Sustainable Soil Amendment: Effect of Reusing Saturated Dolomitic Calcareous Amendment (DCAS) on Chemical Properties of Two Types of Agricultural Soils
Abstract
1. Introduction
2. Materials and Methods
2.1. DCAS Used as a Sustainable Soil Amendment
2.2. Soil Sampling and Analysis
2.3. Experimental Design
3. Results and Discussion
3.1. Soil Analysis
3.2. Application of Treatments to Soil
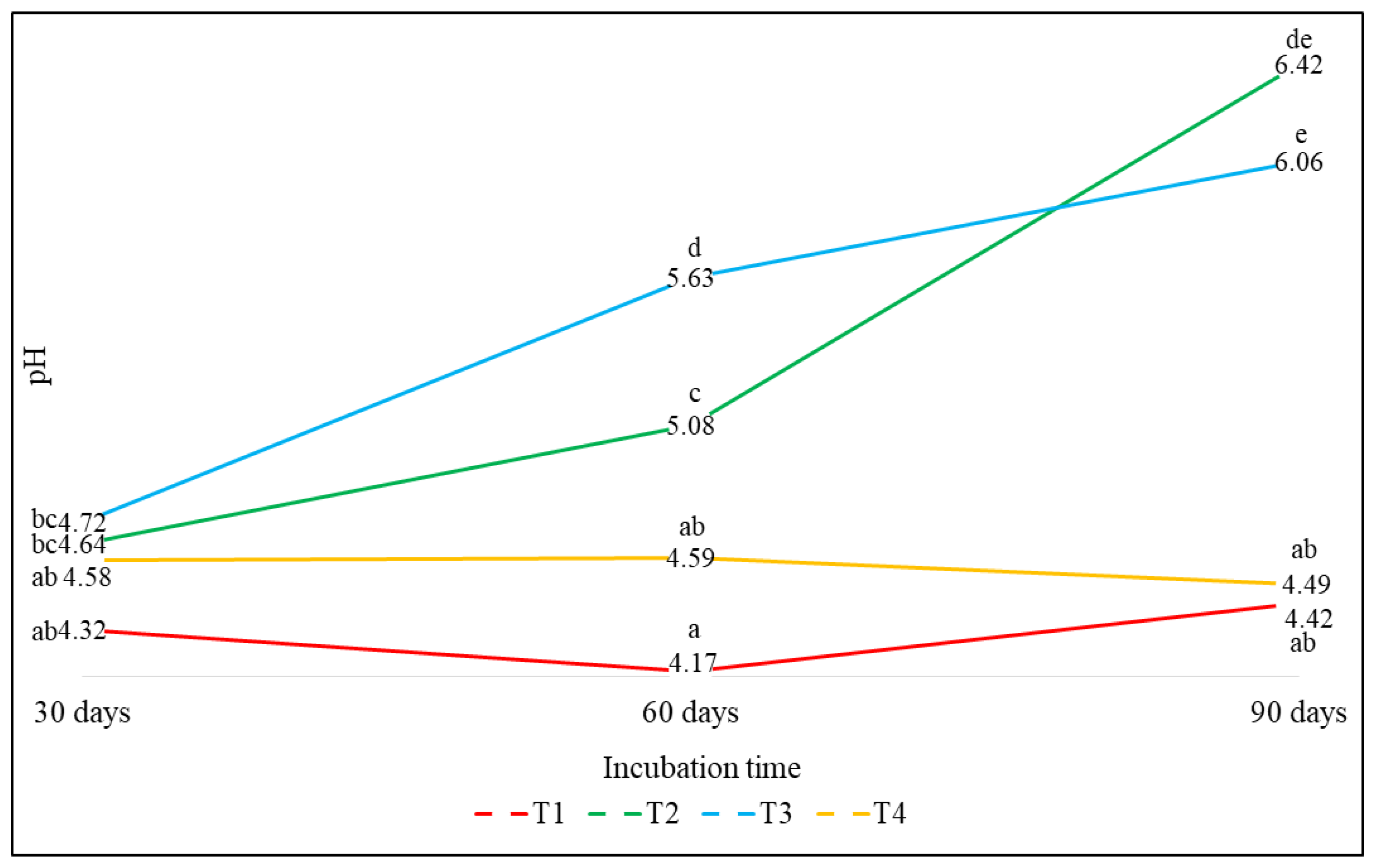
3.2.1. Effect on the pH
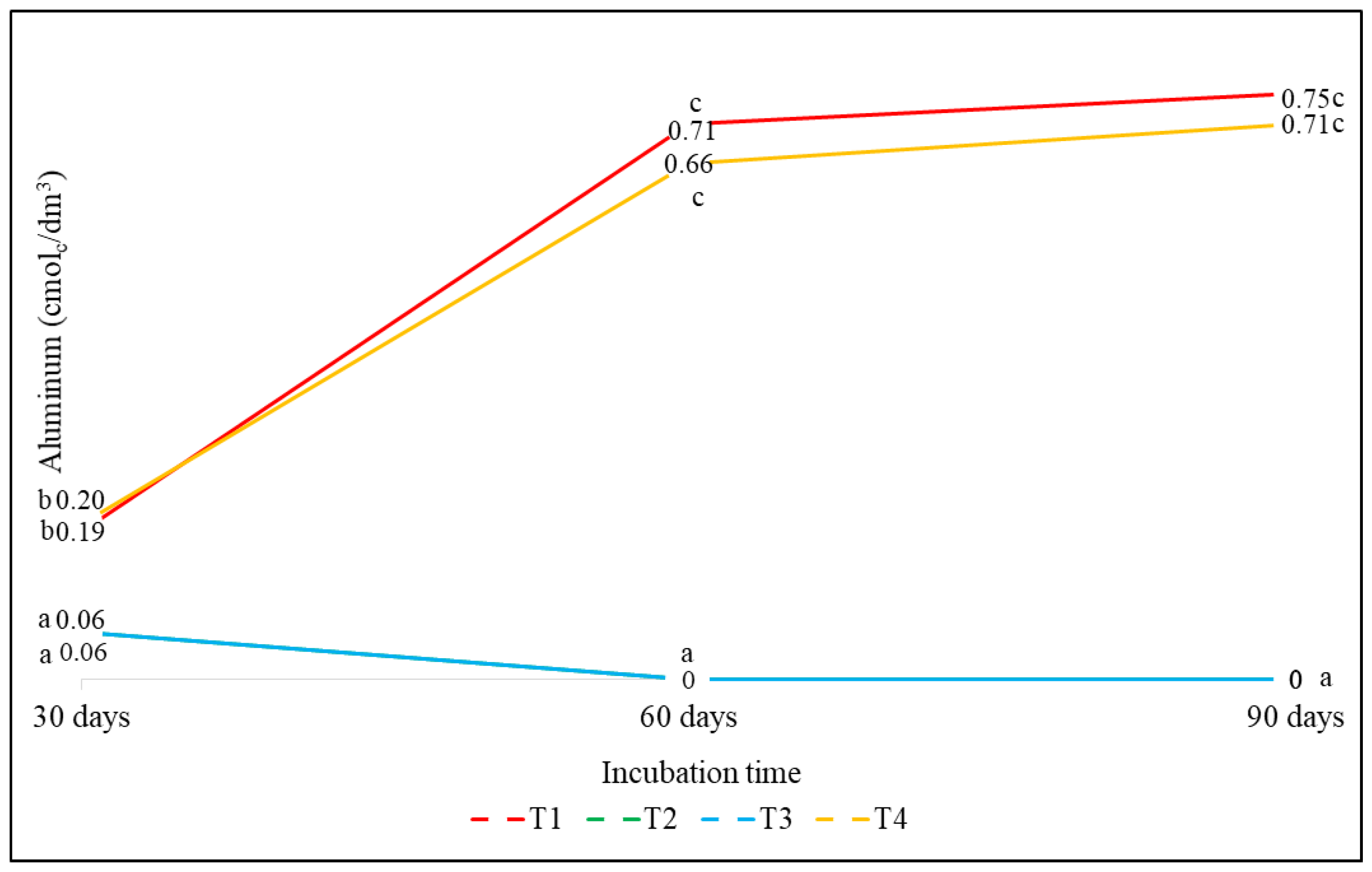
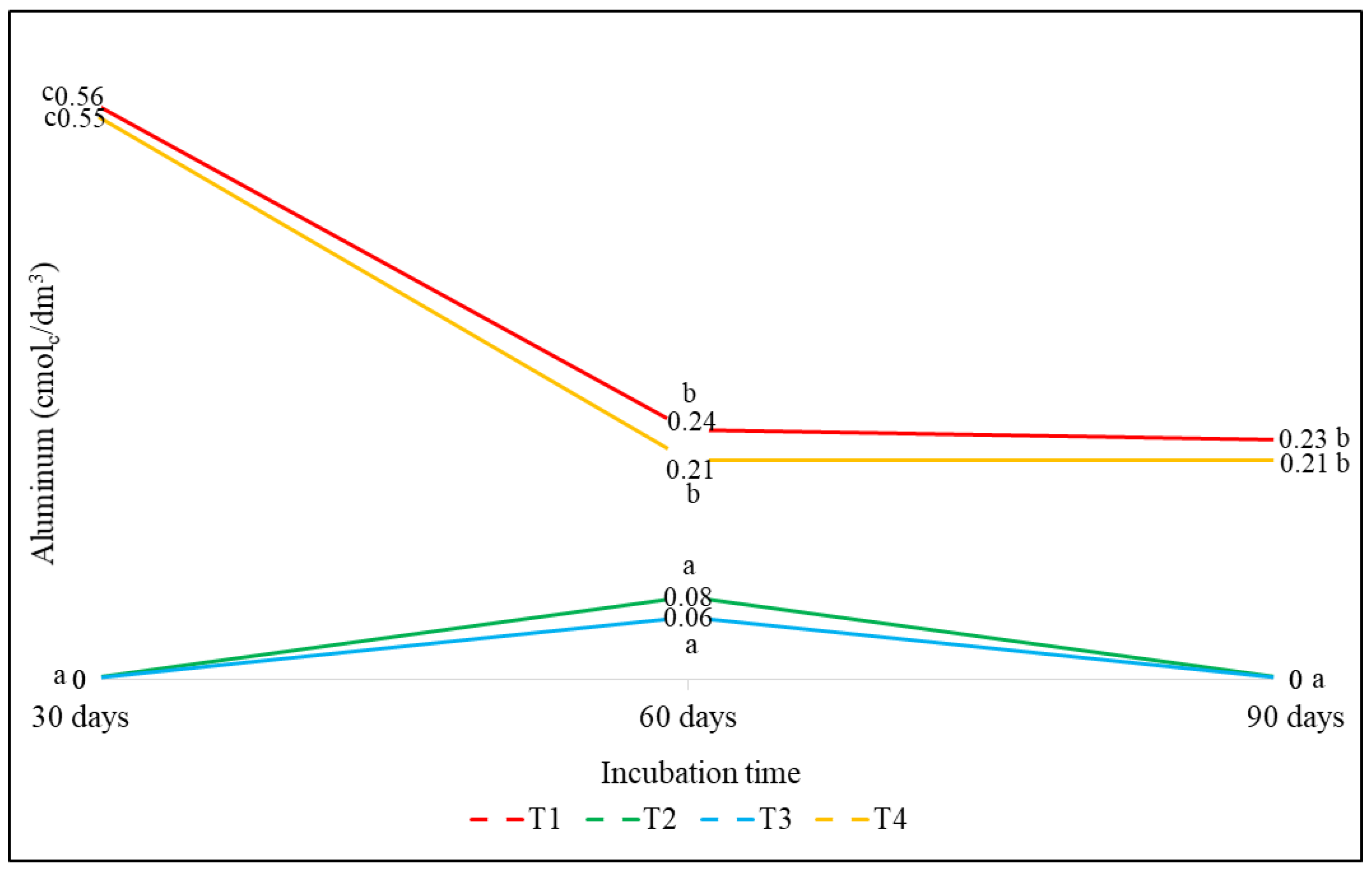
3.2.2. Effect on Aluminum (Al)
3.2.3. Effect on Calcium (Ca)
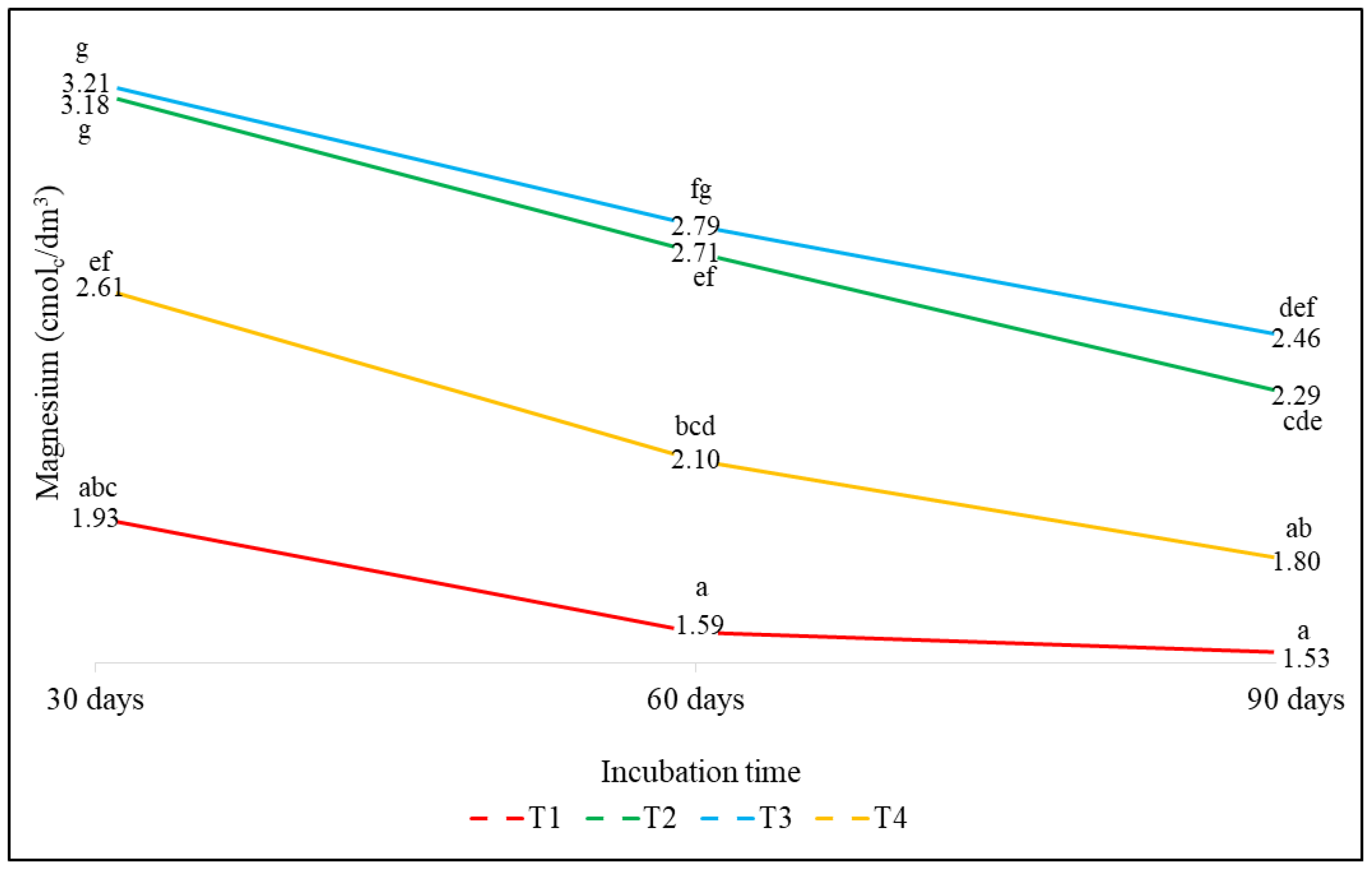
3.2.4. Effect on Magnesium (Mg)
3.2.5. Effect on Phosphorus (P)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, J.B.K.; Craggs, R.J.; Sukias, J.P.S. Treatment of hydroponic wastewater by denitrification filters using plant prunings as the organic carbon source. Bioresour. Technol. 2008, 99, 2711–2716. [Google Scholar] [CrossRef]
- Prystay, W.; Lo, K.V. Treatment of greenhouse wastewater using constructed wetlands. J. Environ. Sci. Health B 2001, 36, 341–353. [Google Scholar] [CrossRef] [PubMed]
- Kwon, M.J.; Hwang, Y.; Lee, J.; Ham, B.; Rahman, A.; Azam, H.; Yang, J.S. Waste nutrient solutions from full-scale open hydroponic cultivation: Dynamics of effluent quality and removal of nitrogen and phosphorus using a pilot-scale sequencing batch reactor. J. Environ. Manag. 2021, 281, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Koide, S.; Satta, N. Separation Performance of Ion-exchange Membranes for Electrolytes in Drainage Nutrient Solutions subjected to Electrodialysis. Biosyst. Eng. 2004, 87, 89–97. [Google Scholar] [CrossRef]
- Dabrowski, A. Adsorption from theory to Practice. Adv. Colloid Interface. 2001, 93, 135–224. [Google Scholar] [CrossRef] [PubMed]
- An, Q.; Li, Z.; Zhou, Y.; Meng, F.; Zhao, B.; Miao, Y.; Deng, S. Ammonium removal from groundwater using peanut shell based modified biochar: Mechanism analysis and column experiments. J. Water Process Eng. 2021, 43, 102219. [Google Scholar] [CrossRef]
- Feng, Q.; Chen, M.; Wu, P.; Zhang, X.; Wang, S.; Yu, Z.; Wang, B. Simultaneous reclaiming phosphate and ammonium from aqueous solutions by calcium alginate-biochar composite: Sorption performance and governing mechanisms. Chem. Eng. J. 2022, 429, 132166. [Google Scholar] [CrossRef]
- Ma, Z.; Li, Q.; Yue, Q.; Gao, B.; Li, W.; Xu, X.; Zhong, Q. Adsorption removal of ammonium and phosphate from water by fertilizer controlled release agent prepared from wheat straw. Chem. Eng. J. 2011, 171, 1209–1217. [Google Scholar] [CrossRef]
- García Rojas, N.; Villanueva Díaz, P.; Campos Medina, E.; Velázquez Rodríguez, A. Análisis de adsorción como métodos de pulimiento en el tratamiento de aguas residuales. Quivera 2012, 14, 109–129. Available online: https://www.redalyc.org/pdf/401/40123894007.pdf (accessed on 30 July 2025).
- Boeykens, S.P.; Piol, M.N.; Samudio Legal, L.; Saralegui, A.B.; Vázquez, C. Eutrophication decrease: Phosphate adsorption processes in presence of nitrates. J. Environ. Manag. 2017, 203, 888–895. [Google Scholar] [CrossRef]
- Han, R.; Zou, L.; Zhao, X.; Xu, Y.; Xu, F.; Li, Y.; Wang, Y. Characterization and properties of iron oxide-coated zeolite as adsorbent for removal of copper (II) from solution in fixed bed column. Chem. Eng. J. 2009, 149, 123–131. [Google Scholar] [CrossRef]
- Adibi, N.; Lafhaj, Z.; Yehya, M.; Payet, J. Global Resource Indicator for life cycle impact assessment: Applied in wind turbine case study. J. Clean. Prod. 2017, 165, 1517–1528. [Google Scholar] [CrossRef]
- Borrello, M.; Caracciolo, F.; Lombardi, A.; Pascucci, S.; Cembalo, L. Consumers perspective on circular economy strategy for reducing food waste. Sustainability 2017, 9, 141. [Google Scholar] [CrossRef]
- Leite, R.; Lucheta, A.; Holanda, R.; Pereira Silva, P.; Vilaça Do Carmo, A.; Leite, R.; Amorim De Melo, C.; Viera Da Costa, R.; Montini, M.; Fernandes, A. Bauxite residue valorization–Soil conditioners production through composting with palm oil mill residual biomass. J. Sci. Total Environ. 2022, 835, 155413. [Google Scholar] [CrossRef]
- Kizito, S.; Luo, H.; Lu, J.; Bah, H.; Dong, R.; Wu, S. Role of nutrient-enriched biochar as a soil amendment during maize growth: Exploring practical alternatives to recycle agricultural residuals and to reduce chemical fertilizer demand. Sustainability 2019, 11, 3211. [Google Scholar] [CrossRef]
- Joniec, J.; Kwiatkowska, E.; Kwiatkowski, C.A. Assessment of the Effects of Soil Fertilization with Spent Mushroom Substrate in the Context of Microbial Nitrogen Transformations and the Potential Risk of Exacerbating the Greenhouse Effect. Agriculture 2022, 12, 1190. [Google Scholar] [CrossRef]
- Hamanaka, A.; Sasaoka, T.; Shimada, H.; Matsumoto, S. Amelioration of acidic soil using fly Ash for Mine Revegetation in Post-Mining Land. Int. J. Coal. Sci. Technol. 2022, 9, 33. [Google Scholar] [CrossRef]
- Mabrouk, O.; Hamdi, H.; Sayadi, S.; Al-Ghouti, M.A.; Abu-Dieyeh, M.H.; Zouari, N. Reuse of Sludge as Organic Soil Amendment: Insights into the Current Situation and Potential Challenges. Sustainability 2023, 15, 6773. [Google Scholar] [CrossRef]
- Rosas-Patiño, G.; Puentes-Páramo, Y.J.; Menjivar-Flores, J.C. Relación entre el pH y la disponibilidad de nutrientes para cacao en un entisol de la Amazonia colombiana. Cienc. Y Tecnol. Agropecu. 2017, 18, 529–541. [Google Scholar] [CrossRef]
- Yan, L.; Riaz, M.; Li, S.; Cheng, J.; Jiang, C. Harnessing the power of exogenous factors to enhance plant resistance to aluminum toxicity; a critical review. Plant Physiol. Biochem. 2023, 203, 108064. [Google Scholar] [CrossRef]
- Chiapini, M.; Schellekens, J.; Calderi, O.J.; Calegari, R.; Vidal, P. Pedogenesis in very deep autochthonous Ferralsols of the Paraná Igneous Province (Brazil). CATENA 2023, 224, 106981. [Google Scholar] [CrossRef]
- Jaskowiak, J.; Tkaczyk, O.; Slota, M.; Kwasniewska, J.; Szarejko, I. Analysis of aluminum toxicity in Hordeum vulgare roots with an emphasis on DNA integrity and cell cycle. PLoS ONE 2018, 13, 2. [Google Scholar] [CrossRef] [PubMed]
- Cui, Q.; Xia, J.; Yang, H.; Liu, J.; Shao, P. Biochar and effective microorganisms promote Sesbania cannabina growth and soil quality in the coastal saline-alkali soil of the Yellow River Delta, China. Sci. Total Environ. 2021, 756, 143801. [Google Scholar] [CrossRef] [PubMed]
- Rodrighero, M.B.; Barth, G.; Fávero Caires, E. Aplicação superficial de calcário com diferentes teores de magnésio e granulometrias em sistema plantio direto. Rev. Bras. Ciênc. Solo 2015, 39, 1723–1736. [Google Scholar] [CrossRef]
- European Parliament. Boosting the Use of Organic and Safer Fertilisers in the EU; Press Releases Number: 20171020IPR86544; News European Parliament: 2017. Available online: https://www.europarl.europa.eu/news/en/press-room/20171020IPR86544/boosting-the-use-of-organic-and-safer-fertilisers-in-the-eu (accessed on 1 August 2025).
- Samudio Legal, L.E.; Aguayo Trinidad, S.; Piol, M.N.; Gamarra Alfonso, P.G.; Pires Frigo, J.; Furtado, A.C. Recovery and Reuse of Nutrients from Hydroponic Effluent in the Context of Circular Agriculture. Sustainability 2025, 17, 6045. [Google Scholar] [CrossRef]
- ASTM E11; Standard Specification for Woven Wire Test Sieve Cloth and Test Sieves. ASTM International: West Conshohocken, PA, USA, 2009; p. 15.
- APHA (American Public Health Association); AWWA (American Water Works Association); WEF (Water Environment Federation). Standard Methods for the Examination of Waters and Wastewaters, 23rd ed.; Rice, E.W., Baird, R., Eaton, A., Clesceri, L., Eds.; American Public Health Association: Washington, DC, USA, 2017. [Google Scholar]
- Legal, L.E.S.; Trinidad, S.A.; Frigo, J.P.; Furtado, A.C. Reuso da dolomita saturada por nutrientes como corretivo em solos agrícolas. Cad. Pedagógico 2023, 20, 1066–1091. [Google Scholar] [CrossRef]
- López, O.; González, E.; De Llamas, P.; Molinas, A.; Franco, S.; García, S.; Ríos, E. Estudio de Reconocimiento de Suelos, Capacidad de uso de la Tierra y Propuesta de Ordenamiento Territorial Preliminar de la Región Oriental del Paraguay; Proyecto de Racionalización del uso de la tierra SSERNMA/MAG/Banco Mundial: Asunción, Paraguay, 1995; p. 246. [Google Scholar]
- Da Silva, F.C. Manual de Análises Químicas de Solos, Plantas e Fertilizantes, 2nd ed.; Embrapa Informação Tecnológica: Brasilia, Brasil, 2009; p. 627. [Google Scholar]
- Quintero, D.R. Interpretación del Análisis de Suelo y Recomendaciones de Fertilizantes para la caña de Azúcar; Centro De Investigación De La Caña De Azúcar De Colombia: Cali, Colombia, 1993; p. 20. [Google Scholar]
- Lopes, A.; Silva, M.; Guilherme, L.R. Acidez do solo e Calagem, 3rd ed.; ANDA Associação Nacional para Difusão de Adubos: São Paulo, Brazil, 1991; p. 22. [Google Scholar]
- Meneghetti, A.M. Manual de Procedimentos de Amostragem e Análise Química de Plantas, Ssolo e Fertilizantes; Universidade Tecnológica Federal do Paraná: Curitiba, Brazil, 2018; p. 252. Available online: http://repositorio.utfpr.edu.br/jspui/handle/1/4071 (accessed on 1 August 2025).
- Peralta Moreno, G.A. Efecto de Diferentes Dosis de Material Calcáreo Sobre el pH y Acidez Intercambiable en Suelos ácidos de Textura Arcillosa y Arenosa en los Distritos de Hernandarias y Nueva Italia. Bachelor’s Thesis, National University of Asunción, San Lorenzo, Paraguay, 2019; p. 89. [Google Scholar]
- Díaz-Poveda, V.; Sadeghian, K.S. Eficiencia de enmiendas utilizadas como correctivos de la acidez del suelo en el cultivo de café en Colombia. Revista Cenicafé. 2022, 73, e73103. [Google Scholar] [CrossRef]
- Viadé, A.; Fernández-Marcos, M.; Hernández-Nistal, J.; Álvarez, E. Effect of particle size of calcareous on Ca, Mg and K contents in soil and in sward plants. Sci Agri. 2011, 68, 200–208. [Google Scholar] [CrossRef]
- Deus, A.C.; Büll, L.; Corrêa, J.; Boas, R. Determination of reactivity rates of silicate particle-size fractions. Rev Ceres. 2014, 61, 265–272. [Google Scholar] [CrossRef]
- Espinosa, J.; Molina, E. Acidez y Encalado de los Suelos, 1st ed.; International Plant Nutrition Institute IPNI: Quito, Ecuador, 1999; p. 49. [Google Scholar]
- Kinraid, T.B. Identity of the rhizotoxic aluminum species. Plant Soil. 1991, 134, 167–178. [Google Scholar] [CrossRef]
- Melo, R.F.; Ferreira, P.A.; Matos, A.T.; Ruiz, H.A.; Oliveira, L.B. Deslocamento miscível de cátions básicos provenientes da água residuária de mandioca em colunas de solo. Rev. Bras. Eng. Agríc. Ambient. 2006, 10, 456–465. [Google Scholar] [CrossRef]
- Vitti, G.C.; Lima, E.; Cicarone, F. Cálcio, Magnésio e Enxofre. In Nutrição Mineral de Plantas; Fernandes, M.S., Ed.; SBCS: Viçosa, Brazil, 2006; pp. 299–326. [Google Scholar]
- Samudio, L.F. Efecto de Enmiendas Calcáreas Sobre las Propiedades Químicas y Biológicas en Suelos Incubados de Alto Paraná, Paraguay. Master’s Thesis, National University of Asunción, San Lorenzo, Paraguay, 2020; p. 63. [Google Scholar]
- Cano-Betancur, S.M.; Gallego-Becerra, M.; Chavarriaga-Montoya, W. Efecto de la aplicación de calcio y fósforo en un suelo ácido y la respuesta en el cultivo de tomate chonto (Solanum lycopersicum (L.) Mill). Agronomy 2011, 19, 77–87. [Google Scholar]
- Kass, D. Fertilidad de Suelos; EUNED: San José, Costa Rica, 1996; p. 272. [Google Scholar]
- Jing, T.; Li, J.; He, Y.; Shankar, A.; Saxena, A.; Tiwari, A.; Chaitanya Maturi, K.; Kumar Solanki, M.; Singh, V.; A. Eissa, M.; et al. Role of calcium nutrition in plant Physiology: Advances in research and insights into acidic soil conditions—A comprehensive review. Plant Physiol. Biochem. 2024, 210, 108602. [Google Scholar] [CrossRef] [PubMed]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 14th ed.; Pearson Education; Prentice-Hall: Upper Saddle River, NJ, USA, 2008; p. 1071. [Google Scholar]
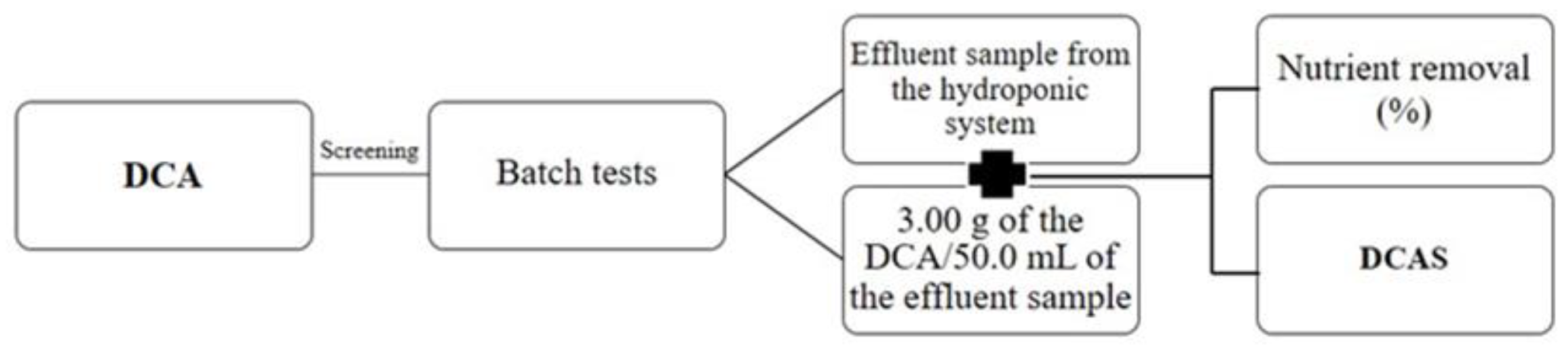






| Nutrient | P | Ca | Mg | Na | Fe | Zn | Mn |
|---|---|---|---|---|---|---|---|
| Initial Concentrations (mg/L) | 2.67 | 100.50 | 30.00 | 4.52 | 4.24 | 0.48 | 0.05 |
| Final Concentrations (mg/L) | 0.68 | 80.65 | 30.00 | 4.52 | 4.24 | 0.42 | 0.02 |
| Removal (%) | 74.53 | 19.75 | - | - | - | 12.50 | 60.00 |
| Standard Deviation | 0.05 | 1.80 | - | - | - | 1.08 | 3.31 |
| Treatment | |
|---|---|
| T1 (Control) | Soil without DCA. |
| T2 (DCAN) | Soil with DCA in natura. |
| T3 (DCAS) | Soil with saturated DCA. |
| T4 (DCAG) | Soil with granulated DCA. |
| pH H2O | Al | Ca | Mg | K | CEC | P | |
|---|---|---|---|---|---|---|---|
| cmolc/dm3 | mg/dm3 | ||||||
| Sandy Soil | 4.55 | 0.70 | 0.24 | 0.10 | 0.05 | 4.49 | 3.11 |
| Clay Soil | 5.00 | 0.30 | 3.20 | 2.34 | 0.64 | 11.85 | 4.77 |
| Treatment | Sandy Soil | ||||
| pH H2O | Al | Ca | Mg | P | |
| cmolc/dm3 | mg/dm3 | ||||
| T1(control) | 4.42 (90 d) | 0.75 (90 d) | 0.46 (90 d) | 0.14 (30 d) | 6.52 (90 d) |
| T2 (DCAN) | 6.42 (90 d) | 0 (60 d) | 1.81 (90 d) | 0.97 (60 d) | 6.55 (60 d) |
| T3 (DCAS) | 6.06 (90 d) | 0 (60 d) | 1.41 (60 d) | 0.80 (30 d) | 6.59 (60 d) |
| T4 (DCAG) | 4.59 (60 d) | 0.71 (90 d) | 0.64 (60 d) | 0.11 (90 d) | 6.03 (60 d) |
| Treatment | Clayey Soil | ||||
| pH H2O | Al | Ca | Mg | P | |
| cmolc/dm3 | mg/dm3 | ||||
| T1(control) | 4.73 (90 d) | 0.23 (90 d) | 3.49 (30 d) | 1.93 (30 d) | 7.24 (60 d) |
| T2 (DCAN) | 5.45 (30 d) | 0 (30 d) | 3.80 (30 d) | 3.18 (30 d) | 5.93 (60 d) |
| T3 (DCAS) | 5.58 (30 d) | 0 (30 d) | 4.18 (30 d) | 3.21 (30 d) | 6.64 (60 d) |
| T4 (DCAG) | 4.61 (90 d) | 0.21 (90 d) | 3.34 (30 d) | 2.61 (30 d) | 6.64 (60 d) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Samudio Legal, L.E.; Aguayo Trinidad, S.; Gamarra Alfonso, P.G.; Piol, M.N.; Saralegui, A.B.; Frigo, J.P.; Furtado, A.C. Sustainable Soil Amendment: Effect of Reusing Saturated Dolomitic Calcareous Amendment (DCAS) on Chemical Properties of Two Types of Agricultural Soils. Sustainability 2025, 17, 8557. https://doi.org/10.3390/su17198557
Samudio Legal LE, Aguayo Trinidad S, Gamarra Alfonso PG, Piol MN, Saralegui AB, Frigo JP, Furtado AC. Sustainable Soil Amendment: Effect of Reusing Saturated Dolomitic Calcareous Amendment (DCAS) on Chemical Properties of Two Types of Agricultural Soils. Sustainability. 2025; 17(19):8557. https://doi.org/10.3390/su17198557
Chicago/Turabian StyleSamudio Legal, Lisa Eliana, Simeón Aguayo Trinidad, Pedro Gabriel Gamarra Alfonso, María Natalia Piol, Andrea Beatriz Saralegui, Jiam Pires Frigo, and Andréia Cristina Furtado. 2025. "Sustainable Soil Amendment: Effect of Reusing Saturated Dolomitic Calcareous Amendment (DCAS) on Chemical Properties of Two Types of Agricultural Soils" Sustainability 17, no. 19: 8557. https://doi.org/10.3390/su17198557
APA StyleSamudio Legal, L. E., Aguayo Trinidad, S., Gamarra Alfonso, P. G., Piol, M. N., Saralegui, A. B., Frigo, J. P., & Furtado, A. C. (2025). Sustainable Soil Amendment: Effect of Reusing Saturated Dolomitic Calcareous Amendment (DCAS) on Chemical Properties of Two Types of Agricultural Soils. Sustainability, 17(19), 8557. https://doi.org/10.3390/su17198557








