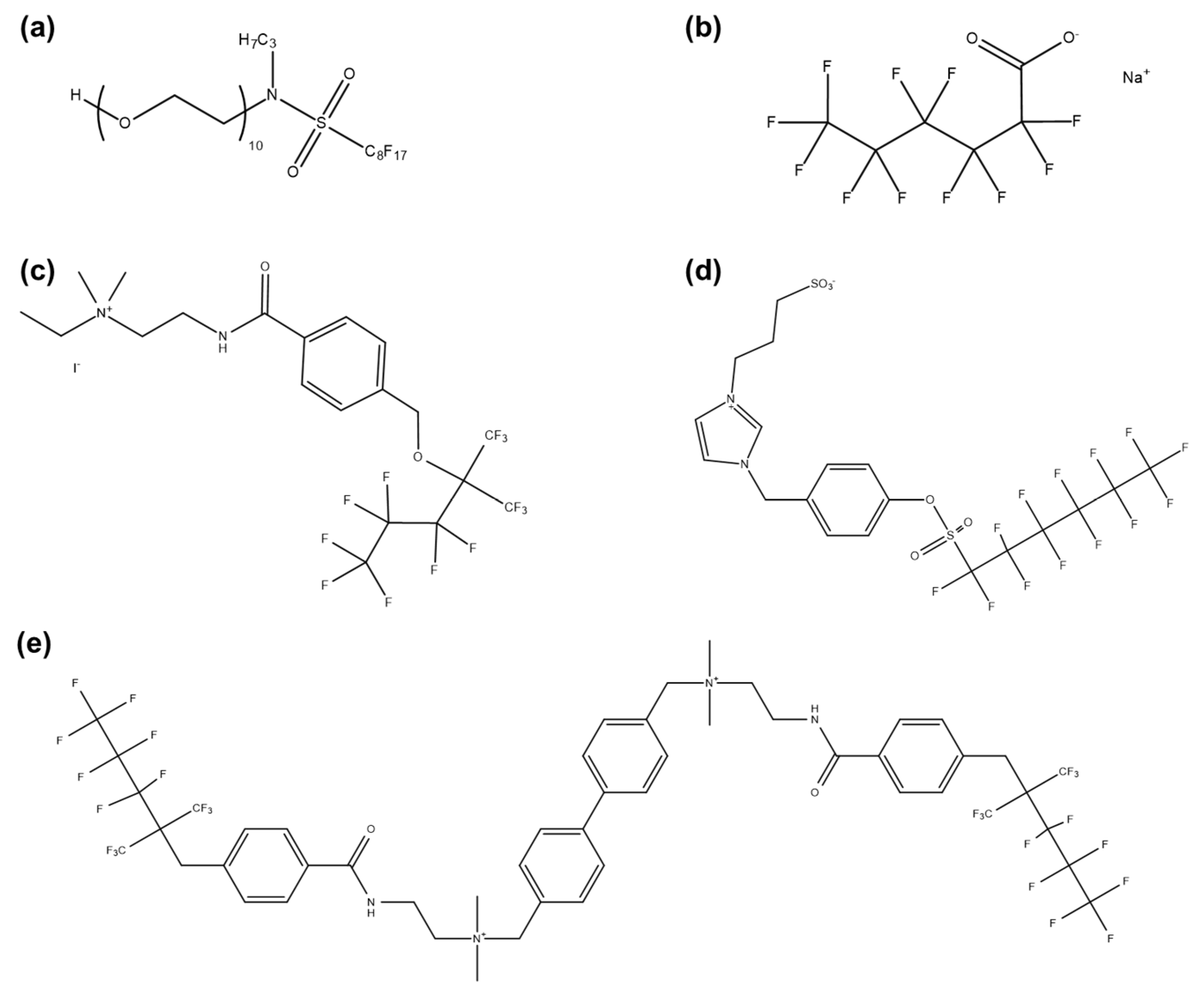
- Fluorocarbon Surfactants
- 2.
- Alkylphenol Ethoxylate Surfactants
2.2. Oil Transportation Chemicals
2.2.1. Viscosity Reducer
2.2.2. Paraffin Wax Inhibitor and Remover
2.2.3. Crude Oil Demulsifier
2.2.4. Anti-Corrosion Lining
2.3. Petroleum Refining Chemicals
2.4. Petroleum Product Additives
2.4.1. Detergents and Dispersants
2.4.2. Extreme-Pressure Wear Agents
2.5. Auxiliary Equipment Chemicals
2.6. Summary of the Entire Chain
3. Environmental Concentrations
3.1. Petroleum Extraction
3.2. Petroleum Refining and Chemical Production
4. Ecological and Health Risks
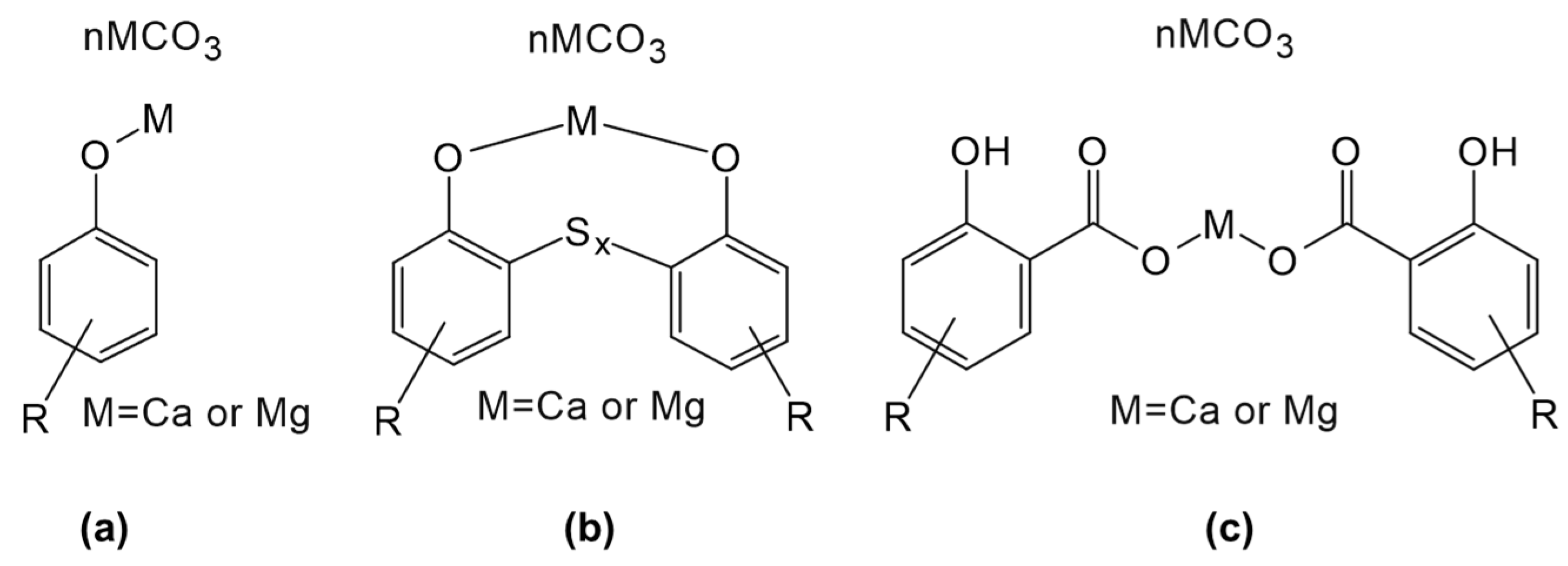
4.1. Alkylphenols
4.2. Per- and Polyfluoroalkyl Substances
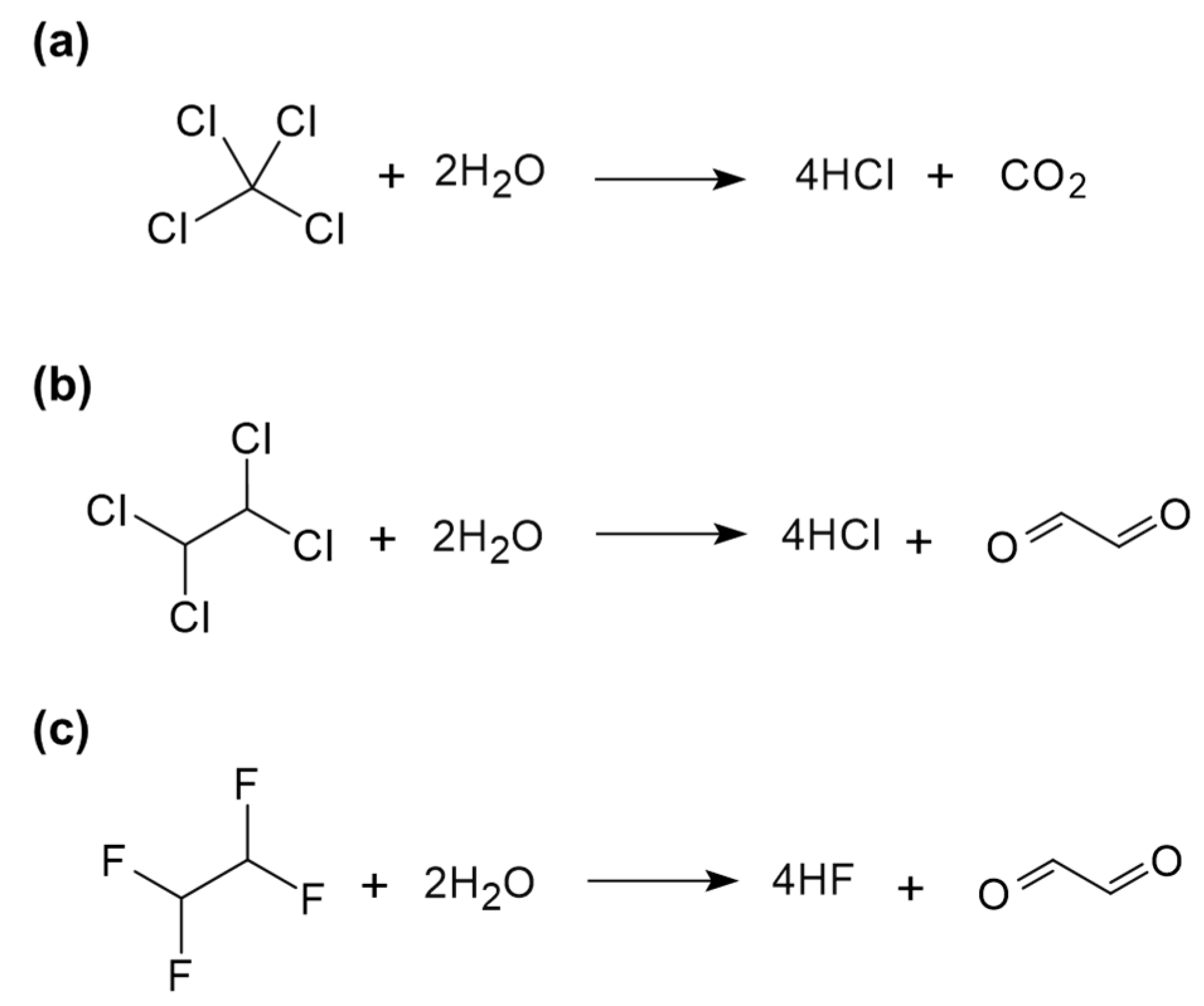
4.3. Halogenated Hydrocarbons
4.4. Short-Chain Chlorinated Paraffins
5. Mitigation Strategies
5.1. Green Oil Recovery Technologies
5.2. Green Surfactants
5.3. Green Solvents
6. Global Governance Frameworks
7. Conclusions and Future Perspectives
- (1)
- Screen chemical additives for EPs. Petrochemical enterprises should utilize advanced analytical methods to identify and quantify EPs in chemical additives, facilitating targeted substitution or phase-out to support sustainable production practices.
- (2)
- Develop pollutant fingerprinting techniques for source apportionment. Characteristic EPs should be identified based on operational profiles of petrochemical facilities to enhance traceability and support region-specific pollution control strategies.
- (3)
- Expand comprehensive monitoring programs. Research should prioritize mixed contamination of multiple EPs beyond conventional single target, and extend to under-represented regions (e.g., the Middle East) and processes (e.g., transportation) to enable full life-cycle assessment.
- (4)
- Track the global transport of EPs via the petroleum trade. Studies should focus on residual EPs in petroleum and assess their movement along international trade routes to improve understanding of transboundary pollution and inform global sustainability initiatives.
- (5)
- Enhance assessment of combined toxicity and health impacts. Mixture toxicity studies of complex chemical additives are essential to accurately evaluate ecological and health risks, supporting science-based and sustainable management decisions.
- (6)
- Accelerate green petrochemical technology deployment. Reducing chemical input through alternative technologies (e.g., CO2-enhanced oil recovery) can significantly lower the use of EPs, contributing to cleaner and more sustainable industrial operations.
- (7)
- Promote the development and assessment of green substitutes. Replacement of EPs with safe substitutes is key to sustainable source control; however, comprehensive environmental and health assessments of substitutes must be conducted to avoid regrettable substitutions.
- (8)
- Strengthen international cooperation under sustainability frameworks. As petroleum is a globally traded commodity, coordinated efforts under conventions such as the Stockholm Convention are essential to advance equitable and effective global pollution control and sustainable industry practices.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Yu, Y.; Wang, S.; Yu, P.; Wang, D.; Hu, B.; Zheng, P.; Zhang, M. A Bibliometric Analysis of Emerging Contaminants (ECs) (2001–2021): Evolution of Hotspots and Research Trends. Sci. Total Environ. 2024, 907, 168116. [Google Scholar] [CrossRef]
- Pastorino, P.; Barcelo, D.; Prearo, M. Alps at Risk: High-Mountain Lakes as Reservoirs of Persistent and Emerging Contaminants. J. Contam. Hydrol. 2024, 264, 104361. [Google Scholar] [CrossRef]
- Malnes, D.; Waara, S.; Figuiere, R.; Ahrens, L.; Wiberg, K.; Kohler, S.J.; Golovko, O. Hazard Screening of Contaminants of Emerging Concern (CECs) in Sweden’s Three Largest Lakes and Their Associated Rivers. J. Hazard. Mater. 2023, 453, 131376. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Guo, C.; Yang, Q.; Liu, W.; Zhang, H.; Luo, Y.; Zhang, Y.; Wang, L.; Chen, C.; Xu, J. Comprehensive Monitoring and Prioritizing for Contaminants of Emerging Concern in the Upper Yangtze River, China: An Integrated Approach. J. Hazard. Mater. 2024, 480, 135835. [Google Scholar] [CrossRef]
- Sousa, J.C.G.; Ribeiro, A.R.; Barbosa, M.O.; Ribeiro, C.; Tiritan, M.E.; Pereira, M.F.R.; Silva, A.M.T. Monitoring of the 17 EU Watch List Contaminants of Emerging Concern in the Ave and the Sousa Rivers. Sci. Total Environ. 2019, 649, 1083–1095. [Google Scholar] [CrossRef] [PubMed]
- Dai, S.; Zhang, G.; Dong, C.; Yang, R.; Pei, Z.; Li, Y.; Li, A.; Zhang, Q.; Jiang, G. Occurrence, Bioaccumulation and Trophodynamics of per- and Polyfluoroalkyl Substances (PFAS) in Terrestrial and Marine Ecosystems of Svalbard, Arctic. Water Res. 2025, 271, 122979. [Google Scholar] [CrossRef] [PubMed]
- Mammo, F.K.; Amoah, I.D.; Gani, K.M.; Pillay, L.; Ratha, S.K.; Bux, F.; Kumari, S. Microplastics in the Environment: Interactions with Microbes and Chemical Contaminants. Sci. Total Environ. 2020, 743, 140518. [Google Scholar] [CrossRef]
- Wang, F.; Xiang, L.; Sze-Yin Leung, K.; Elsner, M.; Zhang, Y.; Guo, Y.; Pan, B.; Sun, H.; An, T.; Ying, G.; et al. Emerging Contaminants: A One Health Perspective. Innovation 2024, 5, 100612. [Google Scholar] [CrossRef]
- Li, X.; Shen, X.; Jiang, W.; Xi, Y.; Li, S. Comprehensive Review of Emerging Contaminants: Detection Technologies, Environmental Impact, and Management Strategies. Ecotoxicol. Environ. Saf. 2024, 278, 116420. [Google Scholar] [CrossRef]
- Rahman, A.; Murad, S.M.W.; Mohsin, A.K.M.; Wang, X. Does Renewable Energy Proactively Contribute to Mitigating Carbon Emissions in Major Fossil Fuels Consuming Countries? J. Clean. Prod. 2024, 452, 142113. [Google Scholar] [CrossRef]
- Johnson, E.; Vadenbo, C. Modelling Variation in Petroleum Products’ Refining Footprints. Sustainability 2020, 12, 9316. [Google Scholar] [CrossRef]
- Statistical Review of World Energy 2025; Energy Institute: London, UK, 2025.
- Sajid, M.J.; Yu, Z.; Rehman, S.A. The Coal, Petroleum, and Gas Embedded in the Sectoral Demand-and-Supply Chain: Evidence from China. Sustainability 2022, 14, 1888. [Google Scholar] [CrossRef]
- Li, Q. Reservoir Science: A Multi-Coupling Communication Platform to Promote Energy Transformation, Climate Change and Environmental Protection. Reserv. Sci. 2025, 1, 1–2. [Google Scholar]
- Yao, Y.; Meng, Y.; Chen, H.; Zhu, L.; Sun, H. Non-Target Discovery of Emerging PFAS Homologues in Dagang Oilfield: Multimedia Distribution and Profiles in Crude Oil. J. Hazard. Mater. 2022, 437, 129300. [Google Scholar] [CrossRef]
- Zhao, M.; Yao, Y.; Dong, X.; Baqar, M.; Fang, B.; Chen, H.; Sun, H. Nontarget Identification of Novel Per- and Polyfluoroalkyl Substances (PFAS) in Soils from an Oil Refinery in Southwestern China: A Combined Approach with TOP Assay. Environ. Sci. Technol. 2023, 57, 20194–20205. [Google Scholar] [CrossRef]
- Yang, J.; Chen, Y.; Zhao, X.; Ma, C.; Li, Y.; He, X. Controlled-Release Chemicals in Oilfield Application: A Review. J. Pet. Sci. Eng. 2022, 215, 110616. [Google Scholar] [CrossRef]
- Vogt, E.T.C.; Weckhuysen, B.M. The Refinery of the Future. Nature 2024, 629, 295–306. [Google Scholar] [CrossRef] [PubMed]
- Naghmash, A.A.; Jabbar, Z.M.; Salman, N.A.A.; Ali, A.A. Dewatering of Crude Oil Emulsion Using Synthetic Polymeric Surfactant. Pet. Chem. 2024, 64, 1008–1015. [Google Scholar] [CrossRef]
- Liu, K.; Du, H.; Zheng, T.; Liu, H.; Zhang, M.; Zhang, R.; Li, H.; Xie, H.; Zhang, X.; Ma, M.; et al. Recent Advances in Cellulose and Its Derivatives for Oilfield Applications. Carbohydr. Polym. 2021, 259, 117740. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Wen, Z.; Khan, M.A.; Lv, K.; Shen, H.; Dai, L.; Li, Y.; Ding, Y.; Liu, C.; Li, M.-C. A Review of Cellulose Nanomaterial-Stabilized Pickering Foam: Formation, Properties, and Emerging Oilfield Applications. Int. J. Biol. Macromol. 2024, 281, 136274. [Google Scholar] [CrossRef]
- Hussain, S.M.S.; Adewunmi, A.A.; Mahboob, A.; Murtaza, M.; Zhou, X.; Kamal, M.S. Fluorinated Surfactants: A Review on Recent Progress on Synthesis and Oilfield Applications. Adv. Colloid Interface Sci. 2022, 303, 102634. [Google Scholar] [CrossRef] [PubMed]
- Fogang, L.T.; Kamal, M.S.; Mahmoud, M. Development of Viscosified Acid-Surfactant Solutions for Oilfield Applications: Rheological Properties. J. Energy Resour. Technol.-Trans. ASME 2021, 143, 023001. [Google Scholar] [CrossRef]
- Guo, K.; Li, H.; Yu, Z. In-Situ Heavy and Extra-Heavy Oil Recovery: A Review. Fuel 2016, 185, 886–902. [Google Scholar] [CrossRef]
- Li, P.; Zhang, F.; Zhu, T.; Zhang, C.; Liu, G.; Li, X. Synthesis and Properties of the Active Polymer for Enhanced Heavy Oil Recovery. Colloids Surf.-Physicochem. Eng. Asp. 2021, 626, 127036. [Google Scholar] [CrossRef]
- Li, J.; Ma, Y.; Sun, J.; Li, X.; Lu, W.; Bi, J. Using Aged Oil to Produce Drilling-Fluid Lubricants. Geoenergy Sci. Eng. 2024, 234, 212635. [Google Scholar] [CrossRef]
- Almubarak, T.; Ng, J.H.; Ramanathan, R.; Nasr-El-Din, H.A. Chelating Agents for Oilfield Stimulation: Lessons Learned and Future Outlook. J. Pet. Sci. Eng. 2021, 205, 108832. [Google Scholar] [CrossRef]
- Santos, N.B.C.; Fagundes, F.M.; de Oliveira Arouca, F.; Damasceno, J.J.R. Sedimentation of Solids in Drilling Fluids Used in Oil Well Drilling Operations. J. Pet. Sci. Eng. 2018, 162, 137–142. [Google Scholar] [CrossRef]
- Li, Z.; Zhao, G.; Xiang, C. Synthesis and Properties of a Gel Agent with a High Salt Resistance for Use in Weak-Gel-Type Water-Based Drilling Fluid. Arab. J. Sci. Eng. 2022, 47, 12045–12055. [Google Scholar] [CrossRef]
- Ma, J.; Xia, B.; Yu, P.; An, Y. Comparison of an Emulsion- and Solution-Prepared Acrylamide/AMPS Copolymer for a Fluid Loss Agent in Drilling Fluid. ACS Omega 2020, 5, 12892–12904. [Google Scholar] [CrossRef]
- Sun, J.; Zhang, X.; Lv, K.; Liu, J.; Xiu, Z.; Wang, Z.; Huang, X.; Bai, Y.; Wang, J.; Jin, J. Synthesis of Hydrophobic Associative Polymers to Improve the Rheological and Filtration Performance of Drilling Fluids under High Temperature and High Salinity Conditions. J. Pet. Sci. Eng. 2022, 209, 109808. [Google Scholar] [CrossRef]
- Li, P.; Shen, Y.; Yang, X. Solution Properties and Flocculation of Hydrophobically Associating Cationic Fluorinated Polyacrylamide. Polym. Bull. 2011, 67, 961–973. [Google Scholar] [CrossRef]
- Li, F.; Liu, J.; Luo, Y.; Li, D.; Zhang, F.; Liu, S. Synthesis, Evaluation and Aqueous Solution Behavior of the Cationic Fluorinated Hydrophobically Associating Polyacrylamide. J. Polym. Res. 2019, 26, 35. [Google Scholar] [CrossRef]
- Wen, X.; Li, X.; Ding, L.; Lu, L.; Wang, L.; Gao, J. Synthesis of Fluorohydrophobically Associatingpolyacrylamide and Its Solution Properties. Appl. Chem. Ind. 2019, 48, 267–271+275. [Google Scholar] [CrossRef]
- Solomon, M.M.; Uzoma, I.E.; Olugbuyiro, J.A.; Ademosun, O.T. A Censorious Appraisal of the Oil Well Acidizing Corrosion Inhibitors. J. Pet. Sci. Eng. 2022, 215, 110711. [Google Scholar] [CrossRef]
- Martyushev, D.A.; Vinogradov, J. Development and Application of a Double Action Acidic Emulsion for Improved Oil Well Performance: Laboratory Tests and Field Trials. Colloids Surf.-Physicochem. Eng. Asp. 2021, 612, 125998. [Google Scholar] [CrossRef]
- Ghany, N.A.A.; Shehata, M.F.; Saleh, R.M.; El Hosary, A.A. Novel Corrosion Inhibitors for Acidizing Oil Wells. Mater. Corros.-Werkst. Korros. 2017, 68, 355–360. [Google Scholar] [CrossRef]
- Du, X.; Xi, C.; Shi, L.; Wang, B.; Qi, Z.; Liu, T.; Zhou, Y.; Lee, J.; Babadagli, T.; Li, H. A Review on the Use of Chemicals as Steam Additives for Thermal Oil Recovery Applications. J. Energy Resour. Technol.-Trans. ASME 2022, 144, 113004. [Google Scholar] [CrossRef]
- Li, P.; Hu, M. A Study on the Effect of New Surfactant Proportions on the Recovery Improvement of Carbonate Reservoir. Appl. Sci. 2024, 14, 4028. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Wang, F.; Wu, J.; Wang, Y. The Carrying Behavior of Water-Based Fracturing Fluid in Shale Reservoir Fractures and Molecular Dynamics of Sand-Carrying Mechanism. Processes 2024, 12, 2051. [Google Scholar] [CrossRef]
- Ding, X.; Wang, K.; Luo, Z.; Fu, Q.; Wang, T.; Xu, Q.; Yi, X.; Dai, R.; Jiang, H.; Xing, Z. The Investigation of Wetting and Agglomerating Mechanism of Short-Chain Fluorocarbon Surfactant Suppressing Coal Dust from Macro and Molecular Scales. Chem. Eng. J. 2024, 487, 150475. [Google Scholar] [CrossRef]
- Glüge, J.; Scheringer, M.; Cousins, I.T.; DeWitt, J.C.; Goldenman, G.; Herzke, D.; Lohmann, R.; Ng, C.A.; Trier, X.; Wang, Z. An Overview of the Uses of Per- and Polyfluoroalkyl Substances (PFAS). Environ. Sci. Process. Impacts 2020, 22, 2345–2373. [Google Scholar] [CrossRef]
- Zhang, D.; Sha, M.; Xing, P.; Pan, R.; Lin, X.; Jiang, B. Synthesis of Novel Oil-Soluble Fluorinated Surfactants via Wittig-Horner Reaction. Tetrahedron 2019, 75, 1652–1657. [Google Scholar] [CrossRef]
- Shen, Y.; Jin, Y.; Lai, S.; Shi, L.; Du, W.; Zhou, R. Synthesis, Surface Properties and Cytotoxicity Evaluation of Nonionic Urethane Fluorinated Surfactants with Double Short Fluoroalkyl Chains. J. Mol. Liq. 2019, 296, 111851. [Google Scholar] [CrossRef]
- Chen, L.; Shi, H.; Wu, H.; Xiang, J. Synthesis and Combined Properties of Novel Fluorinated Anionic Surfactant. Colloids Surf. Physicochem. Eng. Asp. 2011, 384, 331–336. [Google Scholar] [CrossRef]
- Kondo, Y.; Yokochi, E.; Mizumura, S.; Yoshino, N. Syntheses of Novel Fluorocarbon Surfactants with Oxyethylene Groups. J. Fluor. Chem. 1998, 91, 147–151. [Google Scholar] [CrossRef]
- Wu, H.-K.; Zhong, J.-Q.; Shen, H.-M.; Shi, H.-X. Synthesis of a Novel Branch Fluorinated Cationic Surfactant and Its Surface Activity. J. Fluor. Chem. 2013, 156, 5–8. [Google Scholar] [CrossRef]
- Yake, A.; Corder, T.; Moloy, K.; Coope, T.; Taylor, C.; Hung, M.; Peng, S. Fluorinated Pyridinium and Ammonium Cationic Surfactants. J. Fluor. Chem. 2016, 187, 46–55. [Google Scholar] [CrossRef]
- Lin, C.; Pan, R.; Xing, P.; Jiang, B. Synthesis and Surface Activity Study of Novel Branched Zwitterionic Heterogemini Fluorosurfactants with CF3CF2CF2C(CF3)2 Group. J. Fluor. Chem. 2018, 214, 35–41. [Google Scholar] [CrossRef]
- Du, F.; Guo, Y.; Huang, M.; Chen, Q.; Yang, H.; Xie, W.; Cao, W.; Wu, C.; Wang, M. Gemini Cationic Surfactants with Flexible Perfluorinated-Ether Chains. J. Fluor. Chem. 2020, 239, 109632. [Google Scholar] [CrossRef]
- Chen, C.-L.; Liao, Y.-F.; Lu, F.; Zheng, Y.-S.; Peng, Y.-Y.; Ding, C.-W.; Tong, Q.-X. Facile Synthesis, Surface Activity, Wettability and Ultrahigh Foaming Properties of Novel Nonionic Gemini Fluorocarbon Surfactants. J. Mol. Liq. 2020, 302, 112469. [Google Scholar] [CrossRef]
- Cattelaens, F.; Myrdek, T. Study of the Efficiency of Technical Grade Nonionic Surfactants. J. Surfactants Deterg. 2023, 26, 53–60. [Google Scholar] [CrossRef]
- Liu, M.; Wu, Y.; Zhang, L.; Rong, F.; Yang, Z. Mechanism of Viscosity Reduction in Viscous Crude Oil with Polyoxyethylene Surfactant Compound System. Pet. Sci. Technol. 2019, 37, 409–416. [Google Scholar] [CrossRef]
- Huang, J.; Zhao, Z.; Liu, J.; Li, S. The Transformation of Per- and Polyfluoroalkyl Substances in the Aquatic Environment of a Fluorochemical Industrial Park. Water 2024, 16, 1513. [Google Scholar] [CrossRef]
- Zhang, F.; Ouyang, J.; Feng, X.; Zhang, H.; Xu, L. Paraffin Deposition Mechanism and Paraffin Inhibition Technology for High-Carbon Paraffin Crude Oil From the Kazakhstan PK Oilfield. Pet. Sci. Technol. 2014, 32, 488–496. [Google Scholar] [CrossRef]
- Raya, S.A.; Saaid, I.M.; Ahmed, A.A.; Umar, A.A. A Critical Review of Development and Demulsification Mechanisms of Crude Oil Emulsion in the Petroleum Industry. J. Pet. Explor. Prod. Technol. 2020, 10, 1711–1728. [Google Scholar] [CrossRef]
- Chen, X.; Patel, V.; Liu, J.; Liu, S.; Trivedi, J. Surfactant Synergy on Rheological Properties, Injectivity, and Enhanced Oil Recovery of Viscoelastic Polymers. Phys. Fluids 2025, 37, 023107. [Google Scholar] [CrossRef]
- Wang, D.; Yang, D.; Huang, C.; Huang, Y.; Yang, D.; Zhang, H.; Liu, Q.; Tang, T.; El-Din, M.G.; Kemppi, T.; et al. Stabilization Mechanism and Chemical Demulsification of Water-in-Oil and Oil-in-Water Emulsions in Petroleum Industry: A Review. Fuel 2021, 286, 119390. [Google Scholar] [CrossRef]
- Abullah, M.M.S.; Al-Lohedan, H.A.; Attah, A.M. Synthesis and Application of Amphiphilic Ionic Liquid Based on Acrylate Copolymers as Demulsifier and Oil Spill Dispersant. J. Mol. Liq. 2016, 219, 54–62. [Google Scholar] [CrossRef]
- Qi, J.; Ge, Y.; Li, Q.; Lv, X.; Li, X.; Wang, N.; Lu, H. Sustainable and Cleaner Approach for Paraffin Wax Treatment via CO2-Induced Polarity Transformation of Switchable Solvents. J. Clean. Prod. 2023, 396, 136500. [Google Scholar] [CrossRef]
- Adewunmi, A.A.; Hussain, S.M.S.; Patil, S.; Kamal, M.S. Influence of Varying Oil-Water Contents on the Formation of Crude Oil Emulsion and Its Demulsification by a Lab-Grown Nonionic Demulsifier. ACS Omega 2024, 9, 19620–19626. [Google Scholar] [CrossRef]
- Zhu, D.; Bai, B.; Hou, J. Polymer Gel Systems for Water Management in High-Temperature Petroleum Reservoirs: A Chemical Review. Energy Fuels 2017, 31, 13063–13087. [Google Scholar] [CrossRef]
- Li, J.; Liu, Y.; Wang, Z.; Wang, J.; Yu, T. Fluorocarbon Coating Provides Corrosion Protection in Oilfield Flooding System. Mater. Perform. 2016, 55, 40–44. [Google Scholar] [CrossRef]
- Islam Sazzad, M.R.; Rahman, M.M.; Hassan, T.; Al Rifat, A.; Al Mamun, A.; Adib, A.R.; Meraz, R.M.; Ahmed, M. Advancing Sustainable Lubricating Oil Management: Re-Refining Techniques, Market Insights, Innovative Enhancements, and Conversion to Fuel. Heliyon 2024, 10, e39248. [Google Scholar] [CrossRef]
- Bolden, A.L.; Kwiatkowski, C.F.; Colborn, T. New Look at BTEX: Are Ambient Levels a Problem? Environ. Sci. Technol. 2015, 49, 5261–5276. [Google Scholar] [CrossRef]
- Zainal, N.A.; Zulkifli, N.W.M.; Gulzar, M.; Masjuki, H.H. A Review on the Chemistry, Production, and Technological Potential of Bio-Based Lubricants. Renew. Sustain. Energy Rev. 2018, 82, 80–102. [Google Scholar] [CrossRef]
- Hosseinzadeh-Bandbafha, H.; Tabatabaei, M.; Aghbashlo, M.; Khanali, M.; Demirbas, A. A Comprehensive Review on the Environmental Impacts of Diesel/Biodiesel Additives. Energy Convers. Manag. 2018, 174, 579–614. [Google Scholar] [CrossRef]
- Ahmed, N.S.; Abdel-Hameed, H.S.; El-Kafrawy, A.F.; Nassar, A.M. Synthesis and Evaluation of Ashless Detergent/Dispersant Additives for Lubricating Engine Oil. Ind. Lubr. Tribol. 2015, 67, 622–629. [Google Scholar] [CrossRef]
- Nassar, A.M.; Ahmed, N.S.; Abdel-Hameed, H.S.; El-Kafrawy, A.F. Synthesis and Utilization of Non-Metallic Detergent/Dispersant and Antioxidant Additives for Lubricating Engine Oil. Tribol. Int. 2016, 93, 297–305. [Google Scholar] [CrossRef]
- Mamedova, A.K.; Farzaliev, V.M.; Kyazim-zade, A.K. New Sulfur-, Nitrogen-, and Boron-Containing Multifunctional Alkylphenolate Additives for Motor Oils. Pet. Chem. 2017, 57, 718–721. [Google Scholar] [CrossRef]
- Xu, C.; Gao, L.; Zheng, M.; Qiao, L.; Cui, L.; Wang, K.; Huang, D. Short- and Medium-Chain Chlorinated Paraffins in Commercial Rubber Track Products and Raw Materials. J. Hazard. Mater. 2019, 380, 120854. [Google Scholar] [CrossRef]
- Ning, W.; Yang, Y.; Zhang, D.; Pan, R. Surface Modification of Sodium Bicarbonate Ultrafine Powder Extinguishing Agent by Environmental Friendly Fluorinated Acrylate Copolymers. Polym. Degrad. Stab. 2021, 187, 109558. [Google Scholar] [CrossRef]
- Xiao, G.; Lei, L.; Chen, C.; Li, Y.; Hu, W. Optimization and Performance Evaluation of an Environmentally Friendly Fluorine-Free Foam Extinguishing Agent. J. Nanoelectron. Optoelectron. 2020, 15, 884–893. [Google Scholar] [CrossRef]
- Yan, L.; Guan, J.; Wang, N.; Wei, Z.; Xu, Z.; Niu, G. Design of Fluorine-Free Foam Extinguishing Agent with High Surface Activity, Foam Stability and Pool Fire Suppression by Optimization of Surfactant Composition and Foam System. Colloids Surf.-Physicochem. Eng. Asp. 2024, 693, 134094. [Google Scholar] [CrossRef]
- Delanka-Pedige, H.M.K.; Zhang, Y.; Young, R.B.; Wang, H.; Hu, L.; Danforth, C.; Xu, P. Safe Reuse of Treated Produced Water Outside Oil and Gas Fields? A Review of Current Practices, Challenges, Opportunities, and a Risk-Based Pathway for Produced Water Treatment and Fit-for-Purpose Reuse. Curr. Opin. Chem. Eng. 2023, 42, 100973. [Google Scholar] [CrossRef]
- Vengosh, A.; Jackson, R.B.; Warner, N.; Darrah, T.H.; Kondash, A. A Critical Review of the Risks to Water Resources from Unconventional Shale Gas Development and Hydraulic Fracturing in the United States. Environ. Sci. Technol. 2014, 48, 8334–8348. [Google Scholar] [CrossRef]
- Scanlon, B.R.; Reedy, R.C.; Xu, P.; Engle, M.; Nicot, J.P.; Yoxtheimer, D.; Yang, Q.; Ikonnikova, S. Can We Beneficially Reuse Produced Water from Oil and Gas Extraction in the U.S.? Sci. Total Environ. 2020, 717, 137085. [Google Scholar] [CrossRef]
- Gao, J.; Zou, C.; Zhang, X.; Guo, W.; Yu, R.; Ni, Y.; Liu, D.; Kang, L.; Liu, Y.; Kondash, A.; et al. The Water Footprint of Hydraulic Fracturing for Shale Gas Extraction in China. Sci. Total Environ. 2024, 907, 168135. [Google Scholar] [CrossRef]
- Bakke, T.; Klungsoyr, J.; Sanni, S. Environmental Impacts of Produced Water and Drilling Waste Discharges from the Norwegian Offshore Petroleum Industry. Mar. Environ. Res. 2013, 92, 154–169. [Google Scholar] [CrossRef]
- Boitsov, S.; Mjøs, S.A.; Meier, S. Identification of Estrogen-like Alkylphenols in Produced Water from Offshore Oil Installations. Mar. Environ. Res. 2007, 64, 651–665. [Google Scholar] [CrossRef]
- Zhang, Y.; Yang, K.; Dong, Y.; Nie, Z.; Li, W. Chemical Characterization of Non-Volatile Dissolved Organic Matter from Oilfield-Produced Brines in the Nanyishan Area of the Western Qaidam Basin, China. Chemosphere 2021, 268, 128804. [Google Scholar] [CrossRef]
- McLaughlin, M.C.; McDevitt, B.; Miller, H.; Amundson, K.K.; Wilkins, M.J.; Warner, N.R.; Blotevogel, J.; Borch, T. Constructed Wetlands for Polishing Oil and Gas Produced Water Releases. Environ. Sci.-Process. Impacts 2021, 23, 1961–1976. [Google Scholar] [CrossRef]
- Akyon, B.; McLaughlin, M.; Hernandez, F.; Blotevogel, J.; Bibby, K. Characterization and Biological Removal of Organic Compounds from Hydraulic Fracturing Produced Water. Environ. Sci.-Process. Impacts 2019, 21, 279–290. [Google Scholar] [CrossRef]
- Chen, H.; Carter, K.E. Characterization of the Chemicals Used in Hydraulic Fracturing Fluids for Wells Located in the Marcellus Shale Play. J. Environ. Manag. 2017, 200, 312–324. [Google Scholar] [CrossRef]
- Stringfellow, W.T.; Camarillo, M.K.; Domen, J.K.; Sandelin, W.L.; Varadharajan, C.; Jordan, P.D.; Reagan, M.T.; Cooley, H.; Heberger, M.G.; Birkholzer, J.T. Identifying Chemicals of Concern in Hydraulic Fracturing Fluids Used for Oil Production. Environ. Pollut. 2017, 220, 413–420. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Chen, H.; Xing, Q.; Xu, X. Emerging and Legacy Per- and Polyfluoroalkyl Substances from Offshore Oilfields and Receiving Water in China. Environ. Res. 2025, 280, 121865. [Google Scholar] [CrossRef]
- Guo, C.; Wang, X.; Li, C.; Zhang, K.; Cai, Z. The Application of the Yield Approach to Study Slurry Migration in Drill Cuttings Waste Underground Disposal. J. Clean. Prod. 2020, 254, 120144. [Google Scholar] [CrossRef]
- Xue, M.; Dai, Z.; Li, Z.; Li, Z.; Wan, C.; Wang, Y.; Liu, Z.; Yang, X.; Cai, J. Environmentally Friendly Comprehensive Recycling Utilization Technology of Foundation Engineering Slurry. Constr. Build. Mater. 2023, 368, 130400. [Google Scholar] [CrossRef]
- Costa, L.C.; Carvalho, C.F.; Soares, A.S.F.; Souza, A.C.P.; Bastos, E.F.T.; Guimarães, E.C.B.T.; Santos, J.C.; Carvalho, T.; Calderari, V.H.; Marinho, L.S.; et al. Physical and Chemical Characterization of Drill Cuttings: A Review. Mar. Pollut. Bull. 2023, 194, 115342. [Google Scholar] [CrossRef] [PubMed]
- Sundt, R.C.; Ruus, A.; Jonsson, H.; Skarphéðinsdóttir, H.; Meier, S.; Grung, M.; Beyer, J.; Pampanin, D.M. Biomarker Responses in Atlantic Cod (Gadus morhua) Exposed to Produced Water from a North Sea Oil Field: Laboratory and Field Assessments. Mar. Pollut. Bull. 2012, 64, 144–152. [Google Scholar] [CrossRef]
- McLaren, D.E.K.; Rawlins, A.J. Occurrence of Alkylphenols and Alkylphenol Ethoxylates in North Sea Sediment Samples Collected across Oil and Gas Fields. Mar. Pollut. Bull. 2022, 178, 113655. [Google Scholar] [CrossRef]
- Xu, L.; Shi, Y.; Li, C.; Song, X.; Qin, Z.; Cao, D.; Cai, Y. Discovery of a Novel Polyfluoroalkyl Benzenesulfonic Acid around Oilfields in Northern China. Environ. Sci. Technol. 2017, 51, 14173–14181. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Yao, Y.; Chen, H.; Li, Q.; Sun, H. Legacy and Emerging Per- and Polyfluoroalkyl Substances (PFASs) in Dagang Oilfield: Multimedia Distribution and Contributions of Unknown Precursors. J. Hazard. Mater. 2021, 412, 125177. [Google Scholar] [CrossRef] [PubMed]
- Reinikainen, J.; Perkola, N.; Aysto, L.; Sorvari, J. The Occurrence, Distribution, and Risks of PFAS at AFFF-Impacted Sites in Finland. Sci. Total Environ. 2022, 829, 154237. [Google Scholar] [CrossRef]
- Che, S.; Liu, J.; Wang, J.; Sui, L.; Liu, J.; Zhnag, S.; Sui, Q. Research progress and perspectives on the application, occurrence, and treatment of per-and polyfluoroalkyl substances in petrochemical industries. Chin. J. Appl. Environ. Biol. 2025, 31, 768–779. [Google Scholar] [CrossRef]
- Huang, D.; Gao, L.; Qiao, L.; Cui, L.; Xu, C.; Wang, K.; Zheng, M. Concentrations of and Risks Posed by Short-Chain and Medium-Chain Chlorinated Paraffins in Soil at a Chemical Industrial Park on the Southeast Coast of China. Environ. Pollut. 2020, 258, 113704. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Chen, J.; Sun, T.; Zhao, R.-S.; Chen, X.; Fu, J. Removal Efficiency and Fate of Chlorinated Paraffins (CPs) in a Municipal Sewage Treatment Plant near a Typical Petrochemical Park. ACS EST Water 2023, 3, 2535–2543. [Google Scholar] [CrossRef]
- Zhou, G.-J.; Li, X.-Y.; Leung, K.M.Y. Retinoids and Oestrogenic Endocrine Disrupting Chemicals in Saline Sewage Treatment Plants: Removal Efficiencies and Ecological Risks to Marine Organisms. Environ. Int. 2019, 127, 103–113. [Google Scholar] [CrossRef]
- Franska, M.; Franski, R.; Szymanski, A.; Lukaszewski, Z. A Central Fission Pathway in Alkylphenol Ethoxylate Biodegradation. Water Res. 2003, 37, 1005–1014. [Google Scholar] [CrossRef]
- Guo, Z.; Cheng, Y.; Zhu, L.; Li, F.; Liang, H.; Huang, Q.; Wang, L. Dermal Exposure to Alkylphenol Ethoxylates in As-Purchased Garments: Human Health Risk Assessment. AATCC J. Res. 2024, 11, 456–463. [Google Scholar] [CrossRef]
- Venkatesan, A.K.; Halden, R.U. National Inventory of Alkylphenol Ethoxylate Compounds in U.S. Sewage Sludges and Chemical Fate in Outdoor Soil Mesocosms. Environ. Pollut. 2013, 174, 189–193. [Google Scholar] [CrossRef]
- Liu, Q.; Li, F.; Liu, W.; Huang, B.; Li, L.; Wang, X.; Sang, X.; Dong, J.; Ma, J.; Chen, J.; et al. Transcriptional Expression Analysis Reveals Multiple Effects of Nonylphenol Exposure on Scallop Immune System. Fish Shellfish. Immunol. 2022, 123, 290–297. [Google Scholar] [CrossRef]
- Arslan, O.C.; Parlak, H.; Oral, R.; Katalay, S. The Effects of Nonylphenol and Octylphenol on Embryonic Development of Sea Urchin (Paracentrotus lividus). Arch. Environ. Contam. Toxicol. 2007, 53, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Chikae, M.; Ikeda, R.; Hasan, Q.; Morita, Y.; Tamiya, E. Effect of Alkylphenols on Adult Male Medaka: Plasma Vitellogenin Goes up to the Level of Estrous Female. Environ. Toxicol. Pharmacol. 2003, 15, 33–36. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Dai, X.; Wei, J. Toxicity of the Xenoestrogen Nonylphenol and Its Biodegradation by the Alga Cyclotella Caspia. J. Environ. Sci. 2013, 25, 1662–1671. [Google Scholar] [CrossRef]
- Ademollo, N.; Patrolecco, L.; Matozzo, V.; Marin, M.G.; Valsecchi, S.; Polesello, S. Clam Bioaccumulation of Alkylphenols and Polyciclic Aromatic Hydrocarbons in the Venice Lagoon under Different Pressures. Mar. Pollut. Bull. 2017, 124, 121–129. [Google Scholar] [CrossRef]
- Xie, W.; Zhao, J.; Zhang, Q.; Ye, C.; Zheng, G.; Shan, Q.; Li, L.; Shao, X. Occurrence, Distribution and Bioaccumulation of Alkylphenols in the Pearl River Networks, South China. Ecol. Indic. 2020, 110, 105847. [Google Scholar] [CrossRef]
- Klecka, G.M.; Staples, C.A.; Naylor, C.G.; Woodburn, K.B.; Losey, B.S. C8- and C9-Alkylphenols and Ethoxylates: II. Assessment of Environmental Persistence and Bioaccumulation Potential. Hum. Ecol. Risk Assess. 2008, 14, 1025–1055. [Google Scholar] [CrossRef]
- Lalonde, B.; Garron, C. NP, OP and Derivatives in Freshwater Sediment Downstream of Textile Associated Municipal Wastewater Discharges. Arch. Environ. Contam. Toxicol. 2024, 86, 375–382. [Google Scholar] [CrossRef] [PubMed]
- Ying, G.G. Fate, Behavior and Effects of Surfactants and Their Degradation Products in the Environment. Environ. Int. 2006, 32, 417–431. [Google Scholar] [CrossRef]
- Petrovic, M.; Solé, M.; de Alda, M.J.L.; Barceló, D. Endocrine Disruptors in Sewage Treatment Plants, Receiving River Waters, and Sediments:: Integration of Chemical Analysis and Biological Effects on Feral Carp. Environ. Toxicol. Chem. 2002, 21, 2146–2156. [Google Scholar] [CrossRef]
- Ahn, C.; Jeung, E.-B. Endocrine-Disrupting Chemicals and Disease Endpoints. Int. J. Mol. Sci. 2023, 24, 5342. [Google Scholar] [CrossRef]
- Romanelli, A.M.; Montefusco, A.; Sposito, S.; Scafuri, B.; Caputo, I.; Paolella, G. In Vitro Investigation of Biological and Toxic Effects of 4-Octylphenol on Human Cells. Int. J. Mol. Sci. 2024, 25, 13032. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.-L.; Chang, C.-H.; Minatoya, M. Bisphenols and Alkylphenols. In Health Impacts of Developmental Exposure to Environmental Chemicals; Kishi, R., Grandjean, P., Eds.; Springer: Singapore, 2020; pp. 405–437. ISBN 978-981-15-0520-1. [Google Scholar]
- Park, H.; Kim, K. Urinary Levels of 4-Nonylphenol and 4-t-Octylphenol in a Representative Sample of the Korean Adult Population. Int. J. Environ. Res. Public. Health 2017, 14, 932. [Google Scholar] [CrossRef]
- Shekhar, S.; Sood, S.; Showkat, S.; Lite, C.; Chandrasekhar, A.; Vairamani, M.; Barathi, S.; Santosh, W. Detection of Phenolic Endocrine Disrupting Chemicals (EDCs) from Maternal Blood Plasma and Amniotic Fluid in Indian Population. Gen. Comp. Endocrinol. 2017, 241, 100–107. [Google Scholar] [CrossRef]
- Xu, Y.; Jang, J.H.; Gye, M.C. 4-Octylphenol Induces Developmental Abnormalities and Interferes the Differentiation of Neural Crest Cells in Xenopus Laevis Embryos. Environ. Pollut. 2021, 274, 116560. [Google Scholar] [CrossRef]
- de Souza, I.R.; Iulini, M.; Galbiati, V.; Silva, E.Z.M.; Sivek, T.W.; Rodrigues, A.C.; Gradia, D.F.; Pestana, C.B.; Leme, D.M.; Corsini, E. An Integrated in Silico-in Vitro Investigation to Assess the Skin Sensitization Potential of 4-Octylphenol. Toxicology 2023, 493, 153548. [Google Scholar] [CrossRef]
- Magnifico, M.C.; Xhani, M.; Popov, M.; Saso, L.; Sarti, P.; Arese, M. Nonylphenol and Octylphenol Differently Affect Cell Redox Balance by Modulating the Nitric Oxide Signaling. Oxidative Med. Cell. Longev. 2018, 2018, 1684827. [Google Scholar] [CrossRef]
- Paolella, G.; Romanelli, A.M.; Martucciello, S.; Sposito, S.; Lepretti, M.; Esposito, C.; Capaldo, A.; Caputo, I. The Mechanism of Cytotoxicity of 4-Nonylphenol in a Human Hepatic Cell Line Involves ER-Stress, Apoptosis, and Mitochondrial Dysfunction. J. Biochem. Mol. Toxicol. 2021, 35, e22780. [Google Scholar] [CrossRef] [PubMed]
- Zhou, K.; Ding, X.; Yang, J.; Hu, Y.; Song, Y.; Chen, M.; Sun, R.; Dong, T.; Xu, B.; Han, X.; et al. Metabolomics Reveals Metabolic Changes Caused by Low-Dose 4-Tert-Octylphenol in Mice Liver. Int. J. Environ. Res. Public. Health 2018, 15, 2686. [Google Scholar] [CrossRef] [PubMed]
- Lohmann, R.; Abass, K.; Bonefeld-Jorgensen, E.C.; Bossi, R.; Dietz, R.; Ferguson, S.; Fernie, K.J.; Grandjean, P.; Herzke, D.; Houde, M.; et al. Cross-Cutting Studies of per- and Polyfluorinated Alkyl Substances (PFAS) in Arctic Wildlife and Humans. Sci. Total Environ. 2024, 954, 176274. [Google Scholar] [CrossRef]
- Sackett, D.K.; Anderson, D.; Henry, T.; Sweetman, A.K.; Yonkos, L. Persistent Organic Pollutant Accumulation in Pacific Abyssal Plain Sediments and Biota: Implications for Sources, Transport, and Deep-Sea Mining. Elem.-Sci. Anthr. 2024, 12, 00042. [Google Scholar] [CrossRef]
- Kang, J.S.; Ahn, T.-G.; Park, J.-W. Perfluorooctanoic Acid (PFOA) and Perfluooctane Sulfonate (PFOS) Induce Different Modes of Action in Reproduction to Japanese Medaka (Oryzias latipes). J. Hazard. Mater. 2019, 368, 97–103. [Google Scholar] [CrossRef]
- Pietropoli, E.; Bardhi, A.; Simonato, V.; Zanella, M.; Iori, S.; Barbarossa, A.; Giantin, M.; Dacasto, M.; De Liguoro, M.; Pauletto, M. Comparative Toxicity Assessment of Alternative versus Legacy PFAS: Implications for Two Primary Trophic Levels in Freshwater Ecosystems. J. Hazard. Mater. 2024, 477, 135269. [Google Scholar] [CrossRef]
- Song, C.; Gu, Q.; Zhang, D.; Zhou, D.; Cui, X. Prediction of PFAS Bioaccumulation in Different Plant Tissues with Machine Learning Models Based on Molecular Fingerprints. Sci. Total Environ. 2024, 950, 175091. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Yun, X.; McKenzie, E.R.; Heron, C.G.; Field, J.A.; Salice, C.J. Spatial and Temporal Variability of Per- and Polyfluoroalkyl Substances (PFAS) in Environmental Media of a Small Pond: Toward an Improved Understanding of PFAS Bioaccumulation in Fish. Sci. Total Environ. 2023, 880, 163149. [Google Scholar] [CrossRef] [PubMed]
- Dickman, R.A.; Aga, D.S. A Review of Recent Studies on Toxicity, Sequestration, and Degradation of per- and Polyfluoroalkyl Substances (PFAS). J. Hazard. Mater. 2022, 436, 129120. [Google Scholar] [CrossRef]
- Wasel, O.; King, H.; Choi, Y.J.; Lee, L.S.; Freeman, J.L. Differential Developmental Neurotoxicity and Tissue Uptake of the Per- and Polyfluoroalkyl Substance Alternatives, GenX and PFBS. Environ. Sci. Technol. 2023, 57, 19274–19284. [Google Scholar] [CrossRef]
- Huang, J.; Wang, Q.; Liu, S.; Zhang, M.; Liu, Y.; Sun, L.; Wu, Y.; Tu, W. Crosstalk between Histological Alterations, Oxidative Stress and Immune Aberrations of the Emerging PFOS Alternative OBS in Developing Zebrafish. Sci. Total Environ. 2021, 774, 145443. [Google Scholar] [CrossRef]
- Guo, H.; Chen, J.; Zhang, H.; Yao, J.; Sheng, N.; Li, Q.; Guo, Y.; Wu, C.; Xie, W.; Dai, J. Exposure to GenX and Its Novel Analogs Disrupts Hepatic Bile Acid Metabolism in Male Mice. Environ. Sci. Technol. 2022, 56, 6133–6143. [Google Scholar] [CrossRef]
- Shi, Y.; Vestergren, R.; Xu, L.; Zhou, Z.; Li, C.; Liang, Y.; Cai, Y. Human Exposure and Elimination Kinetics of Chlorinated Polyfluoroalkyl Ether Sulfonic Acids (Cl-PFESAs). Environ. Sci. Technol. 2016, 50, 2396–2404. [Google Scholar] [CrossRef] [PubMed]
- Lv, G.; Sun, X. The Molecular-Level Understanding of the Uptake of PFOS and Its Alternatives (6:2 Cl-PFESA and OBS) into Phospholipid Bilayers. J. Hazard. Mater. 2021, 417, 125991. [Google Scholar] [CrossRef]
- Tachachartvanich, P.; Singam, E.R.A.; Durkin, K.A.; Furlow, J.D.; Smith, M.T.; La Merrill, M.A. In Vitro Characterization of the Endocrine Disrupting Effects of per- and Poly-Fluoroalkyl Substances (PFASs) on the Human Androgen Receptor. J. Hazard. Mater. 2022, 429, 128243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Yao, Y.; Tu, L.; Luan, T.; Chen, B. Non-Targeted Metabolomics of Multiple Human Cells Revealing Differential Toxic Effects of Perfluorooctanoic Acid. J. Hazard. Mater. 2021, 409, 125017. [Google Scholar] [CrossRef] [PubMed]
- Huang, Q.; Luo, L.; Han, X.; Li, F.; Zhang, X.; Tian, M. Low-Dose Perfluorooctanoic Acid Stimulates Steroid Hormone Synthesis in Leydig Cells: Integrated Proteomics and Metabolomics Evidence. J. Hazard. Mater. 2022, 424, 127656. [Google Scholar] [CrossRef]
- Ojo, A.F.; Peng, C.; Ng, J.C. Combined Effects of Mixed Per- and Polyfluoroalkyl Substances on the Nrf2-ARE Pathway in ARE Reporter-HepG2 Cells. J. Hazard. Mater. 2022, 421, 126827. [Google Scholar] [CrossRef]
- Xu, C.; Yin, S.; Liu, Y.; Chen, F.; Zhong, Z.; Li, F.; Liu, K.; Liu, W. Prenatal Exposure to Chlorinated Polyfluoroalkyl Ether Sulfonic Acids and Perfluoroalkyl Acids: Potential Role of Maternal Determinants and Associations with Birth Outcomes. J. Hazard. Mater. 2019, 380, 120867. [Google Scholar] [CrossRef]
- Metcalfe, C.D.; Bayen, S.; Desrosiers, M.; Muñoz, G.; Sauvé, S.; Yargeau, V. An Introduction to the Sources, Fate, Occurrence and Effects of Endocrine Disrupting Chemicals Released into the Environment. Environ. Res. 2022, 207, 112658. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; Tao, L.; Wu, Q.; Tu, S.; Liu, B.; Lin, T.; Yang, L.; Li, C.; Liu, G. Global Squid Contamination by Halogenated Polycyclic Aromatic Hydrocarbons and Its Trade Induced Risk Transfer. Environ. Int. 2023, 179, 108163. [Google Scholar] [CrossRef]
- Lee, J.; Kim, Y.; Cha, J.; Kim, D.; Jang, K.; Kim, J.-H.; Nam, S.-I.; Hong, S. Distributions and Potential Sources of Polychlorinated Biphenyls and Polycyclic Aromatic Hydrocarbons in the Glacimarine Sediments of Arctic Svalbard. Mar. Pollut. Bull. 2023, 189, 114740. [Google Scholar] [CrossRef]
- Chen, M.-Y.; Liu, H.-Y.; Luo, X.-J.; Mai, B.-X.; Lu, F.-H. Investigating the Spatial Distribution of Polychlorinated Biphenyls in Sediment in the Pearl River Delta, South China. Environ. Monit. Assess. 2021, 193, 321. [Google Scholar] [CrossRef]
- Olaguer, E.J. The Distribution of the Chlorinated Solvents Dichloromethane, Perchloroethylene, and Trichloroethylene in the Global Atmosphere. Environ. Sci. Pollut. Res. 2002, 9, 175–182. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Zhang, Q.; Gao, M.; Li, H.; Yang, Z.; Wang, Y.; Sun, H. Polycyclic Aromatic Hydrocarbons and Their Halogenated Derivatives in Soil from Yellow River Delta: Distribution, Source Apportionment, and Risk Assessment. Mar. Pollut. Bull. 2024, 202, 116308. [Google Scholar] [CrossRef]
- He, C.Q.; Zou, Y.; Lv, S.J.; Flores, R.M.; Yan, X.L.; Deng, T.; Deng, X.J. The Importance of Photochemical Loss to Source Analysis and Ozone Formation Potential: Implications from in-Situ Observations of Volatile Organic Compounds (VOCs) in Guangzhou, China. Atmos. Environ. 2024, 320, 120320. [Google Scholar] [CrossRef]
- Joshi, S.; Rastogi, N.; Singh, A. Insights into the Formation of Secondary Organic Aerosols from Agricultural Residue Burning Emissions: A Review of Chamber-Based Studies. Sci. Total Environ. 2024, 952, 175932. [Google Scholar] [CrossRef]
- Rocha, A.C.S.; Reis-Henriques, M.A.; Galhano, V.; Ferreira, M.; Guimaraes, L. Toxicity of Seven Priority Hazardous and Noxious Substances (HNSs) to Marine Organisms: Current Status, Knowledge Gaps and Recommendations for Future Research. Sci. Total Environ. 2016, 542, 728–749. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.; Liu, J.; Zhang, X.; Richardson, S.D. Comparative Toxicity of Chlorinated Saline and Freshwater Wastewater Effluents to Marine Organisms. Environ. Sci. Technol. 2015, 49, 14475–14483. [Google Scholar] [CrossRef]
- Ding, J.; Luo, L.; Luan, T. Characteristics and Source Analysis of Polycyclic Aromatic Hydrocarbons and Their Derivatives in Marine Environment. Huanjing Huaxue-Environ. Chem. 2023, 42, 893–903. [Google Scholar] [CrossRef]
- Ji, Y.; Zhang, H.; Yan, X.; Li, Y.; Chai, F.; Li, H. A Review on Atmospheric Volatile Halogenated Hydrocarbons in China: Ambient Levels, Trends and Human Health Risks. Air Qual. Atmos. Health 2024, 17, 389–400. [Google Scholar] [CrossRef]
- Zhang, H.; Ji, Y.; Wu, Z.; Peng, L.; Bao, J.; Peng, Z.; Li, H. Atmospheric Volatile Halogenated Hydrocarbons in Air Pollution Episodes in an Urban Area of Beijing: Characterization, Health Risk Assessment and Sources Apportionment. Sci. Total Environ. 2022, 806, 150283. [Google Scholar] [CrossRef]
- Huang, N.-C.; Wann, S.-R.; Chang, H.-T.; Lin, S.-L.; Wang, J.-S.; Guo, H.-R. Arsenic, Vinyl Chloride, Viral Hepatitis, and Hepatic Angiosarcoma: A Hospital-Based Study and Review of Literature in Taiwan. BMC Gastroenterol. 2011, 11, 142. [Google Scholar] [CrossRef]
- Suehiro, Y.; Uchida, T.; Tsuge, M.; Murakami, E.; Miki, D.; Kawaoka, T.; Imamura, M.; Aikata, H.; Arihiro, K.; Oka, S. Acute Liver Injury in a Non-Alcoholic Fatty Liver Disease Patient with Chloroform Exposure: A Case Report. Clin. J. Gastroenterol. 2023, 16, 250–253. [Google Scholar] [CrossRef]
- Ozdemir, A.; Tumkaya, L.; Kalcan, S.; Uyan, M.; Karakaya, A.; Demiral, G.; Samanci, T.C.; Mercantepe, T.; Cure, M.C.; Cure, E. The Effects of TNF-α Inhibitors on Carbon Tetrachloride-Induced Nephrotoxicity. Clin. Exp. Hypertens. 2022, 44, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Fan, R.; Xu, Y.; Gao, Y.-Z.; Bizimana, A.; Naidoo, A.R.; Han, B.-C.; Meng, X.-Z. Occurrence, Distribution and Health Risk of Short-Chain Chlorinated Paraffins (SCCPs) in China: A Critical Review. Separations 2022, 9, 208. [Google Scholar] [CrossRef]
- Chen, S.; Ren, X.; Yu, Y.; Cheng, L.; Ding, G.; Yang, H.; Zhang, H.; Chen, J.; Geng, N. Metabolic Disturbance of Short- and Medium-Chain Chlorinated Paraffins to Zebrafish Larva. Sci. Total Environ. 2024, 923, 171372. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, Q.; Wu, Y.; Yang, C.; Luo, X.; Mai, B. Bioaccumulation of short- and medium-chain chlorinated paraffins in aquatic insects from an e-waste recycling site. Huanjing Huaxue-Environ. Chem. 2021, 40, 3037–3045. [Google Scholar] [CrossRef]
- Cui, Q.; Han, D.; Qin, H.; Li, H.; Liu, Y.; Guo, W.; Song, M.; Li, J.; Sun, Y.; Luo, J.; et al. Investigating the Levels, Spatial Distribution, and Trophic Transfer Patterns of Short-Chain Chlorinated Paraffins in the Southern Bohai Sea, China. Water Res. 2024, 253, 121337. [Google Scholar] [CrossRef]
- Guan, K.-L.; Liu, Y.; Luo, X.-J.; Zeng, Y.-H.; Mai, B.-X. Short- and Medium-Chain Chlorinated Paraffins in Aquatic Organisms from an e-Waste Site: Biomagnification and Maternal Transfer. Sci. Total Environ. 2020, 708, 134840. [Google Scholar] [CrossRef]
- Hu, H.; Qu, J.; Zhao, M.; Wu, P.; Zhu, W.; Zhou, Y.; Jin, H. Bioaccumulation and Trophic Magnification of Short Chain Chlorinated Paraffins in Marine Organisms from East China Sea. Mar. Pollut. Bull. 2021, 173, 113049. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; Luo, N.; Liu, Y.; Fang, X.; Zhang, S.; Li, S.; Jiang, W.; Zhao, N. Short and Medium-Chain Chlorinated Paraffins in Serum from Residents Aged from 50 to 84 in Jinan, China: Occurrence, Composition and Association with Hematologic Parameters. Sci. Total Environ. 2020, 728, 137998. [Google Scholar] [CrossRef]
- Yu, S.; Gao, Y.; Zhu, X.-H.; Geng, N.-B.; Dai, Y.-B.; Hong, J.-Y.; Chen, J.-P. Determination of short- and medium-chain chlorinated paraffins in different components of human blood using gas chromatography-electron capture negative ion-low resolution mass spectrometry. Chin. J. Chromatogr. 2023, 41, 698–706. [Google Scholar] [CrossRef]
- Chen, C.; Li, L.; Wania, F. Exploring the Long-Term Human Exposure to Short-, Medium-, and Long-Chain Chlorinated Paraffins under Variant Environmental Release Trends and Patterns. Environ. Sci. Technol. 2025, 59, 16304–16313. [Google Scholar] [CrossRef]
- Mu, Y.-W.; Yao, W.-H.; Li, H.; Zhang, C.-L.; Zhang, Y.-J.; Zhang, X.-N.; Song, G.; Zhao, X.-L.; Zeng, T. Short-Chain Chlorinated Paraffins at Environmentally Relevant Doses Disrupt Glucose Homeostasis and Insulin Signaling by Macrophage-Mediated Inflammation. J. Hazard. Mater. 2025, 495, 139144. [Google Scholar] [CrossRef]
- Li, J.; Wang, Z.; Zhang, Y.; Li, Y.; Feng, L.; Wang, J.; Zhang, J.; Zhou, Z.; Zhang, Y.; Chang, X. Effects of Environmentally Relevant Concentration of Short-Chain Chlorinated Paraffins on BV2 Microglia Activation and Lipid Metabolism, Implicating Altered Neurogenesis. Environ. Res. 2024, 251, 118602. [Google Scholar] [CrossRef]
- Xia, P.; Peng, Y.; Fang, W.; Tian, M.; Shen, Y.; Ma, C.; Crump, D.; O’Brien, J.M.; Shi, W.; Zhang, X. Cross-Model Comparison of Transcriptomic Dose-Response of Short-Chain Chlorinated Paraffins. Environ. Sci. Technol. 2021, 55, 8149–8158. [Google Scholar] [CrossRef]
- Xue, Z.; Zhu, J.; Wang, X.; Yang, C.; Fu, Z. Evaluation of the Immunomodulatory Effects of C9-13-CPs in Macrophages. Acta Biochim. Biophys. Sin. 2021, 53, 1154–1165. [Google Scholar] [CrossRef]
- Li, Q.; Li, Q.; Wang, F.; Xu, N.; Wang, Y.; Bai, B. Settling Behavior and Mechanism Analysis of Kaolinite as a Fracture Proppant of Hydrocarbon Reservoirs in CO2 Fracturing Fluid. Colloids Surf.-Physicochem. Eng. Asp. 2025, 724, 137463. [Google Scholar] [CrossRef]
- Zhang, H.; Gong, H.; He, X.; Wu, J.; Sun, H.; Xu, L.; Dong, M. Simultaneous Adjustment on the CO2/Oil and CO2/Water Interface Tensions Using Hydrophilic, Lipophilic and CO2-Philic Surfactants during CO2 Flooding to Enhance Shale Oil Recovery after Hydraulic Fracturing. Chem. Eng. J. 2024, 486, 150244. [Google Scholar] [CrossRef]
- Dai, J.-C.; Wang, T.-Y.; Weng, J.-T.; Tian, K.-J.; Zhu, L.-Y.; Li, G.-S. CO2 Flooding in Shale Oil Reservoir with Radial Borehole Fracturing for CO2 Storage and Enhanced Oil Recovery. Pet. Sci. 2024, 21, 519–534. [Google Scholar] [CrossRef]
- Soltanbekova, K.; Ramazanova, G.; Zhapbasbayev, U. Potential of Gas-Enhanced Oil Recovery (EOR) Methods for High-Viscosity Oil: A Core Study from a Kazakhstani Reservoir. Energies 2025, 18, 4182. [Google Scholar] [CrossRef]
- Purwasena, I.A.; Amaniyah, M.; Astuti, D.I.; Firmansyah, Y.; Sugai, Y. Production, Characterization, and Application of Pseudoxanthomonas Taiwanensis Biosurfactant: A Green Chemical for Microbial Enhanced Oil Recovery (MEOR). Sci. Rep. 2024, 14, 10270. [Google Scholar] [CrossRef] [PubMed]
- Raouf, S.; Ismail, Y.; Gamaleldin, N.; Aboelkhair, H.; Attia, A. Screening of Sustainable Biosurfactant Produced by Indigenous Bacteria Isolated from Egyptian Oil Fields for Microbial Enhanced Oil Recovery. Geoenergy Sci. Eng. 2024, 239, 212974. [Google Scholar] [CrossRef]
- Yernazarova, A.; Shaimerdenova, U.; Akimbekov, N.; Kaiyrmanova, G.; Shaken, M.; Izmailova, A. Exploring the Use of Microbial Enhanced Oil Recovery in Kazakhstan: A Review. Front. Microbiol. 2024, 15, 1394838. [Google Scholar] [CrossRef]
- Moradpour, N.; Pourafshary, P.; Zivar, D. Experimental Analysis of Hybrid Low Salinity Water Alternating Gas Injection and the Underlying Mechanisms in Carbonates. J. Pet. Sci. Eng. 2021, 202, 108562. [Google Scholar] [CrossRef]
- Agi, A.; Junin, R.; Jaafar, M.Z.; Amin, N.A.S.; Sidek, M.A.; Nyakuma, B.B.; Yakasai, F.; Gbadamosi, A.; Oseh, J.; Azli, N.B. Ultrasound-Assisted Nanofluid Flooding to Enhance Heavy Oil Recovery in a Simulated Porous Media. Arab. J. Chem. 2022, 15, 103784. [Google Scholar] [CrossRef]
- Liu, R.; Gao, S.; Peng, Q.; Pu, W.; Shi, P.; He, Y.; Zhang, T.; Du, D.; Sheng, J.J. Experimental and Molecular Dynamic Studies of Amphiphilic Graphene Oxide for Promising Nanofluid Flooding. Fuel 2022, 330, 125567. [Google Scholar] [CrossRef]
- Al-Yaari, A.; Ching, D.L.C.; Sakidin, H.; Muthuvalu, M.S.; Zafar, M.; Alyousifi, Y.; Saeed, A.A.H.; Haruna, A. Optimum Volume Fraction and Inlet Temperature of an Ideal Nanoparticle for Enhanced Oil Recovery by Nanofluid Flooding in a Porous Medium. Processes 2023, 11, 401. [Google Scholar] [CrossRef]
- Amrouche, F.; Blunt, M.J.; Iglauer, S.; Aiouache, F.; Short, M. A Novel Hybrid Enhanced Oil Recovery Technique to Enhance Oil Production from Oil-Wet Carbonate Reservoirs by Combining Electrical Heating with Nanofluid Flooding. Mater. Today Sustain. 2024, 27, 100915. [Google Scholar] [CrossRef]
- Ahmad, F.; Cao, W.; Zhang, Y.; Pan, R.; Zhao, W.; Liu, W.; Shuai, Y. Oil Recovery from Microwave Co-Pyrolysis of Polystyrene and Polypropylene Plastic Particles for Pollution Mitigation. Environ. Pollut. 2024, 356, 124240. [Google Scholar] [CrossRef] [PubMed]
- De Araujo, F.G.; Bauerfeldt, G.F.; Cid, Y.P. Nonylphenol: Properties, Legislation, Toxicity and Determination. An. Da Acad. Bras. De Ciencias 2018, 90, 1903–1918. [Google Scholar] [CrossRef] [PubMed]
- Kwiatkowski, C.F.; Andrews, D.Q.; Birnbaum, L.S.; Bruton, T.A.; DeWitt, J.C.; Knappe, D.R.U.; Maffini, M.; Miller, M.F.; Pelch, K.E.; Reade, A.; et al. Scientific Basis for Managing PFAS as a Chemical Class. Environ. Sci. Technol. Lett. 2020, 7, 532–543. [Google Scholar] [CrossRef]
- Jansen, L.M.; den Bakker, P.C.; Venbrux, N.; van Rijbroek, K.W.M.; Klaassen-Heshof, D.J.; Lenferink, W.B.; Lucker, S.; Ranoux, A.; Raaijmakers, H.W.C.; Boltje, T.J. Synthesis and Performance of Bio-Based Amphoteric Surfactants. Chem. Eur. J. 2024, 30, e202400986. [Google Scholar] [CrossRef] [PubMed]
- Yavrukova, V.I.; Danov, K.D.; Slavova, T.G.; Stanimirova, R.D.; Ung, Y.W.; Suan, A.T.K.; Xu, H.; Petkov, J.T. Enhanced Solubility of Methyl Ester Sulfonates below Their Krafft Points in Mixed Micellar Solutions. J. Colloid Interface Sci. 2024, 660, 896–906. [Google Scholar] [CrossRef]
- Zhou, Y.; Yang, X.; Bai, L.; Wu, Z.; Zhang, J.; Qin, Z.; Fan, J. Investigation on the Phase Behaviors of Aqueous Surfactant Two-Phase Systems Containing Alkyl Polyglucosides (APG) and Alkyl Polyglucoside Sulfosuccinate (APGSS). J. Mol. Liq. 2022, 368, 120630. [Google Scholar] [CrossRef]
- Pal, S.; Chatterjee, N.; Roy, S.S.; Chattopadhyay, B.; Acharya, K.; Datta, S.; Dhar, P. Valorization of Oil Refinery By-Products: Production of Sophorolipids Utilizing Fatty Acid Distillates and Their Potential Antibacterial, Anti-Biofilm, and Antifungal Activities. World J. Microbiol. Biotechnol. 2024, 40, 344. [Google Scholar] [CrossRef]
- Lavanya, M. Rhamnolipids: An Insight to the Overall Characteristics of These Extraordinary Biomolecules. Green Chem. Lett. Rev. 2024, 17, 2371012. [Google Scholar] [CrossRef]
- Padrtova, T.; Marvanova, P.; Mokry, P. Quaternary Ammonium Salts—Synthesis and Use. Chem. Listy 2017, 111, 197–205. [Google Scholar]
- Levashova, V.I.; Nikonorova, N.I. Synthesis and Properties of Quaternary Ammonium Salts Based on N,N′-Tetramethyldiaminomethane and 4-Chloro-2-Pentene. Pet. Chem. 2009, 49, 250–253. [Google Scholar] [CrossRef]
- Wang, W.; Liang, M.-Y.; Lang, J.-Q.; Mtui, H.I.; Yang, S.-Z.; Mu, B.-Z. A New Bio-Based Zwitterionic Surfactant with Strong Interfacial Activity at High Temperature for Enhanced Oil Recovery. Green Mater. 2023, 12, 50–59. [Google Scholar] [CrossRef]
- Afolabi, F.; Mahmood, S.M.; Sharifigaliuk, H.; Bin Kamarozaman, M.I.H.; Mansor, F.N.N.B.M. Investigations on the Enhanced Oil Recovery Capacity of Novel Bio-Based Polymeric Surfactants. J. Mol. Liq. 2022, 368, 120813. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, C.; Shi, Y.; Huang, M.; Song, Z.; Simal-Gandara, J.; Li, N.; Shi, J. Classification, Application, Multifarious Activities and Production Improvement of Lipopeptides Produced by Bacillus. Crit. Rev. Food Sci. Nutr. 2024, 64, 7451–7464. [Google Scholar] [CrossRef]
- Raaijmakers, J.M.; de Bruijn, I.; Nybroe, O.; Ongena, M. Natural Functions of Lipopeptides from Bacillus and Pseudomonas: More than Surfactants and Antibiotics. Fems Microbiol. Rev. 2010, 34, 1037–1062. [Google Scholar] [CrossRef]
- Sanati, A.; Malayeri, M.R.; Busse, O.; Weigand, J.J. Utilization of Ionic Liquids and Deep Eutectic Solvents in Oil Operations: Progress and Challenges. J. Mol. Liq. 2022, 361, 119641. [Google Scholar] [CrossRef]
- Rashid, Z.; Wilfred, C.D.; Gnanasundaram, N.; Arunagiri, A.; Murugesan, T. Screening of Ionic Liquids as Green Oilfield Solvents for the Potential Removal of Asphaltene from Simulated Oil: COSMO-RS Model Approach. J. Mol. Liq. 2018, 255, 492–503. [Google Scholar] [CrossRef]
- Wang, N.; Xu, A.; Liu, K.; Zhao, Z.; Li, H.; Gao, X. Performance of Green Solvents in Microwave-Assisted Pretreatment of Lignocellulose. Chem. Eng. J. 2024, 482, 148786. [Google Scholar] [CrossRef]
- Dolzhenko, A.V. Ethyl Lactate and Its Aqueous Solutions as Sustainable Media for Organic Synthesis. Sustain. Chem. Pharm. 2020, 18, 100322. [Google Scholar] [CrossRef]
- Moser, B.R.; Haas, M.J.; Winkler, J.K.; Jackson, M.A.; Erhan, S.Z.; List, G.R. Evaluation of Partially Hydrogenated Methyl Esters of Soybean Oil as Biodiesel. Eur. J. Lipid Sci. Technol. 2007, 109, 17–24. [Google Scholar] [CrossRef]
- Clark, J.H.; Farmer, T.J.; Hunt, A.J.; Sherwood, J. Opportunities for Bio-Based Solvents Created as Petrochemical and Fuel Products Transition towards Renewable Resources. Int. J. Mol. Sci. 2015, 16, 17101–17159. [Google Scholar] [CrossRef]
- Niu, A.; Xu, H.; Yuan, Q.; Wu, F.; Wei, X. Metal-Based Ionic Liquids and Solid-Loaded Catalysts in Fuel Oil Desulfurization: A Review. Mini-Rev. Org. Chem. 2024, 21, 704–716. [Google Scholar] [CrossRef]
- Liu, Q.; Gui, C.; Li, G.; Lei, Z. Investigation of Ionic Liquids as Extraction Solvents for Separating Bicyclic Aromatic S/N-Compounds from FCC Diesel. ACS Sustain. Chem. Eng. 2023, 11, 7573–7585. [Google Scholar] [CrossRef]
- Abbas, Z.; Jung, S.M. Green and Selective Recovery Process of Mo, V, and Ni from Spent Hydrodesulfurization Catalysts via Novel Ionic Liquids and Deep Eutectic Solvents Technology. Dye. Pigment. 2024, 346, 127450. [Google Scholar] [CrossRef]
- Yu, G.; Dai, C.; Liu, N.; Xu, R.; Wang, N.; Chen, B. Hydrocarbon Extraction with Ionic Liquids. Chem. Rev. 2024, 124, 3331–3391. [Google Scholar] [CrossRef]
- Wu, H.; Li, X.; Chen, Q.; Liu, Z.; Wang, X.; Zhang, W.; Miao, Y.; Yao, C. Molecular Mechanism and Extraction Performance Evaluation of Choline Chloride-Based Deep Eutectic Solvents for Phenol Separation from Oil: Different Glycols as Hydrogen Bond Donors. Fuel 2024, 367, 131530. [Google Scholar] [CrossRef]
- Wu, X.; Ning, Q.; Tu, Y.; Du, C.; Ren, Z. Extractive Desulfurization of Fuel Oil with Dual Imidazolium-Based Deep Eutectic Solvents. Chem. Eng. Sci. 2025, 317, 122092. [Google Scholar] [CrossRef]
- Guan, S.; Li, Z.; Xu, B.; Wu, J.; Han, J.; Guan, T.; Wang, J.; Li, K. Deep Eutectic Solvents with Excellent Catalytic Ability for Extractive and Oxidative Desulfurization under Room Temperature. ACS Sustain. Chem. Eng. 2023, 11, 6292–6301. [Google Scholar] [CrossRef]
- Li, N.; Zhang, H.; Ren, X.; Wang, J.; Yu, J.; Jiang, C.; Zhang, H.; Li, Y. Development Status of Supercritical Carbon Dioxide Thickeners in Oil and Gas Production: A Review and Prospects. Gas Sci. Eng. 2024, 125, 205312. [Google Scholar] [CrossRef]
- Fiedler, H.; Sadia, M.; Krauss, T.; Baabish, A.; Yeung, L.W.Y. Perfluoroalkane Acids in Human Milk under the Global Monitoring Plan of the Stockholm Convention on Persistent Organic Pollutants (2008–2019). Front. Environ. Sci. Eng. 2022, 16, 132. [Google Scholar] [CrossRef]
- Guida, Y.; Capella, R.; Kajiwara, N.; Babayemi, J.O.; Machado Torres, J.P.; Weber, R. Inventory Approach for Short-Chain Chlorinated Paraffins for the Stockholm Convention Implementation in Brazil. Chemosphere 2022, 287, 132344. [Google Scholar] [CrossRef] [PubMed]
- Egorova, T.; Sedlacek, J.; Sukhodolov, T.; Karagodin-Doyennel, A.; Zilker, F.; Rozanov, E. Montreal Protocol’s Impact on the Ozone Layer and Climate. Atmos. Chem. Phys. 2023, 23, 5135–5147. [Google Scholar] [CrossRef]
- Albiac, J.; Calvo, E.; Esteban, E. Review Paper: A Quarter of a Century of the European Water Framework Directive—The Slow Path Towards Sustainable Water Management. Water Econ. Policy 2024, 10, 2430001. [Google Scholar] [CrossRef]
- Galert, W.; Hassold, E. Environmental Risk Assessment of Technical Mixtures Under the European Registration, Evaluation, Authorisation and Restriction of Chemicals-A Regulatory Perspective. Integr. Environ. Assess. Manag. 2021, 17, 498–506. [Google Scholar] [CrossRef]
- Gabbert, S.; Hilber, I. Socio-Economic Analysis in REACH Restriction Dossiers for Chemicals Management: A Critical Review. Ambio 2020, 49, 1394–1411. [Google Scholar] [CrossRef] [PubMed]
- Maruzzo, A.J.; Hernandez, A.B.; Swartz, C.H.; Liddie, J.M.; Schaider, L.A. Drinking Water Contaminants in US Public Water Systems. Environ. Health Perspect. 2025, 133, 017002. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Sui, Q.; Liu, H.; Yu, G.; Qu, J. Promoting Environmental Risk Assessment and Control of Emerging Contaminants in China. Engineering 2024, 37, 13–17. [Google Scholar] [CrossRef]

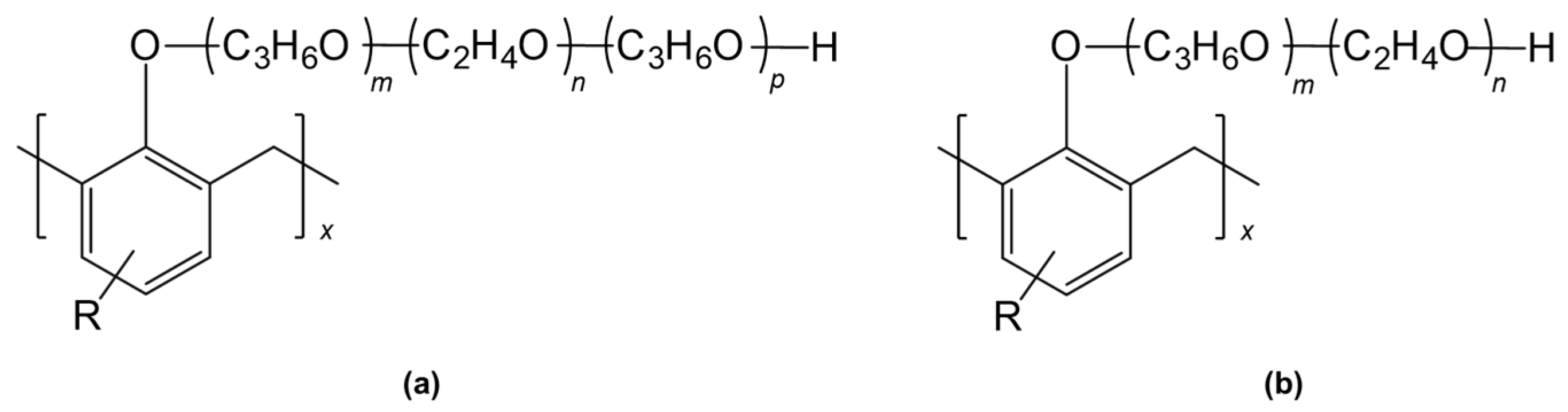

| Number | Group | Application | Chemicals | Major EPs |
|---|---|---|---|---|
| C-1-1 | Oil well drilling | Filtration reducer | Polyacrylamide | PFAS |
| C-1-2 | Shale inhibitor | Polymer Cationic surfactant | PFAS | |
| C-2-1 | Oil recovery | Oil well acidifier | Inorganic acid Potential acid | HHCs |
| C-2-2 | Thickener | Polyacrylamide Surfactant | PFAS | |
| C-2-3 | Retarder | Surfactant (soluble in acid) Polymer | PFAS | |
| C-2-4 | Fracturing agent | Emulsion fracturing agent Foam fracturing agent | PFAS | |
| C-2-5 | Foaming agent | Surfactant | PFAS AP | |
| C-2-6 | Defoamer | Surfactant | AP | |
| C-2-7 | Oil well water-plugging agent | Surfactant | PFAS | |
| C-2-8 | Tracer | Tracer | HHCs | |
| C-3-1 | Chemical oil displacement agent | Polymer oil-displacing agent | Modified polyacrylamide | PFAS |
| C-3-2 | Surfactant oil-displacing agent | Highly chemically resistant surfactant | PFAS |
| Number | Phenotype | Solubility | Major Applications |
|---|---|---|---|
| NPE-4 | Colorless or yellowish transparent oily liquid | Oil-soluble | Polymeric emulsifiers, industrial emulsifiers, metal degreasers, and cleaning agents |
| NPE-7 | Colorless or yellowish transparent oily liquid | Water-soluble | Industrial emulsifier, wetting agent |
| NPE-9 | Colorless, transparent oily liquid | Water-soluble | Antistatic agent, cleaning agent |
| NPE-10 | Colorless, transparent oily liquid | Water-soluble | Emulsifier, detergent |
| NPE-15 | White paste | Water-soluble | Emulsifier, high temperature dispersant, detergent |
| NPE-40 | White wax | Water-soluble | Emulsifier, dispersant, viscosity reducer |
| Number | Group | Application | Chemicals | Major EPs |
|---|---|---|---|---|
| S-1-1 | Improving crude oil liquidity | Polymer viscosity reducer | Modified polyacrylamide | PFAS |
| S-1-2 | Surface-active viscosity reducer | surfactant | PFAS | |
| S-1-3 | Mixed-type viscosity reducer | / | / | |
| S-2-1 | Paraffin wax remover and inhibitor | Paraffin wax remover | Organic Solvent | HHCs |
| S-2-2 | Paraffin wax inhibitor | Polycyclic aromatic hydrocarbons Surfactants Polymer agents | PFAS AP | |
| S-3-1 | Crude oil demulsifier | W/O demulsifier | Sulfonate Phenolic resins Polyoxyethylene ether Phenolamine Resins | PFOS NP |
| S-3-2 | O/W demulsifier | Polymer Emulsion Breaker Macromolecule emulsion breakers | NP |
| Number | Group | Application | Chemicals | Major EPs |
|---|---|---|---|---|
| L-0-1 | Universal agent | Corrosion inhibitor | Surfactant | PFAS |
| L-0-2 | Defoaming agent | Surfactant | PFAS | |
| L-1-1 | Oil Processing | Crude Oil Demulsifies | Sulfonate and other anionic types Polyether type | NP |
| L-2-1 | Oil Distillation | Crude oil distillation enhancer | Aromatic concentrates Surfactant Complex activator | PFAS NP |
| Number | Application | Chemicals | Major EPs |
|---|---|---|---|
| H-1 | Emulsifier | Nonylphenol polyoxyethylene ether sulfate amine salt Nonylphenol polyoxyethylene ether carboxylate salt Betaine amphoteric surfactants | PFOA NP |
| H-2 | Dispersant | PFOA | PFOA |
| H-3 | Polymerization terminator | Bisphenol A Hydroquinone | BPA |
| H-4 | Polymerization inhibitor | Hydroquinone NP | NP |
| H-5 | Antioxidant | Bisphenol A phosphite | BPA |
| H-6 | Antistatic agent | Perfluorobutanesulfonates Salicylates Bisphenol A Bis(salicylate) Benzophenones | PFAS BPA |
| Number | Group | Application | Chemicals | Major EPs |
|---|---|---|---|---|
| T-1-1 | Lubricant Additives | Detergents and dispersants | Alkyl phenate Sulfurized alkyl phenate Alkyl salicylate | APs |
| T-1-2 | Extreme-pressure antiwear agent | Chlorine-based extreme-pressure antiwear agent | SCCPs | |
| T-1-3 | Pour point depressant | Alkylphenol | APs | |
| T-2-1 | Fuel Additives | Antioxidant and anti-gum agent | Alkylphenol | APs |
| T-2-2 | Antistatic agent | Perfluorobutyl sulfonate | PFAS | |
| T-2-3 | Antiwear agent | Chlorine-based antiwear agent | SCCPs PCPs | |
| T-2-4 | Oxidizer | Tritolyl phosphate | ||
| T-2-5 | Detergents and dispersants | Alkyl phenol salts and their derivatives | APs |
| Location | Media | Types of EPs | Concentration | References |
|---|---|---|---|---|
| North Sea Oilfield, Norway | Sediment | AP/APEOs | 4.1–137,000 μg/g | [91] |
| Daqing Oilfield, China | Surface water | ΣPFASs | 560–5000 ng/L | [92] |
| Dagang Oilfield, China | Surface water | ΣPFASs (C ≥ 4) | 201–12,036 ng/L | [93] |
| Sediment | ΣPFASs (C ≥ 4) | 4.65–87.4 ng/g | ||
| Soil | ΣPFASs (C ≥ 4) | 2.57–32.3 ng/g | ||
| Bohai Sea oilfields, China | Receiving water | ΣPFASs (C ≥ 4) | 46.2–99.7 ng/L | [86] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, L.; Long, Z.; Gu, T.; Ju, F.; Zhen, H.; Luan, H.; Xiu, G.; Tang, Z. Emerging Pollutants as Chemical Additives in the Petroleum Industry: A Review of Functional Uses, Environmental Challenges and Sustainable Control Strategies. Sustainability 2025, 17, 8559. https://doi.org/10.3390/su17198559
Wang L, Long Z, Gu T, Ju F, Zhen H, Luan H, Xiu G, Tang Z. Emerging Pollutants as Chemical Additives in the Petroleum Industry: A Review of Functional Uses, Environmental Challenges and Sustainable Control Strategies. Sustainability. 2025; 17(19):8559. https://doi.org/10.3390/su17198559
Chicago/Turabian StyleWang, Limin, Zi Long, Tao Gu, Feng Ju, Huajun Zhen, Hui Luan, Guangli Xiu, and Zhihe Tang. 2025. "Emerging Pollutants as Chemical Additives in the Petroleum Industry: A Review of Functional Uses, Environmental Challenges and Sustainable Control Strategies" Sustainability 17, no. 19: 8559. https://doi.org/10.3390/su17198559
APA StyleWang, L., Long, Z., Gu, T., Ju, F., Zhen, H., Luan, H., Xiu, G., & Tang, Z. (2025). Emerging Pollutants as Chemical Additives in the Petroleum Industry: A Review of Functional Uses, Environmental Challenges and Sustainable Control Strategies. Sustainability, 17(19), 8559. https://doi.org/10.3390/su17198559






