Microbiological Water Quality and Structural Determinants in Preschools of Matehuala, Mexico: Implications for Sustainability and Equity in Safe Water Access
Abstract
1. Introduction
2. Materials and Methods
2.1. Contextual Variables
2.2. Sample Selection
2.3. Water Sample Collection and Microbiological Analysis
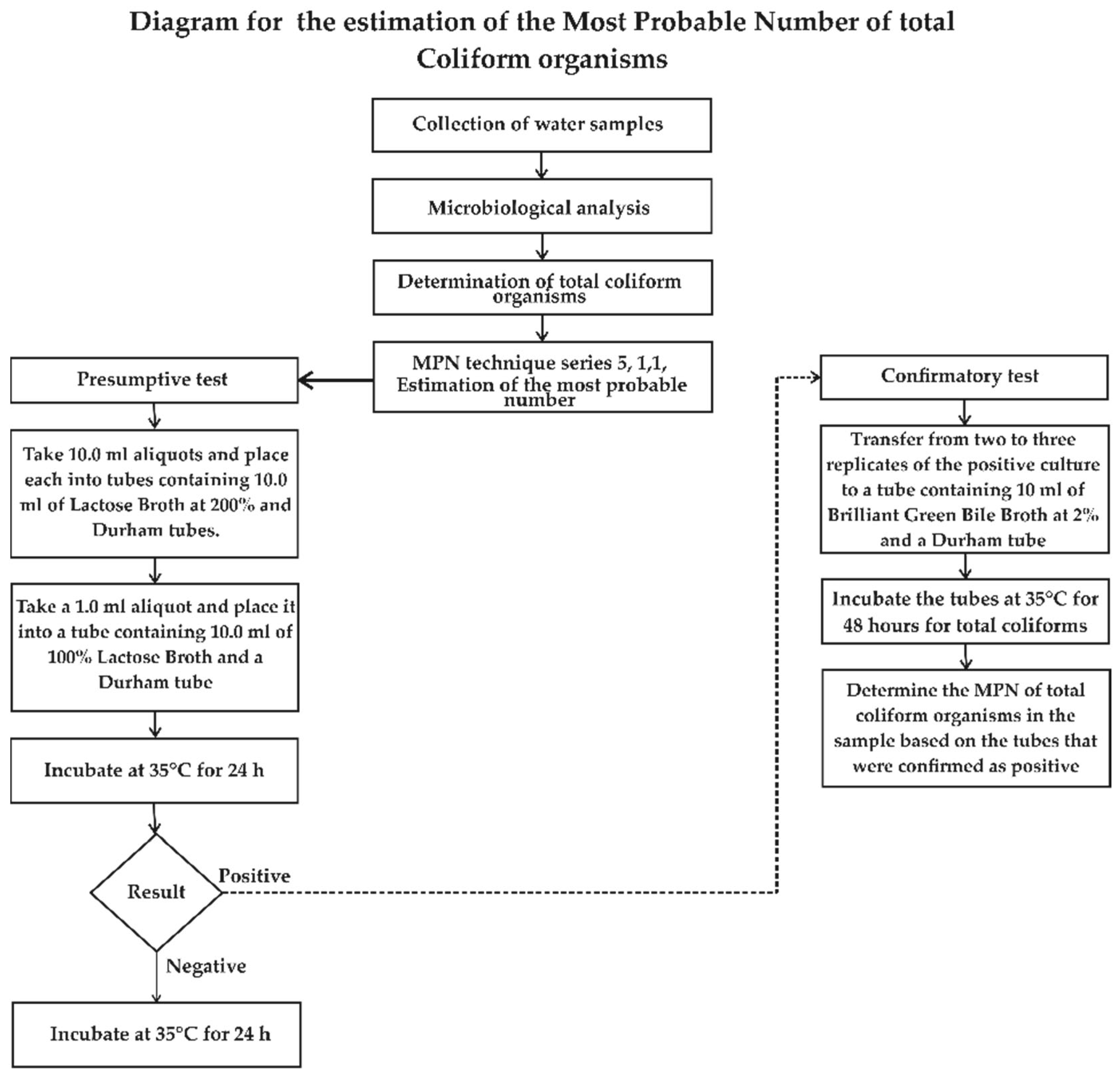
2.4. MPN 5-Tube, 1:1:1 Technique for Estimating the Most Probable Number of Total Coliforms
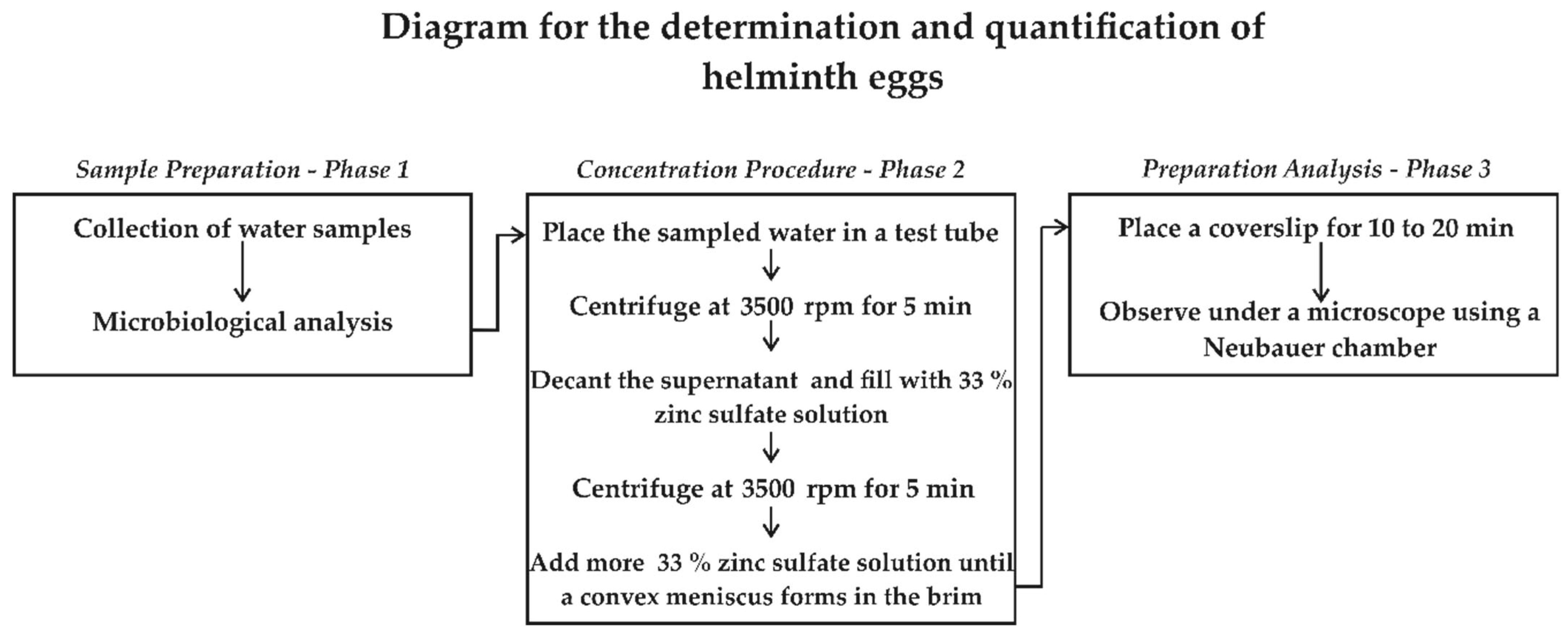
2.5. Technique for Quantification and Identification of Helminth Eggs
2.6. Isolation and Identification of Bacteria
2.7. Statistical Analysis
3. Results
3.1. Characterization of Water Access in Preschool Facilities
3.2. Microbiological Analysis of Available Water
3.3. Identification of Isolated Bacteria
3.4. Relationship Between Contextual Variables and Microbiological Contamination
4. Discussion
5. Conclusions
- The mode of water supply is a key predictor of microbiological risk in the school environment. 64.3% of rural schools relied on storage in tanks, and in 100% of these cases, total coliforms exceeded regulatory limits (up to >16,000 MPN/100 mL), while 35.7% showed fecal coliforms at levels up to 311 MPN/100 mL, classifiable as high or very high risk according to WHO guidelines. In contrast, none of the urban schools (all with piped water supply) exhibited microbiological indicators beyond the permissible limits, underscoring a critical structural disparity in child health protection.
- Qualitative analysis results confirm an association between water storage type and the presence of pathogenic bacteria. The exclusive detection of Escherichia spp., Klebsiella spp., Pseudomonas spp., and Proteus spp. in rural schools with tank water supply suggests that this storage system represents a significant exposure risk to microbiological agents, reinforcing the need to improve sanitary infrastructure conditions in rural areas.
- From a contextual perspective, schools testing positive for fecal coliforms also shared other structural characteristics: 100% stored water in tanks, 80% lacked sanitary drainage, 60% exhibited poor cleaning conditions of storage containers, and 80% of school staff perceived the water quality as “poor” (p < 0.05 for all associations). These conditions, beyond being statistically significant, reflect a sustained and systematically overlooked pattern of structural risk.
- Inferential analysis using non-parametric tests (Mann–Whitney U) demonstrated significant differences (p = 0.000) in total and fecal coliform levels across geographic zones and types of water supply, confirming that microbiological risk is not randomly distributed but determined by structural environmental conditions.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- WHO (World Health Organization). Guidelines for Drinking-Water Quality: Fourth Edition Incorporating the First Addendum; WHO Press: Geneva, Switzerland, 2017. [Google Scholar]
- Bain, R.; Cronk, R.; Wright, J.; Yang, H.; Slaymaker, T.; Bartram, J. Fecal contamination of drinking-water in low- and middle-income countries: A systematic review and meta-analysis. PLoS Med. 2014, 11, e1001644. [Google Scholar] [CrossRef]
- OMS Drinking-Water. World Health Organization. 2022. Available online: https://www.who.int/news-room/fact-sheets/detail/drinking-water (accessed on 15 May 2024).
- Morales, F.; La Paz, M.; Leon, M.; Rivero-Pino, F. Effects of Malnutrition on the Immune System and Infection and the Role of Nutritional Strategies Regarding Improvements in Children’s Health Status: A Literature Review. Nutrients 2023, 16, 1. [Google Scholar] [CrossRef]
- Chiabi, A.; Obadeyi, B.; Nguefack, F.; Chiabi, R.; Berinyuy, E.; Chiabi, E.; Mbang, T.; Obama, M. The Vicious Cycle of Malnutrition and Childhood Infections—What are the policy implications? Arch. Pediatr. Neonatol. 2018, 1, 21–25. [Google Scholar] [CrossRef]
- Ashour, F. Impact of Different Exposures, Including Environmental Enteropathies, on Gut Flora and Integrity. In The Biology of the First 1000 Days; CRC Press: Boca Raton, FL, USA, 2017; pp. 303–320. [Google Scholar] [CrossRef]
- Syed, S.; Ali, A.; Duggan, C. Environmental Enteric Dysfunction in Children. J. Pediatr. Gastroenterol. Nutr. 2016, 63, 6–14. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Acosta, A.M.; De Burga, R.R.; Chavez, C.B.; Flores, J.T.; Olotegui, M.P.; Pinedo, S.R.; Salas, M.S.; Trigoso, D.R.; Vasquez, A.O.; Ahmed, I.; et al. Early childhood cognitive development is affected by interactions among illness, diet, enteropathogens and the home environment: Findings from the MAL-ED birth cohort study. BMJ Glob. Health 2018, 11, e000752. [Google Scholar] [CrossRef]
- Andrade, M.; Barros, L.; Rodrigues, T.; De Lima Santos, M.; Lima, D. Microbiological evaluation of drinking water available in schools in Cruz das Almas, Brazil. Afr. J. Microbiol. Res. 2016, 10, 1759–1766. [Google Scholar] [CrossRef][Green Version]
- Rodriguez-Alvarez, M.; Weir, M.; Pope, J.; Seghezzo, L.; Rajal, V.; Salusso, M.; Moraña, L. Development of a relative risk model for drinking water regulation and design recommendations for a peri urban region of Argentina. Int. J. Hyg. Environ. Health 2015, 218, 627–638. [Google Scholar] [CrossRef]
- Iñiguez-Muñoz, L.E.; Anaya-Esparza, L.M.; Castañeda-Villanueva, A.A.; Martínez-Esquivias, F.; Carvajal-Hernández, M.; Méndez Robles, M.D. Calidad microbiológica del agua potable utilizada en escuelas públicas de la ciudad de Tepatitlán, Jalisco. Boletín Cienc. Agropecu. ICAP 2022, 8, 33–39. [Google Scholar] [CrossRef]
- INEGI. Censo de Población y Vivienda 2020: Servicios Básicos. Instituto Nacional de Estadística y Geografía. 2022. Available online: https://www.inegi.org.mx (accessed on 8 May 2024).
- UNICEF. Progress on Drinking Water, Sanitation and Hygiene in Schools: 2000–2023 Data Update. WHO/UNICEF Joint Monitoring Programme (JMP). 2023. Available online: https://washdata.org/ (accessed on 15 June 2025).
- Coswosk, É.; Neves-Silva, P.; Modena, C.; Heller, L. Having a toilet is not enough: The limitations in fulfilling the human rights to water and sanitation in a municipal school in Bahia, Brazil. BMC Public Health 2019, 19, 137. [Google Scholar] [CrossRef] [PubMed]
- Odiyo, J.; Mathoni, M.; Makungo, R. Health Risks and Potential Sources of Contamination of Groundwater Used by Public Schools in Vhuronga 1, Limpopo Province, South Africa. Int. J. Environ. Res. Public Health 2020, 17, 6912. [Google Scholar] [CrossRef] [PubMed]
- Shrestha, G.; Shrestha, M.; Byanju, R. Assessment of Bacterial Contamination in Drinking water of Schools of Tokha Municipality, Kathmandu. J. Environ. Sci. 2022, 8, 94–106. [Google Scholar] [CrossRef]
- Khalil, R.; Mahmoud, N. Assessment of drinking water quality at public schools at Jenin Directorate of Education, Palestine. Desalination Water Treat. 2022, 275, 196–206. [Google Scholar] [CrossRef]
- Morgan, C.; Bowling, J.; Bartram, J.; Kayser, G. Attributes of drinking water, sanitation, and hygiene associated with microbiological water quality of stored drinking water in rural schools in Mozambique and Uganda. Int. J. Hyg. Environ. Health 2021, 236, 113804. [Google Scholar] [CrossRef]
- Waideman, M.; Teixeira, V.; Uemura, E.; Stamford, T.; Leal, D.; Stangarlin-Fiori, L.; Ferreira, S.; Taconeli, C.; Beux, M. Enterococci used as complementary indicator of fecal contamination to assess water quality from public schools in the city of Curitiba, Paraná, Brazil. Braz. J. Food Technol. 2020, 23, e2019155. [Google Scholar] [CrossRef]
- Ahmed, J.; Wong, L.; Chua, Y.; Channa, N.; Mahar, R.; Yasmin, A.; Vanderslice, J.; Garn, J. Quantitative Microbial Risk Assessment of Drinking Water Quality to Predict the Risk of Waterborne Diseases in Primary-School Children. Int. J. Environ. Res. Public Health 2020, 17, 2774. [Google Scholar] [CrossRef]
- Speak, A.; Escobedo, F.; Russo, A.; Zerbe, S. Comparing convenience and probability sampling for urban ecology applications. J. Appl. Ecol. 2018, 55, 2332–2342. [Google Scholar] [CrossRef]
- NOM-230-SSA1-2002; Environmental Health. Sanitary Requirements for Public and Private Water Supply Systems, as Well as Requirements for Water Purification. Secretaría de Salud: Mexico City, Mexico, 2002. Available online: https://www.dof.gob.mx (accessed on 25 April 2024).
- Manga, M.; Balanji, E.; Nkhata, R. Storage tanks and water handling practices: A systematic review of their effects on household water quality. Environ. Syst. Res. 2021, 10, 18. [Google Scholar] [CrossRef]
- Fall, M.; Kane, C.; Niang, A. Multilevel analysis of access to drinking water in rural communes in the south of the Kaffrine region, Senegal. Discov. Water 2024, 4, 41. [Google Scholar] [CrossRef]
- Kouamé, P.K.; Galli, A.; Peter, M.; Loss, G.; Wassa, D.; Bonfoh, B.; Utzinger, J.; Winkler, M.S. Access to Water and Sanitation Infrastructures for Primary Schoolchildren in the South-Central Part of Côte d’Ivoire. Int. J. Environ. Res. Public Health 2021, 18, 8863. [Google Scholar] [CrossRef]
- NOM-127-SSA1-1994; Environmental Health. Water for Use and Human Consumption. Permissible Quality Limits and Treatments that Water Must Undergo for Its Purification. Secretaría de Salud: Mexico City, Mexico, 1994. Available online: https://www.dof.gob.mx (accessed on 1 October 2024).
- NOM-092-SSA1-1994; Goods and Services. Method for the Plate Count of Aerobic Bacteria. Secretaría de Salud: Mexico City, Mexico, 1995.
- MacFaddin, J.F. Biochemical Tests for Identification of Medical Bacteria, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- World Health Organization (WHO) and the United Nations Children’s Fund (UNICEF). Progress on Drinking Water, Sanitation and Hygiene: 2017 Update and SDG Baselines. 2017. Available online: https://data.unicef.org/resources/progress-drinking-water-sanitation-hygiene-2017-update-sdg-baselines/ (accessed on 21 August 2024).
- Fentie, M.; Abebe, T.; Alemayehu, E.; Mengesha, S.; Melaku, T. Determinant factors of microbial drinking water quality at the point of use in rural Ethiopia: A case study of the South Gondar Zone. Water 2024, 16, 1651. [Google Scholar] [CrossRef]
- Diagbouga, S.; Nadembega, C.; Zabre, H.; Kabore, A.; Tarnagda, G.; Angulo, P.; Sinare, L.; Cisse, G. Microbiological quality of schoolchildren’s drinking water in the rural communes of Coalla and Manni in the Eastern region of Burkina Faso. SOJ Microbiol. Infect. Dis. 2018, 6, 1–8. [Google Scholar] [CrossRef]
- Srećković, M.; Čapo, N.; Dugandžija, T.; Vujkovic, B.; Dragičević, I.; Damnjanović, B.; Đekić-Malbaša, J. Assessment of microbiological quality of drinking water and sanitary-hygienic conditions in rural primary schools of the Mačva District, Serbia. Glas. Javnog Zdr. 2024, 98, 280–295. [Google Scholar] [CrossRef]
- Nowicki, S.; O’Leary, C.; Mutua, A.; Kiiru, J.; Crump, J.A.; Egorov, A.I. The utility of Escherichia coli as a contamination indicator for rural drinking water: Evidence from whole-genome sequencing of isolates from Kitui County, Kenya. PLoS ONE 2021, 16, e0245910. [Google Scholar] [CrossRef]
- Salamandane, A.; Vila-Boa, F.; Malfeito-Ferreira, M.; Brito, L. High Fecal Contamination and High Levels of Antibiotic-Resistant Enterobacteriaceae in Water Consumed in the City of Maputo, Mozambique. Biology 2021, 10, 558. [Google Scholar] [CrossRef] [PubMed]
- Khanna, R.; Bhushan, K. Microbial safety and antibiotic resistance profiles of drinking water sources in Ludhiana, Punjab, India. Int. J. Environ. Health Res. 2025, 35, 1–15. [Google Scholar] [CrossRef]
- Otorkpa, O.J. Overview of major bacterial contaminants of drinking water in Nigeria: A review. Int. J. Pathog. Res. 2019, 2, 1–9. [Google Scholar] [CrossRef][Green Version]
- Judah, L.A.; Beckerman, A.P.; Rakotoarisoa, A.; Randriamanantena, D.; Brown, J. Occurrence and mitigation of bacterial regrowth in stored household water in eastern coastal Madagascar. Water 2024, 16, 1592. [Google Scholar] [CrossRef]
- Aw, T.; Scott, L.; Jordan, K.; Ra, K.; Ley, C.; Whelton, A. Prevalence of opportunistic pathogens in a school building plumbing during periods of low water use and a transition to normal use. Int. J. Hyg. Environ. Health 2022, 241, 113945. [Google Scholar] [CrossRef]
- Rubino, F.; Corona, Y.; Jiménez Pérez, J.G.; Smith, C. Bacterial contamination of drinking water in Guadalajara, Mexico. Int. J. Environ. Res. Public Health 2019, 16, 67. [Google Scholar] [CrossRef]
- Poague, K.; Blanford, J.; Martinez, J.; Anthonj, C. Water, sanitation and hygiene (WASH) in schools in Brazil pre-and peri-COVID-19 pandemic: Are schools making any progress? Int. J. Hyg. Environ. Health 2022, 247, 114069. [Google Scholar] [CrossRef]
- Colín Carreño, R.; Hernández, G.; López, M. Human health risk and quality assessment of spring water associated with fecal coliforms in rural Mexico. Water 2023, 15, 1863. [Google Scholar] [CrossRef]
- Prendergast, A.; Kelly, P. Enteropathies in the developing world: Neglected effects on global health. Am. J. Trop. Med. Hyg. 2012, 86, 756–763. [Google Scholar] [CrossRef] [PubMed]
- Tickell, K.D.; Atlas, H.E.; Walson, J.L. Environmental enteric dysfunction: A review of potential mechanisms, consequences and management strategies. BMC Med. 2019, 17, 181. [Google Scholar] [CrossRef]
- Petek, O.; Aydın, N. Evaluation of the Drinking Water Parameters in Primary and Secondary Schools Located in the Gaziantep Province Center. J. Water Health 2024, 22, 2423–2430. [Google Scholar] [CrossRef]
- Budge, S.; Parker, A.H.; Hutchings, P.T.; Garbutt, C. Environmental enteric dysfunction and child stunting. Nutr. Rev. 2019, 77, 240–253. [Google Scholar] [CrossRef]
- Gilmartin, A.A.; Petri, W.A. Exploring the role of environmental enteropathy in malnutrition, infant development, and oral vaccine response. Philos. Trans. R. Soc. B Biol. Sci. 2015, 370, 20140143. [Google Scholar] [CrossRef]
- Kabir, F.; Iqbal, J.; Jamil, Z.; Iqbal, N.; Mallawaarachchi, I.; Aziz, F.; Kalam, A.; Muneer, S.; Hotwani, A.; Ahmed, S.; et al. Impact of enteropathogens on faltering growth in a resource-limited setting. Front. Nutr. 2023, 9, 1081833. [Google Scholar] [CrossRef]
- Vonaesch, P.; Vonaesch, P.; Morien, E.; Andrianonimiadana, L.; Sanke, H.; Mbecko, J.; Huus, K.; Naharimanananirina, T.; Gondje, B.; Nigatoloum, S.; et al. Stunted childhood growth is associated with decompartmentalization of the gastrointestinal tract and overgrowth of oropharyngeal taxa. Proc. Natl. Acad. Sci. USA 2018, 115, E8489–E8498. [Google Scholar] [CrossRef] [PubMed]
- Khabo-Mmekoa, C.; Momba, M. The Impact of Social Disparities on Microbiological Quality of Drinking Water Supply in Ugu District Municipality of Kwazulu-Natal Province, South Africa. Int. J. Environ. Res. Public Health 2019, 16, 2972. [Google Scholar] [CrossRef]
- Naylor, C.; Lu, M.; Haque, R.; Mondal, D.; Buonomo, E.; Nayak, U.; Mychaleckyj, J.C.; Kirkpatrick, B.; Colgate, R.; Carmolli, M.; et al. Environmental enteropathy, oral vaccine failure and growth faltering in infants in Bangladesh. eBioMedicine 2015, 2, 1759–1766. [Google Scholar] [CrossRef]
- Lorntz, B.; Soares, A.M.; Moore, S.R.; Pinkerton, R.; Gansneder, B.; Bovbjerg, V.E.; Guyatt, H.; Lima, A.A.; Guerrant, R.L. Early childhood diarrhea predicts impaired school performance: A long-term prospective cohort study. Pediatr. Infect. Dis. J. 2006, 25, 513–520. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Wang, K.; Su, N.; Yuan, C.; Zhang, N.; Hu, X.; Fu, Y.; Zhao, F. Microbiota–gut–brain axis in health and neurological disease: Interactions between gut microbiota and the nervous system. J. Cell. Mol. Med. 2024, 28, e70099. [Google Scholar] [CrossRef]
- Interino, N.; Vitagliano, R.; D’Amico, F.; Lodi, R.; Porru, E.; Turroni, S.; Fiori, J. Microbiota–Gut–Brain Axis: Mass-Spectrometry-Based Metabolomics in the Study of Microbiome Mediators—Stress Relationship. Biomolecules 2025, 15, 243. [Google Scholar] [CrossRef]
- Sistema de Agua Potable, Alcantarillado y Saneamiento de Matehuala (SAPSAM). Informe Del Uso de Agua 2024. SAPSAM. Available online: http://sapsam-matehuala.com.mx/Informe%20Anual%202024.pdf (accessed on 21 January 2025).
- Ávila-Díaz, J.A.; Arciniega-Galaviz, M.A.; Moreno-Rentería, K.J.; Llanes-Cárdenas, O. Contaminación microbiológica en agua potable de localidades rurales en el municipio de Ahome, Sinaloa, México. Rev. Iberoam. Investig. Desarro. Educ. RIDE 2024, 15, e781. [Google Scholar] [CrossRef]
- Torres-Rivera, S.; Torres-Hernández, J.R.; Carranco-Lozada, S.E.; García-Arreola, M.E.; López-Doncel, R.A.; Montenegro-Ríos, J.A. Anthropogenic Contamination in the Free Aquifer of the San Luis Potosí Valley. Int. J. Environ. Res. Public Health 2023, 20, 6152. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Oviedo, A.; Monterrubio-Martínez, E.; Tuxpan-Vargas, J. Assessing the water contaminants in San Luis Potosi and its effects on its inhabitants: An interdisciplinary study on environmental contamination and public health. J. Hazard. Mater. 2024, 464, 132828. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Hernández, A.; Hernández-Martínez, J.L.; Meza-Figueroa, D.; Martínez-Villegas, N.; SenGupta, B. Arsenic transport from groundwater to soil solid phase in Matehuala (Mexico). In Arsenic Research and Global Sustainability; Sengupta, B., Ed.; CRC Press: Boca Raton, FL, USA, 2023; pp. 44–46. [Google Scholar] [CrossRef]


| Variable | Total (n = 32) | Urban (n = 18) | Rural (n = 14) |
|---|---|---|---|
| Average number of students (mean ± SD) * | 87.9 ± 53.1 | 133.9 ± 26.8 | 27.0 ± 8.6 |
| Average distance to municipal center (km) | - | - | 23.3 ± 14.8 |
| Access to piped water (%) | 71.9% (23/32) | 100% (18/18) | 35.7% (5/14) |
| Water supply via rooftop tank (%) | 28.1% (9/32) | 0% | 64.3% (9/14) |
| Presence of sanitary sewer system (%) | 71.9% (23/32) | 94.4% (17/18) | 35.7% (5/14) |
| School | Area | Water Supply Type | AMC (CFU/100 mL) | TC (MPN/100 mL) | FC (MPN/100 mL) | Helminth Eggs |
|---|---|---|---|---|---|---|
| PE19 | Rural | Rooftop tank | 5,000,000 | >16,000 | 311 | Absent |
| PE20 | Rural | Rooftop tank | 2,509,000 | >16,000 | 210 | Absent |
| PE21 | Rural | Piped | – | 12 | – | Absent |
| PE22 | Rural | Rooftop tank | 5,000,000 | >16,000 | 56 | Absent |
| PE23 | Rural | Piped | – | >16,000 | – | Absent |
| PE24 | Rural | Piped | – | >16,000 | – | Absent |
| PE25 | Rural | Rooftop tank | – | >16,000 | – | Absent |
| PE26 | Rural | Rooftop tank | 18,000 | >16,000 | – | Absent |
| PE27 | Rural | Rooftop tank | 18,000 | 16,000 | 14 | Absent |
| PE28 | Rural | Rooftop tank | 18,000 | 38 | – | Absent |
| PE29 | Rural | Rooftop tank | 18,000 | 12 | – | Absent |
| PE30 | Rural | Rooftop tank | 18,000 | >16,000 | 220 | Absent |
| PE31 | Rural | Piped | – | 4.4 | – | Absent |
| PE32 | Rural | Piped | – | 38 | – | Absent |
| PE01–PE18 | Urban | Piped | Not detectable | Not detectable | Not detectable | Absent |
| Bacterial Genus | No. of Strains Isolated | Sample Type (Rooftop Tank/Piped) | Geographic Area (Urban/Rural) | Relevant Observations |
|---|---|---|---|---|
| Escherichia spp. | 8 | Rooftop tank | Rural | Lactose +, indole +, motile |
| Enterobacter spp. | 5 | Rooftop tank | Rural | Lactose +, citrate + |
| Klebsiella spp. | 4 | Rooftop tank | Rural | Encapsulated, indole – |
| Citrobacter spp. | 3 | Rooftop tank | Rural | Variable lactose, citrate + |
| Proteus spp. | 3 | Rooftop tank | Rural | Lactose –, motility + |
| Pseudomonas spp. | 3 | Rooftop tank | Rural | Lactose –, oxidase + |
| Variable | Total (n = 32) | Urbana (n = 18) | Rural (n = 14) | p-Value |
|---|---|---|---|---|
| Water supply via rooftop tank (%) | 28.1% | 0% | 64.3% | <0.001 * |
| Presence of sanitary sewer system (%) | 71.9% | 94.4% | 35.7% | 0.008 * |
| Hydraulic installations in good condition (%) | 59.4% | 83.3% | 28.6% | 0.005 * |
| Proper rooftop tank cleaning (%) | 31.3% | N/A | 45.5% | - |
| Perception of water quality as “good” (%) | 65.6% | 88.8% | 28.6% | 0.004 * |
| Variable | Positive FC (n = 5) | Negative FC (n = 27) | p-Value |
|---|---|---|---|
| Use of rooftop tank (%) | 100% | 18.5% | 0.001 * |
| Absence of sanitary sewer system (%) | 80% | 22.2% | 0.009 * |
| Rooftop tank without proper cleaning (%) | 60% | 25.9% | 0.047 * |
| Perception of water as “poor” (%) | 80% | 18.5% | 0.011 * |
| Microorganism | Geographic Area (Urban vs. Rural) | Water Supply Type (Piped vs. Rooftop Tank) |
|---|---|---|
| Aerobic mesophilic bacteria (AMB) | p = 0.051 | p = 0.000 * |
| Total coliforms (TC) | p = 0.000 * | p = 0.000 * |
| Fecal coliforms (FC) | p = 0.000 * | p = 0.000 * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terrones-Gurrola, M.C.d.R.; Alvarez-Macias, H.A.; Compeán-Martinez, I.; Hernandez-Rangel, F.J.; Cruz-Alcantar, P. Microbiological Water Quality and Structural Determinants in Preschools of Matehuala, Mexico: Implications for Sustainability and Equity in Safe Water Access. Sustainability 2025, 17, 8488. https://doi.org/10.3390/su17188488
Terrones-Gurrola MCdR, Alvarez-Macias HA, Compeán-Martinez I, Hernandez-Rangel FJ, Cruz-Alcantar P. Microbiological Water Quality and Structural Determinants in Preschools of Matehuala, Mexico: Implications for Sustainability and Equity in Safe Water Access. Sustainability. 2025; 17(18):8488. https://doi.org/10.3390/su17188488
Chicago/Turabian StyleTerrones-Gurrola, María Cruz del Rocío, Héctor A. Alvarez-Macias, Isaac Compeán-Martinez, Francisco J. Hernandez-Rangel, and Pedro Cruz-Alcantar. 2025. "Microbiological Water Quality and Structural Determinants in Preschools of Matehuala, Mexico: Implications for Sustainability and Equity in Safe Water Access" Sustainability 17, no. 18: 8488. https://doi.org/10.3390/su17188488
APA StyleTerrones-Gurrola, M. C. d. R., Alvarez-Macias, H. A., Compeán-Martinez, I., Hernandez-Rangel, F. J., & Cruz-Alcantar, P. (2025). Microbiological Water Quality and Structural Determinants in Preschools of Matehuala, Mexico: Implications for Sustainability and Equity in Safe Water Access. Sustainability, 17(18), 8488. https://doi.org/10.3390/su17188488







