Ammonia Nitrogen Removal and MAP Crystal Morphology Affected by Reaction Conditions in High-Concentration Wastewater
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
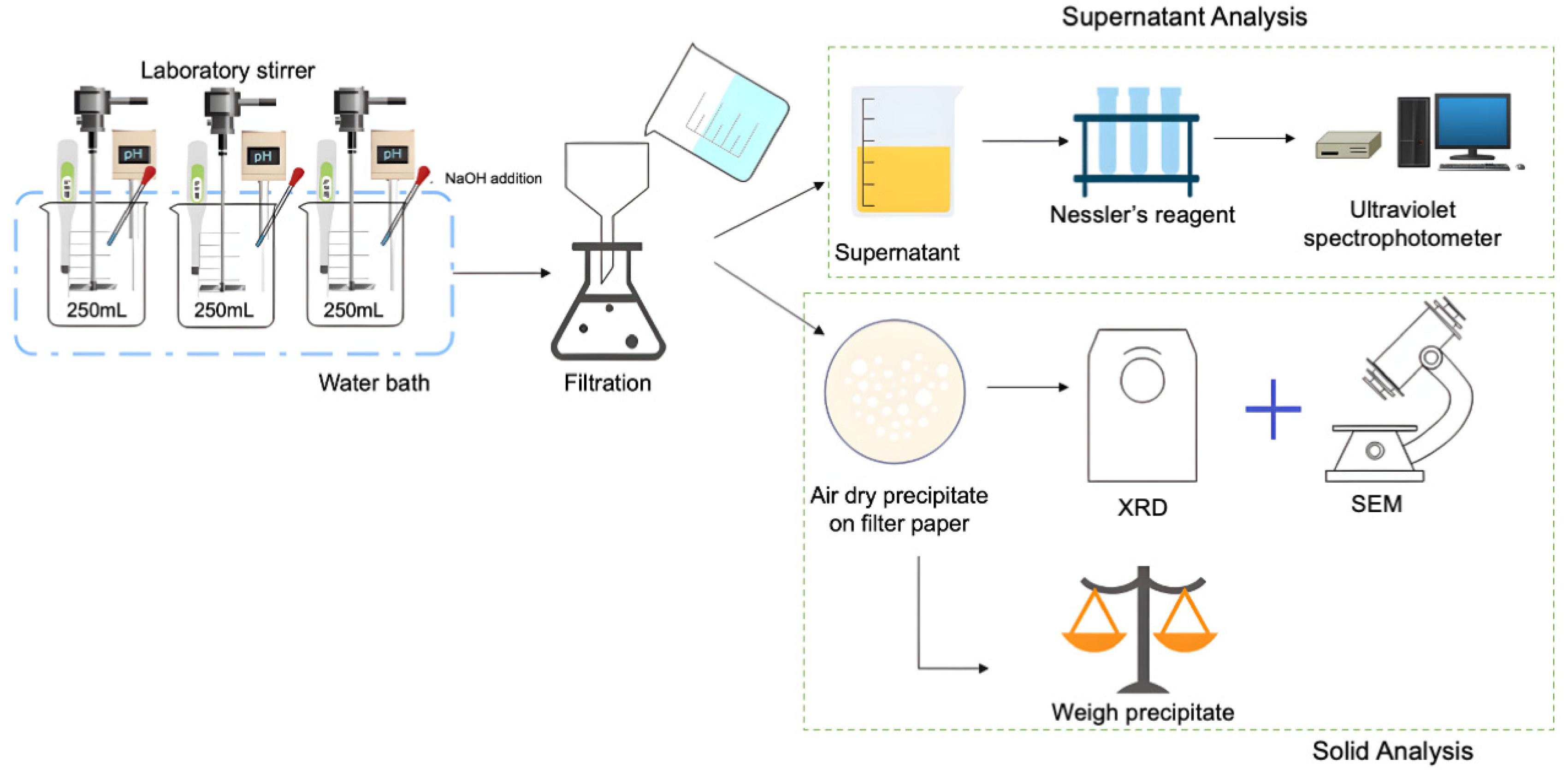
2.2. Experimental Procedures
2.3. Experimental Design
2.3.1. Single-Factor Experimental Design
2.3.2. Machine Learning Models
2.4. Analysis Methods
2.4.1. Nitrogen Removal Efficiency (Φ, %)
2.4.2. Calculation of MAP Purity
2.4.3. Morphological Analysis Methods
2.4.4. Model Development
- 1.
- Dataset Size
- 2.
- Handling of Outliers
- 3.
- Data Splitting Strategy
- 4.
- Feature Selection Rationale
- 5.
- Model hyperparameters
- 6.
- Cross-Validation Procedure
3. Results
3.1. Effects of Parameters on N Removal Efficiency and Precipitate Characteristics
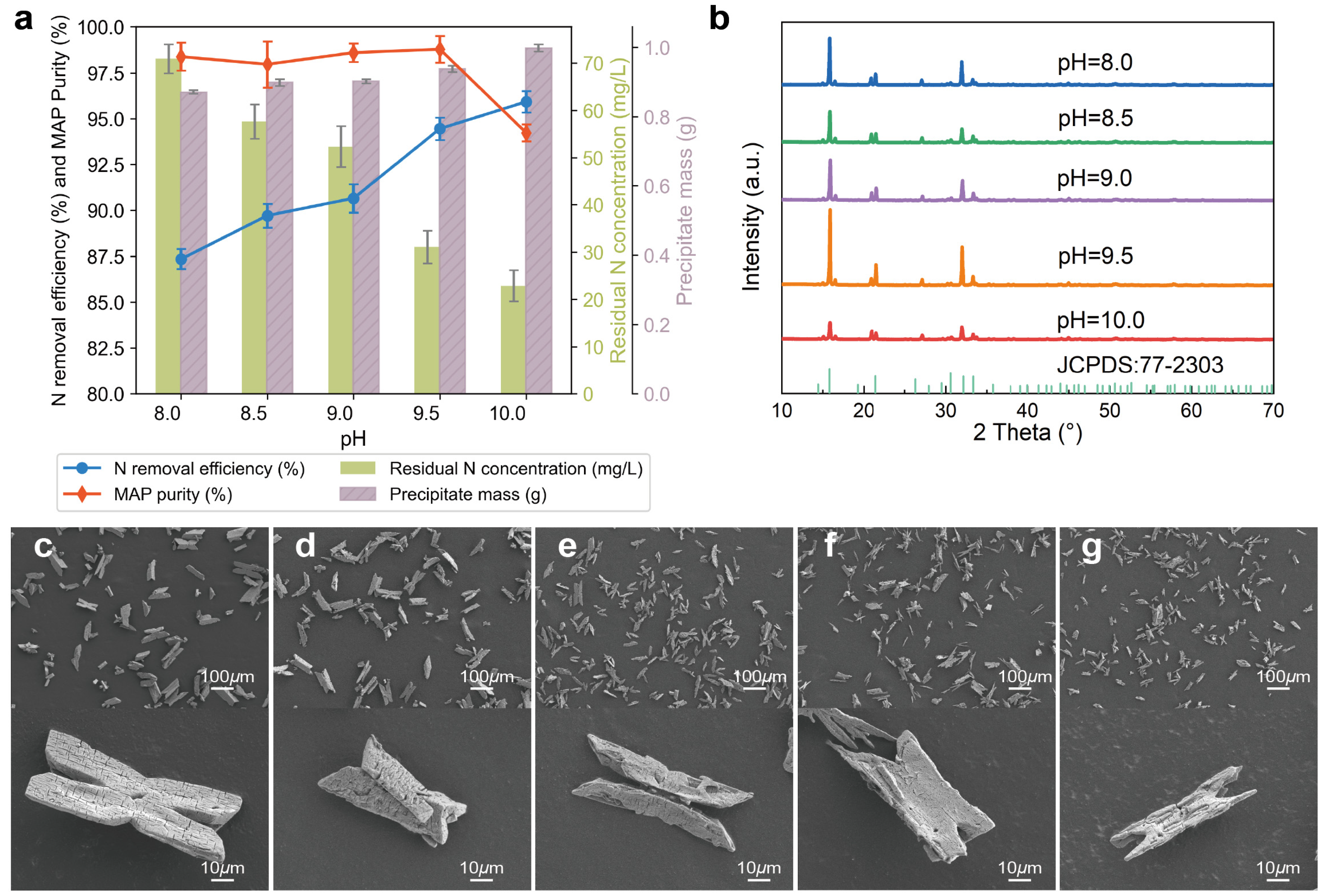
3.1.1. Effects of pH
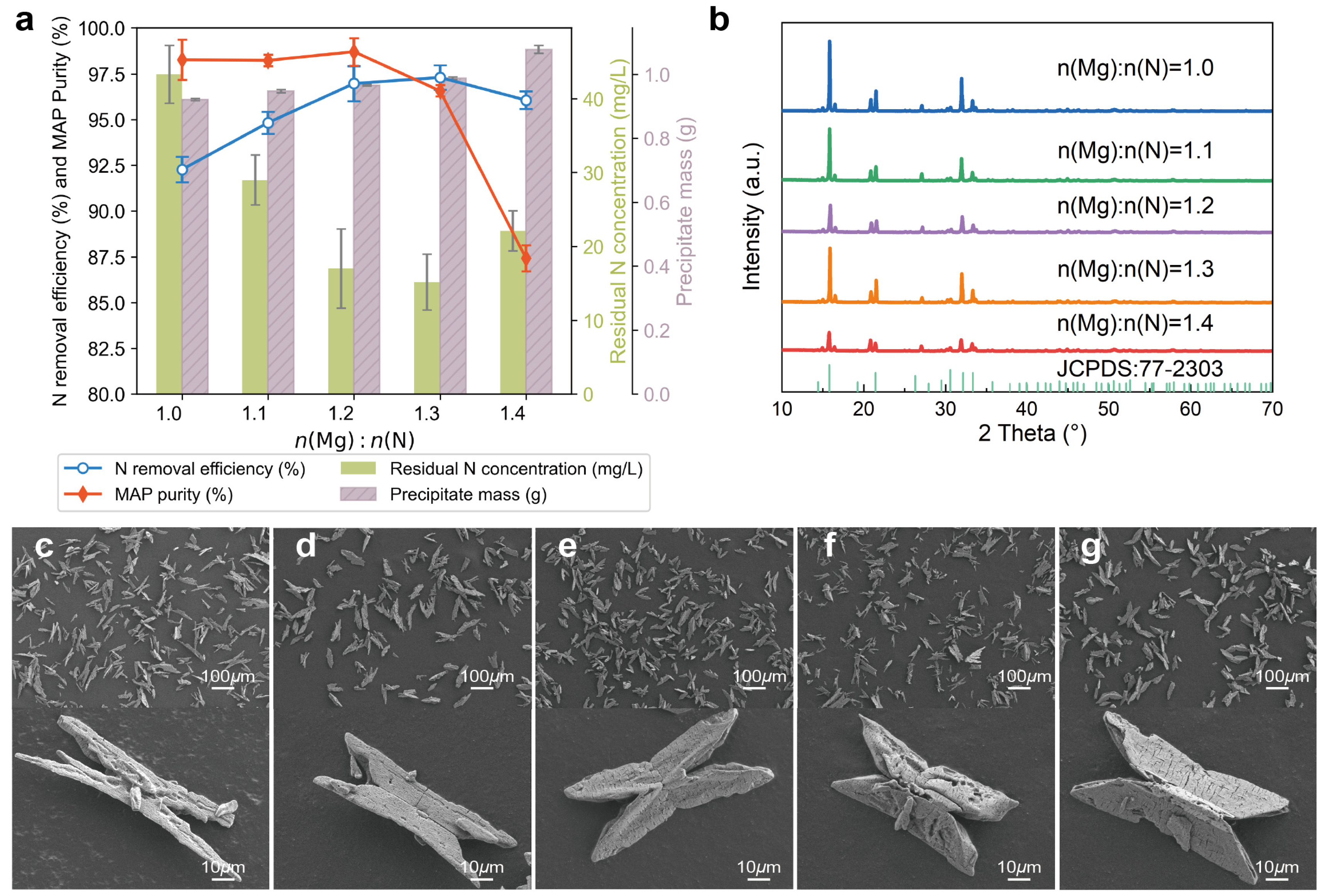
3.1.2. Effects of n(Mg):n(N)
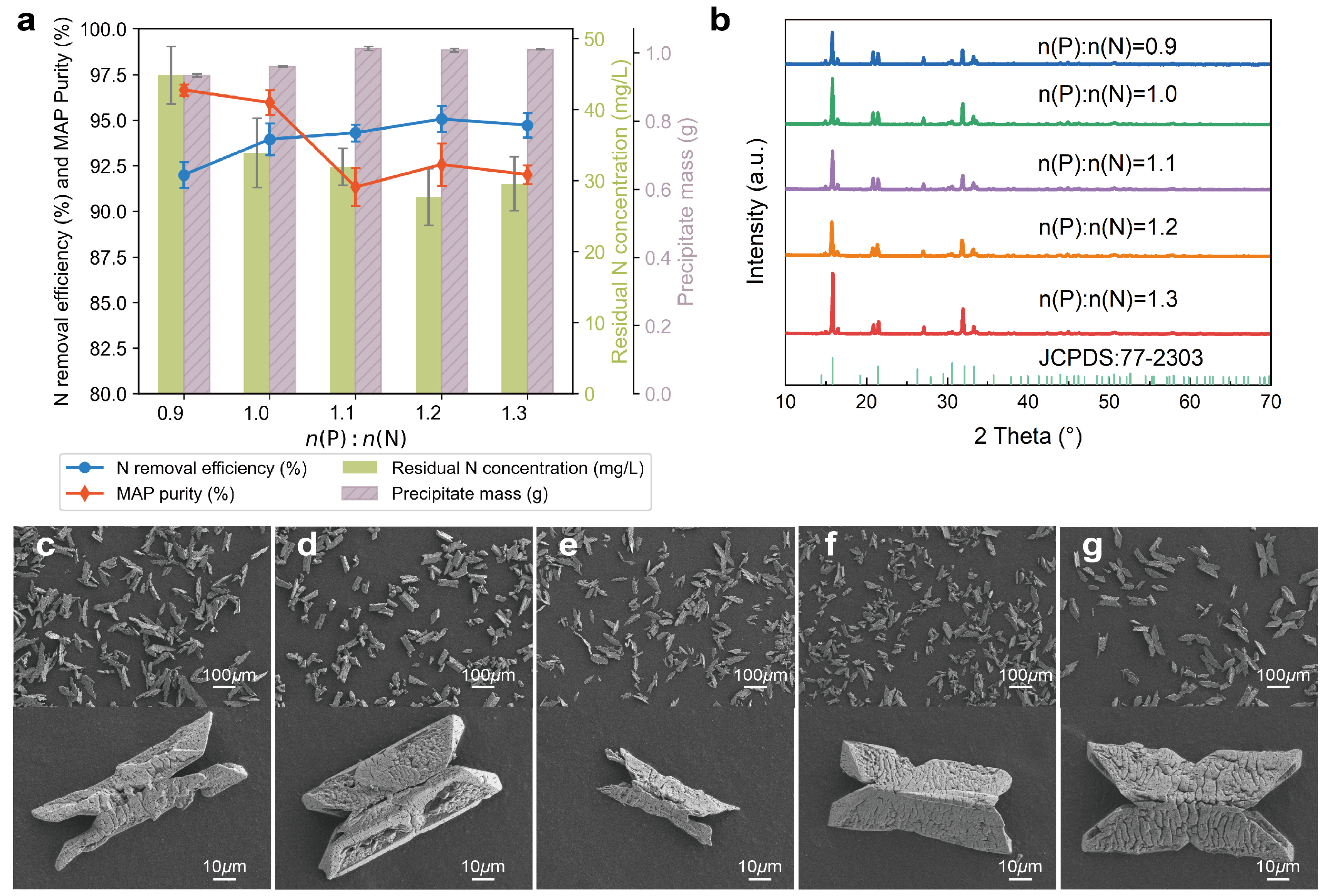
3.1.3. Effects of n(P):n(N)
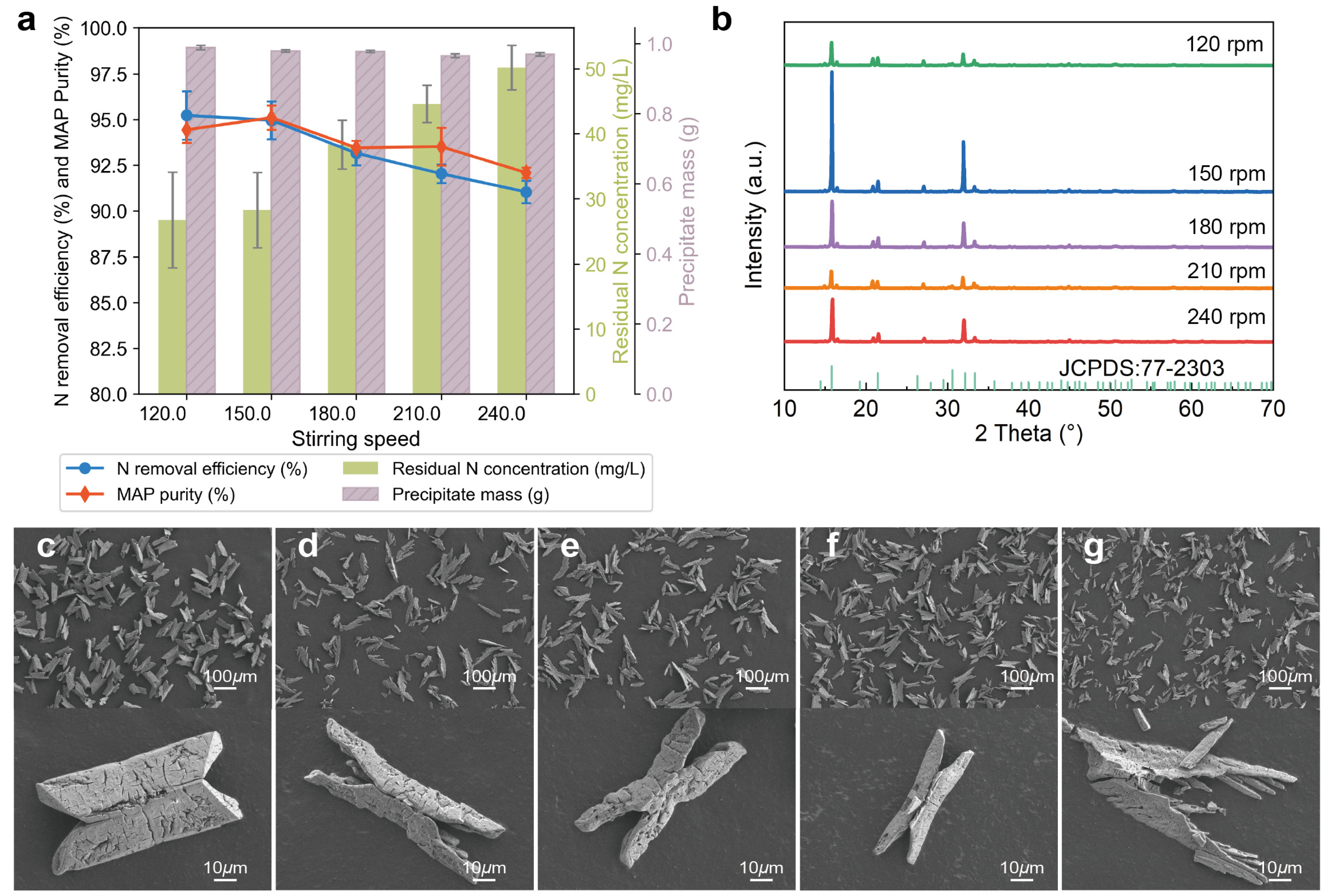
3.1.4. Effects of Stirring Speed
3.1.5. Effects of Reaction Time
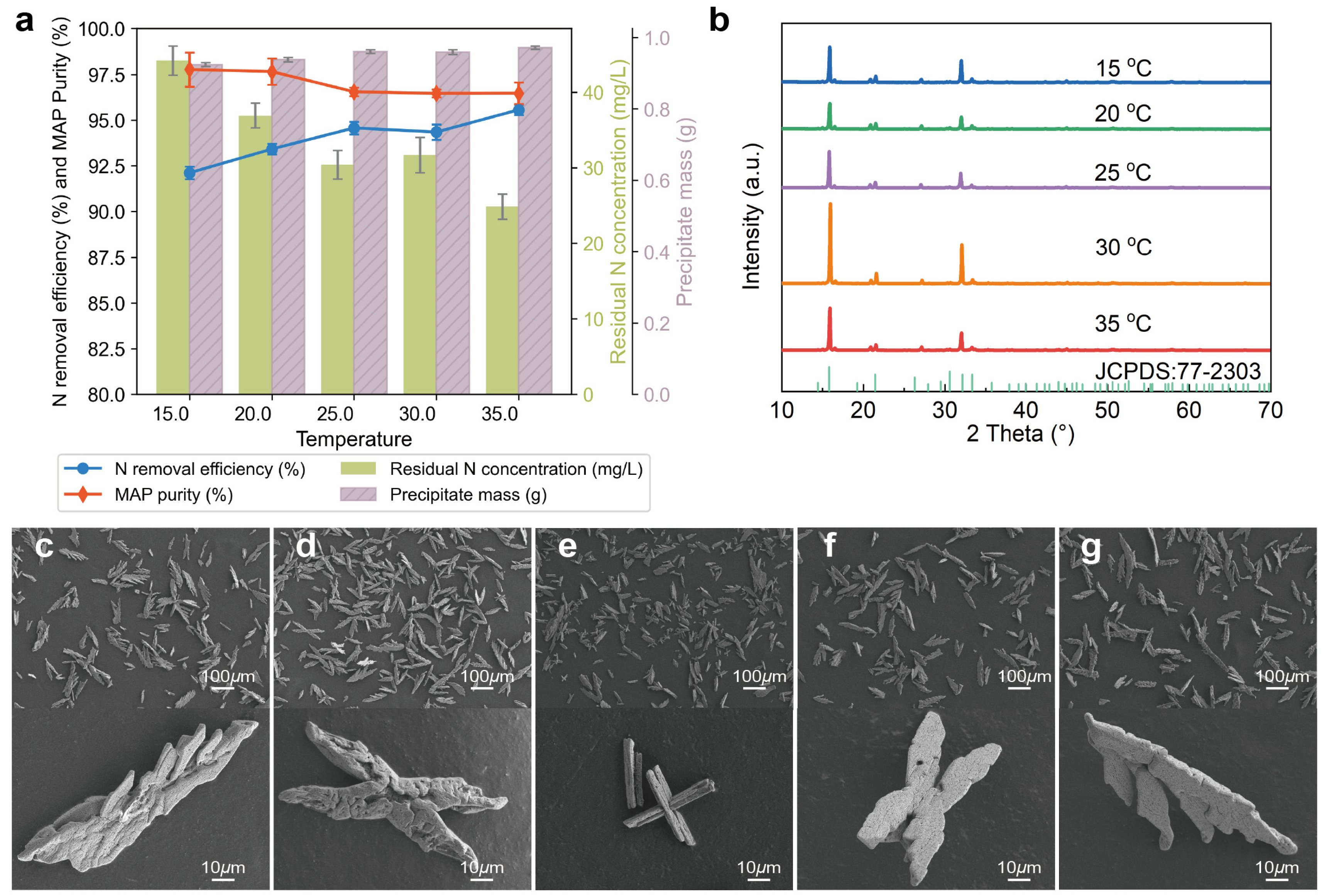
3.1.6. Effects of Temperature
3.2. Regression Model Equations
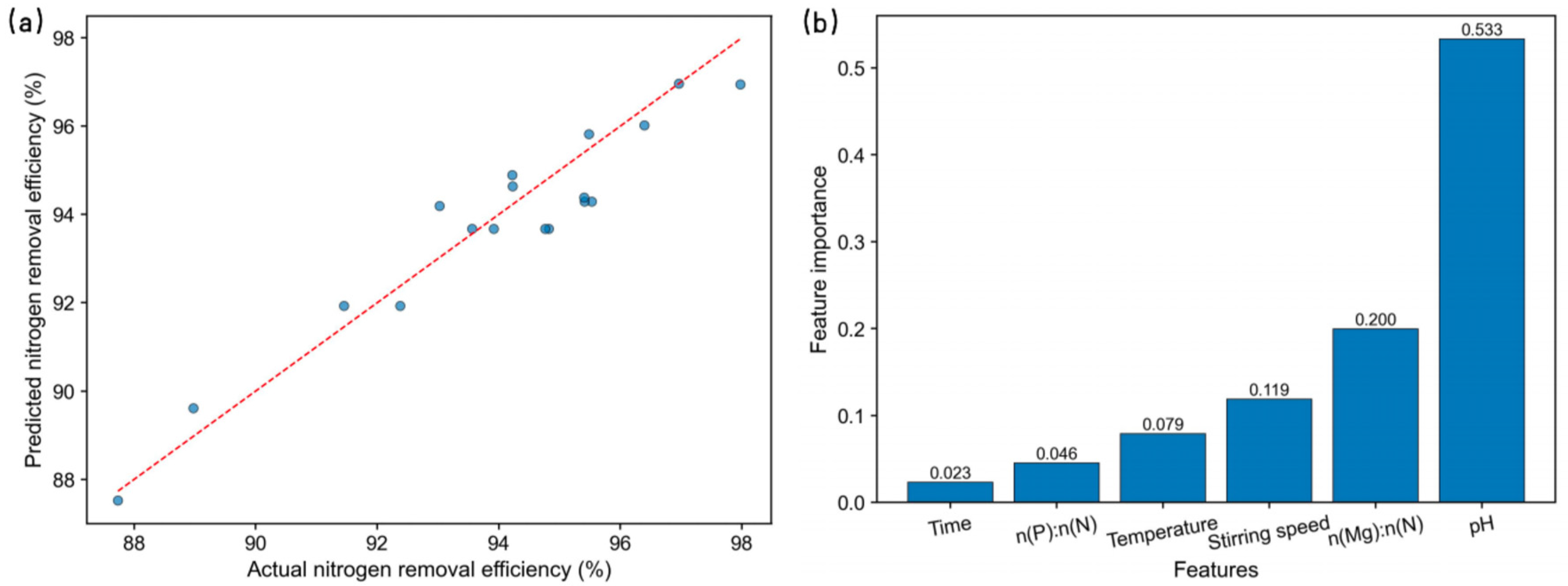
3.3. Factor Importance Analysis for Nitrogen Removal
4. Discussion
4.1. Effects of Operational Parameters on Struvite Formation
4.2. Scalability Considerations for MAP Process in HANW Treatment
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HANW | High-concentration ammonia nitrogen wastewater |
| MAP | Magnesium ammonium phosphate |
| n(Mg):n(N) | Magnesium–nitrogen mole ratio |
| n(P):n(N) | Phosphorus–nitrogen mole ratio |
| N removal (%) | Efficiency of nitrogen removal |
| PMAP (%) | Purity of MAP |
| LR | Linear regression |
| PR | Polynomial regression |
| RFR | Random Forest Regression |
| Coefficient of determination | |
| RMSE | Root mean square error |
| MAE | Mean absolute error |
Appendix A
| Method | Applicable NH4+-N Conc. (mg/L) | Removal Efficiency (%) | Cost-Effectiveness | Advantages | Disadvantages | References |
|---|---|---|---|---|---|---|
| Air Stripping | 500–5000 | 80–95% | Moderate to High | Simple process, easy operation Effective for high concentrations Relatively low equipment investment | Requires pH adjustment to 10.5–11.5 Prone to scaling and clogging Poor performance in winter Cause secondary air pollution | [66,67] |
| Breakpoint Chlorination | 50–1000 | 85–99% | Low to Moderate | Stable and reliable treatment effect Short reaction time Unaffected by temperature | High consumption of chlorinating agents High operating costs Generates by-products like chloramines Uneconomical for high concentrations | [68] |
| Ion Exchange | 20–500 | 90–98% | Moderate | High-quality effluent Simple operation Allows for ammonia nitrogen recovery | Resin is susceptible to fouling/poisoning Requires frequent regeneration Limited application scope High pretreatment requirements | [69,70] |
| Struvite Precipitation (MAP) | 200–8000 | 85–95% | Moderate to High | Recovers nitrogen and phosphorus resources Low sludge production Effective for high concentrations By-product can be used as fertilizer | Requires addition of Mg2+ and PO43− Strict pH control is necessary Potential for scaling issues Higher investment costs | [71,72] |
| Biological Nitrification-Denitrification | 20–200 | 85–95% | High | Low operating costs Environmentally friendly Simultaneously remove COD Mature and established technology | Long start-up time Sensitive to high ammonia concentrations Requires an external carbon source Large footprint | [73,74] |
| Electrochemical Oxidation | 100–2000 | 70–90% | Low | Mild reaction conditions No chemical reagents needed Small footprint High degree of automation | High electricity consumption Electrode passivation is common High investment costs Short electrode lifespan | [75] |
| Membrane Separation | 50–1000 | 90–99% | Low to Moderate | Excellent separation efficiency Small footprint Stable effluent quality Can achieve ammonia recovery | Severe membrane fouling High operating costs Concentrate stream requires further treatment High pretreatment requirements | [76] |
| Catalytic Oxidation | 100–5000 | 80–95% | Moderate | Fast reaction speed High treatment efficiency Strong adaptability Can handle high concentrations | Catalyst is prone to deactivation Relatively high operating costs Complex process Pretreatment is often required | [77,78] |
| Chemical Formula | Mass | Volume | Concentration |
|---|---|---|---|
| NH4Cl | 1.07 g | 500 mL | 560 mg/L |
| MgCl2 | Based on experimental conditions | - | - |
| Na2HPO4 | Based on experimental conditions | - | - |
| NaOH | - | - | 5.0 M |
| KNaC4H4O6·4H2O | 50 g | 100 mL | 500 g/L |
| No. | pH | n(Mg):n(N) | n(P):n(N) | Stirring Speed (rpm) | Time (min) | Temperature (°C) |
|---|---|---|---|---|---|---|
| A1 | 8 | 1 | 1 | 150 | 30 | 30 |
| A2 | 8.5 | 1 | 1 | 150 | 30 | 30 |
| A3 | 9 | 1 | 1 | 150 | 30 | 30 |
| A4 | 9.5 | 1 | 1 | 150 | 30 | 30 |
| A5 | 10 | 1 | 1 | 150 | 30 | 30 |
| B1 | 9.5 | 1 | 1 | 150 | 30 | 30 |
| B2 | 9.5 | 1.1 | 1 | 150 | 30 | 30 |
| B3 | 9.5 | 1.2 | 1 | 150 | 30 | 30 |
| B4 | 9.5 | 1.3 | 1 | 150 | 30 | 30 |
| B5 | 9.5 | 1.4 | 1 | 150 | 30 | 30 |
| C1 | 9.5 | 1 | 0.9 | 150 | 30 | 30 |
| C2 | 9.5 | 1 | 1 | 150 | 30 | 30 |
| C3 | 9.5 | 1 | 1.1 | 150 | 30 | 30 |
| C4 | 9.5 | 1 | 1.2 | 150 | 30 | 30 |
| C5 | 9.5 | 1 | 1.3 | 150 | 30 | 30 |
| D1 | 9.5 | 1 | 1 | 120 | 30 | 30 |
| D2 | 9.5 | 1 | 1 | 150 | 30 | 30 |
| D3 | 9.5 | 1 | 1 | 180 | 30 | 30 |
| D4 | 9.5 | 1 | 1 | 210 | 30 | 30 |
| D5 | 9.5 | 1 | 1 | 240 | 30 | 30 |
| E1 | 9.5 | 1 | 1 | 150 | 20 | 30 |
| E2 | 9.5 | 1 | 1 | 150 | 30 | 30 |
| E3 | 9.5 | 1 | 1 | 150 | 40 | 30 |
| E4 | 9.5 | 1 | 1 | 150 | 50 | 30 |
| E5 | 9.5 | 1 | 1 | 150 | 60 | 30 |
| F1 | 9.5 | 1 | 1 | 150 | 30 | 15 |
| F2 | 9.5 | 1 | 1 | 150 | 30 | 20 |
| F3 | 9.5 | 1 | 1 | 150 | 30 | 25 |
| F4 | 9.5 | 1 | 1 | 150 | 30 | 30 |
| F5 | 9.5 | 1 | 1 | 150 | 30 | 35 |
| No. | N Removal (%)-1 | N Removal (%)-2 | N Removal (%)-3 |
|---|---|---|---|
| A1 | 87.7332 | 86.7154 | 87.5769 |
| A2 | 90.0473 | 88.9712 | 90.1234 |
| A3 | 91.0124 | 91.1908 | 89.7693 |
| A4 | 95.1281 | 93.9163 | 94.3044 |
| A5 | 95.4836 | 95.6868 | 96.5900 |
| B1 | 91.5708 | 92.2728 | 92.9749 |
| B2 | 94.2192 | 94.8262 | 95.4331 |
| B3 | 96.0131 | 96.9656 | 97.9180 |
| B4 | 96.6214 | 97.2976 | 97.9737 |
| B5 | 95.5013 | 96.3917 | 96.2822 |
| C1 | 91.2695 | 91.9950 | 92.7205 |
| C2 | 93.0769 | 93.9505 | 94.8242 |
| C3 | 93.8342 | 94.3003 | 94.7663 |
| C4 | 95.4077 | 95.5266 | 94.2454 |
| C5 | 94.0481 | 94.7256 | 95.4032 |
| D1 | 93.9089 | 95.2225 | 96.5361 |
| D2 | 93.9192 | 94.9542 | 95.9892 |
| D3 | 92.3916 | 93.4934 | 93.5953 |
| D4 | 92.2981 | 92.3742 | 91.4503 |
| D5 | 90.4435 | 91.0191 | 91.6715 |
| E1 | 92.1112 | 92.6441 | 93.1770 |
| E2 | 92.0842 | 92.8235 | 93.5627 |
| E3 | 93.1244 | 93.7442 | 94.3640 |
| E4 | 94.3803 | 93.0267 | 94.0731 |
| E5 | 94.7114 | 93.0746 | 94.4377 |
| F1 | 92.4551 | 92.1105 | 91.7658 |
| F2 | 93.2710 | 93.7442 | 93.2174 |
| F3 | 94.2298 | 94.5693 | 94.9087 |
| F4 | 93.9234 | 94.3421 | 94.7608 |
| F5 | 95.4491 | 95.8965 | 95.3421 |
References
- Liu, X.; Yue, F.-J.; Li, L.; Zhou, F.; Wen, H.; Yan, Z.; Wang, L.; Wong, W.W.; Liu, C.-Q.; Li, S.-L. Chronic nitrogen legacy in the aquifers of China. Commun. Earth Environ. 2025, 6, 58. [Google Scholar] [CrossRef]
- Yi, H.; Gu, W.; Wang, Q.; Liu, B.; Zhang, B. Cost of raising discharge standards: A plant-by-plant assessment from wastewater sector in China. J. Environ. Manag. 2022, 308, 114642. [Google Scholar] [CrossRef]
- Gonzalez-Salgado, I.; Bounouba, M.; Dubos, S.; Mengelle, E.; Guigui, C.; Sperandio, M. Influence of feed salinity on ammonia recovery from high-strength effluents in transmembrane chemical absorption process. J. Membr. Sci. 2023, 687, 122086. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, H.; Chen, Z.; Hu, H.; Deng, C.; Wang, X. The benefits of autotrophic nitrogen removal from high concentration of urea wastewater through a process of urea hydrolysis and partial nitritation in sequencing batch reactor. J. Environ. Manag. 2021, 292, 112762. [Google Scholar] [CrossRef]
- Liu, J.; Ren, N.; Qu, C.; Lu, S.; Xiang, Y.; Liang, D. Recent Advances in the Reactor Design for Industrial Wastewater Treatment by Electro-Oxidation Process. Water 2022, 14, 3711. [Google Scholar] [CrossRef]
- Galeano, M.B.; Sulonen, M.; Ul, Z.; Baeza, M.; Baeza, J.A.; Guisasola, A. Bioelectrochemical ammonium recovery from wastewater: A review. Chem. Eng. J. 2023, 472, 144855. [Google Scholar] [CrossRef]
- Ma, H.; Chen, G.; Huang, F.; Li, Y.; Zhang, L.; Jin, Y. Catalytic ozonation of ammonia nitrogen removal in wastewater: A review. J. Water Process Eng. 2023, 52, 103542. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Chen, Z.; Hu, C.; Qu, J. Recovering nutrients and unblocking the cake layer of an electrochemical anaerobic membrane bioreactor. Nat. Commun. 2024, 15, 9111. [Google Scholar] [CrossRef]
- Zheng, M.; Hu, Z.; Liu, T.; Sperandio, M.; Volcke, E.I.P.; Wang, Z.; Hao, X.; Duan, H.; Vlaeminck, S.E.; Xu, K.; et al. Pathways to advanced resource recovery from sewage. Nat. Sustain. 2024, 7, 1395–1404. [Google Scholar] [CrossRef]
- Ronteltap, M.; Maurer, M.; Gujer, W. The behaviour of pharmaceuticals and heavy metals during struvite precipitation in urine. Water Res. 2007, 41, 1859–1868. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Lee, W.; Choe, J.K.; Choi, Y. Non-evaporative solid phase ammonium sulfate separation from ammonia-stripped sulfuric acid solution by solvent-driven fractional crystallization. Sep. Purif. Technol. 2023, 318, 123869. [Google Scholar] [CrossRef]
- Yellezuome, D.; Zhu, X.; Wang, Z.; Liu, R. Mitigation of ammonia inhibition in anaerobic digestion of nitrogen-rich substrates for biogas production by ammonia stripping: A review. Renew. Sustain. Energy Rev. 2022, 157, 112043. [Google Scholar] [CrossRef]
- Yuan, M.-H.; Trinh, M.V.; Chen, Y.-H.; Lu, Y.-J.; Wang, L.-P.; Cheng, S.; Li, Z.; Santikunaporn, M.; Asavatesanupap, C. Steam Stripping for Recovery of Ammonia from Wastewater Using a High-Gravity Rotating Packed Bed. Environments 2024, 11, 206. [Google Scholar] [CrossRef]
- Zhan, L.; Hou, D.; Chen, H.; Wang, Y.; Zheng, S.; Li, Z.; Wu, H.; Chen, H.; Ma, X.; Yang, L. Occurrence states and migration of water during the evaporation of desulfurization wastewater. Desalination 2024, 578, 117435. [Google Scholar] [CrossRef]
- Zhou, S.; Dong, M.; Ding, X.; Xue, X.; Yang, H. Application of RSM to optimize the recovery of ammonia nitrogen from high chromium effluent produced in vanadium industry using struvite precipitation. J. Environ. Chem. Eng. 2021, 9, 106318. [Google Scholar] [CrossRef]
- Farghali, M.; Chen, Z.; Osman, A.I.; Ali, I.M.; Hassan, D.; Ihara, I.; Rooney, D.W.; Yap, P.-S. Strategies for ammonia recovery from wastewater: A review. Environ. Chem. Lett. 2024, 22, 2699–2751. [Google Scholar] [CrossRef]
- Kirinovic, E.; Leichtfuss, A.R.; Navizaga, C.; Zhang, H.; Schuttlefield Christus, J.D.; Baltrusaitis, J. Spectroscopic and Microscopic Identification of the Reaction Products and Intermediates during the Struvite (MgNH4PO4·6H2O) Formation from Magnesium Oxide (MgO) and Magnesium Carbonate (MgCO3) Microparticles. ACS Sustain. Chem. Eng. 2017, 5, 1567–1577. [Google Scholar] [CrossRef]
- Huang, Y.; Cai, J.; Ye, Z.L.; Lin, L.; Hong, Z. Morphological crystal adsorbing tetracyclines and its interaction with magnesium ion in the process of struvite crystallization by using synthetic wastewater. Water Res. 2022, 215, 118253. [Google Scholar] [CrossRef]
- Iftikhar, A.; Tao, W. Recovery of struvite for organic production: Mineral-based magnesium supplementation and pH elevation. J. Clean. Prod. 2024, 452, 142244. [Google Scholar] [CrossRef]
- Chen, Y.; Long, J.; Chen, S.; Xie, Y.; Xu, Z.; Ning, Z.; Zhang, G.; Xiao, T.; Yu, M.; Ke, Y.; et al. Multi-step purification of electrolytic manganese residue leachate using hydroxide sedimentation, struvite precipitation, chlorination and coagulation: Advanced removal of manganese, ammonium, and phosphate. Sci. Total Environ. 2022, 805, 150237. [Google Scholar] [CrossRef]
- Wang, G.; Diao, J.; Liu, L.; Li, M.; Li, H.; Li, G.; Xie, B. Highly efficient utilization of hazardous vanadium extraction tailings containing high chromium concentrations by carbothermic reduction. J. Clean. Prod. 2019, 237, 117832. [Google Scholar] [CrossRef]
- Sena, M.; Seib, M.; Noguera, D.R.; Hicks, A. Environmental impacts of phosphorus recovery through struvite precipitation in wastewater treatment. J. Clean. Prod. 2021, 280, 124222. [Google Scholar] [CrossRef]
- Shaddel, S.; Ucar, S.; Andreassen, J.-P.; Østerhus, S.W. Engineering of struvite crystals by regulating supersaturation—Correlation with phosphorus recovery, crystal morphology and process efficiency. J. Environ. Chem. Eng. 2019, 7, 102918. [Google Scholar] [CrossRef]
- Corona, F.; Hidalgo, D.; Martin-Marroquin, J.M.; Antolin, G. Study of the influence of the reaction parameters on nutrients recovering from digestate by struvite crystallisation. Environ. Sci. Pollut. Res. Int. 2021, 28, 24362–24374. [Google Scholar] [CrossRef]
- Ronteltap, M.; Maurer, M.; Hausherr, R.; Gujer, W. Struvite precipitation from urine—Influencing factors on particle size. Water Res. 2010, 44, 2038–2046. [Google Scholar] [CrossRef]
- Jia, G. Nutrient Removal and Recovery by the Precipitation of Magnesium Ammonium Phosphate; The university of Adelaide: Adelaide, Australia, 2014. [Google Scholar]
- Luo, Z.; Wang, D.; Yang, J.; Huang, H.; Su, G. Nitrogen removal from digested piggery wastewater using fermented superphosphate within the pretreatment stage and an MAP fertilizer pot test. J. Clean. Prod. 2019, 212, 372–380. [Google Scholar] [CrossRef]
- Tünay, O.; Kabdasli, I.; Orhon, D.; Ates, E. Characterization and pollution profile of leather tanning industry in Turkey. Water Sci. Technol. 1995, 32, 1–9. [Google Scholar] [CrossRef]
- Draper, N.R.; Smith, H. Applied Regression Analysis; McGraw-Hill. Inc.: New York, NY, USA, 1998; Volume 326. [Google Scholar]
- Ambler, G.; Royston, P. Fractional polynomial model selection procedures: Investigation of type i error rate. J. Stat. Comput. Simul. 2001, 69, 89–108. [Google Scholar] [CrossRef]
- Hoerl, A.E.; Kennard, R.W. Ridge regression: Biased estimation for nonorthogonal problems. Technometrics 1970, 12, 55–67. [Google Scholar] [CrossRef]
- Tibshirani, R. Regression Shrinkage and Selection Via the Lasso. J. R. Stat. Soc. Ser. B Stat. Methodol. 1996, 58, 267–288. [Google Scholar] [CrossRef]
- Biau, G. Analysis of a random forests model. J. Mach. Learn. Res. 2012, 13, 1063–1095. [Google Scholar]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef]
- Friedman, J.H. Greedy function approximation: A gradient boosting machine. Ann. Stat. 2001, 29, 1189–1232. [Google Scholar] [CrossRef]
- Racz, A.; Bajusz, D.; Heberger, K. Effect of Dataset Size and Train/Test Split Ratios in QSAR/QSPR Multiclass Classification. Molecules 2021, 26, 1111. [Google Scholar] [CrossRef]
- Leng, L.; Kang, B.; Xu, D.; Kapusta, K.; Xiong, T.; Xu, Z.; Fan, L.; Liu, T.; Peng, H.; Li, H. Machine-learning-aided prediction and optimization of struvite recovery from synthetic wastewater. J. Water Process Eng. 2024, 58, 104896. [Google Scholar] [CrossRef]
- Burzykowski, T.; Geubbelmans, M.; Rousseau, A.J.; Valkenborg, D. Validation of machine learning algorithms. Am. J. Orthod. Dentofac. Orthop. 2023, 164, 295–297. [Google Scholar] [CrossRef]
- Yadav, S.; Shukla, S. Analysis of k-fold cross-validation over hold-out validation on colossal datasets for quality classification. In Proceedings of the 2016 IEEE 6th International Conference on Advanced Computing (IACC), Bhubaneswar, India, 27–28 February 2016; pp. 78–83. [Google Scholar] [CrossRef]
- Wu, H.; Hao, B.; Zhou, Q.; Xu, K.; Cai, Y.; Liu, G. Contribution of various categories of environmental factors to sediment nitrogen-removal in a low C/N ratio river. Ecol. Eng. 2021, 159, 106121. [Google Scholar] [CrossRef]
- Qiu, H.; Geng, J.; Ren, H.; Ding, L.; Xu, K.; Zhang, Y. Aquatic transformation of phosphite under natural sunlight and simulated irradiation. Water Res. 2017, 109, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Du, H.; Ren, X.; Pan, D.; An, Y.; Wei, Y.; Liu, X.; Hou, L.; Liu, B.; Liu, M.; Guo, Z. Effect of phosphating solution pH value on the formation of phosphate conversion coatings for corrosion behaviors on AZ91D. Adv. Compos. Hybrid. Mater. 2021, 4, 401–414. [Google Scholar] [CrossRef]
- Qiu, L.; Shi, L.; Liu, Z.; Xie, K.; Wang, J.; Zhang, S.; Song, Q.; Lu, L. Effect of power ultrasound on crystallization characteristics of magnesium ammonium phosphate. Ultrason. Sonochem. 2017, 36, 123–128. [Google Scholar] [CrossRef] [PubMed]
- Quintana, M.; Sánchez, E.; Colmenarejo, M.F.; Barrera, J.; García, G.; Borja, R. Kinetics of phosphorus removal and struvite formation by the utilization of by-product of magnesium oxide production. Chem. Eng. J. 2005, 111, 45–52. [Google Scholar] [CrossRef]
- Polat, S.; Sayan, P. Application of response surface methodology with a Box–Behnken design for struvite precipitation. Adv. Powder Technol. 2019, 30, 2396–2407. [Google Scholar] [CrossRef]
- Wei, L.; Zhang, T.; Hong, T.; Dong, Y.; Ji, D.; Luo, L.; Li, R.; Li, Z.; Tang, Y. Revealing and quantifying the effect of cattiite coprecipitation on the purity of K-struvite in aqueous solution. J. Environ. Chem. Eng. 2023, 11, 109764. [Google Scholar] [CrossRef]
- Le Corre, K.S.; Valsami-Jones, E.; Hobbs, P.; Parsons, S.A. Phosphorus Recovery from Wastewater by Struvite Crystallization: A Review. Crit. Rev. Environ. Sci. Technol. 2009, 39, 433–477. [Google Scholar] [CrossRef]
- Yetilmezsoy, K.; Sapci-Zengin, Z. Recovery of ammonium nitrogen from the effluent of UASB treating poultry manure wastewater by MAP precipitation as a slow release fertilizer. J. Hazard. Mater. 2009, 166, 260–269. [Google Scholar] [CrossRef]
- Ronteltap, M.; Maurer, M.; Gujer, W. Struvite precipitation thermodynamics in source-separated urine. Water Res. 2007, 41, 977–984. [Google Scholar] [CrossRef]
- Wang, Y.; Mou, J.; Liu, X.; Chang, J. Phosphorus recovery from wastewater by struvite in response to initial nutrients concentration and nitrogen/phosphorus molar ratio. Sci. Total Environ. 2021, 789, 147970. [Google Scholar] [CrossRef]
- Nunes, A.P.P.; Santos, C.F.; Guelfi, D. Interfaces between biodegradable organic matrices coating and MAP fertilizer for improve use efficiency. Sci. Total Environ. 2022, 804, 149896. [Google Scholar] [CrossRef]
- Chai, L.-y.; Peng, C.; Min, X.-b.; Tang, C.-j.; Song, Y.-x.; Zhang, Y.; Zhang, J.; Ali, M. Two-sectional struvite formation process for enhanced treatment of copper–ammonia complex wastewater. Trans. Nonferrous Met. Soc. China 2017, 27, 457–466. [Google Scholar] [CrossRef]
- Ariyanto, E.; Sen, T.K.; Ang, H.M. The influence of various physico-chemical process parameters on kinetics and growth mechanism of struvite crystallisation. Adv. Powder Technol. 2014, 25, 682–694. [Google Scholar] [CrossRef]
- Hutnik, N.; Kozik, A.; Mazienczuk, A.; Piotrowski, K.; Wierzbowska, B.; Matynia, A. Phosphates (V) recovery from phosphorus mineral fertilizers industry wastewater by continuous struvite reaction crystallization process. Water Res. 2013, 47, 3635–3643. [Google Scholar] [CrossRef]
- Zhao, T.L.; Li, H.; Jiang, H.F.; Yao, Q.Z.; Huang, Y.; Zhou, G.T. Morphogenesis and evolution mechanisms of bacterially-induced struvite. Sci. Rep. 2021, 11, 170. [Google Scholar] [CrossRef]
- Kozik, A.; Hutnik, N.; Piotrowski, K.; Matynia, A. Continuous reaction crystallization of struvite from diluted aqueous solution of phosphate(V) ions in the presence of magnesium ions excess. Chem. Eng. Res. Des. 2014, 92, 481–490. [Google Scholar] [CrossRef]
- Krishnamoorthy, N.; Dey, B.; Unpaprom, Y.; Ramaraj, R.; Maniam, G.P.; Govindan, N.; Jayaraman, S.; Arunachalam, T.; Paramasivan, B. Engineering principles and process designs for phosphorus recovery as struvite: A comprehensive review. J. Environ. Chem. Eng. 2021, 9, 105579. [Google Scholar] [CrossRef]
- Bah, A.T.; Shen, Z.; Yan, J.; Li, F. Phosphorous recovery from water via batch adsorption enrichment combined with struvite crystallization in a fluidized bed reactor. J. Environ. Chem. Eng. 2023, 11, 110180. [Google Scholar] [CrossRef]
- Sugiyama, S.; Yokoyama, M.; Ishizuka, H.; Sotowa, K.; Tomida, T.; Shigemoto, N. Removal of aqueous ammonium with magnesium phosphates obtained from the ammonium-elimination of magnesium ammonium phosphate. J. Colloid. Interface Sci. 2005, 292, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Yu, R.; Geng, J.; Ren, H.; Wang, Y.; Xu, K. Struvite pyrolysate recycling combined with dry pyrolysis for ammonium removal from wastewater. Bioresour. Technol. 2013, 132, 154–159. [Google Scholar] [CrossRef]
- Wang, D.; Chen, J.; Li, Y.; Feng, S. Solid State Chemistry and Inorganic Synthetic Chemistry—Novel Structures and Accurate Syntheses of Inorganic Materials. Small Methods 2025, 9, e2401491. [Google Scholar] [CrossRef] [PubMed]
- Whitehead, C.B.; Watzky, M.A.; Finke, R.G. “Burst Nucleation” vs. Autocatalytic, “Burst” Growth in Near-Monodisperse Particle-Formation Reactions. J. Phys. Chem. C 2020, 124, 24543–24554. [Google Scholar] [CrossRef]
- Barbosa, S.G.; Peixoto, L.; Meulman, B.; Alves, M.M.; Pereira, M.A. A design of experiments to assess phosphorous removal and crystal properties in struvite precipitation of source separated urine using different Mg sources. Chem. Eng. J. 2016, 298, 146–153. [Google Scholar] [CrossRef]
- Mugwili, M.E.; Waanders, F.B.; Masindi, V.; Fosso-Kankeu, E. Effective removal of ammonia from aqueous solution through struvite synthesis and breakpoint chlorination: Insights into the synergistic effects of the hybrid system. J. Environ. Manag. 2023, 334, 117506. [Google Scholar] [CrossRef]
- Rahman, M.M.; Salleh, M.A.M.; Rashid, U.; Ahsan, A.; Hossain, M.M.; Ra, C.S. Production of slow release crystal fertilizer from wastewaters through struvite crystallization—A review. Arab. J. Chem. 2014, 7, 139–155. [Google Scholar] [CrossRef]
- Hasar, H.; Unsal, S.A.; Ipek, U.; Karatas, S.; Cinar, O.; Yaman, C.; Kinaci, C. Stripping/flocculation/membrane bioreactor/reverse osmosis treatment of municipal landfill leachate. J. Hazard. Mater. 2009, 171, 309–317. [Google Scholar] [CrossRef]
- Ukwuani, A.T.; Tao, W. Developing a vacuum thermal stripping—Acid absorption process for ammonia recovery from anaerobic digester effluent. Water Res. 2016, 106, 108–115. [Google Scholar] [CrossRef]
- Pressley, T.A.; Bishop, D.F.; Roan, S.G. Ammonia-nitrogen removal by breakpoint chlorination. Environ. Sci. Technol. 2002, 6, 622–628. [Google Scholar] [CrossRef]
- Maghfiroh, M.; Park, N.; Chang, H.; Lim, H.; Kim, W. Ammonium removal and recovery from greywater using the combination of ion exchange and air stripping: The utilization of NaOH for the regeneration of natural zeolites. J. Water Process Eng. 2023, 52, 103581. [Google Scholar] [CrossRef]
- Arias-Paić, M.S.; Korak, J.A. Forward Osmosis for Ion Exchange Waste Brine Management. Environ. Sci. Technol. Lett. 2020, 7, 111–117. [Google Scholar] [CrossRef]
- Song, W.; Li, Z.; Liu, F.; Ding, Y.; Qi, P.; You, H.; Jin, C. Effective removal of ammonia nitrogen from waste seawater using crystal seed enhanced struvite precipitation technology with response surface methodology for process optimization. Environ. Sci. Pollut. Res. Int. 2018, 25, 628–638. [Google Scholar] [CrossRef] [PubMed]
- Tansel, B.; Lunn, G.; Monje, O. Struvite formation and decomposition characteristics for ammonia and phosphorus recovery: A review of magnesium-ammonia-phosphate interactions. Chemosphere 2018, 194, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Miao, L.; Yang, G.; Tao, T.; Peng, Y. Recent advances in nitrogen removal from landfill leachate using biological treatments—A review. J. Environ. Manag. 2019, 235, 178–185. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, G.; Jeison, D.; Chamy, R. Nitrification with high nitrite accumulation for the treatment of wastewater with high ammonia concentration. Water Res. 2003, 37, 1371–1377. [Google Scholar] [CrossRef]
- Li, L.; Liu, Y. Ammonia removal in electrochemical oxidation: Mechanism and pseudo-kinetics. J. Hazard. Mater. 2009, 161, 1010–1016. [Google Scholar] [CrossRef]
- Darestani, M.; Haigh, V.; Couperthwaite, S.J.; Millar, G.J.; Nghiem, L.D. Hollow fibre membrane contactors for ammonia recovery: Current status and future developments. J. Environ. Chem. Eng. 2017, 5, 1349–1359. [Google Scholar] [CrossRef]
- Huang, T.L.; Macinnes, J.M.; Cliffe, K.R. Nitrogen removal from wastewater by a catalytic oxidation method. Water Res. 2001, 35, 2113–2120. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Ren, D.; Harle, G.; Qin, Q.; Guo, L.; Zheng, T.; Yin, X.; Du, J.; Zhao, Y. Ammonia removal in selective catalytic oxidation: Influence of catalyst structure on the nitrogen selectivity. J. Hazard. Mater. 2021, 416, 125782. [Google Scholar] [CrossRef] [PubMed]

| Model | Empirical Regression Equation |
|---|---|
| Linear Regression | y = 39.7778 + 4.4016x1 + 8.8262x2 + 4.9330x3 − 0.0312x4 + 0.0118x5 + 0.0939x6 |
| Ridge Regression | y = 50.8501 + 4.1982x1 + 3.7786x2 + 1.2674x3 − 0.0341x4 + 0.0033x5 + 0.1108x6 |
| Lasso Regression | y = 97.7371 + 0.0000x1 + 0.0000x2 + 0.0000x3 − 0.0284x4 + 0.0000x5 + 0.0103x6 |
| Model | RMSE | MAE | ||
|---|---|---|---|---|
| Linear Regression | 0.890061 | 0.838068 | 0.736886 | 0.830094 |
| Polynomial Regression | 0.890863 | 0.835005 | 0.730308 | 0.831334 |
| Ridge Regression | 0.889255 | 0.841135 | 0.735733 | 0.828849 |
| Lasso Regression | 0.865746 | 0.926121 | 0.767374 | 0.792516 |
| Random Forest | 0.910994 | 0.754074 | 0.651988 | 0.862445 |
| Gradient Boosting | 0.889809 | 0.839027 | 0.720085 | 0.829705 |
| Optimized RF | 0.908239 | 0.765655 | 0.650953 | 0.858187 |
| Parameter | Experimental Optimal Conditions | Model-Predicted Optimal Conditions |
|---|---|---|
| Experiment No. | 27 | — |
| pH | 9.5 | 9.8 |
| n(Mg):n(N) | 1.3 | 1.2 |
| n(P):n(N) | 1.0 | 1.0 |
| Stirring speed (rpm) | 150 | 150 |
| Reaction time (min) | 30 | 30 |
| Temperature (°C) | 30 | 25 |
| N removal (%) | 97.97 | 96.98 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, S.; Xie, Y.; Gao, H.; Xue, X.; Zhou, H.; Dong, M.; Sun, X.; Chen, X. Ammonia Nitrogen Removal and MAP Crystal Morphology Affected by Reaction Conditions in High-Concentration Wastewater. Sustainability 2025, 17, 8550. https://doi.org/10.3390/su17198550
Zhou S, Xie Y, Gao H, Xue X, Zhou H, Dong M, Sun X, Chen X. Ammonia Nitrogen Removal and MAP Crystal Morphology Affected by Reaction Conditions in High-Concentration Wastewater. Sustainability. 2025; 17(19):8550. https://doi.org/10.3390/su17198550
Chicago/Turabian StyleZhou, Suying, Ying Xie, Hui Gao, Xiangxin Xue, Haofei Zhou, Mengge Dong, Xiaohui Sun, and Xiangsheng Chen. 2025. "Ammonia Nitrogen Removal and MAP Crystal Morphology Affected by Reaction Conditions in High-Concentration Wastewater" Sustainability 17, no. 19: 8550. https://doi.org/10.3390/su17198550
APA StyleZhou, S., Xie, Y., Gao, H., Xue, X., Zhou, H., Dong, M., Sun, X., & Chen, X. (2025). Ammonia Nitrogen Removal and MAP Crystal Morphology Affected by Reaction Conditions in High-Concentration Wastewater. Sustainability, 17(19), 8550. https://doi.org/10.3390/su17198550








